Abstract
V(D)J recombination at Igh and Igk loci takes place sequentially during successive stages in B cell development. Using 3-dimensional DNA fluorescence in situ hybridization (FISH), we identified a lineage- and stage-specific interchromosomal association between these two loci which marks the transition between Igh and Igk recombination. Co-localization occured between pericentromerically located alleles in pre-B cells, and was mediated by the 3’ Igk enhancer. Deletion of this regulatory element prevented association of the Igh and Igk loci, inhibited pericentromeric recruitment and locus decontraction of an Igh allele, and resulted in increased distal VH gene rearrangement. Our data indicate a role for the Igk locus and its 3’ enhancer in directing the Igh locus to a repressive nuclear subcompartment, and in rendering the Igh locus unable to contract.
Introduction
B and T cells express lineage-specific antigen receptors that mediate humoral and cellular immunity, respectively. Immunity depends upon the regulated recombination of variable (V), diversity (D), and joining (J) gene-segments at B cell receptor (BCR) and T cell receptor (TCR) loci to generate sufficient receptor diversity to enable recognition of a near-limitless array of potential antigens. Each antigen receptor consists of heavy and light chains, each of which are encoded by distinct loci. As common factors are required for V(D)J recombination at all immune receptor loci, developmentally regulated changes in locus accessibility are crucial for regulating this process 1.
Regulation of accessibility is exerted at a number of levels to ensure lineage specificity and sequential rearrangement of gene-segments at immunoglobulin heavy chain (Igh) and light chain (Igk and Igl) loci 2. Tight regulation is also essential for protecting the genome from these rearrangement events, which require generation of inherently unstable and potentially dangerous double stranded DNA breaks. Allelic exclusion, the process through which successful production of one Ig chain suppresses further rearrangement at the other allele of the same Ig locus, forms an integral part of this regulation and ensures clonality and monospecific recognition by the B cell receptor on individual lymphocytes—a basic tenet of the acquired immune response.
Recombination starts at the pro-B cell stage with D-J rearrangement of both Igh alleles. Synapse formation of gene segments separated by a large distance is facilitated by looping which results in locus contraction in cells undergoing rearrangement 3,4. Functional V-D-J rearrangement at one Igh allele leads to expression of immunoglobulin µ-chain as part of the pre-B cell receptor (pre-BCR). Signaling through this receptor enforces cessation of further Igh rearrangement, and triggers a burst of proliferation of large pre-B cells which subsequently differentiate into small pre-B cells in which Igk rearrangement takes place 5. Locus contraction mediated by looping occurs prior to the onset of Igk germline transcription 6. Igk rearrangement occurs after the onset of transcription of the unrearranged cluster of J gene-segments and depends upon well-characterized enhancers located in the J-C intron (MiEκ) and 3’ of the constant region exon (3’Eκ). Deletion of the two enhancers, individually or simultaneously, diminishes or abrogates V-J Igk rearrangement, respectively 7–9.
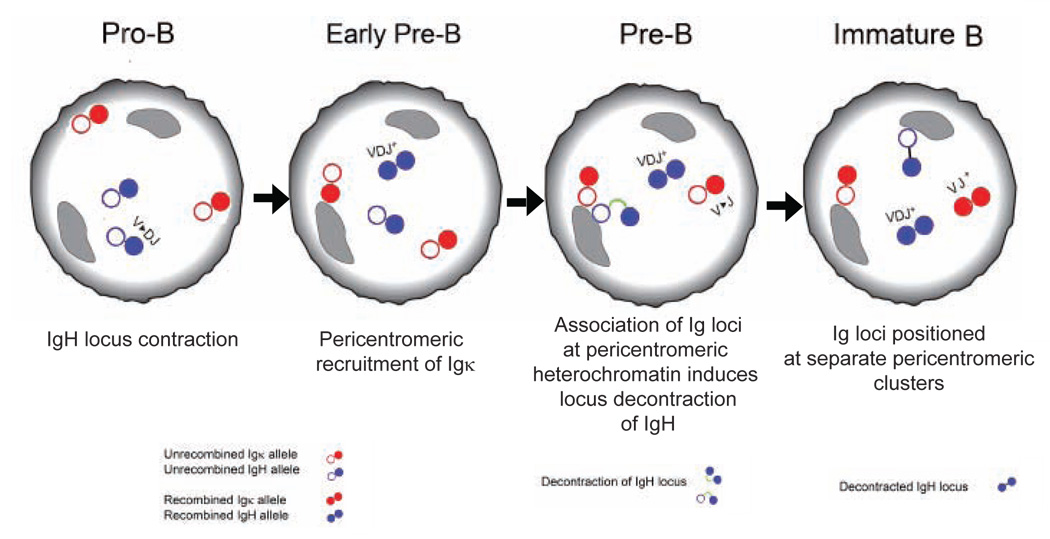
Allelic exclusion at the Igh locus, established at the pre-B cell stage of development by changes in chromatin accessibility 10, is thought to be crucial for preventing ongoing rearrangement of the second partially assembled (DJ-rearranged) Igh allele when the V(D)J recombinase is re-expressed for the purpose of Igk rearrangement. Accumulating evidence supports a “feedback inhibition” model for establishing allelic exclusion of the Igh locus but the detailed molecular basis of this model has yet to be defined 2. However, we know that pericentromeric recruitment plays a role in establishing and maintaining allelic exclusion of all Ig loci 4,11,12. Following successful recombination of one Igh allele, repositioning of the second allele to pericentromeric heterochromatin, a repressive compartment of the nucleus, reduces Igh accessibility to the recombinase during Igk rearrangement 4. In contrast, repositioning of the Igk allele to pericentromeric clusters occurs at the pre-B cell stage, prior to the onset of Igk rearrangement, and may limit recombinase accessibility to a single (euchromatic) allele 12. In addition, decontraction of the Igh locus occurs at the same developmental stage. This process contributes to allelic exclusion by physically separating distal and middle VH gene segments from the proximal D-J domain of the locus, thereby preventing further synapse formation and ongoing rearrangement between these regions 4.
Recruitment of the not-yet-rearranged Igk allele and the partially rearranged Igh allele to pericentromeric heterochromatin, and decontraction of the partially rearranged Igh allele occur at the same developmental stage, suggesting the existence of a coordinated event. This prompted us to examine the locations of these two loci relative to each other and to further investigate the factors required for changes in conformation that occur at the Igh locus during B cell development.
Results
Interchromosomal association between Ig loci
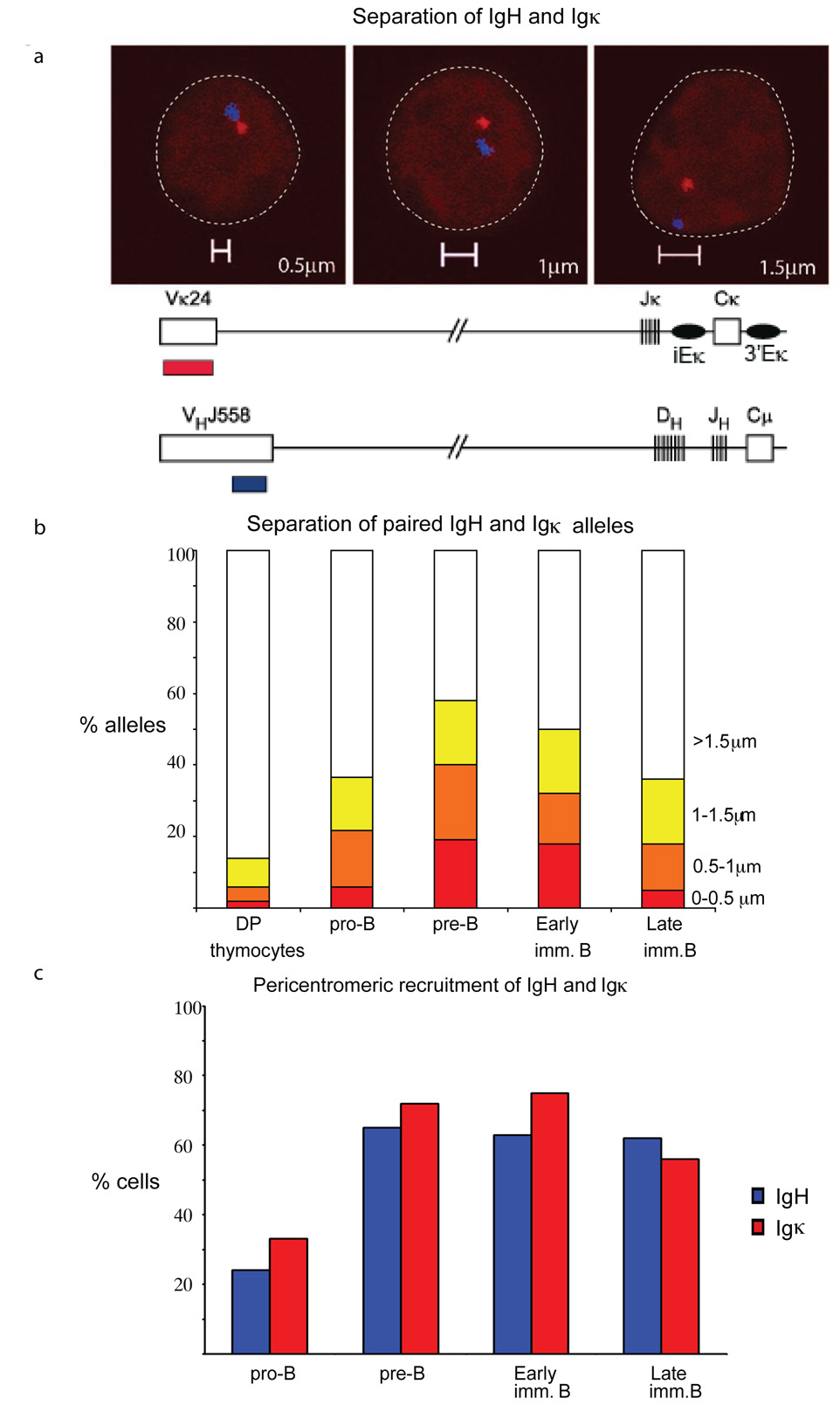
To examine the positions of the Igh and Igk loci, we performed two-color 3-dimensional DNA fluorescence in situ hybridization (FISH) using DNA probes that were generated from two bacterial artificial chromosomes (BACs)--CT7-526A21 and RP23-101G13--which map to the 5’ end of the Igh locus on chromosome 12 and the 5’ end of the Igk locus on chromosome 6, respectively. In each cell, the two alleles of both loci were either well separated, or one Igh and one Igk allele were found in close spatial proximity. Measurements of the distance separating the two loci were grouped into one of the following four categories: <0.5µm apart, 0.5–1µm apart, 1–1.5µm apart and >1.5µm apart (Fig. 1a). Our measurements relate to the distance separating the pair of co-localized alleles in each cell. Where no close association between Igh and Igk alleles was observed in an individual cell (>1.5µm apart), this was scored as separation of a single pair of alleles. We also used probes mapping to the Igh and Igk constant regions to rule out the possibility that deletion of distal VH gene regions affected observations of the frequency of association of the two loci (Supplementary Figure 1, online). Using different subsets of B cells sorted from mouse bone marrow, we examined the positions of the two loci relative to pericentromeric heterochromatin as described previously 4. Interchromosomal association occured at low frequency in pro-B cells, but increased and peaked during the pre-B cell stage--as evidenced by the substantial increase in loci separated by <0.5µm--and declined during the late immature B cell stage (Fig. 1b, Supp. Table 1, online. Co-localization was lineage specific as we observed no significant Igh-Igk pairing in CD4+CD8+ thymocytes (Fig. 1b). In addition, the Igh-Igk association coincided temporally with pericentromeric recruitment of Igh and Igk (Fig. 1c, Supp Tables 2a, b, online).
Figure 1.
Interchromosomal association between one Igh and one Igk allele coincides with pericentromeric recruitment of the two loci. (a) Top, confocal sections showing distances (0.5µm, 1µm and 1.5µm) separating the 5’ ends of the Igh and Igk alleles. Bottom, schematic representation of the position of probes used for detecting Igh and Igk. (b) Distance (in µm) separating the 5’ ends of the Igh and Igk loci was evaluated in indicated subsets of sorted bone marrow cells and thymocytes. The percentage and sample sizes are shown in Supplementary Table 1, online. Cells in which no close association between Igh and Igk alleles was observed (>1.5µm) were scored as separation of a single pair of alleles. (c) Pericentromeric location of Igh and Igk alleles during B cell development. Cells at the indicated developmental stages were analyzed by three-colour3-D DNA FISH with the Igk probes RP23-101G13 (Vκ24) and RP24-387E13 (Igk constant region) in combination with a γ-satellite probe and separately with the Igh probes CT7-526A21 (VHJ558) and CT7-34H6 (CH) in combination with a γ-satellite probe. The data represent the percentage of cells showing monoallelic association of Igh (blue bars) and Igk (red bars) with pericentromeric clusters. The percentages and sample sizes of this data are shown in Supplementary Table 2, online. Data are representative of three experiments.
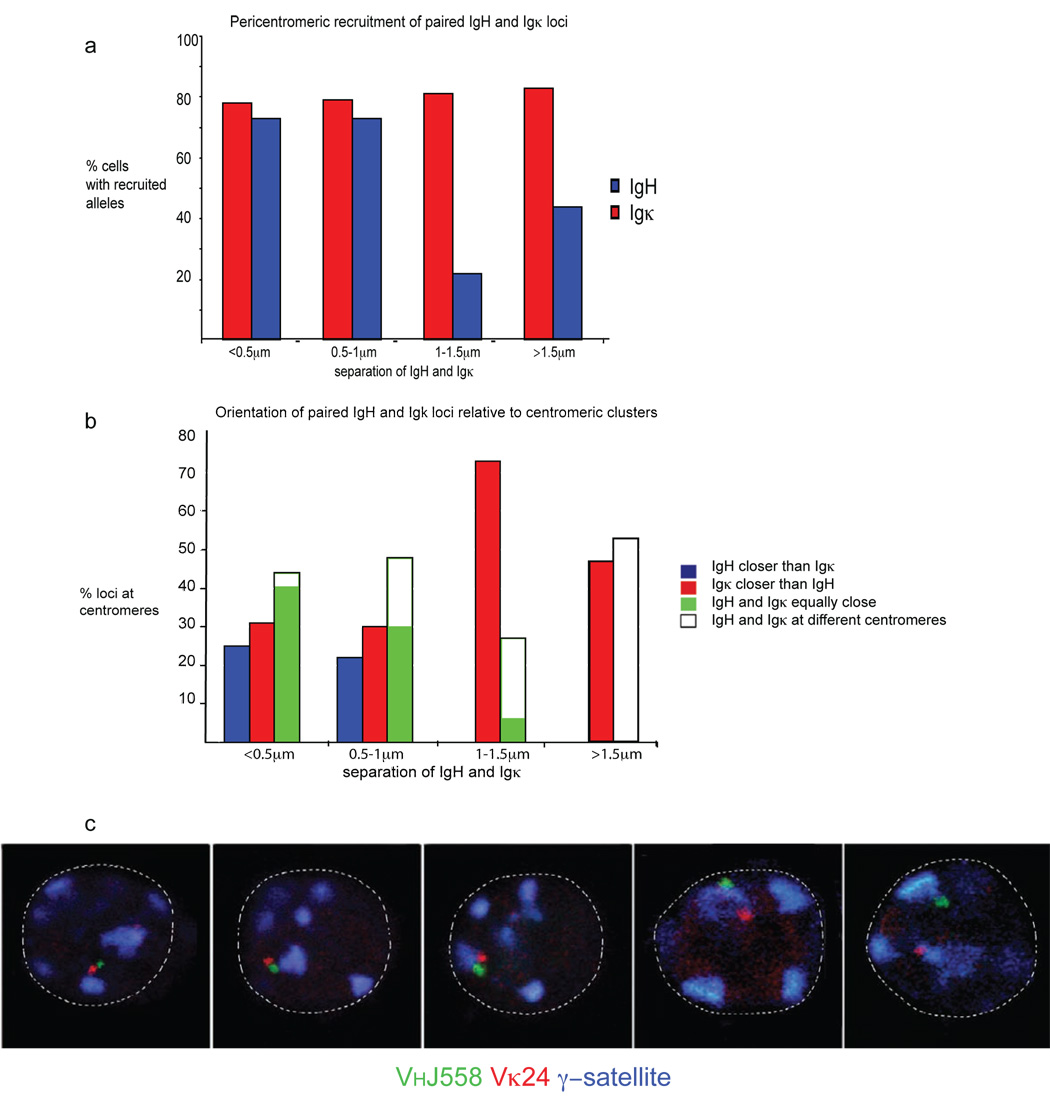
Figure 2.
Association between Igh and Igk occurs on the pericentromerically recruited allele. (a) Pre-B cells were analyzed by three-color 3-dimensional DNA FISH with the Igk probe described in Fig. 1. The data represent the localization Igh and Igk alleles at different distances apart (<0.5µm, 0.5–1µm, 1–1.5µm and >1.5µm) relative to pericentromeric heterochromatin. The percentage and sample sizes are shown in Supplementary Table 3, online. (b) Evaluation of the orientation of Igh and Igk alleles at different distances apart (.0.5, 0.5–1, 1–1.5 and <1.5µm) relative to pericentromeric heterochromatin. The percentage and sample sizes are shown in Supplementary Table 4, online. (c) Confocal sections demonstrating different facets of interacting loci relative to pericentromeric heterochromatin. Data are representative of three experiments.
Association on pericentromerically-recruited alleles
Co-localization between Igh and Igk occurred on one pair of alleles within each cell at a stage in development during which one allele from each locus is often repositioned at pericentromeric heterochromatin. This led us ask which alleles participated in the interchromosomal association. To address this question we carried out three-color 3-dimensional DNA FISH with Igh and Igk probes, in addition to a γ-satellite probe mapping to pericentromeric heterochromatin, in sorted pre-B cells. The most closely associated Igh and Igk alleles were positioned in heterochromatic regions (Fig. 2, Supp. Table 3, online). Where paired recruited alleles were separated by >1µm, the Igk allele frequently co-localized with pericentromeric heterochromatin and was scored as recruited to heterochromatic regions, whereas the Igh allele was positioned at a distance from the pericentromeric cluster and was not scored as recruited to heterochromatic regions (Fig. 2a, Supp. Table 3, online). This suggests that the two Ig loci do not simultaneously go to pericentromeric clusters, but that instead recruitment of Igk might precede that of Igh. To analyze this hypothesis in greater detail we compared the distance between the two loci with the orientation of Igh and Igk alleles in pericentromeric regions. When the two loci were separated by less than 1µm, either Igh (blue bars) or Igk (red bars) was positioned closer to pericentromeric heterochromatin but more often than not they were both equally close to the same pericentromeric cluster (green bars) or equally close to a different pericentromeric cluster (white bars) (Fig. 2b, c, Supp. Table 4, online). Where Ig loci were separated by >1.5µm all cells had either one Igk allele alone at pericentromeric heterochromatin (red bars) or both Igh and Igk at different pericentromeric clusters (white bars). Igh was positioned at heterochromatic regions only when it associated with Igk at the same pericentromeric cluster (blue and white bars) or when both Ig loci were recruited to different pericentromeric clusters (white bars). Igh was never seen at heterochromatic regions in pre-B cells in the absence of Igk recruitment. These observations suggest that pericentromeric recruitment of Igk was the initial event. The frequency of association declined at the immature B cell stage of development, when Igh and Igk were predominantly positioned at different pericentromeric clusters (Fig. 1b, c). These findings suggest that association takes place between the two loci at a shared pericentromeric cluster following recruitment of one Igk allele, and that the two loci subsequently move apart to reposition at different pericentromeric clusters. Thus pericentromeric recruitment of Igk may promote pericentromeric recruitment of Igh, perhaps via a transient association between the two loci. Thus, although Ig alleles may be constrained to a repressive subcompartment of the nucleus they do not necessarily remain static and can move from one pericentromeric cluster to another.
Sequential recruitment to pericentromeric regions
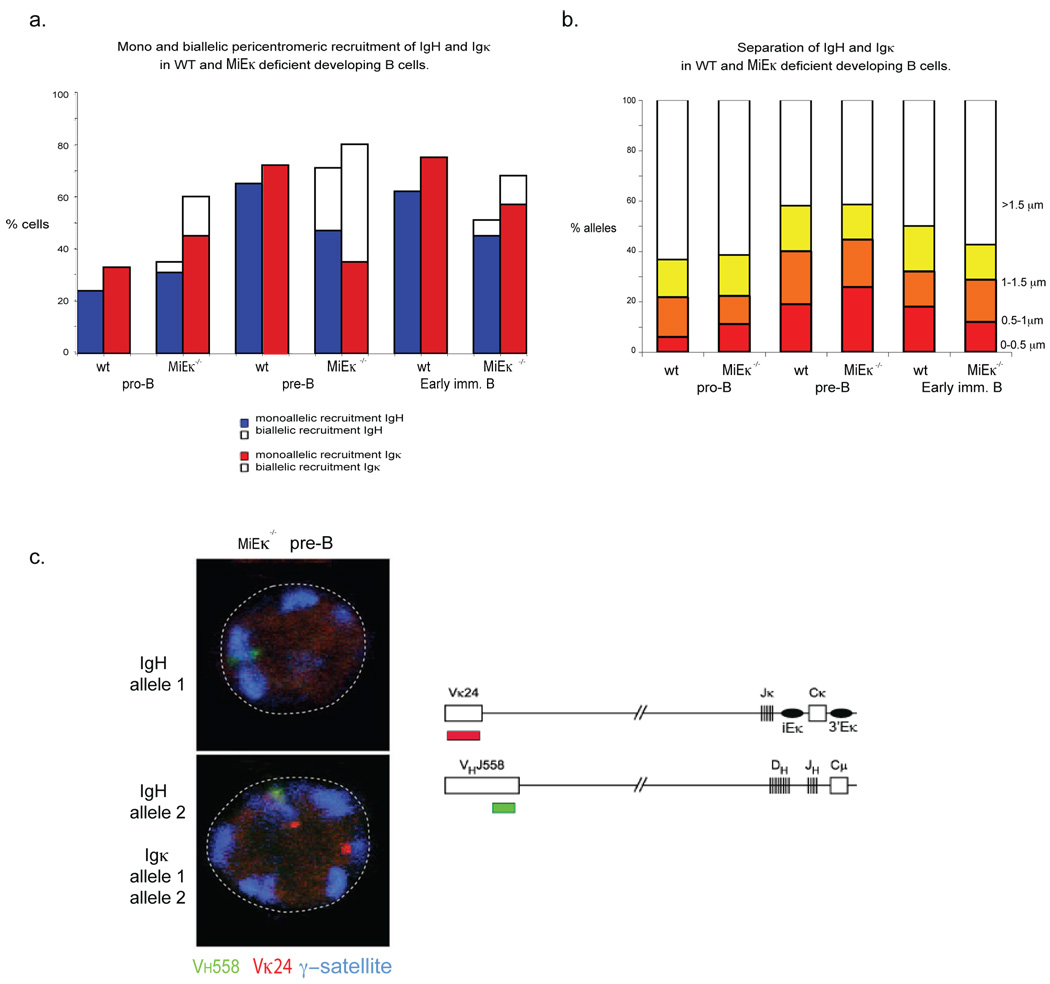
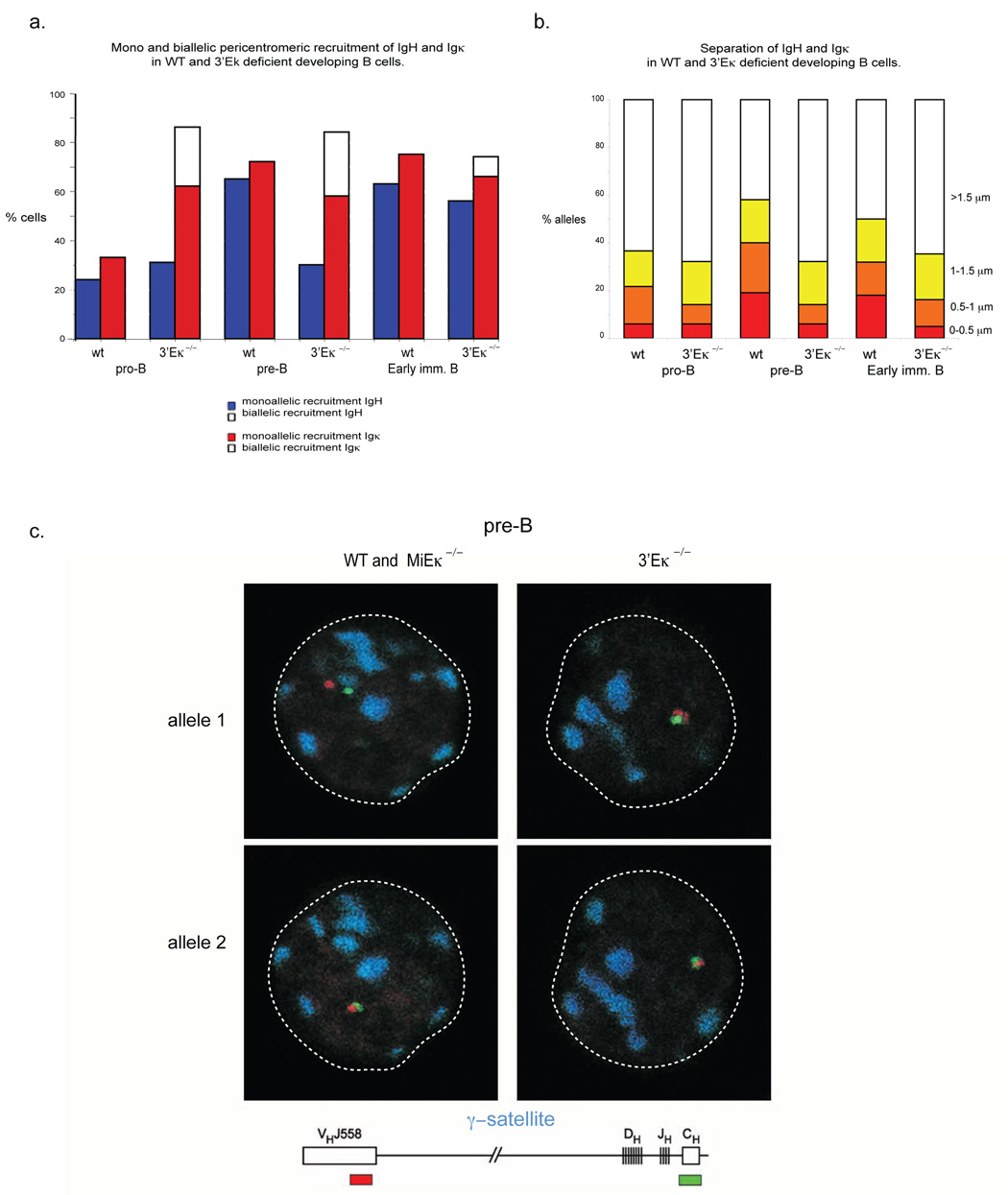
To further test the idea that the pericentromeric Igk allele is involved in recruitment of an Igh allele to pericentromeric heterochromatin, we examined nuclear positioning in mice with targeted deletions of MiEκ or 3’Eκ. Previous analyses of pre-B cells from these mice demonstrated a significant reduction in Vκ and Jκ germline transcription and in Vκ-Jκ recombination 7,9. 3-dimensional DNA FISH analysis of sorted developing B cell subsets from MiEκ-KO mice revealed that pericentromeric recruitment of Igk occurred prematurely at the pro-B cell stage of development and, at the subsequent pre-B cell stage, nearly half the cells exhibited an atypical appearance with both Igk alleles located at pericentromeric heterochromatin (Fig. 3a). These results support previous data showing that the absence of the MiEκ leads to silencing of Igk transcription 9. Interestingly we noted no defect in Igk locus contraction in MiEκ-KO pre-B cells (Supp. Table 5, online). As a test of our model, we determined whether increased pericentromeric recruitment of Igk alleles in these mutants led to an increase in co-localization with Igh and an increased frequency of Igh recruitment to heterochromatin. In pre-B cells from MiEκ-KO mice, close association between the Igh and Igk loci (<0.5µm) increased slightly (Fig. 3b, Supp. Table 1, online) and this change correlated with the unusual appearance of a percentage of pre-B cells in which both IgH alleles were repositioned at pericentromeric heterochromatin (Fig. 3a, Supp. Table 2, online). This data further suggests that recruitment of Igk influences recruitment of Igh to pericentromeric heterochromatin. To verify this conclusion, we examined the cells in which both Igh alleles were repositioned at pericentromeric clusters to determine whether biallelic recruitment of Igh was facilitated by biallelic recruitment of Igk. We used three-color 3-dimensional DNA FISH to examine the position of the two loci in relation to pericentromeric heterochromatin. Analysis of the small number of cells in which both Igh alleles are located at pericentromeric heterochromatin revealed that, in 20/24 cells, both Igh and Igk were biallelically recruited to pericentromeric heterochromatin (Fig. 3c).
Figure 3.
Increased pericentromeric recruitment of Igk promotes an increased frequency of interchromosomal association between Igh and Igkκ and increased pericentromeric recruitment of Igh dependent on 3’Eκ. (a) Pericentromeric location of Igh and Igk alleles. Cells sorted from WT and MiEκ-KO bone marrow at the indicated developmental stages were analyzed by three-color 3-dimensional DNA FISH as described for Fig. 1. The percentages and sample sizes of this data are shown in Supplementary Table 2, online.(b) The distance (in µm) separating the 5’ ends of the Igh and Igk loci was evaluated in pro-B, pre-B, IgMlow (early immature B) and IgMhigh (late immature B) cell subsets of sorted bone marrow cells from WT and MiEκ-KO mice. No IgMhigh cells were present in the mutant mice. The percentage and sample sizes are shown in Supplementary Table 1, online. (c) Top, confocal sections showing biallelically recruited Igh and Igk in pre-B cells from MiEκ-KO mice Bottom, schematic representation of the position of probes used for detecting Igh and Igk. Data are representative of three experiments.
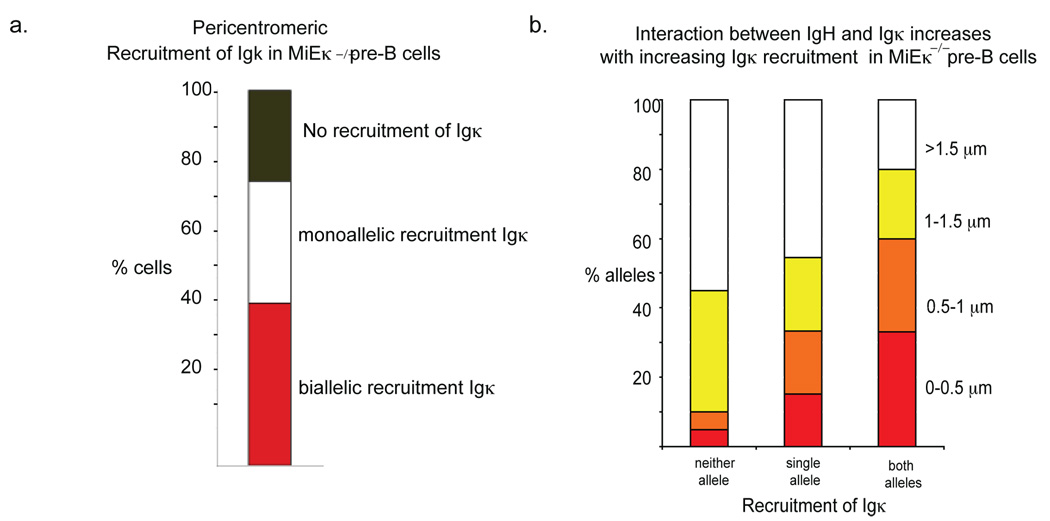
As the observed increase in frequency of closely associating (<0.5µm) alleles in MiEκ-KO pre-B cells was quite subtle, to definitively assess the affect of Igk pericentromeric recruitment on the association between the two loci we separately analyzed the frequency of association between Igh and Igk loci in the following populations from MiEκ-KO pre-B cells: (i) cells in which neither Igk allele was recruited; (ii) cells in which a single Igk allele was recruited; (iii) cells in which Igk was biallelically recruited (Fig. 4a). Igh-Igk loci association clearly increased with increased Igk recruitment to pericentromeric heterochromatin, and we detected negligible close association in the absence of Igk localization in pericentromeric regions (Fig. 4b, Supp. Table 6, online). Together these data indicate that pericentromeric localization of Igk promotes Igk-Igh association and Igh recruitment to pericentromeric heterochromatin
Figure 4.
Association of Igh and Igk increases with increasing Igk recruitment to pericentromeric regions. (a) Percentage of pre-B cells with monoallelically and biallelically pericentromeric Igk alleles, and cells where neither Igk allele is pericentromeric, in MiEκ-KO mice. The pre-B cells analyzed here were from a different batch of mice as those analyzed in the experiments shown in Fig. 3. (b) Analysis of Igh and Igk association in cells with monoallelically and biallelically pericentromeric Igk alleles, and in cells where neither Igk allele is pericentromeric. The percentage and sample sizes are shown in Supplementary Table 5, online. Data are representative of three experiments.
3’Eκ is required for Igh-Igk close association
Our analysis of allelic association and pericentromeric recruitment of Igh in 3’Eκ-KO progenitor B cells provided a strikingly different result. Igk loci in these cells behaved in a manner similar to the Igk loci in MiEκ-KO developing B cells--in both cell types Igk was prematurely repositioned to pericentromeric clusters at the pro-B cell stage of development, and at this and later stages many cells had both alleles located within this repressive compartment (Fig. 5a). As in MiEκ-KO pre-B cells we observed no defect in Igk locus contraction in 3’Eκ-KO pre-B cells (Supp. Table 5, online). As the absence of either enhancer had a similar effect on Igk locus localization, we expected to find that deletion of 3’Eκ would increase the frequency of association between Igh and Igk loci and increase pericentromeric recruitment of the Igh locus. However, we observed just the opposite (Fig. 5a–c, Supp. Tables 1, 2, online). The extent of association between the two loci did not increase beyond the pro-B cell stage and the Igh locus remained positioned away from pericentromeric clusters at the pre-B cell stage, and was not repositioned to pericentromeric heterochromatin until the early immature B cell stage. Furthermore, pericentromeric recruitment of Igh at the immature B cell stage was not linked with an interchromosomal association, as the two loci were positioned at different pericentromeric clusters in immature B cells. We repeated these experiments using Igh and Igk constant region probes to confirm that deletion of distal V gene regions did not affect the frequency of association of the two loci (Supp. Fig. 1, online). These results indicated that the 3’Eκ is important for the association between the Igh and Igk loci and that in the absence of Igh-Igk association recruitment of the Igh locus to heterochromatin is delayed. This observation suggested that the Igh locus in 3’Eκ-KO mice might remain accessible to the V(D)J recombinase during the pre-B cell stage of development.
Figure 5.
3’Eκ mediates association of Igh and Igk, and Igh pericentromeric recruitment at the pre-B cell stage in development. (a) Cells sorted from WT and 3’Eκ-KO bone marrow at the indicated developmental stages were analyzed by three-color 3-dimensional DNA FISH as described for Fig. 1. The percentages and sample sizes of this data are shown in Supplementary Table 2, online. (b) The distance (in µm) separating the 5’ ends of the Igh and Igk loci in pro- and pre-B cell subsets of sorted bone marrow cells from WT and 3’Eκ-KO mice. The percentage and sample sizes are shown in Supplementary Table 1, online. (c) Confocal sections showing the location of Igh alleles relative to pericentromeric clusters in pre-B cells from WT, MiEκ-KO and 3’Eκ- KO mice. The Igh probes used were CT7-526A21 (VHJ558) and CT7-34H6 (CH) in combination with a γ-satellite probe. Individual alleles from the same cell are shown in different confocal sections. Data are representative of three experiments.
Igh decontraction mediated by association with Igk
Locus contraction, mediated by looping, is required for rearrangement of distal and middle VH gene segments to DJH segments, and subsequent decontraction at the pre-B cell stage of development is likely to contribute to allelic exclusion by preventing ongoing rearrangement of these VH gene families 4,13. Decontraction and pericentromeric recruitment occur at the pre-B cell stage of development and to date observations suggest that these two processes occur simultaneously and may be linked 4. As pericentromeric recruitment of Igh is delayed and occurs in early immature B cells of 3’Eκ-KO mice, independently of Igk association, we next asked whether Igh locus decontraction was also affected by the absence of the 3’Eκ. To address this question we measured the separation of the two ends of the Igh locus in sorted developing B cells from WT, MEiκ-KO and 3’Eκ-KO mice. Two-color 3-dimensional DNA FISH was carried out using the Igh probes CT7-526A21 and CT7-34H6 which map to the 5’ end of the Igh locus and the 3’ Igh constant region, respectively (Fig. 6, Supp. Table 7, online). Separation of the two ends of the Igh locus was grouped into the following three categories: 0–0.5µm, 0.5–1µm and 1–1.5µm. Decontraction occurred in developing B cells from MEiκ-KO mice, in which Igh-Igk association and pericentric repositioning of Igh were documented. In contrast, locus decontraction was impaired in 3’Eκ-KO cells, in which Igh-Igk association was impaired and no significant pericentric recruitment of Igh at the pre-B cell stage of development was observed. Furthermore Igh locus decontraction did not occur when Igh was repositioned to pericentromeric regions independent of Igk association in 3’Eκ-KO immature B cells. These results suggest that Igh locus decontraction depends on both repositioning to pericentromeric regions and association with Igk.
Figure 6.
Association of Igh and Igk mediates Igh locus decontraction. The distance (in µm) separating the VHJ5558 and CH gene segments in cells sorted from WT, MiEκ-KO and 3’Eκ-KO bone marrow at the indicated developmental stages were analyzed by two-color 3-dimensional DNA FISH. The Igh probes used were CT7-526A21 (VHJ558) and CT7-34H6 (CH). Data are representative of three experiments.
Enhanced distal VH segment usage in 3’Eκ-KO cells
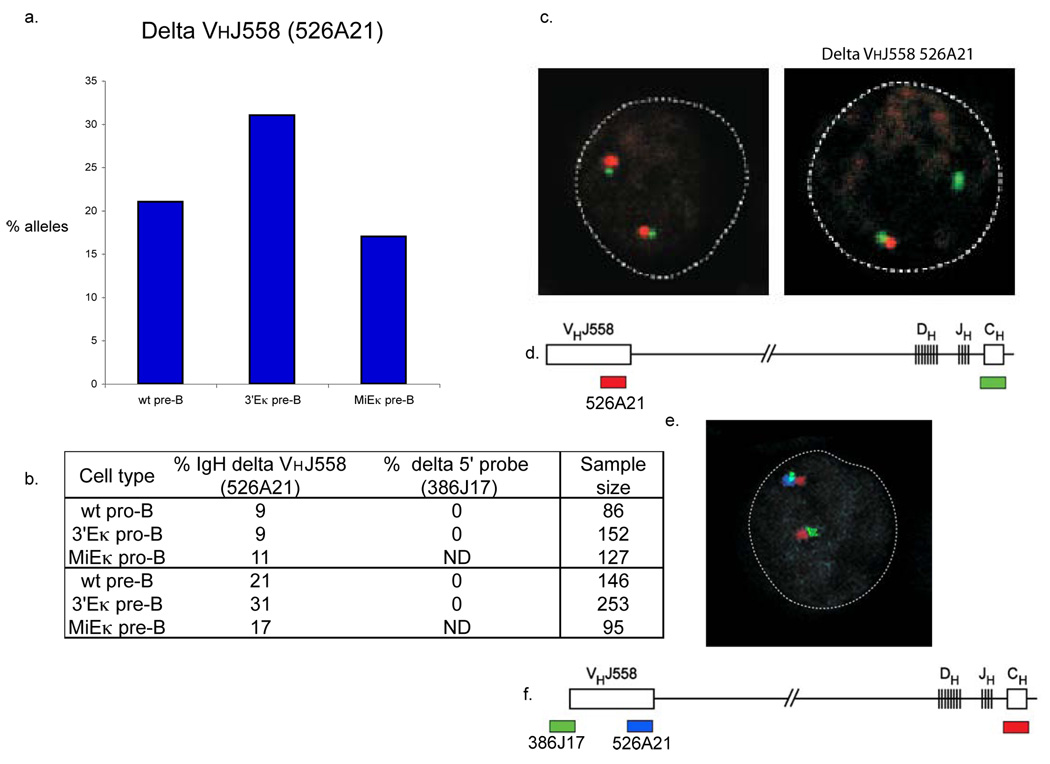
In our FISH analyses of WT, 3’Eκ-KO and MiEκ-KO developing B cells we used the probe CT7-526A21, which hybridizes to the 3’ end of the VHJ558 genes. Deletion of this BAC signal will occur upon rearrangement of VH genes located 5’ of this region. We observed such deletion in approximately 9% of WT and 3’Eκ-KO pro-B cells and 11% of MiEκ-KO pro-B cells. Deletion increased in WT and MiEκ-KO pre-B cells to 21% and 17%, respectively, and in 3’Eκ-KO pre-B cells to 31% (Fig. 7a, b and Table 1.) As a control, in addition to the constant region probe, CT7-34H6, we used a probe located outside of the 5’ end of the Igh locus, CT7-386J17. Signals from these two probes were always present even in the absence of the CT7-526A21 signal (Fig. 7c). These data indicate that distal VH gene rearrangement was increased in pre-B cells from 3’Eκ-KO mice where both Igh alleles remained in a contract conformation and were positioned within euchromatic regions. This type of rearrangement would not be expected if the locus had undergone decontraction 4.
Figure 7.
Deletion of the VHJ558 CT7-526A1 probe occurs more frequently in 3’Eκ-KO than in WT or MiEκ-KO pre-B cells. 3-dimensional DNA FISH was performed using the Igh probes CT7-526A21 (VHJ558) and CT7-34H6 (CH) in combination with a probe located outside of the 5’region of the Igh locus, RP24-386J17. (a) Graph showing frequency of deletion of VHJ558 526A1 in WT, 3’E-KO and MiEκ-KO pre-B cells. Table 1. showing frequency of deletion of the VHJ558 CT7-526A21 and RP24-386J17 signal in WT, 3’E κ−/− and MiE κ−/− pro-B and pre-B cells. (b) Top, confocal sections showing the presence of both CT7-526A21 (VHJ558) and CT7-34H6 (CH) signals on both Igh alleles (left) and in a different nucleus, the absence of the VHJ558 CT7-526A21 signal on one allele (right). Bottom, position and colors of the probes used. (e). Top, confocal section showing the presence of CT7-526A21 (VHJ558), CT7-34H6 (CH) and RP24-386J17 signals on one Igh allele and the absence of the VHJ558 CT7-526A21 signal on the other allele. Bottom, position and colors of the probes used. Data are representative of three experiments.
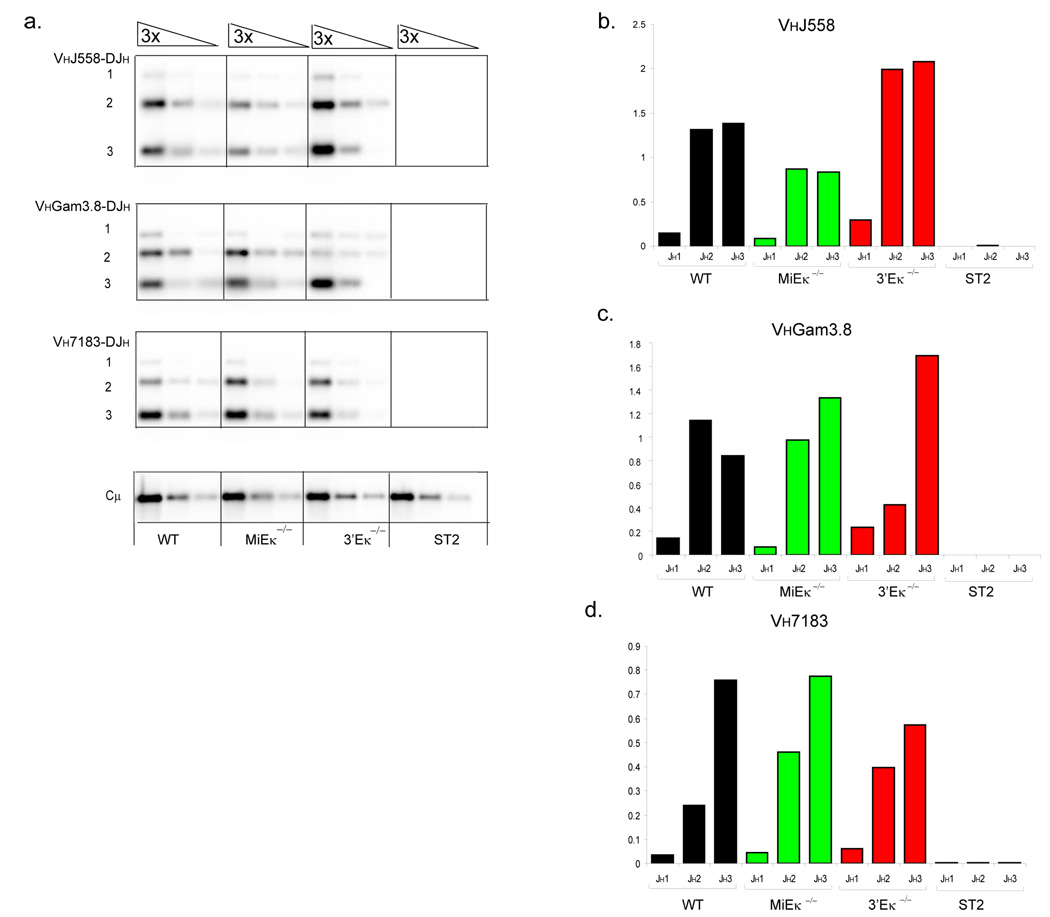
In order to verify that the differences in rearrangement at the pre-B cell stage are seen in peripheral B cells, we analyzed the rearrangement status of the Igh locus in mature resting splenic B cells. PCR analyses and Southern blotting was performed to assess the relative amounts of proximal versus distal VH- DJH rearrangements in 3’Eκ-KO compared to WT and MiEκ-KO mice. Distal Igh rearrangements were increased in 3’Eκ-KO splenic B cells compared to WT splenic B cells (Fig. 8a). Quantification of VHJ558 rearrangements normalized to Cµ, which was used as a PCR control, demonstrated that distal VH rearrangement to the JH3 gene segment are increased by 50% (or 1.5-fold) in the absence of 3’Eκ (Fig. 8b). Rearrangement of the proximal VH7183 family was slightly reduced in 3’Eκ-KO peripheral B cells, in particular those involving the JH3 gene segment (Fig. 8d). Distal VHJ558 rearrangements involving the JH3 gene segment were reduced by 40% (or 0.6-fold) in MiEκ-KO splenic B cells whereas proximal VH7183 rearrangements were largely unchanged. Rearrangements to the middle VH gene family VHGam3.8 demonstrate a 2-fold increase in rearrangement to the furthest JH3 gene segment in B cells lacking 3’Eκ compared to WT B cells. These data indicate that in the absence of 3’Eκ, ongoing rearrangement at the pre-B cell stage of development changes the distribution of VH rearrangement in mature B cells so that distal VH gene rearrangement is increased.
Figure 8.
An altered spectrum of proximal versus distal VH gene segment rearrangements in 3’Eκ-KO splenic B cells. (a) PCR and Southern blot analysis of Igh rearrangements in CD19+ splenic B cells. Genotypes are indicated beneath each set of serial three-fold-diluted DNA samples. PCR primers were chosen to detect VH558 (top), VHGam3.8 (middle) and VH7183 (bottom) family rearrangements to DJH 1, 2, and 3 segments. Input DNA was normalized by amplification of a PCR fragment from the Igh Cµ region and DNA of stromal ST2 cells was used as a negative control. The numbers to the left of bands indicate rearrangements involving JH1, JH2 and JH3 gene segments. (b–d) Quantification of rearrangements shown in (a). The recombination signal is shown as a proportion of Cµ amounts for each DNA dilution and as an average across the two highest dilutions of DNA.
Discussion
Although it is known that regulatory elements within Igh and Igk play key roles in promoting recombinase accessibility, little is known about how accessibility of partially-assembled DJ-rearranged Igh alleles is reduced and how they become refractory to the recombinase during the period of Igk rearrangement in pre-B cells. The interchromosomal associations we observe between Igh and Igk alleles suggest a mechanism for coordinating rearrangement and allelic exclusion at a stage in development when targeting of the recombinase is directed away from one locus and towards the other. Our study demonstrates a previously-unappreciated aspect of immunoglobulin gene regulation and indicates that association of two loci can induce conformational changes which could subsequently alter the function of these loci.
We know from previous studies that dynamic movement of immunoglobulin loci exerts control of recombination at a number of levels. Enrichment of these loci at the nuclear periphery is important for restricting recombination to the B cell lineage 3. Relocation to the center of the nucleus occurs at the pro-B cell stage of development and correlates with increased accessibility of the two loci 3,13,14.
Conformational changes involving contraction of the two loci occur following relocation. At the Igh locus, contraction, mediated by looping, correlates with recombination of distal and middle VH gene rearrangement and may function to facilitate long range RSS interactions 4. It is now known that all antigen receptor loci undergo similar conformational changes during recombination 15. However, the mechanisms underlying locus contraction are not understood, although Pax5 and another unknown factor are required at the Igh locus 13. It may be that the underlying mechanism regulating contraction of the Igk locus is similar to that of the Igh locus and Pax5 could have a common role in this context. Our data from the two Igk enhancer knockout mice indicated that, individually, MiEκ and 3’Eκ are not required for locus contraction to occur, as in the absence of either enhancer element, Igk loci adopt conformations similar to those seen in WT developing B cells. However, as the two enhancer elements have overlapping roles, we cannot rule out that an absence of both enhancer elements could affect this process.
At the pre-B cell stage of development, pericentromeric recruitment of one allele of Igh and Igk has been implicated in establishing allelic exclusion 16. The data we report here indicate that recruitment of the Igk locus is the initiating event, and influences subsequent recruitment of the Igh locus. The Igk locus exerts its effect on the Igh locus through transient association at pericentromeric regions. Igh-Igk close association adds a further dimension to the control exerted by nuclear organization in regulating the process of recombination at these two loci. Our data indicate that Igh locus decontraction is dependent on association with the Igk locus at pericentromeric regions and that pericentromeric repositioning in the absence of Igk association does not induce decontraction. This conclusion is supported by our finding that locus decontraction did not occur in 3’Eκ-KO immature B cells, in which delayed pericentromeric repositioning of Igh occurred, independent of association with Igk. Thus, the association we observe in our FISH analysis may indeed be a physical interaction. It could be that repressive factors bound to the pericentromeric Igk allele are shared with the associated Igh allele and this induces changes in chromatin that disrupt contraction. Such possibilities add a further layer of complexity to immunoglobulin gene regulation and indicate that association of the two loci brings about an epigenetic change to alter locus conformation.
Our microscopy-based investigations provide us with information on events that occur within individual cells from sorted developmental populations. As we use fixed cells, our analysis can only provide us with an incomplete picture of the dynamic movements of these loci. However, it is clear from our current studies that the immunoglobulin loci are not static. After they have repositioned to pericentromeric regions at the pre-B cell stage of development, they do not remain in the same nuclear location as associated alleles, but move apart to different pericentromeric clusters at the immature B cell stage. The snapshot in time that we analyze is therefore likely to be a low estimate for the frequency with which Igh-Igk close association occurs. This could also explain why only 60–70% of pre-B and immature cells have one Igh and one Igk allele repositioned to pericentromeric regions. In the remaining cells from these populations, where no pericentromeric recruitment is seen, we have observed that one allele is always located at the periphery (J.S., unpublished observations). It may be that alleles shuttle between the periphery and pericentromeric heterochromatin, two environments which are thought to be repressive.
Our analysis of cells lacking 3’Eκ or MiEκ identified a shared role for these regulatory elements in diminishing pericentromeric repositioning of the Igk locus, in line with their overlapping roles in activating transcription and rearrangement 7,8. It is likely that binding of transcription factors to the two enhancers at the pre-B cell stage of development prevents association with centromeres, and that a limited supply of activating factors within the cell could result in relocalization of one Igk allele to heterochromatic regions. Analysis of developing B cells from 3’Eκ-KO mice identified a role for this regulatory element in mediating an interchromosomal Igh-Igk association which alters the conformation of the Igh locus at the pre-B cell stage of development. The absence of activating factors or the presence of repressive factors at the 3’Eκ region on the pericentromeric Igk allele may facilitate binding of repressive factors at the Igh locus. Sharing of common repressive factors could mediate association of pericentromeric Igk with the non-productive or DJ-rearranged Igh allele, thereby reducing accessibility and altering the conformation of the latter.
If this model is correct then locus decontraction should only take place on the pericentromeric Igh allele, mediated by interaction with 3’Eκ. This is difficult to conclude from an analysis of contracted and decontracted alleles in developing B cells, as pericentromeric recruitment of immunoglobulin loci is a transient process, the percentage of alleles found at these regions declines at later stages of development, and no pericentromeric recruitment of immunoglobulin loci is observed in mature resting cells 11. As a result, although contracted loci are mostly identified within euchromatic regions, decontracted alleles are also present in these locations.
As we observed pericentromeric repositioning of the Igh locus at the immature B cell stage in 3’Eκ-KO mice, it is likely that at later stages of development additional mechanisms enforce silencing of the Igh locus. An alternative pathway is likely to involve a reduction in interleukin 7 (IL-7), and subsequent deacetylation, a modification known to reduce accessibility at the Igh locus 17. Early repression of Igh by Igk at the pre-B cell stage of development is important for inducing locus decontraction. IL-7R signaling may act as an important back-up mechanism to reduce accessibility of the Igh locus preventing ongoing rearrangement of distal VH segments on the second DJH-rearranged Igh allele during Igk rearrangement.
Both enhancers play overlapping roles and individually have been shown to be quantitatively important, but not essential, for Igk rearrangement. Prior to our study, the only clear functional difference identified between the two enhancers was a role for MiEκ in regulating monoallelic demethylation of Igk 8. Our studies indicate that absence of the two enhancers have substantially different effects on the Igh locus.
Our data showing increased deletion of the VHJ558 associated signal in 3’Eκ-KO but not MiEκ-KO cells suggests that the Igh locus can continue to undergo distal VH gene rearrangement only when the Igh locus remains in a contracted conformation within euchromatic regions of the nucleus. Despite the alterations in Igh locus conformation observed in the Igk enhancer mutant pre-B cells, flow cytometric analyses of allotypically marked Igh alleles failed to detect evidence of allelic inclusion (data not shown). Thus, additional mechanisms must exist to enforce Igh allelic exclusion.
Several recent studies have shown that transcription of genes located on one chromosome can be controlled by regulatory elements located on a different chromosome. These include an association between the promoter of the T helper type 1 (TH1) cytokine gene, Ifng and the locus control region (LCR) of the TH2 cytokine genes 18. In addition, a nonallelic association has been reported between the imprinting control region of the Igf2/H19 locus and the Wsb1/Nf1 gene, mediated by CTCF, a factor that is involved in DNA methylation 19. Interchromosomal pairing has also been demonstrated between the two X chromosomes, and this association is implicated in silencing of the genes on one X chromosome during X-inactivation 20,21. This contrasts with the interchromosomal associations involved in olfactory receptor choice which may be important for promoting gene expression 22. All these interchromosomal associations, including the one that we have identified, involve coordinating decisions involving changes in gene accessibility and/or expression. It could be that physical association of loci or alleles within the nucleus has an essential role in ensuring that the timing of coordinated changes in gene expression is correctly regulated. Previous analyses of interchromosomal associations have not examined association relative to pericentromeric heterochromatin. Our analysis indicates that association between Igh and Igk is a transient event that is important for early silencing of one Igh allele in pre-B cells and for changing the conformation of this allele. This is the first demonstration of an interchromosomal association occurring at pericentromeric heterochromatin. The challenge now is to define the precise regions of Igh which mediate association with Igk and to identify the proteins which mediate this association.
Methods
FACS sorting and analysis
The following fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), allophycocyanin (APC), PE-Cy7 (from BD Biosciences) or PE-Alexa610 (Invitrogen)-coupled antibodies were used for flow cytometry: CD19 (1D3), CD25 (PC61), CD117 (2B8), IgM (II/411), IgD (11-26c.2a), CD4 (RM4-5), CD8α (53-6.7) and Thy1.2 (53-2.1). Isolated bone marrow cells were stained with the appropriate antibody combination, and pro-B cells were isolated on a Becton Dickinson FACSAria or a Dako MoFlo Flow Cytometer as CD19+c-Kit+ IgM− cells, pre-B cells as CD19+ CD25+ IgM− cells, early immature B cells as CD19+ IgMlow IgD− and late immature B cells as CD19+ IgMhigh IgD− cells. The purity of the sorted cells was verified by FACS reanalysis.
3D DNA-FISH and confocal analysis
FACS-sorted cells were washed three times in PBS and then fixed onto poly-L-lysine-coated slides for two- and three-color 3D DNA-FISH analysis as described previously in detail 13. Probes were directly labeled by nick translation with ChromaTide® Alexa Fluor® 488-5-dUTP, ChromaTide® Alexa Fluor® 594-5-dUTP (Molecular Probes/Invitrogen) or dUTP-Cy5 (GE Healthcare). The γ-satellite probe was prepared from a plasmid containing eight copies of the γ-satellite repeat sequence 11 and was directly labeled with dUTP-FITC (Roche/Enzo Biochem) or dUTP-Cy5. Cells were analyzed by confocal microscopy on a Leica SP2 and Leica SP5 AOBS (Acousto-Optical Beam Splitter) system. Optical sections separated by 0.3 µm were collected, and only cells with signals from both alleles (typically 90%) were analyzed.
Statistical analyses of separation of locus probe signals or subnuclear location of probe signals was carried out by applying the χ2 test to observed and expected frequencies. The original data of cell numbers were used rather than percentages. Expected frequencies were calculated according to standard methods 23 and χ2 probabilities were calculated in Microsoft Excel.
Activation of splenic B cells
Splenic B cells were purified by CD43 depletion and activated in culture with 10 µg/ml anti-CD40 as described previously 11.
V(D)J recombination analysis
Splenic B cells were isolated by CD19+ MACS selection and digested with proteinase K. DNA was isolated by phenol extraction and ethanol precipitation. PCR analyses of immunoglobulin genes were performed with published primers 13,24. PCR cycle numbers were adjusted to be in the linear range, based on the analysis of serially diluted DNA. PCR products were separated on agarose gels, transferred to a Hybond-XL membrane and analyzed by Southern blotting using a published upstream JH3 oligonucleotide probe or purified Cµ PCR product 13,24. Quantification of hybridization products was carried out using a Phosphor imager and Quantity One software (Bio-Rad). DNA amounts were normalized to Cµ. The average of the two highest dilutions of DNA is represented.
Mice
3’Eκ-KO mice were provided by F. Alt (Harvard University)7 9. All animal experiments were done in accordance with the guidelines of the IACUC of the NYU School of Medicine.
Supplementary Material
Figure 9.
Acknowledgements
This work is supported by a Wellcome Trust Project grant and New York University School of Medicine start up funds. M.S.S. acknowledges the support of NIH RO1 awards HL48702 and AI40227. We are grateful to Peter Lopez for his expertise and contribution to the flow cytometry.
Footnotes
Competing Interests Statement
The authors declare that they have no competing financial interests.
References
- 1.Alt FW, et al. Ordered rearrangement of immunoglobulin heavy chain variable region segments. Embo J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 3.Kosak ST, et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 4.Roldan E, et al. Locus 'decontraction' and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geier JK, Schlissel MS. Pre-BCR signals and the control of Ig gene rearrangements. Semin Immunol. 2006;18:31–39. doi: 10.1016/j.smim.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Fitzsimmons SP, Bernstein RM, Max EE, Skok JA, Shapiro MA. Dynamic changes in accessibility, nuclear positioning, recombination, and transcription at the Igkappa locus. J Immunol. 2007;179:5264–5273. doi: 10.4049/jimmunol.179.8.5264. [DOI] [PubMed] [Google Scholar]
- 7.Gorman JR, et al. The Ig(kappa) enhancer influences the ratio of Ig(kappa) versus Ig(lambda) B lymphocytes. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]
- 8.Inlay M, Alt FW, Baltimore D, Xu Y. Essential roles of the kappa light chain intronic enhancer and 3' enhancer in kappa rearrangement and demethylation. Nat Immunol. 2002;3:463–468. doi: 10.1038/ni790. [DOI] [PubMed] [Google Scholar]
- 9.Inlay MA, Lin T, Gao HH, Xu Y. Critical roles of the immunoglobulin intronic enhancers in maintaining the sequential rearrangement of IgH and Igk loci. J Exp Med. 2006;203:1721–1732. doi: 10.1084/jem.20052310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanhope-Baker P, Hudson KM, Shaffer AL, Constantinescu A, Schlissel MS. Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell. 1996;85:887–897. doi: 10.1016/s0092-8674(00)81272-6. [DOI] [PubMed] [Google Scholar]
- 11.Skok JA, et al. Nonequivalent nuclear location of immunoglobulin alleles in B lymphocytes. Nat Immunol. 2001;2:848–854. doi: 10.1038/ni0901-848. [DOI] [PubMed] [Google Scholar]
- 12.Goldmit M, et al. Epigenetic ontogeny of the Igk locus during B cell development. Nat Immunol. 2005;6:198–203. doi: 10.1038/ni1154. [DOI] [PubMed] [Google Scholar]
- 13.Fuxa M, et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato H, Saito-Ohara F, Inazawa J, Kudo A. Pax-5 is essential for kappa sterile transcription during Ig kappa chain gene rearrangement. J Immunol. 2004;172:4858–4865. doi: 10.4049/jimmunol.172.8.4858. [DOI] [PubMed] [Google Scholar]
- 15.Skok JA, et al. Reversible contraction by looping of the Tcra and Tcrb loci in rearranging thymocytes. Nat Immunol. 2007;8:378–387. doi: 10.1038/ni1448. [DOI] [PubMed] [Google Scholar]
- 16.Rolink A, Grawunder U, Winkler TH, Karasuyama H, Melchers F. IL-2 receptor alpha chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury D, Sen R. Transient IL-7/IL-7R signaling provides a mechanism for feedback inhibition of immunoglobulin heavy chain gene rearrangements. Immunity. 2003;18:229–241. doi: 10.1016/s1074-7613(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 18.Spilianakis CG, Lalioti MD, Town T, Lee GR, Flavell RA. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637–645. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- 19.Ling JQ, et al. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science. 2006;312:269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- 20.Xu N, Tsai CL, Lee JT. Transient homologous chromosome pairing marks the onset of X inactivation. Science. 2006;311:1149–1152. doi: 10.1126/science.1122984. [DOI] [PubMed] [Google Scholar]
- 21.Bacher CP, et al. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat Cell Biol. 2006;8:293–299. doi: 10.1038/ncb1365. [DOI] [PubMed] [Google Scholar]
- 22.Lomvardas S, et al. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126:403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 23.Campbell R. Statistics for Biologists. Cambridge University Press; 1989. [Google Scholar]
- 24.Schlissel MS, Corcoran LM, Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J Exp Med. 1991;173:711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.