Abstract
The dimethylated ribosomal nucleoside m4Cm and its monomethylated analogues Cm and m4C were synthesized. The conformations (syn versus anti) of the three modified nucleosides and cytidine were determined by CD and 1D NOE difference spectroscopy. The ribose sugar puckers were determined by the use of proton coupling constants. The position of modification (e.g., O versus N methylation) was found to have an effect on the sugar pucker of cytidine.
1. Introduction
Nucleotide modification is a characteristic feature of most ribosomal and transfer RNAs. More than 100 different modified nucleotides are found in various RNAs.1 The most frequent modifications are pseudouridylation, base methylation, and 2′-O-sugar methylation.2 These alterations to the nucleoside bases or ribose moieties may enhance the diverse structural or functional properties of RNA.3 The ribosome is the molecular machinery for protein synthesis in all domains of life.4 Ribosomal RNAs (rRNAs) contain a wide range of modified nucleotides, which are present near or at the key functional sites of the ribosome.5 The modified cytidine m4Cm (N4, 2′-O-dimethylcytidine) is a rare nucleoside, in which both the base and sugar are methylated. To date, m4Cm has only been found in bacterial rRNA.1, 6–9 It is located in one of the most important functional sites of the small subunit 16S rRNA, the decoding region, which is involved in decoding the messenger RNA (mRNA) through interactions with mRNA, transfer RNAs (tRNAs), and the large subunit 23S rRNA.10–12 A number of known antibiotics also bind to the decoding region.13
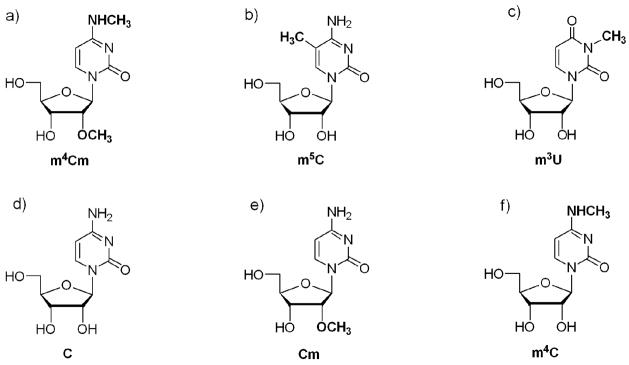
There are three methylated nucleotides in the decoding region of bacterial 16S rRNA, namely m4Cm, 5-methylcytidine (m5C), and 3-methyluridine (m3U) at positions 1402, 1407, and 1498, respectively (E. coli numbering) (Figure 1, a-c).9 The nucleoside m4Cm was isolated from E. coli and characterized by Nichols and Lane in 1966.14, 15 The methytransferase enzyme for E. coli m4Cm1402 in 16S rRNA is currently unknown.
Figure 1.
The structures of the modified nucleosides a) N4, 2′-O-dimethylcytidine (m4Cm), b) 5-methylcytidine (m5C), c) 3-methyluridine (m3U), d) cytidine (C), e) 2′-O-methylcytidine (Cm), and f) N4, methylcytidine (m4C) are shown.
Two of the decoding region modified nucleosides, m3U and m5C, are commercially available.16 In contrast, m4Cm is not available from commercial sources, and only two chemical syntheses of this modified nucleoside have been reported.17, 18 The Robins and Naik method requires only a few steps, but has relatively low overall yields (20% in four steps). The Nyilas and Chattopadhyaya method is facile (five steps) and can be carried out on a large scale with higher yields (~35% overall), but has one major drawback. This synthesis employs the relatively expensive TIPDSiCl2 reagent for 3′, 5′-O-ribose protection. For detailed structural studies of m4Cm, as well as its monomethylated analogues, N4-methylcytidine (m4C) and 2′-O-methylcytidine (Cm) (Figure 1, e and f), an inexpensive and versatile chemical method was desired in order to synthesize large quantities of the modified cytidine nucleosides and incorporate them into RNA. Cm is relatively expensive and m4C is not commercially available, although several synthetic methods have been reported.17–23 Thus, the other rationale for developing a new synthetic route for m4Cm was to generate the intermediates of interest, m4C and Cm. Herein, we report on the synthesis of m4Cm, m4C, and Cm using a convenient method for the generation of all three modified cytidine residues. One-dimensional 1H NMR spectroscopy was used to determine the solution conformations of m4Cm, m4C, Cm, and cytidine. A comparison of the solution conformations of m4Cm and its analogues provides insight into the possible roles of the modifications at the RNA level.
2. Results and Discussion
2.1 Synthesis of N4, 2′-O-dimethylcytidine and its analogues
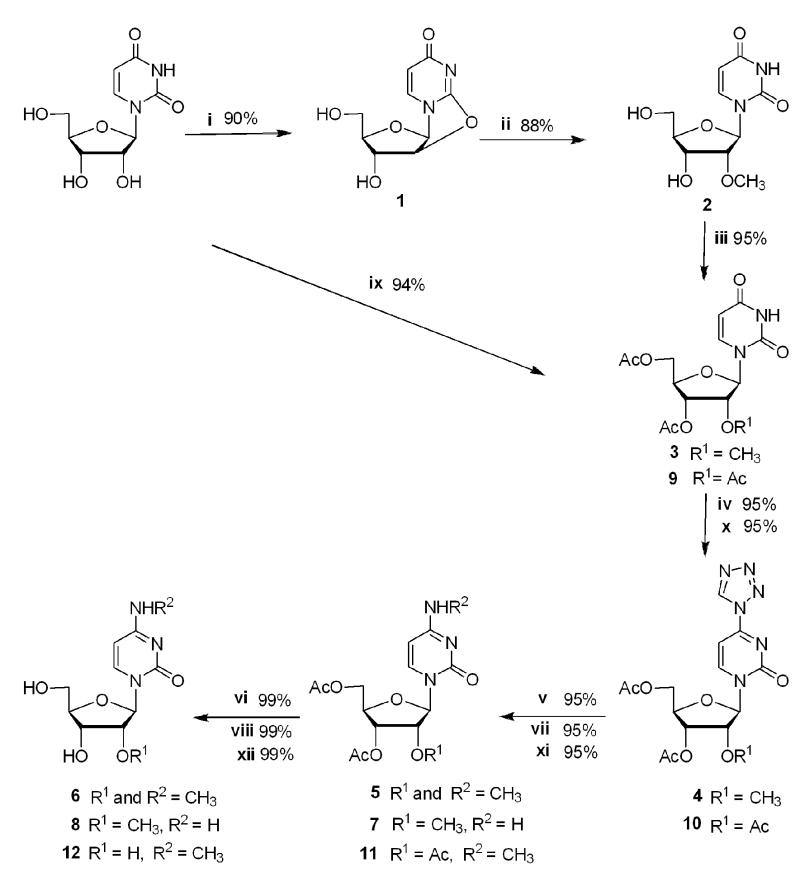
Uridine was converted to the intermediate 2,2′-anhydrouridine, 1, and then reacted with magnesium methoxide to generate 2′-O-methyluridine, 2, using a method developed by Roy and Tang (79% yield for two steps; Scheme 1).24, 25 Compound 2 was acetylated using catalytic amounts of DMAP in pyridine with acetic anhydride to afford 3′, 5′-diacetyl-2′-O-methyluridine, 3, in 95% yield.26 The C-4 position of methyluridine was then activated with tetrazole using the method developed by Reese and Ubasawa to give intermediate 4 in 95% yield.27, 28
Scheme 1. Synthesis of N4, 2′-O-dimethylcytidine and its analogues.
Reagents and conditions: i) (PhO)2CO, NaHCO3, DMF, 80 °C, 3 h; ii) Mg(OCH3)2, CH3OH, reflux, 5 h; iii) (CH3CO)2O, DMAP, pyridine, rt, overnight; iv) tetrazole, TsCl, diphenyl phosphate, pyridine, rt, 36 h; v) CH3NH3+Cl−, KOH, (C2H5)3N, CH3CN-H2O, rt, 24 h; vi) NH3 in CH3OH, rt, overnight; vii) NH4Cl, KOH, (C2H5)3N, CH3CN-H2O, rt, 24 h viii) NH3 in CH3OH, rt, overnight; ix) (CH3CO)2O, DMAP, pyridine, rt, overnight; x) tetrazole, TsCl, diphenyl phosphate, pyridine, RT, 36 h; xi) CH3NH3+Cl−, KOH, (C2H5)3N, CH3CN-H2O, rt, 24 h; xii) NH3 in CH3OH, rt, overnight.
The tetrazoyl derivative 4 can be substituted with a variety of nucleophiles to generate a range of cytidine analogues. Methylamine was employed as the nucleophile to give 3′, 5′-diacetyl-N4, 2′-O-dimethylcytidine, 5, in 95% yield. Removal of the acetyl protecting groups by treatment with 2.0 M NH3 in methanol yielded N4, 2′-O-dimethylcytidine, 6, in 99% yield. The overall yield of m4Cm by this method (Scheme 1) was 67% in six steps from uridine. The reagents used in this procedure are relatively inexpensive, and the synthesis can be carried out on a multigram scale. This method is versatile in that it produces intermediates for other modified nucleotide syntheses.
Ammonium hydrochloride was used as the nucleophile (Scheme 1, vii) in place of methylamine hydrochloride (step v) to generate 3′, 5′-diacetyl-2′-O-methylcytidine, 7, followed by removal of the acetyl groups with 2.0 M NH3 in methanol to give 2′-O-methylcytidine, 8, in 99% yield.
To generate m4C (Scheme 1), uridine was acetylated using catalytic amounts of DMAP in pyridine with acetic anhydride to afford 2′, 3′, 5′-triacetyl uridine, 9, in 94% yield. Compound 9 was reacted with tetrazole (step x) to give intermediate 10 in 95% yield. Methylamine was employed as the nucleophile (step xi) to produce 2′, 3′, 5′-tri-O-acetyl, N4-methycytidine, 11, in 95% yield. Deprotection of the acetyl groups was carried out by treatment with 2.0 M NH3 in methanol to yield N4-methylcytidine, 12, in 99% yield. The overall yield was 84% in four steps from uridine.
2.2. Circular dichroism studies
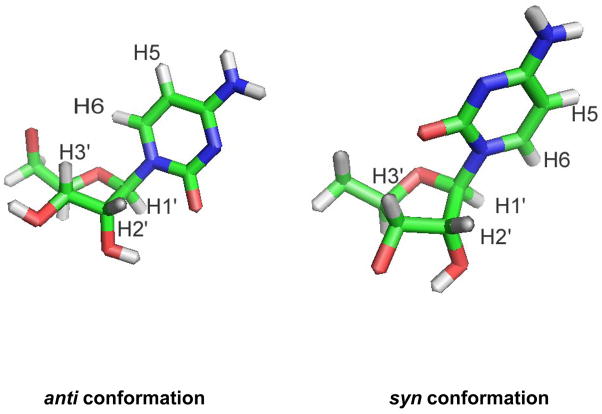
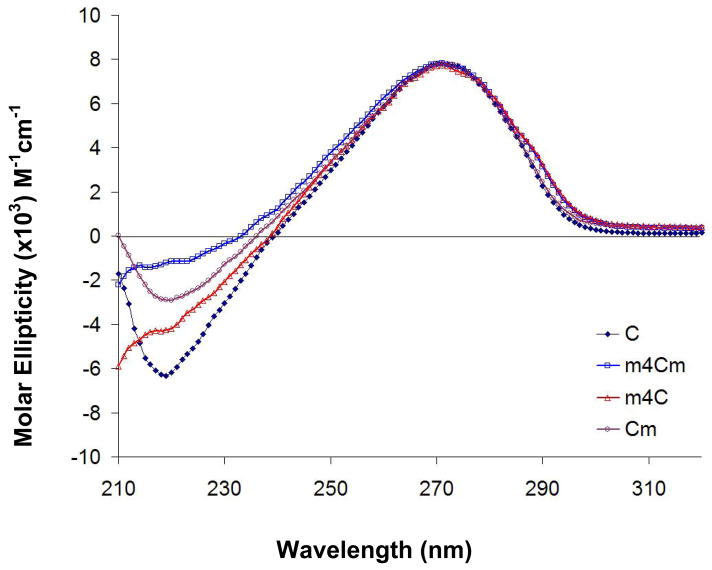
The nucleoside base can exist in two main conformations relative to the ribose sugar due to rotation about the glycosidic C1′-N1 bond, referred to as the anti and syn conformers (Figure 2).29, 30 Circular dichroism (CD) spectroscopy can be used to determine the solution conformations of nucleosides.31 The m4Cm and its analogues all have peak maxima at 271 nm. This feature indicates that they all prefer the anti conformation in solution (Figure 3). There are subtle effects on the solution conformation of cytidine nucleoside due to methylation, which is evident by slight differences in the CD spectra of cytidine and the three analogues. The CD spectra of cytidine is consistent with prior work by Miles et al., even though the solvents in that study were different.31, 32
Figure 2.
The anti and syn conformations of the cytidine nucleoside are represented by stick models.
Figure 3.
The circular dichroism (CD) spectra of cytidine and its methylated analogues at room temperature are shown. Each curve represents the average of five scans.
2.3. One-dimensional 1H NMR studies
Nuclear magnetic resonance (NMR) spectroscopy can be used to determine the solution conformations of nucleosides.33–35 In particular, 1D 1H NOE experiments are valuable for examining relative proton positions and deducing the anti or syn conformations of the nucleoside.33, 34 The distance between two protons is inversely proportional to the magnitude of the NOE signal; therefore, relative proton-proton distances in the nucleoside can be determined (Figure 2). In an anti conformation, the base H6 (pyrimidine) or H8 (purine) protons are closest to the sugar H2′ and H3′ protons; therefore, irradiation of pyrimidine H6 gives a strong NOE to H2′ and H3′. In contrast, if the nucleoside has a syn conformation, base H6 or H8 protons are closest to the sugar H1′ proton, and irradiation of H6 gives a strong NOE to H1′.34 Hence, the magnitude of the sum of H2′ and H3′ NOEs compared to the H1′ NOE is the basis for determining the anti to syn ratio of the nucleoside.34
When H6 of cytidine was irradiated in D2O at 25 °C, combined NOEs of 9.5% for H2′ and H3′ were determined, compared to 4.9% for the H1′ NOE (summarized in Table 1). This data confirms that cytidine prefers the anti conformation. The reverse experiment in which H2′ and H3′ were irradiated gave a 9.0% combined NOE at H6; whereas, irradiation of H1′ gave a 3.1% NOE at H6. This data is consistent with prior work of Rosemeyer and coworkers (8.5% for H2′ and H3′; 3.6% for H1′ in (CD3)2SO4).34 In the case of m4Cm, the H1′ and H5 proton signals overlapped in D2O; therefore, the NOE to H1′ could not be measured accurately. Reverse studies (i.e., irradiation of H2′, H3′, and H1′) showed a preference for the anti conformation (Table 1). To further confirm these results, NOE studies of m4Cm were carried out in CD3OD. Each proton signal was irradiated to determine the NOEs (Table 1). Similar results were obtained, in which irradiation of H6 gave an 8.7% NOE at H2′ and H3′, but only a 3.4% NOE at H1′. The two monomethylated cytidine analogues, m4C and Cm, also prefer the anti conformation (at 25 °C, the combined NOEs at H2′ and H3′ with H6 irradiation were 12.2% and 8.4% NOE for m4C and Cm, respectively; Table 1). One-dimensional 1H NOE experiments were also done at 4 and 37 °C. Only minor variations in the conformation populations were observed with varying temperature (Table 1).
Table 1.
Irradiation and NOE data for cytidine and its methylated analogues at 4, 25, and 37 °C.
| Compound | Irradiated proton | Enhanced proton | NOE % | ||
|---|---|---|---|---|---|
| 4 °C | 25 °C | 37 °C | |||
| C | H6 | H1′ | 3.3 | 4.9 | 4.3 |
| (D2O) | H2′ + H3′ | 8.6 | 9.5 | 8.1 | |
| H5 | 7.3 | 11.4 | 10.1 | ||
| H1′ | H6 | 1.8 | 3.1 | 3.1 | |
| H2′ | H6 | 5.6 | 6.0 | 5.2 | |
| H3′ | H6 | 2.8 | 3.0 | 2.6 | |
|
| |||||
| Cm | H6 | H1′ | 3.5 | 3.7 | 3.6 |
| (D2O) | H2′ + H3′ | 6.7 | 8.4 | 7.6 | |
| H5 | 9.2 | 10.8 | 9.4 | ||
| H1′ | H6 | 2.2 | 4.1 | 2.8 | |
| H2′ | H6 | 5.5 | 4.9 | ||
| H3′ | H6 | 2.9 | 3.7 | 2.6 | |
|
| |||||
| m4C | H6 | H1′ | 4.7 | 8.5 | 4.8 |
| (D2O) | H2′ + H3′ | 10.5 | 12.2 | 10.7 | |
| H5 | 8.2 | 11.6 | 9.2 | ||
| H1′ | H6 | 1.7 | 2.3 | 1.9 | |
| H2′ | H6 | 6.2 | 5.8 | 4.5 | |
| H3′ | H6 | 2.2 | 1.9 | 1.9 | |
|
| |||||
| m4Cm | H6 | H1′ + H5 | 5.4 | 17.6 | 16.1 |
| (D2O) | H2′ + H3′ | 7.2 | 11.9 | 10.9 | |
| H1′ | H6 | 1.5 | 4.8 | 4.2 | |
| H2′ | H6 | 4.3 | 6.0 | 5.2 | |
| H3′ | H6 | 1.9 | 2.8 | 2.4 | |
|
| |||||
| m4Cm | H6 | H1′ | 3.4 | 3.4 | 3.3 |
| (CD3OD) | H2′ + H3′ | 11.0 | 8.7 | 8.5 | |
| H5 | 11.2 | 9.3 | 8.9 | ||
| H1′ | H6 | 2.7 | 2.1 | 2.3 | |
| H2′ | H6 | 3.9 | 3.4 | 3.3 | |
| H3′ | H6 | 5.0 | 3.5 | 3.1 | |
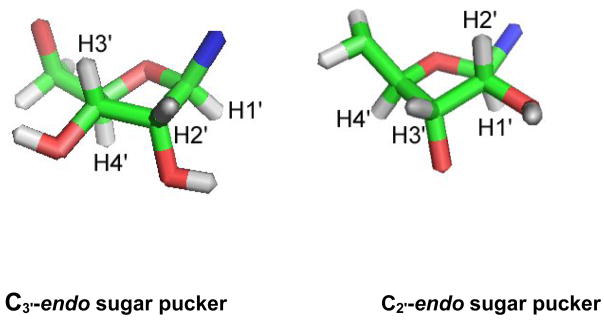
The ribose moieties of nucleosides most often adopt either a C3′-endo (north, N) or a C2′-endo (south, S) orientation (Figure 4).36 These two preferred sugar puckering modes refer to the movement of the carbon atoms out of the plane (i. e., C3′ is above the plane for C3′-endo and C2′ is above the plane for C2′-endo). Altona and Sundaralingam characterized the C3′-endo and C2′-endo sugar puckers using coupling constants of the sugar protons, JH1′-JH2′ and JH3′-JH4′.37 The levels of C2′-endo and C3′-endo sugar pucker can be calculated from the following equations: [%C2′-endo (S)] = 100 × J1′2′/(J1′2′ + J3′4′); [%C3′-endo (N)] = 100 − [% C2′-endo].37 Coupling constants for m4Cm and the monomethylated analogues are given in Table 2. The data reveal that cytidine and the three analogues all prefer the C3′-endo conformation. The N/S ratio (Keq) is similar for cytidine and m4Cm (1.6–1.7), but slightly lower for m4C (1.3) and slightly higher for Cm (1.9–2.0) (Table 3). Thus, 2′-O-methylation more strongly favors the C3′-endo (N) sugar pucker in contrast to N4-methylation, in which C3′-endo is slightly less favored. The differences appear to be balanced such that dimethylated m4Cm is similar to unmodified cytidine in the preference for the C3′-endo sugar conformation.
Figure 4.
Two types of sugar puckers, C3′-endo (north) and C2′-endo (south), are represented by stick models.
Table 2.
1H-1H coupling constants of m4Cm and its analogues at 4, 25, and 37 °C.
| Compound | Temperature | J1′,2′ | J2′,3 | J3′,4′ | J4′,5′ | J4′,5″ |
|---|---|---|---|---|---|---|
| C | 4° C | 3.8 | 5.1 | 6.3 | 2.8 | 4.3 |
| 25° C | 3.9 | 5.3 | 6.1 | 2.8 | 4.4 | |
| 37° C | 4.0 | 5.4 | 6.1 | 2.9 | 4.5 | |
| Cm | 4° C | 3.3 | 5.2 | 6.8 | 2.6 | 4.2 |
| 25° C | 3.5 | 5.3 | 6.7 | 2.8 | 4.4 | |
| 37° C | 3.5 | 5.4 | 6.5 | 2.8 | 4.4 | |
| m4C | 4° C | 4.3 | 5.4 | 5.5 | 2.8 | 4.4 |
| 25° C | 4.4 | 5.4 | 5.6 | 2.9 | 4.6 | |
| 37° C | 4.4 | 5.4 | 5.6 | 2.9 | 4.6 | |
| m4Cm | 4° C | 3.8 | 5.3 | 6.6 | 2.8 | 4.3 |
| 25° C | 3.9 | 5.4 | 6.2 | 2.8 | 4.3 | |
| 37° C | 3.9 | 5.4 | 6.2 | 2.9 | 4.5 |
Table 3.
Type of sugar puckered of m4Cm and its analogues at 4, 25, and 37 °C.
| Compound | Temperature (° C) | % C3′-endo (N) | % C2′-endo (S) | Keq (N/S) |
|---|---|---|---|---|
| C | 4 | 62 | 38 | 1.6 |
| 25 | 61 | 39 | 1.6 | |
| 37 | 60 | 40 | 1.5 | |
| Cm | 4 | 67 | 33 | 2.0 |
| 25 | 66 | 34 | 1.9 | |
| 37 | 65 | 35 | 1.9 | |
| m4C | 4 | 56 | 44 | 1.3 |
| 25 | 56 | 44 | 1.3 | |
| 37 | 56 | 56 | 1.3 | |
| m4Cm | 4 | 63 | 37 | 1.7 |
| 25 | 61 | 39 | 1.6 | |
| 37 | 61 | 39 | 1.6 |
3. Conclusions
The modified nucleosides m4Cm, Cm, and m4C were synthesized in good overall yields (67%, 67%, and 84%, respectively) on multigram scales. Circular dichroism and one-dimensional NOE difference spectroscopy were employed for determination of the syn and anti preferences of the cytidine analogues. All of the cytidine nucleosides examined here (m4Cm, Cm, m4C, and cytidine) prefer the anti conformation. The sugar puckers were determined by using sugar proton coupling constants from the 1D 1H NMR spectra. The 2′-O and N4 methylations have opposing effects in the ribose sugar pucker. Sugar methylation leads to a slight enhanced preference for the C3′-endo (north) sugar pucker; whereas, base methylation leads to a slight decreased preference for the C3′-endo conformation. These results show that the effect of modifications on nucleoside conformation are subtle, which is consistent with previous studies on methylated or alkylated uridine and pseudouridine monomers.38, 39 Nonetheless, the methylations may have significant effects on RNA conformation when present in certain sequences or structural motifs due to long-range tertiary contacts within the RNA. Hence, we are currently examining the effects of m4Cm and its analogues at the oligonucleotide level (in RNA model systems) and full-length rRNAs. Multiple modifications may have additive effects on RNA conformation and play a role in fine-tuning the RNA structure and function.
4. Experimental
4.1. Synthesis of modified nucleosides
4.1.1. General
Tetrazole was purchased from Glen Research. All NMR solvents were from Cambridge Isotope Laboratories, Inc. Dry methanol and dimethylformamide were purchased from Acros. Methylene chloride (CH2Cl2) was purchased from Fisher, distilled over CaH2. All other chemicals were purchased from Sigma-Aldrich or Fisher and used without further purifications. Moisture-sensitive reactions were performed under dry argon. Flame-dried equipment (syringes, round-bottom flasks, etc.) was used in those reactions. The compounds were azeotroped using benzene or toluene.
ESI mass spectra and high resolution mass spectra (HRMS) were obtained on Waters Micromass Zq and Micromass GCT spectrometers, respectively. Flash column chromatography was carried out on silica gel 60 (240-400 mesh). 1H and 13C NMR spectra were recorded on a Varian Unity 500 spectrometer.
4.1.2. 3′,5′-Diacetyl-2′-O-methyluridine (3)
Compounds 1 (2, 2′-anhydrouridine) and 2 (2′-O-methyluridine) were prepared according to literature methods.25 Compound 2 (1.80 g, 7.0 mmol) was placed in 24 mL of pyridine and stirred for 15 min. DMAP (214 mg, 1.75 mmol) was added, followed by 2.3 mL (24.5 mmol) of acetic anhydride. The reaction mixture was stirred overnight at room temperature. The reaction was quenched with saturated NaHCO3. The compound was purified by column chromatography (hexane:ethyl acetate, 8:2) to give a yellow oil (2.27 g, 95%). 1H NMR (500 MHz, CD3Cl) δ (ppm) 2.13 (s, 3H), 2.15 (s, 3H), 3.47 (s, 3H), 4.04 (m, 1H), 4.36 (m, 3H), 4.98(m, 1H), 5.75 (d, 1H), 5.91 (dd, 1H),7.52 (d, 1H), 9.10 (s, 1H), 13C NMR (500 MHz, CD3Cl) δ (ppm) 20.86, 59.30, 62.65, 70.08, 79.40, 81.77, 88.81, 102.94, 127.23, 139.43, 145.69, 150.18, 163.06, 170.37, HRMS calculated for C14H18N2O8 342.1063, found 342.1067.
4.1.3. 4-(Tetrazol-1-yl)-1-(3′, 5′-di-O-acetyl-2′-O-methyl-β-D-ribofuranosyl) pyrimidine-2-(1H)-one (4)
In a round-bottom flask containing 15 mL of pyridine, compound 3 (2.05 g, 6 mmol), tetrazole (0.84 g, 12 mmol), tosyl chloride (2.29 g, 12 mmol), and diphenyl phosphate (2.25 g, 9 mmol) were added. The mixture was stirred for 36 h at room temperature, and then 3.75 mL of water was added. The solution was poured over saturated NaHCO3 solution. The desired product was extracted with methylene chloride. The solvent was dried over Na2SO4 and concentrated on a rotary evaporator. The product was purified by column chromatography (CH2Cl2:CH3OH, 95:5) to give a yellow foam (2.25 g, 95%). 1H NMR (500 MHz, CD3Cl) δ (ppm) 2.11(s, 3H), 2.14(s, 3H), 3.6(s,3H), 4.17(d, 1H), 4.42(m, 2H), 4.54(m, 1H), 4.77(m, 1H), 5.97(s, 1H), 7.21(d, 1H), 8.56(d, 1H), 9.59(s, 1H) 13C NMR (500 MHz, CD3Cl) δ (ppm) 20.75, 21.07, 59.43, 61.8, 69.24, 79.50, 81.75, 90.89, 95.16, 140.95, 147.5, 153.79, 157.69, 170.21, 170.317 ESI-MS (ES+) calculated for C15H18N6O7 394.1, found 395.1 (MH+).
4.1.4. 3′,5′-Diacetyl-N4, 2′-O-dimethylcytidine (5)
Potassium hydroxide (89%, 0.32 g, 5 mmol) and CH3NH3Cl (0.34 g, 5 mmol) were added to a 50 mL round bottom flask. The flask was sealed with a septum. Water (10 mL), acetonitrile (10 mL), triethylamine (770 μL), and a solution of compound 4 (1.97 g, 5 mmol) in acetonitrile (20 mL) were added sequentially by syringe. The mixture was stirred vigorously for 24 h. The solvent was removed with a rotary evaporater. The crude product was purified by column chromatography (CH2Cl2:CH3OH, 9:1) to give a yellow oil (1.69 g, 95%). 1H NMR (500 MHz, CD3Cl) δ (ppm) 2.11(s, 3H), 2.13 (s, 3H), 3.06 (s, 3H), 3.55 (s, 3H), 4.10(m, 1H), 4.40 (m, 3H), 4.88 (m, 1H), 5.75 (d, 1H), 6.00 (d, 1H), 7.50 (d, 1H), 13C NMR (500 MHz, CD3Cl) δ (ppm) 20.85, 20.99, 28.07, 59.15, 62.85, 70.43, 78.43, 78.66, 81.93, 89.93, 96.20,138.63, 164.52, 170.35, 170.52 HRMS calculated for C15H21N3O7 355.1380, found 355.1383.
4.1.5. N4,2′-O-Dimethylcytidine (6)
Compound 5 (1.60 g, 4.5 mmol) was placed in a dry 50 mL round-bottom flask. The flask was fitted with a septum. Next, 25 mL of 2 M NH3 in methanol was added, and the mixture was stirred overnight at room temperature. The mixture was dried on a rotary evaporator. The residue was coevaporated twice with CH3OH and once with CH3OH–CH2Cl2 (1:1) under a vacuum. The residue was heated to 100 °C under vacuum for 2 h to give 6 as a white powder (1.20 g, 99%). 1H NMR (500 MHz, D20) δ (ppm) 2.73 (s, 3H), 3.34 (s, 3H), 3.63 (dd, 1H), 3.75 (dd, 1H), 3.82 (m, 1H), 3.91 (m, 1H), 4.13 (m, 1H), 5.78 (m, 2H), 7.52 (d, 1H),13C NMR (500 MHz, D2O) δ (ppm) 27.27, 58.29, 60.54, 68.42, 82.90, 83.13, 88.31, 97.48, 139.45, 157.78, 164.59, HRMS calculated for C11H17N3O5 271.1168, found 271.1176.
4.1.6. 3′,5′-Diacetyl-2′-O-methycytidine (7)
Potassium hydroxide (89%, 0.32 g, 5 mmol) and NH4Cl (0.27 g, 5 mmol) were added to a round-bottom flask. The flask was sealed with a septum. Water (10 mL), acetonitrile (10 mL), triethylamine (770 μL), and solution of compound 4 (1.97 g, 5 mmol) in acetonitrile (20 mL) were added sequentially with a syringe. The mixture was stirred vigorously for 24 h. The solvent was removed with a rotary evaporator. The crude product was purified by column chromatography (CH2Cl2:CH3OH, 9:1) to give a yellow oil (1.62 g, 95%). 1H NMR (500 MHz, CD3Cl) δ (ppm) 2.09 (s, 3H), 2.11 (s, 3H), 3.47 (s, 3H), 4.01 (d, 1H), 4.32 (m, 1H), 4.34 (m, 1H), 4.35 ( m, 1H), 4.95 (m, 1H), 5.88 (m, 1H), 6.01 (d, 1H), 7.55 (d, 1H) 13C NMR (500 MHz, CD3Cl) δ (ppm) 20.89, 21.08, 58.18, 62.71, 78.84, 81.84, 90.31, 95.59, 125.96,140.82, 155.48, 165.84, 170.41,170.58 ESI-MS (ES+) calculated for C14H19N3O7 341.3, found 342.3 (MH+).
4.1.7. 2′-O-Methycytidine (8)
Compound 7 (1.54 g, 4.5 mmol) was placed in a dry round-bottom flask fitted with a septum. Next, 25 mL of 2 M NH3 in methanol was added, and the mixture was stirred overnight at room temperature. The mixture was dried on a rotary evaporator. The residue was coevaporated twice with CH3OH and once with CH3OH–CH2Cl2 under vacuum. The residue was heated to 100 °C under a vacuum for 2 h to give 8 as a white powder (1.15 g, 99%). The NMR data matched that in the litrature.25 ESI-MS (ES+) calculated for C10H15N3O5 257.1, found 258.1 (MH+).
4.1.8. 4-(Tetrazol-1-yl)-1-(2′, 3′, 5′-Tri-O-acetyl-β-D-ribofuranosyl)pyrimidine-2-(1H)-one (10)
Compound 9 (2′,3′,5′-triacetyluridine) was prepared according to literature methods.26 Compound 9 (2.22 g, 6 mmol), tetrazole (0.84 g, 12 mmol), tosyl chloride ( 2.29 g, 12 mmol), and diphenyl phosphate (2.25 g, 9 mmol) were added to a round bottom flask containing 15 mL of pyridine. The mixture was stirred for 36 h at room temperature, and then 3.75 mL of water was added. The solution was poured over a saturated sodium carbonate solution. The desired product was extracted with methylene chloride. The solvent was dried over Na2SO4 and concentrated on a rotary evaporator. The product was purified by column chromatography (CH2Cl2:CH3OH, 95:5) to give a yellow foam (2.40 g, 95%). 1H NMR (500 MHz, CD3Cl) δ (ppm) 2.07 (s, 3H), 2.12 (s, 3H), 2.14 (s, 3H), 4.40 (m, 3H), 5.27 (m, 1H), 5.48 (m, 1H), 6.09 (d, 1H), 7.24 (d, 1H), 8.38 (d, 1H), 9.60 (s, 1H), 13C NMR (500 MHz, CD3Cl) δ (ppm) 20.66, 20.68, 21.05, 62.66, 69.65, 73.99, 80.51, 90.31, 95.72,141.04, 147.69, 153.98, 157.84, 169.76, 169.76, 170.33, ESI-MS (ES+) calculated for C16H18N6O8 422.1, found 423.1 (MH+).
4.1.9. 2′,3′,5′-Tri-O-acetyl-N4-methylcytidine (11)
Potassium hydroxide (89%, 0.32 g, 5 mmol) and CH3NH3Cl (0.34 g, 5 mmol) were added in a 50 mL round-bottom flask. The flask was sealed with a septum. Water (10 mL), acetonitrile (10 mL), triethylamine (770 μL), and a solution of the compound 10 (2.11 g, 5 mmol) in acetonitrile (20 mL) were added sequentially by syringe. The mixture was stirred vigorously for 24 h. The solvent was removed with a rotary evaporater. The crude product was purified by column chromatography (CH2Cl2:CH3OH, 9:1) to give a yellow oil (1.82 g, 95%). 1H NMR (500 MHz, CD3Cl) δ (ppm), 2.09 (s, 3H), 2.10 (s, 3H), 2.12 (s, 3H), 4.34 (m, 2H), 5.40 (dd, 1H), 5.90 (d, 1H), 5.99 (d, 1H), 6.71 (d, 1H), 7.39 (d, 1H), (s, 3H) 8.27 (d, 1H),13C NMR (500 MHz, CD3Cl) δ (ppm), 20.48, 20.53, 20.68, 63.0, 70.04, 73.41, 79.12, 89.96, 96.11, 141.03, 155.59, 166.10, 169.58, 169.71, 170.38, ESI-MS (ES+) calculated for C16H21N3O8 383.1, found 384.1 (MH+).
4.1.10. N4-Methylcytidine (12)
Compound 11 (1.53 g, 4 mmol) was added to a round-bottom flask fitted with a septum. Next, 25 mL of 2 M NH3 in methanol was added, and the mixture was stirred overnight at room temperature. The mixture was dried on a rotary evaporater. The residue was coevaporated twice with CH3OH and once with CH3OH–CH2Cl2 (1:1) under vacuum. The residue was heated at 100 °C under vacuum for 2 h to give 12 as a white powder (1.01 g, 99%). 1H NMR (500 MHz, D20) δ (ppm) 2.75 (s, 3H), 3.63 (dd, 1H), 3.75 (dd, 1H), 3.98 (m, 1H), 4.05 (m, 1H), 4.17 (m, 1H), 5.77 (d, 1H), 5.83 (d, 1H), 7.54(d, 1H), 13C NMR (500 MHz, D2O) δ (ppm) 27.30, 60.99, 69.60, 73.89, 83.94, 90.13, 97.51, 139.62, 158.17, 164.71 ESI-MS (ES+) calculated for C10H15N3O5 257.1, found 258.2 (MH+).
4.2. Circular dichroism and NMR studies of modified nucleosides
4.2.1. Sample preparation
The nucleosides were dissolved in a 20 mM sodium cacodylate buffer, pH 7.0. The concentrations were calculated by using the Beer-Lambert equation A= ε·C·λ, in which ε, C and λ are extinction coefficient, concentration, and pathlength, respectively. The extinction coefficients (ε) are 11,000 cm−1M−1, 11,700 cm−1M−1, and 9,700 cm−1M−1 for m4Cm, m4C, and Cm, respectively.17, 20, 40
4.2.2. Circular dichroism and NMR spectroscopy
CD spectra were acquired on a Chirascan circular dichroism spectrometer equipped with a water bath to control the temperature. The molar ellipticities were normalized from a concentration of 1.83 mM, 1.45 mM, 1.01 mM, and 1.24 mM for cytidine, m4Cm, m4C, and Cm, respectively. 1H NMR spectra were recorded on a Varian Unity 500 MHz spectrometer. NMR experiments were performed in 99.99% D2O. 1H NMR and NOE experiments were done at 4, 25, and 37 °C.
Supplementary Material
Acknowledgments
We are thankful to Dr. B. Ksebati and Dr. J.-P. Desaulniers for their technical assistance and helpful discussions. This work was supported by the National Institutes of Health (AI061192 and AI055496).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Limbach PA, Crain PF, McCloskey JA. Nucleic Acids Res. 1994;22:2183–2196. doi: 10.1093/nar/22.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decatur WA, Fournier MJ. Trends Biochem Sci. 2002;27:344–351. doi: 10.1016/s0968-0004(02)02109-6. [DOI] [PubMed] [Google Scholar]
- 3.Agris PF, Vendeix FA, Graham WD. J Mol Biol. 2007;366:1–13. doi: 10.1016/j.jmb.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Nierhaus KH, Franceschi F, Subramanian AR, Erdmann VA, Wittmann-Liebold B, editors. The Translational Apparatus: Structure, Function, Regulation, Evolution. 1993. pp. 1–746. [Google Scholar]
- 5.Chow CS, Lamichhane TN, Mahto SK. ACS Chem Biol. 2007;2:610–619. doi: 10.1021/cb7001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fellner P, Sanger F. Nature. 1968;219:236–238. doi: 10.1038/219236a0. [DOI] [PubMed] [Google Scholar]
- 7.Rozenski J, Crain PF, McCloskey JA. Nucleic Acids Res. 1999;27:196–197. doi: 10.1093/nar/27.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCloskey JA, Rozenski J. Nucleic Acids Res. 2005;33:D135–138. doi: 10.1093/nar/gki015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmerechts G, Barbe S, Herdewijn P, Anne J, Rozenski J. Nucleic Acids Res. 2007;35:3494–3503. doi: 10.1093/nar/gkm248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogle JM, Carter AP, Ramakrishnan V. Trends Biochem Sci. 2003;28:259–266. doi: 10.1016/S0968-0004(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 11.Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JHD. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- 12.Rackham O, Wang K, Chin JW. Nat Chem Biol. 2006;2:254–258. doi: 10.1038/nchembio783. [DOI] [PubMed] [Google Scholar]
- 13.Moazed D, Noller HF. Nature. 1987;327:389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 14.Nichols JL, Lane BG. Biochim Biophys Acta. 1966;119:649–651. doi: 10.1016/0005-2787(66)90147-x. [DOI] [PubMed] [Google Scholar]
- 15.Nichols JL, Lane BG. Biochim Biophys Acta. 1968;166:605–615. doi: 10.1016/0005-2787(68)90367-5. [DOI] [PubMed] [Google Scholar]
- 16.Dharmacon Modifications. http://www.dharmacon.com/rna/pricing.aspx.
- 17.Robins MJ, Naik SR. Biochemistry. 1971;10:3591–3597. doi: 10.1021/bi00795a017. [DOI] [PubMed] [Google Scholar]
- 18.Nyilas A, Chattopadhyaya J. Acta Chem Scand. 1986;B40:826–830. doi: 10.3891/acta.chem.scand.40b-0826. [DOI] [PubMed] [Google Scholar]
- 19.Fox JJ, Van Praag D, Wempen I, Doerr IL, Cheong L, Knoll JE, Eidinoff ML, Bendich A, Brown GB. J Am Chem Soc. 1959;81:178–187. [Google Scholar]
- 20.Szer W, Shugar D. Acta Biochim Polon. 1966;13:177–192. [PubMed] [Google Scholar]
- 21.Miah A, Reese CB, Song Q. Nucleosides Nucleotides. 1997;16:53–65. [Google Scholar]
- 22.Zhang J, Chang HM, Kane RR. Synlett. 2001;5:643–645. [Google Scholar]
- 23.Beier M, Pfleiderer W. Helv Chim Acta. 2003;86:2533–2545. [Google Scholar]
- 24.Hampton A, Nichol AW. Biochemistry. 1966;5:2076–2082. doi: 10.1021/bi00870a040. [DOI] [PubMed] [Google Scholar]
- 25.Roy SK, Tang J-y. Org Process Res Develp. 2000;4:170–171. [Google Scholar]
- 26.Brown DM, Todd AR, Varadarajan S. 1956:2388–2393. [Google Scholar]
- 27.Reese CB, Ubasawa A. Nucleic Acids Sym Series. 1980:5–21. [PubMed] [Google Scholar]
- 28.Ariza X, Vilarrasa J. J Org Chem. 2000;65:2827–2829. doi: 10.1021/jo9918706. [DOI] [PubMed] [Google Scholar]
- 29.Donohue J, Trueblood KN. J Mol Biol. 1960;2:363–371. doi: 10.1016/s0022-2836(60)80047-2. [DOI] [PubMed] [Google Scholar]
- 30.Haschemeyer AEV, Rich A. J Mol Biol. 1967;27:369–384. doi: 10.1016/0022-2836(67)90026-5. [DOI] [PubMed] [Google Scholar]
- 31.Miles DW, Robins RK, Eyring H. Proc Natl Acad Sci U S A. 1967;57:1138–1145. doi: 10.1073/pnas.57.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles DW, Robins MJ, Robins RK, Winkley MW, Eyring H. J Am Chem Soc. 1969;91:831–838. doi: 10.1021/ja01032a006. [DOI] [PubMed] [Google Scholar]
- 33.Neuhaus D, Williamson M. The Nuclear Overhauser Effect in Structural and Conformational Analysis. VCH Publisher: New York; 1989. [Google Scholar]
- 34.Rosemeyer H, Toth G, Golankiewicz B, Kazimierczuk Z, Bourgeois W, Kretschmer U, Muth HP, Seela F. J Org Chem. 1990;55:5784–5790. [Google Scholar]
- 35.Davis DR. Biophysical and conformational properties of modified nucleosides in RNA. In: Grosjean H, Benne R, editors. Modification and Editing of RNA. ASM Press; Wasington, D. C.: 1998. pp. 85–102. [Google Scholar]
- 36.Saenger W. Principles of Nucleic Acid Structure. 2. Springer-Verlag; New York: 1988. [Google Scholar]
- 37.Altona C, Sundaralingam M. J Am Chem Soc. 1973;95:2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- 38.Desaulniers JP, Chui HMP, Chow CS. Bioorg Med Chem. 2005;13:6777–6781. doi: 10.1016/j.bmc.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 39.Chang YC, Herath J, Wang THH, Chow CS. Bioorg Med Chem. 2008;16:2676–2686. doi: 10.1016/j.bmc.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall RH. The Modified Nucleosides in Nucleic Acid. Columbia University Press; New York: 1971. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.