Abstract
The results of analyses of Z, RNA-dependent RNA polymerase, glycoprotein precursor, and nucleocapsid protein gene sequence data suggested that Guanarito virus was the most common cause of Venezuelan hemorrhagic fever in a 7-year period in the 1990’s and that the evolution of Pirital virus in association with Sigmodon alstoni (Alston’s cotton rat) has occurred at a significantly higher rate than the evolution of Guanarito virus in association with Zygodontomys brevicauda (short-tailed cane mouse) on the plains of western Venezuela. The results of analyses of the primary structures of the glycoproteins of the 8 strains of Guanarito virus isolated from humans suggested that these strains would be highly cross-reactive in neutralization assays. Thus, passive antibody therapy may prove beneficial in the treatment of human disease caused by strains of Guanarito virus that are enzootic in the region in which Venezuelan hemorrhagic fever is endemic.
Keywords: Arenaviridae, Guanarito virus, Pirital virus, Venezuelan hemorrhagic fever, arenaviral hemorrhagic fever, passive antibody therapy
Introduction
The virus family Arenaviridae comprises 2 serocomplexes and 22 species (Salvato et al., 2005). The lymphocytic choriomeningitis-Lassa (Old World) complex includes Ippy virus (IPPYV), Lassa virus (LASV), lymphocytic choriomeningitis virus (LCMV), Mobala virus (MOBV), and Mopeia virus (MOPV). The Tacaribe (New World) complex includes Bear Canyon virus (BCNV), Tamiami virus (TAMV), and Whitewater Arroyo virus (WWAV) in the United States, Tacaribe virus (TCRV) on Trinidad in the Caribbean Sea, Pichindé virus (PICV) in Colombia, Allpahuayo virus (ALLV) in Peru, Amapari virus (AMAV), Cupixi virus (CPXV), Flexal virus (FLEV), and Sabiá virus (SABV) in Brazil, Junín virus (JUNV) and Oliveros virus (OLVV) in Argentina, Latino virus (LATV) and Machupo virus (MACV) in Bolivia, Paraná virus (PARV) in Paraguay, and Guanarito virus (GTOV) and Pirital virus (PIRV) in Venezuela.
Four New World arenaviruses naturally cause severe disease in humans (Peters, 2002). Junín virus, MACV, and GTOV are the etiological agents of Argentine hemorrhagic fever (AHF), Bolivian hemorrhagic fever (BHF), and Venezuelan hemorrhagic fever (VHF), respectively. These diseases are endemic in rural areas of the countries for which they were named. Sabiá virus was the etiological agent in a fatal case of hemorrhagic fever that died in a hospital in São Paulo, Brazil (Lisieux et al., 1994). The infection in this case was presumed to have been acquired from a natural source in Brazil.
Specific members of the order Rodentia (Musser and Carleton, 2005) are the principal hosts of the arenaviruses for which natural host relationships have been well characterized (Childs and Peters, 1993). For example, the drylands vesper mouse (Calomys musculinus) in central Argentina is the principal host of JUNV (Mills et al., 1992), a vesper mouse (Calomys species) in northeastern Bolivia is the principal host of MACV (Johnson et al., 1966; Salazar-Bravo et al., 2002), and the short-tailed cane mouse (Zygodontomys brevicauda) and Alston’s cotton rat (Sigmodon alstoni) in western Venezuela are the principal hosts of GTOV and PIRV, respectively (Fulhorst et al., 1997, 1999). It is assumed that humans usually become infected with arenaviruses by inhalation of virus in aerosolized droplets of secretions or excretions from infected rodents.
Previous studies (Fulhorst et al., 1997; Weaver et al., 2000) established that the geographical range of GTOV in association with Z. brevicauda includes 5 states in western Venezuela: Apure, Barinas, Cojedes, Guárico, and Portuguesa. Previous studies (Fulhorst et al., 1997; Weaver et al., 2001) also established that PIRV is enzootic in Apure, Barinas, Cojedes, Guárico, and Portuguesa.
Venezuelan hemorrhagic fever was first recognized as a distinct clinical entity in 1989 during an outbreak of hemorrhagic fever that began in the Municipality of Guanarito in southern Portuguesa (Salas et al., 1991). From September 1989 through December 2006, the State of Portuguesa recorded 618 VHF cases, with a case-fatality rate of 23.1% (unpublished data, N. M. C. de Manzione and H. Paredes). Almost all (98.7%) of these cases lived or worked in rural areas in the Municipality of Guanarito at the time that they became ill with VHF.
Guanarito virus is presumed to be the only agent of VHF. However, the majority of the arenaviruses isolated from VHF cases have been identified as strains of GTOV based solely on the results of a fluorescent antibody test in which there can be extensive cross-reactivity between PIRV and GTOV (Fulhorst et al., 1999). Thus, the arenaviruses isolated from some VHF cases may be strains of PIRV. The first objective of this study was to investigate whether PIRV is an agent of VHF.
The genomes of arenaviruses comprise 2 single-stranded RNA segments, designated large (L) and small (S) (Salvato et al., 2005). The L segment (~7.5 kb) consists of a 5′ non-coding region (NCR), the Z gene, an intergenic region (IR) that separates the Z gene from the RNA-dependent RNA polymerase (RdRp) gene, the RdRp gene, and a 3′ NCR. Similarly, the S segment (~3.5 kb) consists of a 5′ NCR, the glycoprotein precursor (GP-C) gene, an IR that separates the GP-C gene from the nucleocapsid (N) protein gene, the N protein gene, and a 3′ NCR.
Analyses of complete GP-C sequences and complete N protein sequences in previous studies (Archer and Rico-Hesse, 2002; Charrel et al., 2002) delineated 5 major phylogenetic lineages within the Arenaviridae: Old World (LASV and LCMV), North American (BCNV, TAMV, and WWAV), South American lineage A (ALLV, FLEV, PARV, PICV, and PIRV), South American lineage B (AMAV, CPXV, GTOV, JUNV, MACV, SABV, and TCRV), and South American lineage C (LATV and OLVV). Note that GTOV and PIRV were included in lineage B and lineage A, respectively. Also note that the analyses of the GP-C sequence data placed the North American lineage in a sister relationship to the South American lineage B whereas the analyses of the N protein sequence data placed the North American lineage in a sister relationship to the South American lineage A. This difference between the phylograms generated from GP-C sequence data and the phylograms generated from N protein sequence data was interpreted in previous studies (Archer and Rico-Hesse, 2002; Charrel et al., 2001, 2002) as evidence that the S genomic segments of the North American arenaviruses are a product of homologous recombination between the S segment of a lineage A virus and the S segment of a lineage B virus.
The GTOV prototype strain INH-95551 originally was isolated from a 20-year-old farm worker who succumbed to VHF in November 1990 (Tesh et al., 1994). The PIRV prototype strain VAV-488 originally was isolated from an Alston’s cotton rat captured in 1994 in southern Portuguesa (Fulhorst et al., 1997). Analyses of complete Z, RdRp, GP-C, and N protein gene sequence data in a previous study (Cajimat and Fulhorst, 2004) established that strain INH-95551 is phylogenetically distinct from strain VAV-488.
Studies done in Portuguesa in the 1990’s revealed that GTOV-infected short-tailed cane mice sometimes live in close physical association with PIRV-infected Alston’s cotton rats (Fulhorst et al., 1997), the short-tailed cane mouse is a natural host of PIRV (Weaver et al., 2001) as well as the principal host of GTOV, and Alston’s cotton rat is a natural host of GTOV (Weaver et al., 2000) as well as the principal host of PIRV. Hypothetically, cotton rat-to-cane mouse or cane mouse-to-cotton rat virus transmission could result in dual infections in individual rodents and ultimately the exchange of genetic elements between GTOV and PIRV. Thus, some VHF cases could have been caused by arenaviruses that are a product of genetic recombination between GTOV and PIRV. The second objective of this study was to increase our knowledge of the phylogenetic origins of arenaviruses causally associated with VHF.
There is no specific therapy for any of the human diseases caused by the South American arenaviruses; however, studies done in Argentina (Maiztegui et al., 1979; Ruggiero et al., 1986) demonstrated that immune plasma administered early in the course of disease can markedly reduce the mortality of AHF. Studies to assess the effect of immune plasma on the course and outcome of VHF, BHF, and hemorrhagic fever caused by SABV have not been done.
The benefit of immune plasma in the treatment of AHF has been positively associated with the capacity of such plasma to neutralize the infectivity of JUNV in vitro (Enria et al., 1984). The results of a study on the specificity of monoclonal antibodies raised against JUNV indicated that neutralization of JUNV in vitro is exclusively associated with the viral glycoprotein(s) (Sanchez et al., 1989), which are generated from the GP-C by proteolytic cleavage within infected cells.
Several structural features of the GP-C are conserved among the arenaviruses (reviewed in Buchmeier, 2002). These features include a signal peptidase cleavage site after amino acid residue 58 (numbered from the initiating methionine), a subtilase SKI-1/S1P cleavage site in the middle of the GP-C, and a highly hydrophobic membrane-spanning domain near the carboxyl terminus of the GP-C.
Co-translational cleavage of the GP-C by a cellular signal peptidase yields a signal peptide (SP) and G1-G2 polypeptide. Post-translational cleavage of the G1-G2 polypeptide by a cellular SKI-1/S1P protease yields the amino-terminal G1 and carboxy-terminal G2. The G1 is peripheral to the lipid envelope of the mature arenavirion, non-covalently associated with G2, and contains binding site(s) for the cellular receptor(s). The G2 is an integral membrane protein and, as such, anchors the G1-G2 heteromer in the lipid envelope of the mature arenavirion.
Previous studies established that 1) the dominant neutralizing epitopes on an arenavirion are associated with G1 (Buchmeier et al., 1981), 2) antibody-mediated neutralization in vitro usually is specific to an arenavirus species (Sanchez et al., 1989) and can vary from strain to strain within an arenavirus species (Jahrling and Peters, 1984; Parekh and Buchmeier, 1986), and 3) formation of intramolecular disulfide bonds and glycosylation during biosynthesis of the GP-C can affect the capacity of neutralizing antibodies to bind G1 (Wright et al., 1989). The third objective of this study was to examine the diversity of the primary structures of the GTOV glycoproteins, in particular – G1, as a prelude to the use of immune plasma, monoclonal antibodies, or synthetic immunomolecules (e.g., diabodies) for therapy of VHF.
Results
The nucleotide sequences of a 332- to 434-nt fragment near the 5′ end of the L segments, a 2132- to 2156-nt fragment near the 3′ end of the L segments, and a 3302- to 3352-nt fragment of the S segments of 7 previously uncharacterized arenaviruses from VHF cases, 4 GTOV strains from short-tailed cane mice, and 4 PIRV strains from Alston’s cotton rats were determined in this study (Table 1). The fragment of the 5′ half of the L segment extended from within the 5′ NCR through the stop codon of the Z gene, the fragment of the 3′ half of the L segment extended from within the RdRp gene through the 5′ end of the 3′ NCR, and the fragment of the S segment extended from within the 5′ NCR, through the GPC gene, IR, and N protein gene, and into the 3′ NCR. The GTOV strains from the short-tailed cane mice were selected to represent a large area within the geographical range of GTOV (Figure 1). Each GTOV strain from a short-tailed cane mouse was matched on locality with a PIRV strain from an Alston’s cotton rat. For example, GTOV strain VHF-3990 and PIRV strain 3945 were isolated from rodents captured in January 1997 on a farm near the town of San Carlos in northern Cojedes (Weaver et al., 2000, 2001).
Table 1.
Histories of the 17 Venezuelan arenaviruses
| Virus species | Strain | Host speciesa | Date of sample (month-year) | Clinical outcomeb | Statec | Locality | Map locationd |
|---|---|---|---|---|---|---|---|
| Guanarito virus | INH-95551 | Hsap | Nov 1990 | Fatal | POR | La Capilla | 8 |
| Guanarito virus | CVH-950801 | Hsap | Aug 1995 | Not fatal | POR | Caño Venado | 10 |
| Guanarito virus | CVH-960101 | Hsap | Jan 1996 | Fatal | POR | La Espinalúa | 5 |
| Guanarito virus | CVH-960102 | Hsap | Jan 1996 | Fatal | POR | Hoja Blanca | 9 |
| Guanarito virus | CVH-960103 | Hsap | Jan 1996 | Fatal | POR | La Cevereña | 6 |
| Guanarito virus | CVH-960302 | Hsap | Mar 1996 | Not fatal | POR | Morrones | 4 |
| Guanarito virus | CVH-961104 | Hsap | Nov 1996 | Not fatal | POR | Botucal | 2 |
| Guanarito virus | S-56764 | Hsap | Sep 1997 | Not fatal | POR | Banco de Marones | 3 |
| Guanarito virus | AV 97021119 | Zbre | Jan 1997 | n/a | POR | Caño Delgadito | 1 |
| Guanarito virus | VHF-1608 | Zbre | Mar 1995 | n/a | APE | Centro de Recriá | 13 |
| Guanarito virus | VHF-3990 | Zbre | Jan 1997 | n/a | COJ | Caño Hondo | 11 |
| Guanarito virus | VHF-1750 | Zbre | Mar 1995 | n/a | GRC | Palo Seco | 12 |
| Pirital virus | VAV-488 | Sals | Feb 1994 | n/a | POR | Pirital | 7 |
| Pirital virus | AV 97021016 | Sals | Feb 1997 | n/a | POR | Caño Delgadito | 1 |
| Pirital virus | 1645 | Sals | Mar 1995 | n/a | APE | Centro de Recriá | 13 |
| Pirital virus | 3945 | Sals | Jan 1997 | n/a | COJ | Caño Hondo | 11 |
| Pirital virus | 1743 | Sals | Mar 1995 | n/a | GRC | Palo Seco | 12 |
Hsap, Homo sapiens; Sals, Sigmodon alstoni; Zbre, Zygodontomys brevicauda.
n/a, not applicable.
APE, Apure; COJ, Cojedes; GRC, Guárico; POR, Portuguesa.
See Figure 1.
Figure 1.
Map of the study area showing the locations of the communities in which the VHF cases lived and the locations of the localities at which the Guanarito virus-infected short-tailed cane mice and Pirital virus-infected Alston’s cotton rats were captured. The rodents were captured at locations 1, 7, 11, 12, and 13. The inset shows the location of the study area within Venezuela.
Genetic diversity between the South American arenavirus species
Nonidentities (uncorrected distances) between the sequences of the Z genes and between the sequences of the RdRp genes of strains of different species ranged from 29.8% (PICV strain Co An 3739 and PIRV strain 1743) to 56.6% (PIRV strain 1645 and TCRV strain TRVL 11573) and from 32.7% (JUNV strain XJ13 and MACV stain Carvallo) to 54.5% (GTOV strain AV 97021119 and PIRV strain 3945), respectively. Similarly, nonidentities between the sequences of the GP-C genes and between the sequences of the N protein genes of strains of different species ranged from 27.3% (PICV strain Co An 3739 and PIRV strain VAV-488) to 50.7% (JUNV strain XJ13 and PICV strain Co An 3739, MACV strain Carvallo and PICV strain Co An 3739, and AMAV strain BeAn 70563 and PIRV strain 1645) and from 23.9% (JUNV strain XJ13 and MACV strain Carvallo) to 44.0% (PIRV strain 1743 and SABV strain SPH 114202), respectively.
Phylogenetic relationships among South American arenaviruses
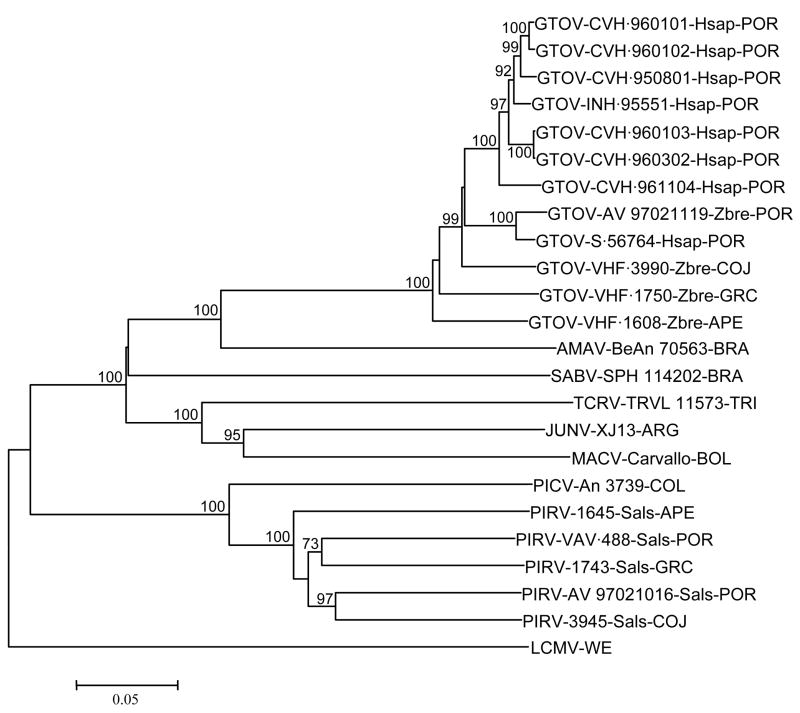
The neighbor-joining analysis of uncorrected genetic distances generated from the GP-C gene sequence alignment indicated that the 7 previously uncharacterized viruses from VHF cases are strains of GTOV (Figure 2). The neighbor-joining analysis of the uncorrected genetic distances generated from the GP-C gene sequence alignment also indicated that 1) GTOV is phylogenetically most closely related to AMAV, 2) PIRV strains 1645, 1743, 3945, AV 97021016, and VAV-488 are monophyletic, and 3) PIRV is phylogenetically most closely related to PICV (Figure 2). Monophyly of the 12 GTOV strains and monophyly of the 5 PIRV strains were strongly supported by the results of the bootstrap analysis.
Figure 2.
Phylogenetic relationships among 23 South American arenaviruses based on neighbor-joining analyses of uncorrected genetic distances generated from alignments of complete glycoprotein precursor (GP-C) gene sequences. The lengths of the horizontal branches are proportional to nucleotide sequence divergence, the length of the scale bar is equivalent to a sequence divergence of 0.05, and the numerical values at the nodes indicate the percentage of 1000 bootstrap replicates that supported the interior branches. Bootstrap support values less than 70% are not listed. The branch labels include (in the following order) virus species, strain, and country or state of origin. The branch labels for the GTOV strains and PIRV strains also include the species of the hosts from which these viruses were isolated. The lymphocytic choriomeningitis virus (LCMV) strain WE is an Old World arenavirus and was included in the analysis to infer the ancestral node within the group of New World arenaviruses. AMAV, Amapari virus; GTOV, Guanarito virus; JUNV, Junín virus; MACV, Machupo virus; PICV, Pichindé virus; PIRV, Pirital virus; SABV, Sabiá virus; TCRV, Tacaribe virus. Hsap, Homo sapiens; Sals, Sigmodon alstoni; Zbre, Zygodontomys brevicauda. ARG, Argentina; BRA, Brazil; BOL, Bolivia; COL, Colombia; TRI, Trinidad. APE, Apure; COJ, Cojedes; GRC, Guárico; POR, Portuguesa.
The results of the neighbor-joining analyses of the uncorrected genetic distances generated from the Z, RdRp, and N protein gene sequence alignments (not shown) were essentially identical to the results of the neighbor-joining analysis of the uncorrected genetic distances generated from the GP-C gene sequence alignment (Figure 2). Monophyly of the 12 GTOV strains and monophyly of the 5 PIRV strains in all the phylograms were strongly supported by the results of the bootstrap analyses. Differences between phylograms with respect to relationships within the GTOV lineage were few and not supported by the results of the bootstrap analyses. Differences between phylograms with respect to relationships within the PIRV lineage also were not supported by the results of the bootstrap analyses.
Genetic diversity between and within the Venezuelan arenavirus species
The Z genes of the 7 previously uncharacterized viruses from VHF cases and the Z genes of the 4 GTOV strains from short-tailed cane mice are identical in length to the Z gene of GTOV strain INH-95551 (Appendix 1). The GP-C genes and N protein genes of the previously uncharacterized viruses from VHF cases and GTOV strains from short-tailed cane mice also are identical in length to the homologous genes of GTOV strain INH-95551 (Appendix 2). The Z genes of PIRV strains 1645, 1743, 3945, and AV 97021016 are identical in length to the Z gene of PIRV strain VAV-488 (Appendix 1). However, the GP-C genes and N protein genes of strains 1645, 1743, 3945, and AV 97021016 are different in length from the homologous genes of strain VAV-488 (Appendix 2).
Nonidentities between the sequences of the Z genes and between the sequences of the RdRp genes of the 12 GTOV strains and 5 PIRV strains ranged from 47.3 to 53.0% and from 51.6 to 54.5%, respectively (Table 2). Nonidentities between the sequences of the GP-C genes and between the sequences of the N protein genes of the 12 GTOV strains and 5 PIRV strains ranged from 48.2 to 50.1% and from 39.9 to 41.3%, respectively (Table 3).
Table 2.
Nonidentities between the nucleotide sequences of the Z genes and between the nucleotide sequences of a fragment of the RNA-dependent RNA polymerase genes of 8 strains of Guanarito virus from humans, 4 strains of Guanarito virus from short-tailed cane mice, and 5 strains of Pirital virus from Alston’s cotton ratsa
| Z gene (% sequence nonidentity)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Strain(s) | from humans | AV 97021119 | VHF-1608 | VHF-3990 | VHF-1750 | VAV-488 | AV 97021016 | 1645 | 3945 | 1743 |
| Guanarito virus | from humansb | c | 4.9–7.4 | 11.6–14.0 | 7.4–10.2 | 10.5–11.9 | 48.4–50.5 | 48.4–49.8 | 49.8–50.9 | 51.3–52.7 | 48.7–50.9 |
| Guanarito virus | AV 97021119 | 3.8–4.7 | -- | 12.3 | 7.7 | 11.9 | 49.5 | 47.7 | 50.2 | 50.9 | 48.4 |
| Guanarito virus | VHF-1608 | 10.5–11.1 | 11.0 | -- | 12.6 | 10.9 | 53.0 | 49.8 | 52.7 | 52.3 | 50.5 |
| Guanarito virus | VHF-3990 | 6.8–8.1 | 6.8 | 11.6 | -- | 13.7 | 48.7 | 47.3 | 49.8 | 50.5 | 48.7 |
| Guanarito virus | VHF-1750 | 7.7–8.7 | 8.3 | 10.6 | 8.5 | -- | 50.9 | 48.7 | 50.9 | 52.7 | 51.6 |
| Pirital virus | VAV-488 | 52.0–52.5 | 52.1 | 51.6 | 51.7 | 52.2 | -- | 22.8 | 18.2 | 23.5 | 18.2 |
| Pirital virus | AV 97021016 | 52.5–53.0 | 53.4 | 53.3 | 52.5 | 52.5 | 31.6 | -- | 20.4 | 19.6 | 20.7 |
| Pirital virus | 1645 | 52.9–53.7 | 53.4 | 53.1 | 53.6 | 53.2 | 29.5 | 32.8 | -- | 21.8 | 16.8 |
| Pirital virus | 3945 | 53.9–54.3 | 54.5 | 54.1 | 54.3 | 53.9 | 29.8 | 24.1 | 31.8 | -- | 18.6 |
| Pirital virus | 1743 | 52.3–53.0 | 53.1 | 51.7 | 51.8 | 52.9 | 25.3 | 29.9 | 31.9 | 31.0 | -- |
|
|
|||||||||||
| RNA-dependent RNA polymerase gene (% sequence nonidentity) | |||||||||||
Nonidentities (p-model distances) between the nucleotide sequences of the Z genes and between the nucleotide sequences of a fragment of the RNA-dependent RNA polymerase (RdRp) genes are listed above and below the diagonal, respectively.
The strains of Guanarito virus from humans included the prototype strain INH-95551 and strains CVH-950801, CVH-960101, CVH-960102, CVH-960103, CVH-960302, CVH-961104, and S-56764. Guanarito virus strains AV 97021119, VHF-1608, VHF-3990, and VHF-1750 were from short-tailed cane mice captured in Portuguesa, Apure, Cojedes, and Guárico, respectively. Pirital virus strains VAV-488 and AV 97021016 and strains 1645, 3945, and 1743 were from Alston’s cotton rats captured in Portuguesa and Apure, Cojedes, and Guárico, respectively.
Nonidentities between the nucleotide sequences of the Z genes and between the nucleotide sequences of the RdRp gene sequences of the 8 Guanarito virus strains from humans ranged from 0.0 to 7.4% and from 0.0 to 5.6%, respectively.
Table 3.
Nonidentities between the sequences of the glycoprotein precursor genes and between the sequences of the nucleocapsid protein genes of 8 strains of Guanarito virus from humans, 4 strains of Guanarito virus from short-tailed cane mice, and 5 strains of Pirital virus from Alston’s cotton ratsa
| Glycoprotein precursor gene (% sequence nonidentity)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Strain(s) | from humans | AV 97021119 | VHF-1608 | VHF-3990 | VHF-1750 | VAV-488 | AV 97021016 | 1645 | 3945 | 1743 |
| Guanarito virus | from humansb | c | 2.4–7.8 | 9.5–10.0 | 6.6–7.9 | 9.3–10.0 | 48.5–49.3 | 48.2–49.2 | 48.5–49.7 | 48.7–49.4 | 48.7–49.9 |
| Guanarito virus | AV 97021119 | 2.5–7.3 | -- | 10.2 | 8.0 | 10.4 | 48.9 | 48.3 | 48.5 | 49.4 | 48.5 |
| Guanarito virus | VHF-1608 | 8.1–9.3 | 9.2 | -- | 9.3 | 9.7 | 49.7 | 48.9 | 49.5 | 48.5 | 48.9 |
| Guanarito virus | VHF-3990 | 6.0–7.1 | 6.8 | 8.9 | -- | 9.9 | 48.9 | 48.7 | 48.8 | 48.7 | 49.0 |
| Guanarito virus | VHF-1750 | 7.9–9.4 | 8.9 | 9.3 | 8.6 | -- | 50.1 | 50.1 | 49.4 | 48.7 | 49.0 |
| Pirital virus | VAV-488 | 40.8–41.3 | 41.0 | 41.2 | 40.9 | 41.0 | -- | 21.3 | 21.6 | 20.6 | 19.5 |
| Pirital virus | AV 97021016 | 40.4–41.3 | 40.1 | 41.0 | 40.7 | 41.1 | 21.2 | -- | 23.5 | 18.3 | 21.1 |
| Pirital virus | 1645 | 39.9–41.0 | 40.4 | 40.7 | 40.1 | 40.5 | 21.2 | 21.9 | -- | 23.5 | 21.8 |
| Pirital virus | 3945 | 40.1–40.4 | 40.3 | 40.8 | 40.0 | 40.8 | 21.6 | 17.9 | 21.7 | -- | 20.6 |
| Pirital virus | 1743 | 39.9–40.4 | 40.3 | 40.3 | 40.0 | 40.4 | 18.7 | 21.3 | 20.2 | 22.5 | -- |
|
|
|||||||||||
| Nucleocapsid protein gene (% sequence nonidentity) | |||||||||||
Nonidentities (p-model distances) between the sequences of the glycoprotein precursor (GP-C) genes and between the sequences of the nucleocapsid (N) protein genes are listed above and below the diagonal, respectively.
The strains of Guanarito virus from humans included the prototype strain INH-95551 and strains CVH-950801, CVH-960101, CVH-960102, CVH-960103, CVH-960302, CVH-961104, and S-56764. Guanarito virus strains AV 97021119, VHF-1608, VHF-3990, and VHF-1750 were from short-tailed cane mice captured in Portuguesa, Apure, Cojedes, and Guárico, respectively. Pirital virus strains VAV-488 and AV 97021016 and strains 1645, 3945, and 1743 were from Alston’s cotton rats captured in Portuguesa and Apure, Cojedes, and Guárico, respectively.
Nonidentities between the sequences of the GP-C genes and between the sequences of the N protein genes of the 8 Guanarito virus strains from humans ranged from 0.1 to 7.1% and from 0.2 to 7.5%, respectively.
Nonidentities between the sequences of the Z genes and between the sequences of the RdRp genes of the 12 GTOV strains ranged from 0.0 to 14.0% and from 0.0 to 11.6%, respectively (Table 2). Nonidentities between the sequences of the GP-C genes and between the sequences of the N protein genes of the 12 GTOV strains ranged from 0.1 to 10.4% and from 0.2 to 9.4%, respectively (Table 3).
Nonidentities between the sequences of the Z genes and between the sequences of the RdRp genes of the 5 PIRV strains ranged from 16.8% to 23.5% and from 24.1 to 32.8%, respectively (Table 2). Nonidentities between the sequences of the GP-C genes and between the sequences of the N protein genes of the 5 PIRV strains ranged from 18.3 to 23.5% and from 17.9 to 22.5%, respectively (Table 3).
The mean of the nonidentities between the nucleotide sequences of the GTOV strains from the short-tailed cane mice varied by gene and ranged from 8.6% (N protein gene) to 11.5% (Z gene) (Table 4). The mean of the nonidentities between the nucleotide sequences of PIRV strains 1645, 1743, 3945, and AV 97021016 also varied by gene but ranged from 19.7% (Z gene) to 30.3% (RdRp gene) (Table 4). By gene, the mean of the sequence nonidentities between the 4 GTOV strains from short-tailed cane mice was significantly less than the mean of the sequence nonidentities between PIRV strains 1645, 1743, 3945, and AV 97021016 (rank sum test, α = 0.05).
Table 4.
Mean genetic sequence nonidentities between 4 strains of Guanarito virus from rodents and between 4 strains of Pirital virus, by genea
| Geneb | Guanarito virus | Pirital virus |
|---|---|---|
| GP-C | 9.6% (8.0–10.4%) | 21.5% (18.3–23.5%) |
| N protein | 8.6% (6.8–9.3%) | 20.9% (17.9–22.5%) |
| Z | 11.5% (7.7–13.7%) | 19.7% (16.8–21.8%) |
| RdRp | 9.5% (6.8–11.6%) | 30.3% (24.1–32.8%) |
Guanarito virus strains AV 97021119, VHF-1608, VHF-3990, and VHF-1750 were from Portuguesa, Apure, Cojedes, and Guárico, respectively, and Pirital virus strains AV 97021016, 1645, 3945, and 1743 were from Portuguesa, Apure, Cojedes, and Guárico, respectively.
GP-C, glycoprotein precursor; N protein, nucleocapsid protein; RdRp, RNA-dependent RNA polymerase.
Diversity of the primary structures of the Guanarito virus glycoproteins
The GP-C of GTOV strain INH-95551 and the GP-C of the 11 GTOV strains characterized in this study each are 479 amino acid residues in length and contain a putative signal peptidase cleavage site after residue 58 (SCS58↓) and a putative SKI-1/S1P cleavage site after residue 245 (RKPL245↓ or RRPL245↓). Thus, the SP, G1, and G2 of these 12 viruses extend from the initiating methionine through residue 58, from residue 59 through residue 245, and from residue 246 through residue 479, respectively.
The hydropathy plot of the GP-C of GTOV strain INH-95551 included a hydrophilic region in G1 that extends from near residue 130 to near residue 192, 2 hydrophilic regions in G2 (1 from near residue 254 to near residue 416 and the other from near residue 447 to near residue 476), a highly hydrophobic region in G2 from near residue 416 to near residue 446, and a highly charged area at the carboxyl terminus of G2 (Figure 3). The hydrophobic region “between” residues 416 and 446 likely is the transmembrane domain in G2 and the highly charged area at the carboxyl terminus should be the cytoplasmic tail of G2. The hydropathy plots of the GP-C of the 7 previously uncharacterized GTOV strains from VHF cases and the hydropathy plots of the GP-C of the 4 GTOV strains from the short-tailed cane mice were visually indistinguishable from the hydropathy plot of the GP-C of strain INH-95551.
Figure 3.
Hydropathy plot of the glycoprotein precursor (GP-C) of Guanarito virus strain INH-95551. The plot was generated by using the Hopp-Woods scale and a window size of 7 residues. Analysis of the GP-C amino acid sequence suggested that the signal peptide (SP), G1, and G2 extend from the initiating methionine through residue 58, from residue 59 through residue 245, and from residue 246 through residue 479, respectively. It was expected that neutralizing epitopes would be clustered in hydrophilic regions with peak size > 0.5.
Nonidentities between the amino acid sequences of the GP-C (479 aa) and SP (58 aa) of the 12 GTOV strains ranged from 0.0 to 8.8% and from 0.0 to 12.1%, respectively, and nonidentities between the amino acid sequences of the G1 (187 aa) and G2 (234 aa) of the 12 GTOV strains ranged from 0.0 to 15.5% and from 0.0 to 4.7%, respectively (Table 5). There was at least 1 difference from the consensus of the 12 GP-C sequences at 74 positions: 9 in the SP, 45 in G1, 10 in the ectodomain of G2 (i.e., the region that extends from near residue 246 to near residue 416), 2 in the transmembrane domain, and 8 in the cytoplasmic tail of G2. Further, there was at least 1 difference from the consensus of the 12 sequences at 23 positions in the hydrophilic region of G1 (i.e., the region that extends from near residue 130 to near residue 192). The differences at 13 positions in the hydrophilic region of G1 were not conservative (Table 6).
Table 5.
Nonidentities between the amino acid sequences of the G1 glycoproteins and between the amino acid sequences of the G2 glycoproteins of 12 strains of Guanarito virusa
| G1 (% amino acid sequence nonidentity, 187 amino acids)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Sourceb | INH-95551 | CVH-950801 | CVH-960101 | CVH-960102 | CVH-960103 | CVH-960302 | CVH-961104 | S-56764 | AV 97021119 | VHF-1608 | VHF-3990 | VHF-1750 |
| INH-95551 | Hsap, POR | -- | 2.1 | 1.1 | 1.1 | 1.6 | 1.6 | 3.2 | 4.3 | 6.4 | 9.6 | 7.5 | 12.8 |
| CVH-950801 | Hsap, POR | 1.3 | -- | 1.1 | 1.1 | 3.7 | 3.7 | 3.7 | 5.9 | 8.0 | 11.2 | 8.6 | 13.9 |
| CVH-960101 | Hsap, POR | 0.9 | 0.9 | -- | 0.0 | 2.7 | 2.7 | 3.7 | 4.8 | 7.0 | 10.2 | 8.0 | 13.4 |
| CVH-960102 | Hsap, POR | 0.9 | 0.9 | 0.0 | -- | 2.7 | 2.7 | 3.7 | 4.8 | 7.0 | 10.2 | 8.0 | 13.4 |
| CVH-960103 | Hsap, POR | 0.4 | 0.9 | 0.4 | 0.4 | -- | 0.0 | 4.8 | 4.8 | 7.0 | 10.2 | 8.0 | 13.4 |
| CVH-960302 | Hsap, POR | 0.4 | 0.9 | 0.4 | 0.4 | 0.0 | -- | 4.8 | 4.8 | 7.0 | 10.2 | 8.0 | 13.4 |
| CVH-961104 | Hsap, POR | 2.1 | 2.6 | 2.1 | 2.1 | 1.7 | 1.7 | -- | 7.0 | 9.1 | 8.6 | 8.0 | 11.2 |
| S-56764 | Hsap, POR | 2.6 | 3.0 | 2.6 | 2.6 | 2.1 | 2.1 | 3.8 | -- | 2.1 | 10.7 | 8.6 | 14.4 |
| AV 97021119 | Zbre, POR | 3.4 | 3.4 | 3.4 | 3.4 | 3.0 | 3.0 | 4.7 | 2.6 | -- | 12.8 | 10.7 | 15.5 |
| VHF-1608 | Zbre, APE | 2.1 | 2.6 | 2.1 | 2.1 | 1.7 | 1.7 | 3.4 | 3.0 | 3.8 | -- | 10.7 | 13.4 |
| VHF-3990 | Zbre, COJ | 2.1 | 2.6 | 2.1 | 2.1 | 1.7 | 1.7 | 3.4 | 3.0 | 3.8 | 2.1 | -- | 13.9 |
| VHF-1750 | Zbre, GRC | 1.3 | 1.7 | 1.3 | 1.3 | 0.9 | 0.9 | 2.6 | 2.1 | 3.0 | 0.9 | 1.3 | -- |
|
|
|||||||||||||
| G2 (% amino acid sequence nonidentity, 234 amino acids) | |||||||||||||
Nonidentities (p-model distances) between the amino acid sequences of the G1 glycoproteins and between the amino acid sequences of the G2 glycoproteins are listed above and below the diagonal, respectively.
Hsap, Homo sapiens; Zbre, Zygodontomys brevicauda; POR, Portuguesa; APE, Apure; COJ, Cojedes; GRC, Guárico.
Table 6.
Non-conservative differences between the predicted amino acid sequences of the hydrophilic region in the G1 glycoproteins of 12 strains of Guanarito virus
| Amino acid residuea |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Sourceb | 130 | 131 | 132 | 164 | 167 | 170 | 179 | 186 | 200 | 203 | 204 | 205 | 206 |
| INH-95551 | Hsap-POR | I | K | G | T | A | T | P | S | I | D | S | Y | S |
| CVH-950801 | Hsap-POR | I | K | G | T | T | T | P | S | I | D | S | Y | S |
| CVH-960101 | Hsap-POR | I | K | G | T | T | T | P | S | I | D | S | Y | S |
| CVH-960102 | Hsap-POR | I | K | G | T | T | T | P | S | I | D | S | Y | S |
| CVH-960103 | Hsap-POR | I | K | G | T | A | T | P | S | I | D | S | Y | S |
| CVH-960302 | Hsap-POR | I | K | G | T | A | T | P | S | I | D | S | Y | S |
| CVH-961104 | Hsap-POR | I | E | G | A | I | T | P | S | I | D | S | Y | S |
| S-56764 | Hsap-POR | I | K | G | T | V | T | V | G | I | D | S | Y | P |
| AV 97021119 | Zbre-POR | I | K | D | T | V | T | V | G | I | D | S | Y | P |
| VHF-1608 | Zbre-APE | I | E | G | S | I | T | P | S | I | D | S | C | S |
| VHF-3990 | Zbre-COJ | T | M | G | S | V | R | P | S | I | D | S | Y | S |
| VHF-1750 | Zbre-GRC | T | E | G | R | I | K | L | S | T | G | N | H | S |
Positions of the residues are numbered from the initiating methionine of the glycoprotein precursor (GP-C).
Hsap, Homo sapiens; Zbre, Zygodontomys brevicauda; APE, Apure; COJ, Cojedes; GRC, Guárico; POR, Portuguesa.
The GP-C of all 12 GTOV strains contain cysteine residues at positions 57, 85, 129, 158, 159, 221, 265, 278, 287, 296, 350, 371, 420, 449, 461, and 463 and potential N-glycosylation sites at positions 88, 125, 174, 214, 314, 359, 376, and 381. With the exception of strain AV 97021119, each GP-C contains a sixteenth cysteine residue at position 116 and a ninth potential N-linked glycosylation site at position 351. The GP-C of strain VHF-1608 contains a seventeenth cysteine residue at position 205.
Statistical association between Guanarito virus and Venezuelan hemorrhagic fever
The 8 VHF cases in this study were 4.8% of the 166 VHF cases recorded by the State of Portuguesa in the 7-year period from November 1990 through September 1997. Note that these 8 cases represented a large portion of the geographical region in which VHF is endemic and that none of the cases were members of the same immediate family. Under the assumption that GTOV was the cause of 108 (65.1%) of the 166 VHF cases in the period from November 1990 through September 1997, the probability that all 8 isolates from the VHF cases in this study were strains of GTOV was 0.0035. Thus, GTOV likely was the cause of more than 65.1% (a majority) of the 166 VHF cases recorded by the State of Portuguesa in the 7-year period from November 1990 through September 1997.
Discussion
Together, the GTOV prototype strain INH-95551 and 7 other GTOV strains from humans in this study suggest that GTOV was the most common cause of VHF in the period from November 1990 through September 1997 (Table 1). Clearly, arenaviruses from a much greater number of VHF cases should be characterized to species level before it is concluded that PIRV is not pathogenic in humans and that GTOV is the only cause of arenaviral hemorrhagic fever in western Venezuela. The characterization of these viruses should include analyses of Z, RdRp, GP-C, and N protein gene sequence data to ensure that GTOV-PIRV chimeras are not mistaken for strains of GTOV or PIRV.
The short-tailed cane mouse is native to the plains of western Venezuela and can reach high densities in tall grassy (weedy) areas alongside roadways, cultivated agricultural fields, human dwellings, and outbuildings. The presence of GTOV-infected short-tailed cane mice in Apure, Barinas, Cojedes, and Guárico indicates that GTOV was enzootic in Portuguesa long before 1989. As such, the emergence of VHF likely was a consequence of demographic and/or ecological changes in rural areas of Portuguesa that ultimately resulted in a significant increase in the frequency of contact between humans and GTOV-infected rodents. Note that the density of the human population in rural areas of Portuguesa increased from 7.6 to 14.3 persons/km2 in the 10-year period that ended in 1990 (Table 7) and that this increase coincided with an increase in the utilization of native grasslands and forested areas in Portuguesa for agricultural purposes (Tesh et al., 1993).
Table 7.
Human population densities (persons/km2) in rural areas of Portuguesa and 4 other states in western Venezuelaa
| Census year
|
||||
|---|---|---|---|---|
| State | Area (km2) | 1980 | 1990 | 2000 |
| Portuguesa | 15,200 | 7.6 | 14.3 | 21.3 |
| Apure | 76,500 | 2.0 | 2.3 | 2.5 |
| Barinas | 35,200 | 2.4 | 3.8 | 5.0 |
| Cojedes | 14,800 | 1.9 | 2.1 | 3.4 |
| Guárico | 64,986 | 2.0 | 2.4 | 2.6 |
Source: Instituto Nacional de Estadistica Republica Bolivariana de Venezuela
The low level of nucleotide sequence nonidentity between GTOV strain INH-95551 and GTOV strains VHF-1608, VHF-3990, and VHF-1750 suggests that GTOV strains pathogenic for humans are enzootic in Apure, Cojedes, and Guárico. Yet all 618 VHF cases recorded by the State of Portuguesa in the period from September 1989 through December 2006 lived or worked in the Municipality of Guanarito, 5 of the 13 other municipalities in Portuguesa, or rural areas in Barinas near southern Portuguesa at the time that they became ill with VHF (unpublished data, N. M. C. de Manzione and H. Paredes).
Venezuelan hemorrhagic fever in Apure, Cojedes, and Guárico may be unrecognized because few persons in these states are exposed to GTOV-infected rodents. Note that the densities of the human populations in rural areas in these states in 2000 were significantly less than the density of the human population in rural areas in Portuguesa in 1990 (Table 7). Hypothetically, VHF will become a significant public health problem in Apure, Cojedes, and Guárico if the number of persons living or working in rural areas in these states increases and/or changes in the usage of land in these states favors large populations of short-tailed cane mice in close proximity to human dwellings or work activities. Future studies on the epidemiology of arenaviral hemorrhagic fever in Venezuela should include active surveillance for VHF in areas in Apure, Cojedes, and Guárico in which GTOV-infected rodents are enzootic.
The results of this study indicate that the primary structure of the hydrophilic region in the GTOV G1 and the number and locations of cysteine residues and potential N-linked glycosylation sites in the GTOV GP-C are highly conserved among GTOV strains that are enzootic in southern Portuguesa. Thus, immune plasma from VHF cases and monoclonal antibodies that neutralize the infectivity of GTOV strain INH-95551 in vitro may prove beneficial in the treatment of VHF caused by GTOV strains enzootic in southern Portuguesa. Clinical studies to assess the benefit of immune plasma in the treatment of VHF should be preceded by laboratory studies to assess the capacity of such plasma to neutralize the infectivity of arenavirus strains causally associated with VHF sensu lato. Note that convalescent sera from 6 of 7 putative VHF cases neutralized the infectivity of strain INH-95551 in Vero E6 cells and that the neutralizing titers in the positive sera ranged from 160 to 640 (unpublished data, C. F. Fulhorst).
The potential for autochthonous VHF to emerge in Apure, Cojedes, and Guárico should prompt development of safe, effective curative therapies for hemorrhagic fever caused by GTOV strains that are enzootic in these states. Obviously, the use of immune plasma from VHF cases in Portuguesa to treat VHF in Apure, Cojedes, or Guárico should be preceded by assessment of the capacity of such plasma to neutralize the infectivity of GTOV strains VHF-1608, VHF-3990, and VHF-1750, and other GTOV strains from Apure, Cojedes, and Guárico.
The results of studies done in Argentina (Enria and Maiztegui, 1994; Maiztegui et al., 1979) indicated that early administration of immune plasma (within 8 days from the onset of symptoms) is highly effective in reducing the mortality of AHF. Thus, the proper utilization of immune plasma, monoclonal antibodies, and synthetic immunomolecules in the treatment of VHF will require rapid, accurate assays for GTOV-specific RNA, antigen, or IgM in acute-phase clinical specimens. Together, the nucleotide sequences of GTOV strain INH-95551 and the 11 other GTOV strains in this study should prove useful in the development of assays for GTOV-specific RNA in acute-phase clinical specimens from VHF cases who live or work in Apure, Cojedes, or Guárico as well as VHF cases who live or work in Portuguesa or Barinas. Likewise, the nucleotide sequences of PIRV strain VAV-488 and the 4 other PIRV strains in this study should prove useful in the development of nucleotide sequence-based assays to distinguish PIRV infections from GTOV infections during the acute phase of severe febrile illnesses caused by PIRV.
Previously, our knowledge of genetic diversity within GTOV and within PIRV was based on nucleotide sequences of a 619-nt and 616-nt fragment of the N protein gene, respectively (Fulhorst 1997, 1999; Weaver 2000, 2001). Pairwise comparisons of the GTOV sequences revealed up to 10% nonidentity between strains of GTOV from different localities in western Venezuela (Weaver et al., 2000). In contrast, pairwise comparisons of the PIRV sequences revealed up to 26% nonidentity between strains of PIRV from different localities in western Venezuela (Weaver et al., 2001).
In this study, the highest level of nucleotide sequence nonidentity between PIRV strains was at least 60% greater than the highest level of nucleotide sequence nonidentity between GTOV strains isolated from short-tailed cane mice, regardless of gene examined (Table 4) and even though each PIRV strain was matched to a GTOV strain on locality and date of “collection”. Thus, the difference between PIRV and GTOV in level of within-species genetic diversity is not unique to the N protein gene.
Pairwise comparisons of N protein gene sequences in a previous study (Fulhorst et al., 1997) revealed up to 4.7% nonidentity between PIRV strains isolated from Alston’s cotton rats captured in 1994 on a small farm near Pirital in Portuguesa. Accordingly, maximum genetic diversity between PIRV strains from Pirital, Caño Delgadito, Centro de Recriá, Caño Hondo, and Palo Seco could be significantly higher than the value estimated from the nucleotide sequences of strains VAV-488, AV 97021016, 1645, 3945, and 1743.
The PIRV strains in this study were isolated from Alston’s cotton rats captured in a 3-year period and on an area slightly less than 30,000-km2. The factors that may have enabled phylogenetically distinct forms of PIRV to coexist on this small geographical area without competitive exclusion were detailed in a previous study (Weaver et al., 2001) and include geographical isolation of allopatric S. alstoni populations. Ecological changes that affect the movement of PIRV-infected rodents on the plains of western Venezuela now or in the future may significantly alter the magnitude or complexity of genetic diversity within PIRV in its association with Alston’s cotton rat and thereby affect the public health significance of PIRV.
The high level of genetic diversity within PIRV compared to GTOV suggests that the radiation of PIRV in association with S. alstoni over the plains in western Venezuela occurred much earlier than the radiation of GTOV in association with Z. brevicauda over the same geographical region. Alternatively, PIRV in association with S. alstoni evolved at a significantly higher rate than GTOV in association with Z. brevicauda or the movement of PIRV-infected rodents between allopatric populations of S. alstoni has been more restricted than the movement of GTOV-infected rodents between allopatric populations of Z. brevicauda. Studies to compare gene flow between allopatric populations of S. alstoni and between allopatric populations of Z. brevicauda may provide insight into the contribution of geographical isolation to the difference between PIRV and GTOV in level of within-species genetic diversity.
Materials and methods
Safety
All work with infectious GTOV was done inside the Maximum Containment Laboratory, which is a biosafety level 4 facility located on the Roybal Campus of the Centers for Disease Control and Prevention. All work with infectious PIRV was done inside a biosafety level 3 laboratory located on the campus of the University of Texas Medical Branch, Galveston.
Isolation of RNA and synthesis of first-strand cDNA
Monolayers of Vero E6 cells in 12.5- cm2 or 25.0-cm2 plastic flasks were inoculated with 0.2-mL of a 1:10 or 1:100 v:v dilution of stock virus. The inoculated cultures were maintained under a fluid medium at 37°C. Total RNA was isolated from adherent cells on the seventh or ninth day after inoculation, using TRIzol® Reagent (Invitrogen Life Technologies, Inc., Carlsbad, CA) or TriPure Isolation Reagent (Roche Applied Science, Indianapolis, IN). Reverse transcription of arenavirus-specific RNA was carried out as described previously (Cajimat and Fulhorst, 2004), using SuperScript II RNase H− Reverse Transcriptase (Invitrogen Life Technologies, Inc.) in conjunction with oligonucleotide 19C-cons (5′-CGCACMGWGGATCCTAGGC-3′). This oligonucleotide is a derivative of oligonucleotide ARE3′-END (Gonzalez et al., 1995) and was expected to prime synthesis of arenavirus-specific first-strand cDNA from 4 different templates: the 3′ end of the S segment, the 3′ end of the S segment replicative intermediate, the 3′ end of the L segment, and the 3′ end of the L segment replicative intermediate (Cajimat and Fulhorst, 2004).
PCR amplification of first-strand cDNA and sequencing reactions
The nucleotide sequence of the fragment at the 5′ end of the L segment was determined from a single PCR product. This PCR product was synthesized by using the Triple Master PCR System (Eppendorf North America, Inc., Westbury, NY) in conjunction with 19C-cons and an oligonucleotide designed to anneal to the L segment downstream from the IR. The nucleotide sequence of the fragment at the 3′ end of the L segment was determined from 2 large PCR products, designated “L3-1” and “L3-2”. The L3-1 product was synthesized by using the Triple Master PCR System in conjunction with 19C-cons and an oligonucleotide designed to anneal to the L segment first-strand cDNA upstream from the start codon of the RdRp gene. The L3-2 product was synthesized by using the Triple Master PCR System in conjunction with an oligonucleotide designed to anneal to a fragment within the L3-1 product and an oligonucleotide designed based on nucleotide sequences available from the GenBank nucleotide sequence database.
The nucleotide sequence of the S segment was determined from 3 overlapping PCR products, designated “S1”, “S2”, and “S3”. The S1 product extended from within the 5′ NCR to a site upstream from the stop codon of the GP-C gene, the S2 product extended from within the GP-C gene to a site downstream from the stop codon of the N protein gene, and the S3 product extended from a site immediately downstream from the stop codon of the N protein gene, through the start codon of the N protein gene, and into the 3′ NCR. The S1 and S3 products were generated by using 19C-cons in combination with oligonucleotides designed to anneal to GP-C and N protein gene-specific sequences, respectively. The S2 product was generated by using an oligonucleotide designed to anneal to a GP-C-specific sequence in combination with an oligonucleotide designed to anneal to an N protein gene-specific sequence.
All the PCR products for a particular virus were synthesized from first-strand cDNA generated in a single reverse transcriptase reaction. Both strands of the L5-END, L3-1, L3-2, S1, S2, and S3 PCR products were sequenced directly, using the Applied Biosystems Prism sequencing kit (Foster City, CA). The GenBank accession numbers for the sequences of the L segments are listed in Appendix 1, the GenBank accession numbers for the sequences of the S segments are listed in Appendix 2, and the sequences of the oligonucleotides that were used to prime the PCR and sequencing reactions are available from M. N. B. Cajimat at mnbcajimat@yahoo.com.
Data analysis
The Z, RdRp, GP-C, and N protein gene sequences of the 15 viruses characterized in this study were compared to the homologous sequences of AMAV strain BeAn 70563, GTOV strain INH-95551, JUNV strain XJ13, MACV strain Carvallo, PICV strain Co An 3739, PIRV strain VAV-488, SABV strain SPH 114202, and TCRV strain TRVL 11573 (Appendices 1 and 2). The predicted amino acid sequences were aligned by using the computer program Clustal W1.7 (Thompson et al., 1994). The nucleotide sequence alignments were constructed manually based on the alignments of the predicted amino acid sequences. The analyses of the nucleotide sequence data and the GP-C amino acid sequence data were done by using programs in the computer software package PAUP*, version 4.0b10 (Swofford, 2003) and MEGA, version 2.1 (Kumar et al., 2001). Nonidentities between sequences were equivalent to uncorrected (p model) distances, with all 3 nucleotide positions included in the calculation of distances. Neighbor-joining analyses were carried out on uncorrected distances generated from the nucleotide sequence alignments. Bootstrap support (Felsenstein, 1985) for the results of each neighbor-joining analysis was based on 1000 repetitions of the heuristic search, with random resampling of the data. The LCMV strain WE was included in the neighbor-joining analyses in order to infer the ancestral node within the group of New World arenaviruses.
Hydropathy plots of the GP-C of GTOV strain INH-95551, the 7 previously uncharacterized arenaviruses from VHF cases, and the 4 GTOV strains from short-tailed cane mice were generated, using the Hopp-Woods scale (Hopp and Woods, 1983) and a window size of 7 residues. It was expected that neutralizing epitopes would be clustered in hydrophilic regions with peak size > 0.5.
The probability that all 8 isolates from the VHF cases in this study were strains of GTOV was calculated by using the hypergeometric probability distribution, which describes the number of successes in a sequence of draws from a finite population when the draws are done without replacement. In the calculation, 8 was the number of successes, 8 was the number of draws, 166 was the size of the population, and we assumed that GTOV was the cause of 108 (65.1%) of the 166 VHF cases that occurred in the seven-year period from November 1990 through September 1997.
Supplementary Material
Acknowledgments
Guanarito virus strain S-56764 was acquired from Robert B. Tesh (World Reference Center for Emerging Viruses and Arboviruses, University of Texas Medical Branch, Galveston). Natalie A. Prow (University of Texas Medical Branch, Galveston) assisted with the genetic characterization of the viruses. This work was financially supported by National Institutes of Health grant AI-53428, entitled “Rapid, accurate diagnostic assays for arenaviral infections”. A Presidential Leave Award from John D. Stobo (President, University of Texas Medical Branch, Galveston) provided salary for Charles F. Fulhorst while he worked on this study at the Centers for Disease Control and Prevention.
Footnotes
Disclaimer: The findings and conclusions in this report do not necessarily represent the views of the funding agencies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Charles F. Fulhorst, Email: cfulhors@utmb.edu.
Maria N.B. Cajimat, Email: mnbcajimat@yahoo.com.
Mary Louise Milazzo, Email: mamilazz@utmb.edu.
Hector Paredes, Email: hparedes484@hotmail.com.
Rosa A. Salas, Email: salasros@carec.paho.org.
Pierre E. Rollin, Email: pyr3@cdc.gov.
Thomas G. Ksiazek, Email: tgk0@cdc.gov.
References
- Archer AM, Rico-Hesse R. High genetic divergence and recombination in arenaviruses from the Americas. Virology. 2002;304:274–281. doi: 10.1006/viro.2002.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier MJ, Lewicki HA, Tomori O, Oldstone MBA. Monoclonal antibodies to lymphocytic choriomeningitis and Pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981;113:73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier MJ. Arenaviruses: protein structure and function. Curr Top Microbiol Immunol. 2002;262:159–173. doi: 10.1007/978-3-642-56029-3_7. [DOI] [PubMed] [Google Scholar]
- Cajimat MNB, Fulhorst CF. Phylogeny of the Venezuelan arenaviruses. Virus Res. 2004;102:199–206. doi: 10.1016/j.virusres.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Charrel RN, de Lamballerie X, Fulhorst CF. The Whitewater Arroyo virus: natural evidence for genetic recombination among Tacaribe serocomplex viruses (family Arenaviridae) Virology. 2001;283:161–166. doi: 10.1006/viro.2001.0874. [DOI] [PubMed] [Google Scholar]
- Charrel RN, Feldmann H, Fulhorst CF, Khelifa R, de Chesse R, de Lamballerie X. Phylogeny of New World arenaviruses based on the complete coding sequences of the small genomic segment identified an evolutionary lineage produced by intrasegmental recombination. Biochem Biophys Res Commun. 2002;296:1118–1124. doi: 10.1016/s0006-291x(02)02053-3. [DOI] [PubMed] [Google Scholar]
- Childs JE, Peters CJ. Ecology and epidemiology of arenaviruses and their hosts. In: Salvato MS, editor. The Arenaviridae. Plenum Press; New York: 1993. pp. 331–384. [Google Scholar]
- Enria DA, Briggiler AM, Fernandez NJ, Levis SC, Maiztegui JI. Importance of dose of neutralizing antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet. 1984;2:255–256. doi: 10.1016/s0140-6736(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Enria D, Maiztegui JI. Antiviral treatment of Argentine hemorrhagic fever. Antivir Res. 1994;23:23–31. doi: 10.1016/0166-3542(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fulhorst CF, Bowen MD, Salas RA, de Manzione NMC, Duno G, Utrera A, Ksiazek TG, Peters CJ, Nichol ST, de Miller E, Tovar D, Ramos B, Vasquez C, Tesh RB. Isolation and characterization of Pirital virus, a novel South American arenavirus. Am J Trop Med Hyg. 1997;56:548–553. doi: 10.4269/ajtmh.1997.56.548. [DOI] [PubMed] [Google Scholar]
- Fulhorst CF, Bowen MD, Salas RA, Duno G, Utrera A, Ksiazek TG, de Manzione NMC, de Miller E, Vasquez C, Peters CJ, Tesh RB. Natural rodent host associations of Guanarito and Pirital viruses (family Arenaviridae) in central Venezuela. Am J Trop Med Hyg. 1999;61:325–330. doi: 10.4269/ajtmh.1999.61.325. [DOI] [PubMed] [Google Scholar]
- Gonzalez JP, Sanchez A, Rico-Hesse R. Molecular phylogeny of Guanarito virus, an emerging arenavirus affecting humans. Am J Trop Med Hyg. 1995;53:1–6. [PubMed] [Google Scholar]
- Hopp TP, Woods KR. A computer program for predicting protein antigenic determinants. Mol Immunol. 1983;20:483–489. doi: 10.1016/0161-5890(83)90029-9. [DOI] [PubMed] [Google Scholar]
- Jahrling PB, Peters CJ. Passive antibody therapy of Lassa fever in cynomologus monkeys: importance of neutralizing antibody and Lassa virus strain. Infect Immun. 1984;44:528–533. doi: 10.1128/iai.44.2.528-533.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Kuns ML, Mackenzie RB, Webb PA, Yunker CE. Isolation of Machupo virus from wild rodent Calomys callosus. Am J Trop Med Hyg. 1966;15:103–106. doi: 10.4269/ajtmh.1966.15.103. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- Lisieux T, Coimbra M, Nassar ES, Burattini MN, de Souza LT, Ferreira I, Rocco IM, Travassos da Rosa AP, Vasconcelos PF, Pinheiro FP, LeDuc JW, Rico-Hesse R, Gonzalez JP, Jahrling PB, Tesh RB. New arenavirus isolated in Brazil. Lancet. 1994;343:391–392. doi: 10.1016/s0140-6736(94)91226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiztegui JI, Férnandez NJ, Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2:1216–1217. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- Mills JN, Ellis BA, McKee KT, Jr, Calderon GE, Maiztegui JI, Nelson GO, Ksiazek TG, Peters CJ, Childs JE. A longitudinal study of Junin virus activity in the rodent reservoir of Argentine hemorrhagic fever. Am J Trop Med Hyg. 1992;47:749–763. doi: 10.4269/ajtmh.1992.47.749. [DOI] [PubMed] [Google Scholar]
- Musser GG, Carleton MD. Superfamily Muroidea. In: Wilson DE, Reeder DM, editors. Mammal Species of the World. A Taxonomic and Geographic Reference. Vol. 3. Johns Hopkins University Press; Baltimore: 2005. pp. 894–1522. [Google Scholar]
- Parekh BS, Buchmeier MJ. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology. 1986;153:168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Peters CJ. Human infection with arenaviruses in the Americas. Curr Top Microbiol Immunol. 2002;262:65–74. doi: 10.1007/978-3-642-56029-3_3. [DOI] [PubMed] [Google Scholar]
- Ruggiero HA, Perez Isquierdo F, Milani HA, Barri A, Val A, Maglio F, Astarloa L, Gonzalez Cambaceres C, Milani HL, Tallone JC. Treatment of Argentine hemorrhagic fever with convalescent’s plasma. 4433 cases. Presse Med. 1986;15:2239–2242. [PubMed] [Google Scholar]
- Salas R, de Manzione N, Tesh RB, Rico-Hesse R, Shope RE, Betancourt A, Godoy O, Bruzual R, Pacheco ME, Ramos C, Taibo ME, Tamayo JG, Jaimes E, Vazquez C, Araoz F, Querales J. Venezuelan haemorrhagic fever. Lancet. 1991;338:1033–1036. doi: 10.1016/0140-6736(91)91899-6. [DOI] [PubMed] [Google Scholar]
- Salazar-Bravo J, Dragoo JW, Bowen MD, Peters CJ, Ksiazek TG, Yates TL. Natural nidality in Bolivian hemorrhagic fever and the systematics of the reservoir species. Infect Genet Evol. 2002;1:191–199. doi: 10.1016/s1567-1348(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Salvato MS, Clegg JCS, Buchmeier MJ, Charrel RN, Gonzalez JP, Lukashevich IS, Peters CJ, Rico-Hesse R, Romanowski V. Family Arenaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; 2005. pp. 725–733. [Google Scholar]
- Sanchez A, Pifat DY, Kenyon RH, Peters CJ, McCormick JB, Kiley MP. Junin virus monoclonal antibodies: characterization and cross-reactivity with other arenaviruses. J Gen Virol. 1989;70:1125–1132. doi: 10.1099/0022-1317-70-5-1125. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods), version 4. Sunderland, Massachusetts: Sinauer Associates; 2003. [Google Scholar]
- Tesh RB, Wilson ML, Salas R, de Manzione NMC, Tovar D, Ksiazek TG, Peters CJ. Field studies on the epidemiology of Venezuelan hemorrhagic fever: implication of the cotton rat Sigmodon alstoni as the probable rodent reservoir. Am J Trop Med Hyg. 1993;49:227–235. doi: 10.4269/ajtmh.1993.49.227. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Jahrling PB, Salas RA, Shope RE. Description of Guanarito virus (Arenaviridae: Arenavirus), the etiologic agent of Venezuelan hemorrhagic fever. Am J Trop Med Hyg. 1994;50:452–459. doi: 10.4269/ajtmh.1994.50.452. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W (1.7): improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choices. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Salas RA, de Manzione N, Fulhorst CF, Duno G, Utrera A, Mills JN, Ksiazek TG, Tovar D, Tesh RB. Guanarito virus (Arenaviridae) isolates from endemic and outlying localities in Venezuela: sequence comparisons among and within strains isolated from Venezuelan hemorrhagic fever patients and rodents. Virology. 2000;266:189–195. doi: 10.1006/viro.1999.0067. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Salas RA, de Manzione N, Fulhorst CF, Travasos da Rosa APA, Duno G, Utrera A, Mills JN, Ksiazek TG, Tovar D, Guzman H, Kang W, Tesh RB. Extreme genetic diversity among Pirital virus (Arenaviridae) isolates from western Venezuela. Virology. 2001;285:110–118. doi: 10.1006/viro.2001.0954. [DOI] [PubMed] [Google Scholar]
- Wright KE, Salvato MS, Buchmeier MJ. Neutralizing epitopes of lymphocytic choriomeningitis virus are conformational and require both glycosylation and disulfide bonds for expression. Virology. 1989;171:417–426. doi: 10.1016/0042-6822(89)90610-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.