Abstract
Purpose
Venous neointimal hyperplasia in hemodialysis grafts is characterized by both proliferation and migration of vascular smooth muscle cell and adventitial fibroblasts. Since fetuin-A is a cytokine which has multifunctional effects on both vascular smooth muscle cells and fibroblasts; we examined the course of its expression in early venous stenosis formation in a porcine model of chronic renal insufficiency with arteriovenous polytetrafluoroethylene (PTFE) grafts.
Methods and materials
Pigs had chronic renal insufficiency created by complete embolization of the left kidney and partial embolization of the right kidney. Twenty eight days later, arteriovenous PTFE grafts were placed from the carotid artery to the ipsilateral jugular vein and the animals were sacrificed 3 days (N=4), 7 days (N=4), and 14 days (N=4) later. Expression of fetuin-A was determined by Western blotting at the venous stenosis, control veins, and plasma. Immunohistochemical analysis of the venous stenosis and control vein was performed. Blood urea nitrogen (BUN) and creatinine before embolization and at time of graft placement was determined.
Results
The mean BUN and creatinine at graft placement was significantly higher than the pre embolization values (P<0.05). Severe venous neointimal hyperplasia occurred by day 14 which was characterized by primarily α-smooth muscle actin positive cells. By day 14, fetuin-A had increased significantly at the venous stenosis and serum of the animals when compared to control veins and prior to embolization, respectively (P<0.05).
Conclusions
We observed significantly increased expression of fetuin-A in early venous stenosis by day 14 and serum compared to baseline. Understanding the role of fetuin-A in venous neointimal hyperplasia could help in improving outcomes in hemodialysis patients.
Introduction
There are currently more than 400,000 patients with end-stage renal disease (ESRD) in the United States (1). Although arteriovenous fistulas are preferred as vascular access for hemodialysis, expanded polytetrafluoroethylene (ePTFE) arteriovenous (AV) grafts are commonly used for dialysis access in many patients. The enormity of the clinical problem lies in the lack of durability of these grafts as only 50% of the grafts are functioning at 1 year and 25% at 2 years and with patency after angioplasty being 40% at 6-months (2). Estimates suggest that hemodialysis access dysfunction costs more than one billion dollars representing an enormous medical and financial burden on the nation and many patients (1). However, the specific mechanisms initiating venous stenosis caused by venous neointimal hyperplasia and subsequent thrombosis remain unknown.
In patients with failed hemodialysis access, increased vascular smooth muscle cell proliferation and migration with matrix deposition have been felt to be the etiology of venous neointimal hyperplasia (3). Recent data suggests that there is adventitial migration and proliferation of fibroblasts which dedifferentitate into myofibroblasts with translocation of these cells to the endothelium resulting in venous neointimal hyperplasia (4). At a cellular level, many proteins have been identified to be associated with failed hemodialysis access including vascular endothelial growth factor-A (VEGF-A), platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), basic fibroblast growth factor (BFGF), and matrix metalloproteinases (MMPs) (5–8).
Recently there have been many reports in the literature on hemodialysis patients with abnormal fetuin-A levels which have been shown to be the etiology of increased calcification in blood vessels (9). This contention is based on the fact that fetuin-A knockout mice have been shown to develop severe soft-tissue and intravascular calcifications (9). On a cellular level, fetuin-A is a multifunctional molecule and acts as an antagonist of transforming growth factor-β, regulating cytokine-dependent osteogenesis, and inhibiting insulin receptor tyrosine kinase and some protease activities (10). It has been shown recently that it can also increase pro MMP-9 protein secretion by monocytes and it has been localized to arteries of patients on hemodialysis (11). Increased expression of TGF-β1 and MMPs have been observed in failed hemodialysis access grafts (12). Finally, recent experimental data shows that fetuin-A has been shown to have an effect on vascular smooth muscle cells and fibroblasts, the major cell phenotypes observed in venous neointimal hyperplasia (4, 13).
Previous studies have been performed in animals with normal kidney function, (4, 5, 7, 14–18), however, in the experimental animal model used in the present study, chronic renal insufficiency was induced prior to graft placement (19, 20). The purpose of the present study was to determine the expression of fetuin-A in early venous stenosis formation in an experimental porcine model of renal failure with a PTFE arteriovenous hemodialysis graft.
Materials and methods
Study design
Institutional Animal Care and Use Committee approval was obtained prior to performing any procedures on animals. Housing and handling of the animals was performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals revised in 2000. Twelve castrated juvenile male pigs (40–50 kg, domestic swine, Larson Products, Sargeant, MN) had chronic renal insufficiency created by renal artery embolization (19). Twenty eight days later, arteriovenous PTFE grafts were placed from the carotid artery to the ipsilateral jugular vein. Protein expression for fetuin-A was determined by Western blotting at the venous stenosis, inflow artery, and control vessels at day 3 (N=4), day 7 (N=4), and day 14 (N=4) following graft placement. In addition, the expression of fetuin-A was determined in the serum of the blood of the animals before creation of renal insufficiency and at time of graft placement. The BUN and creatinine before renal artery embolization and at time of graft placement were determined.
Creation of chronic renal insufficiency by renal artery embolization
Prior to all procedures, animals were kept NPO (nothing per oral) for 12 h. They were initially anesthetized with a combination of 5 mg/kg tiletamine hydrochloride (50 mg/mL) and zolazepam hydrochloride (50 mg/mL), 2 mg/kg xylazine (Bayer, Shawnee Mission, Kansas), and 0.06 mg/kg glycopyrrolate given intramuscularly. To induce additional anesthesia, an intravenous (IV) fluid line was placed in the ear vein for the delivery of zolazepam hydrochloride (5 mg/kg) as needed. During the procedure, the animals were intubated and placed on a positive-pressure ventilator delivering oxygen (3–5 mL/kg) and isoflurane (1%–3%). The end-tidal CO2 volume, oxygen saturation, heart rate, electrocardiogram, and blood pressure were monitored throughout the surgical procedure.
Chronic renal insufficiency was created by embolizing the renal artery (19, 20). The embolization procedure was standardized so that the left kidney was totally embolized and either the upper or lower artery of the right kidney was embolized. Typically, there was a single renal artery supplying each kidney with one upper and one lower polar branch with each polar branch having 2 to 3 branches. The decision to embolize either the upper or lower pole was made based upon which polar branch supplied the lesser amount of renal parenchyma.
Six F sheaths were placed in the right femoral artery and the left renal artery was selected by using a 5F tapered angled glide catheter (Boston Scientific, Natick, MA). Through this catheter, 150 to 250-µm polyvinyl acrylide (PVA) particles (PVA Contour, Boston Scientific, Boston, MA) were infused until the left renal artery was completely occluded. Next, either the right upper or lower pole artery was selected and embolized completely in a similar fashion. The sheath was removed and hemostasis was obtained by manual compression. The pig was treated for 5 days with antibiotics to prevent infection. Blood urea nitrogen (BUN), creatinine, and blood were determined prior to embolization and at the time of graft placement by removing 10-mL of blood from a peripheral vein. The pig was extubated, monitored postoperatively, and started on normal pig diet (Lean Gain 95, Land O’Lakes, Inc. St. Paul, MN). The expression of fetuin-A was determined in the serum of the blood removed prior to the embolization.
Polytetrafluoroethylene graft placement
Polytetrafluoroethylene grafts were placed twenty eight days after renal artery embolization (5). An arteriovenous PTFE graft (4-mm diameter by 7-cm long, Gore, Flagstaff, AZ) was placed from either the right or left carotid artery to the ipsilateral jugular vein (Fig. 1). The contralateral vessels were isolated surgically at the time of graft placement to serve as controls. Ten-mL of blood was removed at time of graft placement and the serum used for determining fetuin-A expression. Plavix (75 mg by mouth, Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Bridgewater, NJ) was started the night before the graft placement and given daily until the animal was sacrificed.
Figure 1.
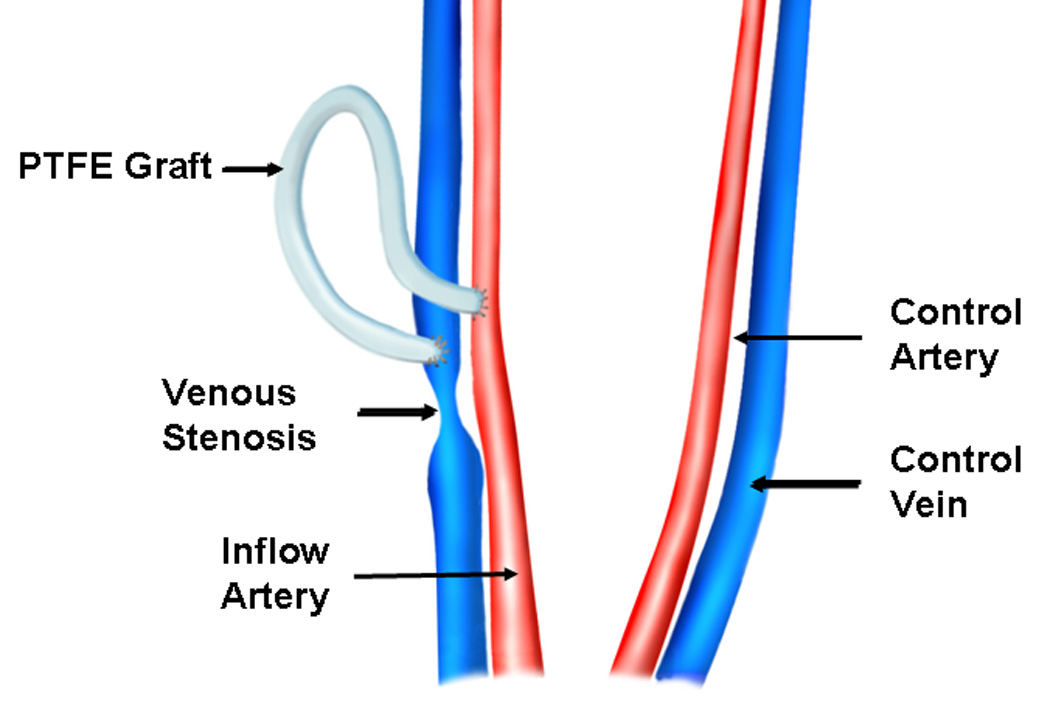
Placement of hemodialysis polytetrafluoroethylene grafts.
Vessel harvesting
The animals were sacrificed at 3 days (N=4), 7 days (N=4), and 14 days (N=4) after graft placement. To harvest the venous stenosis, inflow artery, and control vessels, both the carotid and jugular vessels were dissected free of the surrounding soft tissue and a heparin bolus of 250 units/kg was given intravenously. Venous stenosis formed predictably at the vein-to-graft anastomosis. The vein-to-graft anastomosis approximately 2-cm towards the heart (venous stenosis, Fig. 1), inflow artery, and control vessels were removed as described previously (5). The specimen was sectioned transversely into two equal parts and half was snap frozen in liquid nitrogen and stored at −80 °C for Western blotting and the other half stored in formalin for 24 hours and then embedded in paraffin for immunohistochemical analysis (5).
Immunohistochemical analysis
Hematoxylin and eosin (H & E) and α-smooth muscle staining were performed on specimens embedded in paraffin from the vein-to-graft anastomosis and control vessels as described previously (5).
Sample preparation for Western blot
Specimens were thawed at room temperature (RT) and all graft material was removed by careful dissection and then washed 3 X with 1.0-mL washing buffer (0.45 mM Tris, pH 8.5). The vessels were then sliced with a surgical knife into thin pieces and then put into a denaturing buffer (0.5 mM Tris plus 0.1% SDS). An electronic glass grinder was used to homogenize the blood vessels. The supernatant was separated by centrifugation at 14,000 RPM for 10 min. In a similar fashion, the plasma was separated from the blood removed prior to embolization and at the time of graft placement for Western blots for fetuin-A (see later). The protein concentration of the supernatant was measured with a Bio-Rad (Hercules, CA) protein assay kit and 100-µgs of protein from each of the venous stenosis and control vessels (contralateral vessels) were used for the Western blot.
Western Blot of Fetuin-A
To determine fetuin-A expression, we used Western blotting on whole vessel lysate as previously described (21). The antibody used for fetuin-A was rabbit anti-human (Abcam, Cambridge, MA).
Statistical analysis
The Western blot data, BUN, and creatinine are presented as mean ± SD. Analysis of variance (ANOVA) was used first to compare the means across the day 3, day 7, and day 14 groups. If the ANOVA F-test P-value was statistically significant (P < 0.05) or showed a trend toward significance, a Student t test was performed. The P-values for Western blots for the venous stenosis and control veins were calculated using a one-sample Student t-test to test the null hypothesis that the mean ratio of the venous stenosis to control vein for fetuin-A was different from 1. For the serum samples, this was calculated to determine if there was a difference between the pre embolization values and the values at the time of graft placement. P-value of less than or equal to 0.05 was considered statistically significant. SAS version 9, (SAS Institute Inc., Cary, NC) was used for statistical analyses.
Results
Surgical outcomes
Twelve castrated juvenile male pigs weighing 48.6 ± 1.2 kg underwent total embolization of the left kidney (12) and partial embolization of the right kidney (2 upper pole and 10 lower poles). There were no complications as a result of the embolization procedure other than the expected induction of renal insufficiency. The BUN and creatinine prior to embolization was 8.36 ± 2.01 mg/dL and 1.27 ± 0.18 mg/dL, respectively; and at time of graft placement increased to 17.1 ± 9.12 mg/dL (P < 0.05 when compared to pre) and 2.17 ± 0.57 mg/dl (P < 0.05 when compared to pre), respectively. Twenty eight days later, twelve pigs underwent placement of 12 (4-mm by 7-cm PTFE grafts) - 2 grafts on the left and 10 grafts on the right with the contralateral vessels serving as controls. All grafts were patent on follow-up as documented by auscultation with a bruit over the anastomosis.
Immunohistochemical analysis
Hematoxylin and eosin stained vein-to-graft anastomosis revealed a thickened neointima and media by day 14 (Fig. 2A) when compared to the contralateral (control) vessels (Fig. 2B). The neointima was composed primarily of α-smooth muscle actin positive cells (Fig. 2C) when compared to control vessels (Fig. 2D).
Figure 2.
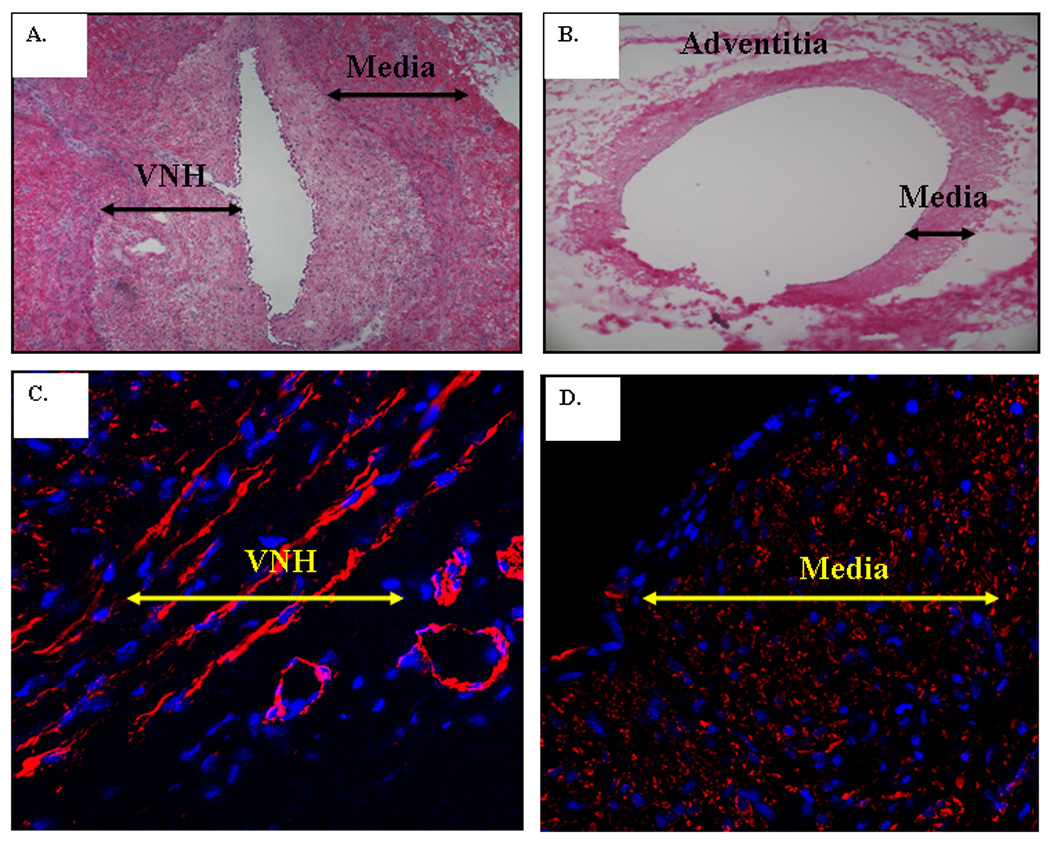
Hematoxylin and eosin staining was performed at the vein to graft anastomosis (A) and contralateral control vessel (B). There is venous neointimal hyperplasia (VNH) and thickened media when compared to the control vein (B). L is the lumen. Alpha smooth muscle actin staining was performed in C and D. Blue is cell nuclei and red is α-smooth muscle actin. Cells (red) in the neointima stained positive for a-smooth muscle actin (C) and the control vein (D) is negative for α-smooth muscle actin.
Western blot of Fetuin-A
We determined protein expression of fetuin-A by Western blot on the venous stenosis, inflow artery, control vessels, and plasma before renal artery embolization, and at time of graft placement. Scanning densitometric values from the immunoblots obtained from protein samples of the stenotic vein were divided by the control vein for each time point and then normalized for differences in protein loading between samples (Fig. 3 and Fig 4). By day 14, the mean fetuin-A at the vein-to-graft anastomosis to control vein was statistically significant (1.43 ± 0.11, P<0.05, Fig. 3). There was no difference in protein expression in fetuin-A between the inflow artery and control artery. Because fetuin-A has been found to be increased in serum of patients with chronic kidney disease, we also determined fetuin-A expression in serum plasma of 12 animals at baseline (before embolization) and in 12 animals at time of graft placement after embolization. There was significant increase in fetuin-A expression in plasma at time of graft placement (33 ± 6.9) when compared to before embolization (23.8 ± 4.5, P<0.05, Fig. 4).
Figure 3.
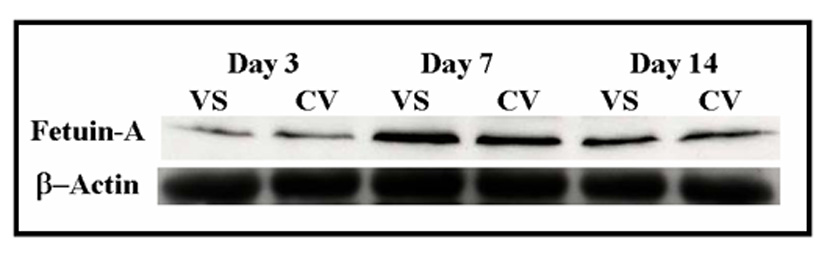
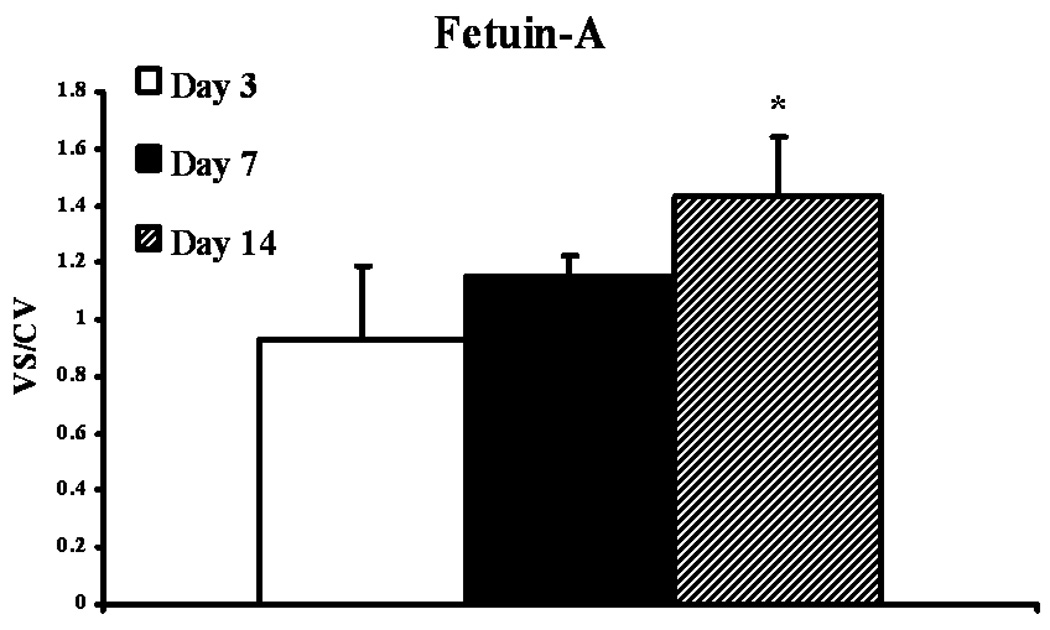
Fetuin-A expression in the venous stenosis compared to control vein. A (upper panel) is the Western blot of fetuin-A at day 3, day 7, and day 14, and lower panel is Western blot for actin showing equal loading of protein. VS is the venous stenosis and CV is the control vein. B is the pooled data of fetuin-A expression at day 3, day 7, and day 14. In PTFE grafts, expression of fetuin-A (*) was significantly higher than the control vein by day 14 (P<0.05). Data are presented as mean ± SD.
Figure 4.
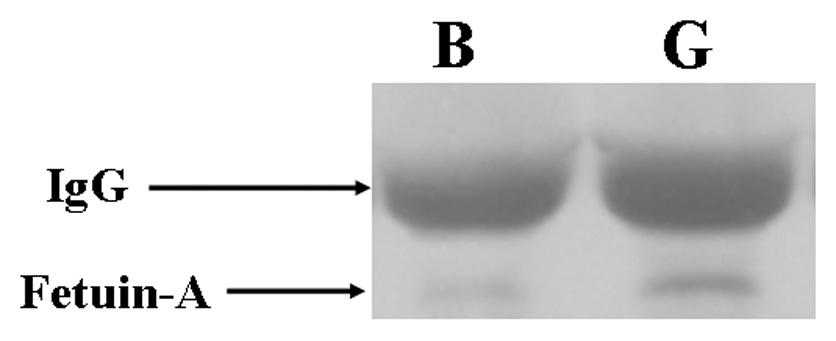
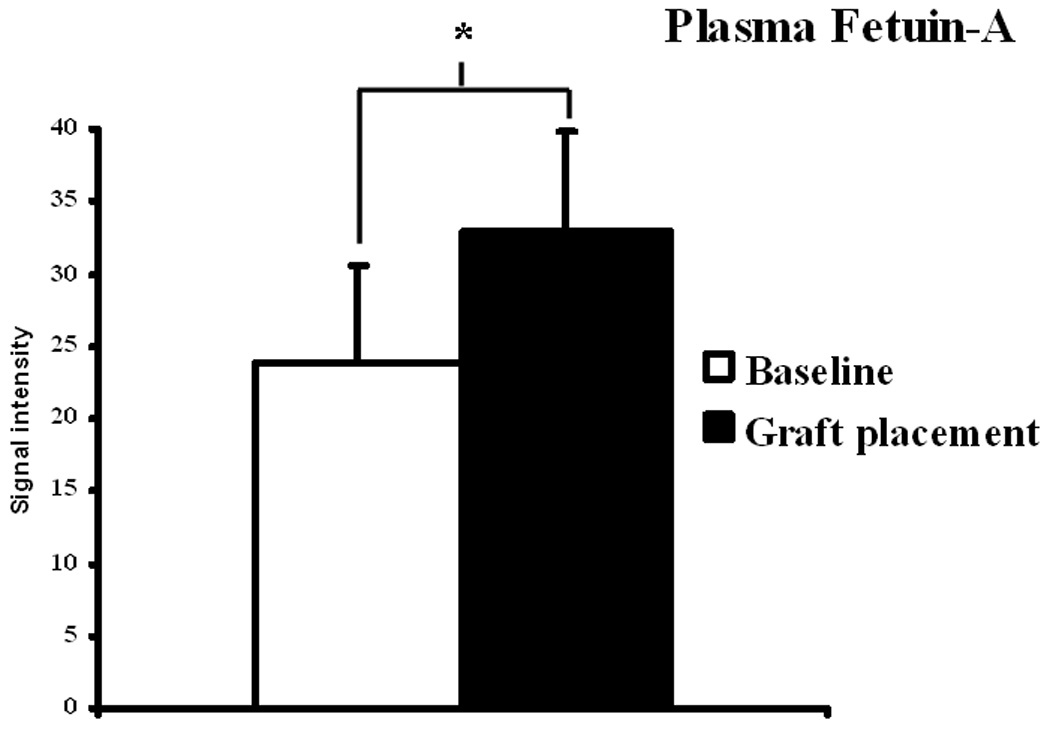
Immunoprecipitation followed by Western blot analysis of fetuin-A in serum at baseline (prior to renal artery embolization) and at graft placement. A is representative blots of fetuin-A in plasma prior to (baseline) renal artery embolization and at PTFE graft placement and controls with IgG loading. B shows pooled data for the fetuin-A. In plasma, there was significant increase in expression of fetuin-A (*) was significantly higher than the baseline plasma value (P<0.05). Data are presented as mean ± SD.
Discussion
Previous experimental porcine models which have been used to study the mechanisms responsible for hemodialysis graft failure have been performed in animals with normal kidney function (4, 5, 7, 14–18). In the present study, a porcine model of renal insufficiency with arteriovenous PTFE graft placement was used to show significantly elevated levels of fetuin-A by day 14 at the venous stenosis when compared to control vessels. In addition, significantly higher expression of fetuin-A was observed in the plasma at time of graft placement which correlated with increased BUN and creatinine when compared to pre embolization values.
At present, factors contributing to hemodialysis graft failure are not well understood, but are hypothesized to include changes in wall shear stress (22) and turbulent flow (23) coupled with vessel hypoxia (6, 20) which cause activation of matrix regulatory proteins (MMPs, VEGF-A, bFGF, TGF-β, and PDGF) resulting in increased cellular proliferation, migration, and matrix deposition. The serum protein fetuin-A was originally described as the major globulin in fetal and newborn calf serum (24). The human homologue was named α2–Heremans-Schmid glycoprotein after its two co discoverers (25). Fetuin-A is a member of the cystatin super family of cysteine protease inhibitors. It is secreted by the liver and found in high concentrations in serum and shown to be involved in vascular disease, atherosclerosis, hemodialysis, and inflammatory conditions (26). There is current controversy in the literature as to the role of fetuin-A in causing cardiovascular mortality in patients with abnormal levels of fetuin-A in serum with some studies suggesting it is associated with metabolic syndrome (26–28). In addition, there appears to be no correlation between the serum creatinine and fetuin-A (28, 32).
On a cellular level, fetuin-A is a multifunctional molecule and acts as an antagonist of transforming growth factor-β, regulating cytokine-dependent osteogenesis, and inhibiting insulin receptor tyrosine kinase and some protease activities (10). Fetuin-A knockout mice have been shown to develop severe soft-tissue and intravascular calcifications (9). It has been shown recently that in monocytes in vitro it can protect pro MMP-9 protein autolysis thus effectively increase local activity of pro MMP-9 (29). Increased expression of MMPs have been observed in failed hemodialysis access grafts and we hypothesize that increased expression of fetuin-A in the venous stenosis can cause an increase in MMP expression which will favor increased cell migration and proliferation leading to venous neointimal hyperplasia formation (5–7, 12, 30).
Previous experimental animal models of hemodialysis graft failure have used animals with normal kidney function (5, 7, 8, 14–16). In the present model, chronic renal insufficiency was induced prior to hemodialysis graft placement. In this animal model, we observed a significant increase in fetuin-A expression in the serum of the animals at time of graft placement twenty eight days later when compared to plasma prior to induction of renal insufficiency (31). This is consistent with animal experiments and observations from clinical studies which have shown that increased fetuin-A expression can occur in chronic kidney disease (32). In clinical samples of arteries removed from patients with hemodialysis, fetuin-A was localized by immunohistochemistry. In the present study, we observed no difference in fetuin-A expression within the inflow artery and control artery by Western blot. A potential explanation for this observation is that the animals had chronic kidney disease with increased serum fetuin-A levels while patients on hemodialysis have decreased fetuin-A levels resulting in calcification of blood vessels (11, 26, 31).
Because the observation of fetuin-A and hemodialysis is relatively new, there are several limitations that must be discussed. There have been no studies performed investigating the amount of fetuin-A in hemodialysis graft duration, frequency of dialysis, or the effect after angioplasty on the vascular access. The findings from the present study need to be validated in different experimental animal models as well as the clinical scenario. It is unclear whether the fetuin-A contributes to venous neointimal hyperplasia or venous neointimal hyperplasia causes the increase in the expression of fetuin-A.
In the present study, we observed that there was significantly increased expression of fetuin-A in venous stenosis formation and in serum of animals prior to graft placement. We hypothesize that increased expression of fetuin-A can cause increased expression of MMPs resulting in increased cell migration and proliferation resulting in venous neointimal hyperplasia formation. The identification of fetuin-A in early venous stenosis formation provides a potential mechanism and therapeutic target for inhibiting hemodialysis graft failure and provide preliminary data for further studies on determining the effect of these proteins on intimal hyperplasia in hemodialysis grafts.
Acknowledgments
This work is supported by NIH grants CA78383, HL072178 and HL70567 and a grant from American Cancer Society to DM. The authors would like to thank Steve Krage for their help with the animal experiments and Dr. Kallmes for the use of his laboratory.
Disclosures: The authors have nothing to declare. The results presented in this paper have not been published previously in whole or part.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2003 Annual Data Report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003;42:A5–A7. [PubMed] [Google Scholar]
- 2.Misra S, Bonan R, Pflederer T, Roy-Chaudhury P. BRAVO I: A pilot study of vascular brachytherapy in polytetrafluoroethylene dialysis access grafts. Kidney Int. 2006;70:2006–2013. doi: 10.1038/sj.ki.5001869. [DOI] [PubMed] [Google Scholar]
- 3.Rekhter M, Nicholls S, Ferguson M, Gordon D. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993;13:609–617. doi: 10.1161/01.atv.13.4.609. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Krishnamoorthy M, Banerjee R, et al. Venous stenosis in a pig arteriovenous fistula model anatomy, mechanisms and cellular phenotypes. Nephrol. Dial. Transplant. 2007;22:3139–3146. doi: 10.1093/ndt/gfm547. [DOI] [PubMed] [Google Scholar]
- 5.Misra S, Doherty MG, Woodrum D, et al. Adventitial remodeling with increased matrix metalloproteinase-2 activity in a porcine arteriovenous polytetrafluoroethylene grafts. Kidney Int. 2005;68:2890–2900. doi: 10.1111/j.1523-1755.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- 6.Misra S, Fu AA, Rajan DK, et al. Increased expression of HIF-1 alpha, MIF, pro MMP-2, pro MMP-9, and TIMP-1 in hemodialysis grafts. J Vasc Interv Radiol. 2008;19:252–259. doi: 10.1016/j.jvir.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Rotmans JI, Velema E, Verhagen HJ, et al. Matrix metalloproteinase inhibition reduces intimal hyperplasia in a porcine arteriovenous-graft model. J Vasc Surg. 2004;39:432–439. doi: 10.1016/j.jvs.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 9.Schafer C, Heiss A, Schwarz A, et al. The serum protein {alpha}2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J. Clin. Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szweras M, Liu D, Partridge EA, et al. alpha 2-HS Glycoprotein/Fetuin, a Transforming Growth Factor-beta /Bone Morphogenetic Protein Antagonist, Regulates Postnatal Bone Growth and Remodeling. J. Biol. Chem. 2002;277:19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 11.Mehrotra R. Emerging role for fetuin-A as contributor to morbidity and mortality in chronic kidney disease. Kidney Int. 2007;72:137–140. doi: 10.1038/sj.ki.5002355. [DOI] [PubMed] [Google Scholar]
- 12.Weiss MF, Scivittaro V, Anderson JM. Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis. 2001;37:970–980. doi: 10.1016/s0272-6386(05)80013-7. [DOI] [PubMed] [Google Scholar]
- 13.Boraldi F, Annovi G, Carraro F, et al. Hypoxia influences the cellular cross-talk of human dermal fibroblasts. A proteomic approach. Biochimica et Biophysica Acta (BBA) - Proteins & Proteomics. 2007;1774:1402–1413. doi: 10.1016/j.bbapap.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Rotmans JI, Pattynama PMT, Verhagen HJM, et al. Sirolimus-Eluting Stents to Abolish Intimal Hyperplasia and Improve Flow in Porcine Arteriovenous Grafts: A 4-Week Follow-Up Study. Circulation. 2005;111:1537–1542. doi: 10.1161/01.CIR.0000159332.18585.B5. [DOI] [PubMed] [Google Scholar]
- 15.Rotmans JI, Verhagen HJ, Velema E, et al. Local overexpression of C-type natriuretic peptide ameliorates vascular adaptation of porcine hemodialysis grafts. Kidney Int. 2004;65:1897–1905. doi: 10.1111/j.1523-1755.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MS, McLennan G, Lalka SG, Whitfield RM, Dreesen RG. The Porcine Hemodialysis Access Model. J Vasc Interv Radiol. 2001;12:969–977. doi: 10.1016/s1051-0443(07)61578-4. [DOI] [PubMed] [Google Scholar]
- 17.Kelly BS, Heffelfinger SC, Whiting JF, et al. Aggressive venous neointimal hyperplasia in a pig model of arteriovenous graft stenosis. Kidney Int. 2002;62:2272–2280. doi: 10.1046/j.1523-1755.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelly B, Melhem M, Zhang J, et al. Perivascular paclitaxel wraps block arteriovenous graft stenosis in a pig model. Nephrol. Dial. Transplant. 2006;21:2425–2431. doi: 10.1093/ndt/gfl250. [DOI] [PubMed] [Google Scholar]
- 19.Misra S, Gordon JD, Fu AA, et al. The Porcine Remnant Kidney Model of Chronic Renal Insufficiency. J. Surg. Res. 2006;135:370–379. doi: 10.1016/j.jss.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Misra S, Fu AA, Pugionni A, et al. Expression of Hypoxia inducible factor-1 in a porcine model of chronic renal insufficiency with arteriovenous polytetrafluoroethylene grafts. J Vasc Interv Radiol. 2008;19:260–265. doi: 10.1016/j.jvir.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Misra S, Lee N, Fu A, et al. Increased expression of a disintegrin and metalloproteinase thrombospondin-1 (ADAMTS-1) in thrombosed hemodialysis grafts. J Vasc Interv Radiol. 2008;19:111–119. doi: 10.1016/j.jvir.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Misra S, Woodrum D, Homburger J, et al. Assessment of Wall Shear Stress Changes in Arteries and Veins of Arteriovenous Polytetrafluoroethylene Grafts Using Magnetic Resonance Imaging. Cardiovasc Intervent Radiol. 2006;29:624–629. doi: 10.1007/s00270-005-0168-z. [DOI] [PubMed] [Google Scholar]
- 23.Abbott WM, Megerman J, Hasson JE, L'Italien G, Warnock DF. Effect of compliance mismatch on vascular graft patency. J. Vasc Surg. 1987;5:376–382. [PubMed] [Google Scholar]
- 24.Pedersen KO. Fetuin, a new globin isolated from serum. Nature. 1944;154 Nature. 154:575. [Google Scholar]
- 25.Schultze HE, Heide K, Haupt H, et al. Charakterisierung eines niedermolekularen 2-Mukoids aus Humanserum. Naturwiss. 1962;49:15–17. [Google Scholar]
- 26.Ketteler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. The Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 27.Ix JH, Shlipak MG, Sarnak MJ, et al. Fetuin-A is not associated with mortality in chronic kidney disease. Kidney Int. 2007;72:1394–1399. doi: 10.1038/sj.ki.5002549. [DOI] [PubMed] [Google Scholar]
- 28.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association Between Human Fetuin-A and the Metabolic Syndrome: Data From the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tajirian T, Dennis J, Swallow C. Regulation of human monocyte proMMP-9 production by fetuin, an endogenous TGF-β antagonist. Journal of Cellular Physiology. 2000;185:174–183. doi: 10.1002/1097-4652(200011)185:2<174::AID-JCP2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 30.Birkedal-Hansen H, Moore W, Bodden M, et al. Matrix metalloproteinases: a review. Crit Rev Oral Bio Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 31.Westenfeld R, Schafer C, Smeets R, et al. Fetuin-A (AHSG) prevents extraosseous calcification induced by uraemia and phosphate challenge in mice. Nephrol. Dial. Transplant. 2007;22:1537–1546. doi: 10.1093/ndt/gfm094. [DOI] [PubMed] [Google Scholar]
- 32.Mehrotra R, Westenfeld R, Christenson P, et al. Serum fetuin-A in nondialyzed patients with diabetic nephropathy: Relationship with coronary artery calcification. Kidney Int. 2005;67:1070–1077. doi: 10.1111/j.1523-1755.2005.00172.x. [DOI] [PubMed] [Google Scholar]


