Abstract
In the past year, the last missing enzyme of the L-galactose pathway, the linear form of which appears to represent the major biosynthetic route to L-ascorbate (vitamin C) in higher plants, has been identified as a GDP-L-galactose phosphorylase. This enzyme catalyzes the first committed step in the synthesis of that vital antioxidant and enzyme cofactor. Here, we discuss how GDP-L-galactose phosphorylase enzymes, encoded in Arabidopsis by the paralogous VTC2 and VTC5 genes, function in concert with the other enzymes of the L-galactose pathway to provide plants the appropriate levels of L-ascorbate. We hypothesize that regulation of L-ascorbate biosynthesis might occur at more than one step and warrants further investigation to allow for the manipulation of vitamin C levels in plants.
Pathways to L-ascorbate in plants
L-Ascorbate (or vitamin C) is an essential enzyme cofactor in hydroxylation and other reactions as well as a primary antioxidant in both plants and animals [1]. Since a few animal species (including primates) have lost the capacity for L-ascorbate synthesis [2], they are dependent upon diet to ensure adequate levels for metabolism and oxidative protection. The high L-ascorbate contents found in plants (which have an average cellular concentration of 2–25 mM or more in the chloroplast [3]) make them the primary source of vitamin C intake for humans. In plants, L-ascorbate has been implicated in processes including growth [4], programmed cell death [5], pathogen responses [6], hormone responses, flowering, and senescence [7], as well as protection against environmental stresses including ozone [8], UV radiation [9], high temperatures [10], and high light intensity [11].
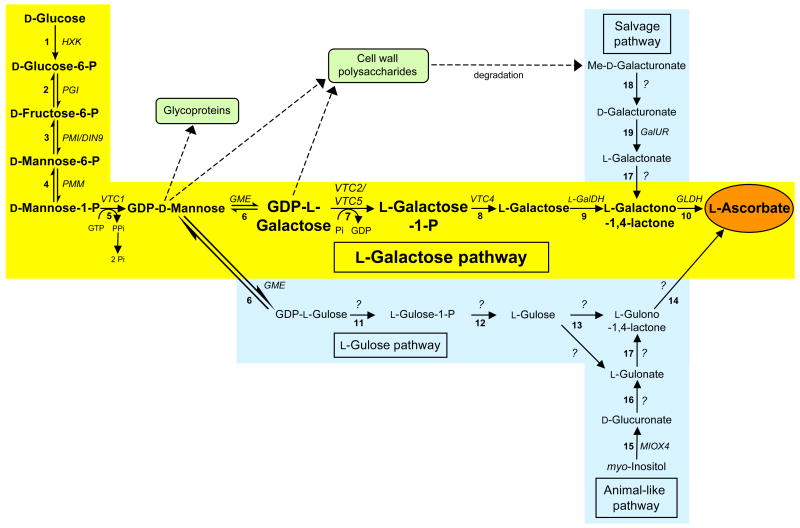
Understanding the biosynthetic pathway(s) for L-ascorbate in plant cells is an important aspect in the effort to elucidate how L-ascorbate levels are maintained in these cells. Surprisingly, it is only recently that the major plant pathway, which is different from the animal L-ascorbate synthesis pathway [2], has been described. It was not until 1998 that, based on tracer studies and biochemical analyses, a pathway (the L-galactose pathway) encompassing a series of earlier observations was proposed [12]. This pathway involves ten enzymatic steps from D-glucose to L-ascorbate via the intermediate formation of GDP-D-mannose and L-galactose (Figure 1). Although alternative pathways have been suggested [13–15], specific enzymes and the corresponding genes have not yet been identified for most of the proposed reactions (Figure 1). In the last few years, a combination of genetic and biochemical approaches have demonstrated that the L-galactose pathway is the dominant biosynthetic route to L-ascorbate in many plants and have allowed us to begin to understand the regulation of the pathway. With the recent characterization of the VTC2 and VTC5 expression products as GDP-L-galactose phosphorylases [16–19], genes have now been identified for all of the reactions involved in this pathway.
Figure 1.
Biosynthetic routes to L-ascorbate in higher plants. The major L-galactose pathway is highlighted in yellow with intermediates in bold type [12]; alternative proposed biosynthetic routes are highlighted in blue [13–15]. Designations are given in italics for genes encoding known enzymes of the pathways; question marks indicate possible reactions where the gene and the specific enzyme have not yet been identified. The central reaction (and the first committed step for L-ascorbate synthesis) in the L-galactose pathway, which is catalyzed by VTC2 and VTC5, is shown in a larger font. Enzymes catalyzing the numbered reactions are: 1, hexokinase; 2, phosphoglucose isomerase; 3, phosphomannose isomerase (PMI); 4, phosphomannomutase (PMM); 5, GDP-D-mannose pyrophosphorylase; 6, GDP-D-mannose 3′,5′-epimerase (GME); 7, GDP-L-galactose phosphorylase; 8, L-galactose-1-P phosphatase; 9, L-galactose dehydrogenase; 10, L-galactono-1,4-lactone dehydrogenase (GLDH); 11, nucleotide pyrophosphatase or sugar-1-P guanylyltransferase; 12, sugar phosphatase; 13, sugar dehydrogenase; 14, L-gulono-1,4-lactone dehydrogenase/oxidase; 15, myo-inositol oxygenase; 16, uronate reductase; 17, aldonolactonase; 18, methylesterase; 19, D-galacturonate reductase.
The first six steps of the L-galactose pathway are used to synthesize activated nucleotide sugars that are also precursors of cell wall polysaccharides and glycoproteins (Figure 1). The committed pathway to L-ascorbate biosynthesis then consists of the sequential conversion of GDP-L-galactose into L-galactose-1-P, L-galactose, L-galactono-1,4-lactone, and L-ascorbate. Since their discovery in 2007, there has been a large interest in the enzymes (VTC2 and VTC5) catalyzing the first reaction of the committed pathway. Here, we discuss this step and the controversies surrounding it after briefly reviewing recent work concerning the other reactions of the L-galactose pathway. On the basis of these studies, we propose that regulation of L-ascorbate synthesis in plants appears to be complex, occurring at the level of GDP-L-galactose phosphorylase and at possible additional steps.
Activities and regulation of the L-galactose pathway enzymes
Three enzymes to produce D-mannose-6-P – an essential part of the pathway?
While hexokinase and phosphoglucose isomerase are well-established players in glycolysis, phosphomannose isomerase (PMI), the enzyme forming D-mannose-6-P, has not been extensively studied. Based on sequence homology, two putative PMI genes can be found in the Arabidopsis thaliana genome, At3g02570 and At1g67070. While little is known about the former gene, transcripts of the latter gene (also named din9) could be detected in leaves only 24 hours after dark treatment [20]; biochemical and genetic evidence has been presented that PMI is either not present or occurs at low levels in plants [21,22]. These observations question the occurrence of the L-galactose pathway as currently proposed (Figure 1) and have prompted Wolucka and Van Montagu [23] to propose the VTC2 cycle described in the next section and in Figure 2b. However, the lower L-ascorbate contents observed in plants with decreased phosphomannomutase (PMM) transcript levels [24] and in plants deficient in GDP-D-mannose pyrophosphorylase activity [25] argue in favor of the involvement of PMI activity in L-ascorbate synthesis. Moreover, this activity has been recently demonstrated in Arabidopsis leaf extracts [18], and strong evidence for the in vivo involvement of PMI activity in GDP-D-mannose synthesis has been provided using a novel dual-radiolabeling strategy in Arabidopsis cell cultures [26]. Although the gene(s) responsible for PMI activity in Arabidopsis still need(s) to be characterized, these recent observations support the idea that PMI activity is present in plants.
Figure 2.
Proposed VTC2 cycles. (a) The original VTC2 cycle proposed by Laing et al. [17] requires only two enzymes, VTC2 and GDP-D-mannose 3′,5′-epimerase (3′,5′-GME), to generate L-galactose-1-P from D-mannose-1-P for L-ascorbate biosynthesis. In this scheme, GDP-D-mannose generated by the transferase activity of VTC2 is recycled to the GDP-L-galactose substrate of VTC2 by 3′,5′-GME. VTC1 activity is only required to compensate for the GDP-D-mannose molecules that are drained from the cycle for polysaccharide and glycoprotein synthesis. (b) The extended VTC2 cycle proposed by Wolucka and Van Montagu [23] involves an additional putative enzyme that interconverts GDP-D-glucose and GDP-D-mannose (2′-GME). Here, D-glucose-1-P, derived from photosynthesis, is converted to L-galactose-1-P by the VTC2 transferase activity. L-Galactose-1-P is used for L-ascorbate synthesis; the other VTC2 product (GDP-D-glucose) can be converted to other GDP-hexoses for polysaccharide and glycoprotein synthesis or can be recycled to GDP-L-galactose through the successive action of the putative 2′-GME and 3′,5′-GME. The priming reaction to start this VTC2 cycle could involve a GDP-D-glucose pyrophosphorylase or VTC1 (not shown). Given the low transferase activities measured with VTC2 and the hypothetical nature of the 2′-GME activity, further work is needed to determine whether either of these cycles is physiologically relevant in plants.
PMM and GDP-D-mannose pyrophosphorylase (VTC1) – regulatory sites for L-ascorbate synthesis?
The activities of the next two enzymes in the L-galactose pathway (PMM and VTC1) result in the formation of GDP-D-mannose (Figure 1). L-ascorbate contents in Nicotiana benthamiana and/or Arabidopsis are modulated by alteration of PMM transcript levels through virus-induced gene silencing and transgenic expression [24] as well as by PMM mutation [27]. These observations suggest that PMM activity might affect the overall rate of L-ascorbate biosynthesis.
An ozone-sensitive Arabidopsis mutant accumulating only 30% of the normal L-ascorbate concentration [28] has been mapped to a locus (VTC1) encoding a GDP-D-mannose pyrophosphorylase [25]. Antisense inhibition of this enzyme in potato also resulted in lowered L-ascorbate levels [29]. Indeed, mRNA levels of GDP-mannose pyrophosphorylase are correlated with L-ascorbate levels in several plant species [30,31]. Finally, jasmonates, which have been shown to increase L-ascorbate levels in Arabidopsis, were also found to induce the expression of VTC1 in this organism [32,33]. Taken together, these results suggest that some control of L-ascorbate synthesis could occur at this step. However, since GDP-D-mannose is also required for cell wall biogenesis and protein glycosylation [34], crucial regulatory control might be expected to occur elsewhere.
GDP-D-mannose 3′,5′-epimerase (GME) – an initiator of a new branch for L-ascorbate biosynthesis?
GME converts GDP-D-mannose not only to GDP-L-galactose, but also to GDP-L-gulose (the C5′ epimer of GDP-D-mannose) [35,36]. The latter has been proposed to initiate an alternative branch (the L-gulose pathway; see Figure 1) for de novo L-ascorbate biosynthesis in plants [35]. The modulation of GME activity by several metabolites [35,36] and the induction of GME expression by methyl jasmonate in tobacco cells [33] suggest the possibility that this step might be a control point in L-ascorbate biosynthesis.
GDP-L-galactose phosphorylase (VTC2, VTC5) – the first committed step in L-ascorbate biosynthesis
The enzyme converting GDP-L-galactose to L-galactose-1-P has only been identified very recently; this step appears to play a major role in the regulation of L-ascorbate synthesis and we will return to focus on it after discussion of the remaining reactions of the pathway.
L-Galactose-1-P phosphatase (VTC4)
VTC4, initially identified from an L-ascorbate-deficient Arabidopsis mutant [37], encodes a specific L-galactose-1-P phosphatase [38,39]. However, the observation that a VTC4 knockout mutant is only partially deficient in L-ascorbate as well as L-galactose-1-P phosphatase activity suggests that VTC4 is not the only enzyme catalyzing this reaction in Arabidopsis [39]. The purple acid phosphatase AtPAP15, whose overexpression has recently been shown to increase foliar L-ascorbate in Arabidopsis [40], might contribute to the hydrolysis of L-galactose-1-P; the latter has, however, not yet been tested as a substrate. Although VTC4 mRNA levels have been correlated with changes in L-ascorbate levels in response to light [41], no change in enzymatic activity was found under such conditions [18].
L-Galactose dehydrogenase – a target for feedback inhibition?
Spinach L-galactose dehydrogenase exhibits reversible competitive inhibition by L-ascorbate with a Ki of 0.13 mM [42], thus making it a possible target for the reported end-product feedback inhibition of L-ascorbate biosynthesis [43]. However, the substantial accumulation of L-ascorbate observed after L-galactose feeding [12] as well as the lack of effect of Arabidopsis L-galactose dehydrogenase overexpression on L-ascorbate contents in tobacco [44] suggest that this inhibition is absent or attenuated in vivo and that L-galactose dehydrogenase exerts little control over flux through the L-galactose pathway.
L-Galactono-1,4-lactone dehydrogenase (GLDH) – in a favorable position for regulation by respiration
The last step of L-ascorbate biosynthesis is catalyzed by a mitochondrial flavin-containing dehydrogenase that is highly specific for L-galactono-1,4-lactone and uses cytochrome c as an electron acceptor in the respiratory chain [13,45,46]. The transcript level and enzyme activity of GLDH have been observed to positively correlate with L-ascorbate content in various plant tissues, although there is species-to-species variation (reviewed in [14]). However, reduction of GLDH activity by RNA interference in tomato, which led to decreased growth rates, did not alter the L-ascorbate synthesis capacity in the transgenic plants [47]. It should be noted that, based on several observations, the existence of different isoforms of GLDH, some of which might use L-gulono-1,4-lactone, has been suggested [45,46]. GLDH appears to play a role in the control of L-ascorbate content by light [18,41,48]; the mechanism is unclear but might involve light-dependent regulation of GLDH expression [49] as well as light-dependent changes in respiration that might directly affect GLDH activity [50,51].
GDP-L-galactose phosphorylase – a central player in plant L-ascorbate biosynthesis?
The first metabolite dedicated only to L-ascorbate biosynthesis is L-galactose-1-P; the enzyme catalyzing its formation thus represents the committed step in the overall pathway. As such, this enzyme has been a focus of intense recent study.
Identification of VTC2 as the enzyme converting GDP-L-galactose to L-galactose-1-P and controversy surrounding its activity
The identity of this enzyme eluded researchers until 2007. Three vitamin C-deficient Arabidopsis mutants were mapped to a locus on chromosome 4 that was designated VTC2 (vitamin C 2) [37]. These mutations were then located in the uncharacterized At4g26850 gene [52]. BLAST (Basic Local Alignment Search Tool; http://blast.ncbi.nlm.nih.gov/Blast.cgi) analyses of the predicted amino acid sequence of VTC2 revealed the existence of a motif characteristic of the histidine triad (HIT) protein superfamily [16,17,18] whose members are hydrolases, phosphorylases, or transferases acting on nucleotide-containing substrates [53]. The conversion of GDP-L-galactose to L-galactose-1-P in the L-galactose pathway represents such a type of reaction and suggested that VTC2 could be the long-sought-after enzyme forming L-galactose-1-P. All known HIT enzymes use the second His residue (His238 in Arabidopsis VTC2) of the HIT motif to attack the α-phosphate of the monophosphonucleoside moiety of their substrates, leading to covalent nucleotidylylation of this His residue [53]. The nucleotidylylated enzyme intermediate can then be broken down either by simple hydrolysis or by nucleotidylyl-transfer to inorganic phosphate (phosphorolysis) or a specific phosphorylated compound (transfer). In the case of VTC2, breakdown of the putative guanylylated enzyme intermediate formed could thus in theory proceed in the absence of a specific guanylyl-acceptor (hydrolysis) or require the presence of inorganic phosphate or a phosphorylated compound.
In three independent studies, Escherichia coli-expressed recombinant Arabidopsis VTC2 [16,18] or a kiwifruit homolog thereof [17] were found to specifically convert GDP-L-galactose to L-galactose-1-P and to require the presence of a guanylyl-acceptor other than water to catalyze the reaction. Moreover, evidence for covalent guanylylation of the second His residue of the HIT motif of Arabidopsis VTC2 has been provided [19]. These results established VTC2 as a novel member of the phosphorylase-transferase (but not hydrolase) branch of the HIT protein superfamily, which catalyzes the last unidentified step of the L-galactose pathway.
However, there is some controversy [23] on whether the physiological guanylyl-acceptor is inorganic phosphate, leading to a phosphorylase activity that generates GDP [16,18], or a hexose-1-P, leading to a transferase activity that generates the corresponding GDP-hexose [17] (Figure 3). This is an important issue that impacts the feasibility of the VTC2 cycles that have been proposed recently as an alternative to the linear L-galactose pathway, allowing maintenance of high-energy phosphate bonds during L-ascorbate synthesis (Figure 2 and see later in this section). Linster et al. [16] and Dowdle et al. [18] found high VTC2 activities in the presence of inorganic phosphate and characterized the enzyme as a GDP-L-galactose phosphorylase; Laing et al. [17] found higher catalytic efficiencies when phosphate was replaced by hexose-1-phosphates such as D-mannose-1-P, D-glucose-1-P, D-galactose-1-P, or L-myo-inositol-1-P and thus described the enzyme as a GDP-L-galactose-hexose-1-P guanylyltransferase. However, a subsequent analysis of the activity of Arabidopsis VTC2 in the presence of different guanylyl-acceptors using a direct high performance liquid chromatography (HPLC)-based assay [19] failed to confirm the results obtained by Laing et al. [17], who utilized an indirect enzyme-coupled assay. On the contrary, VTC2 was found to be more than 100-fold more efficient as a GDP-L-galactose phosphorylase than as a GDP-L-galactose-D-glucose-1-P guanylyltransferase [19]. Phosphate was also preferred over D-glucose-1-P when partially purified extracts of Arabidopsis, Japanese mustard spinach, lemon, spinach, and maize were used as the enzyme source [19]. Moreover, using the HPLC-based assay, conversion of GDP-L-galactose to L-galactose-1-P in the presence of D-mannose-1-P (the compound proposed by Laing et al. [17] to be the endogenous guanylyl-acceptor of VTC2) could be measured neither with recombinant VTC2 nor with partially purified plant extracts [19]. Taken together, these results suggest that in most plant species VTC2 acts predominantly as a phosphorylase.
Figure 3.
Proposed VTC2-catalyzed reactions. The reaction catalyzed by VTC2 proceeds in two steps. In the first step, VTC2 forms a covalent guanylylated active site His intermediate with the -phosphate of GDP-L-galactose, releasing L-galactose-1-P. In the second step, the enzyme could alternatively transfer the guanylyl group to inorganic phosphate, leading to a phosphorylase activity generating GDP (pathway A), or to a hexose-1-P, leading to a transferase activity generating a GDP-hexose (pathway B). Pathway A appears to be the major route in higher plants because the phosphorylase activity of VTC2 was found to be more than 100-fold higher than its transferase activity (see the main text).
Alternative reactions catalyzed by VTC2
Interestingly, VTC2 does not display absolute specificity for its nucleotide sugar substrate; similar catalytic efficiencies were found with GDP-L-galactose and with GDP-D-glucose [16]. Significant phosphorylase activity could also be measured with GDP-L-fucose [19], whereas GDP-D-mannose turned out to be a very poor substrate [16]. No activity was detected in the presence of GDP-L-gulose, a series of UDP-sugars, and ADP-D-glucose [16,18]. VTC2 is thus more specific for the nucleotide moiety than for the sugar moiety of the nucleotide sugar substrate. This specificity (or lack of) is important in formulating the VTC2 cycles shown in Figure 2 and discussed later in this section.
VTC5: a second GDP-L-galactose phosphorylase in Arabidopsis
The VTC2-2 and VTC2-3 mutant alleles resulting in L-ascorbate deficiency lead to G224D and S290F substitutions, respectively [18,52]. No residual activity was detected with recombinant VTC2 containing the aspartate residue at position 224 [18,19]. Compared with the wild-type enzyme, the recombinant VTC2 protein containing the phenylalanine residue at position 290 displayed a higher Km for inorganic phosphate and a lower kcat, leading to a 50-fold decrease in the catalytic efficiency [18]. The relatively high residual L-ascorbate contents (25–50% of wild-type levels) found in these mutants [18] thus suggested the existence of VTC2-independent L-ascorbate biosynthesis pathways and/or of other enzymes catalyzing a VTC2-like reaction.
In fact, a gene (At5g55120) sharing high sequence identity with VTC2 was identified in the Arabidopsis genome and its expression product, designated VTC5, was found to be a GDP-L-galactose phosphorylase whose kinetic properties greatly resemble those of VTC2 [18,19]. Similarly to VTC2, VTC5 is much more efficient as a GDP-L-galactose phosphorylase than as a GDP-L-galactose-hexose-1-P guanylyltransferase [19]. Both VTC2 and VTC5 are expressed in leaf, stem, root, flower, and silique tissue, but the expression level of VTC5 is generally 100- to 1000-times lower than that of VTC2 [18]. Accordingly, the leaf L-ascorbate contents of two homozygous T-DNA insertion mutants (vtc5-1 and vtc5-2) lacking VTC5 transcripts were not markedly different from those found in wild-type plants [18].
Significantly, vtc2 vtc5 double-mutant seedlings stopped growing after initial expansion of the cotyledons, which then bleached within 2 weeks [18]. These seedlings could, however, be rescued by supplementation with L-galactose or L-ascorbate. Larger plants rescued on L-galactose showed bleaching of older leaves one week after transfer to L-galactose-free medium. These results show that the GDP-L-galactose phosphorylase activities of VTC2 and VTC5 are required for seedling viability, but probably also at later growth stages. Importantly, they also demonstrate that the L-galactose pathway is the only significant source of L-ascorbate in Arabidopsis seedlings.
Regulation of VTC2 and VTC5 activities
As GDP-D-mannose and GDP-L-galactose are not only used for L-ascorbate formation but also in the synthesis of cell wall polysaccharides and/or protein glycosylation [21,54], the phosphorylase reaction is the first committed step in the L-galactose pathway and thus VTC2 and VTC5 are good potential targets for the regulation of L-ascorbate synthesis. L-Ascorbate, L-galactono-1,4-lactone, and L-galactose had no effect on VTC2 activity, indicating no feedback regulation of the enzyme by these metabolites [18]. However, L-ascorbate supplementation decreased VTC2 expression in Arabidopsis plants, suggesting feedback inhibition by L-ascorbate at the transcriptional level [18]. The increase in leaf L-ascorbate content measured after a 24 h exposure to high light in Arabidopsis was accompanied by increased expression of VTC2 and VTC5 and by a 20-fold increase in GDP-L-galactose phosphorylase activity [18]. Light induction of VTC2 mRNA has been confirmed in two additional independent studies [41,55]. However, except for a small (2-fold) increase in L-galactono-1,4-lactone dehydrogenase activity, none of the other enzymes of the L-galactose pathway were found to be affected by acclimation to high light [18]. Evidence has been presented that increased L-galactose synthesis, involving a step upstream of L-galactose dehydrogenase, contributes to the light-induced accumulation of L-ascorbate [44]; it appears now very likely that this accumulation is largely mediated by increased VTC2 and/or VTC5 activity. In addition to the response to light intensity, VTC2 and VTC5 expression might also be under the control of the circadian clock [18]. Furthermore, jasmonates induced VTC2 and VTC5 transcription, in addition to VTC1 transcription, in Arabidopsis [32]. Taken together, these observations suggest that regulation of VTC2 and VTC5 expression plays a major role in controlling L-ascorbate biosynthesis. This is further supported by the finding that transient overexpression of the kiwifruit homolog of VTC2 in tobacco leaves led to a 3-fold increase in L-ascorbate content, indicating that this enzyme is rate-limiting for L-ascorbate synthesis [17]. Preliminary evidence for a nuclear localization, in addition to its cytosolic localization, has been provided for the VTC2 protein, suggesting a potential nuclear function for VTC2 [55]; the intracellular localization of VTC5 was not analyzed in this study.
Existence of a VTC2 cycle?
Based on the D-mannose-1-P guanylyltransferase activity they measure, Laing et al. [17] proposed a VTC2 cycle in which the biosynthesis of L-galactose-1-P could be sustained by the action of VTC2 and GME in the absence of VTC1 (Figure 2a), thus avoiding the net hydrolysis of GTP to GDP and inorganic phosphate (Figure 1). In such a cycle, VTC1 is only required to resupply GDP-D-mannose that is taken from the cycle by the reactions leading to polysaccharide and glycoprotein formation.
Wolucka and Van Montagu [23] proposed an extended VTC2 cycle (Figure 2b) that could in addition account for L-ascorbate synthesis in the absence of PMI activity (see previous section). This extended cycle is also dependent upon the use of hexose-phosphates as guanylyl-acceptors by VTC2. Additionally, it requires the presence of an as yet unidentified 2′-epimerase that interconverts GDP-D-glucose and GDP-D-mannose (Figure 2b). In this scheme, the products of photosynthesis could directly enter the extended VTC2 cycle with no need for PMI activity. This cycle would generate, in addition to L-galactose-1-P used for L-ascorbate production, GDP-hexoses for polysaccharide and glycoprotein synthesis and conserve energy by preserving the phosphodiester linkage in the GDP-hexoses.
However, the failure to confirm the initially reported transferase activity of VTC2 [17] in both VTC2 and VTC5 [19] represents an obstacle to the physiological operation of both types of cycles in plants. Furthermore, the recent dual-radiolabeling study described in the previous section [26] provides convincing evidence that, in Arabidopsis cells, GDP-D-mannose is synthesized predominantly via a pathway involving PMI rather than through a pathway involving GDP-D-glucose epimerization. Clearly, further work is needed to experimentally demonstrate the postulated GDP-D-mannose 2′-epimerase activity and to determine whether hexose-phosphates can be guanylyl-acceptors for endogenous VTC2 and/or VTC5 enzymes that might be covalently modified, allosterically regulated, or complexed with other proteins.
Concluding remarks
Control of L-ascorbate steady-state levels in plants potentially involves regulation of biosynthesis, catabolism, recycling, and transport of this compound. Only regulation of biosynthesis is discussed here; current knowledge about catabolism, recycling, and transport of L-ascorbate has recently been reviewed by others [13,14,56]. Although several enzymes of the L-galactose pathway present regulatory aspects, it appears that major control of L-ascorbate synthesis occurs at the step catalyzed by VTC2 and VTC5. Further experimental work is required to understand the mechanisms of this control, including how VTC2 and VTC5 expression is regulated at transcriptional, post-transcriptional, and translational levels, and how their enzymatic activities are potentially modulated by covalent modification, allosteric regulation, and interaction with other proteins. Additionally, studies will be needed to verify the participation of other steps in the control of this pathway, as well as the role of the alternative L-ascorbate biosynthesis pathways that have been proposed. Understanding the regulatory mechanisms could then allow for testing genetic and/or small molecule approaches for manipulating plant L-ascorbate levels. Such intervention could generate plants with increased resistance to oxidative stress, as well as fruits and vegetables with longer shelf life and higher nutritional value for humans.
Acknowledgments
We thank our colleagues Lital Adler, Charles Brenner, and Emile Van Schaftingen for their contributions. The research in our laboratory is supported by National Institutes of Health grants GM026020 to S.G.C., AG018000 to S.G.C., and by a National Science Foundation grant MCB-0448533 to S.G.C.. C.L.L. is supported by a research fellowship (bourse de formation-recherche) from the Luxembourgish Government.
References
- 1.De Tullio MC, Arrigoni O. Hopes, disillusions and more hopes from vitamin C. Cell Mol Life Sci. 2004;61:209–219. doi: 10.1007/s00018-003-3203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linster CL, Van Schaftingen E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 3.Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol. 2000;3:229–235. [PubMed] [Google Scholar]
- 4.Pignocchi C, Foyer CH. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr Opin Plant Biol. 2003;6:379–389. doi: 10.1016/s1369-5266(03)00069-4. [DOI] [PubMed] [Google Scholar]
- 5.de Pinto MC, et al. Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J. 2006;48:784–795. doi: 10.1111/j.1365-313X.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- 6.Barth C, et al. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol. 2004;134:1784–1792. doi: 10.1104/pp.103.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barth C, et al. The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot. 2006;57:1657–1665. doi: 10.1093/jxb/erj198. [DOI] [PubMed] [Google Scholar]
- 8.Conklin PL, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2004;27:959–970. [Google Scholar]
- 9.Gao Q, Zhang L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J Plant Physiol. 2008;165:138–148. doi: 10.1016/j.jplph.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Larkindale J, et al. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138:882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller-Moulé P, et al. Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol. 2004;134:1163–1172. doi: 10.1104/pp.103.032375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler GL, et al. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- 13.Hancock RD, Viola R. Biosynthesis and catabolism of L-ascorbic acid in plants. Crit Rev Plant Sci. 2005;24:167–188. [Google Scholar]
- 14.Ishikawa T, et al. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol Plant. 2006;126:343–355. [Google Scholar]
- 15.Ishikawa T, Shigeoka S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci Biotechnol Biochem. 2008;72:1143–1154. doi: 10.1271/bbb.80062. [DOI] [PubMed] [Google Scholar]
- 16.Linster CL, et al. Arabidopsis VTC2 encodes a GDP-L-galactose phosphorylase, the last unknown enzyme in the Smirnoff-Wheeler pathway to ascorbic acid in plants. J Biol Chem. 2007;282:18879–18885. doi: 10.1074/jbc.M702094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laing WA, et al. The missing step of the L-galactose pathway of ascorbate biosynthesis in plants, an L-galactose guanyltransferase, increases leaf ascorbate content. Proc Natl Acad Sci U S A. 2007;104:9534–9539. doi: 10.1073/pnas.0701625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowdle J, et al. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- 19.Linster CL, et al. A second GDP-L-galactose phosphorylase in Arabidopsis en route to vitamin C: Covalent intermediate and substrate requirements for the conserved reaction. J Biol Chem. 2008;283:18483–18492. doi: 10.1074/jbc.M802594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiki Y, et al. Dark-inducible genes from Arabidopsis thaliana are associated with leaf senescence and repressed by sugars. Physiol Plant. 2001;111:345–352. doi: 10.1034/j.1399-3054.2001.1110312.x. [DOI] [PubMed] [Google Scholar]
- 21.Smirnoff N, et al. Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Biol. 2000;35:291–314. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- 22.Ballester A, et al. Evaluation of selection strategies alternative to nptII in genetic transformation of citrus. Plant Cell Rep. 2008;27:1005–1015. doi: 10.1007/s00299-008-0523-z. [DOI] [PubMed] [Google Scholar]
- 23.Wolucka BA, Van Montagu M. The VTC2 cycle and the de novo biosynthesis pathways for vitamin C in plants: an opinion. Phytochemistry. 2007;68:2602–2613. doi: 10.1016/j.phytochem.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 24.Qian W, et al. Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana. Plant J. 2007;49:399–413. doi: 10.1111/j.1365-313X.2006.02967.x. [DOI] [PubMed] [Google Scholar]
- 25.Conklin PL, et al. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci U S A. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharples SC, Fry SC. Radioisotope ratios discriminate between competing pathways of cell wall polysaccharide and RNA biosynthesis in living plant cells. Plant J. 2007;52:252–262. doi: 10.1111/j.1365-313X.2007.03225.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoeberichts FA, et al. A temperature-sensitive mutation in the Arabidopsis thaliana phosphomannomutase gene disrupts protein glycosylation and triggers cell death. J Biol Chem. 2008;283:5708–5718. doi: 10.1074/jbc.M704991200. [DOI] [PubMed] [Google Scholar]
- 28.Conklin PL, et al. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci U S A. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller R, et al. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J. 1999;19:131–141. doi: 10.1046/j.1365-313x.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- 30.Badejo AA, et al. Cloning and expression of GDP-D-mannose pyrophosphorylase gene and ascorbic acid content of acerola (Malpighia glabra L.) fruit at ripening stages. Plant Physiol Biochem. 2007;45:665–672. doi: 10.1016/j.plaphy.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Badejo AA, et al. Analysis of GDP-D-mannose pyrophosphorylase gene promoter from acerola (Malpighia glabra) and increase in ascorbate content of transgenic tobacco expressing the acerola gene. Plant Cell Physiol. 2008;49:126–132. doi: 10.1093/pcp/pcm164. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki-Sekimoto Y, et al. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 2005;44:653–668. doi: 10.1111/j.1365-313X.2005.02560.x. [DOI] [PubMed] [Google Scholar]
- 33.Wolucka BA, et al. Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J Exp Bot. 2005;56:2527–2538. doi: 10.1093/jxb/eri246. [DOI] [PubMed] [Google Scholar]
- 34.Lukowitz W, et al. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci U S A. 2001;98:2262–2267. doi: 10.1073/pnas.051625798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolucka BA, Van Montagu M. GDP-mannose 3′,5′-epimerase forms GDP-L-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J Biol Chem. 2003;278:47483–47490. doi: 10.1074/jbc.M309135200. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe K, et al. Characterization of a GDP-D-mannose 3″,5″-epimerase from rice. Phytochemistry. 2006;67:338–346. doi: 10.1016/j.phytochem.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Conklin PL, et al. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics. 2000;154:847–856. doi: 10.1093/genetics/154.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laing WA, et al. A highly specific L-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proc Natl Acad Sci U S A. 2004;101:16976–16981. doi: 10.1073/pnas.0407453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conklin PL, et al. Arabidopsis thaliana VTC4 encodes L-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. J Biol Chem. 2006;281:15662–15670. doi: 10.1074/jbc.M601409200. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W, et al. An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol. 2008;146:431–440. doi: 10.1104/pp.107.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yabuta Y, et al. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot. 2007;58:2661–2671. doi: 10.1093/jxb/erm124. [DOI] [PubMed] [Google Scholar]
- 42.Mieda T, et al. Feedback inhibition of spinach L-galactose dehydrogenase by L-ascorbate. Plant Cell Physiol. 2004;45:1271–1279. doi: 10.1093/pcp/pch152. [DOI] [PubMed] [Google Scholar]
- 43.Pallanca JE, Smirnoff N. The control of ascorbic acid synthesis and turnover in pea seedlings. J Exp Bot. 2000;51:669–674. [PubMed] [Google Scholar]
- 44.Gatzek S, et al. Antisense suppression of L-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated L-galactose synthesis. Plant J. 2002;30:541–553. doi: 10.1046/j.1365-313x.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- 45.Smirnoff N, et al. Biosynthesis of ascorbic acid in plants: a renaissance. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:437–467. doi: 10.1146/annurev.arplant.52.1.437. [DOI] [PubMed] [Google Scholar]
- 46.Leferink NG, et al. L-Galactono-gamma-lactone dehydrogenase from Arabidopsis thaliana, a flavoprotein involved in vitamin C biosynthesis. FEBS J. 2008;275:713–726. doi: 10.1111/j.1742-4658.2007.06233.x. [DOI] [PubMed] [Google Scholar]
- 47.Alhagdow M, et al. Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007;145:1408–1422. doi: 10.1104/pp.107.106500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smirnoff N. Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond, B, Biol Sci. 2000;355:1455–1464. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamaoki M, et al. Light-controlled expression of a gene encoding L-galactono-gamma-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci. 2003;164:1111–1117. [Google Scholar]
- 50.Millar AH, et al. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 2003;133:443–447. doi: 10.1104/pp.103.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartoli CG, et al. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot. 2006;57:1621–1631. doi: 10.1093/jxb/erl005. [DOI] [PubMed] [Google Scholar]
- 52.Jander G, et al. Arabidopsis map-based cloning in the post-genome era. Plant Physiol. 2002;129:440–450. doi: 10.1104/pp.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenner C. Hint, Fhit, and GalT: function, structure, evolution, and mechanism of three branches of the histidine triad superfamily of nucleotide hydrolases and transferases. Biochemistry. 2002;41:9003–9014. doi: 10.1021/bi025942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reuhs BL, et al. L-Galactose replaces L-fucose in the pectic polysaccharide rhamnogalacturonan II synthesized by the L-fucose-deficient mur1 Arabidopsis mutant. Planta. 2004;219:147–157. doi: 10.1007/s00425-004-1205-x. [DOI] [PubMed] [Google Scholar]
- 55.Müller-Moulé P. An expression analysis of the ascorbate biosynthesis enzyme VTC2. Plant Mol Biol. 2008;68:31–41. doi: 10.1007/s11103-008-9350-4. [DOI] [PubMed] [Google Scholar]
- 56.DeBolt S, et al. Ascorbate as a biosynthetic precursor in plants. Ann Bot (Lond) 2007;99:3–8. doi: 10.1093/aob/mcl236. [DOI] [PMC free article] [PubMed] [Google Scholar]