Abstract
Alfalfa mosaic virus (AMV) RNA replication requires the viral coat protein (CP). AMV CP is an integral component of the viral replicase; moreover, it binds to the viral RNA 3' termini and induces the formation of multiple new base pairs that organize the RNA conformation. The results described here suggest that AMV coat protein binding defines template selection by organizing the 3'-terminal RNA conformation and by positioning the RNA-dependent RNA polymerase (RdRp) at the initiation site for minus strand synthesis. RNA-protein interactions were analyzed by using a modified northwestern blotting protocol that included both viral coat protein and labeled RNA in the probe solution (“far-northwestern blotting”). We observed that labeled RNA alone bound the replicase proteins poorly; however, complex formation was enhanced significantly in the presence of AMV CP. The RNA-replicase bridging function of the AMV CP may represent a mechanism for accurate de novo initiation in the absence of canonical 3' transfer RNA signals.
Keywords: RNA-protein interactions, RNA-dependent RNA polymerase, protein-protein interactions, conformation, template selection, positive strand RNA virus, viral RNA replication
Introduction
Replication of positive strand virus RNAs requires specific and accurate recognition of genomic RNA 3' termini by the polymerase enzyme, and template selection is enhanced by both RNA sequences and structures and RNA-protein complexes. The 3'-termini of some plant virus RNAs fold into transfer-RNA like structures that include the canonical CCA trinucleotide terminus and the ability to be charged by aminoacyl synthetases (Fechter et al., 2001). Weiner and Maizels have proposed that these tRNA-like termini are molecular fossils that first served to tag the 3' termini of RNAs to be replicated by an RNA enzyme in the RNA world (Maizels and Weiner, 1994; Maizels and Weiner, 1999; Weiner and Maizels, 1987). Today, the 3'-terminal tRNA-like structure is found on only a subset of viral RNAs, and viruses also use RNA-protein complexes to enhance template selectivity. A classic example of RNA-protein complexes in replication initiation is found in bacteriophage Qβ, where the host ribosomal S1 protein and elongation factor Tu are critical for viral template selection (Brown and Gold, 1996). Other examples of proteins facilitating viral RNA replication are poliovirus 3CD (Yang et al., 2004), as well as the 1A protein of brome mosaic virus, and the 126 kDa protein of tobacco mosaic virus (Chen, Noueiry, and Ahlquist, 2001). In recent data, the p33 protein of tomato bushy stunt virus (TBSV) has been shown to bind to both viral RNA and the viral RdRp to provide template specificity (Pogany, White, and Nagy, 2005). These data suggest that host or viral proteins may bridge the interaction between the viral RNA and the replicase, thus providing template specificity and selection.
As a general rule, de novo replication initiation characterizes most viruses that do not use cap-snatched primers or terminal covalently-bound proteins (Kao, Singh, and Ecker, 2001). Even so, in vitro transcription analyses have shown that polymerases that initiate de novo can also use short oligonucleotide primers instead of the initiating rNTP (Kao and Sun, 1996; Nagy, Carpenter, and Simon, 1997). De novo replication introduces a potential telomere problem, wherein nucleotides can be lost from the 5' terminus of the minus-strand if the polymerase does not initiate copying accurately. For RNAs with a tRNA-like 3'-terminus, the CCA terminus can be repaired, possibly by the nucleotidyl transferase enzyme (Rao et al., 1989); moreover, primed initiation using short abortive transcripts may also be a mechanism for maintaining 3' terminal nucleotide sequences (Nagy, Carpenter, and Simon, 1997). Members of the Tombusviridae do not have a tRNA-like terminus, and it has been proposed that the viral replicase may be involved in the repair process (Pogany, White, and Nagy, 2005).
Available evidence suggests that replication of alfalfa mosaic virus (AMV) and ilarvirus RNAs is initiated de novo at the RNA 3' termini. AMV and ilarvirus RNAs lack the canonical tRNA 3'-terminal CCA; moreover, there are no data reported to date suggesting that AMV or ilarviruses initiate replication through a primed mechanism with short abortive transcripts. AMV or ilarvirus coat protein (CP) is implicated in AMV replication because the viral genomic RNAs are not infectious in its absence (Bol, Van Vloten-Doting, and Jaspars, 1971); however, coat protein's exact role in the replication cycle has been debated (Bol, 1999; Guogas, Laforest, and Gehrke, 2005; Guogas et al., 2004; Jaspars, 1999; Neeleman, Linthorst, and Bol, 2004; Neeleman et al., 2001; Olsthoorn, Haasnoot, and Bol, 2004; Petrillo et al., 2005). Defining AMV coat protein's functional role(s) is challenging because, like many viral proteins, it is multifunctional, with proposed roles in transcription or maintenance of the plus/minus RNA strand ratio (Houwing and Jaspars, 1978; van der Kuyl, Neeleman, and Bol, 1991) and translation (Krab et al., 2005; Neeleman, Linthorst, and Bol, 2004; Neeleman et al., 2001). Comparisons among data from different laboratories are also complicated by the fact that at least four different experimental systems have been used; that is, in vitro studies using biochemically purified components (van Rossum et al., 1997), transient expression of viral RNAs expressed from DNA vectors (Vlot et al., 2001), in vivo analyses using wild type plant tissue (Houwing and Jaspars, 2000), and experiments using transgenic plants or protoplasts that overexpress the two polymerase subunits, P1 and P2 (Taschner et al., 1991).
The hypothesis examined here is that AMV and ilarviruses use the RNA-coat protein complex in place of the tRNA-like 3' terminus for template selection and localization of the polymerase on the viral RNA 3' terminus. Several lines of evidence are consistent with this hypothesis; however, the question is an area of controversy in the literature. The unique requirement for coat protein to activate AMV and ilarvirus RNA replication (Bol, Van Vloten-Doting, and Jaspars, 1971), and the cofolding events that occur when coat protein binds the 3' terminus (Guogas et al., 2004) in the minus strand promoter region (van Rossum et al., 1997) suggest that coat protein binding and replication initiation are linked (Houwing and Jaspars, 1978). The AMV coat protein is an integral component of the replicase (Quadt et al., 1991), further suggesting a role in RNA replication. Alternatively, it has been reported that coat protein inhibits viral RNA replication (Bol, 2005; Houwing and Jaspars, 1986) and that coat protein's principal role is to enhance translational efficiency (Bol, 2005; Krab et al., 2005). Structural details of the RNA-coat protein complex (Guogas et al., 2004) do not support the conformational switch model for coat protein function (Olsthoorn et al., 1999); moreover, recent evidence indicates that coat protein strongly stimulates viral RNA replication at low concentrations corresponding to early stages of viral RNA replication, while inhibiting replication at higher coat protein concentrations that correlate with particle assembly (Guogas, Laforest, and Gehrke, 2005).
The experiments described here evaluate AMV RNA-RdRp binding interactions in the presence and absence of the viral coat protein. The data demonstrate that AMV coat protein acts as a bridge to enhance the binding of 3' untranslated region RNAs from AMV and tobacco streak ilarvirus (TSV) to the replicase subunit proteins P1 (helicase-methyltransferase protein) and P2 (RdRp). These interactions are specific because the tRNA-like 3' terminus of tobacco mosaic virus RNA did not bind to the AMV P1 or P2 proteins in the presence or absence of AMV coat protein. Nucleotide substitution experiments provide evidence that disrupting 3'-terminal coat protein binding domains blocks viral RNA replication while permitting coat protein binding to upstream domains that may have a role in enhancing viral mRNA translation. The data suggest that, by binding both the viral RNA 3' termini (Zuidema et al., 1983) and the helicase-methyltransferase/RdRp subunits (Quadt et al., 1991), the AMV coat protein organizes the 3'-terminal RNA structure (Guogas et al., 2004) and positions the polymerase for accurate initiation.
Results
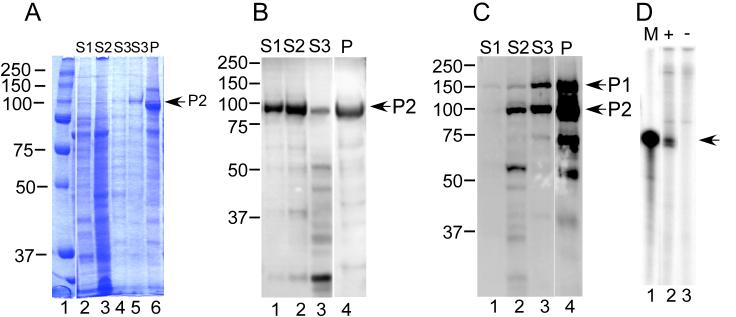
Throughout this report, the term “RNA-dependent RNA polymerase (RdRp)” refers to the AMV P2 protein, while the term “replicase” refers to the RdRp in complex with other macromolecules, such as P1 (helicase-methyltransferase) protein, coat protein, or viral RNA. The P1 and P2 proteins were expressed by infecting insect cells with recombinant baculovirus. The expressed proteins, which are membrane-associated in the insect cells, were released by sequential treatments using buffers containing increasing salt and detergent concentrations (Lohmann et al., 1997). A representative coomassie blue-stained gel from a P2 expression and purification is shown in Figure 1A. Equal proportions of the supernatant fractions S1-S3 and P (pellet) were loaded onto the gel. Bands comigrating with P2 protein were enriched in a second S3 extraction (Figure 1A, lane 5) and in the pellet fraction (Figure 1A, lane 6). Although the expressed proteins carried a 6his tag, affinity purification using nickel column chromatography gave low yields, possibly because the 6His tag was not exposed. However, because of the enrichment provided by the sequential solubilization steps (Figure 1A), as also reported by Lohmann et al. (Lohmann et al., 1997), the extract supernatants or pellet fractions were used without further purification.
Figure 1.
Expression, purification, and activity of expressed AMV P1 and P2 proteins. Recombinant baculoviruses with the 6His-tagged P1 (helicase-methyltransferase) or P2 (RNA-dependent RNA polymerase) protein under the control of the polyhedron promoter were used to infect insect cells. A) Coomassie blue stain pattern of expressed protein fractions. Expressed protein was released from membranes by treatment in buffers containing salt and detergents as described in Methods. Proteins were separated by SDS-polyacrylamide gel electrophoresis, and the gel was fixed and stained with coomassie brilliant blue dye. Lane 1: molecular weight markers; lanes 2-4: equal proportions of fractions S1, S2, S3, from the membrane release protocol as described in Experimental Procedures were loaded. Lane 5 represents a second extraction of the pellet fraction using Buffer LBIII, and lane 6 shows protein in the pellet fraction. B) Immunoblot localizing expressed P2 protein in the fractions. Proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and detected using anti-6His antibodies. Lanes 1-3: soluble fractions S1-S3; lane 4: pellet fraction. The P2 arrow identifies the RNA-dependent RNA polymerase (RdRp) band. C) Immunoblot showing co-expression of the P1 (helicase-methyltransferase) and P2 (RdRp) proteins. Insect cells were co-infected with recombinant baculoviruses expressing the P1 and P2 proteins. The expressed proteins were analyzed as described in (B). D) In vitro transcription reaction. An aliquot of the soluble P2 protein was incubated with and without an RNA template representing the 3' untranslated region of alfalfa mosaic virus RNAs. The transcription products were separated by polyacrylamide gel electrophoresis as described in Methods. Lane 1: molecular size marker showing the migration of a 32P-labeled in vitro-transcription product of the AMV 3' untranslated region RNA (arrow); lanes 2 and 3: incubations performed in the presence (+) and absence (−) of AMV 3' UTR RNA template.
Expressed proteins were readily detected after SDS denaturation by immunoblotting using an anti-6his monoclonal antibody. The solubilized P2 protein was released using buffers LB1 and LB2 (Figure 1B, lanes 1 and 2), although insoluble protein remained in the pellet fraction (lane 4). When the P1 and P2 proteins were co-expressed, membrane release followed a different pattern in the lysis buffers. Although P2 protein expressed alone was released by LBI (Figure 1B, lane 1), little or no co-expressed P1 was similarly released (Figure 1C, lane 1). P1 and P2 were present in roughly equimolar amounts in fraction S3 (Figure 1C, lane 3), suggesting that they form a complex (Van Der Heijden et al., 2001). Approximately one-third of the expressed protein was solubilized in high salt and detergent, while two-thirds remained in the pellet fraction (Figure 1C, lane 4).
To confirm that released proteins retained enzymatic activity, soluble P2 was tested in an in vitro transcription assay. Using positive strand 3' UTR RNA derived from AMV RNA3, template-dependent production of the correct-length product, most likely negative strand RNA, was observed (Figure 1D, compare lanes 2 and 3). Similar results were observed using co-expressed P1 and P2 protein (data not shown). The data presented here demonstrate that soluble and enzymatically active recombinant P2 RdRp was prepared. However, recombinant RNA-dependent RNA polymerase proteins often lack template specificity in the absence of other proteins that contribute to the replicase complex (Kao et al., 2000; Lai, 1998). AMV RNA replication is coat protein-dependent in vivo (Bol, Van Vloten-Doting, and Jaspars, 1971), but in vitro replication systems do not recapitulate coat protein-mediated regulation because added coat protein blocks transcription (Houwing and Jaspars, 1986). As a result, available in vitro replication systems have limited application for revealing mechanistic details of coat protein's role in AMV replication.
RNA-P1/P2 interactions are observed when coat protein is present
The requirement for coat protein in AMV and ilarvirus replication correlates directly with the absence of canonical 3'-terminal CCA transfer RNA-like features across members of the virus family Bromoviridae. Therefore, we reasoned that the AMV RNA-coat protein complex may be involved in template recognition and selection for replication. Coat protein binds specifically to the 3' terminus of the AMV RNAs, generating a structurally uniform population of viral RNA 3' termini (Guogas et al., 2004); moreover, coat protein is integral to the replicase (Quadt et al., 1991). We hypothesized that coat protein, by binding both the viral RNA and the viral RdRp, may bridge the replicase-RNA interaction, providing template selection and specificity in transcription initiation. In a related study, Stork et al. recently reported that the Tombusvirus p33 protein functions in template selection and binds to both the viral RNA and to the RdRp (Stork, Panaviene, and Nagy, 2005).
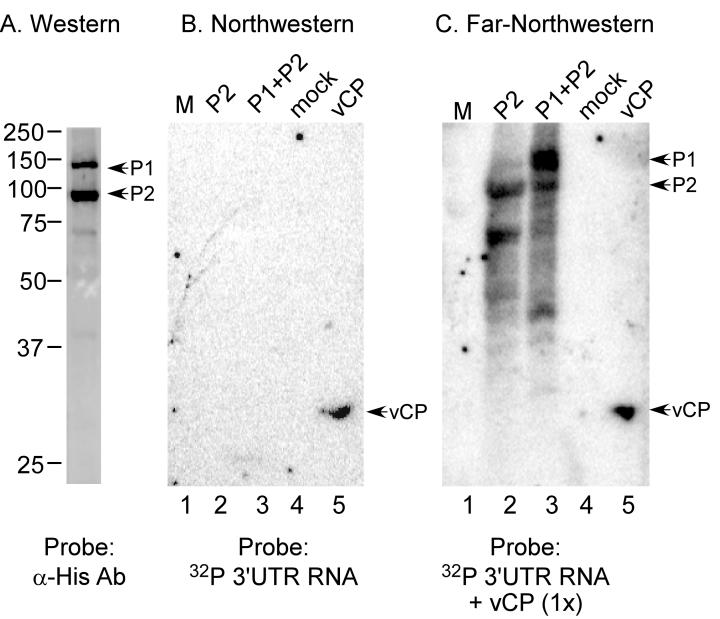
A method, referred to here as far-northwestern blotting, was developed to assay specific protein-protein-RNA or protein-RNA-protein interactions. This technique is similar to a standard northwestern blot, wherein proteins are separated by SDS-PAGE, transferred to a membrane, re-natured on the membrane, and then probed with RNA. The distinguishing feature of the far-northwestern is that the membrane is probed with a combination of RNA and protein; that is, the AMV 3' UTR RNA and viral coat protein. P1 and P2 replicase proteins from pellet fractions (Figure 1) were separated by SDS-PAGE and probed with anti-6His antibody to define their migration positions (Figure 2A). In adjacent lanes, P2 or P1/P2 protein fractions, along with cell extract from mock-infected insect cell and virion coat protein (vCP), were separated and then denatured and renatured on the membrane (Figure 2B, lanes 2-5). To determine if the AMV 3' RNA, containing the promoter for minus strand RNA synthesis (van Rossum et al., 1997), would interact directly with P1 and/or P2 proteins, the blot was probed with radiolabeled 3' UTR RNA. The data (Figure 2B, lanes 1-4) demonstrate that there was no detectable direct association of the labeled RNA with molecular weight marker proteins (M), replicase proteins P1 or P2 (lanes 2 and 3), or proteins of extracts from mock-infected cells (lane 4). As a positive control, the labeled RNA did bind to virion coat protein (Figure 2B, lane 5) as expected (Houser-Scott et al., 1994; Reusken et al., 1997).
Figure 2.
RNA-RdRp binding detected by far-northwestern analysis in the presence of viral coat protein. A) Co-expressed P1 and P2 proteins were separated by electrophoresis into an SDS-polyacrylamide gel and processed for western blot analysis using an anti 6his antibody. The arrows mark the migration positions of the P1 and P2 proteins. B) Northwestern blot analysis of AMV 3' RNA binding to P1, P2, and coat protein. Proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and sequentially denatured and renatured on the membrane as described in Experimental Procedures. Radiolabeled RNA probe was added, followed by incubation, washing, and autoradiography to detect RNA-protein interactions. Lane 1: molecular weight marker proteins; lane 2: P2 protein; lane 3: P1/P2 co-expressed proteins (pellet fractions); lane 4: extract prepared from mock-infected insect cells; lane 5: virion coat protein (vCP). C) Far-northwestern analysis, where the blot is probed with radiolabeled RNA in the presence of soluble coat protein. The lanes are the same as panel B. In lanes 2 and 3, the labeled RNA was found to associate with protein bands that migrate faster than P1/P2, which and are likely to be hydrolysis products of the expressed P1 and P2 proteins.
Coat protein's role in the AMV RNA-P1/P2 interaction was assessed by far-northwestern blotting, wherein the membrane was probed with a solution containing radiolabeled 3' AMV RNA and an equimolar amount of virion coat protein. The resulting blot (Figure 2C) reveals additional interactions as compared to the northwestern analysis (Figure 2B). Bands comigrating with P1 and P2 are evident (Figure 2C, lanes 2 and 3); however, no bands were observed in the molecular weight marker lane (Figure 2C, lane 1), or the lane representing extract prepared from mock-infected insect cells (lane 4). Labeled RNA bound to the vCP control (lane 5). These data demonstrate that, in the presence of the viral coat protein, labeled RNA was found in association with the viral P1 and P2 proteins. When coat protein was present, the 3' UTR RNA associated with either AMV P1 or P2, suggesting that the interaction is not dependent on a native P1/P2 complex. Furthermore, when comparing amounts of proteins on immunoblots to the intensity of bands in the far-northwestern, it appears that 3' UTR RNA associated more efficiently with P1 than with P2 (Figure 2C, lane 3).
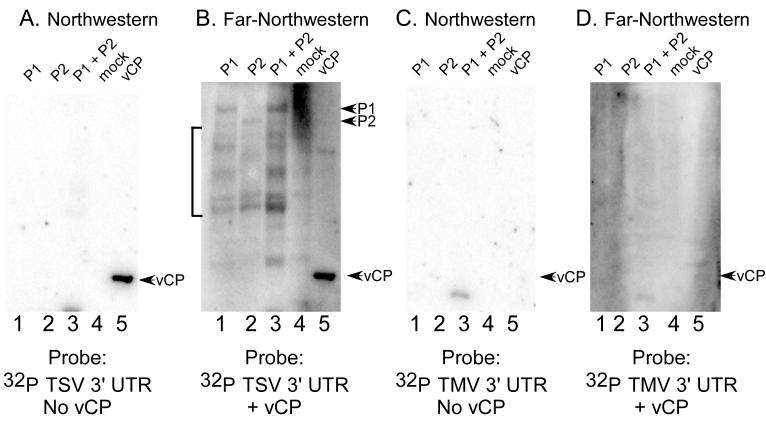
As a further test for binding specificity, northwestern and far-northwestern blotting was performed using other RNAs. Although AMV and ilarvirus coat protein sequences are distinct, they will cross-activate viral RNA replication (Gonsalves and Garnsey, 1975a; Gonsalves and Garnsey, 1975b; van Vloten-Doting, 1975). In other words, AMV coat protein will activate tobacco streak virus (TSV) replication, and vice-versa, suggesting a common role(s) in replication. We hypothesized, therefore, that the 3' untranslated region of the closely-related TSV RNA would complex with the AMV replicase subunits, while that of the distantly-related tobacco mosaic virus (TMV) RNA would not. In a manner similar to that observed using the AMV RNA 3' UTR (Figure 2), the radiolabeled TSV RNA interacted with AMV coat protein in a standard northwestern blot assay (Figure 3A, lane 5); however, there was no detectable interaction with the polymerase P1 and P2 proteins (Figure 3A, lanes 1-3), or with proteins in the mock extracts (lane 4). However, in the presence of AMV coat protein, the TSV RNA associated with P1 and P2 proteins (Figure 3B, lanes 1-3), and also to presumed proteolytic breakdown products of the P1 and P2 proteins (Figure 3B, lanes 1-3, bracket). As predicted, the TMV 3' UTR RNA did not interact with the AMV coat protein (Figures 3C and 3D, lanes 5), or with the polymerase P1 and P2 proteins, irrespective of whether coat protein was present in the probe solution (Figure 3C and 3D, lanes 1-3). These data provide further evidence that template selection and specificity may be enhanced by AMV coat protein's ability to bind specifically to the AMV replicase proteins P1 and P2, and also to the viral RNAs.
Figure 3.
RNA-RdRp interactions detected by far-northwestern blotting are specific to AMV and ilarvirus RNAs. P1, P2, P1/P2, mock extract, and AMV virion coat proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and successively denatured and renatured prior to probing with radiolabeled RNA only (panels A and C) or radiolabeled RNA plus soluble AMV coat protein (panels B and D). The probes used for the experiments were the 3' untranslated region of tobacco streak virus (TSV) RNA 4 (panels A and B) or the 3' untranslated region of tobacco mosaic virus RNA (panels C and D). The bracket indicates labeled RNA associated with protein bands that are likely to be hydrolysis products of the P1 and P2 proteins, which were found to be relatively unstable on storage.
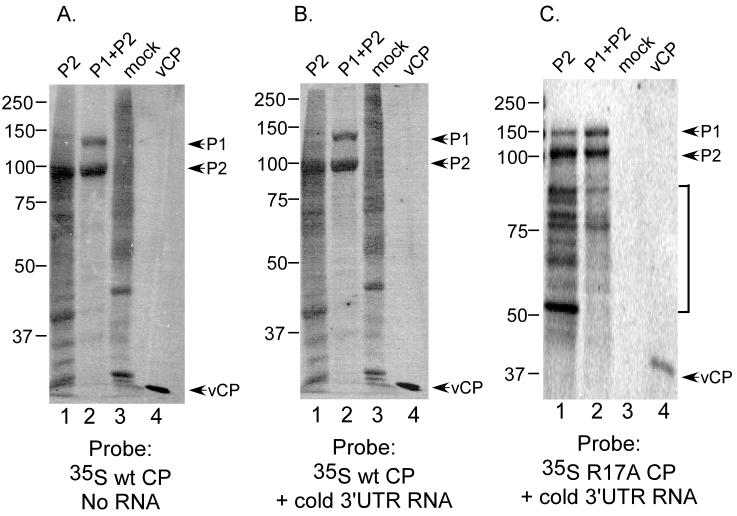
Although the data presented in Figure 2C (lanes 2 and 3) are consistent with coat protein's identification as an integral replicase protein (Quadt et al., 1991), van der Heijden et al. (Van Der Heijden et al., 2001) reported that coat protein-P1/P2 interactions were not detected in a two-hybrid assay. As a further test of direct CP-replicase binding, membranes were probed with 35S-labeled coat protein that was generated by translating viral subgenomic RNA 4 (the coat protein mRNA) in a cell-free translation system. Bands corresponding to P1 and P2 proteins (Figure 4A, lanes 1 and 2), as well as AMV virion coat protein (lane 4) were observed. The interaction of labeled coat protein with the immobilized vCP (Figures 4A-4C, lanes 4) was expected because coat protein forms a homodimer (Kruseman et al., 1971). A pattern of bands similar to those in Figure 4A was observed when the blot was probed using far-northwestern conditions using unlabeled 3' UTR RNA and 35S-labeled coat protein in the probe solution (Figure 4B). Further, this banding pattern was also observed using far-western blot conditions where 35S-labeled coat protein containing an R17A mutation was used as probe (Figure 4C). Coat protein containing the R17A mutation is incapable of binding the viral RNA (Ansel-McKinney et al., 1996). These data suggest that CP binding to P1 and P2 is RNA-independent. Together, these results indicate that coat protein interacts directly with both P1 and P2, suggesting that the addition of coat protein to the far-northwestern analysis (Figure 2C) localizes labeled RNA to the RdRp subunits via RNA-coat protein-replicase binding.
Figure 4.
35S-labeled AMV coat protein binds directly to P1 and P2 polymerase proteins. P2 protein, P1/P2 co-expressed proteins, extract from mock-infected insect cells, and virion coat protein (lanes 1-4 respectively in each panel) were separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane, followed by denaturation and renaturation as described in Experimental Procedures. A) the membrane was incubated with 35S-labeled coat protein generated by cell free translation. B) Similar to (A), except that the unlabeled AMV 3' UTR RNA fragment was included with the 35S-labeled coat protein. C) Similar to (B), except that the cell-free translation extract was programmed with mRNA encoding a variant (R17A) form of the AMV coat protein. The bracket indicates labeled coat protein interacting with protein bands that are likely to be hydrolysis products of the P1 and P2 proteins, which were found to be relatively unstable on storage. The use of translation extracts as probes was sometimes accompanied by high background interactions with proteins from mock-infected cell extracts (panels A and B, lane 3).
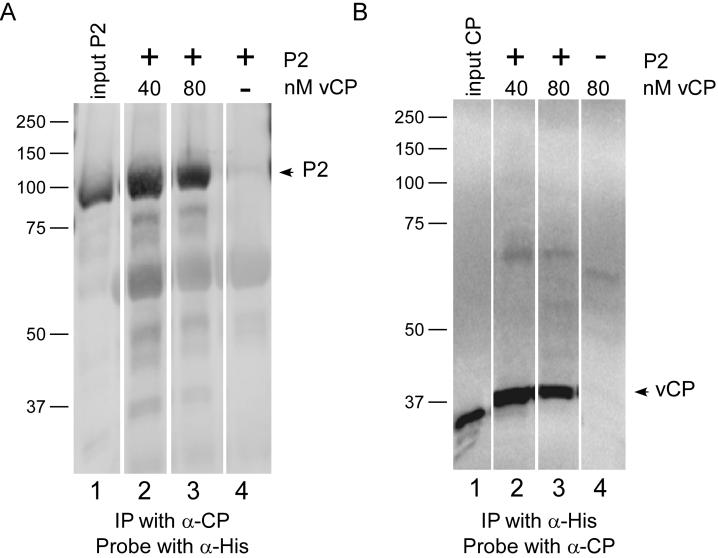
Native immunoprecipitation was used as a second approach to test for coat protein-RdRp interactions. Soluble P2 protein (Figure 1B, lane 2), virion coat protein, and AMV 3' UTR RNA were combined before adding anti-coat protein antiserum. The immune complexes were captured using protein-A Sepharose, and, after washing the matrix, bound proteins were analyzed by SDS-PAGE and western blotting using 6His antiserum. The presence of P2 protein in the input sample was confirmed (Figure 5A, lane 1), and a further control showed that there was no P2 protein pulldown in the absence of added coat protein (lane 4). Coat protein-P2 interactions were observed in the pulldowns (Figure 5A, lanes 2 and 3). The presence or absence of RNA in the pulldown reactions did not alter the results detectably (data not shown). The inverse immunoprecipitation conditions were also tested in parallel. P2 protein extract was added to coat protein and 3' UTR RNA, and the mixtures were immunoprecipitated with anti-6His antibody (Figure 5B, lanes 2 and 3). Following SDS-PAGE and western blotting using the anti-coat protein antiserum, input coat protein (Figure 5B, lane 1) and immunoprecipitated coat protein (Figure 5B, lanes 2 and 3) were observed. Coat protein was not immunoprecipitated in these reactions in the absence of the P2 protein extract (Figure 5B, lane 4). Prior data showed that AMV coat protein co-purifies with replicase (Quadt et al., 1991); however, data presented here provide evidence that AMV coat protein interacts directly with AMV P1 (Figure 4) and P2 (Figures 4 and 5) proteins.
Figure 5.
Analysis of AMV coat protein-polymerase interactions by co-immunoprecipitation. AMV coat protein (40 nM or 80 nM) was added to soluble P2 protein. Complexes were immunoprecipitated with anti-AMV coat protein or anti-6his antibodies. The precipitates were collected, separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed. A) Immunoprecipitation with anti-coat protein antibody, followed by electrophoresis, transfer, and probing with anti-6his antibody to detect P1 and P2 proteins. Lane 1: P2 protein only (input); lanes 2 and 3: P2 protein with 40 nM or 80 nM virion coat protein added, respectively; lane 4: P2 protein extract without added coat protein. B) Immunoprecipitation with anti-6his antibody, followed by electrophoresis, transfer, and probing with anti-coat protein antibody. Lane 1: Virion coat protein only; lane 2: 40 nM virion coat protein with P2 protein; lane 3: 80 nM virion coat protein plus P2 protein; lane 4: 40 nM virion coat protein, without added P2 protein. There was some distortion (“frowning”) of the gel shown in Figure 5B; therefore, the bands in lane 4 do not align perfectly with the other lanes. However, the results show that there is little detectable coat protein signal present when P2 protein was omitted from the immunoprecipitation reaction.
Effects of nucleotide substitutions on coat protein binding and replication
The AMV coat protein is multifunctional in the viral life cycle, and it has been suggested that differential coat protein occupancy at the multiple binding sites may determine coat protein's roles in replication, translation, and assembly (Petrillo et al., 2005). We sought to determine if mutations in individual coat protein binding domains might affect the organization and template selection functions required for viral RNA replication, without an overall block to coat protein binding that may be important for enhancing viral RNA translation (Krab et al., 2005; Neeleman, Linthorst, and Bol, 2004).
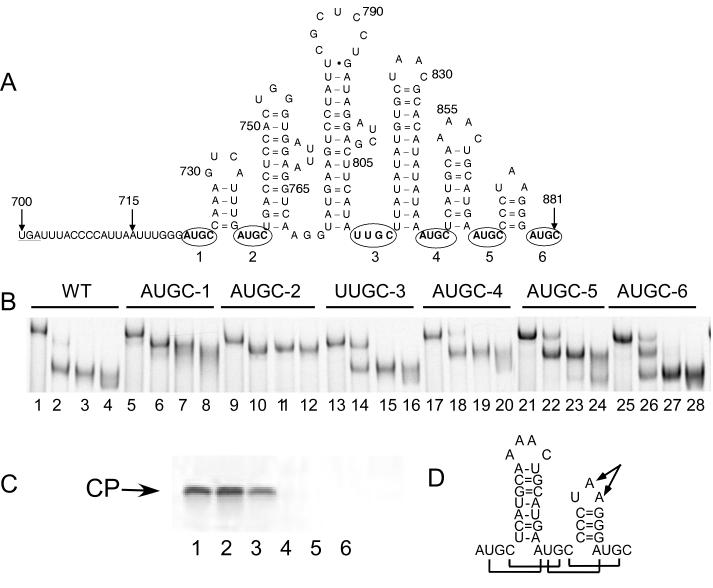
The 3' terminus of the AMV RNAs is characterized by hairpin structures that are separated by (A/U)UGC tetranucleotide repeats, forming multiple coat protein binding sites (Guogas et al., 2004; Houser-Scott et al., 1994; Reusken, Neeleman, and Bol, 1994). A schematic representation of the AMV RNA 3 3' untranslated region shows the positions of the AUGC or UUGC sequences separating proposed hairpin structures (Figure 6A). To assess potential differential functions correlated with coat protein occupancy at the binding sites, individual AUGC or UUGC coat protein binding sites were converted to AAAA in viral genomic RNA 3. The AUGC to AAAA substitutions have been shown previously to disrupt coat protein binding (Guogas et al., 2004; Houser-Scott et al., 1994). Coat protein binding to the 3' UTR RNA was analyzed by electrophoretic mobility shift assay and viral RNA replication from the mutated RNA template was assessed by coat protein accumulation resulting from viral RNA replication in transfected tobacco protoplasts.
Figure 6.
Disrupting individual (A/U)UGC-coat protein binding sites impairs viral RNA replication functions without blocking coat protein binding to the 3' UTR RNA fragment. A) Schematic representation of the sequence and proposed secondary structure of the 3' untranslated region of alfalfa mosaic virus RNA 3 and 4. The AUGC or UUGC sequences that contribute to coat protein binding are numbered and circled. B) Electrophoretic mobility shift analysis of coat protein peptide binding to wild-type and variant 3' UTR RNA fragments containing AUGC to AAAA substitutions. The labels above the lanes refer to the radiolabeled RNA used in the bandshift analysis. WT: wild type RNA. The remaining labels refer to the AUGC or UUGC RNAs with the numbers corresponding to the positions shown in panel A. Lanes 1, 5, 9, 13, 17, 21, 25 are radiolabeled RNA only. The remaining three lanes in each group contain increasing concentrations (50 nM, 100 nM, 500 nM) of the CP26 peptide representing the N-terminal 26 amino acids of the viral coat protein (Ansel-McKinney et al., 1996). The labels above the lanes identify RNA 3 constructs carrying the nucleotide substitutions in AUGC 1-6 (panel A). AUGC positions 1, 2, 4, and 5 (see Figure 5A) were mutated to AAAA. The UUGC at position 3 was changed to UAAA to conserve RNA folding as predicted by MFold (Zuker and Jacobson, 1998), and AUGC #6 (Figure 5A) was changed to AAAC in order to conserve the Sma I restriction enzyme site used to linearize the plasmid DNA for in vitro transcription. C) Viral RNA replication using variant genomic RNA 3 constructs. Tobacco protoplasts were co-transfected with RNA transcripts for genomic RNAs 1 and 2, subgenomic RNA 4 (encoding the viral coat protein) plus wild type (lanes 1-4) or variant RNA 3 RNA transcripts containing AUGC to AAAA substitutions at positions 1-6 (lanes 5-28). The transfected protoplasts were incubated for 48 hours and lysed in SDS-PAGE sample buffer. The lysates were analyzed by SDS-polyacrylamide gel electrophoresis, followed by western blotting using anti-coat protein polyclonal antiserum. CP: viral coat protein. D) Schematic representation of the 3'-terminal 39 nucleotides of AMV RNAs with brackets showing the inter-AUGC basepairs formed upon coat protein binding (Guogas et al., 2004). The arrows indicate nucleotides that are conserved in AMV and ilarvirus RNAs (Houser-Scott et al., 1994), but are not contacted by coat protein (Guogas et al., 2004).
Electrophoretic mobility bandshift data provide evidence that, in the context of the 180-nucleotide 3' UTR sequences, the individual AUGC or UUGC changes to AAAA or UAAA did not prevent coat protein peptide binding (Figure 6B). These results suggest that mutation of individual coat protein binding sites did not prevent binding to other coat protein binding sites on the RNA fragment. The mobility shift patterns associated with each of the RNAs were variable; however, all of the variant RNAs bound the coat protein peptide, whose specificity for the viral RNAs has been demonstrated previously (Ansel-McKinney et al., 1996; Guogas et al., 2004). The negative bandshift, or enhanced mobility shown in Figure 6B, has been discussed previously (Baer et al., 1994), and is likely due to compacting the RNA conformation as a result of forming the RNA-peptide complex (Guogas et al., 2004).
The nucleotide substitutions were engineered into the 3' terminus of genomic RNA 3, and tobacco protoplasts were transfected with RNAs 1, 2, 4 (subgenomic coat protein mRNA) and the variant RNA 3 transcripts. Additional details about the AMV genomic organization are shown in Figure S1. The appearance of coat protein in this assay is evidence of viral RNA replication because translation of input RNA 4 is insufficient for detection by western blotting (Petrillo et al., 2005; Rocheleau et al., 2004). The data (Figure 6C, lanes 1-3) show that the (A/U)UGC to (A/U)AAA at positions 1-3 (see Figure 6A) did not preclude accumulation of viral coat protein. In contrast, coat protein was not detected when AUGC tetranucleotide sequences at positions 4-6 were mutated (Figure 6C, lanes 4-6), suggesting that these downstream AUGC repeats are necessary for viral RNA replication. These data suggest that, despite the fact that coat protein can bind to the 3' UTR of the variant RNAs (Figure 6B), disrupting the downstream AUGC repeats is highly detrimental to viral RNA replication (Figure 6C). These results suggest that the functions of AUGC or UUGC coat protein binding domains 1-3 are distinct from repeats 4-6. The X-ray crystal structure data demonstrated that coat protein stabilizes an unusual pattern of inter-AUGC basepairing in the 3'-terminal thirty-nine nucleotides (Guogas et al., 2004) (Figure 6D). These results may be relevant to explaining how coat protein can influence both viral RNA replication and translation.
Discussion
Viral RNA replication initiation is, in general, either primer-dependent or accomplished de novo (reviewed in (Kao, Singh, and Ecker, 2001)). Theory (Maizels and Weiner, 1994; Maizels and Weiner, 1999; Weiner and Maizels, 1987) and experimental evidence (reviewed in (Fechter et al., 2001)) suggest that amplification of viral genomes requires template selection signals that facilitate polymerase binding and accurate transcription initiation. By classification, AMV and brome mosaic virus are very closely related (Fields, Knipe, and Howley, 1996), yet AMV and the ilarviruses differ from other bromoviruses in several ways: 1) AMV and ilarvirus RNAs lack the canonical features of the tRNA-like 3' terminus, 2) AMV and ilarvirus RNAs require coat protein to replicate their genomes, 3) the viral coat protein is an integral part of the AMV replicase complex, but not of the BMV replicase complex (Quadt et al., 1991), and 4) BMV minus strand synthesis initiates on the penultimate C nucleotide of the template RNA (Miller et al., 1986), while AMV minus strand synthesis presumably initiates on the ultimate C nucleotide. These distinctions, coupled with the data presented in this report, are consistent with a hypothesis stating that AMV and BMV initiate viral RNA replication by distinct mechanisms. The dramatic conformational changes that accompany coat protein binding to the 3' terminus of the viral RNA (Guogas et al., 2004), coupled with the unique requirement for coat protein to initiate viral RNA replication (Bol, Van Vloten-Doting, and Jaspars, 1971; Neeleman and Bol, 1999) are consistent with a hypothesis stating that coat protein binding facilitates RNA-replicase interactions and the positioning of the replicase for accurate initiation.
One conclusion that has had a dominant effect on the AMV literature is that AMV coat protein inhibits minus strand RNA synthesis (Degraaff, Tveld, and Jaspars, 1995; Houwing and Jaspars, 1986; Olsthoorn et al., 1999; van der Kuyl, Neeleman, and Bol, 1991). The possibility of global inhibition of minus strand synthesis by coat protein is inconsistent with arguments presented in this report; that is, that coat protein facilitates replication by organizing the 3' conformation for replicase binding and accurate initiation. A close look at the experimental conditions in published reports may help reconcile the data. In vitro transcription reactions that examined the effects of exogenous coat protein have been done using replicase prepared from infected tissue, wherein coat protein is present as an integral protein (Quadt et al., 1991). Coat protein-free replicase has been used in some experiments; however, it displayed only a few percent of the activity exhibited by coat protein-inclusive replicase preparations (Degraaff, Tveld, and Jaspars, 1995). In recent experiments, coat protein was found to stimulate viral RNA replication when titrated from very low concentrations. In other words, when increasing amounts of coat protein were added to an inoculum containing AMV genomic RNAs 1-3, low coat protein concentrations were strongly stimulatory to replication, while higher coat protein concentrations inhibited replication (Guogas, Laforest, and Gehrke, 2005), presumably by stimulating particle assembly. The potential biological relevance of this observation is that, in a newly-infected cell, coat protein concentrations are correspondingly low, suggesting the potential to stimulate AMV replication. As coat protein accumulates during the infection, coat protein occupancy on the viral RNA 3' terminus could progress, eventually inhibiting replication and triggering particle assembly. The results strongly suggest that coat protein stimulates viral RNA replication during the earliest stages of the infection or in compartmentalized areas of the cell where coat the coat protein concentration is low.
Although the AMV coat protein co-purifies in polymerase preparations (Quadt et al., 1991), and coat protein has been described as an integral component of the polymerase protein (Degraaff, Thorburn, and Jaspars, 1995), the coat protein-polymerase interaction was not detected in yeast two-hybrid experiments (Van Der Heijden et al., 2001). Technical issues with the yeast two hybrid assay might explain the results, because it was also found that yeast growth was not observed when full-length P1 and P2 proteins were used in the binding and activation domain constructs (Van Der Heijden et al., 2001). The highly basic nature of the AMV coat protein may have resulted in its exclusion from the nucleus in the yeast two-hybrid experiments. Although our results demonstrate coat protein-polymerase interactions by co-immunoprecipitation, van Der Heijden et al. reported that co-immunoprecipitations were not observed using in vitro- translated proteins. The basis for the difference in data is not clear; however, the results presented in Figure 4 are consistent with the profile of proteins found in replicase purified from infected tissue (Quadt et al., 1991). One possible explanation is that the low concentration of proteins translated in the in vitro extract might have interfered with immunoprecipitation or, alternatively, that other proteins in the extract interfered with precipitation.
Coat protein has also been reported to stimulate viral mRNA translation, possibly by facilitating mRNA circularization (Krab et al., 2005; Neeleman, Linthorst, and Bol, 2004; Neeleman et al., 2001). Could coat protein enhance both viral mRNA translation and replication? AMV mRNA translation cannot have an absolute requirement for coat protein binding because infections can be initiated by adding the subgenomic RNA 4 (encoding the viral coat protein) to an otherwise coat protein-free transfection mix including the three genomic RNAs (Laforest and Gehrke, 2004; Rocheleau et al., 2004). The conformational switch model (Olsthoorn et al., 1999) proposed that coat protein binding extended the AMV RNA conformation and switched RNA usage from replication to translation. However, the X-ray crystal structure data (Guogas et al., 2004) show that coat protein binding compacts the RNA structure rather than extending it. The conformational switch model also requires viral RNA replication on coat protein-free viral RNA, though there are no mechanisms known at this point that would prevent the coat protein from binding the viral RNA.
It is generally acknowledged that non-packaged viral genomic RNAs are bound either by translating ribosomes, moving in a 5'-3' direction, or by RNA-dependent RNA polymerase, moving 3' to 5'—but not by both concurrently. Gamarnik and Andino's experiments with poliovirus (Gamarnik and Andino, 1998) suggested that polymerase cannot bind to mRNAs being actively translated, and that protein-RNA interactions at the 5' end of the RNA have important roles in regulating translation versus replication. It is conceivable, a priori, that AMV coat protein could have roles in both replication and viral mRNA translation, again providing some reconciliation when evaluating published data. Results presented here and in a previous report (Guogas, Laforest, and Gehrke, 2005) may indicate that minus strand synthesis is facilitated when coat protein concentrations are low while higher coat protein concentrations could trigger assembly. Coat protein's role in regulating transcription and translation could also be influenced by intracellular compartmentalization. AMV replication has been reported to take place at the tonoplast membrane (Van Der Heijden et al., 2001), and membrane specializations (spherules) correlate closely with viral RNA replication sites (Schwartz et al., 2004). Low coat protein concentration within the spherules might enhance viral RNA replication. Clearly, details are lacking at this point and additional study is needed to define the mechanisms.
The three dimensional structures of at least six RNA-dependent RNA polymerases and three initiation complexes have been published (reviewed in (van Dijk, Makeyev, and Bamford, 2004)). None of the Bromovirus polymerase structures has been solved; therefore, it is not known how the tRNA-like 3' termini or the AMV RNA-coat protein complex might look in the initiation complex. The X-ray crystal structure of the AMV RNA-coat protein peptide co-complex, in addition to in vitro genetic selection data (Guogas et al., 2004; Rocheleau et al., 2004) strongly suggest that the coat protein does not make direct contact with the conserved loop nucleotides of the 3'-terminal hairpins (Figure 6D, arrows). We propose that coat protein binding converts a structurally heterogenous population of protein-free 3' RNA ends into a uniform population of structured RNA-protein complexes for presentation to the viral RNA-dependent RNA polymerase. One possibility is that the polymerase may recognize the conserved loop nucleotides and structure as part of its recognition and binding domain.
The number of single-stranded nucleotides associated with the template tunnel of the polymerases has been estimated at about five- to eight (reviewed in (Kao, Singh, and Ecker, 2001)). In the structure of the AMV-coat protein complex (Guogas et al., 2004) the 3'-terminal C residue is unpaired, while the penultimate G is base-paired with an upstream C (Figure 6D). Threading the 3' terminus of the viral RNA into the polymerase template channel may require the helicase activity predicted to be associated with the AMV P1 protein. The status of the bound coat protein at this point of the initiation stage is not known, but one hypothesis is that polymerase binding or the activity of the polymerase/helicase may displace or dissociate coat protein. As stated above, the AMV RNA-coat protein co-complex structure (Guogas et al., 2004) and function of coat protein at low concentration during replication (Guogas, Laforest, and Gehrke, 2005) do not support the conformational switch model (Olsthoorn et al., 1999). However, Olsthoorn and Bol (Olsthoorn et al., 1999) described nucleotides with potential for intermediate-range basepairing between the extreme 3' terminus and mid-3' UTR region. The functional significance of this potential pairing is not clear; however, disruption of 3'-terminal coat protein binding by polymerase binding or movement could potentially permit the formation of these base pairs. If so, a prediction would be that subsequent coat protein binding would convert the RNA back to the compact structure.
This work was initiated to begin to understand how the AMV RNA-dependent RNA polymerase recognizes the 3' terminus of the viral RNA for precise initiation of viral RNA replication. AMV and ilarvirus RNAs are distinct because they lack a 3'-terminal tRNA terminus; moreover, this distinction is coupled with a unique requirement for coat protein to initiate replication. The experimental data presented here show that, in vitro, the AMV coat protein mediates indirect interactions between the AMV 3' UTR RNA and replicase proteins P1 and P2. The results suggest a model wherein coat protein binds to both the polymerase proteins and to the RNA, and thereby bridges their interaction. This interaction is proposed to enhance template selection and the accurate positioning of the polymerase to thread the 3' terminus into the template channel. One approach for testing our model in a more natural experimental system would be to identify and disrupt the coat protein-replicase interaction domains, followed by viral RNA replication analysis. With respect to coat protein's multifunctionality, further study is needed to determine if initiation factor eIF4G {Krab, 2005 #5902}and the viral replicase proteins compete for binding to the viral coat protein, thereby directing function to translation or viral RNA replication.
Materials and Methods
Recombinant baculovirus clones
The coding regions for the alfalfa mosaic virus P1 (presumptive helicase-methyltransferase) and P2 (RNA-dependent RNA polymerase) proteins were amplified from DNA clones by thermal cycling and inserted into the pFastBac HTb vector (Invitrogen), adding a C-terminal 6His affinity tag. The construct DNAs were transformed into DH10Bac cells (Invitrogen) containing a baculovirus shuttle vector bacmid with a recombination target site. Recombinant bacmids generated by recombination events were recovered as high molecular weight DNA and used to transfect insect cells. Recombinant virus was plaque-purified and used to infect insect cells for protein expression.
Protein Expression and purification
TnHi5 cells were infected with recombinant baculoviruses expressing 6His-tagged P1 and P2, or P2 alone. The expressed proteins were released from membranes and solubilized using methods described by Lohmann et al. (Lohmann et al., 1997). Insect cells were sedimented, resuspended in 1× PBS, and re-sedimented by centrifugation for 10 minutes at 1500 × g. Cell pellets were then resuspended in cold Lysis Buffer 1 (LB1) (10 mM Tris-HCl pH 7.5, 10 mM NaCl, 10 mM MgCl2, 10 mM β-mercaptoethanol), and incubated on ice for 30 minutes with vortexing every 5 minutes. The suspension was centrifuged for 5 minutes at 14,000 rpm in a microcentrifuge (Eppendorf) at 4° C, and the supernatant was removed. The pellet was resuspended in Lysis Buffer II (LB2) (20 mM Tris-HCl pH 7.5, 300 mM NaCl, 10 mM MgCl2, 0.5% Triton X-100, 20% glycerol, and 10 mM β-mercaptoethanol). The suspension was sonicated three times for 5 seconds at setting level 2 (Virsonic 100 sonicator, Virtis). The sonicate was centrifuged for 5 minutes at 4° C using a microcentrifuge (Eppendorf, 14,000 × g), and the supernatant was removed. The pellet was then suspended in Lysis Buffer III (LB3) (20 mM Tris-HCl pH 7.5, 500 mM NaCl, 10 mM MgCl2, 2 % Triton X-100, 50% glycerol, and 10 mM β-mercaptoethanol) and sonicated three times for 5 seconds at setting level 2 . The sonicate was centrifuged as described above. Following removal of the supernatant, the pellet was resuspended once again in Lysis Buffer III (LB3), sonicated three times for 5 seconds each at setting level 2. Following a final centrifugation, the fractions containing soluble supernatant protein were either used directly or pooled before use. Pellet fraction protein was used for some of the far-northwestern blot experiments while soluble protein was used for all other experiments.
In vitro translation
Radiolabeled P1 and P2 proteins were produced by in vitro translation using a micrococcal nuclease-treated reticulocyte lysate (Promega Corporation) programmed with in vitro transcripts of the P1 and P2 messenger RNAs and 35S-methionine. Following the incubation period, unincorporated 35S-methionine was removed by size exclusion chromatography on Sepharose G25.
Far-northwestern analysis
This is a method for detecting specific protein-RNA-protein or protein-protein-RNA interactions. The technique is similar to a northwestern blot; however, a combination of protein and RNA is used as probe. In the first step, northwestern analysis was performed essentially as described by Blackwell and Brinton (Blackwell and Brinton, 1997), with modifications (Gomila and Gehrke, 2006). Proteins were separated by SDS-polyacrylamide gel electrophoresis using 10% gels (BioRad, precast). Transfer to nitrocellulose membrane was done overnight at 30 V. Blots were blocked by incubating in 5 % Blotto (Pierce) in PBST (PBS + 0.1% Tween-20) for 1 hr at room temperature. Blots were then washed in HBB buffer (25 mM HEPES-KOH pH 7.5, 25 mM NaCl, 5 mM MgCl2 and 7 mM β-mercaptoethanol) for 10 minutes. Prior to adding the probe, the proteins bound to the nitrocellulose membrane were denatured in guanidinium chloride and subsequently renatured by slowly removing the denaturant. Denaturation was accomplished by two successive washes in HBB buffer containing 6 M guanidinium chloride. Renaturation was performed by washing the nitrocellulose membrane once each (10 minutes) in 3 M, 1.5 M, 0.75 M, 0.375 M and 0.187 M guanidine chloride in HBB. Membranes were then washed in HBB, followed by 2 washes in HYB100 (20 mM HEPES-KOH pH 7.5, 200 mM KCl, 2.5 mM MgCl2, 0.1 mM EDTA, 0.1 % NP40 and 7 mM β-mercaptoethanol). Radioactivity levels were determined by liquid scintillation counting. When membranes were probed with radiolabeled RNA (as for a northwestern), approximately 1 × 106 counts-per-minute of renatured AMV 3' UTR RNA in HYB100 were incubated with the membranes for 4 hours at room temperature. For RNA-coat protein probing (as for a far-northwestern), the labeled RNA and an equimolar amount of virion coat protein were added to the membrane buffer. Following these incubations, blots were washed three times using HYB100 buffer and exposed to X-ray film overnight.
RNAs and RNA transcription
DNAs corresponding to the 3' untranslated regions of alfalfa mosaic virus, tobacco streak virus, and tobacco mosaic virus were subcloned into a transcription vector containing the bacteriophage T7 promoter. RNAs were transcribed using commercial in vitro transcription kits (Ambion). RNA probes were prepared by including 20 μCi of [α-32P]UTP or CTP in a standard 20 μl transcription reaction. Following the transcription, unincorporated nucleotide was separated from transcribed RNA by spin chromatography on a Sepharose G-50 column. Detailed methods for RNA transcription are described in prior publications (Ansel-McKinney and Gehrke, 1998; Petrillo et al., 2005).
Native immunoprecipitation
Duplicate binding reactions were prepared, each containing a solution of 60 μl soluble P2 fraction, 0.1 mM EDTA, 0.015 μg/μl yeast tRNA, and 5 units RNase inhibitor (Qiagen). Virion coat protein (vCP) was added to a final concentration of 40 nM or 80 nM, with and without 10 nM AMV 3' UTR RNA. Reactions were incubated at room temperature for 15 minutes, and then diluted by adding 220 μl of IP Buffer (10 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.05% NP40, 0.5 mM dithiothreitol), and placed on ice. To each pair of duplicate reactions, either 2 μl polyclonal anti-vCP antibody, or 3 μl Qiagen Tetra His monoclonal antibody was added, and the reactions were incubated on a rotator at 4° C for 1 hour. After 1 hour, 20 μl of a 50% slurry of Protein A Sepharose (PAS) was added to each sample, followed by incubation with rotation for an additional hour at 4° C. PAS and bound proteins were washed 3x in IP Buffer and then resuspended in 15 μl SDS-PAGE loading buffer, and the entire sample was loaded onto a 10% (anti-coat protein immunoprecipitation) or 15% (Tetra His immunoprecipitation), and transferred to PVDF membranes. Samples immunoprecipitated with anti-vCP were probed with Tetra His monoclonal antibody, while samples immunoprecipitated with the TetraHis antibody were probed with anti-vCP.
In vitro P2 polymerase activity assay
The activity of soluble P2 protein preparations in minus-strand RNA synthesis was assayed in in vitro transcription reactions containing 50 mM Tris pH 8.0, 10 mM MgCl2, 0.5 mM ATP, GTP, CTP, 10 μM non-radioactive UTP, ∼10 μCi [α-32P]-UTP, 5 units RNase inhibitor (Sigma or Qiagen), 1 pmol RNA template (AMV 3′ UTR RNA), and 2.5 μl P2 soluble protein fraction. In initial experiments, the transcription results were not visibly affected by pre-treating the P2 fractions with micrococcal nuclease, followed by inactivation with EGTA; therefore, subsequent protocols omitted the nuclease treatment. Reactions were incubated at 30° C for 1 hour. Proteins were hydrolyzed by adding proteinase K, and the solution was then extracted using acid phenol-chloroform (Ambion). Nucleic acids were precipitated by adding sodium acetate to 0.3M final concentration along with carrier glycogen (2 μg/μl) and ethanol, added to a final concentration of 70%. The precipitates were sedimentated by centrifugation for 10 minutes at 4° C, washed with 70% ethanol, and resolved by electrophoresis into a 10% denaturing polyacrylamide gel. The labeled transcription products were visualized using a phosphorimager.
Electrophoretic mobility bandshift assay
Coat protein binding to the 3' untranslated region of AMV RNA 4 was assayed using radiolabeled RNA (20 nM) and a 26 amino acid peptide (CP26) corresponding to the N-terminal RNA binding domain (Ansel-McKinney and Gehrke, 1998; Ansel-McKinney et al., 1996). Variant RNAs are as follows: AUGC positions 1, 2, 4, and 5 (see Figure 6A) were changed to to AAAA. The UUGC at position 3 was changed to UAAA to maintain RNA folding as predicted by MFold (Zuker and Jacobson, 1998), and AUGC #6 (Figure 6A) was changed to AAAC in order to conserve the Sma I restriction enzyme site used to linearize the plasmid DNA for in vitro transcription. The 3'-terminal C is not involved in inter-AUGC basepairing (Guogas et al., 2004). Details for the binding assay have been published elsewhere (Ansel-McKinney and Gehrke, 1998; Ansel-McKinney et al., 1996).
Viral RNA replication in transfected protoplasts
The functional effects of AMV coat protein binding domain mutations in genomic RNA 3 were assayed using a virus replication assay in transfected tobacco protoplasts. The AMV genomic organization is presented in Supplemental Data. The AMV genomic RNAs 1-3 are not infectious unless RNA 4 (coat protein mRNA) or coat protein is added to initiate replication. Mutations were introduced into the 3' untranslated region of a DNA clones corresponding to viral genomic RNA 3. Genomic RNAs 1 and 2, variant genomic 3 containing 3' UTR mutations, plus subgenomic RNA 4 were transcribed in vitro, and the RNAs were transfected into protoplasts by electroporation. Replicated genomic RNA 3 and newly-transcribed subgenomic RNA 4 carry the nucleotide substitutions.
Briefly, tobacco cell walls were removed and the protoplasts were transfected by electroporation with genomic RNAs 1-3 plus subgenomic RNA 4 (encoding the viral coat protein). The transfected protoplasts were incubated for 48 hours, followed by lysis and western blotting assay for viral coat protein as a measure of viral RNA replication. Viral coat protein is not detectable in this assay in the absence of viral RNA replication, and coat protein levels reflect replicated viral RNA levels (Rocheleau et al., 2004). Detailed methods for analyzing viral RNA replication in transfected protoplasts are described elsewhere (Laforest and Gehrke, 2004; Petrillo et al., 2005; Rocheleau et al., 2004).
Virion coat protein
Alfalfa mosaic virus coat protein, a gift from Dr. Ed Halk, was isolated from virions (Kruseman et al., 1971).
Supplementary Material
Figure S1. Organization of the alfalfa mosaic virus (AMV) genome. AMV has three genomic RNAs (RNAs 1-3) that encode the helicase-methyltransferase (P1), RdRp (P2), and viral movement protein (MP), respectively. The viral movement protein facilitates cell-to-cell virus movement through plasmodesmata during an infection. Viral RNA 3 is structurally dicistronic, including coding regions for both the viral movement protein and the downstream viral coat protein (stippled pattern), and separated by a translational stop codon and an intergenic region (brick pattern) with the subgenomic promoter domain. However, RNA 3 is functionally monocistronic, and available data indicate that only coat protein is translated from RNA 3. Viral RNA 4 is subgenomic, does not replicate, and is generated by internal initiation of transcription off of RNA 3. Therefore, viral RNAs 3 and 4 are “co-terminal”; that is, subgenomic RNA 4 sequence is identical to the 3' half of genomic RNA 3. For the replication assay, genomic RNAs 1-3 plus RNA 4 are transfected into cells. The RNA 4 translation product; that is, the viral coat protein, is required to initiate viral RNA replication. Genomic RNAs 1-3 are not infectious. The solid filled area at the 3' terminus of each RNA is the 3' untranslated region, which is similar in sequence among the viral RNAs. The 3'-terminal 45 nucleotides of all four RNA sequences are highly conserved in nucleotide sequence.
Acknowledgements
We thank Drs. Siana M. LaForest and Claire van Eenwyk for constructing the DNA plasmids used to prepare the P1 and P2 baculovirus expression vectors, and Drs. Max Nibert and Teresa Broering for expert advice and reagents for baculovirus protein expression. Dr. Ed Halk provided the purified virion coat protein. This research was supported by grants from the U.S. Public Health Service (GM42504) and The Ellison Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrahams MR, Zhang Z, Chien S, Skerns T, Kotwal GJ. The Vaccinia Virus N1L ORF May Encode a Multifunctional Protein Possibly Targeting Different Kinases, One of Which Influences ATP Levels in Vivo. Ann N Y Acad Sci. 2005;1056:87–99. doi: 10.1196/annals.1352.006. [DOI] [PubMed] [Google Scholar]
- Ansel-McKinney P, Gehrke L. RNA determinants of a specific RNA-coat protein peptide interaction in alfalfa mosaic virus: conservation of homologous features in ilarvirus RNAs. J. Molecular Biology. 1998;278:767–785. doi: 10.1006/jmbi.1998.1656. [DOI] [PubMed] [Google Scholar]
- Ansel-McKinney P, Scott SW, Swanson M, Ge X, Gehrke L. A Plant Viral Coat Protein RNA-Binding Consensus Sequence Contains a Crucial Arginine. EMBO Journal. 1996;15:5077–5084. [PMC free article] [PubMed] [Google Scholar]
- Baer M, Houser F, Loesch-Fries LS, Gehrke L. Specific RNA binding by Amino-terminal peptides of alfalfa mosaic virus coat protein. EMBO J. 1994;13:727–735. doi: 10.1002/j.1460-2075.1994.tb06312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell JL, Brinton MA. Translation elongation factor-1 alpha interacts with the 3′ stem-loop region of West Nile virus genomic RNA. J Virol. 1997;71(9):6433–6444. doi: 10.1128/jvi.71.9.6433-6444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol JF. Alfalfa mosaic virus and ilarviruses: involvement of coat protein in multiple steps of the replication cycle. J. General Virology. 1999;82:947–951. doi: 10.1099/0022-1317-80-5-1089. [DOI] [PubMed] [Google Scholar]
- Bol JF. Replication of alfamo- and ilarviruses: role of the coat protein. Annu Rev Phytopathol. 2005;43:39–62. doi: 10.1146/annurev.phyto.43.101804.120505. [DOI] [PubMed] [Google Scholar]
- Bol JF, Van Vloten-Doting L, Jaspars EMJ. A functional equivalence of top component a RNA and coat protein in the initiation of infection by alfalfa mosaic virus. Virology. 1971;46:73–85. doi: 10.1016/0042-6822(71)90007-9. [DOI] [PubMed] [Google Scholar]
- Brown D, Gold L. RNA replication by Q beta replicase: a working model. Proc Natl Acad Sci U S A. 1996;93(21):11558–62. doi: 10.1073/pnas.93.21.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Noueiry A, Ahlquist P. Brome mosaic virus Protein 1a recruits viral RNA2 to RNA replication through a 5′ proximal RNA2 signal. J Virol. 2001;75(7):3207–19. doi: 10.1128/JVI.75.7.3207-3219.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degraaff M, Thorburn C, Jaspars EMJ. Interaction between RNA-dependent RNA polymerase of alfalfa mosaic virus and its template: Oxidation of vicinal hydroxyl groups blocks in vitro RNA synthesis. Virology. 1995;213(2):650–654. doi: 10.1006/viro.1995.0036. [DOI] [PubMed] [Google Scholar]
- Degraaff M, Tveld MRMI, Jaspars EMJ. In vitro evidence that the coat protein of alfalfa mosaic virus plays a direct role in the regulation of plus and minus RNA synthesis: Implications for the life cycle of alfalfa mosaic virus. Virology. 1995;208(2):583–589. doi: 10.1006/viro.1995.1189. [DOI] [PubMed] [Google Scholar]
- Fechter P, Rudinger-Thirion J, Florentz C, Giege R. Novel features in the tRNA-like world of plant viral RNAs. Cell Mol Life Sci. 2001;58(11):1547–61. doi: 10.1007/PL00000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields BN, Knipe DM, Howley PM. Fields Virology. Third ed. Vol. 2. Lippincott-Raven; Philadelphia: 1996. [Google Scholar]
- Gamarnik AV, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12(15):2293–304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomila RC, Gehrke L. Biochemical approaches for characterizing RNA-protein complexes in preparation for high resolution structure analysis. Plant Virology Protocols. 2006;2 doi: 10.1007/978-1-59745-102-4_20. in press. [DOI] [PubMed] [Google Scholar]
- Gonsalves D, Garnsey SM. Functional equivalence of an RNA component and coat protein for infectivity of citrus leaf rugose virus. Virology. 1975a;64:25–31. doi: 10.1016/0042-6822(75)90075-6. [DOI] [PubMed] [Google Scholar]
- Gonsalves D, Garnsey SM. Infectivity of heterologous RNA-protein mixtures from alfalfa mosaic, citrus leaf rugose, citrus variegation, and tobacco streak viruses. Virology. 1975b;67:319–326. doi: 10.1016/0042-6822(75)90433-x. [DOI] [PubMed] [Google Scholar]
- Guogas L, Laforest S, Gehrke L. The activation of replication by alfalfa mosaic virus coat protein is concentration dependent. J. Virology. 2005;79(9):5752–5761. doi: 10.1128/JVI.79.9.5752-5761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guogas LM, Filman DJ, Hogle JM, Gehrke L. Cofolding organizes alfalfa mosaic virus RNA and coat protein for replication. Science. 2004;306(5704):2108–11. doi: 10.1126/science.1103399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser-Scott F, Baer ML, Liem KF, Jr., Cai JM, Gehrke L. Nucleotide sequence and structural determinants of specific binding of coat protein or coat protein peptides to the 3′ untranslated region of alfalfa mosaic virus RNA 4. J. Virology. 1994;68:2194–2205. doi: 10.1128/jvi.68.4.2194-2205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwing C, Jaspars EMJ. Coat protein blocks the in vitro transcription of the virion RNAs of alfalfa mosaic virus. FEBS Letters. 1986;209:284–288. [Google Scholar]
- Houwing CJ, Jaspars EM. Activation of the alfalfa mosaic virus genome by viral coat protein in non-transgenic plants and protoplasts. The protection model biochemically tested. Arch Virol. 2000;145(1):13–35. doi: 10.1007/s007050050002. [DOI] [PubMed] [Google Scholar]
- Houwing CJ, Jaspars EMJ. Coat protein binds to the 3′-terminal part of RNA 4 of alfalfa mosaic virus. Biochemistry. 1978;17:2927–2933. doi: 10.1021/bi00607a035. [DOI] [PubMed] [Google Scholar]
- Houwing CJ, Jaspars EMJ. Coat Protein Stimulates Replication Complexes of Alfalfa Mosaic Virus to Produce Virion RNAs Invitro. Biochimie. 1993;75(7):617–622. doi: 10.1016/0300-9084(93)90068-4. [DOI] [PubMed] [Google Scholar]
- Jaspars EM. Genome activation in alfamo- and ilarviruses. Arch Virol. 1999;144(5):843–63. doi: 10.1007/s007050050551. [DOI] [PubMed] [Google Scholar]
- Jurgens CK, Barton DJ, Sharma N, Morasco BJ, Ogram SA, Flanegan JB. 2A(pro) is a multifunctional protein that regulates the stability, translation and replication of poliovirus RNA. Virology. 2005 doi: 10.1016/j.virol.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Kao CC, Singh P, Ecker DJ. De novo initiation of viral RNA-dependent RNA synthesis. Virology. 2001;287(2):251–60. doi: 10.1006/viro.2001.1039. [DOI] [PubMed] [Google Scholar]
- Kao CC, Sun JH. Initiation of minus-strand RNA synthesis by the brome mosaic virus RNA-dependent RNA polymerase: use of oligoribonucleotide primers. J Virol. 1996;70(10):6826–30. doi: 10.1128/jvi.70.10.6826-6830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CC, Yang X, Kline A, Wang QM, Barket D, Heinz BA. Template requirements for RNA synthesis by a recombinant hepatitis C virus RNA-dependent RNA polymerase. J Virol. 2000;74(23):11121–8. doi: 10.1128/jvi.74.23.11121-11128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krab IM, Caldwell C, Gallie DR, Bol JF. Coat protein enhances translational efficiency of Alfalfa mosaic virus RNAs and interacts with the eIF4G component of initiation factor eIF4F. J Gen Virol. 2005;86(Pt 6):1841–9. doi: 10.1099/vir.0.80796-0. [DOI] [PubMed] [Google Scholar]
- Kruseman J, Kraal B, Jaspars EMJ, Bol JF, Brederode F, Veldstra H. Molecular weight of the coat protein of alfalfa mosaic virus. Biochemistry. 1971;10(3):447–455. doi: 10.1021/bi00779a015. [DOI] [PubMed] [Google Scholar]
- Laforest SM, Gehrke L. Spatial determinants of the alfalfa mosaic virus coat protein binding site. RNA. 2004;10(1):48–58. doi: 10.1261/rna.5154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MM. Cellular factors in the transcription and replication of viral RNA genomes: a parallel to DNA-dependent RNA transcription. Virology. 1998;244(1):1–12. doi: 10.1006/viro.1998.9098. [DOI] [PubMed] [Google Scholar]
- Larralde O, Smith RW, Wilkie GS, Malik P, Gray NK, Clements JB. Direct stimulation of translation by the multifunctional herpesvirus ICP27 protein. J Virol. 2006;80(3):1588–91. doi: 10.1128/JVI.80.3.1588-1591.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann V, Korner F, Herian U, Bartenschlager R. Biochemical properties of hepatitis C virus NS5B RNA-dependent RNA polymerase and identification of amino acid sequence motifs essential for enzymatic activity. J Virol. 1997;71(11):8416–28. doi: 10.1128/jvi.71.11.8416-8428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N, Weiner AM. Phylogeny from function: Evidence from the molecular fossil record that tRNA originated in replication, not translation. Proc Natl Acad Sci USA. 1994;91(15):6729–6734. doi: 10.1073/pnas.91.15.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N, Weiner AM. The genomic tag hypothesis: what molecular fossils tell us about the evolution of tRNA. In: Gesteland R, Cech TR, Atkins J, editors. The RNA World. Second ed. Cold Spring Harbor Press; Cold Spring Harbor: 1999. pp. 79–111. [Google Scholar]
- Miller WA, Bujarski JJ, Dreher TW, Hall TC. Minus-strand initiation by brome mosaic virus replicase within the 3′ tRNA-like structure of native and modified templates. J. Mol. Biol. 1986;187:537–546. doi: 10.1016/0022-2836(86)90332-3. [DOI] [PubMed] [Google Scholar]
- Nagy PD, Carpenter CD, Simon AE. A novel 3′-end repair mechanism in an RNA virus. Proc Natl Acad Sci U S A. 1997;94(4):1113–8. doi: 10.1073/pnas.94.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeleman L, Bol JF. Cis-acting functions of alfalfa mosaic virus proteins involved in replication and encapsidation of viral RNA. Virology. 1999;254(2):324–33. doi: 10.1006/viro.1998.9568. [DOI] [PubMed] [Google Scholar]
- Neeleman L, Linthorst HJ, Bol JF. Efficient translation of alfamovirus RNAs requires the binding of coat protein dimers to the 3′ termini of the viral RNAs. J Gen Virol. 2004;85(Pt 1):231–40. doi: 10.1099/vir.0.19581-0. [DOI] [PubMed] [Google Scholar]
- Neeleman L, Olsthoorn RC, Linthorst HJ, Bol JF. Translation of a nonpolyadenylated viral RNA is enhanced by binding of viral coat protein or polyadenylation of the RNA. Proc Natl Acad Sci U S A. 2001;98(25):14286–91. doi: 10.1073/pnas.251542798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsthoorn RC, Haasnoot PC, Bol JF. Similarities and differences between the subgenomic and minus-strand promoters of an RNA plant virus. J Virol. 2004;78(8):4048–53. doi: 10.1128/JVI.78.8.4048-4053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsthoorn RC, Mertens S, Brederode FT, Bol JF. A conformational switch at the 3′ end of a plant virus RNA regulates viral replication. Embo J. 1999;18(17):4856–64. doi: 10.1093/emboj/18.17.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo JE, Rocheleau G, Kelley-Clarke B, Gehrke L. Evaluation of the conformational switch model for coat protein function in alfalfa mosaic virus replication. J. Virology. 2005;79(9):5743–5751. doi: 10.1128/JVI.79.9.5743-5751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogany J, White KA, Nagy PD. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J Virol. 2005;79(8):4859–69. doi: 10.1128/JVI.79.8.4859-4869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadt R, Rosdorff HJM, Hunt TW, Jaspars EMJ. Analysis of the Protein Composition of Alfalfa Mosaic Virus RNA-Dependent RNA Polymerase. Virology. 1991;182(1):309–315. doi: 10.1016/0042-6822(91)90674-z. [DOI] [PubMed] [Google Scholar]
- Rao ALN, Dreher TW, Marsh LE, Hall TC. Telomeric function of the tRNA-like structure of brome mosaic virus RNA. Proc. Natl. Acad. Sci. USA. 1989;86:5335–5339. doi: 10.1073/pnas.86.14.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken CBEM, Neeleman L, Bol JF. The 3′-untranslated region of alfalfa mosaic virus RNA 3 contains at least two independent binding sites for viral coat protein. Nucleic Acids Res. 1994;22(8):1346–1353. doi: 10.1093/nar/22.8.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken CBEM, Neeleman L, Brederode FT, Bol JF. Mutations in coat protein binding sites of alfalfa mosaic virus RNA 3 affect subgenomic RNA 4 accumulation and encapsidation of viral RNAs. J Virol. 1997;71(11):8385–8391. doi: 10.1128/jvi.71.11.8385-8391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau G, Petrillo J, Guogas L, Gehrke L. Degenerate in vitro genetic selection reveals mutations that diminish alfalfa mosaic virus RNA replication without affecting coat protein binding. J Virol. 2004;78(15):8036–46. doi: 10.1128/JVI.78.15.8036-8046.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Chen J, Lee WM, Janda M, Ahlquist P. Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication. Proc Natl Acad Sci U S A. 2004;101(31):11263–8. doi: 10.1073/pnas.0404157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork J, Panaviene Z, Nagy PD. Inhibition of in vitro RNA binding and replicase activity by phosphorylation of the p33 replication protein of Cucumber necrosis tombusvirus. Virology. 2005;343(1):79–92. doi: 10.1016/j.virol.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Taschner PEM, Vanderkuyl AC, Neeleman L, Bol JF. Replication of an Incomplete Alfalfa Mosaic Virus Genome in Plants Transformed with Viral Replicase Genes. Virology. 1991;181(2):445–450. doi: 10.1016/0042-6822(91)90876-d. [DOI] [PubMed] [Google Scholar]
- Tenllado F, Bol JF. Genetic dissection of the multiple functions of alfalfa mosaic virus coat protein in viral RNA replication, encapsidation, and movement. Virology. 2000;268(1):29–40. doi: 10.1006/viro.1999.0170. [DOI] [PubMed] [Google Scholar]
- Van Der Heijden MW, Carette JE, Reinhoud PJ, Haegi A, Bol JF. Alfalfa mosaic virus replicase proteins P1 and P2 interact and colocalize at the vacuolar membrane. J Virol. 2001;75(4):1879–87. doi: 10.1128/JVI.75.4.1879-1887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kuyl AC, Langereis K, Houwing CJ, Jaspars EM, Bol JF. cis-acting elements involved in replication of alfalfa mosaic virus RNAs in vitro. Virology. 1990;176(2):346–54. doi: 10.1016/0042-6822(90)90004-b. [DOI] [PubMed] [Google Scholar]
- van der Kuyl AC, Neeleman L, Bol JF. Role of alfalfa mosaic virus coat protein in regulation of the balance between viral plus and minus strand RNA synthesis. Virology. 1991;185:496–499. doi: 10.1016/0042-6822(91)90807-n. [DOI] [PubMed] [Google Scholar]
- van Dijk AA, Makeyev EV, Bamford DH. Initiation of viral RNA-dependent RNA polymerization. J Gen Virol. 2004;85(Pt 5):1077–93. doi: 10.1099/vir.0.19731-0. [DOI] [PubMed] [Google Scholar]
- van Rossum CM, Reusken CB, Brederode FT, Bol JF. The 3′ untranslated region of alfalfa mosaic virus RNA3 contains a core promoter for minus-strand RNA synthesis and an enhancer element. J Gen Virol. 1997;78(Pt 11):3045–9. doi: 10.1099/0022-1317-78-11-3045. [DOI] [PubMed] [Google Scholar]
- van Vloten-Doting L. Coat protein is required for infectivity of tobacco streak virus: Biological equivalence of the coat proteins of tobacco streak and alfalfa mosaic virus. Virology. 1975;65:215–225. doi: 10.1016/0042-6822(75)90022-7. [DOI] [PubMed] [Google Scholar]
- Vlot AC, Neeleman L, Linthorst HJ, Bol JF. Role of the 3′-untranslated regions of alfalfa mosaic virus RNAs in the formation of a transiently expressed replicase in plants and in the assembly of virions. J Virol. 2001;75(14):6440–9. doi: 10.1128/JVI.75.14.6440-6449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner AM, Maizels N. tRNA-like structures tag the 3′ ends of genomic RNA molecules for replication: implications for the origin of protein synthesis. Proc. Natl. Acad. Sci. USA. 1987;84:7383–7387. doi: 10.1073/pnas.84.21.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Sampath A, Chao A, Wen D, Nanao M, Chene P, Vasudevan SG, Lescar J. Structure of the Dengue virus helicase/nucleoside triphosphatase catalytic domain at a resolution of 2.4 A. J Virol. 2005;79(16):10278–88. doi: 10.1128/JVI.79.16.10278-10288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rijnbrand R, Watowich S, Lemon SM. Genetic evidence for an interaction between a picornaviral cis-acting RNA replication element and 3CD protein. J Biol Chem. 2004;279(13):12659–67. doi: 10.1074/jbc.M312992200. [DOI] [PubMed] [Google Scholar]
- Zuidema D, Bierhuizen MFA, Cornelissen BJC, Bol JF, Jaspars EMJ. Coat protein binding sites on RNA 1 of alfalfa mosaic virus. Virology. 1983;125:361–369. doi: 10.1016/0042-6822(83)90208-8. [DOI] [PubMed] [Google Scholar]
- Zuker M, Jacobson AB. Using reliability information to annotate RNA secondary structures. RNA. 1998;4(6):669–79. doi: 10.1017/s1355838298980116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Organization of the alfalfa mosaic virus (AMV) genome. AMV has three genomic RNAs (RNAs 1-3) that encode the helicase-methyltransferase (P1), RdRp (P2), and viral movement protein (MP), respectively. The viral movement protein facilitates cell-to-cell virus movement through plasmodesmata during an infection. Viral RNA 3 is structurally dicistronic, including coding regions for both the viral movement protein and the downstream viral coat protein (stippled pattern), and separated by a translational stop codon and an intergenic region (brick pattern) with the subgenomic promoter domain. However, RNA 3 is functionally monocistronic, and available data indicate that only coat protein is translated from RNA 3. Viral RNA 4 is subgenomic, does not replicate, and is generated by internal initiation of transcription off of RNA 3. Therefore, viral RNAs 3 and 4 are “co-terminal”; that is, subgenomic RNA 4 sequence is identical to the 3' half of genomic RNA 3. For the replication assay, genomic RNAs 1-3 plus RNA 4 are transfected into cells. The RNA 4 translation product; that is, the viral coat protein, is required to initiate viral RNA replication. Genomic RNAs 1-3 are not infectious. The solid filled area at the 3' terminus of each RNA is the 3' untranslated region, which is similar in sequence among the viral RNAs. The 3'-terminal 45 nucleotides of all four RNA sequences are highly conserved in nucleotide sequence.