Abstract
Predicting prognosis is the key factor in selecting the proper treatment modality for patients with spinal metastases. Therefore, various assessment systems have been designed in order to provide a basis for deciding the course of treatment. Such systems have been proposed by Tokuhashi, Sioutos, Tomita, Van der Linden, and Bauer. The scores differ greatly in the kind of parameters assessed. The aim of this study was to evaluate the prognostic value of each score. Eight parameters were assessed for 69 patients (37 male, 32 female): location, general condition, number of extraspinal bone metastases, number of spinal metastases, visceral metastases, primary tumour, severity of spinal cord palsy, and pathological fracture. Scores according to Tokuhashi (original and revised), Sioutos, Tomita, Van der Linden, and Bauer were assessed as well as a modified Bauer score without scoring for pathologic fracture. Nineteen patients were still alive as of September 2006 with a minimum follow-up of 12 months. All other patients died after a mean period of 17 months after operation. The mean overall survival period was only 3 months for lung cancer, followed by prostate (7 months), kidney (23 months), breast (35 months), and multiple myeloma (51 months). At univariate survival analysis, primary tumour and visceral metastases were significant parameters, while Karnofsky score was only significant in the group including myeloma patients. In multivariate analysis of all seven parameters assessed, primary tumour and visceral metastases were the only significant parameters. Of all seven scoring systems, the original Bauer score and a Bauer score without scoring for pathologic fracture had the best association with survival (P < 0.001). The data of the present study emphasize that the original Bauer score and a modified Bauer score without scoring for pathologic fracture seem to be practicable and highly predictive preoperative scoring systems for patients with spinal metastases. However, decision for or against surgery should never be based alone on a prognostic score but should take symptoms like pain or neurological compromise into account.
Keywords: Spine, Metastasis, Survival, Vertebral bodies, Cord compression
Introduction
Despite advances in radiotherapy and chemotherapy, metastatic disease of the spine remains a challenging situation for spinal surgeons. An individual therapy should be chosen to provide the maximum palliative effect (reduction of pain, restoration of stability and function) with a minimum of operative morbidity and mortality [4, 19, 21]. Predicting survival is the key factor in selecting the proper treatment modality. While for patients with good prognosis excisional procedures including extensive curettage or en bloc resection of the vertebral body are recommended, for patients with poor prognosis treatment should be mainly limited to posterior instrumentation with or without laminectomy or even to non operative supportive care [7, 14, 15, 19].
Various assessment systems have been designed to predict survival periods and to select the ideal treatment option. Such systems have been proposed by Tokuhashi [14, 15], Sioutos [13], Van der Linden [20], Tomita [16], and Bauer [2]. The scores differ greatly in the kind of parameters assessed and the weight of these factors in the total score. As a result, for the same patient, different survival periods might be calculated and contradictory treatment strategies advised. As surgical treatment for spinal metastatic disease only improves the quality of life in patients with an appropriate indication, the reliability of such scoring systems is essential [6, 21]. To our knowledge, no previous studies have been conducted before comparing more than two assessment systems for patients with spinal metastases. The purpose of the present study was to evaluate which system most accurately predicted survival.
Study participants and methods
Between January 1998 and September 2006, 69 patients (37 male, 32 female) with a mean age at operation of 60 years (median 61, range 30–79 years) were treated for spinal metastases at a single institution. The most frequent site of metastasis was the thoracic spine (41 cases), followed by the lumbar (22), and cervical (6) spine. The patients were referred for treatment due to a neurological deficit (39 patients), pain combined with a present fracture without neurological deficit (7 patients), or pain combined with a progressive osteolysis and impending fracture (23 patients). Thirty-seven patients had already preoperatively received chemotherapy, in 24 patients radiotherapy has been applied preoperatively to site of spinal metastases.
Using the Tokuhashi classification for the extent of the operation [15], 29 patients underwent palliative dorsal procedures while 40 patients underwent excision of vertebral body lesions (excisional procedure group).
Original parameters
Eight parameters were preoperatively assessed for each patient: localisation (cervical vs. thoracic vs. lumbar), primary tumour, pathological fracture, visceral metastases, number of spinal metastases, number of extraspinal bone metastases, Karnofsky score [8], and severity of spinal cord palsy. As the weight of this last parameter is different for Tokuhashi (three groups using Frankel’s scale [5]) and Sioutos score (two groups using MRC motor strength scale [10]), the possible influence was calculated twice. The influence of these eight parameters was analysed by log-rank test for univariate analyses with the software package SPSS, version 14.0 (Chicago, IL). The influence of the seven original parameters used in the scores (excluding location as a parameter) was analysed by Cox proportional hazards model for multivariate analyses. For the multivariate analysis, the MRC motor strength scale was used to score the severity of spinal cord palsy [10]. The grade of malignancy of the primary tumours was thought to be reflected best by the growth speed and therefore, according to Tomita [16] divided into three categories: grade 1, slow growth [breast (12 patients), multiple myeloma (10), prostate (7), metastasizing hemangioendothelioma (1), hemangiopericytoma (1), thyroid (1), non-Hodgkin lymphoma (1)]; grade 2, moderate growth [kidney (15), uterus (2), tonsil (1), epipharynx (1), femoral synovial sarcoma (1), malign thymoma (1)]; and grade 3, rapid growth [lung (6), melanoma (2), malignant teratoma (1), liver (1), stomach (1), colon (1), sigma (1), rectum (1), pancreas (1)]. As only in the original Bauer score, multiple myeloma patients were included [2] but not in the other scoring systems, all analyses were calculated twice—including them (total n = 69) and excluding them (total n = 59).
Evaluation of scores
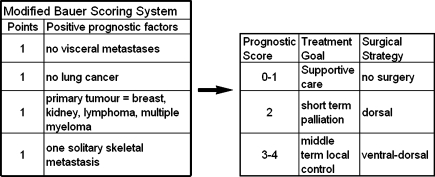
In a prospective manner, for each patient preoperatively, the total number of points was calculated using three scoring systems (Sioutos [13], Tokuhashi [15], and Bauer [2]). In a retrospective manner, for each patient, three other scoring systems were applied—Van der Linden [20], Tomita [16], and Tokuhashi revised [14]. Furthermore, based on the fact that Bauer stated in his publication “the (statistical) impact of pathologic fracture was evident in the extremity group only” [2], we tested a modified Bauer score without scoring for pathologic fracture—proposing three groups with 0–1 versus 2 versus 3–4 positive parameters (Fig. 1). This study was also performed to evaluate the prognostic value of each score and to compare the survival predicted by those scores with the real survival. This has been tested for the Tomita und Tokuhashi score for 37 renal cancer patients by Ulmar et al. in 2006 [18]. Based on the original publications, patients were assigned to the following prognostic groups: Tokuhasi [15]: 1. palliative (scores 0–5), 2. indifferent (patients without a palliative or excisional treatment recommendation, 6–8), 3. excisional (9–12); Tokuhashi revised [14]: 1. no surgery (0–8), 2. palliative (9–11), 3. excisional (12–15); Tomita [16]: 1. long term (2–3), 2. mid term (4–5), 3. palliative (6–7), 4. supportive (8–10); Van der Linden [20]: 1. A (bad, 1), 2. B (middle, 2), 3. C (surgery recommended, 3); Sioutos [13]: 1. excision (0–1), 2. palliative (2–3); Bauer [2]: 1. bad (0–1), 2. intermediate (2–3), 3. good (4–5); and Bauer modified: 1. no surgery (0–1); 2. dorsal (2); 3. ventral–dorsal (3–4).
Fig. 1.
The proposed modified Bauer score excluding pathologic fracture as a prognostic factor
Survival analysis was performed by using the log-rank test. Survival curves were created by using the Kaplan–Meier life-table analysis. A P value < 0.05 (two-tailed) was considered significant. The data were compiled and analysed with the software package SPSS, version 14.0 (Chicago, IL).
Results
Survival
Nineteen patients were still alive as of September 2006 with a minimum follow-up of 12 months. All other patients died after a mean period of 17 months after operation. Excluding the multiple myeloma patients, the mean overall survival was 21 months (median 10 months) (Table 1). For all patients (including the multiple myeloma patients), the mean overall survival was 28 months (median 14 months). Patients with multiple myeloma and breast carcinoma had the best overall survival, with a mean survival of 51 and 35 months, respectively. The mean overall survival was worst for lung cancer patients (mean 3 months), followed by prostate cancer (7 months), other (14 months), and renal cell carcinoma (23 months).
Table 1.
Overall survival in patients with spinal metastases (n = 69)
| Primary tumour | N | Mean | 95% CI | Median | 95% CI |
|---|---|---|---|---|---|
| Lung cancer | 6 | 3 | 1–5 | 2 | 1–3 |
| Prostate cancer | 7 | 7 | 3–11 | 6 | 0–12 |
| Other | 19 | 14 | 7–21 | 7 | 3–12 |
| Renal cell carcinoma | 15 | 23 | 14–31 | 14 | 3–25 |
| Breast cancer | 12 | 35 | 11–58 | 19 | 9–29 |
| Plasmozytoma | 10 | 51 | 37–64 | nd | nd |
N number of patients, Mean/median survival period (months), CI confidence interval
Chemotherapy was continued in 30 cases or started in 12 cases, about 14 days postoperatively. Postoperative radiation was started in 36 patients, 2–6 weeks after surgery while 24 patients had already received this therapy preoperatively.
Original parameters
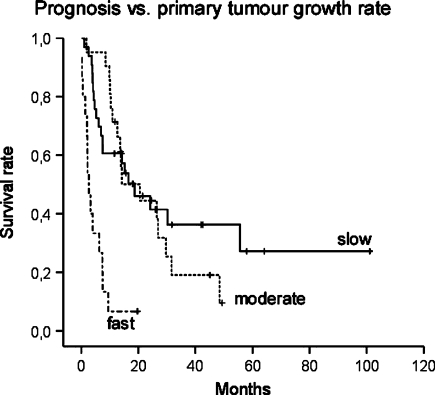
At univariate survival analysis, the type of the primary tumour (Fig. 2) and the presence or absence of visceral metastases were significant parameters (Tables 2, 3). The Karnofsky score was only significant in the group including myeloma patients. In multivariate analysis, including all seven parameters assessed, primary tumour and visceral metastases were the only significant parameters (Tables 4, 5).
Fig. 2.
Survival curves for primary tumour growth rate in 69 patients with spinal metastases, including ten multiple myeloma patients (modified after Tomita et al. [16]: slow growth breast, thyroid, multiple myeloma, etc., moderate growth kidney, uterus, etc., fast growth lung, melanoma, liver, colon, etc.)
Table 2.
Prognostic factors for survival in patients with spinal metastases (including myeloma)—univariate analysis (n = 69)
| Variable | P |
|---|---|
| Localisation (cervical vs. thoracic vs. lumbar) | 0.638 |
| Primary tumour (rapid vs. intermediate vs. slow) | <0.001* |
| Pathological fracture (present vs. absent) | 0.929 |
| Visceral metastases (present vs. absent) | 0.002* |
| No. of spinal metastases (more than one vs. one) | 0.311 |
| No. of extraspinal bone metastases (one or more than one vs. none) | 0.774 |
| Karnofsky score (low vs. intermediate vs. high) | 0.027* |
| Neurol. symptoms (MRC 0/5–3/5 vs. 4/5–5/5) | 0.922 |
| Neurol. symptoms (Frankel’s A–B vs. C–D vs. normal) | 0.930 |
Log-rank test
* Significant at P < 0.05
Table 3.
Prognostic factors for survival in patients with spinal metastases (excluding myeloma)—univariate analysis (n = 59)
| Variable | P |
|---|---|
| Localisation (cervical vs. thoracic vs. lumbar) | 0.371 |
| Primary tumour (rapid vs. intermediate vs. slow) | <0.001* |
| Pathological fracture (present vs. absent) | 0.131 |
| Visceral metastases (present vs. absent) | 0.004* |
| No. of spinal metastases (more than one vs. one) | 0.923 |
| No. of extraspinal bone metastases (one or more than one vs. none) | 0.457 |
| Karnofsky score (low vs. intermediate vs. high) | 0.100 |
| Neurol. symptoms (MRC 0/5–3/5 vs. 4/5–5/5) | 0.982 |
| Neurol. symptoms (Frankel’s A–B vs. C–D vs. normal) | 0.976 |
Log-rank test
* Significant at P < 0.05
Table 4.
Prognostic factors for survival in patients with spinal metastases (including myeloma)—multivariate analysis (n = 69)
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Primary tumour | <0.001* | ||
| (1) Moderate versus slow | 1.07 | 0.51–2.22 | 0.857 |
| (2) Rapid versus slow | 9.32 | 3.87–22.5 | <0.001* |
| Pathological fracturea | 1.3 | 0.68–2.49 | 0.426 |
| Visceral metastasesa | 2.17 | 1.15–4.09 | 0.017* |
| Number of spinal metastasesb | 1.37 | 0.52–3.63 | 0.521 |
| Number of extraspinal bone metastasesa | 0.95 | 0.32–2.73 | 0.916 |
| Karnofsky score | 0.08 | ||
| (1) Intermediate versus high | 1.8 | 0.49–6.55 | 0.375 |
| (2) Low versus high | 3.24 | 0.89–11.8 | 0.075 |
| Neurol. symptomsc | 1.33 | 0.69–2.54 | 0.386 |
Cox proportional hazards model
* Significant at P < 0.05
aPresent versus absent
bMore than one versus one
cMRC 4–5 versus 0–3
HR hazard ratio, CI confidence interval
Table 5.
Prognostic factors for survival in patients with spinal metastases (excluding myeloma)—multivariate analysis (n = 59)
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Primary tumour | <0.001* | ||
| (1) Moderate versus slow | 0.56 | 0.26–1.21 | 0.14 |
| (2) Rapid versus slow | 6.03 | 2.46–14.8 | <0.001* |
| Pathological fracturea | 1.58 | 0.8–3.09 | 0.182 |
| Visceral metastasesa | 2.42 | 1.25–4.64 | 0.008* |
| Number of spinal metastasesb | 0.49 | 0.17–1.4 | 0.185 |
| Number of extraspinal bone metastasesa | 3.16 | 0.96–10.4 | 0.058 |
| Karnofsky score | 0.096 | ||
| (1) Intermediate versus high | 1.35 | 0.35–5.15 | 0.665 |
| (2) Low versus high | 2.54 | 0.68–9.43 | 0.163 |
| Neurol. symptomsc | 1.02 | 0.51–2.02 | 0.952 |
Cox proportional hazards model
* Significant at P < 0.05
aPresent versus absent
bMore than one versus one
cMRC 4–5 versus 0–3
HR hazard ratio, CI confidence interval
Evaluation of scores
The results of survival analyses for each score are shown in Table 6. Mean and median survival periods as well as the 95% confidence intervals are presented for each prognostic group.
Table 6.
Prognostication of survival scores in patients with spinal metastases
| Score (risk groups) | Multiple myeloma excluded | Multiple myeloma included | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | 95% CI | Mean | 95% CI | P | N | Median | 95% CI | Mean | 95% CI | P | |
| Tokukashi | 0.051 | 0.002* | ||||||||||
| Palliative | 15 | 5 | 2–8 | 12 | 4–20 | 15 | 5 | 2–8 | 12 | 4–20 | ||
| Indifferent | 30 | 11 | 6–16 | 14 | 9–18 | 34 | 13 | 9–17 | 17 | 11–23 | ||
| Excisional | 14 | 14 | 0–42 | 46 | 21–71 | 20 | 56 | 12–100 | 53 | 31–76 | ||
| Tokuhashi revised | 0.008* | 0.005* | ||||||||||
| No surgery | 26 | 4 | 2–6 | 11 | 6–17 | 28 | 4 | 1–7 | 15 | 8–21 | ||
| Palliative | 26 | 14 | 11–17 | 18 | 12–23 | 30 | 14 | 12–16 | 22 | 14–30 | ||
| Excisional | 7 | nd | 60 | 26–95 | 11 | 56 | 10–102 | 58 | 29–87 | |||
| Tomita | 0.005* | <0.001* | ||||||||||
| Long-term | 19 | 17 | 3–30 | 46 | 24–69 | 27 | 56 | 0–120 | 54 | 35–73 | ||
| Mid-term | 13 | 19 | 9–29 | 20 | 12–28 | 14 | 19 | 8–29 | 23 | 14–31 | ||
| Palliative | 12 | 6 | 4–9 | 9 | 5–14 | 13 | 7 | 6–9 | 10 | 6–14 | ||
| Supportive | 15 | 3 | 0–5 | 10 | 3–17 | 15 | 3 | 0–5 | 10 | 3–17 | ||
| Van der Linden** | 0.014* | 0.002* | ||||||||||
| A (bad) | 48 | 8 | 5–10 | 14 | 10–18 | 50 | 9 | 5–12 | 15 | 11–20 | ||
| B (middle) | 11 | 24 | 13–35 | 48 | 17–79 | 17 | 24 | 0–70 | 52 | 29–74 | ||
| C (surgery) | 0 | nd | nd | nd | nd | 2 | nd | nd | nd | nd | ||
| Sioutos | 0.584 | 0.364 | ||||||||||
| Excision | 40 | 10 | 6–15 | 23 | 12–34 | 49 | 14 | 7–21 | 30 | 18–42 | ||
| Palliative | 19 | 7 | 3–11 | 16 | 8–24 | 20 | 7 | 2–12 | 19 | 10–27 | ||
| Bauer | <0.001* | <0.001* | ||||||||||
| Bad | 9 | 2 | 2–3 | 3 | 1–5 | 9 | 2 | 2–3 | 3 | 1–5 | ||
| Intermediate | 34 | 10 | 6–14 | 15 | 10–21 | 39 | 10 | 5–15 | 18 | 13–24 | ||
| Good | 16 | 21 | 10–31 | 46 | 23–68 | 21 | 56 | 9–103 | 51 | 31–72 | ||
| Bauer modified | <0.001* | <0.001* | ||||||||||
| No surgery | 14 | 3 | 0–5 | 7 | 1–13 | 14 | 3 | 0–5 | 7 | 1–13 | ||
| Dorsal | 20 | 9 | 3–14 | 13 | 8–18 | 22 | 10 | 2–18 | 15 | 10–20 | ||
| Ventral-dorsal | 25 | 17 | 7–26 | 40 | 22–59 | 33 | 30 | 12–48 | 48 | 31–64 | ||
Log-rank test
* Significant at P < 0.05
** Only two censored multiple myeloma patients in group C
n number of patients, 95% CI 95% confidence interval, mean/median survival period (months), nd not done
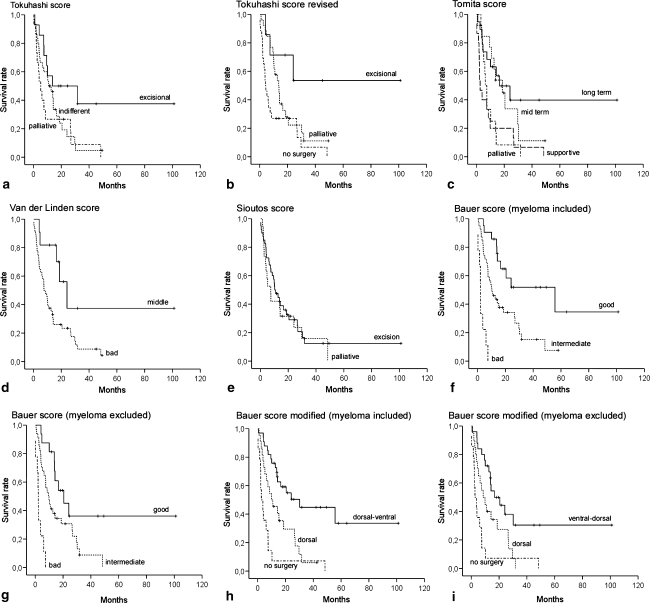
Analysing the difference in survival between each prognostic group the scoring systems by Tokuhashi (revised), Tomita, Bauer, Van der Linden, and the proposed modified Bauer score (without scoring for pathological fracture) provided statistically significant results, independent of including or excluding multiple myeloma patients. The original Tokuhashi score had a significant prognostic value in the group including multiple myeloma patients only. The Sioutos score showed no correlation between predicted and real survival (Fig. 3).
Fig. 3.
Survival curves for the prognostic groups according to seven different scores, a–e, f, h 59 patients excluding multiple myeloma, g, i 69 patients including multiple myeloma: a Tokuhasi [15], b Tokuhashi revised [14], c Tomita [16], d Van der Linden [20], e Sioutos [13], f, g Bauer [2], h, i Bauer modified
Although a statistical significance was calculated for the Van der Linden score, the clinical significance for our series of patients was estimated to be poor, as only two multiple myeloma patients were included in the best group C. Van der Linden proposed that this group alone should be admitted to surgery.
For our series the original Bauer Score and the proposed modified variant alone provided the highest statistical significance (P < 0.001) in both groups (including/excluding multiple myeloma patients).
Discussion
To our knowledge, no previous studies have been conducted before comparing more than two assessment systems for patients with spinal metastases. Ulmar et al. [18] compared the Tomita score and the Tokuhashi score for 37 patients with renal metastases in 2007 and the original Tokuhashi prognosis score with the modified one in 217 patients, also in 2007 [17]. Of all seven scoring systems analysed in the present series, the original Bauer score and a Bauer score modified for pathologic fracture had the best correlation with the survival period (P < 0.001) independently of inclusion or exclusion of multiple myeloma patients.
The original Bauer score was designed to serve both, spinal metastases and extremity metastases. Bauer himself stated in his paper, published in 1995: “…Patent pathologic fracture was related to lower survival compared to patients without fracture. This impact of fracture was evident in the extremity group only…” [2]. As, to the authors’ knowledge, pathologic fracture has never been shown to be a prognostic factor for patients with spinal metastases, we wanted to test if an even more simple assessment system could predict prognosis as good as the original version. The modified Bauer score stays simple and is of a high prognostic value. Four positive prognostic factors are included: (1) absence of visceral metastases, (2) solitary skeletal metastasis, (3) not primary lung cancer, (4) primary tumour breast, kidney, lymphoma or myeloma (Fig. 1). We think that it is a valuable instrument for life expectancy estimation and therefore might help in deciding the treatment strategy for patients with spinal metastases.
Predicting survival stays, the key factor in selecting the proper treatment modality for patients with spinal metastases. While some authors state that patients with a life expectancy less than 3 months (as lung cancer patients in our series) should not be considered for operative treatment [22], other authors extend this group to about 6 months or less [14, 16]. Calculated according to the modified Bauer score the “no surgery” group in the present study had a mean survival period of 7 months (median 3 months). While for patients with good prognosis excisional procedures including extensive curettage or en bloc resection of the vertebral body are recommended and for patients with a middle prognosis treatment should be mainly limited to posterior instrumentation with or without laminectomy [15, 16, 22].
The most obvious limitation of the present study is the low number of patients included (69). However, the original scores have been created after analysing a similar number of patients [Bauer 88, Tomita 67, Tokuhashi (original) 64, and Sioutos 109]. Only Van der Linden et al. published data on 342 patients. Comparison with these patients is difficult, as they have not been operated on and were only treated by radiotherapy. Van der Linden, therefore, presented a totally different subgroup of patients. In our opinion, the Van der Linden score with only three groups (A, B, C) resulting from three prognostic factors (Karnofksy score, primary tumour, visceral metastases) is not applicable for patients that might undergo operation, as the best group (C)—with a Karnofsky score of 80 or better, a slowly growing tumour and no visceral metastases seldom will be admitted to surgery.
It is well accepted and has been demonstrated by several authors that primary tumour site is the most important prognostic factor for surival[2, 6, 14–16]. This major impact is well reflected by scoring for more points in the Tokuhashi revised score [14], the Bauer score [2], as well in part in the Tomita score [16]. The impact on general condition (normally reflected in the Karnofsky score [8]) on a preoperative scoring system is controversially discussed [14–16, 20]. In accordance with Tomita [16], we are of the opinion that spreading of a rapidly growing tumour is normally well-reflected in visceral metastases and often goes hand-in-hand with a decrease of the patients’ general condition. Furthermore, especially patients with a major neurological deficit due to spinal metastases will present with a very low Karnofksy score as they are severely disabled. However, in these patients a low Karnofksy score under 40 could stand in contrast to an otherwise good general condition. In our series of 69 patients the Karnofsky score was not significant in multivariate analysis in contrast to visceral metastases.
Tokuhashi [14, 15] and Sioutos [13] included the presence and grade of a neurological deficit in their preoperative prognostic scoring system? In our series of 69 patients, pre-treatment neurological status has not been identified as a prognostic factor (Tables 2, 3, 4, 5). Again, in accordance with Tomita [16], we are of the opinion that the presence of paralysis per se is not predictive of survival.
Whether patients with multiple myeloma should be included in a preoperative scoring system is discussed controversially. Bauer alone included them in his scoring system. Although per definition, multiple myeloma is a haematological disease and not a metastatic spread of a solid tumour, we propose that these patients should be included in the group with the best prognosis, as there are myeloma cases demanding operative treatment [6].
In agreement with Bauer and Ulmar, we are of the opinion that the decision for or against surgery should never be based alone on a prognostic score but should take symptoms like pain or neurological compromise into account [1, 17]. Various other factors like estrogen receptor-positivity for metastatic breast cancer patients have been shown to be of a high predictive value and should therefore be included in the individual decision process [12]. With some rare exceptions like solitary kidney metastases, we regard treatment of metastatic disease always as palliative, with its sole goal to improve the quality of life [3, 6, 7, 14, 21]. Surgery remains only part of a multimodality treatment [7].
Scores for spinal metastases may furthermore be highly influenced by the effectiveness of chemotherapy present at the time of the creation of the score. In 1991, the mean survival rate for patients with spinal metastases of renal carcinoma was reported to be only 8 months [9], 15 years later a nearly doubled mean survival rate of 14 months was reported for a comparable group [18], mainly due to the introduction of cytokine therapy. In our series of 15 patients with spinal metastases due to renal carcinoma, 7 patients had received this treatment. Further advances like the application of tyrosine kinase inhibitors might increase life expectancy of this group of patients. Spinal kidney cancer metastases might even shift from Tomita’s medium growth group to the slow growth group. In contrast, the prognosis after a diagnosis of carcinoma of unknown primary metastatic to bone remains dismal with reported median survival periods between 2 and 7 months [11].
In conclusion, the data of the present study emphasize that the original Bauer score and a modified Bauer score without scoring for pathologic fracture seem to be practicable and highly predictive preoperative scoring systems for patients with spinal metastases.
Footnotes
A reviewer’s comment on this original article is available at doi:10.1007/s00586-008-0773-z.
Contributor Information
Andreas Leithner, Email: andreas.leithner@aon.at.
Reinhard Windhager, Phone: +43-316-38582849, FAX: +43-316-3854806, Email: reinhard.windhager@meduni-graz.at.
References
- 1.Bauer H. Surgical strategy for spinal metastases. Spine. 2002;27:1124–1125. doi: 10.1097/00007632-200205150-00027. [DOI] [PubMed] [Google Scholar]
- 2.Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand. 1995;66:143–146. doi: 10.3109/17453679508995508. [DOI] [PubMed] [Google Scholar]
- 3.Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine. 2006;31:2849–2856. doi: 10.1097/01.brs.0000245838.37817.40. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JA, Zaveri G, Wai E, Vidmar M, Kreder H, Chow E. A population-based study of surgery for spinal metastases. Survival rates and complications. J Bone Joint Surg Br. 2003;85:1045–1050. doi: 10.1302/0301-620X.85B7.14201. [DOI] [PubMed] [Google Scholar]
- 5.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 6.Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J, Kamimura M, Ohtsuka K, Takaoka K. Clinical outcome and survival after palliative surgery for spinal metastases: palliative surgery in spinal metastases. Cancer. 2003;97:476–484. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 7.Jansson KA, Bauer HC. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J. 2006;15:196–202. doi: 10.1007/s00586-004-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karnofsky DA. Clinical evaluation of anticancer drugs. GANN Monograph. 1967;2:223–231. [Google Scholar]
- 9.King GJ, Kostuik JP, McBroom RJ, Richardson W. Surgical management of metastatic renal carcinoma of the spine. Spine. 1991;16:265–271. doi: 10.1097/00007632-199103000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Aids to the examination of the peripheral nervous system. London: HMSO; 1976. [Google Scholar]
- 11.Pavlidis N. Forty years experience of treating cancer of unknown primary. Acta Oncol. 2007;46:592–601. doi: 10.1080/02841860701243095. [DOI] [PubMed] [Google Scholar]
- 12.Sciubba DM, Gokaslan ZL, Suk I, Suki D, Maldaun MV, McCutcheon IE, Nader R, Theriault R, Rhines LD, Shehadi JA. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J. 2007;16:1659–1667. doi: 10.1007/s00586-007-0380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer. 1995;76:1453–1459. doi: 10.1002/1097-0142(19951015)76:8<1453::AID-CNCR2820760824>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 14.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 15.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 16.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 17.Ulmar B, Huch K, Naumann U, Catalkaya S, Cakir B, Gerstner S, Reichel H. Evaluation of the Tokuhashi prognosis score and its modifications in 217 patients with vertebral metastases. Eur J Surg Oncol. 2007;33:914–919. doi: 10.1016/j.ejso.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Ulmar B, Naumann U, Catalkaya S, Muche R, Cakir B, Schmidt R, Reichel H, Huch K. Prognosis scores of Tokuhashi and Tomita for patients with spinal metastases of renal cancer. Ann Surg Oncol. 2007;14:998–1004. doi: 10.1245/s10434-006-9000-5. [DOI] [PubMed] [Google Scholar]
- 19.Ulmar B, Richter M, Cakir B, Muche R, Puhl W, Huch K. The Tokuhashi score: significant predictive value for the life expectancy of patients with breast cancer with spinal metastases. Spine. 2005;30:2222–2226. doi: 10.1097/01.brs.0000181055.10977.5b. [DOI] [PubMed] [Google Scholar]
- 20.Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer. 2005;103:320–328. doi: 10.1002/cncr.20756. [DOI] [PubMed] [Google Scholar]
- 21.Wai EK, Finkelstein JA, Tangente RP, Holden L, Chow E, Ford M, Yee A. Quality of life in surgical treatment of metastatic spine disease. Spine. 2003;28:508–512. doi: 10.1097/00007632-200303010-00018. [DOI] [PubMed] [Google Scholar]
- 22.Walker MP, Yaszemski MJ, Kim CW, Talac R, Currier BL. Metastatic disease of the spine: evaluation and treatment. Clin Orthop Relat Res. 2003;415:S165–S175. doi: 10.1097/01.blo.0000092977.12414.f9. [DOI] [PubMed] [Google Scholar]