Abstract
This is an experimental study on the distribution of antituberculosis drugs such as rifampin, isoniazid, and pyrazinamide in pathologic vertebrae of spinal tuberculosis in order to provide the regimen of chemotherapy and surgical treatment of spinal tuberculosis. The distribution of antituberculosis drugs in pathologic vertebral tissues matters greatly to the clinical effect of spinal tuberculosis’ treatment. However, few pharmacokinetic studies and clinical reports about the concentrations of antituberculosis drugs in vertebral foci have been published so far. Twenty-four patients with spinal tuberculosis were divided into sclerotic group (n = 15) or non-sclerotic group (n = 9) according to radiographic features of lesion. All patients received chemotherapy with 2HRZ/2·5H2R2Z2 for a duration of 4.5 months. Four weeks after chemotherapy all patients underwent surgery and the specimen of serum, ilium, and pathologic vertebral tissues, including sclerotic wall, subnormal osseous tissue, and foci were obtained during operation in 120–130 and 180–190 min after oral intake in the morning, respectively. The levels of three drugs in the specimen were measured using HPLC method. The concentration levels of isoniazid, rifampin and pyrazinamide varied greatly in different tissues of spinal tuberculosis, of which the bactericidal concentration values of isoniazid and rifampin and fivefold minimal inhibitory concentration (MICs) of pyrazinamide were found in subnormal vertebral bone and self-contrast ilium, the MICs of all drugs were found in sclerotic wall outside foci, and undetected level was found in foci inside the sclerotic wall. To patients without vertebral sclerotic wall around the foci, the isoniazid in foci was of bactericidal level and rifampin and pyrazinamide in foci corresponded to the MICs respectively. The sclerotic bone of affected vertebra plays an important role in blocking the antituberculosis drug’s penetration into tuberculosis focus.
Keywords: Spine, Tuberculosis, Isoniazid, Rifampicin, Pyrazinamide, High performance liquid
Introduction
Pharmacological studies have proved that the clinical effects of medicine are mainly determined by its target tissue’s concentration. Consequently, the distribution of antituberculosis drugs in pathologic vertebral tissues is the key to therapeutic effect of spinal tuberculosis treatment. For spinal tuberculosis patients who were at mid-half or advanced stage, with severe pathologic changes in vertebrae like the sclerotic bone formation around the foci, the focal osseous tissues penetration ability of antituberculosis drugs and its concentration levels in different pathologic vertebral tissues, matter greatly to patients’ focal clearance and postoperative chemotherapy. However, few pharmacokinetic studies and clinical reports so far about the concentrations of antituberculosis drugs in vertebral foci have been published [12, 15]. Therefore, high-pressure liquid chromatography (HPLC) method was adopted on the basis of literatures [10, 17] to determine the concentrations of isoniazid (INH), rifampicin (RFP) and pyrazinamide (PZA) in pathological vertebrae, serum as well as the normal osseous tissue, in order to provide the theoretical basis for the improvement in treating spinal tuberculosis.
Patients and methods
Patients
Twenty-four consecutive cases with spinal tuberculosis, treated by ultrashort-course chemotherapy in conjunction with partial excision of pathologic vertebrae, were studied. There were 18 males and 6 females whose ages ranged from 20 to 54 years, with mean of 44 years. Their weights varied from 55 to 72.5 kgs. According to Jain et al. [6] CT classification and whether or not the sclerotic wall was formed around the foci, among 24 cases, 15 cases were sorted into sclerotic group with the radiographic characters of vertebral sclerosis around the foci (Fig. 1), and nine cases divided into non-sclerotic group (Fig. 2). The chemotherapeutic program was 2SHRZ/2.5H2R2Z2, which was recommended by the State Pulmonary Tuberculosis Short-Course Chemotherapy Coordination Group [2]. Among the scheme, the numbers 2 and 2.5 before the code of drug mean that the lasing time of intensive phase is 2 months and consolidation phase is 2.5 months, respectively, The code H2 means that the patient needs to take “H” twice per week; R2 and Z2 are similar .The parts of scheme before bias stand for intensive phase of chemotherapy and those behind bias stand for consolidation phase of scheme. The codes S, H, R, and Z stand for streptomycin, isoniazid, rifampicin and pyrazinamide, respectively. During the intensive treatment phase, intramuscular injection of 20 mg kg−1 day−1 (maximum dosage: 1.0 g) streptomycin was given once per day; isoniazid was administered of 5 mg kg−1 day−1 (maximum dosage: 300 mg); rifampicin was administered 10 mg kg−1 day−1 (maximum dosage: 600 mg); pyrazinamide was administered 25 mg kg−1 day−1 (maximum dosage: 2.0 g). The course of treatment was 4.5 months. The 2-month intensive treatment phase included the premedication time, and the consolidation time after the intensive treatment phase was 2.5 months. During the consolidation, the dosage was the same as that taken during the intensive treatment phase, which was, however, changed to twice per week.
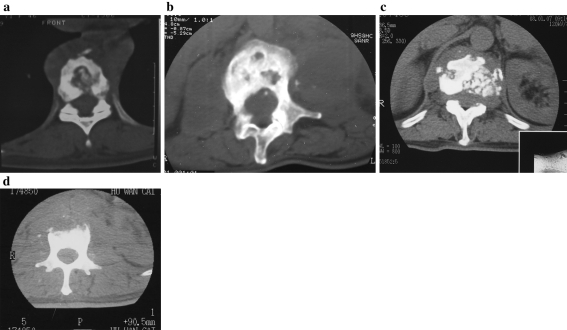
Fig. 1.
Sclerotic group. Patients in this group include localized sclerotic margin type (a) Multi-focus sclerotic margins type (b) fragmentary type (c) subperiosteal type (d). The intraoperative samples include sclerotic wall of vertebrae, subnormal bone around sclerotic wall and foci inside sclerotic wall
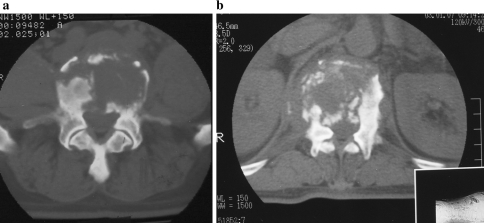
Fig. 2.
Non-sclerotic group. patients in this group mainly include osteolytic type (a) and parts of fragmentary type (b). The intraoperative samples include soft tissues foci of vertebrae and subnormal bone outside foci
All the subjects were diagnostically confirmed to suffer from active spinal tuberculosis, and were patients with surgical indications, based on clinical manifestations, imaging characteristics, laboratory and pathological examinations [4]. The surgical indications include wide lesions of vertebrae, big abscess formations, sinuses, vertebral deformities and neurologic deficits due to spinal tuberculosis. All cases had normal preoperative hepatic and renal function and had no antituberculosis chemotherapy 3 months before hospitalization.
Methods
Sample collection and pre-disposal
All cases underwent surgery 4 weeks after chemotherapy. Before surgery, the antituberculosis drugs were administered as usual early in the morning. With the cooperation of the operators and the anesthetists, the process of drug intake and the time recording after intake as well as the samples’ obtaining were under strict control. All samples were obtained at 120–130 and 180–190 min after drug intake. The vertebral samples were obtained from pathologic vertebrae, by the same surgeon, including sclerotic wall outside the foci, and subnormal vertebra outside the sclerotic wall, sequester and cheesy necrosis tissue inside the foci in sclerotic group and the foci, subnormal bone in non-sclerotic group. According to the given time phases, the blood samples were obtained by cruise nurses and self-contrast bone was obtained from ilium bone graft in both groups.
The blood was centrifuged, and the serum was collected for further determination. Pathologic vertebral tissues and ilium were immediately rinsed with normal saline, blotted dry, and placed in liquid nitrogen for the remainder of the procedure; all serum, soft tissue and bone samples were subsequently stored below −70°C until analysis. To prepare the soft tissue and bone samples for INH, RFP, and PZA extraction, samples were cryodesiccated and pulverized into a fine powder using a SPEX 6700 freezer mill (SPEX, Edison, New Jersey) and then weighed using an analytic balance. Only those samples weighing more than 0.5 g were subsequently used.
Sample determination
INH, RFP, and PZA were extracted accomplished using the method of Ge et al. [5, 17]. From the resulting supernatant, a 200-μl aliquot was used for hemoglobin (Hgb) determination; the hemoglobin in the blood samples was determined using the cyanide method and in the bone extraction with the benzidine-superoxide method. For correction of blood contamination in the bone, the following method as described by Roncoroni et al. was used:
Determined concentration of antituberculosis drugs in bone extraction fluid minus the amount of that in bone sample due to blood contamination, estimated according to the following formula [11]:
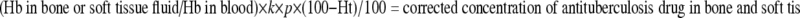 |
where Hb is hemoglobin μmol/l, k the bone dilution factor, p the plasma concentration μg/ml and Ht, the blood hematocrit in percentage.
Statistical analysis
The differences of biodistribution of INH, RFP, and PZA in ilium, pathologic vertebrae tissues as well as serum were analyzed respectively. Analyses were done using SPSS statistical software, version 10 (SPSS UK Ltd., Woking, UK). Data are expressed as mean ± SD (standard deviation). Differences of means within groups were evaluated statistically by one-way ANOVA and Dennett t test. P value <0.05 was considered significant.
Results
The concentration of serum and bone samples in the time phases of 120 and 180 min after the intake of INH, RFP, and PZA were shown in Tables 1, 2, 3 and 4.
Table 1.
The concentration of RFP, INH, PZA in sclerotic group at time phase of 120–130 min 
| Drug | Serum (μg/ml) | Ilium (μg/g) | Sclerotic wall of vertebrae (μg/g) | Subnormal bone (μg/g) | Foci inside sclerotic wall (μg/g) |
|---|---|---|---|---|---|
| RFP | 8.94 ± 2.15** | 4.87 ± 1.65 | 0.47 ± 0.11** | 4.32 ± 1.59 | Undetected |
| INH | 5.48 ± 2.73** | 2.23 ± 0.78 | 0.057 ± 0.011** | 2.14 ± 0.74 | Undetected |
| PZA | 31.54 ± 6.78** | 7.61 ± 2.62 | 0.82 ± 0.26** | 7.58 ± 2.45 | Undetected |
Compared with ilium in same drug group (except focus inside sclerotic wall), *P < 0.05; **P < 0.01
Table 2.
The concentration of RFP, INH, PZA in sclerotic group at time phase of 180–190 min 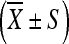
| Drug | Serum (μg/ml) | Ilium (μg/g) | Sclerotic wall of vertebrae (μg/g) | Subnormal bone (μg/g) | Foci inside sclerotic wall (μg/g) |
|---|---|---|---|---|---|
| RFP | 6.82 ± 1.67* | 4.57 ± 1.25 | 0.46 ± 0.08** | 4.10 ± 1.36 | Undetected |
| INH | 2.40 ± 1.63 | 2.12 ± 0.64 | 0.056 ± 0.009** | 2.07 ± 0.62 | Undetected |
| PZA | 25.46 ± 5.72** | 7.10 ± 1.69 | 0.78 ± 0.25** | 6.93 ± 1.98 | Undetected |
Compared with ilium in same drug group (except focus inside sclerotic wall), *P < 0.05; **P < 0.01
Table 3.
The concentration of RFP, INH, PZA in non-sclerotic group at time phase of 120–130 min 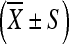
| Drug | Serum (μg/ml) | Ilium (μg/g) | Subnormal bone (μg/g) | Foci inside sclerotic wall (μg/g) |
|---|---|---|---|---|
| RFP | 8.97 ± 2.46** | 4.83 ± 1.58 | 4.30 ± 1.56 | 0.54 ± 012** |
| INH | 5.50 ± 2.61** | 2.25 ± 0.82 | 2.16 ± 0.78 | 0.62 ± 0.07** |
| PZA | 31.78 ± 7.43** | 7.62 ± 2.58 | 7.46 ± 2.40 | 1.64 ± 0.32** |
Compared with ilium in same drug group, *P < 0.05; **P < 0.01
Table 4.
The concentration of RFP, INH, PZA in non-sclerotic group at time phase of 180–190 min 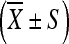
| Drug | Serum (μg/ml) | Ilium (μg/g) | Subnormal bone (μg/g) | Foci inside sclerotic wall (μg/g) |
|---|---|---|---|---|
| RFP | 6.78 ± 1.83* | 4.48 ± 1.24 | 4.22 ± 1.64 | 0.52 ± 0.10** |
| INH | 2.39 ± 1.45 | 2.16 ± 0.61 | 2.05 ± 0.61 | 0.55 ± 0.06** |
| PZA | 26.55 ± 6.02** | 7.35 ± 1.72 | 7.03 ± 1.86 | 1.59 ± 0.28** |
Compared with ilium in same drug group, *P < 0.05; **P < 0.01
Sclerotic group
Compared with the bactericidal concentrations in self-contrast iliac bone, concentrations of INH, RFP, and PZA in subnormal bone of pathologic vertebrae were almost equal to iliac bones’, with the concentrations of 4.32 ± 1.59, 2.14 ± 0.74, 7.58 ± 2.45 μg/g in 120–130 min, and 4.10 ± 1.36, 2.07 ± 0.62, 6.93 ± 1.98 μg/g in 180–190 min, respectively. No statistically significant difference was found between subnormal vertebral bone and iliac bone tissue in two time phases (P > 0.05). However, the concentrations of three drugs in vertebral sclerotic wall were very low. There was a significant difference between sclerotic wall and iliac bone tissue (P < 0.01). The distribution of the three drugs was not detected in foci tissue which located inside sclerotic wall, including granuloma, cheesy necrosis and sequester. Regarding serum, the concentrations of INH, RFP, and PZA in 120–130 min phase were all higher than that of their ilium, respectively, and there was significant difference between serum and iliac tissue (P < 0.01). Except INH, the concentrations of RFP and PZA in 180–190 min phase were still much higher than that of their ilium, respectively (Tables 1 and 2).
The non-sclerotic group
The concentrations of three drugs in subnormal vertebral bone and ilium were all very near to their values in sclerotic group, respectively. There was no statistically significant difference between subnormal bone tissue and ilium (P > 0.05). Different from the sclerotic group, the distribution of INH, PFP, and PZA was determined in foci tissue, with the concentrations far less than normal iliac bone tissue (P < 0.01). Compared with ilium, concentrations of three drugs in serum were all higher than that of their ilium in 120–130 min phase (P < 0.01). Regarding 180–190 min phase, the concentrations of RFP and PZA in serum were higher than that of their ilium, respectively, except INH (Tables 3 and 4).
Clinical effects
All cases received ultrashort-course chemotherapy in conjunction with partial excision of pathologic vertebrae, iliac strut graft and anterior or posterior fixation and had a minimum follow-up of 2 years. The method used to clear foci in sclerotic group is to excise foci by using osteotome layer by layer from inside to outside until the peripheral of focal edges, almost to normal substance of bones; the cutting tissues include all vertebral sclerotic walls, foci inside the sclerotic wall as well as parts of subnormal bone outside the sclerotic wall. Radical clearance of foci was carried out in non-sclerotic group. Results from the 2-year-follow-up showed that all patients recovered to normal with regard to their clinical manifestations, ESR, CRP, as well as radiographic data.
Discussion
The distribution traits of INH, RFP, and PZA in vertebral foci of spinal tuberculosis during chemotherapeutic intensive treatment phase
The extraneous minimal inhibitory concentrations (MIC) of RFP and INH so far have been known as 0.005–0.5 and 0.025–0.05 μg/ml, respectively, and the MIC of PZA related cellular power of hydrogen is 1.5 μg/ml in the condition of pH 5.0 [7]. It was generally accepted that effective bactericidal concentration values should be ten times than MIC [8]. Results from our study showed that concentration levels of INH and PZA in serum were similar to the data of literatures, but the level of RFP was only 75% of that in the literatures [3, 9]. In our studies of 24 patients, the concentrations of three drugs in self-contrast ilium achieved effective bactericidal concentrations, with almost the same concentration levels in both 120–130- and 180–190-min phases, respectively, though they were lower than that of their serums. Besides, their concentration levels in serum in 180–190-min-phase were much lower than those of the former phases. This finding hinted that pharmacokinetics of INH, RFP, and PZA were different from that of their serums, its half-value period prolonged quite a lot, and thus, the curative concentrations of antituberculosis drugs lasted for a longer time after regular chemotherapy, which would benefit the bactericidal of Mycobacterium tuberculosis in foci. Studies proved that the majority of medicine serum concentrations were directly correlated with their normal tissues; however, with the normal tissue changed to pathologic one, drug distribution in pathologic tissues usually distinguished from normal tissue greatly [1]. In our studies of sclerotic group, the distributions of INH, RFP, and PZA in sclerotic walls were much lower than normal bone, with the concentration levels of MIC. In contrast, concentrations of INH and RFP in subnormal vertebral bone outside the sclerotic wall were equal to self-contrast ilium, with the levels of effective bactericidal concentration values, and PZA was fivefold of its MIC in acid cellular condition. Based on the findings in our results, the vertebral sclerotic wall was likely to be a barrier which blocked the antituberculosis drug’s penetration from normal vertebral bone into foci. Meanwhile, it was found that the concentrations of INH, RFP, and PZA in subnormal vertebral bone and ilium of non-sclerotic group achieved bactericidal levels, respectively, and there were no differences between two groups in terms of drugs and time phases. But, totally different from sclerotic group, the distributions of RFP and PZA achieved MICs in foci of non-sclerotic group, and further, the INH achieved bactericidal concentration in foci. The findings further demonstrated that presence of vertebral sclerotic wall was related to the antituberculosis drug penetration in focus and resulted in completely different distribution of chemotherapeutic drugs.
Clinical significance of antituberculosis drug concentration in pathologic vertebrae
With the advent of new factors like gene mutation of M. tuberculosis and host immunity, cases with spinal tuberculosis were more refractory than before, especially in the developing countries. And new radiopathologic characters, which differed from the past, appeared in the majority of cases with spinal tuberculosis. According to Jain CT classification, recent studies by Wang et al. [8, 14] found that several pathological changes of affected vertebrae, namely, foci, vertebral sclerotic bone formation, osteoclasia, osteolytic and fragmentary changes as well as cold abscess, coexist. Among them, the vertebral sclerotic bone formation which existed in other pathological styles, accounted for 69.2–75% of spinal tuberculosis. A recent study by Yang et al. [16] proved that 82% of sclerotic walls had tubercles and it should be considered as the focal tissues of spinal tuberculosis. Owing to the traits of hardness and scattering of sclerotic vertebral bone, it was very difficult to curette all the sclerotic bone outside the foci. Thus, a relatively high relapse rate by using chemotherapy alone and by traditional clearance of foci have been found in treating of spinal tuberculosis. Currently, few clinical reports, so far, about focus clearance of spinal tuberculosis involving in the vertebral sclerotic bone resection have been published. Wang et al. [13] pointed that the reservation or the residual of sclerotic wall after debridement might cause the recurrence of spinal tuberculosis or the lasting of chemotherapy. Results from our studies showed that the sclerotic wall outside the foci blocked the medicine’s penetration from the normal tissue of vertebrae into foci, which resulted in the bactericidal concentration of antituberculosis drugs in normal or subnormal parts of pathologic vertebrae, the minimal bacteriostatic concentration in sclerotic walls, and the concentration far less than minimal bacteriostatic in the foci. Because of the delitescent M. tuberculosis in cheesy necrosis foci were only sensitive to RFP and PZA, the very low antituberculosis drug distribution of focus and its relative enclosed environment, like hypoxia and acidity condition might lead to the sterilizing of slow dividing bacilli and intermission dividing bacilli causing of foci unavailable, thus, a recurrence. Based on the distribution traits of INH, RFP, and PZA in vertebral foci in conjunction with current pathologic characters of spinal tuberculosis, our results suggest that antituberculosis drug’s distribution of pathologic vertebrae is directly related to its pathologic structure, to those with sclerotic radiographic traits; more attention should be paid to removing all sclerotic bone around the foci during the surgery so that the antituberculosis drugs could easily penetrate into the remaining vertebrae and control the pathologic changes. By doing so, the chemotherapeutic term and the curative rate might be improved significantly in treating spinal tuberculosis, which has been achieved in the clinical studies with the chemotherapy period of less than 6 months and with excellent curative rates. However, this is only a primary study involving three major antituberculosis drugs, to completely reveal the distributive characters of antituberculosis drugs in pathologic vertebrae of spinal tuberculosis. More antituberculosis drugs and more cases should be selected for further studies.
Acknowledgments
This work was supported in part by grants from a grant from Ningxia Science Foundation, People’s Republic of China. The technical assistance of Dr. Wei Minji is greatly appreciated.
Contributor Information
Zhaohui Ge, Phone: +86-951-6743242, FAX: +86-951-4082981, Email: gezhaohui@gmail.com.
Zili Wang, Phone: +86-951-6744618, FAX: +86-951-4082981, Email: myovid@126.com.
References
- 1.Both B. Penetration of parenterally administered rifampicin into bone tissue. Chemotherapy. 1984;30:358–365. doi: 10.1159/000238294. [DOI] [PubMed] [Google Scholar]
- 2.Coordination Group of the State Short-Course Chemotherapy for Pulmonary Tuberculosis Clinical comparison studies on a 5 month chemotherapy and 6 month whole course intermittent program in the treatment of pulmonary tuberculosis with positive bacteria. Chin J Tuberc Respir. 1998;21:388–391. [Google Scholar]
- 3.Deng L, Jiang X, Chen J, et al. Study on pharmacokinetics and bioequivalent evaluation of Yif uxianan tablets. Chin Hosp Pharm J. 2003;23:475–478. [Google Scholar]
- 4.Dept of Orthopedics (1974) Tianjin Hospital, Clinical Osteology (II). Tuberculosis, 1st edn. People’s Health Publishing House, Beijing, p. 215
- 5.Ge Z, Wang Z, Wei M. An experimental study of rifampicin’s distribution in different tissues of spinal tuberculosis patients. Chin J Spine Spinal Cord. 2004;14:741–744. [Google Scholar]
- 6.Jain R, Sawhney S, Berry M. Computed tomography of vertebral tuberculosis: patterns of bone destruction. Clin Radiol. 1993;47:196–199. doi: 10.1016/S0009-9260(05)81162-6. [DOI] [PubMed] [Google Scholar]
- 7.Lee J. Clinical pharmacology, 2 edn. Beijing: People’s Health Publishing House; 1997. pp. 692–700. [Google Scholar]
- 8.Li P, Yang W, Zhong Q. Computed tomography of vertebral tuberculosis. CT Theory 7 Appl. 2003;13:33–35. [Google Scholar]
- 9.Liu H, Wang J, Hou J, et al. Pharmacokinetics of rifampin in healthy volunteers after a high oral dose. Chin J Pharm. 2000;35:396–398. [Google Scholar]
- 10.Panchagnula R, Sood A, Sharda N, et al. Determination of rifampicin and its main metabolite in plasma and urine in presence of pyrazinamide and isoniazid by HPLC method. J Pharm Biomed Anal. 1999;18:1013–1020. doi: 10.1016/S0731-7085(98)00112-5. [DOI] [PubMed] [Google Scholar]
- 11.Roncoroni AJ, Manuel C, Nedjar C, et al. Cefamandole bone diffusion in patients undergoing total hip replacement. Chemotherapy. 1981;27:166–172. doi: 10.1159/000237973. [DOI] [PubMed] [Google Scholar]
- 12.Tuli SM, Kumar K, Sen PC. Penetration of antituberculosis drugs in clinical osteoarticular tubercular lesion. Acta Orthop Scand. 1977;48:362–368. doi: 10.3109/17453677708992009. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Ge Z, Jin W, et al. Treatment of spinal tuberculosis with ultrashort-course chemotherapy in conjunction with partial excision of pathologic vertebrae. Spine J. 2007;7:671–681. doi: 10.1016/j.spinee.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Yang W, Jin W, et al. Treatment of spinal tuberculosis with anterior partial vertebrectomy, iliac grafting and internal fixation. Chin J Spine Spinal Cord. 2004;14:716–719. [Google Scholar]
- 15.Wu Q, Duan L, Ling Y, et al. Comparison of three antituberculous drugs in serum and cold abscessed of patients with spinal tuberculosis. Chin J Tuberc Respir. 1998;21:617–619. [PubMed] [Google Scholar]
- 16.Yang W, Wang Z, Qiao Y, et al. Resectable range of anterior partial vertebrectomy for spinal tuberculosis. J Fourth Mil Med Univ. 2006;27:695–697. [Google Scholar]
- 17.Zhuo H, Lei Y, Wang C, et al. Study of pharmacokinetic and bioavailability of a combination rifampicin tablet in healthy volunteers. Chin J Clin Pharm. 1999;15:425–430. [Google Scholar]