Abstract
The objective of this study was to assess the efficacy of paracetamol (acetaminophen) in the treatment of pain and disability in patients with non-specific low back pain. We conducted a systematic review of randomized controlled trials to assess the efficacy of paracetamol in the treatment of pain and disability in patients with non-specific low back pain. A search for randomized controlled trials was conducted using the Medline, Embase and CINAHL databases. Trials were eligible if they were randomized controlled trials comparing paracetamol to no treatment, placebo or another treatment in patients with non-specific low back pain. Two of the authors independently assessed trials for methodological quality on the PEDro Scale and extracted data. Continuous pain and disability data were converted to a common 0–10 scale; ordinal data were dichotomized (e.g., no pain, pain). The data was analyzed using the MIX version 1.61 meta-analysis software. Out of 205 unique articles found in the searches, 7 eligible trials were identified. The trials enrolled a total of 676 participants with 5 investigating acute low back pain, 1 investigating chronic low back pain and 1 investigating both. No trial provided data comparing paracetamol to placebo and only one trial compared paracetamol to no treatment. In general the trials were small (only 1 trial had >25 subjects per group) and of low methodological quality (only 2 had a score above 6 on the quality scale). All but one of the trials provided imprecise estimates of the effects of treatment with confidence intervals spanning clinically important beneficial and also harmful effects of paracetamol. No trial reported a statistically significant difference in favor of paracetamol. There is insufficient evidence to assess the efficacy of paracetamol in patients with low back pain. There is a clear need for large, high quality randomized controlled trials evaluating paracetamol, to provide reliable evidence of paracetamol’s effectiveness in patients with low back pain and to establish the validity of the recommendations in clinical guidelines.
Keywords: Low back pain, Paracetamol, Acetaminophen, Review
Introduction
Paracetamol (World Health Organization International Non Proprietary Name), also known as acetaminophen (United States Adopted Name), has been used as an analgesic for approximately 50 years and has relatively few side effects [7]. A review of clinical practice guidelines from around the world, published up until the year 2000, found that almost all the guidelines recommended paracetamol as part of the first line of care for patients with low back pain [11]. Subsequent clinical practice guidelines for acute low back pain, published in Australia [1], New Zealand [6] and Europe [18] and a French guideline for chronic low back pain [4] have also recommended paracetamol. Therefore, given its prominence in clinical practice guidelines, we believed it was important to review the evidence for the efficacy of paracetamol in low back pain treatment.
The aims of this review were to:
Assess the evidence for the effectiveness of paracetamol compared to placebo or no treatment, in patients with low back pain, for the outcomes of pain and disability.
Assess the evidence for the effectiveness of paracetamol compared to other treatments, in patients with low back pain, for the outcomes of pain and disability.
Materials and methods
Identification and selection of trials
A search of the Medline, Embase and CINAHL databases up to August 2007 was conducted. The search strategies were based upon those recommended by the Cochrane Collaboration Back Review Group [19] using a sensitive search for randomized controlled trials combined with a specific search for low back pain and paracetamol (Appendix). Titles and abstracts were used to exclude clearly irrelevant studies. Full copies were obtained of all other studies. The reference lists of included trials were also screened for relevant trials and the authors’ personal files were reviewed. Handsearching of journals was not undertaken and we did not contact pharmaceutical companies to locate trials.
The inclusion criteria for studies were:
We were able to locate a reviewer who spoke the language the trial was written in.
The trial was a randomized controlled trial.
The participants were aged greater than 16 years and were experiencing non-specific low back pain. Trials with both acute (≤12 weeks) and chronic (≥12 weeks) low back pain were included. Trials of participants with specific pathologies such as fracture, cancer, rheumatological conditions or osteoporosis were excluded from the review.
- The trial contained data about the efficacy of paracetamol. Accepted treatment contrasts were:
- Paracetamol alone versus placebo or no treatment.
- Paracetamol alone versus another treatment regimen not including paracetamol.
- Paracetamol and another treatment versus the other treatment alone.
Trial selection and data extraction was performed independently by two authors with disagreements resolved by discussion and consensus. We did not blind the authors to aspects of study reports, e.g., title or author names.
Description of trials
The methodological quality of the trials was assessed using the ten scoring items of the PEDro scale. The scores were determined independently by two authors (Reece A. Davies and Christopher G. Maher) and any disagreements were resolved by consensus. The PEDro scale has been found to have acceptable reliability [12] and has also been found to provide a more comprehensive measure of methodological quality than the Jadad scale [3]. Trials with scores less than six were considered of low methodological quality.
The comparison interventions were grouped as placebo/no treatment, non-steroidal anti-inflammatory drugs, other drugs and other treatments. The primary outcomes for this review were pain and disability post treatment. For this review, disability could be measured through a scale (for example the Roland Morris disability questionnaire) or through other functional outcomes such as ability to work, mobility or performance of activities of daily living. Other outcomes, including intermediate time points for the primary outcomes, were described where possible.
Data analysis
Baseline demographic data (age and sex of participants and the numbers to follow up) as well as outcome data of post treatment pain and disability scores were extracted from the included trials independently by two authors (Reece A. Davies and Christopher G. Maher) with disagreements resolved by consensus. Continuous pain or disability outcomes, such as visual analogue scales (VAS) numerical rating scales (NRS) or the Roland Morris disability questionnaire, were converted to a common 0–10 scale. Scales with less than seven points were not considered continuous and were dichotomized into ‘no pain’ and ‘pain’ or ‘no disability’ and ‘disability’ [14].
Effect sizes were calculated using MIX version 1.61 meta-analysis software [2] with relative risks calculated for dichotomized data and weighted mean differences for continuous data. If standard deviations for continuous data were not presented in the trial, they were estimated using the standard error, p value or t value if supplied; otherwise they were estimated from the mean of available standard deviations in accordance with the approach recommended in the Cochrane Handbook for Systematic Reviews [10]. In estimating the standard deviation the first option was to use standard deviations for other time-points for that outcome and treatment group from the same trial. When this was not possible, the mean of all the standard deviations for that outcome for all available trials was calculated. Any trials which were homogenous with respect to participants, intervention, methodological quality and outcome measures were to be included in a meta-analysis. Those trials not included in the meta-analysis had their data presented individually in the text and/or tables. Also included in this individual description were any other outcomes, other than the primary ones, presented in the trials which were of interest.
Results
Identification and selection of trials
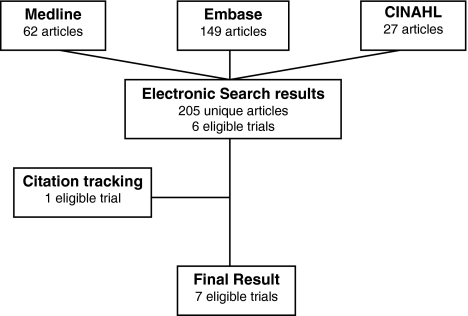
The search retrieved 205 unique articles which resulted in seven trials, reported in six articles [8, 9, 13, 15, 17, 20] being included in the review (Fig. 1). The article by Wiesel et al. [20] reported three trials, of which two met the inclusion criteria. These two trials were considered separately in the analysis for this review.
Fig. 1.
Search results
Description of trials
An overview of each of the included trials is presented in Table 1 with the methodological quality of the trials summarized in Table 2. None of the trials were large, high quality trials. Randomization and between group statistical comparisons were performed by all trials while concealed allocation and intention to treat analysis were each only fulfilled by one trial.
Table 1.
Summary of included trials
| Trial | Overviewa | Paracetamol dosage | Comparison(s) | Length of treatment |
|---|---|---|---|---|
| Hackett et al. [8] | 40 Participants aged 16–60 years with LBP <3 days duration. Outcomes measured baseline, 1, 2 and 6 weeks; follow-up 92% | 2 Tab every 4 h | Electroacupuncture, 2 treatments within 96 h | Not stated |
| Hickey [9] | 30 Participants (26 female, 4 male) aged between 21 and 75 years with chronic LBP >6 months duration. Outcomes measured baseline, 2 and 4 weeks; follow-up 93% | 1,000 mg qid | Diflunisal, 500 mg bid | 4 weeks |
| Milgrom et al. [13] | 70 Army recruits with acute over-exertional LBP. Outcomes measured at the end of basic training; follow-up 100% | 1,000 mg tid | (1) Ibuprofen, 800 mg tid (2) No treatment |
1 week |
| Nadler et al. [15] | 371 Participants (216 female, 155 male) aged 18–55 years with acute LBP >48 h duration. Outcomes measured at baseline and day 1, 2 and 4; follow-up 97% | 1,000 mg qid | (1) Heat wraps, 8 h/day (2) Ibuprofen, 400 mg tid |
2 days |
| Stein et al. [17] | 45 Participants with LBP <6 months duration. Outcomes measured baseline and 1, 2, 3, 4 5 weeks; follow-up was 80% | 500 mg qid | Amitriptyline, 37.5 mg qid | 5 weeks |
| Wiesel et al. [20] (I) | 45 Male army recruits aged 17–34 years with acute LBP. Outcomes measured at baseline and every day for 14 days | 1 Tab bid | (1) Aspirin, 625 mg qid (2) Phenylbutazone, 100 mg qid |
5 days |
| Wiesel et al. [20] (II) | 75 Male army recruits 17–34 years with acute LBP. Outcomes measured at baseline and every day for 14 days | 1 Tab bid | (1) Codeine, 60 mg qid (2) Oxycodone plus aspirin, 1 Tab qid |
5 days |
Tab Tablet(s), qid four times per day, tid three times per day, bid two times per day
aInformation on age, gender, LBP duration, and loss to follow-up was not completely reported in all trials
Table 2.
Trial methodological quality
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hackett et al. [8] | + | − | − | − | − | − | + | − | + | − | 3 |
| Hickey [9] | + | + | − | + | + | + | + | − | + | + | 8 |
| Milgrom et al. [13] | + | − | − | − | − | − | + | − | + | + | 4 |
| Nadler et al. [15] | + | − | + | − | − | − | + | + | + | − | 5 |
| Stein et al. [17] | + | − | + | + | + | + | + | − | + | + | 8 |
| Wiesel et al. [20] (I) | + | − | − | − | − | − | − | − | + | + | 3 |
| Wiesel et al. [20] (II) | + | − | − | − | − | − | − | − | + | + | 3 |
| Sum | 7 | 1 | 2 | 2 | 2 | 2 | 5 | 1 | 7 | 5 |
1 Randomisation, 2 concealed allocation, 3 baseline comparability, 4 subject blinding, 5 therapist/physician blinding, 6 assessor blinding, 7 adequate follow-up (≥85%), 8 intention-to-treat analysis, 9 between group statistical comparisons, 10 point measures and measures of variability, Total total number of fulfilled items (maximum = 10); +, fulfilled; −, not fulfilled
Five of the trials reported on participants with acute low back pain [8, 13, 15, 17, 20] one trial reported on participants with chronic low back pain [9] and one trial included both participants with acute low back pain and with chronic low back pain as defined for this review [17].
None of the trials presented data comparing paracetamol with placebo, one trial compared paracetamol with no treatment [13], four of the trials included a comparison group of NSAIDs [9, 13, 15, 20] two of the trials compared paracetamol with another drug [17, 20] and two of the trials compared paracetamol with a different treatment [8, 15]. All of the trials provided outcome data on pain and five provided data on disability. Due to the heterogeneity of methodological quality, treatment contrast, dosage regimens and outcomes of the trials, a meta-analysis was not performed; however, effect sizes for individual trials were able to be calculated. The data from the trial by Hickey [9] were dichotomized, with a rating of none regarded as recovered for pain and functional disability and all others as not recovered.
Effect of paracetamol versus no treatment
Milgrom and colleagues’ trial [13] provided data comparing paracetamol with no treatment for over-exertional low back pain in military recruits and found no significant difference in the rate of resolution of symptoms between paracetamol and no treatment (Table 3). At the end of treatment paracetamol was found to delay recovery, with a relative risk for recovery of 0.66 (0.44–1.01) compared with no treatment.
Table 3.
Effects of paracetamol on recovery from pain and disability
| Outcome | Comparison | Relative risk (95% CI) | Recovery rate | ||
|---|---|---|---|---|---|
| Week | Paracetamol | Comparison | |||
| Pain | |||||
| Milgrom [13] | Var | No treatment | 0.66 (0.44–1.01) | 13/24 | 18/22 |
| Var | Ibuprofen | 0.81 (0.51–1.29) | 13/24 | 16/24 | |
| Hickey [9] | 2 | Diflunisal | 0.89 (0.18–4.51) | 2/12 | 3/16 |
| 4 | Diflunisal | 0.80 (0.24–2.71) | 3/12 | 5/16 | |
| Disability | |||||
| Hickey [9] | 2 | Diflunisal | 0.67 (0.07–6.52) | 1/12 | 2/16 |
| 4 | Diflunisal | 0.44 (0.11–1.83) | 2/12 | 6/16 | |
Relative risk is for recovery with paracetamol. Recovery rate is number recovered/number in group
N number, 95% CI 95% confidence interval, var variable time to outcome (end of basic training)
Effect of paracetamol versus NSAIDs
Of the four trials that compared paracetamol with a NSAID, two used ibuprofen [13, 15] as the comparison, one used diflunisal [9] and the other used both phenylbutazone and aspirin [20]. One trial investigated participants with chronic low back pain [9], while the other trials all included only participants with acute low back pain.
Pain outcomes
In each of the four trials the effect of treatment on pain outcomes was not statistically significant; however, two trials reported a trend favoring NSAID treatment. For chronic low back pain, Hickey [9] found at both 2 and 4 weeks that there was no significant difference between diflunisal and paracetamol (Table 3). For example at 4 weeks paracetamol provided a relative risk (95% confidence interval) of recovery compared to diflunisal of 0.80 (0.24–2.71). Milgrom and colleagues’ trial [13] investigating acute over-exertional low back pain found no significant difference between paracetamol and ibuprofen in resolving low back pain (Table 3). At the end of treatment this trial found that paracetamol provided a relative risk (95% confidence interval) of recovery compared to ibuprofen of 0.81 (0.51–1.29). Wiesel and colleagues’ trial [20], investigating acute low back pain, did not provide sufficient data to allow calculation of effect sizes; however, the authors reported that there were no significant differences in the relative pain scores between paracetamol and aspirin or phenylbutazone. Nadler and colleagues’ trial [15] also studying people with acute low back pain, comparing paracetamol with ibuprofen also did not provide data that would permit the calculation of effect sizes; however, the largest between group difference was trivially small: 0.2 points on a 6 point pain relief scale.
Disability outcomes
Three trials reported disability outcomes with none reporting statistically significant effects. For chronic low back pain, a single trial [9] found at both 2 and 4 weeks that there was no significant difference between diflunisal and paracetamol in resolving ‘functional disability’ (Table 3). For example at 4 weeks paracetamol provided a relative risk (95% confidence interval) for recovery compared with diflunisal of 0.44 (0.11–1.83). Another trial [20] investigating acute low back pain did not provide sufficient data to permit effect sizes to be calculated. The trial found no significant differences between paracetamol, aspirin and phenylbutazone in number of days before return to full activity (5.7, 5.7 and 5.5 days, respectively). The third trial [15], investigating acute low back pain and comparing paracetamol to ibuprofen, did not provide outcome data to permit effect sizes to be calculated. However, the between group differences for reduction in disability, measured on the Roland Morris disability questionnaire, were too small to be clinically relevant with the largest being 0.2 points (95% confidence intervals unable to be calculated) on a converted 0–10 scale.
Effect of paracetamol versus other drugs
Of the trials comparing paracetamol with other drugs, one used amitriptyline (a tricyclic antidepressant) in a trial that included participants with both acute and chronic low back pain [17] while the other trial used codeine and oxycodone plus aspirin for participants with acute low back pain [20].
Pain outcomes
Stein and colleagues’ trial [17] investigating both acute and chronic low back pain found that paracetamol was less effective in reducing pain than amitriptyline. The between group differences ranged from 1.1 to 1.5 (Table 4) with the difference being statistically significant at week 4: effect size 1.5 (0.1–2.9). Another study [20] investigating acute low back pain supplied no numerical data about codeine and oxycodone plus aspirin compared with paracetamol for pain; however, they stated that both provided greater pain relief than paracetamol, especially in the first 3 days.
Table 4.
Effects of paracetamol on pain and disability outcomes (presented on 0–10 scale)
| Outcome | Comparison | Effect size (95% CI) | Paracetamol | Comparison | |||||
|---|---|---|---|---|---|---|---|---|---|
| Week | Mean | SD | N | Mean | SD | N | |||
| Pain | |||||||||
| Stein [17] | 1 | Amitriptyline | 0.0 (−1.3 to 1.3) | 4.7a | 2.2c | 19 | 4.7a | 1.8c | 20 |
| 2 | Amitriptyline | 1.1 (−0.1 to 2.3) | 3.6a | 1.8b | 19 | 2.5a | 1.9b | 20 | |
| 3 | Amitriptyline | 1.2 (−0.1 to 2.5) | 3.5a | 2.0b | 19 | 2.3a | 2.1b | 20 | |
| 4 | Amitriptyline | 1.5 (0.1 to 2.9) | 3.4a | 2.3b | 19 | 1.9a | 2.3b | 20 | |
| 5 | Amitriptyline | 1.2 (0 to 2.4) | 3.0 | 2.5 | 19 | 1.8 | 1.0 | 20 | |
| Hackett [8] | 1 | Electro-acupt | 0.0 (−1.2 to 1.3) | 2.3 | 2.0c | 20 | 2.3 | 2.0c | 20 |
| 2 | Electro-acupt | 0.4 (−0.9 to 1.6) | 2.2 | 2.0c | 20 | 1.8 | 2.0c | 20 | |
| 6 | Electro-acupt | 1.0 (−0.2 to 2.3) | 1.4 | 2.0c | 20 | 0.3 | 2.0c | 20 | |
| Disability | |||||||||
| Hackett [8] | 1 | Electro-acupt | −0.2 (n/c) | 2.5 | n/c | 20 | 2.7 | n/c | 20 |
| 2 | Electro-acupt | −0.1 (n/c) | 1.7 | n/c | 20 | 1.8 | n/c | 20 | |
| 6 | Electro-acupt | 1.4 (n/c) | 1.6 | n/c | 20 | 0.2 | n/c | 20 | |
Effect size difference of means (negative means paracetamol gives greater reduction), SD standard deviation, N number in group, 95% CI 95% confidence interval, Electro-acupt electroacupuncture, n/c not able to be calculated
aEstimated value from a graph
bCalculated SD from other data
cEstimated SD
Disability outcomes
A trial [20] investigating acute low back pain found no significant difference between paracetamol, codeine and oxycodone plus aspirin for the number of days before return to full activity (5.6, 5.2 and 5.6 days, respectively).
Effect of paracetamol versus other interventions
For the two trials that compared paracetamol with other interventions in participants with acute low back pain, one used electroacupuncture [8] and the other used heat wraps [15].
Pain outcomes
Hackettt and colleagues’ trial [8] investigating acute low back pain reported that electroacupuncture provided greater pain reduction after 6 weeks than paracetamol (Table 4), with the between group difference being 1.0 (−0.2–2.3). Another trial [15] also investigating acute low back pain compared paracetamol to heat wraps but did not provide outcome data to permit effect sizes to be calculated. However, the between group differences for paracetamol and heat wraps, were found to be statistically significant. The largest difference was reported on the second day: 0.8 on a 6 point pain relief scale.
Disability outcomes
Hackett and colleagues’ trial [8] investigating acute low back pain reported that electroacupuncture provided greater improvements in mobility than paracetamol after 6 weeks (Table 4), with the between group difference being 1.4 points on a 0–10 scale (95% confidence intervals unable to be calculated). Nadler and colleagues’ trial [15] investigating acute low back pain did not provide outcome data to permit effect sizes to be calculated. However, the heat wrap group had greater improvements in disability than the paracetamol group and these differences were found to be statistically significant by 4 days: 0.8 (95% confidence intervals unable to be calculated) on the Roland Morris disability questionnaire converted to 0–10 points.
Discussion
This systematic review has failed to find evidence to support the widely held view that paracetamol is effective in the treatment of non-specific low back pain. A number of problems with the previous research have been highlighted by this review. These problems include the lack of large high quality trials, inadequate reporting of methods and results, results that appear implausible and the choice of treatment contrasts used in the trials. The results of this review demonstrate a clear need for further quality research into the efficacy of paracetamol in patients with low back pain.
Considering how widely paracetamol is recommended for low back pain, it is very surprising that there are so few trials investigating its efficacy, and that the quality of the existing trials is so low. The trials included in this review were clearly underpowered with only one trial reporting more than 24 participants per group [15]. The small sample sizes likely contributed to the imprecise estimates of treatment effects in many of the trials. While this problem could potentially be overcome through meta-analysis, the heterogeneity of the trials precluded pooling. Small sample sizes can also lead to biased results if by chance randomization leads to groups with different baseline characteristics or prognosis. This possibility was difficult to assess as the baseline characteristics of the participants were only presented in two of the seven trials in this review.
The trials included in the review appear to be of low methodological quality and this may be a consequence of the age of the trials, with only one having been published within the last 10 years [15]. Methodological features that were particularly problematic were a failure to use concealed allocation of participants and intention to treat analysis, which were each satisfied in only one trial. The poor methodological quality of the included trials means that they are likely to report biased results. Future trials need to overcome these serious methodological flaws to provide less biased estimates of the efficacy of paracetamol in patients with low back pain.
Another problem with the trials included in the review is the poor reporting of methods and results and poor measurement of outcomes. As already mentioned, baseline data were only reported for two of the trials [15, 17] with another two of the trials not even describing how many participants were in each of the groups [8, 20]. These trials also lacked sufficient information about the dosages of paracetamol and some of the other drugs, only stating the number of tablets given but not providing information on the dose of drug in each tablet. Only one of six trials used a validated pain scale with the remainder using their own unvalidated pain scales. For example, Wiesel et al. [20] developed their own measure of relative pain change and Nadler et al. [15] used a scale of pain relief that did not allow the possibility of deterioration, thereby potentially biasing the results. Wiesel et al. [20] also did not provide numerical data to back up their conclusion that analgesic medications reduce the pain experienced in low back pain, meaning there is no way to establish what was the actual degree of benefit.
Some of the results that were reported in the included trials appeared implausible. For example in the trial by Hackett et al. [8] where pain was measured on a 100 mm VAS the mean follow up pain scores for the electroacupuncture group was reported as 3.3 and 1.9: a result that could only occur if nearly all participants gained complete pain relief. Milgrom et al. [13] reported an even odder result with both paracetamol and ibuprofen providing worse (though not statistically significant) outcomes than no treatment. We would advise caution in the interpretation of the results of these two trials until they are replicated in large high quality trials.
The treatment contrasts in the available paracetamol trials are not particularly helpful when attempting to judge the effectiveness of paracetamol as advocated in guidelines. The typical recommendation in guidelines for acute low back pain is that patients be provided with advice and paracetamol as the first line of treatment. Unfortunately only one trial has compared paracetamol to no treatment or placebo and no trials have evaluated whether paracetamol provides an additional benefit to advice alone. There are also no trials that have investigated the optimal way to take paracetamol, e.g., time-contingent or pain-contingent use of paracetamol or the optimum dose. In our view these are important areas for future research.
A limitation of the review was that we were only able to locate a small number of trials and these were typically small and of low quality. This is a potential problem because small low quality studies are thought to over-estimate the effects of treatment compared to large well-conducted studies [16]. Additionally our review is susceptible to publication bias, where trials with negative results are less likely to be published than those with positive results [5]. However, the effects of these two limitations are to produce overly optimistic estimates of the effect of treatment and are less relevant given our conclusion. We do not believe that the review has introduced a language of publication bias [5] because we did not exclude any studies because we could not locate a reviewer who spoke the language the paper was written in.
In conclusion, there is insufficient evidence to make a determination of paracetamol’s effectiveness in low back pain treatment. This is concerning since paracetamol is recommended as part of the baseline care in most clinical guidelines for low back pain. It is unclear how much of an effect, if any, paracetamol provides in improving pain and disability in low back pain. This is due to the absence of any placebo controlled trials and the small number, very low quality and lack of homogeneity of the existing trials comparing paracetamol to other treatments. There is a need for large high quality randomized controlled trials comparing paracetamol to placebo in low back pain to establish the validity of the current recommendations of clinical guidelines from around the world.
Acknowledgments
The authors would like to thank A/Professor Jane Latimer for critical review of the study protocol, A/Professor Rob Herbert for his assistance with statistical analyses and Brendan Wilson and Grant Mackay for assistance with proof reading of the manuscript. Chris Maher’s Research Fellowship is funded by the National Health and Medical Research Council of Australia. Mark Hancock’s Primary Care Post Graduate Scholarship was funded by the National Health and Medical Research council of Australia.
Appendix
Search strategies (adapted from van Tulder et al. [19])
Medline—Ovid Medline
01. randomized controlled trial.pt, 02. controlled clinical trial.pt, 03. Randomized Controlled Trials/, 04. Random Allocation/, 05. Double-Blind Method/, 06. Single-Blind Method/, 07. or/1–6, 08. Animal/not Human/, 09. 7 not 8, 10. clinical trial.pt, 11. explode Clinical Trials/, 12. (clinic$ adj25 trial$).tw, 13. ((singl$ or doubl$ or treb$ or tripl$) adj (mask$ or blind$)).tw, 14. Placebos/, 15. placebo$.tw, 16. random$.tw, 17. Research Design/, 18. (latin adj square).tw, 19. or/10–18, 20. 19 not 8, 21. 20 not 9, 22. Comparative Study/, 23. explode Evaluation Studies/, 24. Follow-Up Studies/, 25. Prospective Studies/, 26. (control$ or prospective$ or volunteer$).tw, 27. Cross-Over Studies/, 28. or/22–27, 29. 28 not 8, 30. 29 not (9 or 21), 31. 9 or 21 or 30, 32. low back pain/, 33. low back pain.tw, 34. backache.tw, 35. lumbago, 36. or/32–35, 37. paracetamol.tw, 38. paracetamol/, 39. acetaminophen.tw, 40. acetaminophen/, 41. APAP.tw, 42. APAP/, 43. chemadol.tw, 44. duatrol.tw, 45. dymadon.tw, 46. febridol.tw, 47. panadol.tw, 48. panamax.tw, 49. parahexal.tw, 50. paralgin.tw, 51. parmol.tw, 52. perfalgan, 53. tylenol.tw, 54. or/37–53, 55. 31 and 36 and 54.
CINAHL—Ovid CINHAL
01. randomized controlled trial.pt, 02. controlled clinical trial.pt, 03. Randomized Controlled Trials/, 04. Random Assignment/, 05. Double-Blind Studies/, 06. Single-Blind Studies/, 07. or/1–6, 08. Animal/not Human/, 09. 7 not 8, 10. clinical trial.pt, 11. exp Clinical Trials/, 12. (clinic$ adj25 trial$).tw, 13. ((singl$ or doubl$ or treb$ or tripl$) adj (mask$ or blind$)).tw, 14. Placebos/, 15. placebo$.tw, 16. random$.tw, 17. Research Design/, 18. (latin adj square).tw, 19. or/10–18, 20. 19 not 8, 21. 20 not 9, 22. Comparative Study/, 23. exp Evaluation Studies/, 24. Follow-Up Studies/, 25 Prospective Studies/, 26. (control$ or prospective$ or volunteer$).tw, 27. Cross-Over Design/, 28. or/22–27, 29. 28 not 8, 30. 29 not (9 or 21), 31. 9 or 21 or 30, 32. low back pain/, 33. low back pain.tw, 34. backache.tw, 35. lumbago, 36. or/32–35, 37. paracetamol.tw, 38. paracetamol/, 39. acetaminophen.tw, 40. acetaminophen/, 41. APAP.tw, 42. APAP/, 43. chemadol.tw, 44. duatrol.tw, 45. dymadon.tw, 46. febridol.tw, 47. panadol.tw, 48. panamax.tw, 49. parahexal.tw, 50. paralgin.tw, 51. parmol.tw, 52. perfalgan, 53. tylenol.tw, 54. or/37–53, 55. 31 and 36 and 54.
Embase—EMBASE.com
(‘low back pain’ or ‘backache’ or lumbago’) and (‘paracetamol’ or ‘acetaminophen’ or ‘APAP’ or ‘chemadol’ or ‘duatrol’ or ‘dymadon’ or ‘febridol’ or ‘panadol’ or ‘panamax’ or ‘parahexal’ or ‘paralgin’ or ‘parmol’ or ‘perfalgan’ or ‘tylenol’).
Selecting the following limits: “Map to preferred terminology”, “Also search as free text”, “Include sub-terms/derivatives”, “All Years”, “Humans”, “EMBASE only”, “Controlled Clinical Trial” and “Randomized Controlled Trial”.
References
- 1.Evidence-based management of acute musculoskeletal pain: a guide for clinicians. Bowen Hills: Australian Academic Press Pty Ltd.; 2003. [Google Scholar]
- 2.Bax L, Yu L-M, Ikeda N, Tsuruta H, Moons KGM. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhogal SK, Teasell RW, Foley NC, Speechley M. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. J Clin Epidemiol. 2005;58:668–673. doi: 10.1016/j.jclinepi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Delcambre B, Jeantet M, Laversin S, et al. Diagnosis, management and follow-up of patients with chronic low back pain (quick reference guide for clinicians) Paris: Agence Nationale d’Accréditation et d’Évaluation en Santé; 2000. [Google Scholar]
- 5.Egger M, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7:1–76. [PubMed] [Google Scholar]
- 6.Gow P, Griffiths R, Grimes P, et al. New Zealand acute low back pain guide. Wellington: ACC; 2004. [Google Scholar]
- 7.Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Ther. 2005;12:46–55. doi: 10.1097/00045391-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hackett GI, Seddon D, Kaminski D. Electroacupuncture compared with paracetamol for acute low back pain. Practitioner. 1988;232:163–164. [PubMed] [Google Scholar]
- 9.Hickey RF. Chronic low back pain: a comparison of diflunisal with paracetamol. N Z Med J. 1982;95:312–314. [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S (eds) (2008) Cochrane handbook for systematic reviews of interventions version 5.0.0. The Cochrane Collaboration. Available from http://www.cochrane-handbook.org
- 11.Koes BW, Tulder MW, Ostelo R, Burton K, Waddell G. Clinical guidelines for the management of low back pain in primary care: an international comparison. Spine. 2001;26:2504–2513. doi: 10.1097/00007632-200111150-00022. [DOI] [PubMed] [Google Scholar]
- 12.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 13.Milgrom C, Finestone A, Lev B, Wiener M, Floman Y. Overexertional lumbar and thoracic back pain among recruits: a prospective study of risk factors and treatment regimens. J Spinal Disord. 1993;6:187–193. doi: 10.1097/00002517-199306030-00001. [DOI] [PubMed] [Google Scholar]
- 14.Miller G. The magical number seven, plus or minus two. Psychol Rev. 1956;63:81–97. doi: 10.1037/h0043158. [DOI] [PubMed] [Google Scholar]
- 15.Nadler SF, Steiner DJ, Erasala GN, et al. Continuous low-level heat wrap therapy provides more efficacy than ibuprofen and acetaminophen for acute low back pain. Spine. 2002;27:1012–1017. doi: 10.1097/00007632-200205150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Schulz K, Chalmers I, Hayes R, Altman D. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 17.Stein D, Peri T, Edelstein E, Elizur A, Floman Y. The efficacy of amitriptyline and acetaminophen in the management of acute low back pain. Psychosomatics. 1996;37:63–70. doi: 10.1016/S0033-3182(96)71600-6. [DOI] [PubMed] [Google Scholar]
- 18.Tulder M, Becker A, Bekkering T, Breen A, Gil del Real MT, Hutchinson A, et al. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15:S169–S191. doi: 10.1007/s00586-006-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tulder M, Furlan A, Bombardier C, Bouter L, The Editorial Board of the Cochrane Collaboration Back Review Group Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine. 2003;28:1290–1299. doi: 10.1097/00007632-200306150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Wiesel SW, Cuckler JM, Deluca F, Jones F, Zeide MS, Rothman RH. Acute low-back pain. An objective analysis of conservative therapy. Spine. 1980;5:324–330. doi: 10.1097/00007632-198007000-00006. [DOI] [PubMed] [Google Scholar]