Abstract
Retinal ganglion cells process the visual signal and transmit it along their axons in the optic nerve to the brain. Molecular, immunohistochemical, and functional analyses indicate that the majority of retinal ganglion cells express the ionotropic P2X7 receptor. Stimulation of the receptor can lead to a rise in intracellular calcium and cell death, although death does not involve the opening of a large diameter pore. Adenosine acting at A3 receptors can attenuate the rise in calcium and death accompanying P2X7 receptor activation, suggesting that dephosphorylation of ATP into adenosine is neuroprotective and that the balance of extracellular purines can influence neuronal survival. Increased intraocular pressure can lead to release of excessive extracellular ATP in the retina and damage ganglion cells by acting on P2X7 receptors, implicating a role for the receptor in the loss of ganglion cell activity in glaucoma. In summary, the activation of P2X7 receptors has both physiologic and pathophysiologic implications for ganglion cell function. These characteristics may also provide an insight into the contributions the P2X7 receptor makes to neurons elsewhere.
Keywords: A3 adenosine receptor, Excitotoxicity, Glaucoma, Neuronal death, Neuroprotection, P2X7 receptor, Retinal ganglion cells
Retinal ganglion cells are responsible for the most complex level of visual processing within the eye. Impulses travel along their axons into the optic nerve and to higher visual centers in the brain for further manipulation. Synaptic interactions in the retina with bipolar and amacrine cells, in addition to communication with the surrounding Müller and astrocytic glial cells, ensure these ganglion cells receive a variety of neurochemical stimulation. Growing evidence suggests that the P2X7 receptor for ATP contributes to the neurochemical mix and influences both the signaling and health of ganglion cells.
The presence of P2X7 receptors on retinal ganglion cells may seem at odds with the traditional view of the receptor as a harbinger of death and destruction, for ganglion cells are postmitotic cells. The neurons cannot be replaced once lost and the visual signal they convey to the brain is consequently gone. However, the recognition that P2X7 receptors are distributed throughout the nervous system and may contribute to the complex signaling between neurons and glial cells suggests these P2X7 receptors normally function in a benign fashion in adult retinal ganglion cells [1, 2].
While their abundant presence on healthy adult ganglion cells implies that the primary contribution of the P2X7 receptors is beneficial, their unique characteristics may bring pathologic consequences when the neurochemical environment in the retina becomes unbalanced. In particular, the slow inactivation of the receptor may allow for prolonged signaling under conditions of excessive agonist availability. At this point we understand more about the potentially detrimental effects of receptor stimulation than its contribution to normal visual signaling. In this regard, the elevation of intraocular pressure (IOP) may be particularly intrusive, and recent evidence suggests that P2X7 receptors may help link the excess pressure found in some forms of glaucoma with the accompanying loss of ganglion cells in the disease.
This review will describe the considerable immunocytochemical and molecular data localizing the P2X7 receptor to retinal ganglion cells, followed by the physiologic evidence for a functional receptor on the plasma membrane of these neurons. Interactions with other receptors present on ganglion cells will be discussed, and evidence for a role of the P2X7 receptor in pressure-dependent damage and retinal development will be covered. While our understanding of the contribution that P2X7 receptors make to the physiology, and pathophysiology, of retinal ganglion cells is still in the early stages, it is hoped that the integration of this information here may propel future investigations and provide lessons for other neuronal systems.
Localization of P2X7 receptor to retinal ganglion cells
The first indications that P2X7 receptors were located on retinal ganglion cells came from molecular and immunologic studies. mRNA for the P2X7 receptor was initially identified in the whole rat retina [3]. Subsequently, message for the P2X7 receptor was demonstrated to be present in material isolated from single ganglion cells with a patch pipette [4]. This implied that P2X7 receptors were present at the molecular level in individual ganglion cells.
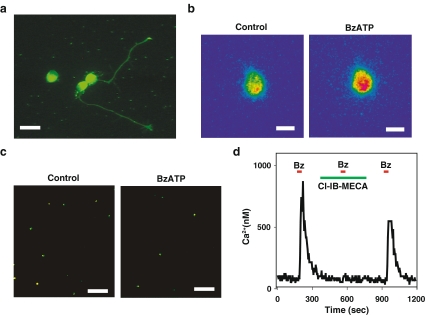
Identification of the molecular message for the P2X7 receptor was supported by immunohistochemical studies localizing the P2X7 receptor to large cells in the ganglion cell layer of the rat retina [3], with most cells on the rat ganglion cell layer staining for the receptor [5]. We have identified the P2X7 receptor to the cell body and neurites of rat ganglion cells after 1–3 days growth on coverslips, with staining for the receptor particularly high on the growth cones of the extending neurites (Fig. 1a).
Fig. 1.
The P2X7 receptor on rat retinal ganglion cells. a Immunohistochemical staining for the P2X7 receptor on rat retinal ganglion cells isolated from the retina at postnatal day (PD) 8 and grown for 2 days on a coverslip treated with poly-L-lysine and laminin. Three cell bodies are in view; neurites projecting from two cells also stained for the P2X7 receptor. Of note, the growth cones on the neurites stained brightly. Scale bar = 20 µm. Background staining produced by reaction with the coverslip coating is seen. b Pseudocolor image of a rat retinal ganglion cell loaded with the calcium indicator fura-2 and excited at 340 nm. Application of 50 µM BzATP increased the signal from the cell (with red indicating a greater intensity), indicative of an increase in intracellular calcium levels. Bar = 10 µm. c Incubation with 50 µM BzATP decreased the number of viable ganglion cells. Ganglion cells were labeled in vivo by injection of fluorogold derivative aminostilbamidine into the superior colliculus followed by retrograde transport of the dye into the ganglion cell bodies, ensuring identification of ganglion cells when dissociated retinal cells were exposed to drugs in vitro. The number of fluorescent ganglion cells remaining after 24 h in the presence of BzATP in vitro was significantly less than in control. Bar = 100 µm. d The A3 adenosine receptor attenuated the calcium rise triggered by stimulation of the P2X7 receptor. A brief application of 50 µM BzATP led to a rapid and reversible rise in calcium within a rat ganglion cell, as calibrated from the ratio of fluorescence excited at 340–380 nm in a fura-2-loaded cell. The response to BzATP was prevented by 1 µM of the A3 adenosine receptor antagonist Cl-IB-MECA, but was restored upon removal of Cl-IB-MECA
The presence of the P2X7 receptor was confirmed immunohistochemically in ganglion cells of the primate retina [6]. Most ganglion cells stained positively for the receptor in primates, with similar staining patterns for peripheral and foveal regions. Ultrastructural analysis confirmed that while membrane staining was present, the P2X7 antigen was also located in the cytoplasm near the nucleus. Many cells in the primate ganglion cell layer containing the P2X7 antigen also co-stained positive for the calcium-binding protein parvalbumin. The lack of overlap with the smaller choline acetyltransferase-stained cells in the ganglion cell layer implied that displaced amacrine cells did not express the P2X7 receptor. The presence of the receptor in retinal neurons of healthy adult eyes was interpreted as implying a role in neural transmission rather than cell death, although their actual contribution to visual processing in the healthy retina remains unclear.
It should be noted when assessing immunologic studies of the P2X7 receptor that the specificity of some antibodies raised against the receptor is uncertain, and neuronal staining has been questioned [7]. Patterns of staining in the hippocampus produced by different antibodies were inconsistent, while staining was similar in P2X7-/- and wild-type mice. However, several lines of evidence support the presence of the receptor in retinal ganglion cells. The molecular analysis of individual ganglion cells described above did not depend on a single antibody [4]. Western blots from the retina of adult rats stained a band of the predicted 70 kD [8]. Genomic analysis indicates that the P2X7 receptor exists as multiple splice variants [9], and it is possible that neuronal splice variants can react differently to particular antibodies. The physiologic data described below also support the molecular and immunologic data and strongly implicate the presence of functioning P2X7 receptors on the plasma membrane of retinal ganglion cells.
While the presence of the P2X7 receptor on retinal ganglion cells is well established, other P2Y and P2X receptors have been detected. Antibodies raised against P2Y4 and P2Y6 receptors stained the rat ganglion cell layer [10], while in situ hybridization identified the P2Y2 receptor in ganglion cells of the primate and rabbit retina [11]. Reverse transcriptase polymerase chain reaction (RT-PCR) amplification of single retinal ganglion cells identified message for P2Y1, P2Y2, P2Y4, and P2Y6 receptors [12]. Identification of these receptors in the ganglion cell layer was supported by in situ hybridization, with immunohistochemical staining confirming the presence of P2Y1, P2Y2, and P2Y4 receptors [13]. The P2X2 receptor was identified in rat retinal ganglion cells using in situ hybridization and immunohistochemistry [14]. Amplification from single rat ganglion cells identified mRNA message for P2X3, P2X4, and P2X5 receptors, with immunohistochemical staining confirmed for P2X3 and P2X4 [4]. In the mouse, staining for P2X3, P2X5, and P2X6 was found in the ganglion cell layer [15]. While the presence of multiple P2 receptors implies that purines and pyrimidines have a complex effect on visual signaling, it also emphasizes the importance of isolating the contribution from individual receptors, as we have done for the P2X7 receptor.
Physiologic effects of P2X7 receptor stimulation on retinal ganglion cells
The initial indications that P2X receptors were physiologically active on retinal ganglion cells were provided using the whole cell patch clamp technique [16]. Postnatal rat retinal ganglion cells exposed to 100–300 µM ATP displayed rapidly activating large inward cation currents, consistent with the opening of ionotropic channels. The response was heterogeneic with respect to agonist sensitivity and kinetics, suggesting multiple P2X receptors were present, although the currents generally displayed little inactivation. The pharmacologic tools available at the time of this study did not allow subtypes of P2X receptors to be accurately distinguished, but some cells showed little or no attenuation to 300 µM ATP during a 10-s exposure, consistent with activity of the P2X7 receptor [17].
A detailed analysis of the ionic currents through cloned P2X receptors indicates that a relatively high proportion of the current passing through the receptors is carried by calcium as compared to other ionotropic receptors [18]. Measurements of intracellular calcium levels in response to agonist stimulation thus provide a reasonable approach to characterizing the physiologic effects of P2X7 receptor stimulation. The P2X7 agonist BzATP produced robust elevations in the levels of calcium inside rat retinal ganglion cells (Fig. 1b) [19]. While BzATP is considered a P2X7 agonist, it is not specific and can stimulate other P2X [20] and P2Y receptors [21]. However, several observations suggest that the calcium elevations in retinal ganglion cells induced by BzATP are mediated by P2X7 receptors. Extended applications of BzATP (up to 2 min) led to sustained responses, while 15-s applications produced reversible, repeatable, and relatively consistent elevations in calcium. Both of these profiles suggested the activation of a ligand-gated channel with little inactivation, such as that displayed by cloned P2X7 receptors [22]. The response to BzATP in rat retinal ganglion cells was dependent upon extracellular calcium, ruling out G protein-linked P2Y receptors [19]. The calcium elevation was considerably higher when cells were stimulated by 10 µM BzATP than by 100 µM ATP; the relative potency of BzATP over ATP is a key characteristic of P2X7 receptors [23, 24]. The threefold increase in the response to BzATP upon removal of magnesium is similar to that described for the cloned P2X7 receptor and rules out a contribution from P2X5 receptors [20, 25]. Pharmacologic identification in rat ganglion cells was supported by the ability of 1 µM Brilliant Blue G (BBG) to completely block the calcium elevations triggered by BzATP; the neuroprotective effects of 100 nM BBG described below, combined with the lack of a contribution from the P2X5 receptor, support the inhibition as specific for the P2X7 receptor.
Stimulation of the P2X7 receptor kills retinal ganglion cells
Sustained elevations in intracellular calcium can frequently be hazardous to neurons, initiating a series of pathologic steps that can lead to the activation of an apoptotic cascade [26]. To determine whether the large, sustained calcium elevations accompanying P2X7 receptor stimulation were capable of killing rat retinal ganglion cells, the effect of BzATP on cell survival was determined [19]. Ganglion cells were labeled by injection of aminostilbamidine into the superior colliculus, with dye delivered to the retinal cell bodies of ganglion cells several days later by retrograde transport. The retina was dissociated and cultured in the presence or absence of drugs for various intervals, with ganglion cell survival determined by comparing the number of fluorescent cells remaining in control solution with those treated (Fig. 1c).
Stimulation of the P2X7 receptor with BzATP killed retinal ganglion cells as compared to control levels [19]. Neuronal loss associated with BzATP was detected within a few hours and increased with exposure time up to 48 h, when 60% of the ganglion cells were lost. The death was dose dependent, with an EC50 of 30 µM BzATP. Increasing the concentration of BzATP to 500 mM only killed 50% of the neurons at 24 h, suggesting that the vulnerable cells represented only a subset of the total ganglion cells. This contrasts with the immunologic identification of the P2X7 receptor on most ganglion cells [6, 5] and suggests that an additional as yet unknown factor is selectively present in the susceptible cells.
Pharmacologic characterization was used to confirm that the lethal actions of BzATP were mediated by the P2X7 receptor [19]. The death triggered by 50 µM BzATP over 24 h was completely inhibited by 100 µM oxidized ATP. While block by oxidized ATP is used to identify P2X7 receptor activity, it is not specific [27]. However, the lethal effects of BzATP were also inhibited by 100 nM BBG. At this concentration, BBG inhibits only 5% of the current through the P2X5 receptor [20] and is generally considered specific for the receptor P2X7 receptor [22]. Further confirmation came from the lack of inhibition by 30 µM suramin [19]. This concentration of suramin inhibits P2Y1, P2Y2, P2X1, P2X2, P2X3, and P2X5 receptors, but the drug has an IC50 of 500 µM at P2X7 receptors [24, 28]. When combined with the characterization of the calcium response, the pharmacologic profile makes it highly likely that the lethal response to BzATP in rat retinal ganglion cell in vitro is mediated by the P2X7 receptor.
These in vitro experiments demonstrating that stimulation of the P2X7 receptor can injure retinal ganglion cells are consistent with in vivo experiments showing a role for the receptor in the loss of ganglion cells in development [5] and in response to elevated pressure [29]. These interesting applications are discussed in more detail below. Further support for a lethal role for the P2X7 receptor in vivo comes from preliminary data demonstrating that the death of rat retinal ganglion cells triggered by intravitreal injection of BzATP can be prevented by BBG [30]. While the elevation of cellular calcium in isolated ganglion cells implies that the pathologic pathways are contained within ganglion cells, it is likely that other cells may contribute to secondary damage in vivo. For example, noxious metabolites released from glial cells in response to excess ATP may themselves damage ganglion cells.
Large pore not involved in death of retinal ganglion cells
In many peripheral cells, stimulation of the P2X7 receptor leads rapidly to the opening of a large pore permeable to fluorescent dyes such as Yo-Pro-1 [25]. This link between channel and pore can be so close that it was once thought the ionotropic channel progressively dilated until its internal diameter was sufficient to accommodate the bulky dyes and that the opening of this pore was necessary for cell death [31]. More recent evidence supports a model where the channel and the pore are distinct entities. The link between P2X7 receptor stimulation and cell death can be interrupted by interfering with second messenger pathways such as MAP kinases and calcium [32]. The C terminus of the P2X7 receptor is necessary for the increased permeability and interacts with epithelial membrane proteins which are themselves capable of killing cells [33]. Of particular interest is the report that stimulation of the P2X7 receptor opens the pannexin-1 hemichannel, suggesting that this pannexin may itself act as the pore in some cases [34].
While the historical association between the P2X7 receptor and the pore in peripheral cells is well established, pore formation may be less relevant to the effects of the receptor in neurons. It is clear that the death of retinal ganglion cells following P2X7 receptor stimulation does not involve pore formation. Stimulation of the P2X7 receptor in a population of mixed retinal cells did not make the ganglion cells permeable to Yo-Pro-1 [19]. The uptake of dye by smaller cells provided a positive control and reinforced the lack of permeability in ganglion cells. The findings of Innocenti et al. support this, as they demonstrate that BzATP added to retinal whole mounts selectively increased the permeability of only microglial cells to Yo-Pro-1 [35]. Although many retinal neurons in their preparation, including ganglion cells, contained the P2X7 receptor, only microglial cells took up dye. This is consistent with an emerging pattern where the P2X7 receptor induces death of neurons in particular without pore formation [1]. The mechanisms connecting the P2X7 receptor with ganglion cell death are currently unclear but may involve the apoptotic pathway, as receptor stimulation led to the activation of caspases-3 [19]. Preliminary evidence implicates the N-methyl-D-aspartate (NMDA) receptor and excitotoxicity downstream from the P2X7 receptor [36], but this remains to be confirmed.
Adenosine acting at A3 receptors prevents P2X7-mediated death
Extracellular ATP is rapidly dephosphorylated to adenosine in the space surrounding retinal ganglion cells [37, 38]. As such, the relative effects of ATP and adenosine must be considered when placing the effects of P2X7 receptor stimulation within a physiologic context. While stimulation of the P2X7 receptor repeatedly killed retinal ganglion cells, incubation with ATP actually increased the proportion of surviving cells as compared to control [39]. This apparent contradiction was explained by the ability of the slowly hydrolyzable ATP analogue ATPγS to mimic the actions of BzATP. This implied that the hydrolysis of ATP not only prevented its lethal actions, but produced a formulation that prevented the ganglion cell loss that occurs normally in neuronal culture. As ATP can be rapidly dephosphorylated into adenosine, and as adenosine has protective actions in many neurons, the contribution of adenosine to this protection was tested directly. Adenosine prevented both the rise in calcium and the cell death accompanying stimulation of the P2X7 receptor [39]. The adenosine deaminase inhibitor EHNA also reduced the number of surviving cells; as adenosine deaminase converts adenosine into inosine this is consistent with a protective action of inosine.
The effects of adenosine to attenuate the responses to P2X7 receptor stimulation were mediated, at least in part, by the A3 receptor. The ability of adenosine to inhibit the calcium rise triggered by BzATP by adenosine was partially reversed by the selective A3 adenosine receptor antagonist MRS1191 [40], suggesting at least some of the neuroprotective actions of adenosine required a functioning A3 receptor [39]. This was supported by experiments with the A3 adenosine receptor agonists IB-MECA and Cl-IB-MECA, which stopped both the calcium rise and the ganglion cell death accompanying stimulation of the P2X7 receptor (Fig. 1d). The relatively selective actions of inosine at A3 receptors, as compared to A1 and A2 receptors, combined with the effects of EHNA discussed above, support a neuroprotective role for the A3 receptor [41].
While the pharmacologic tools clearly implied that the A3 adenosine receptor protected ganglion cells from the detrimental effects of P2X7 receptor activation, it was necessary to identify the A3 adenosine receptor in ganglion cells on a molecular level, as an initial in situ hybridization study failed to find message for the A3 adenosine receptor anywhere in the eye [42]. The A3 adenosine receptor promoter was subsequently localized to retinal ganglion cells [43], and mRNA message for the A3 adenosine receptor itself was confirmed using traditional and real-time PCR in cells obtained from the nerve fiber layer as well as from immunopurified ganglion cells [44]. The A3 adenosine receptor was thus recognized as an appropriate target to attenuate the pathologic actions of the P2X7 receptor in retinal ganglion cells. Although similarities suggest the A1 receptor may also be protective [45], this has yet to be shown directly for P2X7 receptor stimulation.
The precise point where the A3 adenosine receptor intervenes to protect ganglion cells from pathologic effects of P2X7 receptor stimulation is currently unclear, although parallels with the NMDA glutamate receptors and the A1 adenosine receptors suggest adenosine may act at voltage-dependent calcium channels. Activation of the A1 receptor leads to direct binding of the Gβγ protein to the CaVα subunit of the calcium channel, with resulting loss of current flow [46]. The A1 adenosine receptor has been shown to inhibit voltage-dependent calcium channels in salamander retinal ganglion cells [47] and to prevent the influx of calcium after activation of NMDA channels by glutamate in rat cells [45]. As activation of both NMDA [48] and P2X7 [49] channels can lead to a secondary opening of voltage-dependent calcium channels, it is possible that adenosine acts to limit the effects of P2X7 receptor stimulation at this common downstream route, although this has yet to be demonstrated for the A3 receptor. The ability of the L-type calcium channel blocker nifedipine to reduced the death of ganglion cells following exposure to BzATP does implicate calcium channels in the response and make this scenario more likely [19], although additional interactions between A3 and P2X7 receptors cannot be ruled out.
Pressure-dependent activation of P2X7 receptors on ganglion cells
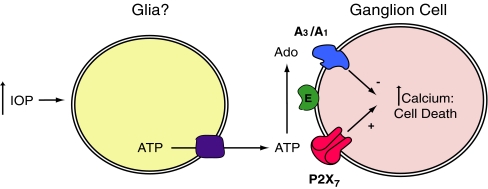
Elevated IOP is frequently associated with the loss of retinal ganglion cells in glaucoma. While the causal connections between pressure rise and neuronal death remain elusive, accumulating evidence suggests that the P2X7 receptor may contribute to ganglion cell loss. Mechanical distention is one of the most effective triggers for release of ATP from multiple cell types [50], with changes in hydrostatic pressure [51] and cell swelling [52] capable of initiating release. Within the retina, slight mechanical pressure on the inner limiting membrane activates the release of ATP from Müller cells in close proximity to retinal ganglion cells [37, 38]. Given that stimulation of the P2X7 receptor can be lethal for ganglion cells, and that elevated pressure can lead to an excessive release of ATP, we have hypothesized that the P2X7 receptor could link the elevated pressure of glaucoma to the pathologic changes and even death of ganglion cells (Fig. 2).
Fig. 2.
Schematic model describing the hypothesized relationship between elevated intraocular pressure (IOP), excess extracellular ATP, P2X7 and adenosine receptors, and ganglion cell death. The increased levels of calcium inside ganglion cells following stimulation of the P2X7 receptor may lead to cell death but may also damage cell signaling under chronic conditions. The dephosphorylation of ATP by ectonucleotidases (E) produces adenosine that is neuroprotective by acting at A3 (and possibly A1) receptors to limit the rise in calcium and cell death. The converse relationship between the harmful effects of excess ATP and the beneficial actions of adenosine suggests the health of retinal ganglion cells is influenced by the balance of purines in extracellular space. The depiction of glial cells as the source of pressure-dependent ATP is based on the findings of Newman [37, 38], although it remains to be determined whether alternative sources contribute to extracellular ATP levels under sustained periods of elevated pressure. The identity of the conduit for ATP exit is presently unknown
Several observations suggest a role of the P2X7 receptor in pressure-related ganglion cell death. In a pivotal study, examination of individual rat ganglion cells labeled within a retinal whole mount demonstrated that rapid elevation in hydrostatic pressure above 50 mmHg led to blebbing of the soma and dendrites [29]. This damage was more conspicuous when pressure was repetitively raised, and the degree of damage to ganglion cells corresponded to the magnitude of pressure increase. The soluble ecto-ATDPase apyrase and oxidized ATP eliminated the ganglion cell damage in response to pressure elevation. Incubation with 500 nM BBG also prevented damage, strongly implicating P2X7 receptor involvement. ATP itself mimicked the damage that accompanied raised pressure, with the injury similarly blocked by BBG.
The link between pressure and ATP seems to be maintained in vivo, as elevation of IOP to 50 mmHg increased the number of retinal ganglion cells permeable to propidium iodide [29]. Increased IOP was accompanied by a fivefold elevation in vitreal levels of ATP, and the number of ganglion cells permeable to propidium iodide was significantly reduced by dephosphorylating ATP with apyrase. Of particular interest, transient pressure increases led to a temporary loss in light-triggered spike activity in the optic tract; the duration of this loss in transmission was significantly reduced by apyrase, even though apyrase did not alter baseline levels of spike activity. Together, these findings imply that levels of extracellular ATP are elevated in response to acute increases in IOP in vivo and that ATP, acting at P2X7 receptors, mediates damage to the soma, dendrites, and axons of retinal ganglion cells. Furthermore, they suggest that excess ATP can interfere with the transmission of the visual signal. This may prove critical as some of the loss in ganglion cell function in glaucoma is reversible upon reduction of IOP [53]. Excessive extracellular ATP, and perhaps stimulation of P2X7 receptors, may thus interfere with transmission of the visual signal long before the neurons actually die.
These findings may well have implications for humans, as patients with acute elevations in IOP had substantially increased levels of ATP in the aqueous chamber [54]. The ATP levels were proportional to the magnitude of the pressure increase and were sustained. While ATP in the aqueous humor is unlikely to diffuse to the retina without considerable dephosphorylation, this does establish that extracellular levels of ATP do rise in the human eye in response to elevated pressure. Whether this response occurs in more chronic elevations in humans remains to be determined. Recent evidence suggesting that ATP is released by the retina in response to pressure increases as low as 20 mmHg supports the link between ocular pressure and ATP at more moderate pressures [55].
P2X7 receptors and ganglion cell age
The stable expression of the P2X7 receptor in adult ganglion cells has been clearly demonstrated, with immunohistochemical localization found on 120-day-old rats [3]. However, levels of the P2X7 receptor in the retina do change with age. While mRNA message for the P2X7 receptor was detected in rat retinas throughout life, expression was higher in neonatal retinas; expression at postnatal day (PD) 2 was higher than on PD 7, although staining at PD 7 was similar to that found at PD 14 and in adult rats, suggesting expression stabilized around PD 7 [35]. While the loss of cholinergic cells in the ganglion cell layer over PD 1–2 was reduced by blocking P2X7 receptors, these cells were likely related to displaced amacrine cells not ganglion cells, and block did not change the number of cells identified as retinal ganglion cells over this period [5].
On the other end of the age spectrum, it will be interesting to determine whether changes in levels of extracellular ATP or the P2X7 receptor contribute to the increased susceptibility older ganglion cells show with regards to elevated IOP. The proportion of ganglion cells lost in response to a given increase in IOP was greater for older rats than young adults [56]. This may have implications for the enhanced damage done by elevated ocular pressure in older humans. While the P2X7 receptor contributed to pressure-induced damage in ganglion cells from young adult rats, these cells recovered from moderate acute rises in pressure [29]. Whether older rats differ in the amount of ATP released by a given amount of pressure, the levels of P2X7 receptor expression, or the damage done by receptor stimulation remains to be determined, but this may be an important avenue for future research.
Conclusions
This review has attempted to summarize our current understanding of the P2X7 receptor in retinal ganglion cells. The confluence of molecular, immunohistochemical, and physiologic data clearly demonstrates that the P2X7 receptor is present in healthy adult ganglion cells. The broad extent of receptor expression implies an important role for the receptor in normal visual processing, and the elucidation of this role should provide a fertile area of research in retinal neuroscience. The evidence from several laboratories for a link between elevated pressure and excess extracellular ATP in the retina, combined with in vitro and in vivo data showing that stimulation of the P2X7 receptor can damage ganglion cells, suggests the receptor may play a role in the pressure-dependent pathologies such as glaucoma. Whether this relationship applies to chronic as well as acute situations is currently being investigated. The effects of P2X7 receptor stimulation on transmission of the visual signal may provide some insight in this regard, given the reversibility of vision loss upon pressure reduction and the long delay between the initial elevation in pressure and permanent vision loss in chronic glaucoma. The neuroprotective effects of adenosine suggest that the dephosphorylation of ATP can act in multiple ways to limit damage from the P2X7 receptor to retinal ganglion cells. Upregulation of ectonucleotidases may be an adaptive response to enhance the neuroprotective actions of adenosine under chronic conditions and may have therapeutic advantages. The combination of both beneficial and pathologic roles of the P2X7 receptor in retinal ganglion cells, of the contrasting effects of ATP and adenosine, and of the role pressure may play in the relationship that make the situation in retinal ganglion cells so fascinating can provide multiple lessons for the P2X7 receptor in neurons elsewhere.
Acknowledgements
This work was supported by grants EY-015537 and EY-013434 from the NIH to CHM, and EY-01583 to the Vision Research Center of the University of Pennsylvania, by a University Research Foundation award from the University of Pennsylvania to CHM, by the Jody Sack Fund to XZ, MZ, and HH. The authors would like to thank Alan Laties for many useful discussions.
References
- 1.Anderson C, Nedergaard M (2006) Emerging challenges of assigning P2X(7) receptor function and immunoreactivity in neurons. Trends Neurosci 29:257–262 [DOI] [PubMed]
- 2.Sperlagh B, Vizi ES, Wirkner K, Illes P (2006) P2X7 receptors in the nervous system. Prog Neurobiol 78:327–346 [DOI] [PubMed]
- 3.Brandle U, Kohler K, Wheeler-Schilling TH (1998) Expression of the P2X7-receptor subunit in neurons of the rat retina. Brain Res Mol Brain Res 62:106–109 [DOI] [PubMed]
- 4.Wheeler-Schilling TH, Marquordt K, Kohler K, Guenther E et al (2001) Identification of purinergic receptors in retinal ganglion cells. Brain Res Mol Brain Res 92:177–180 [DOI] [PubMed]
- 5.Resta V, Novelli E, Di Virgilio F, Galli-Resta L (2005) Neuronal death induced by endogenous extracellular ATP in retinal cholinergic neuron density control. Development 132:2873–2882 [DOI] [PubMed]
- 6.Ishii K, Kaneda M, Li H, Rockland KS et al (2003) Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J Comp Neurol 459:267–277 [DOI] [PubMed]
- 7.Sim JA, Young MT, Sung HY, North RA et al (2004) Reanalysis of P2X(7) receptor expression in rodent brain. J Neurosci 24:6307–6314 [DOI] [PMC free article] [PubMed]
- 8.Puthussery T, Fletcher EL (2004) Synaptic localization of P2X7 receptors in the rat retina. J Comp Neurol 472:13–23 [DOI] [PubMed]
- 9.Cheewatrakoolpong B, Gilchrest H, Anthes JC, Greenfeder S (2005) Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Comm 332:17–27 [DOI] [PubMed]
- 10.Pintor P, Sánchez-Nogueiro J, Irazu M, Mediero A et al (2004) Immunolocalisation of P2Y receptors in the rat eye. Purinergic Signal 1:83–90 [DOI] [PMC free article] [PubMed]
- 11.Cowlen MS, Zhang VZ, Warnock L, Moyer CF et al (2003) Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res 77:77–84 [DOI] [PubMed]
- 12.Fries JE, Wheeler-Schilling TH, Kohler K, Guenther E (2004) Distribution of metabotropic P2Y receptors in the rat retina: a single-cell RT-PCR study. Brain Res Mol Brain Res 130:1–6 [DOI] [PubMed]
- 13.Fries JE, Wheeler-Schilling TH, Guenther E, Kohler K (2004) Expression of P2Y1, P2Y2, P2Y4, and P2Y6 receptor subtypes in the rat retina. Invest Ophthalmol Vis Sci 45:3410–3417 [DOI] [PubMed]
- 14.Greenwood D, Yao WP, Housley GD (1997) Expression of the P2X2 receptor subunit of the ATP-gated ion channel in the retina. Neuroreport 8:1083–1088 [DOI] [PubMed]
- 15.Shigematsu Y, Shimoda Y, Kaneda M (2007) Distribution of immunoreactivity for P2X3, P2X5, and P2X6-purinoceptors in mouse retina. J Mol Histol 38:369–371 [DOI] [PubMed]
- 16.Taschenberger H, Juttner R, Grantyn R (1999) Ca2+-permeable P2X receptor channels in cultured rat retinal ganglion cells. J Neurosci 19:3353–3366 [DOI] [PMC free article] [PubMed]
- 17.North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed]
- 18.Egan TM, Khakh BS (2004) Contribution of calcium ions to P2X channel responses. J Neurosci 24:3413–3420 [DOI] [PMC free article] [PubMed]
- 19.Zhang X, Zhang M, Laties AM, Mitchell CH (2005) Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci 46:2183–2191 [DOI] [PubMed]
- 20.Bo X, Jiang LH, Wilson HL, Kim M et al (2003) Pharmacological and biophysical properties of the human P2X5 receptor. Mol Pharmacol 63:1407–1416 [DOI] [PubMed]
- 21.Fedorov IV, Rogachevskaja OA, Kolesnikov SS (2007) Modeling P2Y receptor-Ca2+ response coupling in taste cells. Biochim Biophys Acta 1768:1727–1740 [DOI] [PubMed]
- 22.Jiang LH, Mackenzie AB, North RA, Surprenant A (2000) Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol 58:82–88 [PubMed]
- 23.Bianchi BR, Lynch KJ, Touma E, Niforatos W et al (1999) Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol 376:127–138 [DOI] [PubMed]
- 24.North RA, Surprenant A (2000) Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40:563–580 [DOI] [PubMed]
- 25.Surprenant A, Rassendren F, Kawashima E, North RA et al (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738 [DOI] [PubMed]
- 26.Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276 [DOI] [PubMed]
- 27.Beigi RD, Kertesy SB, Aquilina G, Dubyak GR (2003) Oxidized ATP (oATP) attenuates proinflammatory signaling via P2 receptor-independent mechanisms. Br J Pharmacol 140:507–519 [DOI] [PMC free article] [PubMed]
- 28.von Kügelgen I (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432 [DOI] [PubMed]
- 29.Resta V, Novelli E, Vozzi G, Scarpa C et al (2007) Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci 25:2741–2754 [DOI] [PubMed]
- 30.Hu H, Lu W, Laties AM, Mitchell CH (2008) Stimulation of P2X7 receptor kills rat retinal ganglion cells in vivo. Invest Ophthalmol Vis Sci 49:ARVO E-Abstract 2063
- 31.Virginio C, MacKenzie A, North RA, Surprenant A (1999) Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol 519:335–346 [DOI] [PMC free article] [PubMed]
- 32.Faria RX, Defarias FP, Alves LA (2005) Are second messengers crucial for opening the pore associated with P2X7 receptor? Am J Physiol Cell Physiol 288:C269–C271 [DOI] [PubMed]
- 33.Wilson HL, Wilson SA, Surprenant A, North RA (2002) Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus. J Biol Chem 277:34017–34023 [DOI] [PubMed]
- 34.Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed]
- 35.Innocenti B, Pfeiffer S, Zrenner E, Kohler K et al (2004) ATP-induced non-neuronal cell permeabilization in the rat inner retina. J Neurosci 24:8577–8583 [DOI] [PMC free article] [PubMed]
- 36.Mitchell CH, Zhang M, Zhang X, Lu W et al (2006) Neuronal death evoked by the P2X7 receptor mediated by the NMDA receptor. Invest Ophthalmol Vis Sci 47:ARVO E-abstract 2589
- 37.Newman EA (2003) Glial cell inhibition of neurons by release of ATP. J Neurosci 23:1659–1666 [DOI] [PMC free article] [PubMed]
- 38.Newman EA (2001) Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J Neurosci 21:2215–2223 [DOI] [PMC free article] [PubMed]
- 39.Zhang X, Zhang M, Laties AM, Mitchell CH (2006) Balance of purines may determine life or death as A3 adenosine receptors prevent loss of retinal ganglion cells following P2X7 receptor stimulation. J Neurochem 98:566–575 [DOI] [PubMed]
- 40.Jacobson KA, Park KS, Jiang JL, Kim YC et al (1997) Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology 36:1157–1165 [DOI] [PMC free article] [PubMed]
- 41.Jin X, Shepherd RK, Duling BR, Linden J (1997) Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest 100:2849–2857 [DOI] [PMC free article] [PubMed]
- 42.Kvanta A, Seregard S, Sejersen S, Kull B et al (1997) Localization of adenosine receptor messenger RNAs in the rat eye. Exp Eye Res 65:595–602 [DOI] [PubMed]
- 43.Yaar R, Lamperti ED, Toselli PA, Ravid K (2002) Activity of the A3 adenosine receptor gene promoter in transgenic mice: characterization of previously unidentified sites of expression. FEBS Lett 532:267–272 [DOI] [PubMed]
- 44.Zhang M, Budak MT, Lu W, Khurana TS et al (2006) Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol Vis 12:937–948 [PubMed]
- 45.Hartwick ATE, Lalonde MR, Barnes S, Baldridge WH (2004) Adenosine A(1)-receptor modulation of glutamate-induced calcium influx in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 45:3740–3748 [DOI] [PubMed]
- 46.Dolphin AC (2003) G protein modulation of voltage-gated calcium channels. Pharmacol Rev 55:607–627 [DOI] [PubMed]
- 47.Sun X, Barnes S, Baldridge WH (2002) Adenosine inhibits calcium channel currents via A1 receptors on salamander retinal ganglion cells in a mini-slice preparation. J Neurochem 81:550–556 [DOI] [PubMed]
- 48.Calton JL, Kang MH, Wilson WA, Moore SD (2000) NMDA-Receptor-dependent synaptic activation of voltage-dependent calcium channels in basolateral amygdala. J Neurophysiol 83:685–692 [DOI] [PubMed]
- 49.Brater M, Li SN, Gorodezkaya IJ, Andreas K et al (1999) Voltage-sensitive Ca2+ channels, intracellular Ca2+ stores and Ca2+-release-activated Ca2+ channels contribute to the ATP-induced [Ca2+]i increase in differentiated neuroblastoma x glioma NG 108–15 cells. Neurosci Lett 264:97–100 [DOI] [PubMed]
- 50.Burnstock G (1999) Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194:335–342 [DOI] [PMC free article] [PubMed]
- 51.Ferguson D, Kennedy I, Burton T (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol 505:503–511 [DOI] [PMC free article] [PubMed]
- 52.Reigada D, Mitchell C (2005) Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol 288:C132–C140 [DOI] [PubMed]
- 53.Ventura LM, Porciatti V (2005) Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: a pilot study. Ophthalmology 112:20–27 [DOI] [PMC free article] [PubMed]
- 54.Zhang X, Li A, Ge J, Reigada D et al (2007) Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res 85:637–643 [DOI] [PubMed]
- 55.Reigada D, Lu W, Zhang M, Mitchell CH (2008) Elevated pressure triggers a physiological release of ATP from the retina: possible role for pannexin hemichannels. Neuroscience doi:10.1016/j.neuroscience.2008.08.036 [DOI] [PMC free article] [PubMed]
- 56.Cepurna WO, Jia L, Johnson EC, Morrison JC (2006) Aging increases susceptibility to intraocular pressure (IOP) and alters retinal gene expression responses in the rat. Invest Ophthalmol Vis Sci 47:ARVO E-abstract 1243