Abstract
Periodontal diseases are initiated by Gram-negative tooth-associated microbial biofilms that elicit a host response, with resultant osseous and soft tissue destruction. In response to endotoxins derived from periodontal pathogens, several osteoclast-related mediators target the destruction of alveolar bone and supporting connective tissues. Major drivers of this aggressive tissue destruction are matrix metalloproteinases (MMPs), cathepsins, and other osteoclast-derived enzymes. This article focuses on the downstream factors of the osteoclast responsible for the degradation of bone and soft tissues around teeth and oral implants. Furthermore, therapeutic approaches that target MMP-2, -8, and -9 inhibition, such as MMP inhibitors, chemically modified tetracyclines, and subantimicrobial formulations of tetracycline analogues, are discussed. The use of rapid, chair-side tests of MMP activity, in particular for MMP-8 and bone collagen fragments, show strong potential as non-invasive measures of tissue health or disease. In addition, studies using other agents for the preservation of bone mass, such as bisphosphonates that inhibit osteoclast recruitment, are highlighted. The application of these bone-preservation strategies to periodontal management and treatment are discussed in the context of high-risk patients susceptible to disease reactivation or disease complications.
Keywords: Bisphosphonates, bone resorption, matrix metalloproteinases, periodontitis
Periodontal diseases, which cause the destruction of the supporting structures of the dentition, are common chronic infectious diseases of the oral cavity. They are initiated by Gram-negative tooth-associated pathogens organized as a biofilm, whose presence elicits a host inflammatory response. Although gingivitis represents the reversible inflammatory reaction to biofilms, periodontitis is the non-reversible destructive stage of a persistent bacterial infection. If left untreated, periodontitis results in soft tissue and progressive bone destruction and leads to tooth mobility and subsequent tooth loss.1 Recently, there has been a great deal of basic and clinical research focusing on the underlying mechanisms of the major enzymatic drivers of this aggressive tissue destruction. Along with briefly discussing the pathology of chronic periodontitis and its main players, this article focuses on promising therapeutic agents for the tissue destruction of periodontitis; i.e., using matrix metalloproteinase (MMP) inhibitors as host modulatory agents, and bisphosphonates as blockers of tooth-supporting alveolar bone destruction. Together, improved appreciation of such therapeutic strategies may ultimately lead to a more individualized targeted treatment for a disease of which 31% of the United States population exhibits mild forms, 13% display moderate severity, and 4% have advanced disease symptoms.2
PATHOGENIC PROCESSES IN PERIODONTAL DISEASE
Acting as the prototypical endotoxin, lipopolysaccharides (LPS), a major component of the outer membrane of Gram-negative bacteria, initiate the cascade of events leading to periodontal tissue destruction.1 Briefly, LPS derived from plaque biofilms on the tooth root surface lead to the recruitment of polymorphonuclear leukocytes (PMNs) to the site. Monocytes and activated macrophages respond by releasing various proinflammatory cytokines, including interleukin (IL)-1β and tumor necrosis factor (TNF)-alpha, which, in turn, direct further destructive processes. Along with cathepsins and other osteoclast-derived mediators of bone resorption, one group of powerful endopeptidases released by fibroblasts and PMNs at this stage is MMPs. Specific members of the MMP family have become attractive targets for therapeutic intervention. As such, it is worth examining their physiological functions in greater detail, because their role in periodontitis is complex.
MMPs: Tissue destruction and beyond
Proteolytic enzymes are implicated in a number of processes in normal bone remodeling, including bone resorption and bone formation.3 The activity of osteoclast-secreted proteolytic enzymes, such as the MMPs, is essential to normal bone homeostasis. Such MMPs are responsible for the destruction of mineralized tissue during bone resorption. In contrast, osteoblasts also secrete MMPs that degrade the nonmineralized osteoid layer on the surface of bone.3
The MMP multigene family encodes 22 structurally related endopeptidases with activity against most extracellular matrix, pericellular, and non-matrix macromolecules.4 As components of the greater human “degradome,” they can be divided into a number of subclasses according to their substrate specificities and physical structure: interstitial collagenases, gelatinases, membrane-type MMPs, and other MMPs that include stromelysins and metalloelastases (Fig. 1). MMPs play key roles in the degradation of various extracellular molecules, including collagen, elastin, proteoglycans, and laminins.5 Although beyond the scope of this article, it is worth noting that MMPs have other significant roles in wound healing and immunity, in the pathology of tumor progression in cancer, and in fibrosis.3,6 A role that has been considered of great clinical importance in periodontitis is the ability of MMPs to activate latent forms of effector proteins, such as antimicrobial peptides, chemokines, and cytokines, as well their role in altering protein function, such as shedding of cell-surface proteins.6 There are a great number of chemokines that are proteolytically processed by various MMPs during wound healing and inflammation, resulting in subsequent modifications to chemokine function(Table1). Forexample,MMP-8 is a critical mediator initiating LPS responsiveness in vivo. MMP-8 cleaves LPS-induced CXC chemokine (LIX). PMN-derived MMP-8 cleaves and activates LIX to execute an in cis PMN-controlled feed-forward mechanism to orchestrate the initial inflammatory response and promote LPS responsiveness in periodontal tissue.7 These processes may include complete degradation of the chemokine, the creation of receptor antagonists, or the stimulation of dramatic increases in chemokine activity.8 Regardless, because MMPs can govern the activity of various effectors and other biologically active molecules by methods such as direct cleavage or by modification or inactivation of their inhibitors, they can be considered host-modulatory agents.4 As such, and depending on the tissue, its complexity, and the exposure to particular pathogens, these actions have great implications on disease progression. For example, in tumor progression, various MMPs directly or indirectly have key roles during growth, survival, angiogenesis, invasion, inflammation, and repair.9 As a result, in part, of this functional complexity, long-term therapies that have been developed to block the activity of MMPs during the progression of cancer have generally failed in the clinic. Nonetheless, because MMPs remain among the key mediators of irreversible tissue destruction in periodontitis, a study10 was undertaken that examined their potential as a biomarker, and thus as a modulator, of disease progression.
Figure 1.
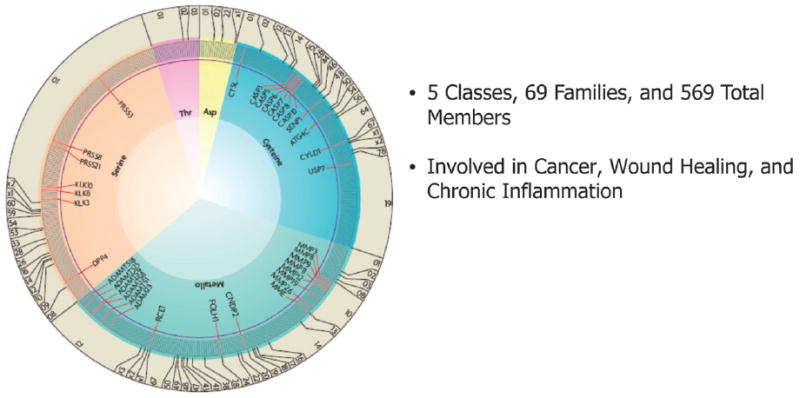
Distribution of proteases in the human degradome involved in tumorogenesis, wound repair, and tissue destruction. A gray line indicates each individual enzyme, and those with tumor-protective properties are shown in red. Numbers at the edge represent different protease families of each catalytic class according to MEROP database numbering. Adapted with permission from Macmillian Publishers, copyright 2007.9
Table 1.
Chemokines Are Proteolytically Processed by MMPs During Wound Healing and Inflammation
| Chemokine | MMP | ADAM | Legend | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 7 | 8 | 9 | 13 | 14 | 10 | 17 | ||
| CCL2(MCP1) | Not tested | ||||||||||
| CCL5 (RANTES) | Not cleaved | ||||||||||
| CCL7 (MCP3) | Unknown effect | ||||||||||
| CCL8 (MCP2) | |||||||||||
| CCL13(MCP4) | Variable effect | ||||||||||
| CXCL1 (KC) | |||||||||||
| CXCL1 (GROα) | Increases activity | ||||||||||
| CXCL2 (MIP2) | |||||||||||
| CXCL2 (GROβ) | Decreases activity | ||||||||||
| CXCL2 (DCIP1) | |||||||||||
| CXCL2 (GROγ) | Degraded | ||||||||||
| CXCL4 (PF4) | Creates receptor antagonist | ||||||||||
| CXCL5 (LIX) | ◦ | ||||||||||
| CXCL5 (ENA78) | ◦ | ||||||||||
| CXCL6 (GCP2) | Creates toxic protein | ||||||||||
| CXCL7 (NAP2) | |||||||||||
| CXCL8 (1L8) | ◦ | ||||||||||
| CXCL12 (SDF1) | • | • = tested in vivo | |||||||||
| CXCL16 | |||||||||||
Reprinted with permission from Elsevier.8
MMPs as biomarkers of periodontal diseases
The number of publications investigating the role of MMPs in periodontal disease progression and expression continue to grow. Similar to the use of collagen telopeptide fragments, such as pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP), as biomarkers of bone degradation,11 a recent focus has been on the diagnostic usefulness of measuring levels of MMPs as a biomarker of periodontal severity and as a response to therapy (Fig. 2).1 Of the several biomarkers that have been studied, one of the strongest potential candidates in point-of-care (POC) tests is MMP-8, the most prevalent MMP in diseased periodontal tissue and saliva.11 Recently, a portable diagnostic device has been developed (based on the principle of rapid saliva diagnosis at the POC) called the Integrated Microfluidic Platform for Oral Diagnostics (IMPOD).12 An early clinical study13 in which the hand-held IMPOD rapidly (3 to 10 minutes) measured the concentrations of MMP-8 and other biomarkers in small amounts (10 μl) of saliva was reported. The mean MMP-8 concentration in the saliva of the periodontally healthy individuals was 10-fold less than that of the periodontally diseased patients. Thus, the use of such immunoassay technologies in measuring a putative biomarker of periodontal disease in saliva may permit rapid accurate POC diagnoses, dynamic monitoring of disease activity, and potentially a more effective treatment.12 Clearly, MMPs are promising candidates for predicting, diagnosing, and, possibly more importantly, assessing the progression of this episodic disease.1,13 Furthermore, the use of rapid, chair-side tests of MMP activity showed strong potential as a non-invasive measure of tissue health or disease, because MMPs are clearly associated with progressive periodontitis.14
Figure 2.
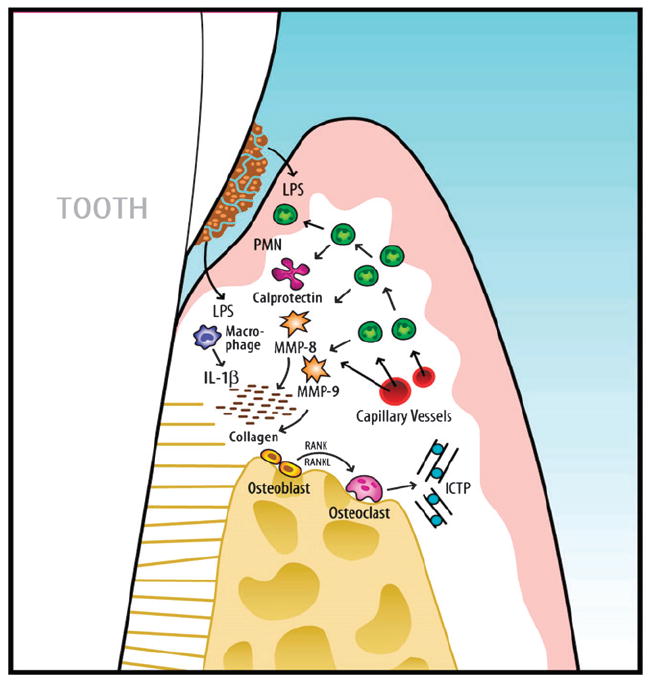
Schematic overview of the key biomarkers related to periodontal disease progression. Initial events are triggered by LPS from Gram-negative plaque biofilms on the periodontal tissues. As a first line of defense, PMNs are recruited to the site. Monocytes and activated macrophages respond to endotoxin by releasing cytokines (TNF and IL-1) that direct further destruction processes. MMPs, which can act as powerful collagen-destroying enzymes, are produced by fibroblasts and PMNs. TNF, IL-1, and receptor activator of nuclear factor-kappa B ligand (RANKL) are elevated in active sites and mediate osteoclastogenesis and bone breakdown. Bone-specific markers, such as ICTP, are released into the surrounding area and transported by way of gingival crevicular fluid into the sulcus or pocket and serve as potential biomarkers for periodontal disease detection. Adapted with permission from Blackwell Publishing.1
POTENTIAL THERAPEUTIC STRATEGIES IN PERIODONTAL DISEASE
After considering the role of MMPs in periodontal disease, it is clear that agents that directly or indirectly block the activity of active osteoclasts may represent potential “bone-sparing” therapies. There are many potential therapeutic targets during the progression of periodontal lesions, from the prestimulated monocyte to the postactivated osteoclast, along with the multitude of cytokines produced by these cells (Fig. 3).15 The unifier of these potential targets is their shared role in the inflammatory response, which has led to the concept of host modulation. Patients with systemic diseases, such as diabetes and cardiovascular diseases, share links between biomarkers of systemic inflammation and periodontitis and, thus, are believed to be at high-risk for periodontal disease, because systemic inflammation is proposed to coinduce periodontal tissue destruction in concert with microbial-secreted LPS.16
Figure 3.
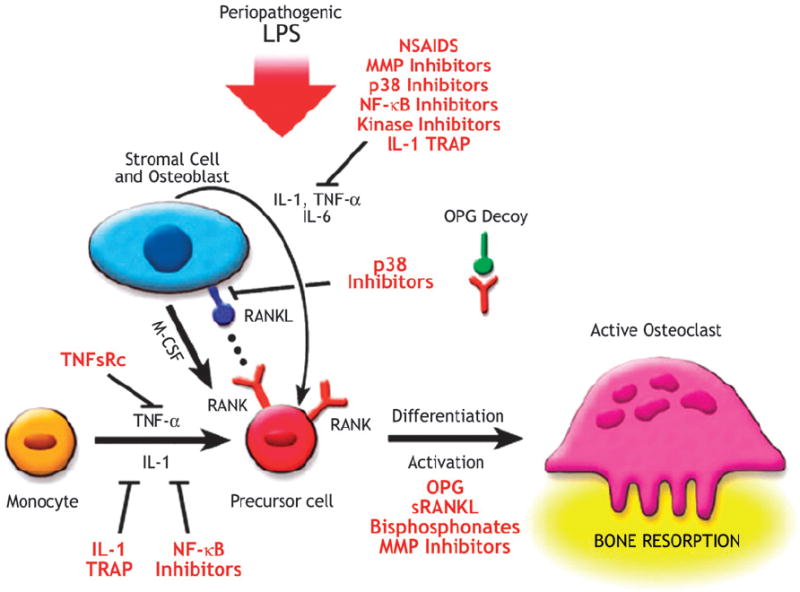
Potential therapeutic strategies to treat bone resorption: agents that block the differentiation or activity of osteoclasts are potential therapeutic agents. Osteoprotegerin (OPG) inhibits the differentiation of osteoclasts through its action as a decoy receptor that blocks receptor activator of nuclear factor-kappa B (NF-κB) ligand (RANKL) and RANK juxtacrine interaction. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory molecules (including p38 mitogen-activated protein kinase inhibitors, c-jun N-terminal kinase inhibitors, NF-κB inhibitors, and the specific, high-affinity IL-1 inhibitor IL-1 [TRAP]) can inhibit the formation of hemato-progenitor cells to preosteoclasts. Antibodies to RANKL can also block this interaction. MMP inhibitors reduce the protease degradation of the organic matrix, and anti-integrins block the initial osteoclast adhesion to the matrix. Bisphosphonates and MMP inhibitors work at the site of the osteoclast adhesion zone to the mineralized matrix in blocking bone resorption. M-CSF = macrophage colony-stimulating factor; sRANKL = soluble RANKL; TNFsRC = TNF soluble receptor. Adapted with permission from Blackwell Publishing.15
First described by Golub et al.17 and later expanded by Williams,18 research on the protective effects of common non-steroidal anti-inflammatory drugs (NSAIDs) and tetracyclines led to the concept of host modulation as a therapy for the cessation of periodontitis progression. Rheumatoid arthritis represents an example of a disease through which an understanding of potential therapeutic strategies for periodontitis has occurred.19 The various commonalities in terms of genesis and progression of the two diseases allow for direct comparisons of the juncture points for blocking downstream molecules, including PMNs, macrophages, and MMPs. Partly as a result of these types of comparisons, a variety of host-modulatory therapies for periodontitis now include the aforementioned NSAIDs; proteinase inhibitors; such as doxycyclines; MMP inhibitors; anabolics, such as parathyroid hormone; TNF antagonists; and a variety of antiresorptive agents represented by the bisphosphonates.15 However, when focusing on the bone-destroying activity of the osteoclast, the bisphosphonates and MMP inhibitors deserve additional discussion.
Inhibition of MMPs
Periodontal disease is generally characterized by an increased presence of subgingival Gram-negative microorganisms resulting in increased secretion of endotoxin, which ultimately leads to increased gingival collagenase activity, collagen destruction, and subsequent connective tissue destruction and bone loss. Currently, clinical therapy inhibiting the mediators of connective tissue breakdown is used for the adjunctive treatment of periodontitis. This is accomplished through the non-antimicrobial activities of low-dose tetracycline and tetracycline analogs via the inhibition of MMP-8 and -13 protease mechanisms.20 The tetracycline analog doxycycline hyclate,‡ available for use specifically in periodontal disease, is the only collagenase inhibitor approved by the United States Food and Drug Administration (FDA) for any human disease.10 To clarify, because the low-dose formulations of these drugs have lost their antimicrobial activity,20 the therapeutic action witnessed is due primarily to the modulation of the host response. This subantimicrobial-dose doxycycline (SDD) approach has become widely established as an effective adjunctive systemic therapy in the management of periodontitis, along with the traditional mechanical therapies of scaling and root planing (SRP). For example, initial Phase III clinical trials21 of MMP inhibition by SDD in subjects with periodontal disease over a 6-month period led to maintained alveolar bone height compared to bone height loss with placebo (as measured by subtraction radiography). In another early clinical study,22 the efficacy and safety of SDD were evaluated in conjunction with SRP in subjects with chronic periodontitis. Here, the more severe the periodontitis, the greater the observed attenuation of disease activity by SDD therapy (Fig. 4). In a recent systematic review,23 the effectiveness of SRP accompanied by MMP inhibition (by SDD), as an adjunctive treatment, showed improved outcomes that persisted for ≥9 months in adults with chronic periodontitis as observed in gains in clinical attachment level (CAL) and probing depth (PD) reduction. Most recently, in a double-masked, randomized, placebo-controlled, multicenter study24 of 266 subjects with periodontal disease, those individuals treated with a modified-release SDD formulation taken once daily as an adjunct to SRP displayed significantly greater clinical benefits (improved CAL gain and PD reduction) than individuals treated with SRP alone.
Figure 4.
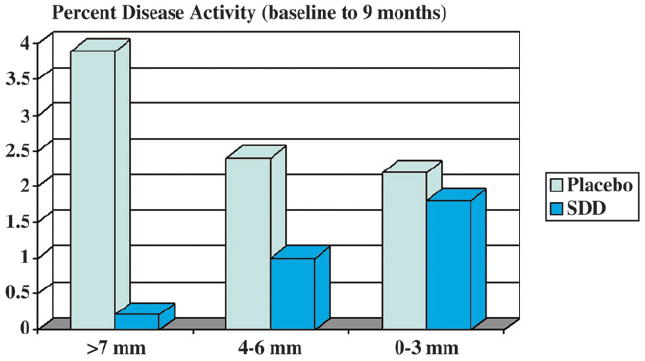
MMP inhibition reduces disease activity in patients with severe periodontitis. Effect of SDD on clinical attachment loss ≥2 mm from baseline to 9 months. Tooth sites were stratified by degree of disease severity, based on PD at baseline. Mean per-patient percentages (± SE) are presented. The more severe the disease state, the greater the observed attenuation of disease activity by SDD therapy. Adapted from reference 22.
There have been other therapeutic approaches that involve SDD in the treatment of periodontitis. Notably, a recent proof-of-principle study25 was designed to examine aspects of the biologic response brought on by SDD combined with access flap surgery (AFS) on the modulation of periodontal wound repair in subjects with severe periodontitis who were not candidates for regenerative therapy. Briefly, the periodontal surgery aimed to remove the microbial biofilm and improve the host environment, in concert with the SDD modification of the host inflammatory response. Together, the goal is augmentation of periodontal wound healing (through improving CAL and PD), increased bone stabilization, and decreased MMP expression. The results of this investigation demonstrated that SDD, in combination with AFS, may improve the response to surgical therapy during drug dosing by reducing PD in cases of severe periodontitis compared to AFS alone (Fig. 5)25 SDD tended to reduce post-surgical bleeding on probing (BOP), PD, and periodontal bone resorption during drug administration, yet it did not affect the periodontal microflora beyond the contribution of surgery alone.
Figure 5.
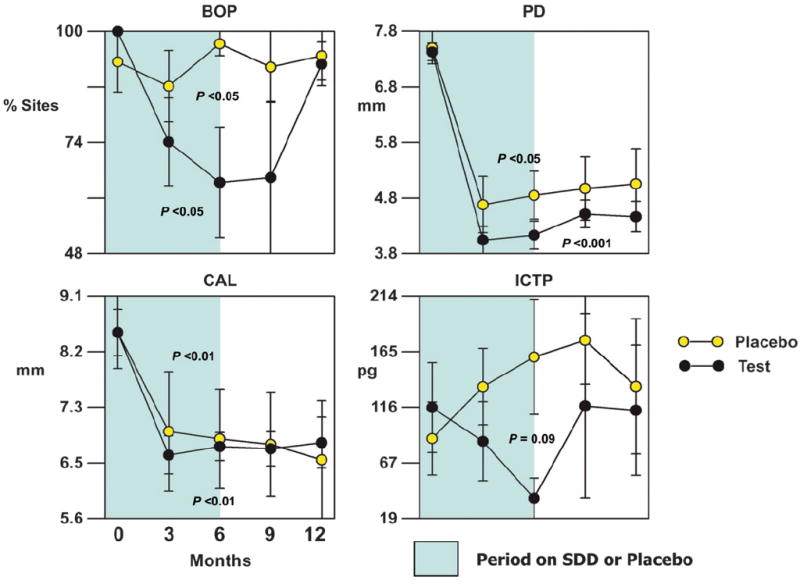
Effect of subantimicrobial-dose doxycycline (SDD) or placebo in combination with surgery on clinical parameters and ICTP for initial pocket ≥7 mm. The bars represent per-patient standard errors. P values indicate significant changes over time within a treatment as determined by the Quade test. Adapted from reference 25.
Of interest, other accumulating evidence demonstrated the ability of MMP inhibitors to be used in the management of periodontal disease in patients with decreased bone mass (as in the situation of post-menopausal osteoporosis). Recent studies26,27 demonstrated the ability of SDD to be used to maintain bone mass while reducing periodontal disease progression. Furthermore, oral fluid–derived (i.e., gingival crevicular fluid) biomarkers, such as collagen telopeptide fragments, were reduced in subjects following SDD dosing.28
Although there is strong evidence to suggest that inhibition of MMPs in patients with periodontal disease clearly offers potential in disease management when coupled with mechanical therapy, such as SRP, there is only preliminary evidence available to suggest the value of MMP inhibitory therapies for patients with peri-implant disease or in those conditions requiring surgical management.23,25 However, a number of questions need to be answered as the management of periodontitis develops. For example, considering these are chronic conditions and the role of MMPs in bone homeostasis and cancer, what are the long-term consequences of extended MMP inhibition in patients? Also, what are the downstream-extended effects on controlling cytokine processing, especially concerning the role of decreasing MMP-8 activity over time? Perhaps through the development of more selective MMP inhibitors for periodontal treatment (to reduce potential side effects) and by examining combination therapy approaches considering antimicrobial and host response targets, we may make further inroads into answering these questions. Encouragingly, a survey of the recent trends in scientific publications suggest a continued interest in understanding the role of MMPs in normal bone homeostasis and periodontal diseases, as well as in other pathologies, particularly tumor metabolism and metastasis. Such continued investigations on MMPs suggest that many of these unanswered questions are being addressed.29
Bisphosphonates as bone-sparing agents
Bisphosphonate drugs have well-characterized modulatory roles on osteoclast function and bone metabolism.30 Notably, at the tissue level, they decrease bone turnover by decreasing bone resorption and by reducing the number of new bone multicellular units. At the cellular level, they decrease osteoclast and osteoblast recruitment, decrease osteoclast adhesion, and decrease the release of cytokines by macrophages (Table 2). Based on these properties, several generations of oral bisphosphonate drugs have been successfully developed for the treatment of postmenopausal osteoporosis, osteopenia, and Paget’s disease of bone.31 Because of these same properties, a possible use for this class of drugs in the management of periodontal disease was put forth.30
Table 2.
Activities of Bisphosphonates on Osteoclast Function at the Tissue, Cellular, and Molecular Levels
| Tissue Level | Cellular Level | Molecular Level |
|---|---|---|
| ↓ bone turnover due to ↓ bone resorption | ↓ osteoclast recruitment | Inhibit mevalonate pathway (can result in perturbated cell activity and induction of apoptosis) |
| ↓ number of new bone multicellular units | ↑ osteoclast apoptosis | ↓ post-translational prenylation of GTP-binding proteins |
| Net positive whole body bone balance | ↓ osteoclast adhesion | |
| ↓ depth of resorption site | ||
| ↓ release of cytokines by macrophages | ||
| ↑ osteoblast differentiation and number |
GTP = guanosine triphosphate.
Adapted from reference 30.
A few clinical studies have been performed to examine a possible use for bisphosphonates in the management of periodontal bone loss.23 In a study32 of 40 subjects with chronic periodontitis, a statistically significant decrease in the proportion of teeth demonstrating bone loss was observed. Two other studies33,34 also demonstrated modest improvements in clinical and/or radiographic bone-preservation measures when a bisphosphonate was combined with conventional periodontal treatments. Together, these studies support the assertion that bisphosphonates may be useful as a host modulator in periodontal disease. A more recent, larger 12-month clinical study35 echoed this contention; 70 subjects randomized to one of two bisphosphonate therapies or placebo demonstrated clinical benefit in moderate-to-severe periodontitis. No differences in the change in periodontal bone mass were recorded among the treatments as measured by standardized radiography. Despite this, bisphosphonate therapy improved CAL, PD, and BOP over the course of the study, providing some evidence that bisphosphonate may be an appropriate adjunctive therapy to preserve periodontal support over time. However, overall, only limited information exists on the potential of bisphosphonates for periodontal treatment.
ADVERSE EFFECTS WITH BISPHOSPHONATE DOSING
Bisphosphonates are administered by intravenous (IV) infusion (in the case of treatment for metastatic bone cancers) or orally (for the treatment of decreased bone density in osteoporosis). Because of a significant rate of non-compliance and the subsequent decrease in clinical efficacy, IV bisphosphonate delivery has been used extensively for malignant bone diseases, as well as in breast, prostate, and lung cancer.36 However, a number of publications documented the retrospective reports associating IV bisphosphonate delivery and osteonecrosis of the jaws (ONJ).36-38 Clinically, ONJ is essentially exposed bone in the maxilla or mandible that does not heal within 8 weeks of identification by health care professionals (HCPs). ONJ is hypothesized to be due to the disruption of the resorption–remodeling cycle of bone inhibition of endothelial cell proliferation caused by high-dose IV administration of bisphosphonates. The resulting poor healing and secondary infections lead to tooth and bone segment loss. A recent report by the American Society for Bone and Mineral Research (ASBMR) addressed bisphosphonate-associated ONJ case definition, epidemiology, risk factors, diagnostic imaging, and clinical management (Table 3).38 Based on its review of the available data on the ranges of ONJ incidence, the ASBMR report concluded that a risk for ONJ is associated with oral bisphosphonate therapy for osteoporosis in less than one in 100,000 patients. However, a much higher risk for ONJ was associated with high-dose IV bisphosphonate therapy in patients with cancer and was reported to be in the range of one to 10 per 100 patients, increasing depending on therapy duration. Although there are few reports specifically on ONJ in periodontal patients, dental professionals have been made aware of the potential adverse effects in their patients under IV bisphosphonate treatment for other bone diseases or cancer. In a study37 that reported nine cases of ONJ in periodontal patients, all nine patients had a history of extractions of periodontally hopeless teeth preceding the onset of ONJ. Along with a predilection for the disease in the mandible, the duration of IV bisphosphonate therapy at presentation ranged from >5 years to as short as 10 months. Of further interest to those HCPs treating patients on oral bisphosphonates is one particular case report36 describing how a patient who had been taking oral bisphosphonates for osteoporosis for >10 years developed unexplained clinical signs of bone necrosis after routine dental implant placement. Briefly, the report noted compromised healing was successfully treated with systemic antibiotics, local microbial mouthrinse, and aggressive defect management via detoxification and a mixture of bone graft and tetracycline. This suggests that dental HCPs should treat patients undergoing long-term oral bisphosphonate treatment with caution.
Table 3.
Treatment Recommendations for Patients With Osteoporosis Receiving Bisphosphonate Therapy
| Patients informed of risks |
| Oral hygiene and dental care emphasized |
| Not necessary to require dental examination prior to bisphosphonate therapy or alter dental management |
For patients on bisphosphonates >3 years:
|
Adapted with permission from the American Society for Bone and Mineral Research.38
PERIODONTAL TREATMENT RECOMMENDATIONS FOR PATIENTS WITH OSTEOPOROSIS
There cent ASBMR report on bisphosphonate-associated ONJ provided a host of treatment recommendations for patients with osteoporosis (Table 3).38 Informing patients of the ONJ risks involved at the initiation of bisphosphonate treatment was recommended, coupled with an emphasis on the importance of good oral hygiene and dental care. The investigators did not deem it necessary for these patients to have a dental examination prior to treatment initiation or to alter their dental management during the treatment. In particular, for those patients on bisphosphonate for >3 years, recommendations included an encouragement for non-surgical or conservative surgical periodontal disease treatment; dental implant placement with informed consent; and a preference for endodontic treatment over extraction or periapical surgery. For invasive procedures, such as grafting, anecdotal evidence suggests that a drug vacation may be helpful given the long-term sequestration of bisphosphonates in bone for up to 10 years or longer.38 In a similar vein, the FDA provided recommendations39 to dental practitioners regarding the treatment of patients presenting with ONJ that the American Academy of Periodontology has incorporated into its own guidance.40 The recommendations of this mostly palliative approach include non-surgical approaches in the oral cavity, as well as bony debridement to reduce sharp edges, both to prevent further osseous injury; protective stents in areas of exposed bone; biopsy performed only if metastasis to the jaw is suspected; the initiation of antibiotic therapy (topical and systemic); and frequent monitoring of these patients.
Overall, the use of bisphosphonates for the management of periodontal diseases has limited promise, especially in affecting alveolar bone loss. However, despite their different mode of action, additional studies are needed to evaluate their potential as alveolar bone–sparing agents.23 Considerations related to the duration of use are relevant, given the reported risks associated with ONJ related to the long-term use of high-dose bisphosphonates, contrasting the potential benefits of the short-term oral use of these drugs. Despite progression in this area of research and a better understanding of the reported risks, a number of questions for future consideration of bisphosphonates in the treatment of periodontal diseases remain, which should be addressed.
CONCLUSIONS
There has been a great deal of basic and clinical research focusing on the underlying mechanisms of the major enzymatic drivers of the aggressive tissue destruction found in periodontitis. Major drivers of this damage are MMPs, cathepsins, and other osteoclast-derived mediators of bone resorption, all of which act as part of the host inflammatory response. Modification of this host response via the use of MMP inhibitors, along with the use of bisphosphonates as blockers of periodontal tissue destruction, has shown promise in the therapeutic treatment of these disease states. Although questions remain regarding optimizing treatment efficacy while limiting any potential adverse effects, the evidence clearly suggests a strong potential for the modulation of the host response in aiding disease management, when coupled with traditional mechanical therapy.
Acknowledgments
This research was supported, in part, by the National Institutes of Health/National Institutes of Dental and Craniofacial Research, Bethesda, Maryland (U01-DE14961 and UL1RR024986). The author has financial interest related to licensed diagnostic technology to BioMimetic Therapeutics, Franklin, Tennessee. The initial draft of this manuscript was developed by a medical writer (Axon Medical Communications Group, Toronto, Ontario) based on content provided solely by the author. The final manuscript submitted was under the sole control of the author.
Footnotes
Periostat, Galderma Labs, Fort Worth, TX.
References
- 1.Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci. 2007;1098:230–251. doi: 10.1196/annals.1384.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albandar JM. Periodontal diseases in North America. Periodontol 2000. 2002;29:31–69. doi: 10.1034/j.1600-0757.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- 3.Woodward JK, Holen I, Coleman RE, Buttle DJ. The roles of proteolytic enzymes in the development of tumour-induced bone disease in breast and prostate cancer. Bone. 2007;41:912–927. doi: 10.1016/j.bone.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Pussinen PJ, Paju S, Mantyla P, Sorsa T. Serum microbial- and host-derived markers of periodontal diseases: A review. Curr Med Chem. 2007;14:2402–2412. doi: 10.2174/092986707781745604. [DOI] [PubMed] [Google Scholar]
- 5.auf dem Keller U, Doucet A, Overall CM. Protease research in the era of systems biology. Biol Chem. 2007;388:1159–1162. doi: 10.1515/BC.2007.146. [DOI] [PubMed] [Google Scholar]
- 6.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tester AM, Cox JH, Connor AR, et al. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS ONE. 2007;2:e312. doi: 10.1371/journal.pone.0000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill SE, Parks WC. Metalloproteinases and their inhibitors: Regulators of wound healing. Int J Biochem Cell Biol. 2007;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Otín C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 10.Sorsa T, Tjäderhane L, Konttinen YT, et al. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306–321. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 11.Golub LM, Lee HM, Greenwald RA, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- 12.Herr AE, Hatch AV, Giannobile WV, et al. Integrated microfluidic platform for oral diagnostics. Ann N Y Acad Sci. 2007;1098:362–374. doi: 10.1196/annals.1384.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herr AE, Hatch AV, Throckmorton DJ, et al. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc Natl Acad Sci USA. 2007;104:5268–5273. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinane DF, Darby IB, Said S, et al. Changes in gingival crevicular fluid matrix metalloproteinase-8 levels during periodontal treatment and maintenance. J Periodontal Res. 2003;38:400–404. doi: 10.1034/j.1600-0765.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- 15.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol 2000. 2007;43:294–315. doi: 10.1111/j.1600-0757.2006.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golub LM, Payne JB, Reinhardt RA, Nieman G. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J Dent Res. 2006;85:102–105. doi: 10.1177/154405910608500201. [DOI] [PubMed] [Google Scholar]
- 17.Golub LM, Suomalainen K, Sorsa T. Host modulation with tetracyclines and their chemically modified analogues. Curr Opin Dent. 1992;2:80–90. [PubMed] [Google Scholar]
- 18.Williams RC. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 19.Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350:2167–2179. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- 20.Ashley RA. Clinical trials of a matrix metalloproteinase inhibitor in human periodontal disease. SDD Clinical Research Team. Ann N Y Acad Sci. 1999;878:335–346. doi: 10.1111/j.1749-6632.1999.tb07693.x. [DOI] [PubMed] [Google Scholar]
- 21.Ciancio S, Ashley R. Safety and efficacy of subantimicrobial-dose doxycycline therapy in patients with adult periodontitis. Adv Dent Res. 1998;12:27–31. doi: 10.1177/08959374980120011501. [DOI] [PubMed] [Google Scholar]
- 22.Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 23.Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- 24.Preshaw PM, Novak MJ, Mellonig J, et al. Modified-release subantimicrobial dose doxycycline enhances scaling and root planing in subjects with periodontal disease. J Periodontol. 2008;79:440–452. doi: 10.1902/jop.2008.070375. [DOI] [PubMed] [Google Scholar]
- 25.Gapski R, Barr JL, Sarment DP, Layher MG, Socransky SS, Giannobile WV. Effect of systemic matrix metalloproteinase inhibition on periodontal wound repair: A proof of concept trial. J Periodontol. 2004;75:441–452. doi: 10.1902/jop.2004.75.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne JB, Stoner JA, Nummikoski PV, et al. Subantimicrobial dose doxycycline effects on alveolar bone loss in post-menopausal women. J Clin Periodontol. 2007;34:776–787. doi: 10.1111/j.1600-051X.2007.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinhardt RA, Stoner JA, Golub LM, et al. Efficacy of subantimicrobial dose doxycycline in post-menopausal women: Clinical outcomes. J Clin Periodontol. 2007;34:768–775. doi: 10.1111/j.1600-051X.2007.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golub LM, Lee HM, Stoner JA, et al. Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol. 2008;79:1409–1418. doi: 10.1902/jop.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuti E, Tuccinardi T, Rossello A. Matrix metalloproteinase inhibitors: New challenges in the era of post broad-spectrum inhibitors. Curr Pharm Des. 2007;13:2087–2100. doi: 10.2174/138161207781039706. [DOI] [PubMed] [Google Scholar]
- 30.Tenenbaum HC, Shelemay A, Girard B, Zohar R, Fritz PC. Bisphosphonates and periodontics: Potential applications for regulation of bone mass in the periodontium and other therapeutic/diagnostic uses. J Periodontol. 2002;73:813–822. doi: 10.1902/jop.2002.73.7.813. [DOI] [PubMed] [Google Scholar]
- 31.Kamel HK. Update on osteoporosis management in long-term care: Focus on bisphosphonates. J Am Med Dir Assoc. 2007;8:434–440. doi: 10.1016/j.jamda.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Jeffcoat MK, Reddy MS. Alveolar bone loss and osteoporosis: Evidence for a common mode of therapy using the bisphosphonate alendronate. In: Davidovitch Z, Norton LA, editors. Biological Mechanisms of Tooth Movement and Craniofacial Adaptation. Vol. 1. Cambridge, MA: Harvard Society for the Advancement of Orthodontics; 1996. pp. 365–373. [Google Scholar]
- 33.Rocha M, Nava LE, Vázquez de la Torre C, Sánchez-Marin F, Garay-Sevilla ME, Malacara JM. Clinical and radiological improvement of periodontal disease in patients with type 2 diabetes mellitus treated with alendronate: A randomized, placebo-controlled trial. J Periodontol. 2001;72:204–209. doi: 10.1902/jop.2001.72.2.204. [DOI] [PubMed] [Google Scholar]
- 34.Takaishi Y, Miki T, Nishizawa Y, Morii H. Clinical effect of etidronate on alveolar pyorrhoea associated with chronic marginal periodontitis: Report of four cases. J Int Med Res. 2001;29:355–365. doi: 10.1177/147323000102900413. [DOI] [PubMed] [Google Scholar]
- 35.Lane N, Armitage GC, Loomer P, et al. Bisphosphonate therapy improves the outcome of conventional periodontal treatment: Results of a 12-month, randomized, placebo-controlled study. J Periodontol. 2005;76:1113–1122. doi: 10.1902/jop.2005.76.7.1113. [DOI] [PubMed] [Google Scholar]
- 36.Wang HL, Weber D, McCauley LK. Effect of long-term oral bisphosphonates on implant wound healing: Literature review and a case report. J Periodontol. 2007;78:584–594. doi: 10.1902/jop.2007.060239. [DOI] [PubMed] [Google Scholar]
- 37.Ficarra G, Beninati F, Rubino I, et al. Osteonecrosis of the jaws in periodontal patients with a history of bis-phosphonates treatment. J Clin Periodontol. 2005;32:1123–1128. doi: 10.1111/j.1600-051X.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 38.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 39.Expert Panel Recommendation for the Prevention, Diagnosis and Treatment of Osteonecrosis of the Jaw. [January 23, 2008]; Available at: http://www.fda.gov/OHRMS/DOCKETS/AC/05/briefing/2005-4095B2_02_12-Novartis-Zometa-App-11.pdf.
- 40.American Academy of Periodontology. AAP statement on bisphosphonates. [May 30,2008]; http://www.perio.org/resources-products/bisphosphonates.htm. Published 2005.


