Abstract
Most wavelengths change hue when mixed with white light. These changes, known as the Abney effect, have been extensively studied to characterize nonlinearities in the neural coding of color, but their potential function remains obscure. We measured the Abney effect in a new way—by varying the bandwidth of the spectrum rather than mixing with white—and this leads to a new interpretation of the role of nonlinear responses in color appearance. Because of the eye’s limited spectral sensitivity, increasing the bandwidth of a spectrum changes the relative responses in the three classes of cone receptor and thus would change hue if the percept were tied to a fixed cone ratio. However, we found that hue is largely independent of bandwidth and thus constant for a constant peak wavelength for stimuli with Gaussian spectra. This suggests that color appearance is compensated for the eye’s spectral filtering, and that this compensation embodies specific perceptual inferences about how natural spectra vary. When a wavelength is instead diluted with white light—which does not bias the cone ratios—then the same compensation predicts changes in hue because the “right” response is made to the “wrong” stimulus. This model generates constant hue loci that are qualitatively consistent with measures of the Abney effect and provides a novel functional account of such effects in color appearance, in which postreceptoral responses are adjusted so that constant hue percepts are tied to consistent physical properties of the environment rather than consistent physiological properties such as the cone ratios.
Keywords: color vision, color appearance, Abney effect, nonlinearities, color spectra
Introduction
Color appearance can be described by the psychological dimensions of brightness, hue, and saturation. A major focus of color science has been to understand the coding mechanisms giving rise to these perceptual dimensions. Many studies have examined the neural bases for hue, and in particular for perceptually unique hues (e.g., a pure yellow untinged by red or green). In standard models of color vision, these hues represent elemental sensations corresponding to special physiological states (Hurvich & Jameson, 1957; Kaiser & Boynton, 1996), yet it has thus far proven difficult to identify physiological constraints on the unique hues. For example, the unique hues are largely unaffected by individual differences in spectral sensitivity or in the relative numbers of the different cone types (Brainard et al., 2000; Miyahara, Pokorny, Smith, Baron, & Baron, 1998; Pokorny & Smith, 1987; Schefrin & Werner, 1990; Webster, Miyahara, Malkoc, & Raker, 2000). This has suggested that the unique hues are more closely tied to properties of the environment than the observer (Mollon, 1982; Pokorny & Smith, 1977). Consistent with this, color judgments can be biased by long term differences in the color environment (Neitz, Carroll, Yamauchi, Neitz, & Williams, 2002; Webster & Mollon, 1997; Webster, Webster, et al., 2002), while remaining stable despite long term changes in vision with aging (Schefrin & Werner, 1990). For example, sensitivity to short wavelengths dramatically decreases with aging because of increasing density of the lens pigment, yet the spectra that appear achromatic instead remain constant across the lifespan (Werner & Schefrin, 1993). This perceptual constancy could be achieved if the sensitivity changes are compensated by normalizing the cone responses for the average spectrum in the environment (Werner, 1996).
In this study, we examined whether hue is more generally compensated for the eye’s spectral sensitivity so that hue percepts can be tied to consistent properties of the stimulus spectrum. To do this, we asked how the hue of a spectrum changes when the stimulus is desaturated. In linear models of color opponency (Hurvich & Jameson, 1957; Kaiser & Boynton, 1996), hue is represented by the ratio of cone signals and thus follows straight lines of constant slope emanating from the achromatic origin in color space (Chichilnisky & Wandell, 1999; Larimer, 1974). However, nonlinearities in color coding are well documented (Abney, 1910; Ayama, Nakatsue, & Kaiser, 1987; Burns, Elsner, Pokorny, & Smith, 1984; Ikeda & Uehira, 1989; Kulp & Fuld, 1995; Kurtenbach, Sternheim, & Spillmann, 1984; Larimer, Krantz, & Cicerone, 1975; Scheibner & Kremer, 1996; Westphal, 1909). When a single wavelength is mixed with white light its hue changes, and thus the loci of constant hue fall along curved contours in color space. These changes in hue with purity are known as the Abney effect (Abney, 1910) and have been extensively studied over the last century to describe the response properties of human color vision. However, the functional implications of the Abney effect have remained obscure. Specifically, it is not known whether the nonlinearities revealed by the Abney effect are merely an epiphenomenon of color coding or whether they play a more deliberate role in the representation of object color.
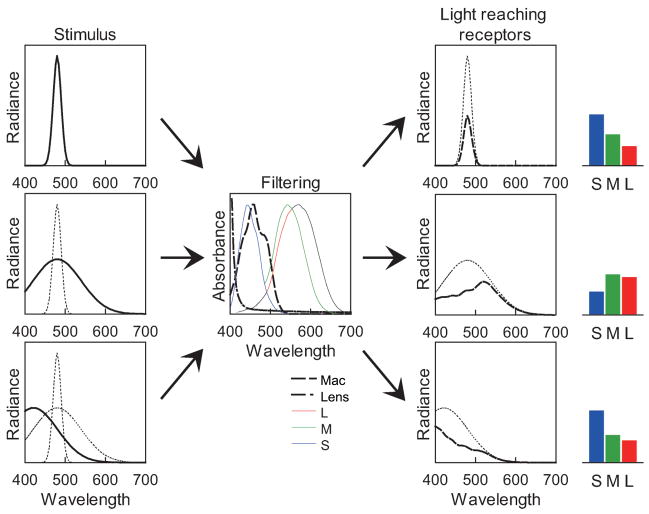
We reexamined the relationship between hue and stimulus purity, but with novel stimuli in which purity was varied by broadening the bandwidth of the spectrum rather than diluting with a fixed desaturant. This allowed us to examine how color appearance depends on the filtering effects inherent in the eye’s spectral sensitivity. The visible spectrum is limited at longer wavelengths by the absorption spectra of the cone photopigments, and at short wavelengths by both the photopigments and by screening from inert pigments in the lens and macular area of the central retina (Stockman & Sharpe, 1999). As a result, the spectral sensitivity of the visual system acts as a band-pass filter for wavelength. Weighting the color signal by the spectral sensitivity function biases the response to broadband stimuli relative to narrowband stimuli because wavelengths longer or shorter than the peak are differently filtered (Figure 1). As a result, for most center wavelengths the ratio of responses across the three classes of cones changes as the bandwidth increases. If constant hue percepts corresponded to constant cone ratios—and thus were not compensated for the effect of bandwidth—then to maintain the hue, the center wavelength of most stimuli must change as the spectrum is broadened. Yet we found that when observers match the hue of narrow and broad spectra, they instead choose roughly the same center wavelengths, consistent with a “correction” for their spectral sensitivity that maintains hue constancy for the spectral peak of the stimulus. These results point to a possible function of nonlinearities in color appearance—to tie hue to the stimulus rather than the observer—and suggest a novel account of the Abney effect, in which the visual system misinterprets the cause of the purity change and thus applies the right correction to the wrong stimulus.
Figure 1.
The band-pass nature of the eye’s spectral sensitivity biases the response to broadband stimuli relative to narrow spectra. Filtering a narrow spectrum primarily affects the height of the curve (top) while a broader spectrum is changed in shape (middle). To match the relative cone excitation to the narrow stimulus, the peak of the broader spectrum must usually be shifted (bottom).
Methods
Predicted color shifts
We modeled the effects of observer spectral sensitivity to predict how cone responses should change as a light’s spectrum varies from narrow to broad and to predict the resulting hue loci based on constant cone ratios. The lights had a Gaussian spectrum with a peak at wavelengths typical of blue, green, or yellow hues. For our analysis, the stimulus spectrum and cone spectral sensitivities were defined in terms of energy on a linear wavelength axis. An alternative would be to express the sensitivity on a linear or log axis of frequency (the reciprocal of wavelength), which has been suggested to more nearly equate the bandwidths of the photoreceptors (Barlow, 1982; Dartnall, 1953; MacLeod & Webster, 1988). The effects we model are independent of the metric for defining the photopigment sensitivities but could differ depending on the axis along which the signal is assumed to be Gaussian because a symmetric spectrum in wavelength is asymmetric in terms of frequency.
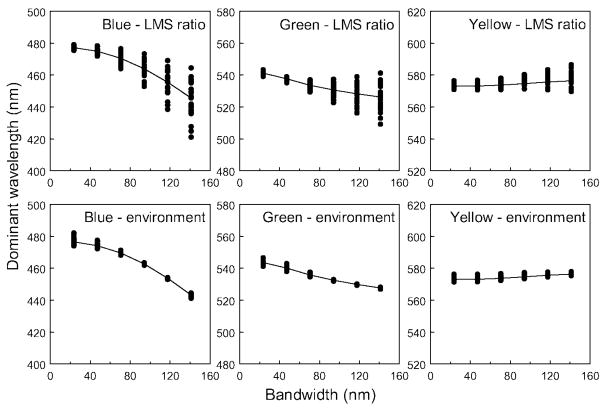
As noted above, increasing the spectral bandwidth alters the cone ratios and thus would require a compensatory change in spectral peak (Figure 1). For the standard observer, wavelengths near blue require a large shift toward shorter values as the bandwidth increases because of the falloff in visual sensitivity at short wavelengths; whereas settings for green and yellow, which are nearer the middle of the visible spectrum, show less pronounced variation (Figure 2).
Figure 2.
Predicted hue loci as a function of spectral bandwidth for observers with different spectral sensitivities. Lines plot the settings for the “standard observer” with standard sensitivity. Predictions are based on adjusting the center wavelength to maintain a constant ratio across the cone receptors. Top panels: settings for a blue, green, or yellow assuming all observers choose the same cone ratio for a given hue. Bottom: hue settings assuming instead that observers choose the same broadband environmental stimulus for a given unique hue.
Specifically how cone responses change with bandwidth depends on the individual’s spectral sensitivity. Observers widely vary in the optical density of the lens and macular pigment and vary in the absorption spectra of the cone photopigments (Neitz & Neitz, 1998; Smith & Pokorny, 1995; Stockman & Sharpe, 1999; Webster & MacLeod, 1988). Individuals also widely differ in their unique hue settings (Kuehni, 2004; Webster et al., 2000). The differential effects of spectral sensitivity on narrow vs. broad spectra have been proposed as a possible basis for individual differences in color appearance (Jordan & Mollon, 1995; Mollon & Jordan, 1997), and this proposal provided the initial motivation for our study. To assess individual differences, we calculated predictions for simulated observers who differed in macular and lens pigments and in the spectral peaks of the cones, based on prior estimates of these variations from a factor analysis of the 49 observers in the Stiles and Burch 10° field color matches with macular pigment adjusted for a 2° field (Webster & MacLeod, 1988).
Notably, because individuals are predicted to show different effects of bandwidth, the differences between individuals also vary with bandwidth. For example, suppose that observers chose the same wavelengths for hues in narrowband stimuli because a given hue corresponded to a particular cone ratio. Their hue settings for broadband stimuli should then disagree (Figure 2, top panels). This is because the stimuli required to maintain a fixed cone ratio should diverge as the spectrum is broadened and as individual differences in spectral sensitivity come to play an increasing role. Conversely, if observers agree about the hue of a broadband stimulus, for example because they learned to associate the hue with a common stimulus in the environment, then individual differences in the hue settings will instead be larger for narrowband stimuli (Jordan & Mollon, 1995; Figure 2, bottom panels). Again, this occurs because each observer must choose a stimulus that preserves the same cone ratio as in the broadband stimulus, and these will diverge as the screening pigments now become less important in shaping the response. Finally, because the macular pigment is only present in the central retina (Snodderly, Auran, & Delori, 1984), similar predictions also apply to the color judgments of a single observer for stimuli viewed at different retinal eccentricities.
Apparatus
To measure how hue actually varies with bandwidth, we used a display modeled after Bonnardel, Bellemare, and Mollon (1996) that allows the light spectrum to be filtered in arbitrary ways (Figure 3). Light from a 300-W Xenon lamp was collimated to illuminate an interference wedge, which yields a continuous spectrum that is linear in wavelength units. The light then passed through an LCD panel directly placed after the wedge. Spatial patterns generated on the LCD panel masked the light from different parts of the wedge and thus controlled the spectrum reaching an integrating sphere, which presented a uniform 2° stimulus to the observer. Participants viewed the field binocularly from a distance of 95 cm.
Figure 3.
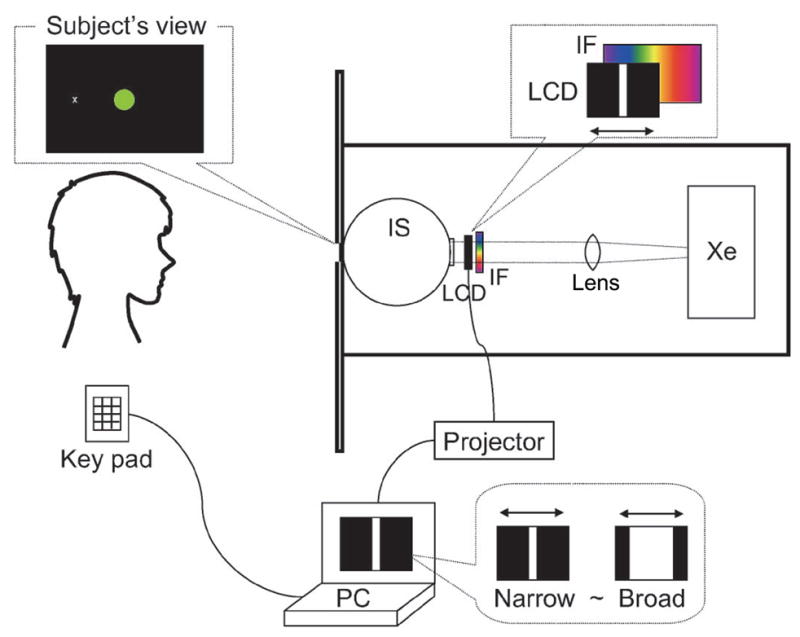
Experimental apparatus. Xe, Xenon lamp; IF, interference wedge; LCD, LCD panel; IS, integrating sphere.
Stimuli
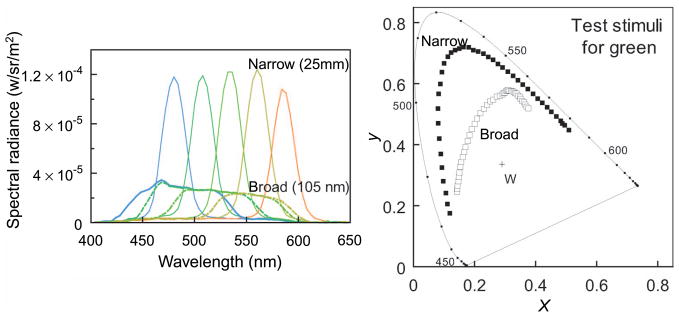
For our display, rectangular slits formed on the LCD produced approximately Gaussian spectral distributions (Figure 4), although limitations of the apparatus including a limited contrast ratio prevented us from precisely defining the spectra. Accordingly, all stimuli were measured with a PR650 spectroradiometer, and all reported results are based on the actual measured spectra. For unique hue settings, two different bandwidths were used for test stimuli: 25 nm (full width at half height) for the narrow stimulus and 105 nm for broad, in the case of unique green and yellow, or 85 nm in the case of unique blue. These ranges were limited by available light at the narrow end and spectral limits at the broad end. The stimuli were generated by changing the slit size on the LCD panel. The luminance of the test stimuli was set near 2.0 cd/m2 for unique green and yellow and 0.5 cd/m2 for unique blue because of the limited power of the lamp. In a second experiment, constant hue loci were measured across different bandwidths by matching the hue of a test stimulus to a reference stimulus with a bandwidth of 105 nm. The center wavelength of the reference was fixed at 480, 490, 500, 510, or 520 nm for different settings. The bandwidths of tests were 25 and 45 nm. A bandwidth of 85 nm was also tested for one participant (YM). Stimuli were matched for brightness to a broadband achromatic adaptation light generated through the same display. The adapting field had a chromaticity of x = 0.290, y = 0.336 (CIE 1931), and a luminance of 2.2 cd/m2 for the green and yellow settings. The specific choice for the achromatic point is unlikely to alter the settings because moderate differences are compensated by von Kries adaptation (Brainard & Wandell, 1992; MacLeod & Golz, 2003; Webster & Mollon, 1995). Note also that the effects we report do not depend on the spectral content of the white point, which in the extremes could have been generated by a flat spectrum or by a pair of complementary wavelengths. These would be filtered in different ways by the eye, but as long as they were chosen to give the same cone excitations they would be indistinguishable and thus subject to the same perceptual inferences.
Figure 4.
Examples of the stimulus spectra used to measure unique green, and their color coordinates shown in the CIE 1931 chromaticity diagram.
Procedure
Observers varied the stimuli either to match the hue of narrow to broad spectra or to determine the stimuli that appeared unique blue, green, or yellow. For the unique hues, test stimuli were shown for 1.5 s following adaptation for 30 s to the achromatic adapting field, and then followed again by the achromatic light. Observers used a keypad to rate the color of the test as either reddish or greenish for unique blue or yellow settings, or as yellowish or bluish for unique green. A staircase method was used to vary subsequent stimuli and the average of 10 reversals was taken as the unique hue setting. For constant hue loci, a similar procedure was followed, but in this case the test was presented 1 s after the fixed broadband reference stimulus (both again shown for 1.5 s each), and the observer varied the center wavelength of the test to try to match the hue of the reference. Observers made the hue settings for narrow and broad stimuli in the fovea or at 10° in the periphery. Each observer made six settings for each condition in random order. All participants had normal color vision as assessed by the Ishihara plate test, and most were undergraduate students participating for class credit. Testing protocols were approved by the University of Nevada Institutional Review Board and informed consent was obtained for all participants.
Results
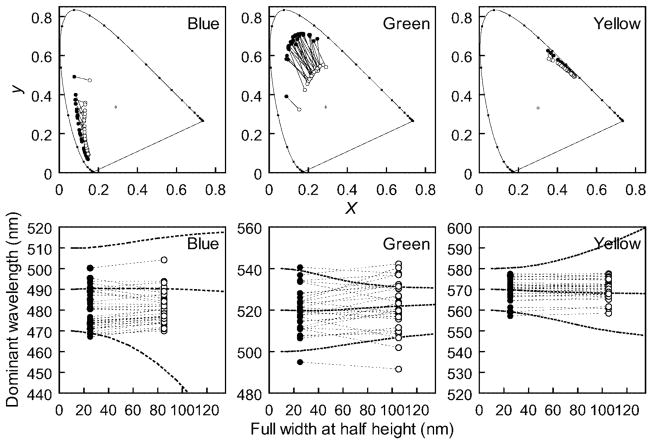
We tested the effects of bandwidth changes on two tasks—unique hue judgments and hue matches; and at two eccentricities—the fovea and 10°, to test the influence of macular pigment. The center wavelengths chosen for the unique hues by individual observers showed little change with bandwidth (Figure 5), and although the difference reached significance for unique blue, it amounted to less than a 2-nm shift and was in the opposite direction of the predicted linear change, which approached 20 nm for the shorter wavelengths (Table 1 and Figure 5). For unique blue in particular, this departs from the predictions for hue based on preserving the cone ratios and suggests that the hue signaled by a given set of cone ratios is instead adjusted to preserve hue constancy for the center wavelength of the stimulus. The range of unique hue settings across observers was large, consistent with previous reports (Kuehni, 2004; Webster et al., 2000). However, this interobserver variance was also largely unaffected by changing the stimulus bandwidth (and for all three unique hues was found in F tests to not significantly differ across the two bandwidths; Table 1). This suggests that differences in spectral sensitivity probably place little constraint on the unique hues because each observer’s settings instead appear already compensated for their spectral sensitivity.
Figure 5.
Stimuli selected by individual observers for unique blue, green, and yellow for foveally viewed stimuli. Top panels plot the chromaticities in the CIE diagram. Bottom panels instead plot the stimuli according to the selected center wavelength. Solid and open symbols indicate narrowband and broadband settings, respectively. Dashed lines show settings predicted if no compensation occurs for the bandwidth change, based on the standard observer.
Table 1.
Means and standard deviations of the center wavelength chosen for unique blue, green, and yellow. Numbers in parentheses specify the number of observers tested. Values in the top panel show settings for narrow vs. broad spectra (foveal viewing), while the lower panel shows settings for foveal vs. peripheral viewing.
| Narrow vs. broad (fovea) | t test (mean), F test (variances) | ||||||
|---|---|---|---|---|---|---|---|
| Blue | Mean (41) | 479.2 vs. 480.5 | t = −2.69 (p = .01) | ||||
| SD (41) | 8.98 | 7.47 | F = 1.45 (p = .12) | ||||
| Green | Mean (30) | 520.1 vs. 521.9 | t = −1.57 (p = .13) | ||||
| SD (30) | 10.23 | 12.07 | F = 1.39 (p = .19) | ||||
| Yellow | Mean (33) | 570.6 vs. 570.7 | t = −0.5 (p = .61) | ||||
| SD (33) | 5.11 | 4.83 | F = 1.12 (p = .37) | ||||
| Fovea vs. periphery (narrow) | t test | Fovea vs. periphery (broad) | t test | ||||
|
| |||||||
| Blue | Mean (9) | 474.9 vs. 472.3 | t = 2.71 (p = .01) | 476.2 vs. 475.8 | t = 0.45 (p = .67) | ||
| SD (9) | 3.51 | 5.23 | 2.88 | 4.62 | |||
| Green | Mean (9) | 520.3 vs. 513.0 | t = 2.54 (p = .03) | 519.0 vs. 517.7 | t = 0.78 (p = .46) | ||
| SD (9) | 13.50 | 10.60 | 14.89 | 13.98 | |||
| Yellow | Mean (9) | 569.4 vs. 560.4 | t = 4.20 (p = .003) | 569.5 vs. 561.7 | t = 4.62 (p = .002) | ||
| SD (9) | 6.10 | 7.25 | 5.87 | 4.99 | |||
Nine observers repeated unique hue settings for both foveal and peripheral (10°) viewing conditions. At either location, differences in unique hue with bandwidth were not significant. There was an overall trend for the peripheral settings to shift toward shorter wavelengths (Table 1). A change in hue with eccentricity is consistent with previous reports (Nerger, Volbrecht, & Ayde, 1995; Volbrecht, Nerger, Imhoff, & Ayde, 2000). However, the difference in macular pigment density is predicted to have largest effects on the blue settings, and these again shifted only slightly (~2 nm) and in the opposite direction to the macular pigment prediction. The relative stability of unique blue across the two loci and across bandwidth at both loci is again consistent with a compensatory adjustment for changes in spectral sensitivity. On the other hand, the fact that hues did change suggests that the visual system does not compensate for all sources of retinal variation (for these Gaussian spectra). One potential source in color appearance is intrusion by rod receptors in the periphery (Buck, 1997; Cao, Pokorny, & Smith, 2005). However, the hue settings were unaffected in control conditions following a rod bleach.
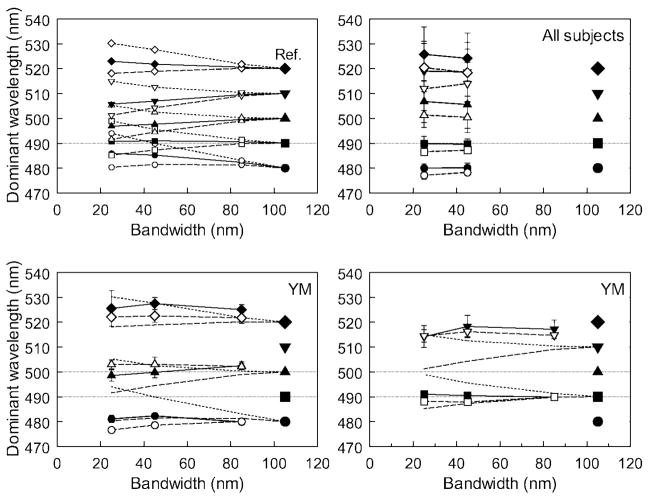
To more finely sample hues in the blue-green region of the spectrum—where effects of preretinal screening should be most evident—we used a hue-matching task, in which observers adjusted the center wavelength of narrowband stimuli to match the hue of a broadband reference. At the 480- to 520-nm range of wavelengths tested, equating the fovea and periphery for the same cone ratios requires a shift toward longer wavelengths in the fovea relative to the periphery as the bandwidth narrows (Figure 6, top left). Average hue settings for 7 observers are plotted in the top right panel of Figure 6. At the shorter wavelengths (480 and 490 nm), these remained similar for the fovea and periphery and again remained close to the center wavelength of the broadband reference. Yet the settings for the longer wavelengths are more equivocal and are difficult to interpret because we did not measure macular pigment density and because some settings (e.g., at 510 nm) fall outside the range of predictions for the different densities. However, the bottom panels of Figure 6 show matches for a further single observer who was found by standard tests (Wooten, Hammond, Land, & Snodderly, 1999) to have an extremely high density of macular pigment (1.3 at 460 nm, compared to an average value of 0.465). Because of this, her matches are predicted to show large differences of 14 nm or more in center wavelength between stimuli of different bandwidths or between the fovea and periphery (Figure 6, top left). Yet at shorter wavelengths the constant hue loci are very close to maintaining a constant center wavelength, and the modest variations at longer wavelength do not follow the large differences predicted by her macular screening.
Figure 6.
(Top left) Predicted center wavelength for narrowband stimuli matched in hue to a broadband reference (large symbols), assuming a standard observer (solid lines), an observer with no macular pigment (dashed lines), or an observer with a high macular density (1.3 at 460 nm; dotted lines). (Top right) Mean hue matches for seven participants for foveal (filled) and peripheral (open) viewing. Error bars are standard deviations across observers. (Bottom panels) Hue matches for one participant (YM) with a measured peak macular density of 1.3. Solid and open symbols show foveal and peripheral settings, respectively. Error bars are ±1 SD of repeated settings.
Discussion
Since the original reports by Westphal (1909) and Abney (1910), changes in hue with colorimetric purity have remained a well-established yet little understood property of color vision (Ayama et al., 1987; Burns et al., 1984; Ikeda & Uehira, 1989; Kulp & Fuld, 1995; Kurtenbach et al., 1984; Scheibner & Kremer, 1996). Like the present results, these represent a nonlinearity in the response to color because the hue of a mixture of lights is not an additive mixture of the hues of the components. Our measurements can therefore be interpreted as a variant of the Abney effect but differ from previous studies because the hue of our stimuli remained constant rather than varying as purity changed. This difference is due to the difference in how we varied stimulus purity. In a linear cone excitation space, changing spectral bandwidth alters the cone ratios and thus the angle of the stimulus relative to the white point so that the coordinates for different purities follow a curved path (Figure 2). A nonlinearity is revealed in our results because the constant hue loci are instead approximately straight lines tied to the constant peak wavelength of the stimulus. Conversely, the Abney effect is conventionally measured by diluting monochromatic light with a fixed broadband white (Ikeda & Uehira, 1989; Kurtenbach et al., 1984) or by mixing a set of fixed primaries (Ayama et al., 1987; Burns et al., 1984). In this case, the chromaticity coordinates do plot along a straight line between the fixed coordinates of the components, and the nonlinearity is manifest as loci of constant hue that are instead curved.
This difference also suggests a novel explanation for nonlinear processing in color appearance. Previous studies of additivity failures have focused on largely mechanistic accounts of the effects in terms of the nonlinear response properties of the cones or of postreceptoral chromatic mechanisms and in most cases have treated these response properties separately for different parts of the spectrum (Ayama et al., 1987; Burns et al., 1984; Chichilnisky & Wandell, 1999; Ikeda & Uehira, 1989; Knoblauch & Shevell, 2001; Kulp & Fuld, 1995; Kurtenbach et al., 1984; Larimer, 1974, 1975; Scheibner & Kremer, 1996; Shklover, 1958). Our findings point instead to a functional interpretation of the Abney effect and one that provides a unified explanation for the hue changes at all wavelengths. The hue constancy we observed suggests that postreceptoral mechanisms compensate color appearance for the filtering effects imposed by the cone sensitivities and preretinal absorption, and that this compensation embodies an inference that under natural viewing conditions purity variations within or across stimuli are most likely to arise from variations in spectral bandwidth. From this perspective, the Abney effect as it is traditionally studied occurs because the visual system has been fooled by an unnatural color signal, in which the purity is instead diluted to create an unnatural spectrum. The desaturated stimulus is “misinterpreted” because it leads to the same triplet of cone excitations as a broadband stimulus, but the hue shift observed is one that would be appropriate for maintaining constant hue percepts for broadband spectra.
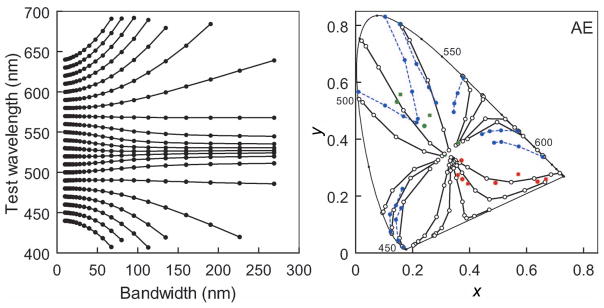
Figure 7 shows that this explanation for the nonlinearities in color appearance in fact captures many of the characteristic color changes reported for the Abney effect (Burns et al., 1984). In particular, it predicts that when mixed with white, both short and long wavelengths appear redder (i.e., shifted toward the ends of the spectrum), and thus lines of constant hue instead curve toward the center of the visible spectrum whereas medium wavelengths show much less shift. The same predictions can also be extended to extraspectral reds (e.g., for stimuli formed by subtracting a Gaussian from a flat spectrum) and again capture the characteristic curvature of unique red loci. Note that these predictions make no assumptions about specific mechanisms of postreceptoral color coding and in particular make no distinction between unique hues and other hues. All wavelengths are simply shifted by the amount required to preserve the hue expected for color signals that vary in bandwidth.
Figure 7.
(Left) Constant hue loci as a function of bandwidth for the standard observer, assuming no compensation for spectral screening. Complete compensation instead predicts straight lines of constant center wavelength. (Right) Corresponding predictions for the Abney effect, shown in the CIE diagram for comparison with observed measurements. In this case, the stimulus is desaturated by mixing a monochromatic light with an equal-energy white. This produces straight hue lines for a linear model, whereas the curved contours shown are predicted if the visual system instead applies a correction for the purity change that would be appropriate if the desaturated chromaticity resulted from a change in spectral bandwidth. For the extraspectral reds, the predictions were instead generated for inverse Gaussian spectra [with increasing purity modeled by subtracting from a flat spectrum a Gaussian of fixed bandwidth (100 nm) but increasing height]. Solid lines, unfilled symbols: predicted constant hue loci; dotted blue lines, blue symbols: observed constant hue loci replotted from Burns et al. (1984) for one participant (AE); unconnected red and green squares: observed unique red and green settings for the same observer.
To the extent that this adjustment removes the filtering effects specific to an individual’s eye—and to how sensitivity within the eye varies at different locations—it removes differences in spectral sensitivity as a likely basis for individual differences in color appearance. On the other hand, the Abney effect itself should be tied to these differences. Large individual differences have been noted in the Abney effect (Burns et al., 1984; Ikeda & Uehira, 1989; Kurtenbach et al., 1984) and have even been seen as a deterrent to understanding it (Ikeda & Uehira, 1989). However, the present account suggests that these differences could again be subsumed under the same principle. In particular, it should be possible to predict an individual’s Abney effect from measurements of their spectral sensitivity because the effect may represent a postreceptoral correction specifically designed to discount their sensitivity.
An important question raised by this analysis is whether natural spectra can be meaningfully approximated by their centroid and dispersion, and whether these are stimulus properties that the visual system might reasonably adopt for representing color. We modeled these parameters using Gaussian spectra. Most treatments of reflectances and illuminants have instead focused on linear models in which the lights and surfaces are defined by mixtures of a small number of basis functions (Hurlbert, 1998; Maloney, 2003b). However, MacLeod and Golz (2003) have recently developed a model of stimulus and cone spectra entirely based on Gaussian functions and have shown that this “Gaussian World” can account for many aspects of the color signal and color coding that pose difficulties for linear models. One example is that the Gaussian model can be readily applied to narrowband spectra, whereas linear models are normally restricted to describing broadband stimuli. In this model, an illuminant or reflectance function is determined by its spectral centroid and curvature (i.e., bandwidth), and for such stimuli a simple and plausible rule for color coding would be to associate hue with the centroid. That is, stimuli with the same centroid should be represented by the same hue. This may more often apply to the problem of how hues are assigned to different surfaces with different (e.g., narrow or broad) reflectance spectra but could also apply to judging the same surface in different contexts (e.g., seen under narrow or broad illuminants). If the cone sensitivities are also approximated by Gaussians, then the constant hue loci (i.e., loci of constant centroid) can be analytically solved and are straight lines in log cone excitation space (MacLeod & Golz, 2003), which again provide a prediction of the Abney effect that is similar in theory to the one we derived (see also Shklover, 1958).
Our results, and the Abney effect itself, are qualitatively consistent with this rule. However, the underlying principle—that constant hues reflect inferences about the stimulus—is a general one and thus not tied to a specific model of the stimulus, and in particular to the specific assumption of a Gaussian model. There are in fact a number of ways in which the Gaussian assumption is an oversimplification. Like linear models, the Gaussian model cannot easily approximate some spectra, such as the cutoff filters characteristic of natural reds and yellows, and it remains an open question whether it provides a more viable formulation of spectral distributions (Maloney, 2003a). A Gaussian assumption also quantitatively fails in our case by predicting too much curvature in the Abney effect around unique yellow and longer wavelengths (Figure 7). Whether models derived from natural color signals yield a better quantitative account of the observed nonlinearities might clarify the specific assumptions that color coding embodies about stimulus spectra. A related limitation is that we have assumed that the visual system fully adopts the inference that differences in purity reflect differences in bandwidth rather than dilution by a fixed desaturant, yet in the natural environment both types of physical events and spectra might occur. Indeed, the weaker observed curvature in the Abney effect compared to the bandwidth prediction might reflect a weighted guess about the possible bases for the sampled chromaticity. In any case, because additive mixtures predict no curvature, the nonlinearities in hue loci again suggest that the visual system gives considerable weight to an inference like spectral bandwidth.
Conclusions
Many perceptual judgments are compensated for sensitivity limitations in the visual system. For example, in spatial vision, perceived contrast (Georgeson & Sullivan, 1975) and subjective focus (Webster, Georgeson, & Webster, 2002) are largely compensated for optical and neural blur, whereas in color vision differences in spectral sensitivity do not predict which stimuli appear achromatic (Beer, Wortman, Horwitz, & MacLeod, 2005; Delahunt, Webster, Ma, & Werner, 2004; Werner & Schefrin, 1993) or the loci of unique hues (Brainard et al., 2000; Miyahara et al., 1998; Pokorny & Smith, 1987; Schefrin & Werner, 1990; Webster et al., 2000). Here we have shown that a similar principle can potentially explain how the hue of a stimulus varies with purity. Paradoxically, the interactions between hue and saturation revealed by the Abney effect may actually reflect compensatory adjustments designed to maintain the hue of a stimulus when its purity is reduced by broadening the spectrum. From this perspective, the very fact that constant hue loci are nonlinear suggests that the visual system does not infer a linear model to interpret stimulus color, and we show that the curvature of hue loci are instead qualitatively consistent with an alternative model in which hue and saturation represent inferences about spectral features such as the centroid and bandwidth of the color signal.
Acknowledgments
We are very grateful to D. I. A. MacLeod and J. D. Mollon for comments on an earlier version of the manuscript. This research was supported by National Institutes of Health Grants EY10834 (MW) and AG04058 (JW).
Footnotes
Commercial relationships: none.
Contributor Information
Yoko Mizokami, Department of Psychology, University of Nevada, Reno, Reno, NV, USA.
John S. Werner, Department of Ophthalmology and Vision Science Section of Neurobiology, Physiology and Behavior, University of CA, Davis, CA, USA
Michael A. Crognale, Department of Psychology, University of Nevada, Reno, Reno, NV, USA
Michael A. Webster, Department of Psychology, University of Nevada, Reno, Reno, NV, USA
References
- Abney W. On the changes in hue of spectrum colors by dilution with white light. Proceedings of the Royal Society of London. 1910;82:120–127. [Google Scholar]
- Ayama M, Nakatsue T, Kaiser PK. Constant hue loci of unique and binary hues at 10, 100, and 1000 Td. Journal of the Optical Society of America A. 1987;4:1136–1144. doi: 10.1364/josaa.4.001136. [DOI] [PubMed] [Google Scholar]
- Barlow HB. What causes trichromacy? A theoretical analysis using comb-filtered spectra. Vision Research. 1982;22:635–643. doi: 10.1016/0042-6989(82)90099-2. [DOI] [PubMed] [Google Scholar]
- Beer D, Wortman J, Horwitz G, MacLeod D. Compensation of white for macular filtering [Abstract] Journal of Vision. 2005;5(8):282a. doi: 10.1167/5.8.282. http://journalofvision.org/5/8/282/ [DOI]
- Bonnardel V, Bellemare H, Mollon JD. Measurements of human sensitivity to comb-filtered spectra. Vision Research. 1996;36:2713–2720. doi: 10.1016/0042-6989(96)00014-4. [DOI] [PubMed] [Google Scholar]
- Brainard DH, Roorda A, Yamauchi Y, Calderone JB, Metha A, Neitz M, et al. Functional consequences of the relative numbers of L and M cones. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2000;17:607–614. doi: 10.1364/josaa.17.000607. [DOI] [PubMed] [Google Scholar]
- Brainard DH, Wandell BA. Asymmetric color matching: How color appearance depends on the illuminant. Journal of the Optical Society of America A, Optics and Image Science. 1992;9:1443–1448. doi: 10.1364/josaa.9.001433. [DOI] [PubMed] [Google Scholar]
- Buck SL. Influence of rod signals on hue perception: Evidence from successive scotopic contrast. Vision Research. 1997;37:1295–1301. doi: 10.1016/s0042-6989(96)00276-3. [DOI] [PubMed] [Google Scholar]
- Burns SA, Elsner AE, Pokorny J, Smith VC. The Abney effect: Chromaticity coordinates of unique and other constant hues. Vision Research. 1984;24:479–489. doi: 10.1016/0042-6989(84)90045-2. [DOI] [PubMed] [Google Scholar]
- Cao D, Pokorny J, Smith VC. Matching rod percepts with cone stimuli. Vision Research. 2005;45:2119–2128. doi: 10.1016/j.visres.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Chichilnisky EJ, Wandell BA. Trichromatic opponent color classification. Vision Research. 1999;39:3444–3458. doi: 10.1016/s0042-6989(99)00033-4. [DOI] [PubMed] [Google Scholar]
- Dartnall HJ. The interpretation of spectral sensitivity curves. British Medical Bulletin. 1953;9:24–30. doi: 10.1093/oxfordjournals.bmb.a074302. [DOI] [PubMed] [Google Scholar]
- Delahunt PB, Webster MA, Ma L, Werner JS. Long-term renormalization of chromatic mechanisms following cataract surgery. Visual Neuroscience. 2004;21:301–307. doi: 10.1017/S0952523804213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgeson MA, Sullivan GD. Contrast constancy: Deblurring in human vision by spatial frequency channels. The Journal of Physiology. 1975;252:627–656. doi: 10.1113/jphysiol.1975.sp011162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert A. Computational models of color constancy. In: Walsh V, Kulikowski J, editors. Perceptual constancy: Why things look as they do. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Hurvich LM, Jameson D. An opponent–process theory of color vision. Psychological Review. 1957;64:384–404. doi: 10.1037/h0041403. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Uehira I. Unique hue loci and implications. Color Research and Application. 1989;6:318–324. [Google Scholar]
- Jordan G, Mollon JD. Rayleigh matches and unique green. Vision Research. 1995;35:613–620. doi: 10.1016/0042-6989(94)00153-d. [DOI] [PubMed] [Google Scholar]
- Kaiser P, Boynton RMB. Human color vision. USA: Optical Society of America; 1996. [Google Scholar]
- Knoblauch K, Shevell SK. Relating cone signals to color appearance: Failure of monotonicity in yellow/blue. Visual Neuroscience. 2001;18:901–906. doi: 10.1017/s0952523801186062. [DOI] [PubMed] [Google Scholar]
- Kuehni RG. Variability in unique hue selection: A surprising phenomenon. Color Research and Application. 2004;29:158–162. [Google Scholar]
- Kulp TD, Fuld K. The prediction of hue and saturation for non-spectral lights. Vision Research. 1995;35:2967–2983. doi: 10.1016/0042-6989(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Kurtenbach W, Sternheim CE, Spillmann L. Change in hue of spectral colors by dilution with white light (Abney effect) Journal of the Optical Society of America A, Optics and Image Science. 1984;1:365–372. doi: 10.1364/josaa.1.000365. [DOI] [PubMed] [Google Scholar]
- Larimer J. Opponent–process additivity: I. Red–green equilibria. Vision Research. 1974;14:1127–1140. doi: 10.1016/0042-6989(74)90209-0. [DOI] [PubMed] [Google Scholar]
- Larimer J, Krantz DH, Cicerone CM. Opponent process additivity: II. Yellow/blue equilibria and nonlinear models. Vision Research. 1975;15:723–731. doi: 10.1016/0042-6989(75)90291-6. [DOI] [PubMed] [Google Scholar]
- MacLeod DIA, Golz J. A computational analysis of colour constancy. In: Mausfeld R, Heyer D, editors. Colour perception: Mind and the physical world. Oxford: Oxford University Press; 2003. pp. 205–242. [Google Scholar]
- MacLeod DI, Webster MA. Direct psychophysical estimates of the cone-pigment absorption spectra. Journal of the Optical Society of America A, Optics and Image Science. 1988;5:1736–1743. doi: 10.1364/josaa.5.001736. [DOI] [PubMed] [Google Scholar]
- Maloney LT. Commentary on MacLeod and Golz: The importance of realistic models of surface and light in the study of human color vision. In: Mausfeld R, Heyer D, editors. Colour perception: Mind and the physical world. Oxford: Oxford University Press; 2003a. pp. 243–246. [Google Scholar]
- Maloney LT. Surface colour perception and environmental constraints. In: Mausfeld R, Heyer D, editors. Colour perception: Mind and the physical world. Oxford: Oxford University Press; 2003b. pp. 279–300. [Google Scholar]
- Miyahara E, Pokorny J, Smith VC, Baron R, Baron E. Color vision in two observers with highly biased LWS/MWS cone ratios. Vision Research. 1998;38:601–612. doi: 10.1016/s0042-6989(97)88334-4. [DOI] [PubMed] [Google Scholar]
- Mollon JD. Color vision. Annual Review of Psychology. 1982;33:41–85. doi: 10.1146/annurev.ps.33.020182.000353. [DOI] [PubMed] [Google Scholar]
- Mollon JD, Jordan G. On the nature of unique hues. In: Dickenson C, Murray I, Carden D, editors. John Dalton’s colour vision legacy. London: Taylor and Francis; 1997. pp. 381–392. [Google Scholar]
- Neitz J, Carroll J, Yamauchi Y, Neitz M, Williams DR. Color perception is mediated by a plastic neural mechanism that is adjustable in adults. Neuron. 2002;35:783–792. doi: 10.1016/s0896-6273(02)00818-8. [DOI] [PubMed] [Google Scholar]
- Neitz J, Neitz MN. Molecular genetics and the biological basis of color vision. In: Backhaus GK, Kliegl R, Werner JS, editors. Color vision: Perspectives from different disciplines. Berlin: Walter de Gruyter; 1998. pp. 101–119. [Google Scholar]
- Nerger JL, Volbrecht VJ, Ayde CJ. Unique hue judgments as a function of test size in the fovea and at 20-deg temporal eccentricity. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1995;12:1225–1232. doi: 10.1364/josaa.12.001225. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Smith VC. Evaluation of single-pigment shift model of anomalous trichromacy. Journal of the Optical Society of America. 1977;67:1196–1209. doi: 10.1364/josa.67.001196. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Smith VC. L/M cone ratios and the null point of the perceptual red/green opponent system. Die Farbe. 1987;34:53–57. [Google Scholar]
- Schefrin BE, Werner JS. Loci of spectral unique hues throughout the life span. Journal of the Optical Society of America A, Optics and Image Science. 1990;7:305–311. doi: 10.1364/josaa.7.000305. [DOI] [PubMed] [Google Scholar]
- Scheibner H, Kremer T. Deuteranomaly studied with four perceptual criteria. Vision Research. 1996;36:3157–3166. doi: 10.1016/0042-6989(95)00020-8. [DOI] [PubMed] [Google Scholar]
- Shklover DA. National Physical Laboratory Symposium No. 8: Visual Problems of Colour Volume II. London: Her Majesty’s Stationary Office; 1958. The equicontrast colorimetric system; pp. 603–614. [Google Scholar]
- Smith VC, Pokorny J. Chromatic-discrimination axes, CRT phosphor spectra, and individual variation in color vision. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 1995;12:27–35. doi: 10.1364/josaa.12.000027. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Auran JD, Delori FC. The macular pigment: II. Spatial distribution in primate retinas. Investigative Ophthalmology & Visual Science. 1984;25:674–685. [PubMed] [Google Scholar]
- Stockman A, Sharpe LT. Cone spectral sensitivities and color matching. In: Gegenfurtner KR, Sharpe LT, editors. Color Vision: From Genes to Perception. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Volbrecht VJ, Nerger JL, Imhoff SM, Ayde CJ. Effect of the short-wavelength-sensitive-cone mosaic and rods on the locus of unique green. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2000;17:628–634. doi: 10.1364/josaa.17.000628. [DOI] [PubMed] [Google Scholar]
- Webster MA, Georgeson MA, Webster SM. Neural adjustments to image blur. Nature Neuroscience. 2002;5:839–840. doi: 10.1038/nn906. [DOI] [PubMed] [Google Scholar]
- Webster MA, MacLeod DI. Factors underlying individual differences in the color matches of normal observers. Journal of the Optical Society of America A, Optics and Image Science. 1988;5:1722–1735. doi: 10.1364/josaa.5.001722. [DOI] [PubMed] [Google Scholar]
- Webster MA, Miyahara E, Malkoc G, Raker VE. Variations in normal color vision: II. Unique hues. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2000;17:1545–1555. doi: 10.1364/josaa.17.001545. [DOI] [PubMed] [Google Scholar]
- Webster MA, Mollon JD. Colour constancy influenced by contrast adaptation. Nature. 1995;373:694–698. doi: 10.1038/373694a0. [DOI] [PubMed] [Google Scholar]
- Webster MA, Mollon JD. Adaptation and the color statistics of natural images. Vision Research. 1997;37:3283–3298. doi: 10.1016/s0042-6989(97)00125-9. [DOI] [PubMed] [Google Scholar]
- Webster MA, Webster SM, Bharadwadj S, Verma R, Jaikumar J, Madan G, et al. Variations in normal color vision: III. Unique hues in Indian and United States observers. Journal of the Optical Society of America A, Optics, Image Science, and Vision. 2002;19:1951–1962. doi: 10.1364/josaa.19.001951. [DOI] [PubMed] [Google Scholar]
- Werner JS. Visual problems of the retina during ageing: Compensation mechanisms and colour constancy across the life span. Progress in Retinal and Eye Research. 1996;15:621–645. [Google Scholar]
- Werner JS, Schefrin BE. Loci of achromatic points throughout the life span. Journal of the Optical Society of America A, Optics and Image Science. 1993;10:1509–1516. doi: 10.1364/josaa.10.001509. [DOI] [PubMed] [Google Scholar]
- Westphal H. Unmittlebare Bestimmung der Urfarben. Zeitschrift fur Sinnesphysiologie. 1909;44:479–486. [Google Scholar]
- Wooten BR, Hammond BR, Jr, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Investigative Ophthalmology & Visual Science. 1999;40:2481–2489. [PubMed] [Google Scholar]