Abstract
Purpose
To study the anatomic details of retinal angiomatous proliferation (RAP) in patients with age-related macular degeneration (AMD) using high-resolution Fourier-domain optical coherence tomography (Fd-OCT) and its three-dimensional reconstructions.
Methods
A Fd-OCT instrument was used to image five patients clinically diagnosed with RAP. A series of 100 raster-scanned B-scans centered over the macula was registered and rendered as a three-dimensional volume. These retinal structures were analyzed for anatomic details of the RAP lesions.
Results
The RAP lesion could be identified within the retina on Fd-OCT in all five cases. Fd-OCT images of the first four cases revealed areas of intraretinal neovascularization (IRN) in the deep retina adjacent to a pigment epithelial detachment (PED). There was neovascular proliferation anteriorly and posteriorly through a break in the retinal pigment epithelium (RPE). In three of the four cases, Bruch membrane remained intact. There was no identifiable choroidal neovascularization (CNV). The fifth case had both subretinal and sub-RPE neovascular membranes without a PED.
Conclusion
Fd-OCT provides unprecedented in vivo detail of the anatomy of RAP lesions that nearly resembles histologic specimens. This study suggests that the initial neovascular process in RAP can originate either within the retina or in the sub-RPE space.
Keywords: chorioretinal anastomosis, exudative age, related macular degeneration, Fourier, domain optical coherence tomography, high resolution optical coherence tomography, intraretinal neovascularization, retinal angiomatous proliferation, staging system, three, dimensional reconstructions
Retinal angiomatous proliferation (RAP) represents a distinct form of neovascular age-related macular degeneration (AMD). Accounting for approximately 10% of newly diagnosed exudative AMD, RAP tends to affect the elderly white population, has a greater associated risk of second eye involvement, and carries a poorer outcome from current treatment modalities.1
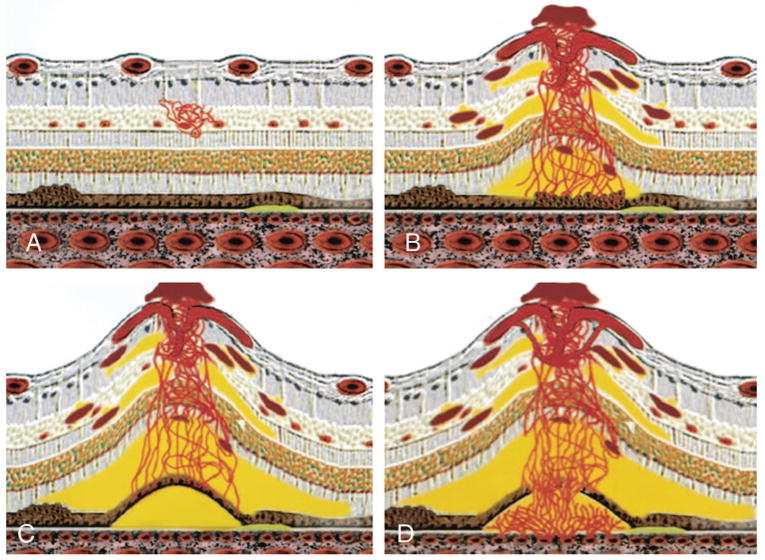
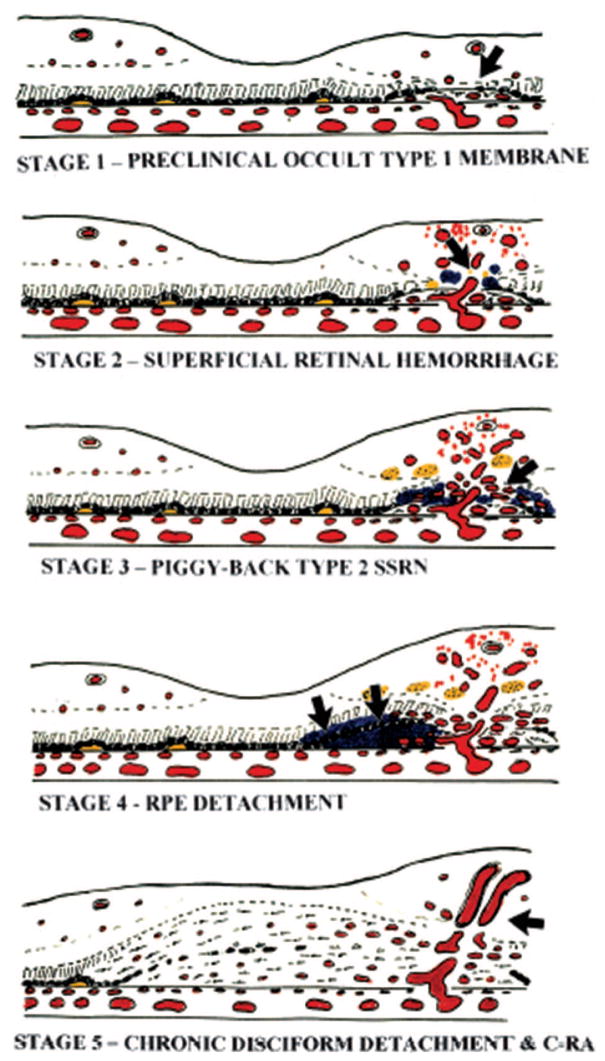
The clinical hallmark of RAP is the presence of an intraretinal vascular lesion. Hartnett et al originally labeled it as a retinal angiomatous lesion,2 then referred to it as a deep retinal vascular anomalous complex,3 based on clinical and angiographic examination of 11 patients. In subsequent studies, this lesion was also known as retinal choroidal anastomosis.4,5 In 2001, Yannuzzi et al6 re-examined this form of neovascular AMD and coined the currently accepted term RAP. They developed a three-stage system based on clinical and angiographic observations (Figure 1). In their model, the neovascularization originated within the deep retinal layers and extended posteriorly to anastomose with a subretinal pigment epithelium (RPE) neovascularization (Type 1 lesion). Thus, a RAP lesion could be classified as Stage I, II, or III when there was intraretinal neovascularization, sub-retinal neovascularization, or sub-RPE neovascularization present, respectively. Concurrently, Gass et al7 independently developed a separate model for RAP that involved five stages based on clinical and angiographic observations (Figure 2). However, Gass et al postulated that the initial neovascular event was an occult choroidal Type 1 neovascular membrane which extended anteriorly to form a Type 2 “piggyback” neovascular membrane. Ultimately, the result was a retinal-choroidal anastomosis.
Fig. 1.
Yannuzzi classification system of retinal angiomatous proliferation. A, Stage I: intraretinal neovascularization. B, Stage II: subretinal neovascularization with a retinal-retinal anastomosis. C, Stage II: subretinal neovascularization with a serous pigment epithelial detachment. D, Stage III: choroidal neovascularization with a vascularized pigment epithelial detachment and a retinal-choroidal anastomosis.6
Fig. 2.
Gass classification system of retinal angiomatous proliferation. Stage 1. Atrophy of the outer retina (arrow) with approximation of retinal capillaries and occult Type 1 subretinal pigment epithelium choroidal new vessels. Stage 2. Anastomosis (arrow) of retinal capillaries with Type 1 new vessels. Stage 3. Proliferation of Type 2 new vessels in the subretinal space (arrow). SSRN, subsensory retinal choroidal neovascularization. Stage 4. Activation of Type 1 vessels causing serous retinal pigment epithelium (RPE) detachment (arrows). Stage 5. Compound piggyback Type 2-Type 1 cicatricial disciform lesion with overt CRA (arrow).7
To date, there have been few histologic studies of RAP lesions. In 2000, Lafaut el al8 performed histologic examination of surgically excised neovascular membranes that were angiographically consistent with RAP. They demonstrated histologically that the neovascular membrane was located in the subretinal space, and was strongly adherent to the outer neurosensory retina. In 2005, Shimada et al9 performed immunohistochemical studies on surgically excised neovascular membranes and demonstrated that intraretinal neovascularization can occur without the presence of a sub-RPE neovascularization, and thereby supported Yannuzzi’s staging system. There are no reports of histologic studies of RAP lesions in whole eyes.
Optical coherence tomography (OCT) provides cross-sectional images of the retina in live patients that resemble histologic analysis.10–12 OCT has emerged as an important diagnostic tool in the evaluation of macular diseases in live patients. There is one report using OCT-3 to image RAP.6 However, the commercially available instrument, OCT-3 (Carl Zeiss, Meditec, Inc., Dublin, CA), employs a time-domain OCT technique that requires long acquisition times and provides axial and lateral resolutions on the order of 15 μm. This slow speed and relatively low resolution of this system does not allow adequate identification of RAP lesions or other neovascular tissue. On the other hand, Fourier-domain optical coherence tomography (Fd-OCT) acquires data up to 20 to 40 times faster and achieves higher resolution (3 μm axial, 10 μm transverse) images using a broader bandwidth lightsource from a superluminescent diode light source.13–16 From the raster-acquired sets of high resolution B-scan images of the macula, three-dimensional reconstructions of the neovascular complex can be created. This high resolution OCT system provides unprecedented in vivo detail of the anatomy of the macula that nearly resembles histologic specimens and has been shown to provide additional information about macular details missed on single OCT-3 scans.16 In this study, we used custom software developed at the Institute for Data Analysis and Visualization (IDAV), University of California, Davis, to visualize and reconstruct three-dimensional images of the macula of patients clinically diagnosed with RAP. These OCT images were then used to study the anatomic details of the RAP lesion and correlate the findings to the two models of RAP proposed by Yannuzzi et al and Gass et al.
Materials and Methods
We studied five eyes of five patients (three male, two female, aged 58–88 years) who were evaluated for neovascular AMD and diagnosed with RAP by the Retina Service at the University of California, Davis Department of Ophthalmology between September 2005 and June 2006. All patients underwent complete ophthalmologic examination with slit-lamp biomicroscopy and fundus photography. All patients underwent fundus fluorescein angiography (FA); one patient also had indocyanine green angiography (ICG). All angiograms were evaluated independently by five of the authors (S.N.T., S.A., D.G.T., S.S.P., and L.S.M.). Written consents were then obtained for Fd-OCT imaging, with approval by the Office of Human Research Protection of the University of California, Davis School of Medicine.
A state-of-the-art Fd-OCT instrument, constructed at the University of California, Davis Medical Center,17 was used to image the five patients. The instrument used a superluminescent diode as a light source, model D855 (855 nm @ 75 mm bandwidth, Superlum Diodes Ltd., Moscow, Russia) and created images with an axial resolution of 4.5 μm and calculated transverse resolution between 10 and 15 μm. A raster series of 100 B-scans (1000 A-scans/frame, 9 frames/s) imaged over a 6 mm × 6 mm × 2 mm volume of retina (lateral × lateral × depth) centered over the RAP lesion was obtained. The total acquisition time for a single macular sweep was 11 seconds. Each consecutive B-scan image was laterally separated by 60 μm on the retina. After the 100 B-scans were acquired, the images were registered using custom software to minimize fine axial motion artifacts. The images of the macula were then reconstructed in three dimensions.
Results
Case 1
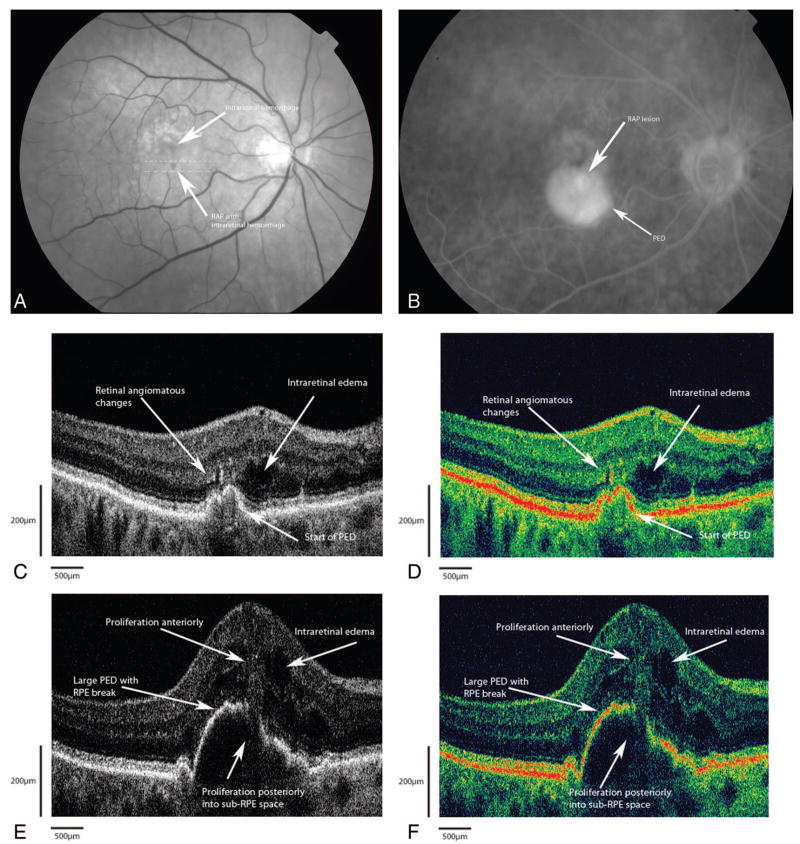
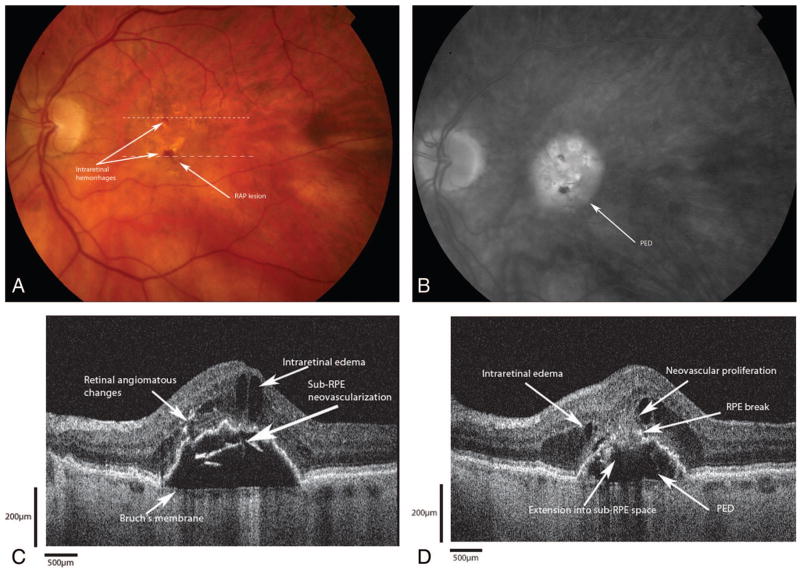
An 80-year-old woman was referred to the retina service for evaluation of decreased vision in both eyes (Figure 3A). Best-corrected visual acuities were 20/400 in both eyes. Anterior segment examinations revealed a well-centered posterior chamber intraocular lens in the right eye with a dense brunescent cataract in the left eye. Fundus examination revealed large, soft drusen in the macula with RPE changes bilaterally. In the right eye, there was a large intraretinal hemorrhage overlying an angiomatous lesion just inferior to the fovea with an adjacent pigment epithelial detachment (PED). FA showed early hyperfluorescence of the angiomatous lesion with leakage and pooling into a large PED in the right eye (Figure 3B). There was no leakage in the left eye. Fd-OCT B-scans, shown in grayscale (Figure 3C) and false color (Figure 3D) renditions, clearly demonstrate diffuse intraretinal edema with cystoid changes in the fovea. There were focal areas of hyper-reflectivity within the outer retina and underneath the retina, which localized to the angiomatous changes seen clinically. The foci of hyper-reflectivity appeared to emanate from these areas posteriorly through a break in the RPE into the sub-RPE space (Figure 3, E and F). Note that the neovascular proliferation into the sub-RPE space is seen slightly clearer on the false-color rendition. No highly reflective lesion suggestive of foci of neovascularization was seen in the sub-RPE space.
Fig. 3.
Patient 1. A, Red free fundus photograph shows the retinal angiomatous proliferation lesion just inferior to the fovea with an intraretinal hemorrhage. The dotted lines correspond to the Fourier-domain optical coherence tomography (Fd-OCT) images presented. B, Fluorescein angiogram showing diffuse hyperfluorescence with pooling consistent with a large pigment epithelial detachment (PED). C, Grayscale rendition of Fd-OCT B-scan (small dashed line on fundus photo) shows highly reflective areas that correspond to the deep angiomatous changes and intraretinal neovascularization with intraretinal edema. D, False-color rendition of same B-scan as in C. E, Grayscale rendition Fd-OCT B-scan (large dashed line on fundus photograph) shows a large PED with proliferation from the deep retina through a break in the retinal pigment epithelium (RPE). The anterior extent of the proliferation approaches the inner retina via a retina-retinal anastomosis. No identifiable sub-RPE hyper-reflectivity suggestive of a choroidal neovascular complex is seen. F, False-color rendition of same B-scan as in E. Note that the sub-RPE extension of the hyperreflective lesion is slightly better visualized in false color.
Case 2
A 72-year-old woman presented to the retina service with complaints of progressive visual loss in her left eye over the previous 6 months (Figure 4A). Best-corrected visual acuities were 20/25 in the right eye and 20/30 in the left eye. Fundus examination revealed large, soft, confluent drusen in both eyes. In the left eye, there were intraretinal hemorrhages with a grayish area of angiomatous change within the retina. FA of the left eye (Figure 4B) showed diffuse leakage subfoveally. Indocyanine green angiography (ICG) of the left eye (Figure 4C) revealed a focal “hot spot” in the area of angiomatous changes. The patient was successfully treated with combination photodynamic therapy (PDT) and intravitreal Kenalog for RAP in the left eye. Eight months after treatment, she presented with a recurrence of the RAP lesion, and received a second round of combination therapy. Seven months after her second treatment, the patient presented with a recurrence of poor vision. FA revealed the presence of leakage, similar to the FA appearance before her treatment. Fd-OCT (Figure 4D) showed the presence of intraretinal edema associated with a PED. Highly reflective lesions within the deep retinal layers and in the subretinal space suggestive of intraretinal and subretinal neovascularization were seen. This highly reflective lesion extended anteriorly towards the superficial retina as well as posteriorly through a break in the RPE (Figure 4E). This is suggestive of an early sub-RPE extension of the highly reflective lesion.
Fig. 4.
Patient 2. A, Color fundus photograph of retinal angiomatous proliferation (RAP) shows intraretinal hemorrhages overlying a grayish mound of deep angiomatous changes just superior to the fovea. The dotted lines correspond to the Fourier-domain optical coherence tomography (Fd-OCT) images presented. B, Fluorescein angiogram shows intense hyperfluorescence of the RAP lesion with ill-defined zone of leakage around the angiomatous changes. C, Indocyanine green angiogram shows a focal area of intense hyperfluorescence (“hot spot”). D, Fd-OCT image (small dashed line) shows multifocal areas of high reflectivity corresponding to the angiomatous changes. Adjacent to the RAP lesions are areas of intraretinal edema. There is tenting up of the retinal pigment epithelium (RPE) under the neovascular proliferation, forming a pocket of subretinal fluid. Note that Bruch’s membrane is intact. E, Fd-OCT B-scan (large dashed line) shows the extension from the intraretinal neovascularization through a break in the RPE to an early lesion in the sub-RPE space. Note that Bruch’s membrane is still intact. F, Three-dimensional reconstruction of the macula with cut through the RAP complex. The B-scans are taken from the x plane.
Case 3
An 88-year-old man was evaluated for decreased vision in his left eye over a 1-week period (Figure 5A). Best-corrected visual acuities of 20/400 in the right eye and 20/60 in the left eye were obtained. Fundus examination revealed an evolving disciform scar in the right eye. The left eye had intraretinal hemorrhages on an elevated mound of intraretinal anomalies. FA (Figure 5B) demonstrated an ill-defined zone of hyperfluorescence around the hemorrhages with an associated PED. Fd-OCT showed evidence of diffuse intraretinal edema with cystic changes (Figure 5C). There were highly reflective areas in the deep retina and the subretinal space above a large PED, suggestive of intraretinal neovascularization. The proliferation extended deep from this area of neovascularization through a break in the RPE (Figure 5D).
Fig. 5.
Patient 3. A, Color photograph shows a retinal angiomatous proliferation (RAP) lesion with intraretinal hemorrhages superior to and inferior to the fovea. The dotted lines correspond to the Fourier-domain optical coherence tomography (Fd-OCT) images presented. B, Fluorescein angiogram shows ill-defined zone of hyperfluorescence pooling into an associated pigment epithelial detachment (PED). C, Fd-OCT image (small dashed line) shows a large PED with several areas of high reflectivity in the deep retina and intraretinal cystic edema overlying a PED. Underneath the retinal pigment epithelium (RPE), there appears to be a sub-RPE highly reflective lesion. Bruch’s membrane remains intact. D, Fd-OCT (large dashed line) shows the extension of the highly reflective lesion into the sub-RPE space through the RPE break. Bruch’s membrane appears intact. Note the shadowing effects from the angiomatous changes overlying the RPE break.
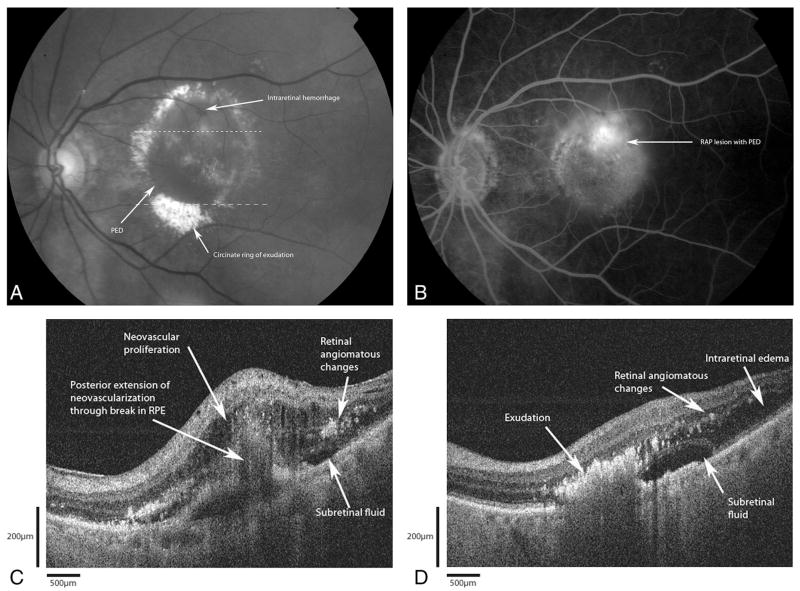
Case 4
An 85-year-old woman presented with complaints of decreased vision and increased metamorphopsia in her left eye over the past year (Figure 6A). Best-corrected visual acuities were 20/25 in the right eye and 20/200 in the left eye. Fundus examination of the right eye revealed diffuse large drusen with RPE changes in the macula. In the left eye, there were intraretinal hemorrhages with a large PED and a circinate ring of exudation. FA (Figure 6B) revealed hyperfluorescence surrounding the areas of hemorrhage with a large PED. The B-scan from Fd-OCT (Figure 6C) revealed intraretinal hyper-reflectivity suggestive of intraretinal proliferation. This lesion extended posteriorly through a break in the RPE into the sub-RPE space. No hyperreflective lesion was seen in the sub-RPE space. A second B-scan from Fd-OCT (Figure 6D) shows fluid within and underneath the retina. There is intense hyper-reflectivity in the deep retina, more intense than those neovascular lesions, corresponding to the immense exudation.
Fig. 6.
Patient 4. A, Red-free photograph of retinal angiomatous proliferation (RAP) shows an area of intraretinal hemorrhage superior to the fovea with a large pigment epithelial detachment (PED). Note the ring of circinate exudation. The dashed lines correspond to the Fourier-domain optical coherence tomography (Fd-OCT) images presented. B, Fluorescein angiogram shows leakage around the angiomatous proliferation. C, Fd-OCT (small dashed line) image shows intraretinal edema with pinpoint hyper-reflectivities representing the angiomatous changes. There is proliferation into the subretinal space with thickening of the retina. The retinal pigment epithelium shows signs of discontinuity and early detachment. Bruch’s membrane is not clearly visualized here. D, Fd-OCT (large dashed line) through areas of exudation reveal dense, intense reflectivity with shadowing corresponding to the exudation.
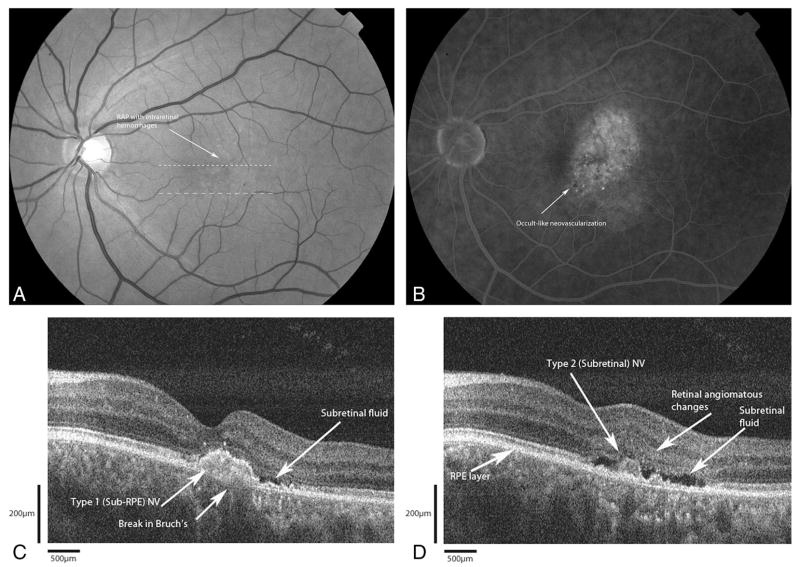
Case 5
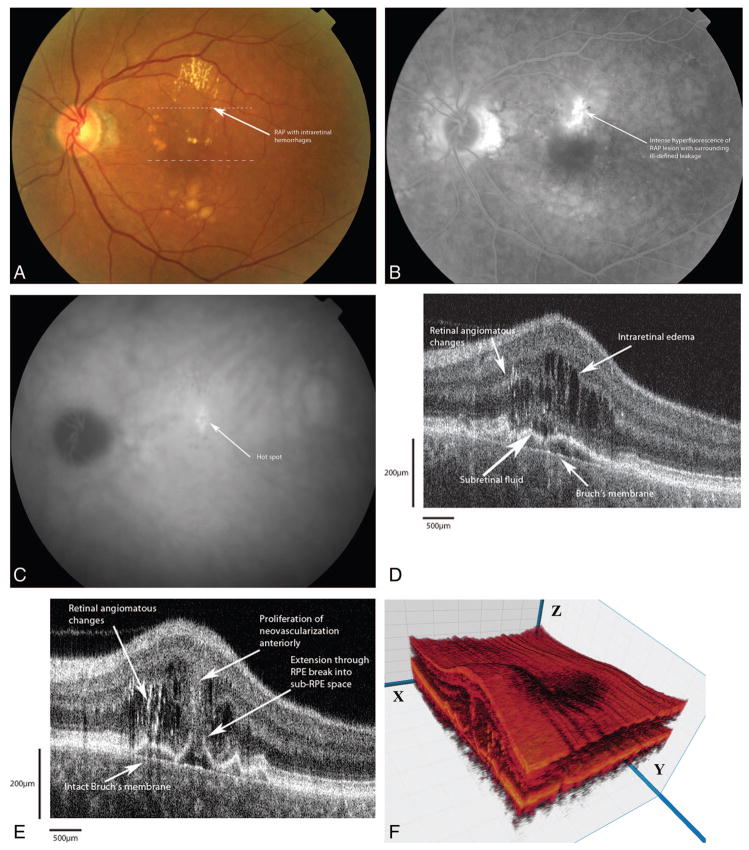
A 58-year-old man was seen with complaints of gradual decreased vision in his left eye over the previous year (Figure 7A). Best-corrected visual acuities measured 20/25 in the right eye and 20/40 in the left eye. Fundus examination of the left eye revealed a focal area of intraretinal hemorrhages over a graying mound of angiomatous changes. FA showed a well-defined area of hyperfluorescence consistent with the angiomatous proliferation with surrounding ill-defined leakage (Figure 7B). There was no associated PED seen either clinically or angiographically. Fd-OCT B-scan (Figure 7C) showed a highly reflective sub-RPE lesion suggestive of a type I neovascular membrane with a break in Bruch’s membrane. There is associated subretinal fluid. However, there is no intraretinal edema. A second Fd-OCT B-scan (Figure 7D) shows a highly reflective lesion overlying intact RPE, suggestive of a type II (subretinal) neovascular membrane, with adjacent area of intraretinal high reflection. Again, there is associated subretinal fluid, but no intraretinal edema.
Fig. 7.
Patient 5. A, Red-free photograph shows the intraretinal hemorrhages with a retinal vessel diving into a mound of angiomatous change. The dashed lines correspond to the Fourier-domain optical coherence tomography (Fd-OCT) images presented. B, Fluorescein angiogram shows an occult-like choroidal neovascularization with diffuse leakage. C, Fd-OCT shows a subretinal pigment epithelium (RPE) (Type 1) lesion under the fovea (small dashed lines) with a break in Bruch’s membrane. No pigment epithelial detachment (PED) or intraretinal edema is seen. There is subretinal fluid. D, Fd-OCT image (large dashed lines) shows a subretinal (Type 2) lesion adjacent to pinpoint areas of hyper-reflectivity corresponding to the angiomas. Again, there is no PED or intraretinal edema. Note the presence of an intact RPE beneath the type II lesion.
Discussion
RAP represents a distinct variant in the spectrum of neovascular AMD. Currently, two models exist that attempt to explain the pathogenesis of this entity. The first model, proposed by Yannuzzi et al, involves a three-stage system in which angiomatous proliferation originates within the outer neurosensory retina. In Stage I RAP, there is intraretinal neovascularization with spontaneous angiomatous proliferation of capillaries within the inner retina. These vessels then extend into the subretinal space, forming subretinal neovascularization (Stage II) with retinal-retinal anastomoses (RRA). There may also be anterior proliferation as well, and preretinal hemorrhaging may occur in 15% of subjects.6 The RRA continues its posterior extension and lifts the RPE, forming a serous PED (Stage II with PED). Finally, a retinal-choroidal anastomosis (RCA) forms through a break in the RPE layer with an underlying choroidal neovascularization (Stage III).
Gass et al proposed an alternate model involving five stages in which RAP results from anastomotic proliferation between a pre-existing type I sub-RPE neovascular membrane and a deep retinal anomalous complex. Stage 1 involves an occult Type 1 (sub-RPE) neovascular membrane associated with a focal area of atrophic outer retina. Stage 2 includes the presence of superficial retinal hemorrhages that occur at the interface of the Type 1 membrane and the outer retinal capillaries. In Stage 3, dilated capillaries of the Type 1 membrane proliferate through the RPE and anastomose with the outer retinal capillaries, forming a “piggyback” Type 2 membrane in the subretinal space. This is the first evidence of a retinal-choroidal anastomosis. This anastomosis is further evident in Stage 4, which occurs when a PED develops. Stage 5 results from further proliferation of the Type 1 and Type 2 membranes, forming a disciform scar.
Since the proposals of the two models, there has been only one histologic study of RAP. Shimada et al performed immunohistochemical studies on surgical excised neovascular membranes and supported the theory proposed by Yannuzzi et al.9 Their studies showed that intraretinal neovascular membranes can exist without the presence of a sub-RPE membrane, and that the sub-RPE membrane appeared once an anastomotic connection evolved between the retina and sub-RPE space. However, no other studies have since supported either model. Our study is the first in vivo study to use Fd-OCT to examine the morphology of the RAP lesion.
All five subjects had Fd-OCT images with highly reflective intraretinal lesions that colocalized with RAP lesions seen clinically. Using Fd-OCT, it was possible to localize the extent of these lesions to the intraretinal, subretinal, or sub-RPE layers. Focal areas of high reflectivity in the deep retinal layers correspond to the deep retinal angiomatous changes and hemorrhage seen clinically and angiographically. In most cases, these areas are seen adjacent to a large PED. These reflective areas extend posteriorly and in most cases, pass through a break in the RPE, and appear to form a retinal-choroidal anastomosis. Pre-retinal hemorrhages were not seen in any of our cases, though there was proliferative extension anteriorly from the deep retina. Intraretinal edema with cystic changes was profound, whereas there was scant sub-retinal fluid. In one case (Case 4), the presence of exudation is evident as intensely reflective areas in the subretinal space that causes a shadowing effect. This potentially could limit visualization of lesions below the exudation in the subretinal or sub-RPE spaces.
One of our cases (Case 2) had prior treatments using combination photodynamic therapy and intravitreal Kenalog for RAP before being imaged with Fd-OCT. One could argue that this treatment may have altered the anatomy of the macula. Clinically, however, the appearance of the lesion after treatment was the same as it was before treatment. It was consistent with RAP, with the presence of intraretinal hemorrhage over a mound of gray subretinal lesion. Additionally, the fluorescein angiogram before treatment and after treatment were identical.
Among our five cases, the first three cases appear to support Yannuzzi’s model for RAP. The B-scans from the Fd-OCT showed discrete, highly reflective areas within the outer retina suggestive of deep retinal angiomatous complexes. Adjacent to these areas, there was a break in the RPE layer with an apparent retinal-choroidal anastomosis passing through it into the sub-RPE space. There was an associated PED. Close examination of the B-scans revealed no clear lesion in the sub-RPE space that would suggest the presence of neovascular tissue. According to the Yannuzzi model, this case would be classified as an advanced Stage II RAP or a pre-Stage III RAP, as the lesion is seen without an identifiable sub-RPE lesion. This absence of type I neovascular lesion would not be in accordance with the Gass model. One could argue that because there is a 60 μm separation between successive B-scans, a sub-RPE neovascular membrane could be missed in our serial scans. However, given that the diameter of a retinal capillary is approximately 20–30 μm and the axial resolution of the Fd-OCT system is 3 μm, this would be unlikely.18 Additionally, in these first three cases, close analysis of all B-scans revealed an intact Bruch’s membrane.
The fourth case also appears to support the Yannuzzi classification scheme. Clinical examination and FA clearly support the diagnosis of a RAP lesion. Fd-OCT findings are consistent intraretinal neovascularization with proliferation posteriorly through a break in the RPE into the sub-RPE space, forming an early sub-RPE neovascular membrane. Similar to the three preceding cases, this case could represent the progression from a Stage II to Stage III RAP, as per Yannuzzi’s model. The interesting finding here is that Bruch’s membrane was not clearly identified in all serial B-scans. It is possible that the sub-RPE extension of the proliferation may have penetrated Bruch’s membrane.
Case 5 seems to better support Gass’ model than Yannuzzi’s model, however. Both clinical examination and FA support the diagnosis of a RAP lesion. Fd-OCT findings are consistent with a combined Type 1 sub-RPE and Type 2 subretinal neovascular membranes, associated with highly reflective areas within the deep retina consistent with RAP. This is in accordance with a stage 3 CRA, based on Gass’ model. One could argue that this could be considered a Yannuzzi Stage III RAP lesion as well. However, based on Yannuzzi’s model, the neovascular proliferation of retinal origin invades the subretinal space and creates a PED before invading the sub-RPE space. If a sub-RPE lesion is present, then a PED, either serous or fibrovascular, should be present. In this case, no PED is seen clinically, angiographically, or by Fd-OCT. It is possible, however, that a PED was present but subsequently collapsed.
In macular degeneration, vascular endothelial growth factor (VEGF) has been shown to be the most potent angiogenic factor, promoting the growth of endothelial cells and enhancing vascular permeability. It is known that the basal secretion of VEGF from damaged and ischemic RPE cells induces angiogenesis,19 with the resultant formation of a choroidal neovascularization. The presence of RAP without a choroidal neovascularization suggests that there could be an apical secretion of VEGF causing the formation of an intraretinal neovascular membrane that could proliferate posteriorly through an already-weakened RPE.
This study is the first to use Fd-OCT findings to aid in the diagnosis of RAP. With the high-resolution images obtained by Fd-OCT, it is now possible to study the anatomy and pathogenesis of the RAP lesions in vivo with detail that approaches that of histology. Associated morphologic changes such as cystoid macular edema, subretinal fluid, or pigment epithelial detachments can be visualized in greater detail. Although the false-color rendition of the OCT images reflects the familiar color scheme of the OCT-3 images commonly used, we chose in our study to use the grayscale coloring rendition. We found the grayscale images to be better at discerning retinal layers than the false-color rendition and also at highlighting the retinal angiomatous changes. The false-color rendition does, however, provide better visualization of some deeper structures, where the signals may not be strong. This is evident in our first case, where the sub-RPE extension of the neovascular proliferation appears more visible with the false-color rendition.
Although there is no histologic correlate confirming that the highly reflective intraretinal lesions seen on Fd-OCT are indeed RAP, these lesions all colocalized with the RAP lesion seen clinically in all five of our subjects. The results of this study suggest that the evolution of RAP can fit into either of the currently accepted models. RAP appears to be a continuum of variants where the initial neovascular process could originate either within the retina or the sub-RPE space. However, our case series is small, with only five patients, and only includes the advanced stages of this disease process. A larger sampling is necessary to further understand the pathogenesis of this subset of neovascular AMD and is underway. Furthermore, refinement of the Fd-OCT by combining the system with adaptive optics and scanning laser ophthalmoscopy may allow us to characterize these lesions in even greater detail.
Acknowledgments
Supported by the National Eye Institute, Bethesda, Maryland (grant no. 014743 [J.S.W.]), and Research to Prevent Blindness, New York, New York (Jules and Doris Stein Professorship [J.S.W.]).
References
- 1.Freund KB, Klais CM, Eandi CM, et al. Sequenced combined intravitreal triamcinolone and indocyanine green angiography-guided photodynamic therapy for retinal angiomatous proliferation. Arch Ophthalmol. 2006;124:487–492. doi: 10.1001/archopht.124.4.487. [DOI] [PubMed] [Google Scholar]
- 2.Hartnett ME, Weiter JJ, Gardts A, Jalkh AE. Classification of retinal pigment epithelium detachments associated with drusen. Graefes Arch Clin Exp Ophthalmol. 1992;230:11–19. doi: 10.1007/BF00166756. [DOI] [PubMed] [Google Scholar]
- 3.Hartnett ME, Weiter JJ, Staurenghi G, Elsner AE. Deep retinal vascular anomalous complexes in advanced age-related macular degeneration. Ophthalmology. 1996;103:2042–2053. doi: 10.1016/s0161-6420(96)30389-8. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn D, Meunier I, Soubrane G, Coscas G. Imaging of chorioretinal anastomoses in vascularized RPE detachments. Arch Ophthalmol. 1995;113:1392–1398. doi: 10.1001/archopht.1995.01100110052025. [DOI] [PubMed] [Google Scholar]
- 5.Slakter JS, Yannuzzi LA, Schneider U, et al. Retinal choroidal anastomoses and occult choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2000;107:742–754. doi: 10.1016/s0161-6420(00)00009-9. [DOI] [PubMed] [Google Scholar]
- 6.Yannuzzi LA, Negrao S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina. 2001;21:416–434. doi: 10.1097/00006982-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Gass JDM, Agarwal A, Lavina AM, Tawansy KA. Focal inner retinal hemorrhages in patients with drusen. An early sign of occult choroidal neovascularization and chorioretinal anastomosis. Retina. 2003;23:741–751. doi: 10.1097/00006982-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Lafaut BA, Aisenbrey S, Vanden Broecke C, et al. Clinicopathological correlation of deep retinal vascular anomalous complex in age related macular degeneration. Br J Ophthalmol. 2000;84:1269–1274. doi: 10.1136/bjo.84.11.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimada H, Kawamura A, Mori R, Yuzawa M. Clinicopathological findings of retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. 2006:1–6. doi: 10.1007/s00417-006-0367-6. (Epub) [DOI] [PubMed] [Google Scholar]
- 10.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fercher AF. Optical coherence tomography. J Biomed Opt. 1996;1:157–173. doi: 10.1117/12.231361. [DOI] [PubMed] [Google Scholar]
- 12.Drexler W, Morgner U, K GR, et al. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat Med. 2001;7:502–507. doi: 10.1038/86589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wojkowski M, Leitgeb R, Kowalczyk A, et al. In vivo human retina imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002;7:457–463. doi: 10.1117/1.1482379. [DOI] [PubMed] [Google Scholar]
- 14.Leitgeb R, Drexler W, Unterhuber A, et al. Ultrahigh resolution Fourier domain optical coherence tomography. Opt Express. 2004;12:2435–2447. doi: 10.1364/opex.12.002156. [DOI] [PubMed] [Google Scholar]
- 15.Nassif N, Cense B, Park B, et al. In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Opt Express. 2004;12:367–376. doi: 10.1364/opex.12.000367. [DOI] [PubMed] [Google Scholar]
- 16.Alam S, Zawadzki RJ, Choi SS, et al. Clinical application of rapid serial Fourier-domain optical coherence tomography for macular imaging. Ophthalmology. 2006;113:1425–1431. doi: 10.1016/j.ophtha.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zawadzki RJ, Bower BA, Zhao M, et al. Exposure time dependence of image quality in high-speed retinal in vivo Fourier-domain OCT. In: Manns F, Soederber PG, Ho A, editors. Ophthalmic Technologies. XV. Bellingham, WA: SPIE; 2005. [Google Scholar]
- 18.Park SS. The anatomy and cell biology of the retina. In: Tasman W, Jaeger FA, editors. Duane’s Foundation of Clinical Ophthalmology. Philadelphia: J P. Lippincott; 2004. pp. 1–66. [Google Scholar]
- 19.Spaide RF, Armstrong D, Browne R. Choroidal neovascularization in age-related macular degeneration—what is the cause? Retina. 2003;23:595–614. doi: 10.1097/00006982-200310000-00001. [DOI] [PubMed] [Google Scholar]