Abstract
Color appearance remains remarkably stable in the aging visual system despite large changes in the spectral distribution of the retinal stimulus and losses in chromatic sensitivity (P. B. Delahunt, J. L. Hardy, K. Okajima, & J. S. Werner, 2005; J. S. Werner, 1996). This stability could reflect adaptive adjustments in peripheral or central chromatic mechanisms that compensate for sensitivity losses in senescence. We asked whether similar compensatory adjustments play a role in maintaining spatial vision—and whether the adaptation itself shows changes with aging—by examining the effects of adaptation on judgments of image focus. Perceptual aftereffects following adaptation to a uniform field and blurred or sharpened images were compared between younger adults and older observers. Subjects adapted to a sequence of blurred or sharpened images for 120 s, and a two-alternative forced-choice staircase task was used to vary the filter exponent of the test to define the subjective point of best focus. There was a small but significant difference between younger and older observers in the level perceived as best focused in all three adaptation conditions, possibly reflecting differences in the ambient blur level the groups are routinely exposed to. However, the magnitude of the blur aftereffect did not differ between the two age groups. These results suggest that although there may be small differences in the long-term adaptation to blur, younger and older observers do not differ in the strength of adaptation to transient changes in blur. The neural processes mediating adaptation to blur thus appear to remain largely intact with aging.
Keywords: blur adaptation, spatial vision, aging, contrast sensitivity
Introduction
Senescent changes in low-level visual mechanisms are well documented (Werner & Schefrin, 2000). Retinal mechanisms suffer not only from age-related optical deterioration but also from neural losses in sensitivity as well. Optical alterations, such as a decreased pupil size (Kadlecová, Peleška, & Vaško, 1958), increased density of the lens (Weale, 1988; Werner, 1982), as well as increased higher order optical aberrations and scatter (Guirao, Redondo, & Artal, 2000; Hennelly, Barbur, Edgar, & Woodward, 1998), will contribute to a reduction in illumination and contrast of the retinal image. When these factors can be accounted for, neural losses become evident. Slowed photopigment regeneration in the foveal cone photoreceptors (Coile & Baker, 1992) as well as rods (Jackson, Owsley, & McGwin, 1999) is believed to underlie a decreased rate of dark adaptation. Schefrin, Shinomori, and Werner (1995) have shown that losses in chromatic discrimination are light dependent, a result consistent with differences between young and old at early stages of retinal processing.
Sensitivity of early stage spatial vision mechanisms also decreases with age. Contrast sensitivity loss appears to be greatest for intermediate to high spatial frequencies, especially at low illumination levels (Owsley, Sekular, & Siemsen, 1983; Sloane, Owsley, & Alvarez, 1988; Tulunay-Keesey, Ver Hoeve, & Terkla-McGrane, 1988). Optical factors can only partially explain losses in contrast sensitivity under photopic conditions (Burton, Owsley, & Sloane, 1993; Owsley et al., 1983); when optical factors are corrected, losses persist and may be attributed to neural factors (Elliott, Whitaker, & MacVeigh, 1990; Higgins, Jafe, Caruso, & deMonasterio, 1988; Sloane, Owsley, & Alvarez, 1988; Sloane, Owsley, & Jackson, 1988).
Although these findings demonstrate losses in detection and discrimination for stimuli at threshold, only minor deficits are found when the same stimuli are above threshold (Werner, Delahunt, & Hardy, 2004). At higher levels of S cone excitation, chromatic discrimination losses wane (Schefrin et al., 1995). Contrast-matching functions show no evidence of sensitivity loss where contrast sensitivity functions show selective losses at high spatial frequencies (Delahunt, Hardy, Okajima, & Werner, 2005; Tulunay-Keesey et al., 1988). One possible explanation for this stability is that mechanisms of adaptation within the visual system adjust neural responses to maintain perceptual constancy for a variety of visual stimuli (Webster, Werner, & Field, 2005). One such adaptive adjustment is the constancy of the achromatic point across a lifetime. Stimuli that appear achromatic or a unique hue remain surprisingly stable despite large age-related losses in the short wavelength light reaching the retina as the lens becomes brunescent (Schefrin & Werner, 1990; Werner & Schefrin, 1993). This example of adaptation occurs over a relatively protracted time course due to slow optical changes, although chromatic adaptation can also be very rapid when the stimulus changes (e.g., when the illuminant changes). The possibility of analogous age-related changes in a “set point” for spatial blur has not been explored. Evaluating the integrity of different adaptation processes in aging is thus fundamental to understanding the perceptual consequences of senescence as well as age-independent normalization processes.
Previous studies have documented changes in the dynamics of adaptation with aging in early retinal processes of light and dark adaptation (Coile & Baker, 1992; Jackson et al., 1999) and have also explored changes in perceptual motor adaptation (Buch, Young, & Contreras-Vidal, 2003; Paige, 1992; Roller, Cohen, Kimball, & Bloomberg, 2002). However, surprisingly little is known about the effects of aging on the types of pattern-selective aftereffects that are thought to be mediated by response changes at cortical levels. Aging may differentially affect visual performance due to senescent losses at either early or late stages of processing, or differences in processing requirements (Faubert, 2002; Habak & Faubert, 2000). For example, a recent study (Rivest, Kim, Intriligator, & Sharpe, 2004) found that older observers exhibited a reduction in shape distortion interactions—which are potentially diagnostic of adaptation in high-level vision (Suzuki, 2005)—although there were no differences in elementary shape perception.
In this study, we used adaptation to image blur in order to assess the integrity of the mechanisms underlying spatial adaptation. Blur is an important and salient feature of natural images and has a well-defined neutral point (i.e., the point of subjective focus). In natural viewing, observers are often exposed for short or long periods to blur either because of blur in the physical stimulus (Field & Brady, 1997), or much more importantly because of blur in the retinal image owing to the optical imperfections of the eye. Brief exposure to image blur has been shown to influence acuity (Pesudovs & Brennen, 1993; Mon-Williams, Tresilian, Strange, Kcohar, & Wann, 1998) as well as contrast sensitivity (Mon-Williams et al., 1998; Webster, 1999; Webster & Miyahara, 1997). In addition, Webster, Georgeson, and Webster (2002) recently demonstrated that adaptation to blur will bias the perceived focus of normally focused images: adaptation to images of faces or outdoor scenes that had been spatially filtered to appear either blurred or sharpened produced strong aftereffects in the opposite direction when viewing “in focus” stimuli. For example, adaptation to a blurred (sharpened) image caused a physically focused image to appear too sharp (blurred). These aftereffects presumably reflect response changes at a cortical locus because they depend on spatial frequency or scale-selective changes in sensitivity, and thus the aftereffects allow us to use an intuitive and functionally important visual judgment that might reveal how adaptation is affected by aging at cortical sites. Accordingly, we compared the magnitude of this aftereffect between younger and older observers to test whether changes in brief blur adaptation reveal senescent changes in short-term pattern-selective adaptation.
Methods
Subjects
Ten younger (mean age of 25.4, range of 20–33, 5 female) and 10 older (mean age of 73.9, range of 67–80; 6 female) observers participated in this experiment. Young observers reported having an eye examination within the last year and were free from ocular disease. Refractive errors did not exceed ±5 D sphere or ±2.5 D cylinder. Younger observers wore their habitual correction during the study.
Prior to testing, each older observer was screened in the Department of Ophthalmology at the University of California, Davis, by slit lamp examination, direct and indirect ophthalmoscopy to rule out the presence of abnormal ocular media and retinal disease. Color fundus photographs of the macula and optic disc (ETDRS Fields 1 and 2) were evaluated by a retinal specialist. No participant had more small (≤63 μm) drusen than is considered normal for their age or any abnormal vascular, retinal, choroidal, or optic nerve findings. Intraocular pressure was <22 mm Hg. Refractive errors did not exceed ±5 D sphere or ±2.5 D cylinder, and corrected visual acuity was 20/25 or better with the use of trial lenses. Older observers were refracted for the test distance by adding +0.87 D to their best optical correction. Written informed consent was obtained following the Tenets of Helsinki and with approval of the Institutional Review Board of the University of California, Davis, School of Medicine.
Apparatus and stimuli
Stimuli were presented on a 17-in. CRT monitor (Sony Trinitron multiscan G220) driven by a PC with 8 bits of color resolution. The experimental software was written in Visual Basic. Net. Observers adapted to a uniform gray field of 15 cd/m2 for 2 min before proceeding with the experiment.
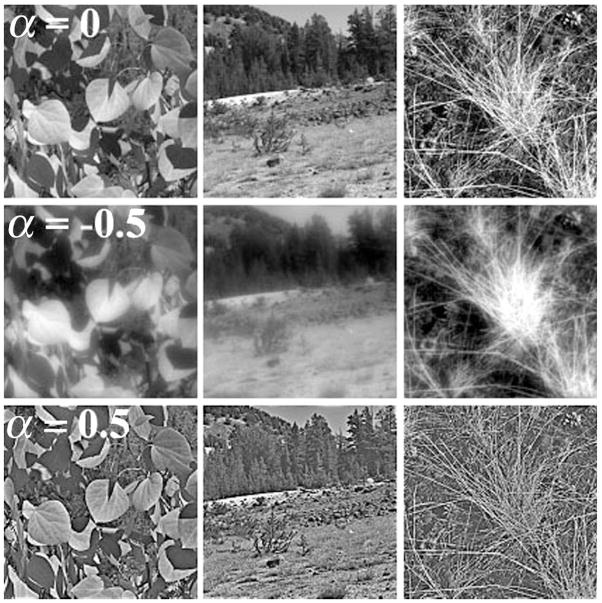
Ten grayscale images of natural scenes were used as stimuli (Webster & Miyahara, 1997); examples are shown in Figure 1. Natural images can be described by a power spectrum where the amplitude falls as the frequency (f) increases, thus defining a slope of −1(1/f) on a log-amplitude versus log-frequency plot (Field, 1987). Each image was filtered by multiplying the original amplitude spectrum by fα, with α controlling the magnitude of change of the spatial frequency distribution (Field & Brady, 1997; Tadmor & Tolhurst, 1994). For all stimuli, α was varied relative to 1/f from −1 (image appears strongly blurred) to +1 (image appears strongly sharpened) in steps of 0.5 for adapting stimuli. Two images chosen at random to be used as test stimuli were filtered in steps of 0.01 to allow for finely graded adjustments in blur magnitude. After filtering, all images had a maximum frequency of 32 c/deg with root mean square contrast equated to be equivalent to the original image to prevent subjects from using image contrast as a cue. Images had a resolution of 256 × 256 pixels and a mean luminance of 20 cd/m2.
Figure 1.
Examples of the natural images used for tests of blur adaptation. α denotes the slope. The top row presents original images before filtering (α = 0). The middle row presents the same images after filtering with attenuating high and amplified low spatial frequencies (α =−.5). The bottom row presents the images after filtering with amplified high and attenuated low spatial frequencies (α = .5).
Procedure
Natural image stimuli were presented in a 4° square patch centered on a uniform gray background with an average luminance of 15 cd/m2. The observer viewed the display binocularly at a distance of 110 cm in a windowless, dark room. The three experimental conditions included adaptation to (1) a random sequence of blurred images (α = −0.5), (2) a random sequence of sharpened images (α = +0.5), and (3) a uniform field (used as the neutral adaptation condition). Each condition was repeated three times. The random sequence included all 10 images, was resampled every 0.25 s, and was used to prevent afterimages from local light adaptation. This initial adaptation period lasted for 120 s, followed by a 0.5 s blank screen. An “in focus” (α = 0) test image was then presented for 0.5 s. The test image was chosen before the session began so it would remain constant for each observer. The subject’s task was to press one of two keys to indicate whether the focused test image appeared too blurry or too sharp as compared to their individual internal reference for image focus. Test images were interleaved with a 0.25 s uniform field followed by a 6 s top-up re-adaptation period. Using a two-alternative forced-choice staircase, this process continued until a point of subjective neutrality (PSN) was found. The PSN was the average α of the last 6 of 8 reversals averaged over the three sessions per condition.
Results
The neutral PSN (best focused filtered slope) is plotted in Figure 2 as a function of age. Younger and older observers are shown by filled and unfilled gray circles, respectively. Black circles (filled for young, unfilled for older) present the mean for each age group ±1 standard error of the mean (SEM). Due to the overlap between younger and older observers and the tendency of both groups toward a PSN that was slightly blurred, the neutral PSN settings were not significantly different for the two groups, t(18) = 1.31, p < .2 two tailed. Compared to the physically focused image, however, the two groups do appear different. The neutral PSN for younger observers was not significantly different from a physically focused image, t(18) = −0.44, p <.6 two tailed; mean PSN = −0.013 ± 0.028 SEM. The neutral PSN for the older age group, however, did show a significant shift toward blur when compared to a physically focused image, t(18) = −2.33, p <.03 two tailed; mean PSN for older = −0.070 ± 0.026 SEM.
Figure 2.
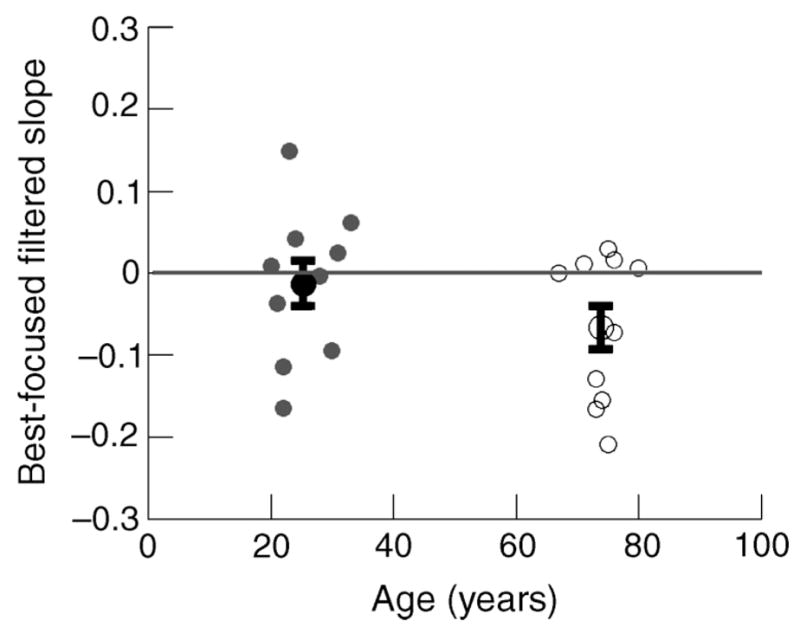
Neutral PSN for younger and older observers are shown by filled and unfilled symbols, respectively. Dark circles represent the mean PSN for younger (filled) and older (unfilled) observers. Error bars are ±1 SEM.
The mean PSN for both age groups for all three adaptation conditions are shown in Figure 3. Strong adaptation effects were observed for both blurred and sharpened adaptation conditions, with PSN settings in both conditions shifting away from a physically focused image, F(2, 17) = 98.02, p <.001 MANOVA. Older observers exhibit an overall shift in perceived focus toward blur when compared to the younger observers, F (2, 17) = 6.21, p <.02 MANOVA; however, the interaction between adaptation condition and the two age groups was not significant.
Figure 3.
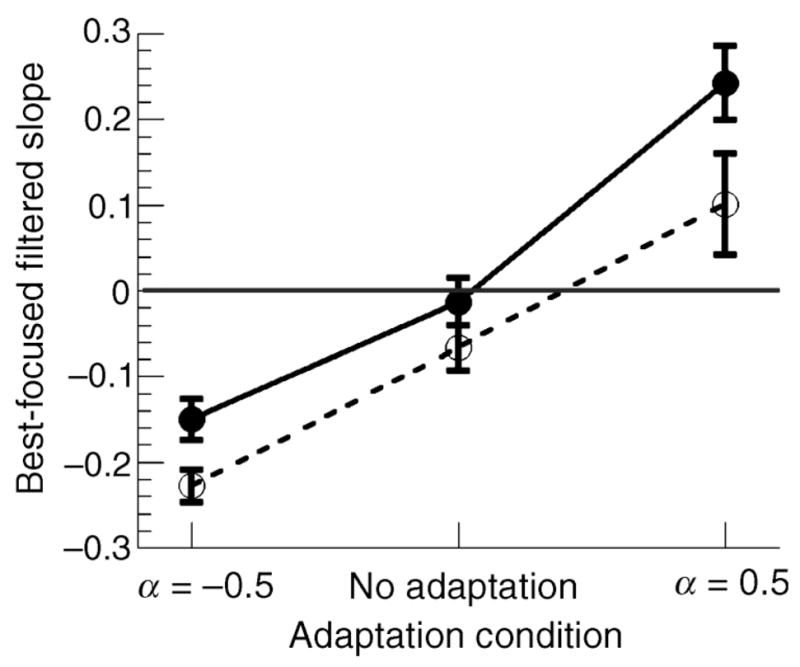
Mean PSN across all adaptation conditions for younger (filled circle, bold line) and older (unfilled circles, dashed line) observers. Error bars are ±1 SEM.
To compare the adaptation for the two age groups more directly, data for each subject were normalized to the image slope that they perceived as in focus, described by their neutral PSN. Individual neutral PSN settings were subtracted from each observer’s blur and sharpened PSN settings. These data are presented in Figure 4 (filled circles for young, unfilled circles for older). With the data normalized, it is evident that the pattern of aftereffects produced by blur adaptation does not differ between the two age groups, F(2, 17) = 1.14, p <.3 MANOVA. The average shift in PSN for all observers after adaptation to blur was −0.149 ± 0.01 SEM (young = −0.137 ± 0.017; older = −0.161 ± 0.025). For the sharp adaptation condition, the average PSN shift was 0.226 ± 0.03 SEM (young = 0.25 ± 0.029; older = 0.17 ± 0.05). The degree of aftereffect produced by adaptation to the sharp images did appear slightly reduced for older observers, but this difference was not significant, t(18) = 1.52, p <.14 two tailed.
Figure 4.
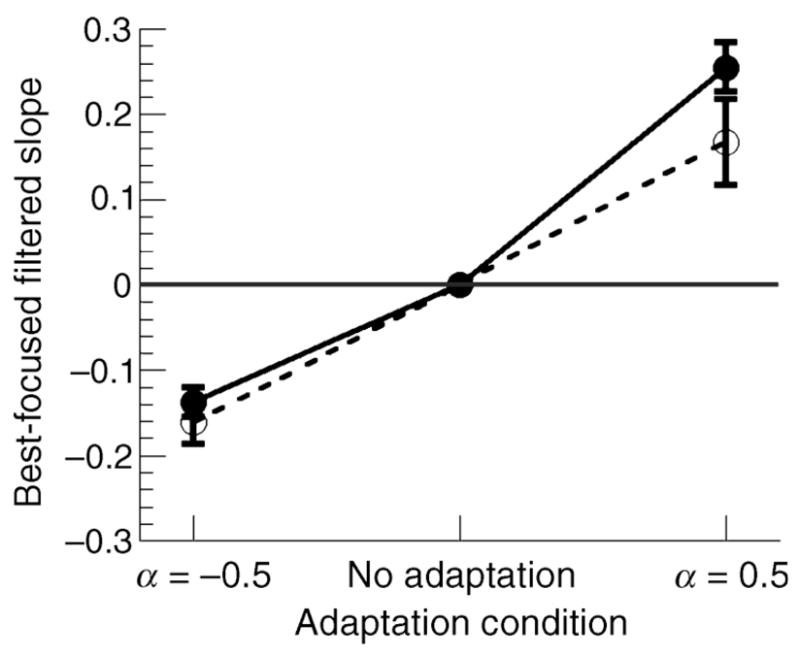
Normalized mean PSN across all adaptation conditions for younger (filled circles, solid line) and older (unfilled circles, dashed line) observers. The data were normalized to each subject’s neutral PSN. Error bars are ±1 SEM.
Control experiment for potential effects of age-related contrast constancy loss
The neural mechanisms responsible for blur adaptation are unknown. We wondered whether the differences in PSN between young and old could simply be an epiphenomenon of age-related changes in the contrast sensitivity function. Owsley et al. (1983) reported that peak contrast sensitivity, usually lying near 4 c/deg in young individuals at photopic levels, shifts to a peak spatial frequency of 2 c/deg near the age of 60. The ability to detect spatial frequencies above 4 c/deg is greatly diminished in older observers, being more pronounced in low illumination levels (Owsley et al., 1983; Sloane, Owsley, & Alvarez, 1988; Tulunay-Keesey et al., 1988). The filtering process used in this study inherently enhances (sharp images) or attenuates (blurred images) high spatial frequencies in the natural image stimuli. It is therefore possible that any results obtained after adaptation to image blur may be simply due to losses in contrast sensitivity for our older observers.
Losses in contrast sensitivity with age are reduced when the stimuli are above threshold, as measured with contrast-matching functions (CMFs) where contrast discrimination losses are absent (Delahunt et al., 2005; Tulunay-Keesey et al., 1988). Contrast sensitivity is thought to reflect early stage cortical processing (Georgeson & Sullivan, 1975); however, CMFs at suprathreshold levels may reflect a different stage of processing where the gain is altered for the range of spatial frequencies where sensitivity is low (Georgeson & Sullivan, 1975; Tulunay-Keesey et al., 1988). This mechanism provides contrast constancy and compensates for losses at threshold. To evaluate any losses in contrast constancy and the influence this may have on our blur adaptation results, we obtained a CMF for each observer.
Apparatus and stimuli
Stimuli were presented on a 17-in. CRT monitor (EIZO FlexScan T566) driven by a Macintosh G4 400-Hz computer with 10 bits of color resolution. The experimental software was written in MATLAB 5.2.1 and used the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). Subjects adapted to a uniform gray field with a mean luminance of 30 cd/m2 for 2 min before proceeding with the experiment.
All stimuli were vertically oriented static sinusoidal gratings subtending 8° visual angle, viewed at 100 cm. A spatial frequency grating of 2 c/deg at 0.1 Michelson contrast was used as the standard. Spatial frequency gratings of 0.25, 0.5, 1, 2, 4, and 8 c/deg were used to make the perceptual match.
Procedure
Sinusoidal gratings modulated in luminance were presented in the center of the monitor and alternated continuously between the standard and the test. Observers adjusted the contrast of the test grating until it matched the contrast of the standard. Test gratings were accompanied with a beep so they were easily distinguished by the observer. Five matches were made for each spatial frequency.
Results
CMFs for both age groups are presented in Figure 5. Each point represents the mean contrast value for the observers in each age group (solid circles, solid lines for younger; unfilled circles, dashed lines for older). There appears to be a small difference between the two age groups, but the effect of age was not significant, F(5, 13) = 1.97, p <.14 MANOVA, with a mean difference of 0.023. This is consistent with previous data on luminance CMFs exhibiting increasingly band-pass characteristics in senescence with the highest sensitivity at 2 c/deg (Delahunt et al., 2005; Georgeson & Sullivan, 1975; Tulunay-Keesey et al., 1988). This is evidence that contrast sensitivity losses are not entirely responsible for the shifted PSNs observed for older observers after adaptation to image blur. The contrast-matching results for these subjects confirm that contrast constancy is maintained in senescence.
Figure 5.
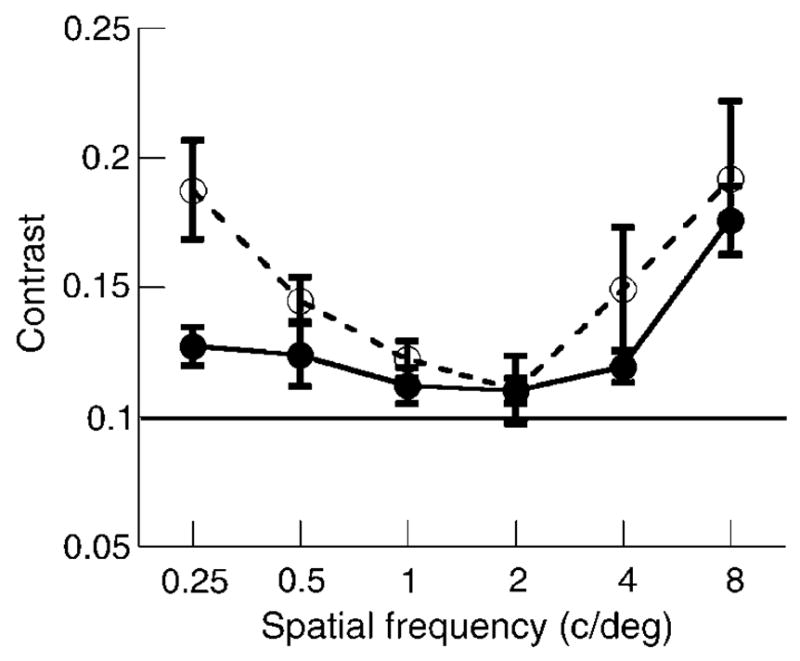
Mean CMFs for younger (filled circles, solid line) and older (unfilled circles, dashed line) observers. The standard grating was 2 c/deg presented at 0.1% Michelson contrast. Error bars are ±1 SEM.
Discussion
The blur aftereffects we observed are strong and consistent with previous studies (Webster et al., 2002). When subjects are adapted to blurred (sharpened) images, they perceive a physically focused image as appearing too sharp (blurred). Here we show that these aftereffects remain strong with aging, albeit starting from somewhat different neutral states. Specifically, when adapted to blurred or sharpened natural images, the changes in subjective focus were similar for both age groups. These results indicate that the mechanisms mediating blur adaptation are robust to senescent changes associated with losses of sensitivity at early stages of processing.
It is interesting to note that a change in the mechanisms of adaptation with aging could potentially have led to either greater or weaker aftereffects in older observers. The neural basis of pattern-selective adaptation remains poorly understood but is thought to involve both intra-cellular and synaptic processes and both fatigue and active recalibration (Carandini, 2000; Ibbotson, 2005). A process based on simple fatigue might predict stronger adaptation in older observers if aging somehow compromised neural function or resources. That is, the cells might fatigue more readily leading to larger perceptual shifts. Conversely, any loss in the efficiency or plasticity of intercellular interactions might be expected to weaken the aftereffects. As such age-related changes with adaptation have the potential to test for alternative models of the adaptation. However, our present results are silent about the possible mechanisms because we did not observe age-dependent adaptation effects.
The fact that blur adaptation remained robust suggests that this adaptation could play an important role in maintaining perceptual constancy across the life span for image focus, analogous to the role that compensatory adjustments are thought to play in color vision. For many stimulus dimensions, the visual system may anchor appearance relative to a norm defined by the distribution of stimuli in the observer’s environment (Webster et al., 2005). When the mean level of stimulation changes, whether due to changes in the observer or in the environment, neural responses are altered to remain consistent with the new mean. In the case of blur, this norm may represent the expected spatial scaling of natural images. Natural images have a characteristic structure, and the sensitivity of cells in primary visual cortex appears well matched to this expectation (Field, 1987). However, the blur caused by the optics of the eye or by environments with altered structure will introduce a mismatch. A gain control mechanism that acts to normalize neural responses to the mean spatial statistics of images would therefore be necessary to preserve the spatial appearance of images. This adaptation may be particularly important for compensating for long-term changes in sensitivity during development and aging. However, the extent to which adaptation renormalizes focus judgments (analogous to the renormalization of the white point in chromatic adaptation) versus merely reducing sensitivity to the adapting blur level (analogous to conventional spatial frequency adaptation; Blakemore & Sutton, 1969) remains uncertain (Elliott, Georgeson, & Webster, 2005).
The current results suggest that this compensation may in fact occur for spatial properties of the stimulus in the same ways that have been demonstrated previously for color appearance. Related mechanisms and their maintained integrity in senescence have already been described in early stage vision for changes in chromatic discrimination along a tritan axis (Schefrin et al., 1995), as well as CMF settings (Delahunt et al., 2005; Tulunay-Keesey et al., 1988). This type of recalibration also appears to maintain color appearance over a life span. The aging lens reduces the amount of short wavelength light reaching the retina and therefore should theoretically cause the achromatic point to shift toward blue. This is not the case, however, as achromatic settings remain similar in older and younger observers (Werner & Schefrin, 1993). The chromatic gain control mechanisms may have a rather protracted time course because renormalization of the achromatic point requires several months following cataract surgery (Delahunt, Webster, Ma, & Werner, 2004). Such a long time course is not expected from retinal mechanisms. It would be interesting to test whether renormalization to blur has a similar time course, as this process is even more likely to be due to sensitivity changes at cortical sites.
Although the results for the two age groups were similar, we did find a small difference in the subjective focus points, with older observers choosing stimuli that were slightly more physically blurred. Note that this means that older observers actually judged the physically focused image to be too sharp, implying if anything an overcompensation for any increased blur in their visual system. (Interestingly, an overcompensation is also seen when observers judge blur in the periphery; Galvin, O’Shea, Squire, & Govan, 1997.) The shifted PSNs toward blur for older observers could have several explanations. It is possible that the differences reflect differences in sensitivity to high spatial frequencies. However, the CMFs obtained from each subject do not reveal a greater loss of sensitivity to high spatial frequencies in senescence for the population we tested. Another alternative is that the stimuli used in this experiment could expose important optical differences between younger and older observers. Such optical errors are largely responsible for losses in contrast sensitivity to luminance patterns under photopic conditions (Burton et al., 1993; Owsley et al., 1983). The effects of decreased pupil size (Kadlecová et al., 1958) and increased ocular media density (Weale, 1988; Werner, 1982) in senescence may lead to a decrease in retinal illuminance under these conditions. In addition, the loss of accommodation could produce prolonged blur adaptation, similar to that seen in chromatic adaptation (Delahunt et al., 2004). That is, if they were already correctly normalized for the blur at near distances so that these distances appeared more nearly focused to them, then our correction for the test distance may have actually caused the test images to appear too sharp for the older observers when compared to their normal visual state. (In this regard it is of note that it is possible to induce blur adaptation effects that are contingent on perceived distance; Battaglia, Jacobs, & Aslin, 2004.) Further evaluation is needed to test this possibility. However, this difference is again small, and what is striking is instead the similarity in the focus settings by observers despite the large presumed differences in vision across the two age groups.
Conclusions
We used adaptation to image blur to investigate the integrity of mechanisms underlying spatial adaptation in senescence. Results indicate that although older subjects chose a slightly blurred subjective point of focus, the pattern of adaptation does not differ between younger and older observers. These results suggest that the mechanisms mediating blur adaptation are robust to senescent changes associated with losses of sensitivity in early stages mechanisms.
Acknowledgments
This work was supported by funds from the National Institute on Aging (AG04058), the National Eye Institute (EY10834), a Research to Prevent Blindness Jules and Doris Stein Professorship, and the University of Nevada, Reno Sanford Center for Aging. We thank Peter Delahunt and Yoko Mizokami for help with software, Susan Garcia for assistance with subject recruitment and screening, and Lothar Spillmann for a critical reading of the manuscript.
Contributor Information
Sarah L. Elliott, Department of Ophthalmology & Vision Science, Department of Psychology, University of California, Davis, CA, USA
Joseph L. Hardy, Posit Science Corporation, San Francisco, CA, USA
Michael A. Webster, Department of Psychology, University of Nevada, Reno, NV, USA
John S. Werner, Department of Ophthalmology & Vision Science, Department of Neurobiology, Physiology & Behavior, University of California, Davis, CA, USA
References
- Battaglia PW, Jacobs RA, Aslin RN. Depth-dependent blur adaptation. Vision Research. 2004;44:113–117. doi: 10.1016/j.visres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Sutton P. Size adaptation: A new aftereffect. Science. 1969;166:245–247. doi: 10.1126/science.166.3902.245. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learning and Memory. 2003;10:55–63. doi: 10.1101/lm.50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton KB, Owsley C, Sloane ME. Aging and neural spatial contrast sensitivity: Photopic vision. Vision Research. 1993;33:939–946. doi: 10.1016/0042-6989(93)90077-a. [DOI] [PubMed] [Google Scholar]
- Carandini M. Visual cortex: Fatigue and adaptation. Current Biology. 2000;10:R605–R607. doi: 10.1016/s0960-9822(00)00637-0. [DOI] [PubMed] [Google Scholar]
- Coile DC, Baker HD. Foveal dark adaptation, photopigment regeneration, and aging. Visual Neuroscience. 1992;8:27–39. doi: 10.1017/s0952523800006465. [DOI] [PubMed] [Google Scholar]
- Delahunt PB, Hardy JL, Okajima K, Werner JS. Senescence of spatial chromatic contrast sensitivity. II. Matching under natural viewing conditions. Journal of the Optical Society of America A, Optics, image science, and vision. 2005;22:60–67. doi: 10.1364/josaa.22.000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahunt PB, Webster MA, Ma L, Werner JS. Long-term renormalization of chromatic mechanism following cataract surgery. Visual Neuroscience. 2004;21:301–307. doi: 10.1017/S0952523804213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D, Whitaker D, MacVeigh D. Neural contributions to spatiotemporal contrast sensitivity decline in healthy ageing eyes. Vision Research. 1990;30:541–547. doi: 10.1016/0042-6989(90)90066-t. [DOI] [PubMed] [Google Scholar]
- Elliott SL, Georgeson MA, Webster MA. Adaptation to blur: Repulsion or renormalization? [Abstract] Journal of Vision. 2005;5(8):246–246a. doi: 10.1167/5.8.246. http://journalofvision.org/5/8/246/ [DOI]
- Field DJ. Relations between the statistics of natural images and the response properties of cortical cells. Journal of the Optical Society of America A, Optics and image science. 1987;4:2379–2394. doi: 10.1364/josaa.4.002379. [DOI] [PubMed] [Google Scholar]
- Field DJ, Brady N. Visual sensitivity, blur and the sources of variability in the amplitude spectra of natural scenes. Vision Research. 1997;37:3367–3383. doi: 10.1016/s0042-6989(97)00181-8. [DOI] [PubMed] [Google Scholar]
- Faubert J. Visual perception and aging. Canadian Journal of Experimental Psychology. 2002;56:164–176. doi: 10.1037/h0087394. [DOI] [PubMed] [Google Scholar]
- Galvin SJ, O’Shea RP, Squire AM, Govan DG. Sharpness overconstancy in peripheral vision. Vision Research. 1997;37:2035–2039. doi: 10.1016/s0042-6989(97)00016-3. [DOI] [PubMed] [Google Scholar]
- Georgeson MA, Sullivan GD. Contrast constancy: Deblurring in human vision by spatial frequency channels. The Journal of Physiology. 1975;252:627–656. doi: 10.1113/jphysiol.1975.sp011162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao A, Redondo M, Artal P. Optical aberrations of the human cornea as a function of age. Journal of the Optical Society of America A, Optics, image science, and vision. 2000;17:1697–1702. doi: 10.1364/josaa.17.001697. [DOI] [PubMed] [Google Scholar]
- Habak C, Faubert J. Larger effects of aging on the perception of higher-order stimuli. Vision Research. 2000;40:943–950. doi: 10.1016/s0042-6989(99)00235-7. [DOI] [PubMed] [Google Scholar]
- Hennelly ML, Barbur JL, Edgar DF, Woodward EG. The effect of age on the light scattering characteristics of the eye. Ophthalmic & Physiological Optics. 1998;18:197–203. [PubMed] [Google Scholar]
- Higgins KE, Jafe MJ, Caruso RC, de Monasterio FM. Spatial contrast sensitivity: Effects of age, test–retest, and psychophysical methods. Journal of the Optical Society of America A, Optics and image science. 1988;5:2173–2180. doi: 10.1364/josaa.5.002173. [DOI] [PubMed] [Google Scholar]
- Ibbotson MR. Physiological mechanisms of adaptation in the visual system. In: Clifford CWG, Rhodes G, editors. Fitting the mind to the world: Adaptation and after-effects in high level vision. Oxford: Oxford University Press; 2005. pp. 15–45. [Google Scholar]
- Jackson GR, Owsley C, McGwin G., Jr Aging and dark adaptation. Vision Research. 1999;39:3975–3982. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- Kadlecová V, Peleška M, Vaško A. Dependence on age of the diameter of the pupil in the dark. Nature. 1958;182:1520–1521. doi: 10.1038/1821520a0. [DOI] [PubMed] [Google Scholar]
- Mon-Williams M, Tresilian JR, Strange NC, Kcohar P, Wann JP. Improving vision: neural compensation for optical defocus. Proceedings of the Royal Society B: Biological Sciences. 1998;265:71–77. doi: 10.1098/rspb.1998.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Siemsen D. Contrast sensitivity throughout adulthood. Vision Research. 1983;23:689–699. doi: 10.1016/0042-6989(83)90210-9. [DOI] [PubMed] [Google Scholar]
- Paige GD. Senescence of human visual–vestibular interactions: I. Vestibulo ocular reflex and adaptive plasticity with aging. Journal of Vestibular Research. 1992;2:133–151. [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Pesudovs K, Brennan NA. Decreased uncorrected vision after a period of distance fixation with spectacle wear. Optometry and Vision Science. 1993;70:528–531. doi: 10.1097/00006324-199307000-00002. [DOI] [PubMed] [Google Scholar]
- Rivest J, Kim JS, Intriligator J, Sharpe JA. Effect of aging on visual shape distortion. Gerontology. 2004;50:142–151. doi: 10.1159/000076776. [DOI] [PubMed] [Google Scholar]
- Roller CA, Cohen HS, Kimball KT, Bloomberg JJ. Effects of normal aging on visuo-motor plasticity. Neurobiology of Aging. 2002;23:117–123. doi: 10.1016/s0197-4580(01)00264-0. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Shinomori K, Werner JS. Contributions of neural pathways to age-related losses in chromatic discrimination. Journal of the Optical Society of America A, Optics, image science, and vision. 1995;12:1233–1241. doi: 10.1364/josaa.12.001233. [DOI] [PubMed] [Google Scholar]
- Schefrin BE, Werner JS. Loci of spectral unique hues throughout the life span. Journal of the Optical Society of America A, Optics and image science. 1990;7:305–311. doi: 10.1364/josaa.7.000305. [DOI] [PubMed] [Google Scholar]
- Sloane ME, Owsley C, Alvarez SL. Aging, senile miosis and spatial contrast sensitivity at low luminance. Vision Research. 1988;28:1235–1246. doi: 10.1016/0042-6989(88)90039-9. [DOI] [PubMed] [Google Scholar]
- Sloane ME, Owsley C, Jackson CA. Aging and luminance-adaptation effects on spatial contrast sensitivity. Journal of the Optical Society of America A, Optics and image science. 1988;5:2181–2190. doi: 10.1364/josaa.5.002181. [DOI] [PubMed] [Google Scholar]
- Suzuki S. High-level pattern coding revealed by brief shape aftereffects. In: Clifford CWG, Rhodes G, editors. Fitting the mind to the world: Adaptation and aftereffects in high level vision. Oxford: Oxford University Press; 2005. pp. 135–172. [Google Scholar]
- Tadmor Y, Tolhurst DJ. Discrimination of changes in the second-order statistics of natural and synthetic images. Vision Research. 1994;34:541–554. doi: 10.1016/0042-6989(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Tulunay-Keesey U, Ver Hoeve JN, Terlka-McGrane C. Threshold and suprathreshold spatiotemporal response throughout adulthood. Journal of the Optical Society of America A, Optics and image science. 1988;5:2191–2200. doi: 10.1364/josaa.5.002191. [DOI] [PubMed] [Google Scholar]
- Weale RA. Age and the transmittance of the human crystalline lens. The Journal of Physiology. 1988;395:577–587. doi: 10.1113/jphysiol.1988.sp016935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MA. Contrast sensitivity under natural states of adaptation. Proceedings of SPIE. 1999;3644:58–70. [Google Scholar]
- Webster MA, Georgeson MA, Webster SM. Neural adjustments to image blur. Nature Neuroscience. 2002;5:839–840. doi: 10.1038/nn906. [DOI] [PubMed] [Google Scholar]
- Webster MA, Miyahara E. Contrast adaptation and the spatial structure of natural images. Journal of the Optical Society of America A, Optics, image science, and vision. 1997;14:2355–2366. doi: 10.1364/josaa.14.002355. [DOI] [PubMed] [Google Scholar]
- Webster MA, Werner JS, Field DJ. Adaptation and the phenomenology of perception. In: Clifford CWG, Rhodes G, editors. Fitting the mind to the world: Adaptation and aftereffects in high level vision. Oxford: Oxford University Press; 2005. pp. 241–277. [Google Scholar]
- Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human ocular media. Journal of the Optical Society of America. 1982;72:247–258. doi: 10.1364/josa.72.000247. [DOI] [PubMed] [Google Scholar]
- Werner JS. Visual problems of the retina during ageing: compensation mechanisms and colour constancy across the life span. Progress in Retinal and Eye Research. 1996;15:621–645. [Google Scholar]
- Werner JS, Delahunt PB, Hardy JL. Chromatic-spatial vision of the aging eye. Optical Review. 2004;11:226–234. doi: 10.1007/s10043-004-0226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JS, Schefrin BE. Loci of achromatic points throughout the life span. Journal of the Optical Society of America A, Optics and image science. 1993;10:1509–1516. doi: 10.1364/josaa.10.001509. [DOI] [PubMed] [Google Scholar]
- Werner JS, Schefrin BE. Optics and vision of the aging eye. In: Bass M, Enoch JM, VanStryland EW, Wolfe WL, editors. OSA Handbook of Optics, Vol. III. Classical, Vision & X-Ray Optics. New York: McGraw-Hill; 2000. pp. 13.1–13.31. [Google Scholar]