Abstract
Objective
To evaluate the progression of change in the cone-driven multifocal electroretinogram (mfERG) responses in patients previously identified as having high-risk, soft drusen 63 μm or greater.
Methods
Seventeen eyes of 14 patients were reevaluated after 28 to 41 months. Fundus changes were graded depending on drusen size and extent. Each of the 103 mfERG responses was analyzed and compared with age-matched normal controls and with the baseline measurement.
Results
Stable visual acuity was found in 12 of the 17 eyes. Drusen size or extent was increased, decreased, and unchanged in 6, 3, and 8 eyes, respectively. The mfERG responses demonstrated a significant progression in the response density loss and in N1 and P1 implicit time delay compared with the baseline evaluation regardless of drusen change. The extent of response deterioration occurred over the entire retinal area tested. Eyes having decreased drusen at follow-up were typically associated with higher response delays at baseline and follow-up than eyes with stable or increased drusen.
Conclusions
Early age-related macular degeneration is associated with a progressive loss in the cone-driven mfERG response despite stable visual acuity. The response deterioration extended beyond the visible drusen area. Implicit times seem to be an important predictor of drusen regression.
Drusen, First Described 150 years ago by Donders,1 are deposits of extracellular material and typically accumulate between the basement membrane of the retinal pigment epithelium (RPE) cell and the inner collagenous layer of Bruch’s membrane.2 Large, soft drusen (≥63 μm), an early sign of age-related macular degeneration (AMD), are known to be a high-risk factor for developing exudative macular degeneration.3–5 Johnson et al6 reported that drusen-associated retinal abnormalities are limited to photoreceptor cells. Cones and rods directly over or next to drusen are characterized by structural changes in the inner and outer segments with alterations in the synaptic architecture. Curcio et al7 showed a higher vulnerability of rods compared with cones in eyes with early AMD. Psychophysical and electrophysiological studies demonstrated different patterns of cone- and rod-mediated sensitivity loss.8–19 Recently, we have shown that localized cone-mediated retinal response losses are evident in patients with large drusen without finding a functional-morphological correlation in this patient group.16 Longitudinal studies revealed a progression in drusen size and drusen number.5 It is also known that drusen sometime regress and disappear2,5,20 and are associated with RPE and photoreceptor atrophy.2
The multifocal electroretinogram (mfERG) allows a localized measurement of retinal responses.21,22 Hood et al23 showed that the first-order kernel response of the mfERG originates from photoreceptor and bipolar cells. The purpose of this study was to determine whether the cone-mediated mfERG response delay and loss would progress over time and whether the response deterioration would differ with retinal topography and associated morphological changes. Because we found reduced or delayed responses over the entire retinal area tested at baseline, we expected a further progression. Feigl et al24 did not find a progression in either the cone- or the rod-mediated mfERG response in patients with early AMD after 1 year. We were able to retest patients with large drusen after a longer follow-up period. We found that response density deteriorated to a larger degree than implicit time in both the central and peripheral regions tested. The mfERG responses varied among the patient groups with increased, decreased, and unchanged drusen.
METHODS
PATIENTS
We retested 14 patients (17 eyes) of the original 20 patients (31 eyes)16 with large drusen. Seven of the 31 eyes were excluded from the follow-up because of prophylactic laser photocoagulation.25 One patient (patient 7 [patient numbers refer to Gerth et al16]) developed exudative AMD with severe visual loss in the eye previously tested. Another patient lost vision in one eye due to giant cell arteritis (patient 14, right eye). Four patients were not able to return because of general health problems and 1 patient was not reachable. Of the 14 patients, 3 were tested in both eyes and 11 were tested in one eye. The patients were reevaluated after 28 to 41 months (mean, 31 months).
Best-corrected visual acuity was checked with the Early Treatment of Diabetic Retinopathy Study chart. All patients received a dilated eye examination. None of the patients showed elevated intraocular pressure. Slitlamp examination did not reveal significant changes in lens opacities compared with the baseline visit (except patient 6). Three of the patients had pseudophakic eyes, including 1 patient (patient 6) who had undergone cataract surgery within the follow-up period.
Written informed consent was obtained from the patients in accordance with the Tenets of Helsinki and with the approval of the Office of Human Research Protection of the University of California, Davis, School of Medicine.
FUNDUS GRADING
Fifty-degree color fundus photographs were obtained with a Topcon retinal camera (Topcon American Corporation, Paramus, NJ). Fundus features in these digital photographs were compared with prior photographs. Analyses are based only on the 30° retinal area (standardized by the optic disc–fovea distance) acquired in the baseline photos. A circular grid containing 9 subfields (single central subfield and inner and outer ring, each divided into 4 subfields)26 was digitally superimposed on the fundus image and centered on the fovea using Imagenet (Topcon Medical Systems, Inc, Paramus). Each of the 9 subfields was first analyzed for the presence of drusen. Maximum drusen size in each subfield was then estimated using circles of defined size (63 μm, 125 μm, 250 μm). Two of us (C.G. and S.A.) performed the gradings independently. The interobserver agreement was 100%. The observers were masked for the mfERG response results at the time of fundus grading.
The definitions of increased, decreased, and unchanged drusen5 are summarized in Table 1. An increase was defined by (1) the presence of new soft drusen (≥63 μm) in 2 or more subfields at follow-up where it was not present at baseline or (2) the maximum size of drusen increase in at least 2 subfields at follow-up. Decreased drusen were defined (1) as the absence of soft drusen (≥63 μm) in 2 or more subfields at follow-up where it was present at baseline or (2) by the maximum size of drusen decrease in at least 2 subfields at follow-up. Eyes were categorized as having unchanged fundus morphological features if they did not meet the criteria of increased or decreased drusen.
Table 1.
Definition of Drusen Change5
| Criteria | Increase | Decrease |
|---|---|---|
| Presence of drusen ≥ 63 μm | In ≥2 additional subfields at follow-up | In ≥2 fewer subfields at follow-up |
| Change of maximum drusen size | 63 μm to ≥125 μm; 125 μm to ≥250 μm; or 250 μm to >250 μm in ≥2 subfields | 125 μm to ≤63 μm; 250 μm to ≤125 μm; or >250 μm to 250 μm in ≥2 subfields |
MULTIFOCAL ERG
The mfERG response recordings were performed with a VERIS (version 4.8) stimulus-refractor unit (frame rate, 75 Hz) (Electro-Diagnostic Imaging, Inc, San Mateo, Calif) under the same protocol described previously.16 The mfERG responses were acquired under room-light conditions on dilated pupils with a bipolar Burian-Allen contact lens electrode (Hansen Ophthalmic Development Laboratory, Coralville, Iowa). Noise-contaminated segments were rejected and repeated.
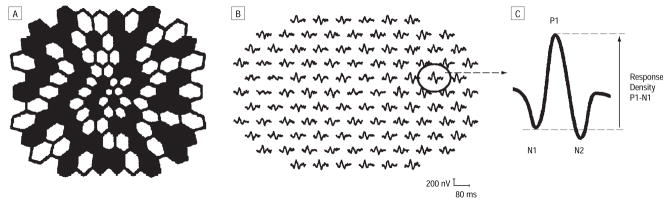
The stimulus consisted of 103 scaled hexagons (Figure 1) flashed pseudorandomly at intervals of 13.3 milliseconds (m-sequence length 214-1) on a dark background (<1 candela [cd] × m−2). The flash intensity was 2.67 cd × sec × m−2 (200 cd × m−2/75 Hz). Signals were sampled at 1200 Hz (ie, 0.83 milliseconds between samples). The data were acquired at a gain of 105 over a frequency range of 10 to 300 Hz (GRASS preamplifier ICP 511; Grass Instruments, West Warwick, RI). A 4.8° black fixation cross was used. Stimulus luminance was calibrated with the autocalibrator (Electro-Diagnostic Imaging, Inc). The recording protocol was chosen according to the recommended guidelines of the International Society for Clinical Electrophysiology of Vision for basic mfERG.27
Figure 1.
Stimulus pattern consisting of 103 scaled hexagons (A) and typical patient multifocal electroretinogram responses (B [all responses] and C [single response]). All of the 103 multifocal electroretinograms were analyzed for N1, P1, and N2 implicit times and P1-N1 response density as illustrated.
All mfERG responses were compared with age-matched normal groups, separately for patients with phakic and pseudophakic eyes (10 subjects with phakic eyes aged 60–69 years, 9 subjects with phakic eyes aged 70–80 years, 9 subjects with pseudophakic eyes aged 68–76 years).28,29 One iteration of artifact rejection but no spatial smoothing was applied to the raw data. First-order kernel responses were analyzed for the following parameters: implicit times N1 (first negative trough), P1 (first positive peak), and N2 (second negative trough) and response density amplitudes P1-N1 (from the first negative trough to the first positive peak) (Figure 1). Amplitudes were measured on the response density scaled regional averages. Waveforms were exported from VERIS 4.8 to MATLAB (The Math Works, Natick, Mass) for graphical and statistical analyses. Response losses or delays of more than 2 SDs from normal were categorized as significantly abnormal. We used 2-tailed t tests to test differences between baseline and follow-up data.
RESULTS
VISUAL ACUITY AND FUNDUS MORPHOLOGICAL CHANGES
Visual acuity remained stable in 12 of the 17 tested eyes. Three of 17 eyes lost 1 line and 2 of 17 eyes lost 2 lines in the Early Treatment of Diabetic Retinopathy Study chart (Table 2). All eyes were classified as AMD category 3 according to the Age-Related Eye Disease Study System.30 Comparison of fundus morphological changes, as listed in Table 2, revealed an increase in drusen number or drusen area in 6 eyes. A decrease in drusen number and area and associated increase in RPE changes was found in 3 eyes. The fundus in the remaining 8 eyes were graded as unchanged.
Table 2.
Patient Data at Baseline and Follow-up
| Patient/Sex/Age, y | Follow-up, mo | Eye | Baseline Visual Acuity | Follow-up Visual Acuity | Drusen |
|---|---|---|---|---|---|
| 1/F/71 | 41 | OD | 20/20 | 20/20 | Increase |
| 5/F/76 | 33 | OS | 20/16 | 20/20 | Decrease, RPE changes |
| 6/F/77 | 33 | OS | 20/16 | 20/25 | No change |
| 8/F/70 | 29 | OD | 20/20 | 20/20 | No change |
| 9/F/77 | 29 | OD | 20/16 | 20/25 | No change |
| 10/F/72 | 29 | OS | 20/25 | 20/25 | No change |
| 12/F/86 | 29 | OS | 20/32 | 20/40 | Decrease, RPE changes |
| 13/F/74 | 28 | OD | 20/25 | 20/20 | No change |
| 28 | OS | 20/20 | 20/20 | Increase | |
| 14/M/87 | 29 | OS | 20/40 | 20/32 | No change |
| 15/F/73 | 28 | OD | 20/25 | 20/20 | Increase |
| 28 | OS | 20/25 | 20/32 | Increase | |
| 16/F/61 | 28 | OD | 20/20 | 20/20 | Decrease, RPE changes |
| 17/M/70 | 29 | OD | 20/40 | 20/40 | No change |
| 29 | OS | 20/32 | 20/32 | Increase | |
| 18/F/80 | 41 | OS | 20/32 | 20/25 | No change |
| 19/F/78 | 40 | OS | 20/20 | 20/16 | Increase |
Abbreviations: OD, right eye; OS, left eye; RPE, retinal pigment epithelium.
mfERG RESPONSE COMPARISON WITH AGE-MATCHED NORMAL CONTROLS
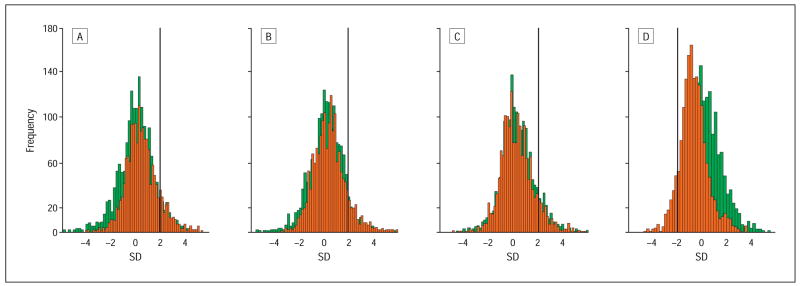
To test the mfERG responses for progression and to take into account the normal variation, we compared all mfERG responses with the age-matched normal control group. All tested eyes showed abnormal mfERG responses. The analysis of 1751 mfERGs (103 areas tested in 17 eyes) revealed delayed N1, P1, and N2 implicit times in 12.7%, 11%, and 10.2% of all mfERGs, respectively. The comparison with the baseline mfERG demonstrated a significant progression for N1 implicit time (baseline, 8.1% abnormal; P<.001) and P1 implicit time (baseline, 7.9% abnormal; P<.001). Response densities were reduced in 10.8% of all mfERGs compared with 2.4% at baseline (P<.001). Figure 2 illustrates the deterioration in implicit times and response densities with a clearly visible shift toward slower and lower responses at the follow-up visit.
Figure 2.
Comparison of the localized multifocal electroretinogram responses with the normal control group at baseline (green) and at the follow-up visit (red) plotted in histograms. Numbers of multifocal electroretinograms are plotted as a function of standard deviations (SDs) from normal control data. Bold lines mark the point of significantly abnormal data (>2 SD for implicit times, <2 SD for response density). Significant changes were found for N1 and P1 implicit times and P1-N1 response density. A, N1 implicit time. Baseline, 8.1% had abnormal response; follow-up visit, 12.7%. B, P1 implicit time. Baseline, 7.9% had abnormal response; follow-up visit, 11.0%. C, N2 implicit time. Baseline, 10.8% had abnormal response; follow-up visit, 10.2%. D, P1-N1 response density. Baseline, 2.4% had abnormal response; follow-up visit, 10.8%.
TOPOGRAPHICAL ANALYSIS
The results at the baseline visit pointed out a general retinal dysfunction in patients with early AMD.16 To evaluate whether the deterioration in retinal function would be confined to the central area, the mfERG data within and outside 15° were compared separately. Longitudinal changes were found in the central 15° as well as in the peripheral stimulated area 15° to 25° retinal eccentricity (not shown). Changes between the baseline and follow-up mfERG were significant in both areas for the N1 and P1 implicit times and for P1-N1 response density.
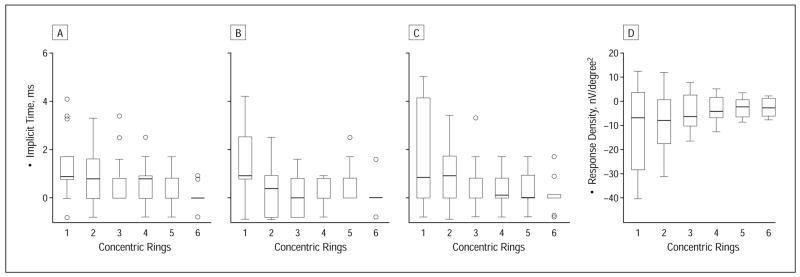
Histological data indicate a higher vulnerability of the parafoveal cones outside of 2.8° in patients with early AMD.7,31 To determine whether the cone-mediated pathways are deteriorating more in the parafoveal than in the foveal area, we analyzed and compared the baseline and the follow-up mfERGs in 6 concentric rings (response average for areas 1°, 2°–5°, 6°–10°, 11°–15°, 16°–20°, and 21°–25° using the VERIS analysis program). Figure 3 demonstrates implicit time increase and response density decrease mostly in the central 1° to 5° retinal eccentricity (central and second concentric rings). Significant differences were found in the central area (1°) for N1 and P1implicit times (the differences in N1 implicit time in rings 2–5, P1 implicit time in ring 5, N2 implicit times in rings 1 and 2, and response density in all concentric rings would be considered statistically significant had we not chosen a P value corrected for the number of statistical tests). Localized changes outside 1° were undetectable owing to the averaging concentric ring analysis.
Figure 3.
Follow-up-baseline differences in implicit times N1 (A), P1 (B), and N2 (C) and response density P1-N1 (D) plotted for 6 different retinal eccentricities (labeled as concentric rings: ring 1, 1°; ring 2, 2°–5°; ring 3, 6°–10°; ring 4, 11°–15°; ring 5,16°–20°; and ring 6, 21°–25° radius retinal eccentricity). Boxes enclose 50% of the data with the median value displayed as a line; the top and bottom of the boxes mark 25%; and the error bars denote the minimum and maximum values of the data set. Values outside the range are displayed as an individual point. Significant changes in N1 and P1 implicit times occurred in the central ring (see text).
mfERG RESPONSES AND MORPHOLOGICAL CHANGE
Fundus morphological features in the 17 eyes exhibited different visible longitudinal change among patients with increased, decreased, or unchanged pathological characteristics. Recently, we and others have shown that there is no direct correlation between visible drusen (by ophthalmoscopy, red-free fundus photography, or fluorescein angiography) and retinal sensitivity in the cone- and rod-driven pathways.8,13,16 Despite those findings, we wanted to know whether the overall longitudinal morphological change is associated with a distinct alteration in the photopic mfERG responses. The phenotype might look similar among those 17 eyes but the course of the disease could be different. We therefore analyzed the mfERGs from the group with (1) drusen progression, (2) stable fundus morphological features, and (3) drusen regression and increased RPE changes separately. Results are presented in Table 3 for the inner 10° and outside of 10° retinal eccentricity. All 3 groups had in common a greater reduction in response density in the central than outside 10° retinal eccentricity. Differences among the 3 groups are obvious in implicit time. Eyes with drusen progression can be characterized by a slight increase of delayed retinal areas. The focal retinal areas with increased drusen were not correlated with localized mfERG alterations. In contrast, eyes with unchanged drusen morphological features showed a shift in N2 implicit time with fewer abnormal areas compared with baseline; this shift was more common in the central 10° than outside 10° retinal eccentricity. Eyes with drusen regression associated with increasing RPE changes typically showed delayed implicit times more than in the other 2 groups at baseline. After 28 to 33 months, those responses were even more delayed, together with a significant loss in response density.
Table 3.
Longitudinal mfERG Changes Depending on Fundus Alterations
| Fundus: Abnormal mfERGs* | Drusen Progression, % | Drusen Unchanged, % | Drusen Regression With RPE Changes, % |
|---|---|---|---|
| Central 10° retinal eccentricity | |||
| P1-N1 response density | 1.8–11.4† | 3.9–21.0† | 0–12.3† |
| N1 implicit time | 4.4–8.8† | 5.3–6.6 | 15.8–28.1 |
| P1 implicit time | 7.0–10.5 | 12.5–13.2 | 31.6–36.8 |
| N2 implicit time | 9.6-7.0 | 19.7-9.2† | 47.4–52.6 |
| Outside 10° retinal eccentricity | |||
| P1-N1 response density | 1.2–6.2† | 4.2–15.0† | 0–2.0† |
| N1 implicit time | 6.9–9.1† | 8.5–11.6† | 10.7–25.0† |
| P1 implicit time | 3.8–6.7† | 6.0-5.5 | 13.5–27.4† |
| N2 implicit time | 5.2–6.0 | 8.6-4.2 | 14.7–27.4† |
Abbreviations: mfERG, multifocal electroretinogram; RPE, retinal pigment epithelium.
Percentage of the mfERGs that are delayed or reduced of ≥2 SD in comparison with the normal controls.
Significant (adjusted P≤.001).
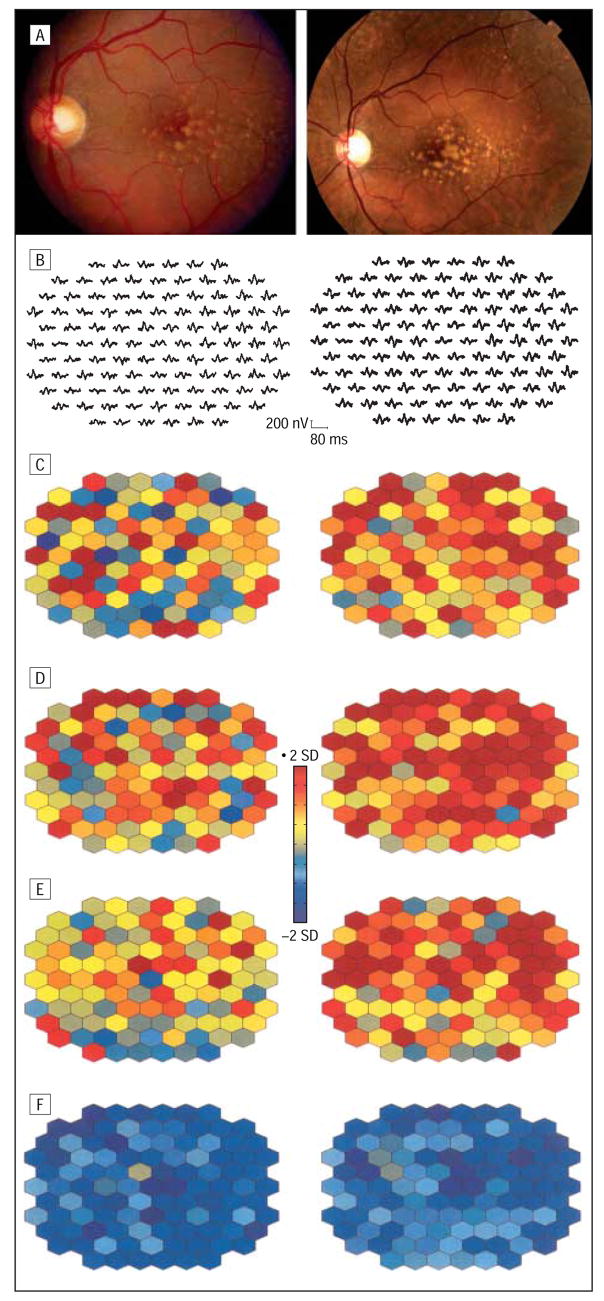
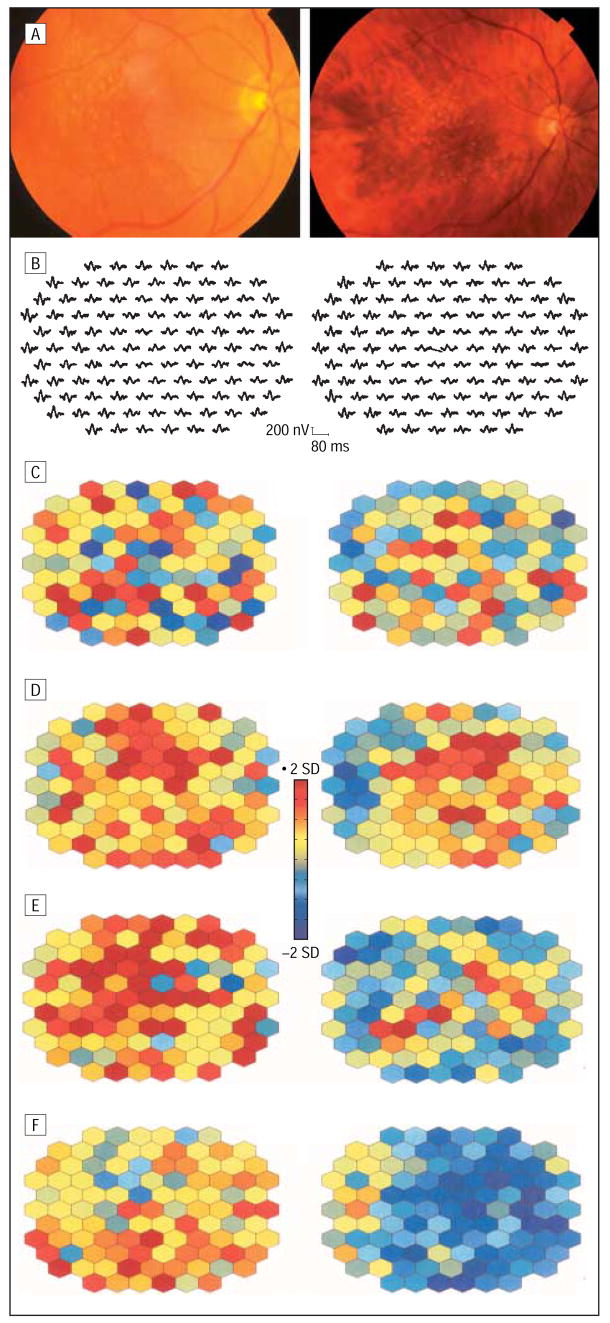
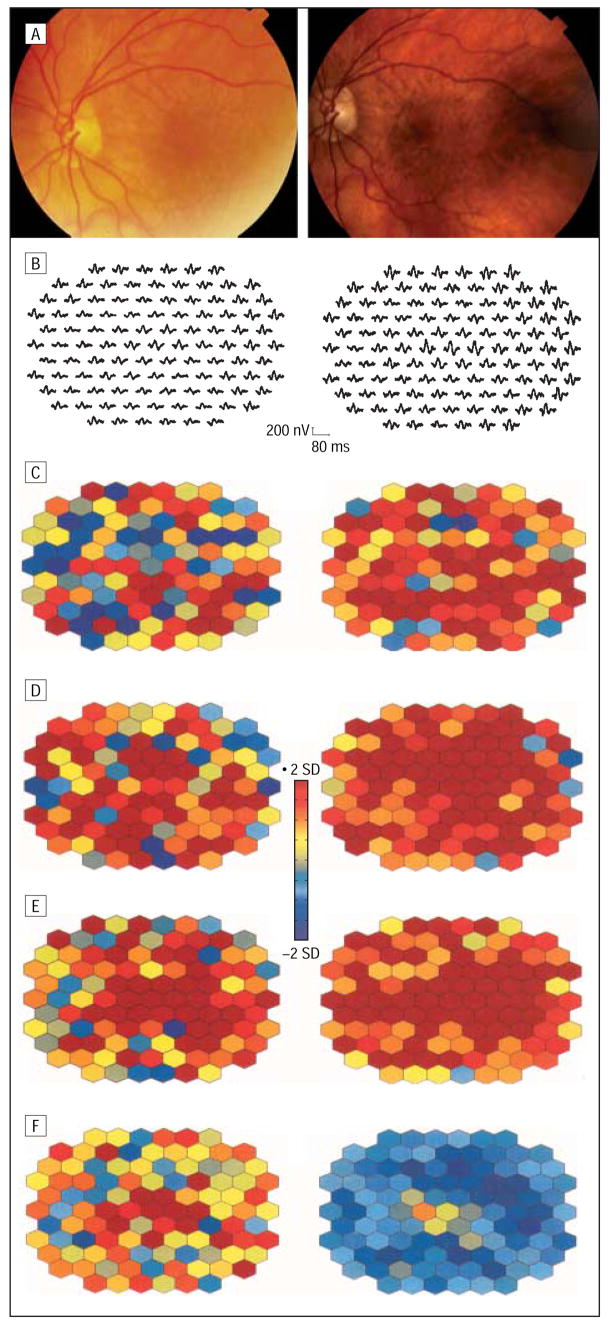
Typical examples for the implicit time alterations in the 3 groups are illustrated in Figures 4, 5, and 6. Figure 4 depicts baseline and follow-up fundus photographs and mfERG responses from a patient with a pseudophakic eye (patient 19) with increased drusen in 2 subfields and increasing maximum drusen size in 3 subfields (group 1). At the follow-up after 40 months, visual acuity was stable but more areas were delayed in N1, P1, and N2 implicit times within and outside the drusen area. In contrast, the right eye of patient 13 (group 2) with unchanged fundus morphological features and visual acuity revealed fewer areas of delayed N2 implicit time (Figure 5). Patient 5, a typical example for group 3, showed 2 fewer subfields with soft drusen, and the maximum drusen size decreased in 2 additional subfields. The mfERG comparison revealed increasing numbers of retinal areas with delayed implicit times after 33 months (Figure 6). At the initial visit, the mfERG of this patient had already shown more areas with delayed responses compared with other patients with large drusen. This patient received cataract surgery prior to the follow-up visit. Therefore, mfERG responses appear larger than at baseline. The color plots can be compared with each other because both mfERG recordings were compared with normal controls with phakic and pseudophakic eyes separately.
Figure 4.
Multifocal electroretinogram response alterations in an eye with drusen progression (patient 19, left eye). A, Color fundus photographs show an increased area of drusen after 40 months. Multifocal electroretinogram traces (B) and corresponding implicit time and response density parameters demonstrate a further progression in retinal function. Parameters are plotted in color-coded units of standard deviation (SD) from normal. Abnormal response densities are coded in dark blue (<2 SDs) and delayed implicit times of more than 2 SDs, in red. Images on the left side of the figure are baseline; images on the right side, at 40 months’ follow-up. C, N1 implicit time. D, P1 implicit time. E, N2 implicit time. F, P1-N1 response density.
Figure 5.
Fundus photographs (A), multifocal electroretinogram traces (B), and analyzed multifocal electroretinogram parameters from the right eye of patient 13, with unchanged drusen after 28 months. Color-coded plots illustrate fewer areas with abnormal N2 implicit time but deterioration in response density. See Figure 4 for plot explanation. Images on the left side of the figure are baseline; images on the right side, at 28 months’ follow-up. C, N1 implicit time. D, P1 implicit time. E, N2 implicit time. F, P1-N1 response density.
Figure 6.
Multifocal electroretinogram response progression in 1 eye with drusen regression associated with retinal pigment epithelium changes after 33 months. More retinal areas show response delays (color-coded as red) and response losses (blue) at the follow-up test compared with baseline. See text for explanation of different multifocal electroretinograms’ trace quality. Images on the left side of the figure are baseline; images on the right side, at 33 months’ follow-up. A, Fundus photographs. B, Multifocal electroretinogram trace. C, N1 implicit time. D, P1 implicit time. E, N2 implicit time. F, P1-N1 response density.
COMMENT
Significant morphological progression in patients with early signs of age-related maculopathy has been demonstrated by longitudinal population-based studies.5,20 Herein, we show that the cone-mediated retinal responses elicited by the mfERG significantly deteriorate over time. The loss was greater in response density than in implicit time. One could argue that those changes are partly due to optical factors (lens opacities exceeding the normal aging). That would result in response density losses but not in implicit time delays.29 Tested patients with pseudophakic eyes showed fewer changes in response density than the patients with phakic eyes. However, the patient who received cataract surgery during the follow-up demonstrated an obvious response density loss. In the tested phakic eyes, the lens properties were graded as unchanged without measuring the actual lens density. An undetected increase in lens opacities would reduce response density. But it would also be associated with faster implicit times due to reduced contrast and reduced retinal illuminance relative to our standard condition.28,29
The cone-mediated response deterioration observed in this study could be caused by structural and metabolic alterations. Johnson et al6 described deflected and truncated or even undetectable outer and inner segments and absent synaptophysin immunolabeling, suggesting altered synaptic architecture in cones overlying drusen. Functionally, that would likely result in reduced quantum catch and changed response characteristics. The progression in mfERG response was not limited to the drusen area. As we demonstrated earlier, the functional changes are not correlated with the visible morphological changes in patients with drusen.16 Basal linear deposits, which are diffuse deposits internal to the RPE basal lamina and of the same membranous material as soft drusen, are specific for early AMD.32 Those deposits play an important role in forming diffusion barriers between the choriocapillaris and the RPE and are associated with delayed choroidal perfusion.33 That led to the retinoid-deficiency hypothesis in the pathogenesis of AMD.34 The end point of the disease is photoreceptor dysfunction and death. The data of Owsley et al13 and Scholl et al18 indicate that rods are more vulnerable and affected earlier than cones. Further progression in RPE dysfunction and a possible change in cone photopigment regeneration might lead to the dysfunction in the cone-mediated pathways. As shown by Remulla et al,35 prolonged choroidal perfusion in nonneovascular AMD was associated with implicit time delays in the foveal cone ERG.
Feigl et al24 could not find a significant progression in the rod- and cone-mediated mfERG response among 13 patients with early AMD after 1 year. The heterogeneous phenotype and pathogenesis of AMD as well as the slow and varied progression might account for their results.
We characterized 3 types of drusen changes by a different implicit time alteration. Retinal pigment epithelium and photoreceptor alteration follow drusen regression2 and can therefore account for the further response density loss and implicit time delays among the 3 eyes with drusen regression. As shown in patients with diabetes mellitus, locally compromised metabolism leads to delayed neural conduction and prolonged mfERG implicit times.36 Whether the markedly delayed implicit times at baseline in our 3 patients are a prognostic marker needs to be tested in a larger patient group.
Stable drusen morphological features do not necessarily preclude further microstructural and functional progression as indicated by response density loss and further delayed N1 implicit times in our patient group. Interestingly, fewer areas showed a delayed N2 implicit time compared with baseline. If the other N1 and P1 implicit times would have shown a similar pattern, then it would likely be related to optical factors. The nature of this implicit time “readjustment” remains unclear and further longitudinal studies might provide an explanation.
This study may have implications for predicting the further outcome in patients with drusen. Patients with significantly delayed implicit times might be at a higher risk for developing nongeographic atrophy.
Acknowledgments
Funding/Support: This study was supported by grant AG04058 from the National Institute on Aging, Bethesda, Md, and a Research to Prevent Blindness Jules and Doris Stein Professorship.
We thank Susan Garcia, BA, COT, for help with the patients and Marc Thomas, BA, CRA, and Eugene Huang, PhD, for support with the imaging analysis.
Footnotes
Financial Disclosure: None.
References
- 1.Donders FC. Beiträge zur pathologischen Anatomie des Auges. Arch Ophthalmol. 1855;1:106–118. [Google Scholar]
- 2.Sarks SH, Sarks JP. Age-related maculopathy: nonneovascular age-related macular degeneration and the evolution of geographic atrophy. In: Schachat AP, editor. Retina. Vol. 2. St Louis, Mo: Mosby-Year Book; 2001. pp. 1064–1099. [Google Scholar]
- 3.Pauleikhoff D, Barondes MJ, Minassian D, et al. Drusen as risk factors in age-related macular disease. Am J Ophthalmol. 1990;109:38–43. doi: 10.1016/s0002-9394(14)75576-x. [DOI] [PubMed] [Google Scholar]
- 4.Bressler SB, Maguire MG, Bressler NM, Fine SL. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration: the Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108:1442–1447. doi: 10.1001/archopht.1990.01070120090035. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Tomany SC, et al. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam eye study. Ophthalmology. 2002;109:1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PT, Lewis GP, Talaga KC, et al. Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci. 2003;44:4481–4488. doi: 10.1167/iovs.03-0436. [DOI] [PubMed] [Google Scholar]
- 7.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–1249. [PubMed] [Google Scholar]
- 8.Sunness JS, Johnson MA, Massof RW, Marcus S. Retinal sensitivity over drusen and nondrusen areas: a study using fundus perimetry. Arch Ophthalmol. 1988;106:1081–1084. doi: 10.1001/archopht.1988.01060140237032. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg MA, Miller S, Gaudio AR. Foveal cone ERGs in fellow eyes of patients with unilateral neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 1993;34:3477–3480. [PubMed] [Google Scholar]
- 10.Midena E, Degli Angeli C, Blarzino MC, et al. Macular function impairment in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 1997;38:469–477. [PubMed] [Google Scholar]
- 11.Falsini B, Fadda A, Iarossi G, et al. Retinal sensitivity to flicker modulation: reduced by early age-related maculopathy. Invest Ophthalmol Vis Sci. 2000;41:1498–1506. [PubMed] [Google Scholar]
- 12.Huang S, Wu D, Jiang F, et al. The multifocal electroretinogram in age-related maculopathies. Doc Ophthalmol. 2000;101:115–124. doi: 10.1023/a:1026587103165. [DOI] [PubMed] [Google Scholar]
- 13.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–273. [PubMed] [Google Scholar]
- 14.Li J, Tso MO, Lam TT. Reduced amplitude and delayed latency in foveal response of multifocal electroretinogram in early age related macular degeneration. Br J Ophthalmol. 2001;85:287–290. doi: 10.1136/bjo.85.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takiura K, Yuzawa M, Miyasaka S. Multifocal electroretinogram in patients with soft drusen in the macula [ARVO abstract] Invest Ophthalmol Vis Sci. 2001;42:S73. [Google Scholar]
- 16.Gerth C, Hauser D, Delahunt PB, et al. Assessment of multifocal electroretinogram abnormalities and their relation to morphologic characteristics in patients with large drusen. Arch Ophthalmol. 2003;121:1404–1414. doi: 10.1001/archopht.121.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phipps JA, Guymer RH, Vingrys AJ. Loss of cone function in age-related maculopathy. Invest Ophthalmol Vis Sci. 2003;44:2277–2283. doi: 10.1167/iovs.02-0769. [DOI] [PubMed] [Google Scholar]
- 18.Scholl HP, Bellmann C, Dandekar SS, et al. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci. 2004;45:574–583. doi: 10.1167/iovs.03-0495. [DOI] [PubMed] [Google Scholar]
- 19.Feigl B, Brown B, Lovie-Kitchin J, Swann P. Cone- and rod-mediated multifocal electroretinogram in early age-related maculopathy. Eye. 2005;19:431–441. doi: 10.1038/sj.eye.6701503. [DOI] [PubMed] [Google Scholar]
- 20.Bressler NM, Munoz B, Maguire MG, et al. Five-year incidence and disappearance of drusen and retinal pigment epithelial abnormalities: Waterman study. Arch Ophthalmol. 1995;113:301–308. doi: 10.1001/archopht.1995.01100030055022. [DOI] [PubMed] [Google Scholar]
- 21.Sutter EE. The fast m-transform: a fast computation of cross-correlations with binary m-sequences. SIAM J Appl Math. 1991;20:686–694. [Google Scholar]
- 22.Sutter EE, Tran D. The field topography of ERG components in man, I: the photopic luminance response. Vision Res. 1992;32:433–446. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- 23.Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–1685. [PubMed] [Google Scholar]
- 24.Feigl B, Brown B, Lovie-Kitchin J, Swann P. Monitoring retinal function in early age-related maculopathy: visual performance after 1 year. Eye. 2005;19:1169–1177. doi: 10.1038/sj.eye.6701711. [DOI] [PubMed] [Google Scholar]
- 25.Olk RJ, Friberg TR, Stickney KL, et al. Therapeutic benefits of infrared (810-nm) diode laser macular grid photocoagulation in prophylactic treatment of nonexudative age-related macular degeneration: two-year results of a randomized pilot study. Ophthalmology. 1999;106:2082–2090. doi: 10.1016/S0161-6420(99)90487-6. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 27.Marmor M, Hood D, Keating D, et al. Guidelines for basic multifocal electroretinography (mfERG) [International Society for Clinical Electrophysiology of Vision Web site] [Accessed October 20, 2004]; doi: 10.1023/a:1022591317907. Available at: www.iscev.org. [DOI] [PubMed]
- 28.Gerth C, Garcia SM, Ma L, et al. Multifocal electroretinogram: age-related changes for different luminance levels. Graefes Arch Clin Exp Ophthalmol. 2002;240:202–208. doi: 10.1007/s00417-002-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner JS, Gerth C. Optical and neural factors mediating senescent changes in multifocal electroretinogram responses. Paper presented at: Annual Meeting of the Association for Research in Vision and Ophthalmology; May 6, 2002; Fort Lauderdale, Fla. [Google Scholar]
- 30.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study System for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 31.Medeiros NE, Curcio CA. Preservation of ganglion cell layer neurons in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:795–803. [PubMed] [Google Scholar]
- 32.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117:329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- 33.Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch’s membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33:334–340. [PubMed] [Google Scholar]
- 34.Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev. 2002;1:381–396. doi: 10.1016/s1568-1637(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 35.Remulla JF, Gaudio AR, Miller S, Sandberg MA. Foveal electroretinograms and choroidal perfusion characteristics in fellow eyes of patients with unilateral neovascular age-related macular degeneration. Br J Ophthalmol. 1995;79:558–561. doi: 10.1136/bjo.79.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Y, Bearse MA, Jr, Schneck ME, et al. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:948–954. doi: 10.1167/iovs.03-1101. [DOI] [PubMed] [Google Scholar]