Abstract
The accurate synthesis of proteins, dictated by the corresponding nucleotide sequence encoded in mRNA, is essential for cell growth and survival. Central to this process are the aminoacyl-tRNA synthetases (aaRSs), which provide amino acid substrates for the growing polypeptide chain in the form of aminoacyl-tRNAs. The aaRSs are essential for coupling the correct amino acid and tRNA molecules, but are also known to associate in higher order complexes with proteins involved in processes beyond translation. Multiprotein complexes containing aaRSs are found in all three domains of life playing roles in splicing, apoptosis, viral assembly, and regulation of transcription and translation. An overview of the complexes aaRSs form in all domains of life is presented, demonstrating the extensive network of connections between the translational machinery and cellular components involved in a myriad of essential processes beyond protein synthesis.
Keywords: aminoacyl-tRNA synthetase, translation, amino acid
Introduction
The faithful translation of mRNA into protein requires aminoacyl-tRNA synthetases (aaRSs), which provide the elongating polypeptide chain with amino acids in the form of aminoacyl-tRNAs (aa-tRNA). The aaRSs are responsible for accurately attaching the correct amino acid onto the cognate tRNA molecule in a two-step reaction. The amino acid is first activated with ATP, forming an aminoacyladenylate intermediate. Once activated, the amino acid is transferred to the 3′ end of its corresponding tRNA molecule to be used for protein synthesis. Although related by this common mechanism of aminoacylation, aaRSs are separated into two classes (I and II) based on the structural topologies of their active sites (Cusack et al., 1990; Eriani et al., 1990).
Class I aaRSs are generally monomeric and composed of a Rossmann-nucleotide-binding fold, containing the highly conserved sequence elements KMSKS and HIGH. Class I enzymes approach tRNA from the minor groove of the tRNA acceptor stem, aminoacylating the terminal adenosine of the tRNA molecule at the 2′-OH position. Class II aaRSs are generally multimeric enzymes that approach the major groove of their respective tRNAs, charging the 3′-OH of the terminal adenosine. Once synthesized, aa-tRNAs are selectively bound by EF-1A in archaea and eukaryotes (homologous to the bacterial EF-Tu) as a ternary complex with GTP and ushered to the ribosome during protein synthesis. Eukaryotic aaRSs are distinguished by the presence of appended domains at either the N- or C-terminus, which are generally absent from their bacterial/archaeal counterparts (Mirande, 1991). These appendages to the catalytic cores of several aaRSs are non-catalytic and instead function to mediate protein–protein interactions or act as general RNA-binding domains (Cahuzac et al., 2000; Robinson et al., 2000; Guigou et al., 2004).
An elaborate network of protein–protein interactions is required for efficient translation in all domains of life. AaRSs must correctly bind a diverse array of molecules to perform their essential function of aminoacylation, including ATP, amino acids, tRNAs, and in some cases other aaRSs. Also, several aaRSs associate with additional or auxiliary protein factors that are primarily used in cellular tasks beyond translation, expanding the repertoire of functions aaRSs may pursue (Fig. 1). Although many of these associations were first described in eukaryotic cells, numerous multi-enzyme complexes containing aaRSs have recently been identified in both bacteria and archaea (Table 1).
Fig. 1.
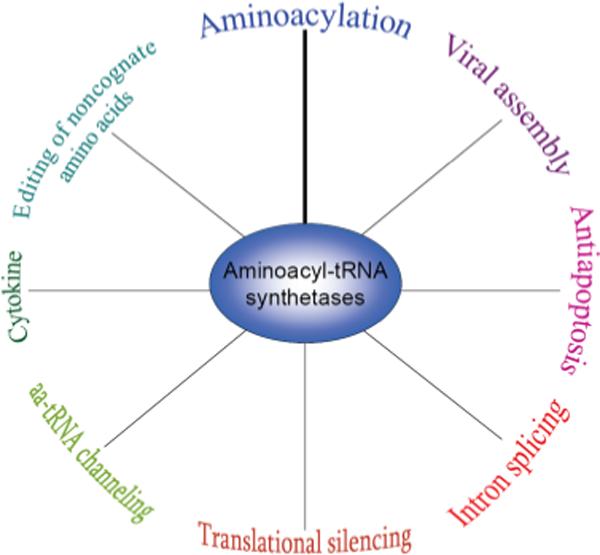
AaRSs impact a diverse range of functions throughout all domains of life.
Table 1.
aaRS-containing complexes in all three domains of life
| Domain | aaRS component(s) (class) | Accessory protein(s) | Species | Function | References |
|---|---|---|---|---|---|
| Bacteria | ProRS (II) | YbaK | H. influenza; E. coli | Hydrolysis of Cys-tRNAPro | An & Musier-Forsyth (2004), Ruan & Söll (2005) |
| AspRS (II) | GatCAB amidotransferase | T. thermophilus | Conversion of Asp-tRNAAsn to Asn-tRNAAsn | Bailly et al. (2007) | |
| TrpRS (II) | Nitric oxide synthase | D. radiodurans | Nitration of tryptophan, producing 4-nitro-Trp | Buddha et al. (2004a), Buddha et al. (2004b), Buddha & Crane (2005) | |
| Archaea | ProRS (II), (LysRS (I), and AspRS (II)) | Mj1338 (metabolic protein) | M. janaschii | Unknown (*enhanced protein stability) | Lipman et al. (2003) |
| LysRS (I), LeuRS (I), ProRS (III) | EF-1A | M. thermautotrophicus | Enhanced aminoacylation | Praetorius-Ibba et al. (2005), Praetorius-Ibba et al. (2007), Hausmann et al. (2007) | |
| Eukarya | MetRS (I), GluRS (I) | Arc1p | S. cerevisiae | Enhanced binding affinity of tRNAMet and tRNAGlu.; subcellular localization | Simos et al. (1996), Simos et al. (1998), Galani et al. (2001), Karanasios et al. (2007) |
| SerRS (II) | Pex21p | S. cerevisiae | Enhanced binding of tRNASer to SerRS | Rocak et al. (2002), Godinic et al. (2007) | |
| TyrRS (I) | Knr4 | S. cerevisiae | Unknown (*dityrosine formation during sporulation) | Dagkessamanskaia et al. (2001), Ivakhno & Kornelyuk (2005) | |
| ValRS (I) | EF-1A | H. sapiens | Enhanced aminoacylation; restriction of associated proteins to the cytoplasm | Motorin et al. (1988), Venema et al. (1991), Bec et al. (1994), Negrutskii et al. (1999) | |
| AspRS (II) | EF-1A | H. sapiens | Asp-tRNAAsp product release | Reed et al. (1994), Reed & Yang (1994) | |
| LysRS (II) and AspRS (II) | EF-1A and p38 | H. sapiens | Enhanced aminoacylation | Guzzo & Yang (2008) | |
| GluProRS (I, II) | L13a, GAPDH, NSAP1 | H. sapiens | Translational silencing | Mazumder et al. (2003b), Sampath et al. (2004), Ray et al. (2007) | |
| LysRS (II) | HIV-1 Gag | H. sapiens | Viral assembly; packaging of tRNALys | Javanbakht et al. (2003), Guo et al. (2005), Kovaleski et al. (2006) | |
| LysRS (II), LeuRS (I), IleRS (I), GluProRS (I,II), MetRS (I), GlnRS (I), ArgRS (I), AspRS (II) | p18, p38, p43 | Chordates | Unknown (*exclusion of aaRSs from the nucleus, enhanced protein stability; control of noncanonical aaRS functions) | Bandyopadhyay & Deutscher (1971), Mirande et al. (1985), Kerjan et al. (1994), Quevillon et al. (1999), Robinson et al. (2000) |
Proposed.
Complexes in bacteria
Bacterial aaRSs are generally thought to function as stand-alone proteins to carry out the fundamental task of aminoacylation. However, a handful of binary complexes comprised of one aaRS and a second protein factor have recently been discovered in bacteria, with one early report suggesting the formation of a higher order multi-aaRS complex (Harris, 1987). These binary complexes are involved in a diverse range of functions, from editing of misacylated tRNAs to metabolite biosynthesis.
Prolyl-tRNA synthetase :YbaK
Accurate translation of the genetic code relies on the correct pairing of amino acids with their cognate tRNAs. In order to preserve the fidelity of protein synthesis, misactivated amino acids and mischarged tRNAs must be efficiently hydrolyzed to avoid infiltration of the genetic code by noncognate amino acids (Baldwin & Berg, 1966; Eldred & Schimmel, 1972; Fersht & Dingwall, 1979; Kim et al., 1993; Schmidt & Schimmel, 1994; Roy et al., 2004; Williams & Martinis, 2006). This may be accomplished by discrete, free-standing editing proteins or by editing domains appended to the catalytic cores of aaRSs (Ahel et al., 2003; Wong et al., 2003; Korencic et al., 2004; Fukunaga & Yokoyama, 2007a, b). Prolyl-tRNA synthetase (ProRS) is known to misactivate tRNAPro with alanine and cysteine (Ambrogelly et al., 2002; An & Musier-Forsyth, 2005). Although most ProRS enzymes have editing domains that efficiently deacylate AlatRNAPro, ProRS is unable to clear Cys-tRNAPro (Beuning & Musier-Forsyth, 2001; An & Musier-Forsyth, 2005; Ruan & Söll, 2005). To overcome this deficiency, bacterial ProRS forms stable interactions with YbaK, a free-standing homologue of the ProRS Ala-tRNAPro-editing domain (An & Musier-Forsyth, 2004, 2005; Ruan & Söll, 2005).
Stable ternary complex formation between bacterial ProRS, tRNAPro, and YbaK results in hydrolysis of mischarged Cys-tRNAPro both in vitro and in vivo (An & Musier-Forsyth, 2005; Ruan & Söll, 2005). YbaK, a general tRNA-binding protein, does not specifically recognize tRNAPro, necessitating protein interactions with ProRS for correct substrate binding. Interestingly, YbaK not only deacylates Cys-tRNAPro, but may also diminish the formation of mischarged Cys-tRNAPro by ProRS, further guarding against the incorporation of noncognate amino acids into the growing polypeptide chain (An & Musier-Forsyth, 2005). In competition studies, YbaK was unable to compete with EF-Tu for Cys-tRNAPro, suggesting that YbaK hydrolyzes mischarged tRNAs before their release from ProRS. The inability of YbaK alone to either specifically recognize tRNAPro or to compete with EF-Tu for binding of the mischarged species means that complex formation between YbaK and ProRS is essential for efficient hydrolysis of mischarged Cys-tRNAPro.
Transamidosome (aspartyl-tRNA synthetase : amidotransferase)
While some mischarged aa-tRNAs are hydrolyzed by appended or discrete editing domains, protein synthesis in many bacterial species rely on the incorrect coupling of certain amino acid and tRNA pairs. Such is the case for the synthesis of Asn-tRNAAsn and Gln-tRNAGln, which proceeds via the specific mischarging of tRNAAsn and tRNAGln by aspartyl- and glutamyl-tRNA synthetases (AspRS and GluRS), respectively (Schon et al., 1988; Feng et al., 2005; Bailly et al., 2007). The indirect synthesis of Asn-tRNAAsn requires a two-step reaction in which first a nondiscriminating AspRS mischarges tRNAAsn with aspartic acid (Becker & Kern, 1998; Tumbula-Hansen et al., 2002; Min et al., 2003; Cardoso et al., 2006). The misacylated Asp-tRNAAsn is then bound by the GatCAB amidotransferase, which functions to convert the aspartic acid moiety to asparagine in the presence of an amide donor. Similarly, Gln-tRNAGln in bacteria and organelles is synthesized via an indirect pathway, which requires misacylation of tRNAGln by GluRS and subsequent conversion to Gln-tRNAGln by the GatCAB amidotransferase (Curnow et al., 1998; Raczniak et al., 2001; Salazar et al., 2001; Oshikane et al., 2006). The synthesis of Asn-tRNAAsn and Gln-tRNAGln via indirect pathways is essential for those bacterial species whose genomes do not code for asparaginyl- or glutaminyl-tRNA synthetases (AsnRS or GlnRS, respectively), providing the sole route for biosynthesis of these amino acid : tRNA pairs.
The rate-limiting step in this tRNA-dependent reaction is amide transfer prior to the formation of Asn-tRNAAsn (Bailly et al., 2007). Recently, it was discovered that the bacterial AspRS stably associates with amidotransferase in the presence of tRNAAsn, forming the transamidosome complex. Within the context of the transamidosome, the rate of Asp-tRNAAsn conversion to Asn-tRNAAsn was significantly enhanced, as compared to free amidotransferase. This suggests that the transfer of Asp-tRNAAsn from AspRS to amidotransferase requires stable complex formation between the two proteins for efficient conversion. Although it has not been observed in vitro, a similar complex between the nondiscriminating GluRS and amidotransferase has also been suggested to occur in the cell (Oshikane et al., 2006; Bailly et al., 2007). The transamidosome harbors two proteins that execute consecutive functions: AspRS and amidotransferase. Protein–protein interactions between the aaRS and amidotransferase may be necessary not only to enhance the synthesis of Asn-tRNAAsn, but may also be important to facilitate the direct transfer of the vital AsptRNAAsn intermediate from AspRS to the amidotransferase, preventing spontaneous hydrolysis in the cytoplasm.
Tryptophanyl-tRNA synthetase : nitric oxide synthase (NOS)
Many bacteria harbor multiple copies of aaRSs encoded in the same genome (Brown et al., 2003; Salazar et al., 2003; Levengood et al., 2004; Ataide et al., 2005; Ataide & Ibba, 2006). One such example is Deinococcus radiodurans, which harbors two genes encoding tryptophanyl-tRNA synthetase (TrpRS I and TrpRS II) (Buddha et al., 2004a, b). Although the two TrpRSs contain the conserved amino acid residues responsible for aminoacylation of tRNATrp with tryptophan (Trp), TrpRS II is unusual in that it is structurally more similar to human TrpRS and is induced in response to damage caused by radiation (Buddha et al., 2004a). In addition, TrpRS II contains an N-terminal extension similar to other proteins involved in stress responses in bacteria and has been shown to associate with nitric oxide synthase (NOS) (Buddha et al., 2004a, b).
In mammals, nitric oxide is synthesized from the oxidation of arginine (Arg), and functions in nerve signal transmission, vasodilation, and the immune system (Kers et al., 2004). Some bacterial species harbor truncated homologues of the mammalian NOS, which have been identified to perform novel functions in bacteria. In complex with TrpRS II and tRNATrp, the bacterial NOS catalyzes the regiospecific nitration of Trp from Arg at the four-position, producing 4-Nitro-Trp (Buddha et al., 2004a). Complex formation between TrpRS II and NOS favored the activities of both associated enzymes. TrpRS II increased NOS solubility and affinity for its arginine substrate, facilitating the specific nitration of Trp from Arg (Buddha et al., 2004a, b). TrpRS II may also supply NOS with adenylated-Trp for subsequent nitration or nitrosylation, as Trp nitration does not occur with free Trp in vitro.
Both TrpRS I and TrpRS II are able to aminoacylate tRNA with Trp, although the efficiency of TrpRS II is about fivefold lower than TrpRS I (Buddha et al., 2004a). In addition to the canonical Trp substrate, TrpRS II has been observed to charge tRNA with 4-nitro-Trp in the presence of ATP with nearly the same aminoacylation efficiency as with Trp (Buddha & Crane, 2005). Although the role of TrpRS II catalyzed 4-nitro-Trp aminoacylation is largely unknown, it may function outside of protein synthesis as D. radiodurans encodes two TrpRSs, with TrpRS II expression only induced in response to specific stimuli such as radiation damage. 4-nitro-Trp may play a role in the synthesis of other metabolites or may perhaps function in a role similar to that of charged tRNA in peptidoglycan production, where the aa-tRNA functions outside of protein synthesis to provide amino acids required for cell membrane biosynthesis (Ibba & Söll, 2004; Kers et al., 2004; Roy & Ibba, 2008).
Complexes in archaea
Although binary complexes containing one aaRS are generally the norm in bacteria, archaea and eukaryotes have been found to harbor higher order complexes composed of multiple aaRS activities in association with a variety of cellular factors as described below.
Prolyl-tRNA synthetase : Mj1338
In an early report, several if not all aaRSs were observed to associate into a large multi-aminoacyl-tRNA synthetase complex (MSC) in the archaeon Haloarcula marismortui, providing precedence for the existence of higher order complexes containing aaRSs in archaea (Goldgur & Safro, 1994). More recently, ProRS has been found to associate in Methanocaldococcus jannaschii with a metabolic protein (Mj1338) predicted to be involved in one-carbon metabolism in archaea (Lipman et al., 2003). Although the function of Mj1338 has not been completely resolved, the metabolic protein has been demonstrated to possess a general affinity for tRNA. The archaeal ProRS bound Mj1338 with a KD of about 1.4 μM, indicating a stable protein–protein interaction. Although the formation of a higher order complex has not been verified, lysyl-tRNA synthetase (LysRS) and AspRS each interacted independently with Mj1338, suggesting a possible association with the archaeal ProRS : Mj1338 complex.
As is the case for the higher order complex of aaRSs discovered in H. marismortui, the biological function of complex formation between archaeal ProRS and Mj1338 remains largely unknown, as complex formation did not stimulate aminoacylation activity. Although further experimentation would be required to investigate the formation of a higher order complex in M. jannaschii, these specific aaRSs (ProRS, LysRS, and AspRS) are noteworthy as their eukaryotic counterparts are all known to associate into a larger MSC in mammalian cells composed of nine aaRS activities and three auxiliary proteins (Mirande et al., 1985; Kerjan et al., 1994; Quevillon et al., 1999; Robinson et al., 2000).
Lysyl-tRNA synthetase : leucyl-tRNA synthetase : prolyl-tRNA synthetase
In the archaeal species Methanothermobacter thermautotrophicus, a MSC composed of LysRS, LeuRS, and ProRS was identified via yeast two-hybrid screening of an M. thermautotrophicus cDNA library with LysRS and ProRS as bait proteins (Praetorius-Ibba et al., 2005, 2007). Formation of a stable complex between LysRS, LeuRS, and ProRS was further confirmed in vitro and in vivo via copurification experiments using both the corresponding His-tagged proteins and archaeal cell-free extracts (Praetorius-Ibba et al., 2007). Pairwise binding affinities were determined from fluorescence anisotropy studies, indicating that LeuRS bound LysRS and ProRS with comparable KDs of about 0.3−0.9 μM, lending support to the formation of a stable MSC in archaea.
The functional consequences of complex formation were investigated by determining the steady-state aminoacylation parameters of each aaRS alone or in the MSC. In the presence of LeuRS, the catalytic efficiencies of aminoacylation by LysRS and ProRS were enhanced three- and fivefold, respectively, while no significant changes in the kinetics of aminoacylation by LeuRS were observed. No further changes were identified upon addition of the aaRSs in alternative combinations. This indicates the possible role of an archaeal MSC comprised of three aaRSs in which LeuRS improves the catalytic efficiencies of tRNA aminoacylation by both LysRS and ProRS. Interestingly, these three aaRSs activities are also found in the macromolecular mammalian MSC, suggesting that the biological role of aaRS complexes in translation may be to enhance aminoacylation by the associated aaRSs.
The archaeon M. thermautotrophicus also harbors a complex between LeuRS and EF-1A, a translation elongation factor that specifically binds and escorts aa-tRNAs to the ribosome. First identified via yeast two-hybrid screening of an M. thermautotrophicus cDNA library, complex formation between EF-1A and LeuRS was confirmed by co-immuno-precipitation and fluorescence anisotropy experiments, from which a KD of about 0.7 μM was determined (Hausmann et al., 2007). In complex with EF-1A, aminoacylation by LeuRS was enhanced threefold, with an eightfold increase in the kcat of Leu-tRNALeu synthesis, suggesting that perhaps a conformational change occurs upon complex formation. The activities of EF-1A, however, remained largely unaffected. Unlike the archaeal EF-1A, the bacterial homolog EF-Tu had no effect on aminoacylation by LeuRS, indicating that the enhanced rate of Leu-tRNALeu synthesis was neither a result of product sequestration by EF-1A (known to protect the labile ester bond of aa-tRNA) nor was it the nonspecific effect of a GTPase. EF-1A has also been suggested to associate in vivo with the M. thermautotrophicus MSC, composed of LysRS, LeuRS, and ProRS, possibly through interactions with LeuRS (Hausmann et al., 2007). Although EF-1A enhances the kcat of Leu-tRNALeu synthesis, no change in aminoacylation by LysRS or ProRS was observed in the presence of EF-1A. Taken together, the association of all four proteins involved in translation results in enhanced aminoacylation by LysRS, LeuRS, and ProRS (Praetorius-Ibba et al., 2005, 2007; Hausmann et al., 2007).
It has been suggested that aminoacylation by class I aaRSs may be rate-limited by aa-tRNA release, while class II enzymes are rate-limited by a step before product release (Zhang et al., 2006). These distinctions between class I and class II aaRSs may compel an association of translation elongation factors with class I aaRSs for efficient aa-tRNA release while class II aaRSs may not necessarily require stable interactions with EF-1A for product release. Stable protein–protein interactions between LeuRS, a class I aaRS, and EF-1A lend support to this notion. Further, aaRSs and EF-1A perform consecutive functions in translation, aminoacylation of tRNA and subsequent delivery to the ribosome. Although the molecular mechanism remains largely unknown, complex formation between the elongation factor and an aaRS may favor the direct hand-off of aa-tRNA from the aaRS to the elongation factor without diffusion into the cytoplasm, which was first suggested in the case of the valyl-tRNA synthetase (ValRS) : EF-1A complex in human cells (Negrutskii & Deutscher, 1991; Stapulionis & Deutscher, 1995; Negrutskii & El'skaya, 1998; Negrutskii et al., 1999; Petrushenko et al., 2002; Yang et al., 2006). In accordance with this proposed model, the association of EF-1A with the archaeal MSC may serve to directly transfer aa-tRNAs synthesized by the associated aaRSs to EF-1A for delivery to the ribosome. Further studies are now necessary to determine if these MSCs are widespread in archaea, which would provide new insights into their possible functions in translation.
Complexes in eukaryotes
A variety of multiprotein complexes containing aaRSs exists throughout all three domains of life. In eukaryotes these complexes tend to be larger than those discovered in bacteria and archaea and also perform a wider range of functions from aminoacylation to noncanonical functions beyond translation. The true identity of complexes that occur in vivo may be masked, however, possibly due to the fragility of the complex, loosely associated peripheral proteins, or transient interactions that disassociate during copurification procedures. With this in mind, a comprehensive overview of the complexes currently known to form in eukaryotes is presented.
Glutamyl-tRNA synthetase : Arc1p : methionyltRNA synthetase
In Saccharomyces cerevisiae, glutamyl- and methionyl-tRNA synthetases (GluRS and MetRS, respectively) stably interact with aminoacyl-tRNA synthetase cofactor I (Arc1p), a nonsynthetase accessory protein (Simos et al., 1996, 1998; Galani et al., 2001; Karanasios et al., 2007). Protein–protein interactions are mediated through the N-terminal domains of each associated component, as observed from biochemical studies and from modeling of two binary complexes of Arc1p with each aaRS (Deinert et al., 2001; Simader et al., 2006). Discrete residues of Arc1p have been identified as critical for binding GluRS and MetRS, mediating ternary complex formation (Galani et al., 2001; Karanasios et al., 2007). Arc1p harbors an EMAPII-like domain at its C-terminus, containing an oligonucleotide-binding (OB) domain, and a positively charged central domain, which enables Arc1p to act as a general tRNA-binding factor (Simos et al., 1996, 1998). As part of the ternary complex, Arc1p preferentially facilitates the binding of the cognate tRNA substrates (tRNAGlu and tRNAMet) to the associated aaRSs, thus increasing the catalytic efficiencies of both GluRS and MetRS.
Bacterial tRNA-binding protein (Trbp111) and human p43 (an auxiliary protein of the mammalian MSC) both harbor tRNA-binding domains similar to that found in the C-terminus of Arc1p and can in fact functionally replace Arc1p to complement a synthetic lethal arc1− los1− strain of S. cerevisiae (Simos et al., 1996; Quevillon et al., 1997; Swairjo et al., 2000; Deinert et al., 2001; Golinelli-Cohen et al., 2004). Based on models of Trbp111, Arc1p has been suggested to interact with the top corner of the tRNA substrates, which is also in agreement with biochemical studies implicating the tRNA D- and TΨC-loops as part of the binding site for Arc1p (Simos et al., 1996; Swairjo et al., 2000). Both GluRS and MetRS are class I aaRSs, known to interact with the inner L portion of the tRNA molecule, which may permit simultaneous associations of Arc1p and aaRSs with the tRNA substrates in complex (Senger et al., 1995; Sekine et al., 2001).
Arc1p in complex with GluRS and MetRS has been observed to preferentially facilitate the binding of tRNAGlu and tRNAMet to each aaRS, possibly by promoting structural rearrangements (Simos et al., 1998; Galani et al., 2001; Simader et al., 2006; Golinelli-Cohen & Mirande, 2007). Although unbound GluRS is able to bind its cognate tRNAGlu with moderate affinity, tRNA binding is greatly influenced by the presence of Arc1p. In complex, the apparent KD for tRNAGlu is reduced about 120-fold to 30 nM (Graindorge et al., 2005). Steady-state aminoacylation kinetic parameters could not be determined, however, because in vitro transcribed tRNAGlu proved a poor substrate for aminoacylation. In the case of MetRS, the kcat for tRNA aminoacylation increased almost fivefold in the presence of Arc1p as compared to MetRS alone (Simos et al., 1996). Arc1p facilitated the binding of tRNAMet to MetRS by significantly decreasing (about 100-fold) the KM of tRNAMet, as compared to the aaRS alone (KM ∼10 μM).
Beyond its role in enhanced binding of cognate tRNAs to MetRS and GluRS, the Arc1p complex also regulates the subcellular localization of each associated component (Galani et al., 2001; Golinelli-Cohen & Mirande, 2007). Arc1p is exclusively found in the cytoplasm due to export by Xpo1p (Galani et al., 2001, 2005). When associated with Arc1p, both GluRS and MetRS localize to the cytoplasm, while disruption of the complex due to N-terminal truncations of each protein allows entrance of the individual aaRSs into the nucleus (Galani et al., 2001, 2005; Karanasios et al., 2007). Arc1p may also interact indirectly with Los1p (a nuclear pore-associated protein involved in tRNA export), as discovered from synthetic lethal screens using a disrupted los1− strain, indicating the involvement of Arc1p in tRNA export from the nucleus (Simos et al., 1996, 1998). Translation factor EF-1A was also discovered in a suppressor screen of a synthetically lethal los1− strain, suggesting that EF-1A may usher newly exported tRNA from the nucleus to the corresponding aaRSs (Sarkar et al., 1999; Grosshans et al., 2000a, b; McGuire & Mangroo, 2007). Taken together, these data reveal how Arc1p coordinates events between protein synthesis and tRNA export in S. cerevisiae.
Seryl-tRNA synthetase : Pex21p
In addition to the Arc1p : GluRS : MetRS complex, S. cerevisiae also harbors a complex between seryl-tRNA synthetase (SerRS) and Pex21p, a protein involved in peroxisome biosynthesis. First identified by yeast two-hybrid analysis, pull-down experiments indicated that Pex21p and SerRS associate in cell-free extracts, suggesting complex formation in vivo (Rocak et al., 2002). The C-terminus of SerRS, which is not essential for cellular viability or aminoacylation, mediates protein–protein interactions with Pex21p (Godinic et al., 2007; Mocibob & Weygand-Durasevic, 2008). In complex, Pex21p promoted the binding of tRNASer to SerRS, slightly enhancing aminoacylation (Rocak et al., 2002; Godinic et al., 2007). Pex21p may induce conformational changes in SerRS to enhance the binding of cognate tRNA, thereby playing a similar role to that of Arc1p in complex with GluRS and MetRS (Simos et al., 1998; Rocak et al., 2002; Simader et al., 2006; Godinic et al., 2007).
Peroxisomes, which are involved in the metabolism of fatty acids and other metabolites, function as part of the secretory pathway to post-translationally import peroxisomal enzymes across the peroxisome membrane. Although the functional consequences of complex formation on the activity of Pex21p remain to be discovered, the SerRS : Pex21p complex provides a connection between translation and peroxisome biosynthesis. SerRS is also involved in the synthesis of diadenosine oligophosphates, which may act as a signal of oxidative stress (Lopez-Huertas et al., 2000). Peroxisome biosynthesis genes are also known to be induced by hydrogen peroxide and other stressors to the cell, suggesting that complex formation between SerRS and Pex21p may contribute to coordination of stress signaling networks in S. cerevisiae (Lee et al., 1983; Belrhali et al., 1995; Godinic et al., 2007).
Tyrosyl-tRNA synthetase (TyrRS) : Knr4
A connection between tyrosyl-tRNA synthetase (TyrRS) and a protein involved in cell wall biosynthesis (Knr4) has been identified in S. cerevisiae from DNA microarray, yeast two-hybrid, and pull-down experiments (Dagkessamanskaia et al., 2001; Ivakhno & Kornelyuk, 2005). DNA microarray analyses demonstrated that TyrRS gene expression during sporulation correlated well with the expression of Knr4, as well as other genes that participate in cell-wall assembly (Ivakhno & Kornelyuk, 2005). Further, disruption of the gene encoding Knr4 reduced glucan synthase activity and resulted in decreased spore formation, which was associated with a concomitant activation of TyrRS during sporulation (Chu et al., 1998). Although the biological function of this cellular association is largely unknown, dityrosine is an essential component of the outermost layer of the spore wall, suggesting a collaborative effect between TyrRS and Knr4 to promote dityrosine formation during sporulation in S. cerevisiae (Briza et al., 1986; Coluccio et al., 2004).
Valyl-tRNA synthetase : EF-1H
Translation elongation requires the correct synthesis of aa-tRNA by aaRSs and their subsequent delivery to the ribosome by EF-1A. In human cells, the GTPase EF-1A associates with the guanine nucleotide recycling machinery, EF-1B, to form the larger elongation factor 1H (EF-1H) complex. Upon codon–anticodon recognition on the ribosome, GTP is hydrolyzed, releasing the GDP-bound form of EF-1A. EF-1B then functions to recycle GDP to GTP, permitting EF-1A to pursue another round of tRNA selection. A stable complex between human ValRS and EF-1H provides a link between these essential steps in translation (Motorin et al., 1988; Venema et al., 1991; Bec et al., 1994; Minella et al., 1998; Negrutskii et al., 1999). The N-terminal appended domain of ValRS was identified to mediate protein : protein interactions, as it strongly associates with the EF-1d subunit of EF-1H (Negrutskii et al., 1999). Although the stoichiometry of the EF-1H : ValRS complex is debated, the presence of excess EF-1A and GTP enhanced the catalytic efficiency of ValRS almost twofold, while the KM for tRNAVal remained unaffected (Negrutskii et al., 1999). This increase in the catalytic efficiency was not observed in the presence of EF-1A : GDP or bacterial EF-Tu : GTP, indicating that the increased levels of ValtRNAVal were due to improved catalysis by ValRS, and not the protective effects of EF-1A.
The EF-1A : ValRS complex correlates well with the ability of the elongation factor to form complexes with and enhance the rate of aminoacylation by class I aaRSs (Kern & Gangloff, 1981; Zhang et al., 2006), as also observed for the EF-1A : LeuRS complex in archaea (Hausmann et al., 2007). EF-1A has been observed to associate with the class II aaRS, human AspRS, and stimulated the rate of AsptRNAAsp release (Reed & Yang, 1994; Reed et al., 1994). More recently, EF-1A has been suggested to interact with class II human LysRS, slightly enhancing aminoacylation by LysRS (Guzzo & Yang, 2008). An interaction between human TrpRS and EF-1A has also been proposed, although stable complex formation has not yet been confirmed (Yang et al., 2006); EF-1A has also been shown to enhance aminoacylation by MetRS (Kaminska et al., 2001).
AaRSs and EF-1A perform two consecutive functions in translation: aminoacylation and the subsequent delivery of aa-tRNA to the ribosome. The link between these two translational enzymes lends support to the notion that protein–protein interactions between aaRSs and EF-1A may serve as a vector, directly transferring aa-tRNA from aaRSs to the ribosome. Further extending this role, EF-1A : GDP may then complete the cycle by accepting uncharged tRNA from the ribosomal E site for another round of aminoacylation by the associated aaRS (Petrushenko et al., 1997). This is evidenced by the unusual complex phenylalanyl-tRNA synthetase (PheRS) forms with EF-1A : GDP and uncharged tRNA (Dibbelt & Zachau, 1981; Petrushenko et al., 1997, 2002). Beyond translation, EF-1A has also been implicated in chaperone-like functions, such as protecting from denaturation and promoting renaturation of PheRS and SerRS (Gonen et al., 1994; Kudlicki et al., 1997; Caldas et al., 1998; Hotokezaka et al., 2002; Lukash et al., 2004). Through a concerted effort, EF-1A may associate with aaRSs in eukaryotes and archaea to facilitate the delivery of aa-tRNA to the ribosome and to promote protein stability.
Mammalian MSC
In mammalian cells, a larger 1.4 MDa multi-aaRS complex exists, composed of nine aaRS activities (leucyl-, lysyl-, prolyl-, isoleucyl-, methionyl-, glutamyl-, glutaminyl-, arginyl-, and aspartyl-tRNA synthetases) (Bandyopadhyay & Deutscher, 1971; Kerjan et al., 1994; Quevillon et al., 1999; Robinson et al., 2000). Three non-aaRS factors p38, p43, and p18 complete the complex, playing roles in tRNA binding and complex stability/assembly. The MSC is composed of a mixture of class I and class II aaRSs, indicating that the distinction between the complexed and free forms does not solely reside in the structural architectures of their active sites nor the amino acids that are activated and attached to their corresponding tRNA molecules. It has been observed that the aaRSs responsible for coupling charged amino acids and hydrophobic, nonaromatic amino acids to tRNA molecules are all present within the complex, while those aminoacylating the smallest and largest amino acids are absent (Wolfson & Knight, 2005). It is unknown why certain aaRSs unite into a larger complex while others exist as free-standing proteins. It is possible, however, that several other, or perhaps even all, aaRSs associate with the complex more transiently in the cell and the current model of the MSC may be a reflection of experimental limitations when copurifying macromolecular complexes.
Although the composition of the proteins stably associated in the mammalian MSC was deciphered nearly 30 years ago, the exact structural arrangement and assembly of the complex remain undefined. Through biochemical, genetic, and cryo-electron microscopic analyses, however, a clearer picture of the MSC has been captured (Norcum, 1989, 1999; Quevillon et al., 1999; Kim et al., 2000; Robinson et al., 2000; Han et al., 2003; Wolfson & Knight, 2005). The MSC particle is compact, as visualized by electron and immunoelectron microscopy, and assembles into a V-shaped complex, possibly via the association of three subcomplexes (Norcum, 1989, 1999; Norcum & Warrington, 1998). Localized in one arm are AspRS, MetRS, and GlnRS, while on the other arm are LysRS and ArgRS. The bifunctional GluProRS, IleRS, and LeuRS are found at the base of the V-shaped macromolecule, and the polypeptides connect the three subdomains, forming the complete mammalian MSC (Wolfe et al., 2005).
The assembly of the MSC is thought to be highly structured and interdependent on other binding events within the complex. Lending support to the notion of ordered assembly, NaSCN or high concentrations of NaCl result in the release of LysRS and AspRS from the complex, respectively, suggesting that these aaRSs are located near the periphery (Cirakoglu et al., 1985; Norcum, 1991; Agou & Mirande, 1997). MetRS, GlnRS, and ArgRS are also relatively easy to remove from the MSC and have been found as free forms in the cell (Ko et al., 2001; Han et al., 2006a, b). Extensive pairwise yeast two-hybrid screening of each aaRS associated in the MSC was performed, providing insight into all 64 combinations of protein–protein interactions within the complex (Rho et al., 1999). Protein–protein interactions within the mammalian MSC may be mediated through appended domains found in the associated aaRSs, as has been observed for MetRS, LeuRS, and ArgRS (Mirande et al., 1982, 1992; Vellekamp & Deutscher, 1987; Robinson et al., 2000; Guigou et al., 2004; Ling et al., 2005). Conversely, several aaRSs may associate with the MSC through their catalytic domains (LysRS, AspRS, and GlnRS), because deletion of the appended extensions did not abolish interactions with the MSC (Agou & Mirande, 1997; Kim et al., 2000; Robinson et al., 2000; Francin et al., 2002). The interdependence of binding events was further evidenced by the fact that the binding of GlnRS to p38 was significantly enhanced in the presence of p43 and ArgRS, indicating that perhaps a discreet quaternary complex is required for the ordered formation of the MSC (Robinson et al., 2000).
In addition to the nine aaRS activities associated within the MSC, three auxiliary polypeptides (p38, p43, and p18) complete the complex, lending stability to the complex and promoting the binding of tRNA. The auxiliary protein p38 has been observed to mediate protein–protein interactions, acting as an essential scaffolding protein of the mammalian MSC (Robinson et al., 2000; Kim et al., 2002; Ahn et al., 2003). p38 interacts with nearly all other proteins associated in the MSC over a range of binding affinities between 0.3 nM–5 μM, emphasizing its role as an indispensable core protein important for assembly and stability of the complex (Quevillon et al., 1999; Robinson et al., 2000; Kim et al., 2002; Ahn et al., 2003; Wolfe et al., 2005). Although the molecular mechanisms are not well-defined, p38 has also been implicated in roles outside of the MSC. For example, p38 has been observed to associate in the nucleus with FUSE-binding protein (a transcriptional activator of c-myc), resulting in ubiquitination of FUSE-binding protein and subsequent down-regulation of c-myc (Kim et al., 2002, 2003). p38 has also been shown to associate with and act as a substrate of Parkin (a protein involved in ubiquitination), which results in the degradation of p38 (Corti et al., 2003; Ko et al., 2005). Autosomal-recessive juvenile parkinsonism is caused by loss-of-function mutations in the gene encoding Parkin, which lead to degeneration of dopaminergic neurons via accumulation of toxic proteins. It has been observed that loss of Parkin function results in the accumulation of nonubiquitinated p38, which leads to the formation of aggresome-like inclusions. Although the mechanism remains largely unknown, this accumulation of p38 may contribute to dopaminergic neurodegeneration, providing a link between a protein normally associated in the mammalian MSC and the neurode-generative disorder Parkinson's disease.
The p38 polypeptide associates with a second auxiliary protein, p43, which is also an integral part of the MSC (Wolfe et al., 2003). The C-terminus of p43 harbors an endothelial monocyte-activating polypeptide II (EMAPII)-like domain, which confers a general tRNA-binding property (Simos et al., 1996; Quevillon et al., 1997; Swairjo et al., 2000; Deinert et al., 2001). This domain is homologous to the yeast Arc1p protein that facilitates the binding of cognate tRNA substrates to MetRS and GluRS (Golinelli-Cohen & Mirande, 2007). The EMAPII-like domain, which may be removed from the full-length p43 following apoptotic cleavage, is involved in altering endothelial functions as a proinflammatory cytokine (Behrensdorf et al., 2000; Shalak et al., 2001; Faisal et al., 2007). The p43 EMAPII domain has also been shown to upregulate proinflammatory genes and increase chemotactic migration of polymorphonuclear leukocytes upon cleavage and removal from the MSC (Behrensdorf et al., 2000; Park et al., 2002). The multifunctionality of these proteins involved in the mammalian MSC emphasizes the interconnection between translation and cellular processes outside of protein synthesis.
Although the role of the third auxiliary factor p18 remains largely unknown, p18 shares homology with the N-terminus of human ValRS, which is responsible for mediating complex formation with EF-1H (Quevillon & Mirande, 1996; Sang et al., 2002; Park et al., 2005a). EF-1A also associates with LysRS, a member of the mammalian MSC, suggesting that EF-1A may transiently associate with the macromolecular complex to provide an efficient means to shuttle aa-tRNA directly from the MSC to the ribosome during translation (Sang et al., 2002; Guzzo & Yang, 2008). AaRSs have also been observed to copurify with polysomes (Popenko et al., 1994). Whether aaRSs form specific complexes with polysomes or rather associate transiently remains to be determined. Similar to the smaller complex discovered in M. thermautotrophicus, aaRSs may join to form a macromolecular complex in mammalian cells to enhance the aminoacylation activities of the associated aaRSs (Haus-mann et al., 2007; Praetorius-Ibba et al., 2007). Taken together, MSCs characterized from archaea and eukaryotes improve the efficiency of early steps of translation, aa-tRNA synthesis and perhaps subsequent delivery to the ribosome.
Further, increased protein stability or restriction of the associated aaRSs to the cytoplasm, as was observed for the yeast Arc1p : GluRS : MetRS complex, may entice aaRSs to associate into the higher order MSC complex (Galani et al., 2001). The functional association of nine aaRS activities has also been suggested to provide a sequestered pool of aa-tRNAs specifically for utilization in protein synthesis (Negrutskii & Deutscher, 1991, 1992). Radiolabeled aatRNA, introduced into permeabilized CHO cells, were unable to be used as substrates for protein synthesis, while radiolabeled amino acids were efficiently incorporated during translation. This suggests that endogenously aminoacylated tRNAs, synthesized within the MSC, are required for efficient protein synthesis and that the pools of aa-tRNAs within the cell do not mix freely. This is supported by studies of ArgRS, which exists as both complexed (full-length) and free (truncated at its N-terminus) forms, translated from alternative start codons (Sivaram & Deutscher, 1990; Zhang et al., 2006). It was observed that the pool of aa-tRNAs synthesized by ArgRS complexed with the MSC was preferentially utilized as substrates for protein synthesis in vivo (Kyriacou & Deutscher, 2008). Although the precise molecular mechanism remains to be deciphered, the work by Deutscher and colleagues lends support to the notion that the mammalian MSC is intimately involved in protein synthesis by directly providing the elongating ribosome with substrates synthesized within the MSC (Negrutskii & Deutscher, 1991; Negrutskii et al., 1999). A larger complex of aaRSs was also discovered in the nucleus, possibly functioning in a role similar to the cytoplasmic MSC counterpart via association with nuclear pore-associated EF-1α or to promote tRNA export (Popenko et al., 1994; Ko et al., 2002). Taken together, the mammalian MSC has been implicated in a variety of functions including protein stability, restriction of aaRSs to the cytoplasm, coordination of events during translation elongation.
Noncanonical activities of aaRS complexes
IFN-γ activated inhibitor of translation (GAIT) : GluProRS complex
Apart from their primary role of coupling cognate amino acid and tRNA pairs, it has recently become increasingly evident that aaRSs are not limited to protein synthesis (Table 2). In fact, the MSC may act as a repository for aaRSs where in response to cellular changes, aaRSs are subsequently liberated from the complex to participate in noncanonical tasks beyond translation (Lee et al., 2004a; Park et al., 2005b; Ray et al., 2007). The bifunctional GluProRS, which harbors two different aaRS catalytic activities separated by three tandem linker domains, lends support to this notion by participating in the translational silencing of ceruloplasmin (a protein linked to the inflammatory response) (Rho et al., 1998; Fox et al., 2000; Sampath et al., 2004; Ray et al., 2007). Although generally associated with the MSC, GluProRS is phosphorylated in response to IFN-γ by an unknown mechanism, departs from the MSC, and is involved in mRNA silencing of ceruloplasmin via the GAIT complex composed of GluProRS, NS1-associated protein (NSAP1), glyceraldehydes-3-phosphate dehydrogenase (GAPDH), and ribosomal protein L13a (Cirakoglu et al., 1985; Norcum, 1991; Agou & Mirande, 1997). Within the context of this heterotetrameric complex, translation is inhibited by the binding of the GluProRS linker region to a regulatory stem-loop GAIT mRNA element located within the 3′-untranslated region (UTR) of ceruloplasmin.
Table 2.
Examples of noncanonical functions of aaRSs
| Domain | aaRS component (Class) | Location | Species | Function | References |
|---|---|---|---|---|---|
| Bacteria | ThrRS (II) | Cytoplasm | E. coli | Translational control | Brunel et al. (1993), Romby et al. (1996), Torres-Larios et al. (2002) |
| AlaRS (II) | Cytoplasm | E. coli | Transcriptional control | Putney & Schimmel (1981) | |
| Eukarya | LeuRS (I) | Mitochondria | S. cerevisiae | Group I intron splicing | Herbert et al. (1988), Labouesse (1990), Rho et al. (2002) |
| TyrRS (I) | Mitochondria | N. crassa | Group I intron splicing | Akins & Lambowitz (1987), Cherniack et al. (1990), Paukstelis et al. (2005) | |
| MetRS (I) | Nucleus | H. sapiens | Transcription of rRNA | Ko et al. (2000) | |
| GlnRS (I) | Cytoplasm | H. sapiens | Antiapoptosis | Ko et al. (2001) | |
| TrpRS (I) | Extracellular | H. sapiens | Angiostatic cytokine (targets endothelial cells) | Otani et al. (2002), Wakasugi et al. (2002b), Kise et al. (2004) | |
| TyrRS (I) | Extracellular | H. sapiens | Angiogenic cytokine (targets endothelial cells) | Wakasugi et al. (2002a), Greenberg et al. (2008) | |
| LysRS (II) | Extracellular | H. sapiens | Inflammatory cytokine | Park et al. (2005c) | |
| LysRS (II) | Plasma membrane | H. sapiens | Viral assembly; packaging of tRNALys | Javanbakht et al. (2003), Guo et al. (2005), Kovaleski et al. (2006) | |
| LysRS (II) | Nucleus | H. sapiens | Transcriptional control | Lee et al. (2004b) | |
| HisRS (II) | Cytoplasm | H. sapiens | Chemotactic for leukocytes | Howard et al. (2002) | |
| GluProRS (I, II) | Cytoplasm | H. sapiens | Translational silencing | Sampath et al. (2004), Ray et al. (2007) |
First, a pre-GAIT complex of GluProRS and NSAP1 is formed that is unable to bind the 3′-UTR region of ceruloplasmin and silence translation, possibly due to the inhibitory effects of NSAP1 (Jia et al., 2008). The association of L13a and GAPDH complete the heterotetrameric GAIT complex, inducing a conformational change that abrogates the inhibitory effects of NSAP1. The GAIT complex is then able to bind the GAIT mRNA element of ceruloplasmin, mediated by the GluProRS WHEP domains, resulting in translation inhibition in response to IFN-γ (Mazumder et al., 2003a, b; Sampath et al., 2004; Kapasi et al., 2007). Although the precise molecular mechanism of translational silencing via the GAIT complex is not well-defined, GAIT mRNA elements are found in more than 30 human mRNAs, suggesting a coordinated regulation of the inflammatory response via a complex involving the multifunctional GluProRS (Mazumder et al., 2003a, b; Sampath et al., 2004).
Lysl-tRNA synthetase (LysRS) : Gag in HIV1
AaRSs have also been implicated in a number of functions separate from translation, including involvement in HIV type 1 (HIV-1) viral assembly (Stark & Hay, 1998; Halwani et al., 2004; Kleiman et al., 2004; Cen et al., 2001, 2004a, b, c). Human HIV-1, an RNA retrovirus, enters the target cell and converts its RNA genome into DNA using a virally encoded reverse transcriptase (Chan et al., 1999; Dobard et al., 2007; McCulley & Morrow, 2007). Initiation of reverse transcription requires the specific packaging of tRNALys into the HIV-1 virions, which functions as a primer for reverse transcriptase by binding near the 5′ end of the HIV-1 genomic RNA (Barat et al., 1989; Kleiman & Cen, 2004; McCulley & Morrow, 2007). Following minus strand transfer and reverse transcription of the minus strand DNA, the plus strand DNA is synthesized and the double-stranded DNA copy is translocated into the nucleus of the target cell where it is integrated into the host's chromosomal DNA (Liu et al., 2005; Zeng et al., 2007).
Human LysRS is also selectively packaged into the HIV-1 virion, independent of tRNALys incorporation, which is evidenced by the fact that LysRS mutants unable to bind tRNA retain the ability to be packaged (Javanbakht et al., 2003). Incorporation of LysRS into the virion is accomplished via specific interactions with HIV-1 Gag (a dimeric protein involved in assembly of viral particles), which interacts stably with a KD of about 0.3 μM (Javanbakht et al., 2003; Guo et al., 2005; Kovaleski et al., 2006).
LysRS is believed to contribute positively to the selective incorporation of tRNALys. Lending support to this notion was the discovery that inhibition of LysRS synthesis resulted in reduced tRNALys packaging and annealing to the HIV-1 viral RNA (Guo et al., 2003).
The interaction interface between LysRS, a dimeric class II aaRS, and Gag has been mapped to the dimerization domains of each protein, although the precise stoichiometry and multimeric form of each enzyme remain largely unknown (Javanbakht et al., 2003; Kovaleski et al., 2006). It has been postulated that newly synthesized LysRS associates with Gag rapidly before joining the MSC (Halwani et al., 2004). This demonstrates that LysRS, which is an essential part of the mammalian MSC required for efficient protein synthesis, has been recruited from its primary role in translation to take part in the auxiliary function of HIV-1 viral assembly and packaging of its cognate tRNA.
Concluding remarks
Accurate translation of mRNA into protein is essential for cellular viability. Central to this process are the aaRSs, providing amino acid substrates to the ribosome in the form of aminoacyl-tRNAs. It has become increasingly evident that these indispensable enzymes have also accepted tasks in other cellular processes, acting outside of their canonical roles. The aaRSs are capable of acquiring new functions by associating into higher order complexes with proteins used in a variety of cellular tasks in all three domains of life. Most evident in archaea and eukaryotes, these macromolecular complexes are believed to share the common feature of promoting the direct hand-off of aa-tRNA from aaRSs to the ribosome during protein synthesis or connecting translation to other processes in the cell. By communicating with additional protein factors, aaRSs gain roles in alternative cellular processes such as translational silencing and viral assembly.
Multiprotein complexes harboring aaRSs vary in size from binary to macromolecular. This diversity may indicate that other cellular proteins associate with these known complexes, but have remained undiscovered due to the experimental limitations of purification. Providing new insight into previously unknown protein factors beyond the easily purified core may be the key to unlock the biological function of complex formation. Insight into subcellular localization and dynamic assays in vivo will provide further evidence to reveal the physiological role of protein–protein interactions. Another avenue of experimentation to be addressed is how protein complexes respond to physiological changes in the cell. Under different conditions of environmental stress or progression through the cell cycle, it will be of interest to investigate the effects on complex formation, the possible association of other protein factors, and the essentiality of the complex. Advances in proteomics and mass spectrometry may also be used to complement existing genetic and biochemical tools and will be essential to deconvolute the functions of known macromolecular complexes and to aid in the discovery of new complexes, perhaps revealing novel functions.
Acknowledgements
We thank S. Ataide, J. Ling, and N. Reynolds for critical reading of the manuscript. This work was supported by Grant GM 65183 from the National Institutes of Health.
References
- Agou F, Mirande M. Aspartyl-tRNA synthetase from rat: in vitro functional analysis of its assembly into the multisynthetase complex. Eur J Biochem. 1997;243:259–267. doi: 10.1111/j.1432-1033.1997.0259a.x. [DOI] [PubMed] [Google Scholar]
- Ahel I, Korencic D, Ibba M, Söll D. Trans-editing of mischarged tRNAs. Proc Natl Acad Sci USA. 2003;100:15422–15427. doi: 10.1073/pnas.2136934100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn HC, Kim S, Lee BJ. Solution structure and p43 binding of the p38 leucine zipper motif: coiled-coil interactions mediate the association between p38 and p43. FEBS Lett. 2003;542:119–124. doi: 10.1016/s0014-5793(03)00362-4. [DOI] [PubMed] [Google Scholar]
- Akins RA, Lambowitz AM. A protein required for splicing group I introns in Neurospora mitochondria is mitochondrial tyrosyl-tRNA synthetase or a derivative thereof. Cell. 1987;50:331–345. doi: 10.1016/0092-8674(87)90488-0. [DOI] [PubMed] [Google Scholar]
- Ambrogelly A, Ahel I, Polycarpo C, et al. Methanocaldococcus jannaschii prolyl-tRNA synthetase charges tRNAPro with cysteine. J Biol Chem. 2002;277:34749–34754. doi: 10.1074/jbc.M206929200. [DOI] [PubMed] [Google Scholar]
- An S, Musier-Forsyth K. Trans-editing of Cys-tRNAPro by Haemophilus influenzae YbaK protein. J Biol Chem. 2004;279:42359–42362. doi: 10.1074/jbc.C400304200. [DOI] [PubMed] [Google Scholar]
- An S, Musier-Forsyth K. Cys-tRNAPro editing by Haemophilus influenzae YbaK via a novel synthetase. YbaK.tRNA ternary complex. J Biol Chem. 2005;280:34465–34472. doi: 10.1074/jbc.M507550200. [DOI] [PubMed] [Google Scholar]
- Ataide SF, Ibba M. Small molecules: big players in the evolution of protein synthesis. ACS Chem Biol. 2006;1:285–297. doi: 10.1021/cb600200k. [DOI] [PubMed] [Google Scholar]
- Ataide SF, Jester BC, Devine KM, Ibba M. Stationary-phase expression and aminoacylation of a transfer-RNA-like small RNA. EMBO Rep. 2005;6:742–747. doi: 10.1038/sj.embor.7400474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly M, Blaise M, Lorber B, Becker HD, Kern D. The transamidosome: a dynamic ribonucleoprotein particle dedicated to prokaryotic tRNA-dependent asparagine biosynthesis. Mol Cell. 2007;28:228–239. doi: 10.1016/j.molcel.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Baldwin AN, Berg P. Transfer ribonucleic acid-induced hydrolysis of valyladenylate bound to isoleucyl ribonucleic acid synthetase. J Biol Chem. 1966;241:839–845. [PubMed] [Google Scholar]
- Bandyopadhyay AK, Deutscher MP. Complex of aminoacyl-transfer RNA synthetases. J Mol Biol. 1971;60:113–122. doi: 10.1016/0022-2836(71)90451-7. [DOI] [PubMed] [Google Scholar]
- Barat C, Lullien V, Schatz O, Keith G, Nugeyre MT, Gruninger-Leitch F, Barre-Sinoussi F, LeGrice SF, Darlix JL. HIV-1 reverse transcriptase specifically interacts with the anticodon domain of its cognate primer tRNA. EMBO J. 1989;8:3279–3285. doi: 10.1002/j.1460-2075.1989.tb08488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bec G, Kerjan P, Waller JP. Reconstitution in vitro of the valyl-tRNA synthetase-elongation factor (EF) 1 beta gamma delta complex. Essential roles of the NH2-terminal extension of valyl-tRNA synthetase and of the EF-1 delta subunit in complex formation. J Biol Chem. 1994;269:2086–2092. [PubMed] [Google Scholar]
- Becker HD, Kern D. Thermus thermophilus: a link in evolution of the tRNA-dependent amino acid amidation pathways. Proc Natl Acad Sci USA. 1998;95:12832–12837. doi: 10.1073/pnas.95.22.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrensdorf HA, van de CM, Knies UE, Vandenabeele P, Clauss M. The endothelial monocyte-activating polypeptide II (EMAP II) is a substrate for caspase-7. FEBS Lett. 2000;466:143–147. doi: 10.1016/s0014-5793(99)01777-9. [DOI] [PubMed] [Google Scholar]
- Belrhali H, Yaremchuk A, Tukalo M, Berthet-Colominas C, Rasmussen B, Bosecke P, Diat O, Cusack S. The structural basis for seryl-adenylate and Ap4A synthesis by seryl-tRNA synthetase. Structure. 1995;3:341–352. doi: 10.1016/s0969-2126(01)00166-6. [DOI] [PubMed] [Google Scholar]
- Beuning PJ, Musier-Forsyth K. Species-specific differences in amino acid editing by class II prolyl-tRNA synthetase. J Biol Chem. 2001;276:30779–30785. doi: 10.1074/jbc.M104761200. [DOI] [PubMed] [Google Scholar]
- Briza P, Winkler G, Kalchhauser H, Breitenbach M. Dityrosine is a prominent component of the yeast ascospore wall. A proof of its structure. J Biol Chem. 1986;261:4288–4294. [PubMed] [Google Scholar]
- Brown JR, Gentry D, Becker JA, Ingraham K, Holmes DJ, Stanhope MJ. Horizontal transfer of drug-resistant aminoacyl-transfer-RNA synthetases of anthrax and Gram-positive pathogens. EMBO Rep. 2003;4:692–698. doi: 10.1038/sj.embor.embor881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel C, Romby P, Moine H, Caillet J, Grunberg-Manago M, Springer M, Ehresmann B, Ehresmann C. Translational regulation of the Escherichia coli threonyl-tRNA synthetase gene: structural and functional importance of the thrS operator domains. Biochimie. 1993;75:1167–1179. doi: 10.1016/0300-9084(93)90016-l. [DOI] [PubMed] [Google Scholar]
- Buddha MR, Crane BR. Structure and activity of an aminoacyl-tRNA synthetase that charges tRNA with nitrotryptophan. Nat Struct Mol Biol. 2005;12:274–275. doi: 10.1038/nsmb907. [DOI] [PubMed] [Google Scholar]
- Buddha MR, Keery KM, Crane BR. An unusual tryptophanyl tRNA synthetase interacts with nitric oxide synthase in Deinococcus radiodurans. Proc Natl Acad Sci USA. 2004a;101:15881–15886. doi: 10.1073/pnas.0405483101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddha MR, Tao T, Parry RJ, Crane BR. Regioselective nitration of tryptophan by a complex between bacterial nitric-oxide synthase and tryptophanyl-tRNA synthetase. J Biol Chem. 2004b;279:49567–49570. doi: 10.1074/jbc.C400418200. [DOI] [PubMed] [Google Scholar]
- Cahuzac B, Berthonneau E, Birlirakis N, Guittet E, Mirande M. A recurrent RNA-binding domain is appended to eukaryotic aminoacyl-tRNA synthetases. EMBO J. 2000;19:445–452. doi: 10.1093/emboj/19.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas TD, El Yaagoubi A, Richarme G. Chaperone properties of bacterial elongation factor EF-Tu. J Biol Chem. 1998;273:11478–11482. doi: 10.1074/jbc.273.19.11478. [DOI] [PubMed] [Google Scholar]
- Cardoso AM, Polycarpo C, Martins OB, Söll D. A nondiscriminating aspartyl-tRNA synthetase from Halobacterium salinarum. RNA Biol. 2006;3:110–114. doi: 10.4161/rna.3.3.3116. [DOI] [PubMed] [Google Scholar]
- Cen S, Khorchid A, Javanbakht H, Gabor J, Stello T, Shiba K, Musier-Forsyth K, Kleiman L. Incorporation of lysyl-tRNA synthetase into human immunodeficiency virus type 1. J Virol. 2001;75:5043–5048. doi: 10.1128/JVI.75.11.5043-5048.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen S, Javanbakht H, Niu M, Kleiman L. Ability of wild-type and mutant lysyl-tRNA synthetase to facilitate tRNALys incorporation into human immunodeficiency virus type 1. J Virol. 2004a;78:1595–1601. doi: 10.1128/JVI.78.3.1595-1601.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen S, Niu M, Kleiman L. The connection domain in reverse transcriptase facilitates the in vivo annealing of to HIV-1 genomic RNA. Retrovirology. 2004b;1:33. doi: 10.1186/1742-4690-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen S, Niu M, Saadatmand J, Guo F, Huang Y, Nabel GJ, Kleiman L. Incorporation of pol into human immunodeficiency virus type 1 Gag virus-like particles occurs independently of the upstream Gag domain in Gag-pol. J Virol. 2004c;78:1042–1049. doi: 10.1128/JVI.78.2.1042-1049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B, Weidemaier K, Yip WT, Barbara PF, Musier-Forsyth K. Intra-tRNA distance measurements for nucleocapsid protein dependent tRNA unwinding during priming of HIV reverse transcription. Proc Natl Acad Sci USA. 1999;96:459–464. doi: 10.1073/pnas.96.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniack AD, Garriga G, Kittle JD, Jr, Akins RA, Lambowitz AM. Function of Neurospora mitochondrial tyrosyl-tRNA synthetase in RNA splicing requires an idiosyncratic domain not found in other synthetases. Cell. 1990;62:745–755. doi: 10.1016/0092-8674(90)90119-y. [DOI] [PubMed] [Google Scholar]
- Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, Herskowitz I. The transcriptional program of sporulation in budding yeast. Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- Cirakoglu B, Mirande M, Waller JP. A model for the structural organization of aminoacyl-tRNA synthetases in mammalian cells. FEBS Lett. 1985;183:185–190. [PubMed] [Google Scholar]
- Coluccio A, Bogengruber E, Conrad MN, Dresser ME, Briza P, Neiman AM. Morphogenetic pathway of spore wall assembly in Saccharomyces cerevisiae. Eukaryot Cell. 2004;3:1464–1475. doi: 10.1128/EC.3.6.1464-1475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti O, Hampe C, Koutnikova H, et al. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum Mol Genet. 2003;12:1427–1437. doi: 10.1093/hmg/ddg159. [DOI] [PubMed] [Google Scholar]
- Curnow AW, Tumbula DL, Pelaschier JT, Min B, Söll D. Glutamyl-tRNAGln amidotransferase in Deinococcus radiodurans may be confined to asparagine biosynthesis. Proc Natl Acad Sci USA. 1998;95:12838–12843. doi: 10.1073/pnas.95.22.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack S, Berthet-Colominas C, Hartlein M, Nassar N, Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 Å. Nature. 1990;347:249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Dagkessamanskaia A, Martin-Yken H, Basmaji F, Briza P, Francois J. Interaction of Knr4 protein, a protein involved in cell wall synthesis, with tyrosine tRNA synthetase encoded by TYS1 in Saccharomyces cerevisiae. FEMS Microbiol Lett. 2001;200:53–58. doi: 10.1111/j.1574-6968.2001.tb10692.x. [DOI] [PubMed] [Google Scholar]
- Deinert K, Fasiolo F, Hurt EC, Simos G. Arc1p organizes the yeast aminoacyl-tRNA synthetase complex and stabilizes its interaction with the cognate tRNAs. J Biol Chem. 2001;276:6000–6008. doi: 10.1074/jbc.M008682200. [DOI] [PubMed] [Google Scholar]
- Dibbelt L, Zachau HG. On the rate limiting step of yeast tRNAPhe aminoacylation. FEBS Lett. 1981;129:173–176. doi: 10.1016/0014-5793(81)80783-1. [DOI] [PubMed] [Google Scholar]
- Dobard CW, Briones MS, Chow SA. Molecular mechanisms by which human immunodeficiency virus type 1 integrase stimulates the early steps of reverse transcription. J Virol. 2007;81:10037–10046. doi: 10.1128/JVI.00519-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldred EW, Schimmel PR. Rapid deacylation by isoleucyl transfer ribonucleic acid synthetase of isoleucine-specific transfer ribonucleic acid aminoacylated with valine. J Biol Chem. 1972;247:2961–2964. [PubMed] [Google Scholar]
- Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990;347:203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Faisal W, Symonds P, Panjwani S, Heng Y, Murray JC. Cell-surface associated p43/endothelial-monocyte-activating-polypeptide-II in hepatocellular carcinoma cells induces apoptosis in T-lymphocytes. Asian J Surg. 2007;30:13–22. doi: 10.1016/S1015-9584(09)60122-6. [DOI] [PubMed] [Google Scholar]
- Feng L, Sheppard K, Tumbula-Hansen D, Söll D. Gln-tRNAGln formation from Glu-tRNAGln requires cooperation of an asparaginase and a Glu-tRNAGln kinase. J Biol Chem. 2005;280:8150–8155. doi: 10.1074/jbc.M411098200. [DOI] [PubMed] [Google Scholar]
- Fersht AR, Dingwall C. Evidence for the double-sieve editing mechanism in protein synthesis, Steric exclusion of isoleucine by valyl-tRNA synthetases. Biochemistry. 1979;18:2627–2631. doi: 10.1021/bi00579a030. [DOI] [PubMed] [Google Scholar]
- Fox PL, Mazumder B, Ehrenwald E, Mukhopadhyay CK. Ceruloplasmin and cardiovascular disease. Free Radic Biol Med. 2000;28:1735–1744. doi: 10.1016/s0891-5849(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Francin M, Kaminska M, Kerjan P, Mirande M. The N-terminal domain of mammalian lysyl-tRNA synthetase is a functional tRNA-binding domain. J Biol Chem. 2002;277:1762–1769. doi: 10.1074/jbc.M109759200. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Yokoyama S. Structure of the AlaX-M trans-editing enzyme from Pyrococcus horikoshii. Acta Crystallogr D Biol Crystallogr. 2007a;63:390–400. doi: 10.1107/S090744490605640X. [DOI] [PubMed] [Google Scholar]
- Fukunaga R, Yokoyama S. The C-terminal domain of the archaeal leucyl-tRNA synthetase prevents misediting of isoleucyl-tRNAIle. Biochemistry. 2007b;46:4985–4996. doi: 10.1021/bi6024935. [DOI] [PubMed] [Google Scholar]
- Galani K, Grosshans H, Deinert K, Hurt EC, Simos G. The intracellular location of two aminoacyl-tRNA synthetases depends on complex formation with Arc1p. EMBO J. 2001;20:6889–6898. doi: 10.1093/emboj/20.23.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani K, Hurt E, Simos G. The tRNA aminoacylation co-factor Arc1p is excluded from the nucleus by an Xpo1p-dependent mechanism. FEBS Lett. 2005;579:969–975. doi: 10.1016/j.febslet.2004.11.112. [DOI] [PubMed] [Google Scholar]
- Godinic V, Mocibob M, Rocak S, Ibba M, Weygand-Durasevic I. Peroxin Pex21p interacts with the C-terminal noncatalytic domain of yeast seryl-tRNA synthetase and forms a specific ternary complex with tRNASer. FEBS J. 2007;274:2788–2799. doi: 10.1111/j.1742-4658.2007.05812.x. [DOI] [PubMed] [Google Scholar]
- Goldgur Y, Safro M. Aminoacyl-tRNA synthetases from Haloarcula marismortui: an evidence for a multienzyme complex in a procaryotic system. Biochem Mol Biol Int. 1994;32:1075–1083. [PubMed] [Google Scholar]
- Golinelli-Cohen MP, Mirande M. Arc1p is required for cytoplasmic confinement of synthetases and tRNA. Mol Cell Biochem. 2007;300:47–59. doi: 10.1007/s11010-006-9367-4. [DOI] [PubMed] [Google Scholar]
- Golinelli-Cohen MP, Zakrzewska A, Mirande M. Complementation of yeast Arc1p by the p43 component of the human multisynthetase complex does not require its association with yeast MetRS and GluRS. J Mol Biol. 2004;340:15–27. doi: 10.1016/j.jmb.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Gonen H, Smith CE, Siegel NR, Kahana C, Merrick WC, Chakraburtty K, Schwartz AL, Ciechanover A. Protein synthesis elongation factor EF-1 alpha is essential for ubiquitin-dependent degradation of certain N alpha-acetylated proteins and may be substituted for by the bacterial elongation factor EF-Tu. Proc Natl Acad Sci USA. 1994;91:7648–7652. doi: 10.1073/pnas.91.16.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graindorge JS, Senger B, Tritch D, Simos G, Fasiolo F. Role of Arc1p in the modulation of yeast glutamyl-tRNA synthetase activity. Biochemistry. 2005;44:1344–1352. doi: 10.1021/bi049024z. [DOI] [PubMed] [Google Scholar]
- Greenberg Y, King M, Kiosses WB, Ewalt K, Yang X, Schimmel P, Reader JS, Tzima E. The novel fragment of tyrosyl tRNA synthetase, mini-TyrRS, is secreted to induce an angiogenic response in endothelial cells. FASEB J. 2008;22:1597–1605. doi: 10.1096/fj.07-9973com. [DOI] [PubMed] [Google Scholar]
- Grosshans H, Hurt E, Simos G. An aminoacylation-dependent nuclear tRNA export pathway in yeast. Genes Dev. 2000a;14:830–840. [PMC free article] [PubMed] [Google Scholar]
- Grosshans H, Simos G, Hurt E. Review: transport of tRNA out of the nucleus-direct channeling to the ribosome? J Struct Biol. 2000b;129:288–294. doi: 10.1006/jsbi.2000.4226. [DOI] [PubMed] [Google Scholar]
- Guigou L, Shalak V, Mirande M. The tRNA-interacting factor p43 associates with mammalian arginyl-tRNA synthetase but does not modify its tRNA aminoacylation properties. Biochemistry. 2004;43:4592–4600. doi: 10.1021/bi036150e. [DOI] [PubMed] [Google Scholar]
- Guo F, Cen S, Niu M, Javanbakht H, Kleiman L. Specific inhibition of the synthesis of human lysyl-tRNA synthetase results in decreases in tRNALys incorporation, annealing to viral RNA, and viral infectivity in human immunodeficiency virus type 1. J Virol. 2003;77:9817–9822. doi: 10.1128/JVI.77.18.9817-9822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Gabor J, Cen S, Hu K, Mouland AJ, Kleiman L. Inhibition of cellular HIV-1 protease activity by lysyl-tRNA synthetase. J Biol Chem. 2005;280:26018–26023. doi: 10.1074/jbc.M502454200. [DOI] [PubMed] [Google Scholar]
- Guzzo CM, Yang DC. Lysyl-tRNA synthetase interacts with EF1alpha, aspartyl-tRNA synthetase and p38 in vitro. Biochem Biophys Res Commun. 2008;365:718–723. doi: 10.1016/j.bbrc.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Halwani R, Cen S, Javanbakht H, Saadatmand J, Kim S, Shiba K, Kleiman L. Cellular distribution of lysyl-tRNA synthetase and its interaction with Gag during human immunodeficiency virus type 1 assembly. J Virol. 2004;78:7553–7564. doi: 10.1128/JVI.78.14.7553-7564.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Kim JY, Kim S. Molecular network and functional implications of macromolecular tRNA synthetase complex. Biochem Biophys Res Commun. 2003;303:985–993. doi: 10.1016/s0006-291x(03)00485-6. [DOI] [PubMed] [Google Scholar]
- Han JM, Lee MJ, Park SG, Lee SH, Razin E, Choi EC, Kim S. Hierarchical network between the components of the multi-tRNA synthetase complex: implications for complex formation. J Biol Chem. 2006a;281:38663–38667. doi: 10.1074/jbc.M605211200. [DOI] [PubMed] [Google Scholar]
- Han JM, Park SG, Lee Y, Kim S. Structural separation of different extracellular activities in aminoacyl-tRNA synthetase-interacting multi-functional protein, p43/AIMP1. Biochem Biophys Res Commun. 2006b;342:113–118. doi: 10.1016/j.bbrc.2006.01.117. [DOI] [PubMed] [Google Scholar]
- Harris CL. An aminoacyl-tRNA synthetase complex in Escherichia coli. J Bacteriol. 1987;169:2718–2723. doi: 10.1128/jb.169.6.2718-2723.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann CD, Praetorius-Ibba M, Ibba M. An aminoacyl-tRNA synthetase: elongation factor complex for substrate channeling in archaeal translation. Nucleic Acids Res. 2007;35:6094–6102. doi: 10.1093/nar/gkm534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert CJ, Labouesse M, Dujardin G, Slonimski PP. The NAM2 proteins from S. cerevisiae and S. douglasii are mitochondrial leucyl-tRNA synthetases, and are involved in mRNA splicing. EMBO J. 1988;7:473–483. doi: 10.1002/j.1460-2075.1988.tb02835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotokezaka Y, Tobben U, Hotokezaka H, Van Leyen K, Beatrix B, Smith DH, Nakamura T, Wiedmann M. Interaction of the eukaryotic elongation factor 1A with newly synthesized polypeptides. J Biol Chem. 2002;277:18545–18551. doi: 10.1074/jbc.M201022200. [DOI] [PubMed] [Google Scholar]
- Howard OM, Dong HF, Yang D, et al. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med. 2002;196:781–791. doi: 10.1084/jem.20020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibba M, Söll D. Aminoacyl-tRNAs: setting the limits of the genetic code. Genes Dev. 2004;18:731–738. doi: 10.1101/gad.1187404. [DOI] [PubMed] [Google Scholar]
- Ivakhno SS, Kornelyuk AI. Bioinformatic analysis of changes in expression level of tyrosyl-tRNA synthetase during sporulation process in Saccharomyces cerevisiae. Mikrobiol Z. 2005;67:37–49. [PubMed] [Google Scholar]
- Javanbakht H, Halwani R, Cen S, Saadatmand J, Musier-Forsyth K, Gottlinger H, Kleiman L. The interaction between HIV-1 Gag and human lysyl-tRNA synthetase during viral assembly. J Biol Chem. 2003;278:27644–27651. doi: 10.1074/jbc.M301840200. [DOI] [PubMed] [Google Scholar]
- Jia J, Arif A, Ray PS, Fox PL. WHEP domains direct noncanonical function of glutamyl-prolyl tRNA synthetase in translational control of gene expression. Mol Cell. 2008;29:679–690. doi: 10.1016/j.molcel.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska M, Shalak V, Mirande M. The appended C-domain of human methionyl-tRNA synthetase has a tRNA-sequestering function. Biochemistry. 2001;40:14309–14316. doi: 10.1021/bi015670b. [DOI] [PubMed] [Google Scholar]
- Kapasi P, Chaudhuri S, Vyas K, Baus D, Komar AA, Fox PL, Merrick WC, Mazumder B. L13a blocks 48S assembly: role of a general initiation factor in mRNA-specific translational control. Mol Cell. 2007;25:113–126. doi: 10.1016/j.molcel.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanasios E, Simader H, Panayotou G, Suck D, Simos G. Molecular determinants of the yeast Arc1p-aminoacyl-tRNA synthetase complex assembly. J Mol Biol. 2007;374:1077–1090. doi: 10.1016/j.jmb.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Kerjan P, Cerini C, Semeriva M, Mirande M. The multienzyme complex containing nine aminoacyl-tRNA synthetases is ubiquitous from Drosophila to mammals. Biochim Biophys Acta. 1994;1199:293–297. doi: 10.1016/0304-4165(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Kern D, Gangloff J. Catalytic mechanism of valyl-tRNA synthetase from baker's yeast. Reaction pathway and rate-determining step in the aminoacylation of tRNAVal. Biochemistry. 1981;20:2065–2074. doi: 10.1021/bi00511a001. [DOI] [PubMed] [Google Scholar]
- Kers JA, Wach MJ, Krasnoff SB, Widom J, Cameron KD, Bukhalid RA, Gibson DM, Crane BR, Loria R. Nitration of a peptide phytotoxin by bacterial nitric oxide synthase. Nature. 2004;429:79–82. doi: 10.1038/nature02504. [DOI] [PubMed] [Google Scholar]
- Kim HY, Ghosh G, Schulman LH, Brunie S, Jakubowski H. The relationship between synthetic and editing functions of the active site of an aminoacyl-tRNA synthetase. Proc Natl Acad Sci USA. 1993;90:11553–11557. doi: 10.1073/pnas.90.24.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Kang YS, Lee JW, Kim HJ, Ahn YH, Park H, Ko YG, Kim S. p38 is essential for the assembly and stability of macromolecular tRNA synthetase complex: implications for its physiological significance. Proc Natl Acad Sci USA. 2002;99:7912–7916. doi: 10.1073/pnas.122110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Park BJ, Kang YS, et al. Downregulation of FUSE-binding protein and c-myc by tRNA synthetase cofactor p38 is required for lung cell differentiation. Nat Genet. 2003;34:330–336. doi: 10.1038/ng1182. [DOI] [PubMed] [Google Scholar]
- Kim T, Park SG, Kim JE, Seol W, Ko YG, Kim S. Catalytic peptide of human glutaminyl-tRNA synthetase is essential for its assembly to the aminoacyl-tRNA synthetase complex. J Biol Chem. 2000;275:21768–21772. doi: 10.1074/jbc.M002404200. [DOI] [PubMed] [Google Scholar]
- Kise Y, Lee SW, Park SG, Fukai S, Sengoku T, Ishii R, Yokoyama S, Kim S, Nureki O. A short peptide insertion crucial for angiostatic activity of human tryptophanyl-tRNA synthetase. Nat Struct Mol Biol. 2004;11:149–156. doi: 10.1038/nsmb722. [DOI] [PubMed] [Google Scholar]
- Kleiman L, Cen S. The tRNALys packaging complex in HIV-1. Int J Biochem Cell Biol. 2004;36:1776–1786. doi: 10.1016/j.biocel.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Kleiman L, Halwani R, Javanbakht H. The selective packaging and annealing of primer tRNALys3 in HIV-1. Curr HIV Res. 2004;2:163–175. doi: 10.2174/1570162043484988. [DOI] [PubMed] [Google Scholar]
- Ko HS, von Coelln R, Sriram SR, et al. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J Neurosci. 2005;25:7968–7978. doi: 10.1523/JNEUROSCI.2172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YG, Kang YS, Kim EK, Park SG, Kim S. Nucleolar localization of human methionyl-tRNA synthetase and its role in ribosomal RNA synthesis. J Cell Biol. 2000;149:567–574. doi: 10.1083/jcb.149.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YG, Kim EY, Kim T, Park H, Park HS, Choi EJ, Kim S. Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:6030–6036. doi: 10.1074/jbc.M006189200. [DOI] [PubMed] [Google Scholar]
- Ko YG, Park H, Kim S. Novel regulatory interactions and activities of mammalian tRNA synthetases. Proteomics. 2002;2:1304–1310. doi: 10.1002/1615-9861(200209)2:9<1304::AID-PROT1304>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Korencic D, Ahel I, Schelert J, Sacher M, Ruan B, Stathopoulos C, Blum P, Ibba M, Söll D. A freestanding proofreading domain is required for protein synthesis quality control in Archaea. Proc Natl Acad Sci USA. 2004;101:10260–10265. doi: 10.1073/pnas.0403926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovaleski BJ, Kennedy R, Hong MK, Datta SA, Kleiman L, Rein A, Musier-Forsyth K. In vitro characterization of the interaction between HIV-1 Gag and human lysyl-tRNA synthetase. J Biol Chem. 2006;281:19449–19456. doi: 10.1074/jbc.M601189200. [DOI] [PubMed] [Google Scholar]
- Kudlicki W, Coffman A, Kramer G, Hardesty B. Renaturation of rhodanese by translational elongation factor (EF) Tu. Protein refolding by EF-Tu flexing. J Biol Chem. 1997;272:32206–32210. doi: 10.1074/jbc.272.51.32206. [DOI] [PubMed] [Google Scholar]
- Kyriacou SV, Deutscher MP. An important role for the multienzyme aminoacyl-tRNA synthetase complex in mammalian translation and cell growth. Mol Cell. 2008;29:419–427. doi: 10.1016/j.molcel.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse M. The yeast mitochondrial leucyl-tRNA synthetase is a splicing factor for the excision of several group I introns. Mol Gen Genet. 1990;224:209–221. doi: 10.1007/BF00271554. [DOI] [PubMed] [Google Scholar]
- Lee PC, Bochner BR, Ames BN. Diadenosine 5′,5‴-P1,P4-tetraphosphate and related adenylylated nucleotides in Salmonella typhimurium. J Biol Chem. 1983;258:6827–6834. [PubMed] [Google Scholar]
- Lee SW, Cho BH, Park SG, Kim S. Aminoacyl-tRNA synthetase complexes: beyond translation. J Cell Sci. 2004a;117:3725–3734. doi: 10.1242/jcs.01342. [DOI] [PubMed] [Google Scholar]
- Lee YN, Nechushtan H, Figov N, Razin E. The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcεRI-activated mast cells. Immunity. 2004b;20:145–151. doi: 10.1016/s1074-7613(04)00020-2. [DOI] [PubMed] [Google Scholar]
- Levengood J, Ataide SF, Roy H, Ibba M. Divergence in noncognate amino acid recognition between class I and class II lysyl-tRNA synthetases. J Biol Chem. 2004;279:17707–17714. doi: 10.1074/jbc.M313665200. [DOI] [PubMed] [Google Scholar]
- Ling C, Yao YN, Zheng YG, Wei H, Wang L, Wu XF, Wang ED. The C-terminal appended domain of human cytosolic leucyl-tRNA synthetase is indispensable in its interaction with arginyl-tRNA synthetase in the multi-tRNA synthetase complex. J Biol Chem. 2005;280:34755–34763. doi: 10.1074/jbc.M413511200. [DOI] [PubMed] [Google Scholar]
- Lipman RS, Chen J, Evilia C, Vitseva O, Hou YM. Association of an aminoacyl-tRNA synthetase with a putative metabolic protein in archaea. Biochemistry. 2003;42:7487–7496. doi: 10.1021/bi0344533. [DOI] [PubMed] [Google Scholar]
- Liu HW, Cosa G, Landes CF, Zeng Y, Kovaleski BJ, Mullen DG, Barany G, Musier-Forsyth K, Barbara PF. Single-molecule FRET studies of important intermediates in the nucleocapsid-protein-chaperoned minus-strand transfer step in HIV-1 reverse transcription. Biophys J. 2005;89:3470–3479. doi: 10.1529/biophysj.105.065326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Huertas E, Charlton WL, Johnson B, Graham IA, Baker A. Stress induces peroxisome biogenesis genes. EMBO J. 2000;19:6770–6777. doi: 10.1093/emboj/19.24.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukash TO, Turkivska HV, Negrutskii BS, El'skaya AV. Chaperone-like activity of mammalian elongation factor eEF1A: renaturation of aminoacyl-tRNA synthetases. Int J Biochem Cell Biol. 2004;36:1341–1347. doi: 10.1016/j.biocel.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Mazumder B, Sampath P, Seshadri V, Maitra RK, DiCorleto PE, Fox PL. Regulated release of L13a from the 60S ribosomal subunit as a mechanism of transcript-specific translational control. Cell. 2003a;115:187–198. doi: 10.1016/s0092-8674(03)00773-6. [DOI] [PubMed] [Google Scholar]
- Mazumder B, Seshadri V, Fox PL. Translational control by the 3′-UTR: the ends specify the means. Trends Biochem Sci. 2003b;28:91–98. doi: 10.1016/S0968-0004(03)00002-1. [DOI] [PubMed] [Google Scholar]
- McCulley A, Morrow CD. Nucleotides within the anticodon stem are important for optimal use of as the primer for HIV-1 reverse transcription. Virology. 2007;364:169–177. doi: 10.1016/j.virol.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AT, Mangroo D. Cex1p is a novel cytoplasmic component of the Saccharomyces cerevisiae nuclear tRNA export machinery. EMBO J. 2007;26:288–300. doi: 10.1038/sj.emboj.7601493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Kitabatake M, Polycarpo C, Pelaschier J, Raczniak G, Ruan B, Kobayashi H, Namgoong S, Söll D. Protein synthesis in Escherichia coli with mischarged tRNA. J Bacteriol. 2003;185:3524–3526. doi: 10.1128/JB.185.12.3524-3526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minella O, Mulner-Lorillon O, Bec G, Cormier P, Belle R. Multiple phosphorylation sites and quaternary organization of guanine-nucleotide exchange complex of elongation factor-1 (EF-1βγδ/ValRS) control the various functions of EF-1α. Biosci Rep. 1998;18:119–127. doi: 10.1023/a:1020140527930. [DOI] [PubMed] [Google Scholar]
- Mirande M. Aminoacyl-tRNA synthetase family from prokaryotes and eukaryotes: structural domains and their implications. Prog Nucleic Acid Res Mol Biol. 1991;40:95–142. doi: 10.1016/s0079-6603(08)60840-5. [DOI] [PubMed] [Google Scholar]
- Mirande M, Kellermann O, Waller JP. Macromolecular complexes from sheep and rabbit containing seven aminoacyl-tRNA synthetases. II. Structural characterization of the polypeptide components and immunological identification of the methionyl-tRNA synthetase subunit. J Biol Chem. 1982;257:11049–11055. [PubMed] [Google Scholar]
- Mirande M, Le Corre D, Waller JP. A complex from cultured Chinese hamster ovary cells containing nine aminoacyl-tRNA synthetases. Thermolabile leucyl-tRNA synthetase from the tsH1 mutant cell line is an integral component of this complex. Eur J Biochem. 1985;147:281–289. doi: 10.1111/j.1432-1033.1985.tb08748.x. [DOI] [PubMed] [Google Scholar]
- Mirande M, Lazard M, Martinez R, Latreille MT. Engineering mammalian aspartyl-tRNA synthetase to probe structural features mediating its association with the multisynthetase complex. Eur J Biochem. 1992;203:459–466. doi: 10.1111/j.1432-1033.1992.tb16570.x. [DOI] [PubMed] [Google Scholar]
- Mocibob M, Weygand-Durasevic I. The proximal region of a noncatalytic eukaryotic seryl-tRNA synthetase extension is required for protein stability in vitro and in vivo. Arch Biochem Biophys. 2008;470:129–138. doi: 10.1016/j.abb.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Motorin Y, Wolfson AD, Orlovsky AF, Gladilin KL. Mammalian valyl-tRNA synthetase forms a complex with the first elongation factor. FEBS Lett. 1988;238:262–264. doi: 10.1016/0014-5793(88)80492-7. [DOI] [PubMed] [Google Scholar]
- Negrutskii BS, Deutscher MP. Channeling of aminoacyl-tRNA for protein synthesis in vivo. Proc Natl Acad Sci USA. 1991;88:4991–4995. doi: 10.1073/pnas.88.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrutskii BS, Deutscher MP. A sequestered pool of aminoacyl-tRNA in mammalian cells. Proc Natl Acad Sci USA. 1992;89:3601–3604. doi: 10.1073/pnas.89.8.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrutskii BS, El'skaya AV. Eukaryotic translation elongation factor 1 α: structure, expression, functions, and possible role in aminoacyl-tRNA channeling. Prog Nucleic Acid Res Mol Biol. 1998;60:47–78. doi: 10.1016/s0079-6603(08)60889-2. [DOI] [PubMed] [Google Scholar]
- Negrutskii BS, Shalak VF, Kerjan P, El'skaya AV, Mirande M. Functional interaction of mammalian valyl-tRNA synthetase with elongation factor EF-1alpha in the complex with EF-1H. J Biol Chem. 1999;274:4545–4550. doi: 10.1074/jbc.274.8.4545. [DOI] [PubMed] [Google Scholar]
- Norcum MT. Isolation and electron microscopic characterization of the high molecular mass aminoacyl-tRNA synthetase complex from murine erythroleukemia cells. J Biol Chem. 1989;264:15043–15051. [PubMed] [Google Scholar]
- Norcum MT. Structural analysis of the high molecular mass aminoacyl-tRNA synthetase complex. Effects of neutral salts and detergents. J Biol Chem. 1991;266:15398–15405. [PubMed] [Google Scholar]
- Norcum MT. Ultrastructure of the eukaryotic aminoacyl-tRNA synthetase complex derived from two dimensional averaging and classification of negatively stained electron microscopic images. FEBS Lett. 1999;447:217–222. doi: 10.1016/s0014-5793(99)00287-2. [DOI] [PubMed] [Google Scholar]
- Norcum MT, Warrington JA. Structural analysis of the multienzyme aminoacyl-tRNA synthetase complex: a three-domain model based on reversible chemical crosslinking. Protein Sci. 1998;7:79–87. doi: 10.1002/pro.5560070108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshikane H, Sheppard K, Fukai S, et al. Structural basis of RNA-dependent recruitment of glutamine to the genetic code. Science. 2006;312:1950–1954. doi: 10.1126/science.1128470. [DOI] [PubMed] [Google Scholar]
- Otani A, Slike BM, Dorrell MI, Hood J, Kinder K, Ewalt KL, Cheresh D, Schimmel P, Friedlander M. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc Natl Acad Sci USA. 2002;99:178–183. doi: 10.1073/pnas.012601899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BJ, Kang JW, Lee SW, et al. The haploinsufficient tumor suppressor p18 upregulates p53 via interactions with ATM/ATR. Cell. 2005a;120:209–221. doi: 10.1016/j.cell.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Park H, Park SG, Kim J, Ko YG, Kim S. Signaling pathways for TNF production induced by human aminoacyl-tRNA synthetase-associating factor, p43. Cytokine. 2002;20:148–153. doi: 10.1006/cyto.2002.1992. [DOI] [PubMed] [Google Scholar]
- Park SG, Ewalt KL, Kim S. Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci. 2005b;30:569–574. doi: 10.1016/j.tibs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Park SG, Kim HJ, Min YH, Choi EC, Shin YK, Park BJ, Lee SW, Kim S. Human lysyl-tRNA synthetase is secreted to trigger proinflammatory response. Proc Natl Acad Sci USA. 2005c;102:6356–6361. doi: 10.1073/pnas.0500226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukstelis PJ, Coon R, Madabusi L, Nowakowski J, Monzingo A, Robertus J, Lambowitz AM. A tyrosyl-tRNA synthetase adapted to function in group I intron splicing by acquiring a new RNA binding surface. Mol Cell. 2005;17:417–428. doi: 10.1016/j.molcel.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Petrushenko ZM, Negrutskii BS, Ladokhin AS, Budkevich TV, Shalak VF, El'skaya AV. Evidence for the formation of an unusual ternary complex of rabbit liver EF-1α with GDP and deacylated tRNA. FEBS Lett. 1997;407:13–17. doi: 10.1016/s0014-5793(97)00242-1. [DOI] [PubMed] [Google Scholar]
- Petrushenko ZM, Budkevich TV, Shalak VF, Negrutskii BS, El'skaya AV. Novel complexes of mammalian translation elongation factor eEF1A. GDP with uncharged tRNA and aminoacyl-tRNA synthetase. Implications for tRNA channeling. Eur J Biochem. 2002;269:4811–4818. doi: 10.1046/j.1432-1033.2002.03178.x. [DOI] [PubMed] [Google Scholar]
- Popenko VI, Ivanova JL, Cherny NE, Filonenko VV, Beresten SF, Wolfson AD, Kisselev LL. Compartmentalization of certain components of the protein synthesis apparatus in mammalian cells. Eur J Cell Biol. 1994;65:60–69. [PubMed] [Google Scholar]
- Praetorius-Ibba M, Rogers TE, Samson R, Kelman Z, Ibba M. Association between archaeal prolyl- and leucyl-tRNA synthetases enhances tRNAPro aminoacylation. J Biol Chem. 2005;280:26099–26104. doi: 10.1074/jbc.M503539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius-Ibba M, Hausmann CD, Paras M, Rogers TE, Ibba M. Functional association between three archaeal aminoacyl-tRNA synthetases. J Biol Chem. 2007;282:3680–3687. doi: 10.1074/jbc.M609988200. [DOI] [PubMed] [Google Scholar]
- Putney SD, Schimmel P. An aminoacyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature. 1981;291:632–635. doi: 10.1038/291632a0. [DOI] [PubMed] [Google Scholar]
- Quevillon S, Mirande M. The p18 component of the multisynthetase complex shares a protein motif with the β and γ subunits of eukaryotic elongation factor 1. FEBS Lett. 1996;395:63–67. doi: 10.1016/0014-5793(96)01005-8. [DOI] [PubMed] [Google Scholar]
- Quevillon S, Agou F, Robinson JC, Mirande M. The p43 component of the mammalian multi-synthetase complex is likely to be the precursor of the endothelial monocyte-activating polypeptide II cytokine. J Biol Chem. 1997;272:32573–32579. doi: 10.1074/jbc.272.51.32573. [DOI] [PubMed] [Google Scholar]
- Quevillon S, Robinson JC, Berthonneau E, Siatecka M, Mirande M. Macromolecular assemblage of aminoacyl-tRNA synthetases: identification of protein–protein interactions and characterization of a core protein. J Mol Biol. 1999;285:183–195. doi: 10.1006/jmbi.1998.2316. [DOI] [PubMed] [Google Scholar]
- Raczniak G, Becker HD, Min B, Söll D. A single amidotransferase forms asparaginyl-tRNA and glutaminyl-tRNA in Chlamydia trachomatis. J Biol Chem. 2001;276:45862–45867. doi: 10.1074/jbc.M109494200. [DOI] [PubMed] [Google Scholar]
- Ray PS, Arif A, Fox PL. Macromolecular complexes as depots for releasable regulatory proteins. Trends Biochem Sci. 2007;32:158–164. doi: 10.1016/j.tibs.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Reed VS, Yang DC. Characterization of a novel N-terminal peptide in human aspartyl-tRNA synthetase. Roles in the transfer of aminoacyl-tRNA from aminoacyl-tRNA synthetase to the elongation factor 1 α. J Biol Chem. 1994;269:32937–32941. [PubMed] [Google Scholar]
- Reed VS, Wastney ME, Yang DC. Mechanisms of the transfer of aminoacyl-tRNA from aminoacyl-tRNA synthetase to the elongation factor 1 alpha. J Biol Chem. 1994;269:32932–32936. [PubMed] [Google Scholar]
- Rho SB, Lee JS, Jeong EJ, Kim KS, Kim YG, Kim S. A multifunctional repeated motif is present in human bifunctional tRNA synthetase. J Biol Chem. 1998;273:11267–11273. doi: 10.1074/jbc.273.18.11267. [DOI] [PubMed] [Google Scholar]
- Rho SB, Kim MJ, Lee JS, Seol W, Motegi H, Kim S, Shiba K. Genetic dissection of protein–protein interactions in multi-tRNA synthetase complex. Proc Natl Acad Sci USA. 1999;96:4488–4493. doi: 10.1073/pnas.96.8.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho SB, Lincecum TL, Jr, Martinis SA. An inserted region of leucyl-tRNA synthetase plays a critical role in group I intron splicing. EMBO J. 2002;21:6874–6881. doi: 10.1093/emboj/cdf671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JC, Kerjan P, Mirande M. Macromolecular assemblage of aminoacyl-tRNA synthetases: quantitative analysis of protein–protein interactions and mechanism of complex assembly. J Mol Biol. 2000;304:983–994. doi: 10.1006/jmbi.2000.4242. [DOI] [PubMed] [Google Scholar]
- Rocak S, Landeka I, Weygand-Durasevic I. Identifying Pex21p as a protein that specifically interacts with yeast seryl-tRNA synthetase. FEMS Microbiol Lett. 2002;214:101–106. doi: 10.1111/j.1574-6968.2002.tb11331.x. [DOI] [PubMed] [Google Scholar]
- Romby P, Caillet J, Ebel C, et al. The expression of E. coli threonyl-tRNA synthetase is regulated at the translational level by symmetrical operator-repressor interactions. EMBO J. 1996;15:5976–5987. [PMC free article] [PubMed] [Google Scholar]
- Roy H, Ibba M. RNA-dependent lipid remodeling by bacterial multiple peptide resistance factors. Proc Natl Acad Sci USA. 2008;105:4667–4672. doi: 10.1073/pnas.0800006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy H, Ling J, Irnov M, Ibba M. Post-transfer editing in vitro and in vivo by the β subunit of phenylalanyl-tRNA synthetase. EMBO J. 2004;23:4639–4648. doi: 10.1038/sj.emboj.7600474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan B, Söll D. The bacterial YbaK protein is a Cys-tRNAPro and Cys-tRNACys deacylase. J Biol Chem. 2005;280:25887–25891. doi: 10.1074/jbc.M502174200. [DOI] [PubMed] [Google Scholar]
- Salazar JC, Zuniga R, Raczniak G, Becker H, Söll D, Orellana O. A dual-specific Glu-tRNAGln and Asp-tRNAAsn amidotransferase is involved in decoding glutamine and asparagine codons in Acidithiobacillus ferrooxidans. FEBS Lett. 2001;500:129–131. doi: 10.1016/s0014-5793(01)02600-x. [DOI] [PubMed] [Google Scholar]
- Salazar JC, Ahel I, Orellana O, Tumbula-Hansen D, Krieger R, Daniels L, Söll D. Coevolution of an aminoacyl-tRNA synthetase with its tRNA substrates. Proc Natl Acad Sci USA. 2003;100:13863–13868. doi: 10.1073/pnas.1936123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath P, Mazumder B, Seshadri V, et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell. 2004;119:195–208. doi: 10.1016/j.cell.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Sang LJ, Gyu PS, Park H, Seol W, Lee S, Kim S. Interaction network of human aminoacyl-tRNA synthetases and subunits of elongation factor 1 complex. Biochem Biophys Res Commun. 2002;291:158–164. doi: 10.1006/bbrc.2002.6398. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Azad AK, Hopper AK. Nuclear tRNA aminoacylation and its role in nuclear export of endogenous tRNAs in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1999;96:14366–14371. doi: 10.1073/pnas.96.25.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E, Schimmel P. Mutational isolation of a sieve for editing in a transfer RNA synthetase. Science. 1994;264:265–267. doi: 10.1126/science.8146659. [DOI] [PubMed] [Google Scholar]
- Schon A, Kannangara CG, Gough S, Soll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988;331:187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- Sekine S, Nureki O, Shimada A, Vassylyev DG, Yokoyama S. Structural basis for anticodon recognition by discriminating glutamyl-tRNA synthetase. Nat Struct Biol. 2001;8:203–206. doi: 10.1038/84927. [DOI] [PubMed] [Google Scholar]
- Senger B, Aphasizhev R, Walter P, Fasiolo F. The presence of a D-stem but not a T-stem is essential for triggering aminoacylation upon anticodon binding in yeast methionine tRNA. J Mol Biol. 1995;249:45–58. doi: 10.1006/jmbi.1995.0279. [DOI] [PubMed] [Google Scholar]
- Shalak V, Kaminska M, Mitnacht-Kraus R, Vandenabeele P, Clauss M, Mirande M. The EMAPII cytokine is released from the mammalian multisynthetase complex after cleavage of its p43/proEMAPII component. J Biol Chem. 2001;276:23769–23776. doi: 10.1074/jbc.M100489200. [DOI] [PubMed] [Google Scholar]
- Simader H, Hothorn M, Suck D. Structures of the interacting domains from yeast glutamyl-tRNA synthetase and tRNA-aminoacylation and nuclear-export cofactor Arc1p reveal a novel function for an old fold. Acta Crystallogr D Biol Crystallogr. 2006;62:1510–1519. doi: 10.1107/S0907444906039850. [DOI] [PubMed] [Google Scholar]
- Simos G, Segref A, Fasiolo F, Hellmuth K, Shevchenko A, Mann M, Hurt EC. The yeast protein Arc1p binds to tRNA and functions as a cofactor for the methionyl- and glutamyl-tRNA synthetases. EMBO J. 1996;15:5437–5448. [PMC free article] [PubMed] [Google Scholar]
- Simos G, Sauer A, Fasiolo F, Hurt EC. A conserved domain within Arc1p delivers tRNA to aminoacyl-tRNA synthetases. Mol Cell. 1998;1:235–242. doi: 10.1016/s1097-2765(00)80024-6. [DOI] [PubMed] [Google Scholar]
- Sivaram P, Deutscher MP. Existence of two forms of rat liver arginyl-tRNA synthetase suggests channeling of aminoacyl-tRNA for protein synthesis. Proc Natl Acad Sci USA. 1990;87:3665–3669. doi: 10.1073/pnas.87.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapulionis R, Deutscher MP. A channeled tRNA cycle during mammalian protein synthesis. Proc Natl Acad Sci USA. 1995;92:7158–7161. doi: 10.1073/pnas.92.16.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark LA, Hay RT. Human immunodeficiency virus type 1 (HIV-1) viral protein R (Vpr) interacts with Lys-tRNA synthetase: implications for priming of HIV-1 reverse transcription. J Virol. 1998;72:3037–3044. doi: 10.1128/jvi.72.4.3037-3044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swairjo MA, Morales AJ, Wang CC, Ortiz AR, Schimmel P. Crystal structure of trbp111: a structure-specific tRNA-binding protein. EMBO J. 2000;19:6287–6298. doi: 10.1093/emboj/19.23.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Larios A, Dock-Bregeon AC, Romby P, et al. Structural basis of translational control by Escherichia coli threonyl tRNA synthetase. Nat Struct Biol. 2002;9:343–347. doi: 10.1038/nsb789. [DOI] [PubMed] [Google Scholar]
- Tumbula-Hansen D, Feng L, Toogood H, Stetter KO, Söll D. Evolutionary divergence of the archaeal aspartyl-tRNA synthetases into discriminating and nondiscriminating forms. J Biol Chem. 2002;277:37184–37190. doi: 10.1074/jbc.M204767200. [DOI] [PubMed] [Google Scholar]
- Vellekamp G, Deutscher MP. A basic NH2-terminal extension of rat liver arginyl-tRNA synthetase required for its association with high molecular weight complexes. J Biol Chem. 1987;262:9927–9930. [PubMed] [Google Scholar]
- Venema RC, Peters HI, Traugh JA. Phosphorylation of valyl-tRNA synthetase and elongation factor 1 in response to phorbol esters is associated with stimulation of both activities. J Biol Chem. 1991;266:11993–11998. [PubMed] [Google Scholar]
- Wakasugi K, Slike BM, Hood J, Ewalt KL, Cheresh DA, Schimmel P. Induction of angiogenesis by a fragment of human tyrosyl-tRNA synthetase. J Biol Chem. 2002a;277:20124–20126. doi: 10.1074/jbc.C200126200. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Slike BM, Hood J, Otani A, Ewalt KL, Friedlander M, Cheresh DA, Schimmel P. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci USA. 2002b;99:173–177. doi: 10.1073/pnas.012602099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Martinis SA. Mutational unmasking of a tRNA-dependent pathway for preventing genetic code ambiguity. Proc Natl Acad Sci USA. 2006;103:3586–3591. doi: 10.1073/pnas.0507362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CL, Warrington JA, Davis S, Green S, Norcum MT. Isolation and characterization of human nuclear and cytosolic multisynthetase complexes and the intracellular distribution of p43/EMAPII. Protein Sci. 2003;12:2282–2290. doi: 10.1110/ps.03147903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CL, Warrington JA, Treadwell L, Norcum MT. A three-dimensional working model of the multienzyme complex of aminoacyl-tRNA synthetases based on electron microscopic placements of tRNA and proteins. J Biol Chem. 2005;280:38870–38878. doi: 10.1074/jbc.M502759200. [DOI] [PubMed] [Google Scholar]
- Wolfson A, Knight R. Occurrence of the aminoacyl-tRNA synthetases in high-molecular weight complexes correlates with the size of substrate amino acids. FEBS Lett. 2005;579:3467–3472. doi: 10.1016/j.febslet.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Wong FC, Beuning PJ, Silvers C, Musier-Forsyth K. An isolated class II aminoacyl-tRNA synthetase insertion domain is functional in amino acid editing. J Biol Chem. 2003;278:52857–52864. doi: 10.1074/jbc.M309627200. [DOI] [PubMed] [Google Scholar]
- Yang XL, Otero FJ, Ewalt KL, et al. Two conformations of a crystalline human tRNA synthetase-tRNA complex: implications for protein synthesis. EMBO J. 2006;25:2919–2929. doi: 10.1038/sj.emboj.7601154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Liu HW, Landes CF, Kim YJ, Ma X, Zhu Y, Musier-Forsyth K, Barbara PF. Probing nucleation, reverse annealing, and chaperone function along the reaction path of HIV-1 single-strand transfer. Proc Natl Acad Sci USA. 2007;104:12651–12656. doi: 10.1073/pnas.0700350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CM, Perona JJ, Ryu K, Francklyn C, Hou YM. Distinct kinetic mechanisms of the two classes of aminoacyl-tRNA synthetases. J Mol Biol. 2006;361:300–311. doi: 10.1016/j.jmb.2006.06.015. [DOI] [PubMed] [Google Scholar]


