Abstract
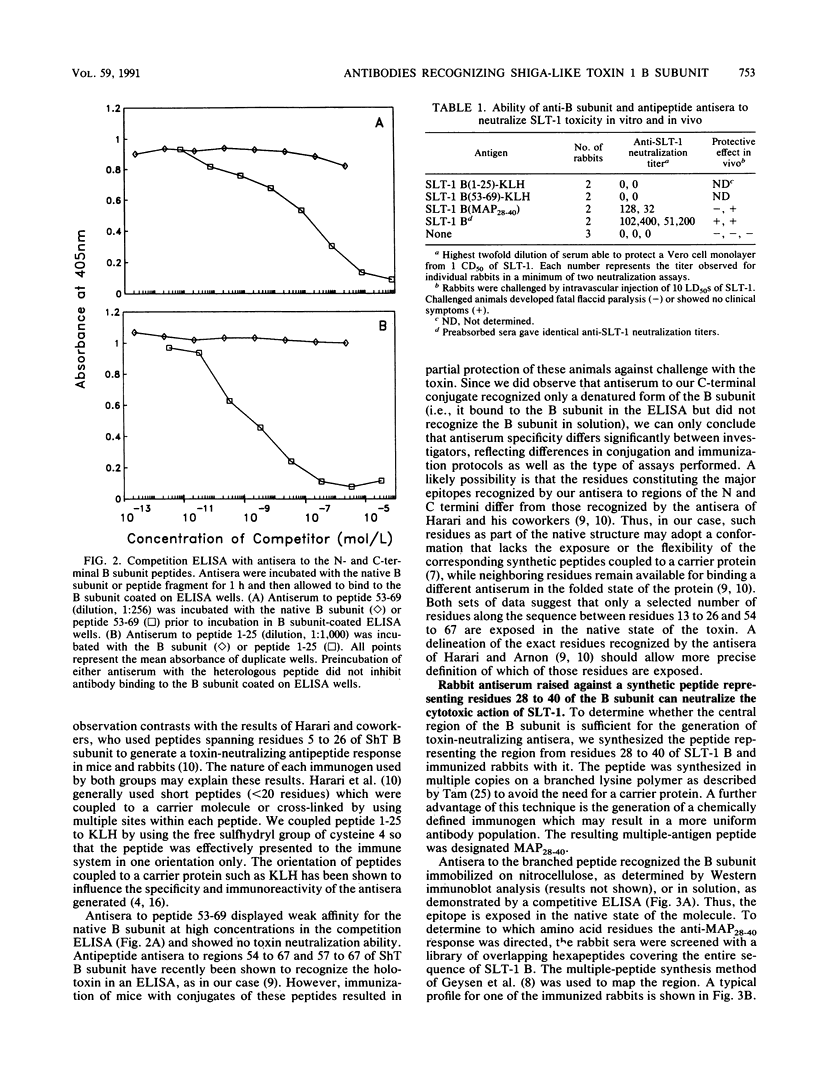
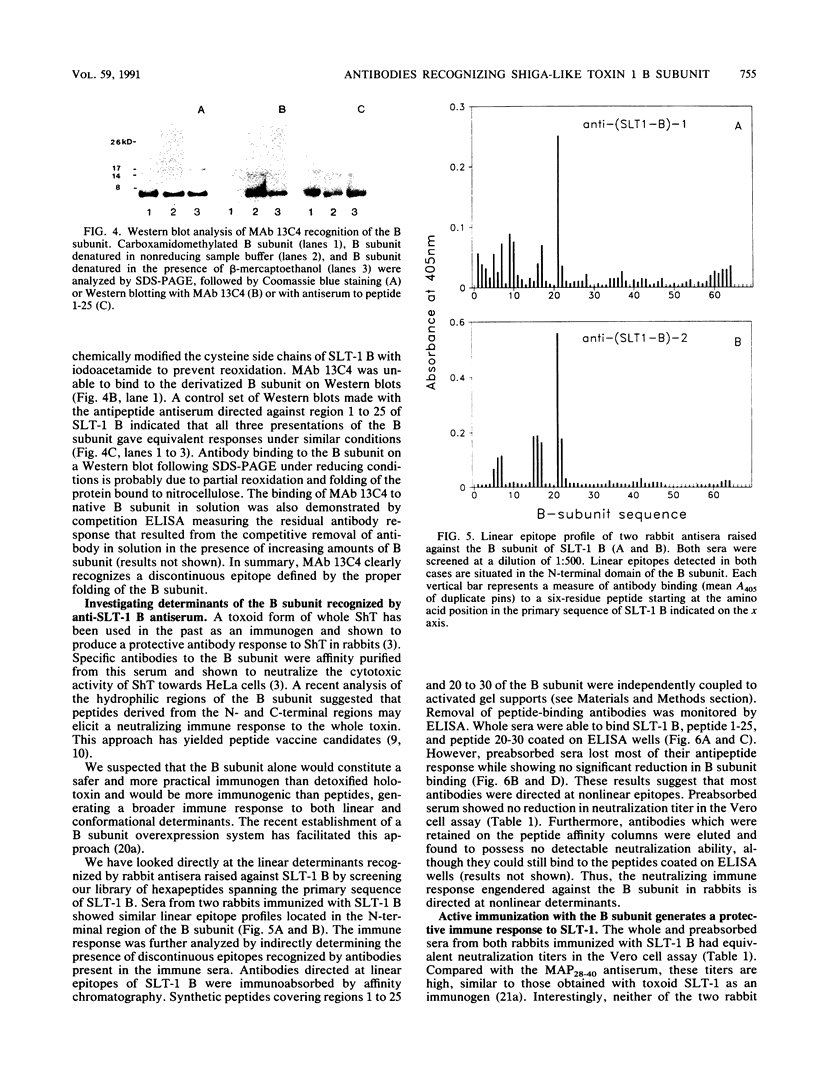
The B subunit of Shiga toxin and Shiga-like toxin (SLT-1) and its fragments are potentially immunogenic and may generate protective humoral responses against the action of these toxins. We have analyzed the antibody response of rabbits immunized with pure B subunit of SLT-1 or synthetic fragments of the subunit. The immune response to the native B subunit was found to be largely directed at conformational epitopes. More importantly, rabbits immunized with the B subunit were protected from a lethal challenge with SLT-1, indicating that the B subunit represents an excellent vaccine candidate to counter the effects of Shiga toxin and SLT-1 in humans. Polyclonal antibodies against a synthetic peptide corresponding to residues 28 to 40 of the B subunit neutralized the cytotoxicity of SLT-1 towards Vero cells. This region is thus exposed in the native state of the B subunit. The sequence specificity of other antipeptide antisera also provides clues to the state of folding and assembly of the B subunit. Antisera to synthetic peptides representing the N- and C-terminal regions of the SLT-1 B subunit did not cross-react with native B subunit but strongly recognized denatured forms of the protein. Finally, the monoclonal antibody 13C4 was shown to bind to a discontinuous epitope expressed only on the native form of the protein. These immunological reagents can be used to probe the conformational state of the B subunit and the holotoxin as it relates to their functional properties.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd B., Lingwood C. Verotoxin receptor glycolipid in human renal tissue. Nephron. 1989;51(2):207–210. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Donohue-Rolfe A., Keusch G. T., Edson C., Thorley-Lawson D., Jacewicz M. Pathogenesis of Shigella diarrhea. IX. Simplified high yield purification of Shigella toxin and characterization of subunit composition and function by the use of subunit-specific monoclonal and polyclonal antibodies. J Exp Med. 1984 Dec 1;160(6):1767–1781. doi: 10.1084/jem.160.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrberg T., Oldstone M. B. Peptides as antigens. Importance of orientation. J Exp Med. 1986 Oct 1;164(4):1344–1349. doi: 10.1084/jem.164.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieser T. M., Tainer J. A., Geysen H. M., Houghten R. A., Lerner R. A. Influence of protein flexibility and peptide conformation on reactivity of monoclonal anti-peptide antibodies with a protein alpha-helix. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8568–8572. doi: 10.1073/pnas.84.23.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine A., Arondel J., Sansonetti P. J. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988 Dec;56(12):3099–3109. doi: 10.1128/iai.56.12.3099-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy J., Mietzner T. A., Schoolnik G. K. Peptide antisera as sequence-specific probes of protein conformational transitions: calmodulin exhibits calcium-dependent changes in antigenicity. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8888–8892. doi: 10.1073/pnas.83.23.8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari I., Arnon R. Carboxy-terminal peptides from the B subunit of Shiga toxin induce a local and parenteral protective effect. Mol Immunol. 1990 Jul;27(7):613–621. doi: 10.1016/0161-5890(90)90003-i. [DOI] [PubMed] [Google Scholar]
- Harari I., Donohue-Rolfe A., Keusch G., Arnon R. Synthetic peptides of Shiga toxin B subunit induce antibodies which neutralize its biological activity. Infect Immun. 1988 Jun;56(6):1618–1624. doi: 10.1128/iai.56.6.1618-1624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Brown J. E., Strömberg N., Westling-Ryd M., Schultz J. E., Karlsson K. A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987 Feb 5;262(4):1779–1785. [PubMed] [Google Scholar]
- Lingwood C. A., Law H., Richardson S., Petric M., Brunton J. L., De Grandis S., Karmali M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987 Jun 25;262(18):8834–8839. [PubMed] [Google Scholar]
- Lipkin W. I., Schwimmbeck P. L., Oldstone M. B. Antibody to synthetic somatostatin-28(1-12): immunoreactivity with somatostatin in brain is dependent on orientation of immunizing peptide. J Histochem Cytochem. 1988 Apr;36(4):447–451. doi: 10.1177/36.4.2450123. [DOI] [PubMed] [Google Scholar]
- Ludwig D. S., Holmes R. K., Schoolnik G. K. Chemical and immunochemical studies on the receptor binding domain of cholera toxin B subunit. J Biol Chem. 1985 Oct 15;260(23):12528–12534. [PubMed] [Google Scholar]
- Moyer M. P., Dixon P. S., Rothman S. W., Brown J. E. Cytotoxicity of Shiga toxin for primary cultures of human colonic and ileal epithelial cells. Infect Immun. 1987 Jun;55(6):1533–1535. doi: 10.1128/iai.55.6.1533-1535.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig T. G., Del Vecchio P. J., Brown J. E., Moran T. P., Rowland B. M., Judge T. K., Rothman S. W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect Immun. 1988 Sep;56(9):2373–2378. doi: 10.1128/iai.56.9.2373-2378.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisbig R., Olsnes S., Eiklid K. The cytotoxic activity of Shigella toxin. Evidence for catalytic inactivation of the 60 S ribosomal subunit. J Biol Chem. 1981 Aug 25;256(16):8739–8744. [PubMed] [Google Scholar]
- Rothbard J. B., Fernandez R., Schoolnik G. K. Strain-specific and common epitopes of gonococcal pili. J Exp Med. 1984 Jul 1;160(1):208–221. doi: 10.1084/jem.160.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Holmes R. K., O'Brien A. D. Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun. 1985 Dec;50(3):695–700. doi: 10.1128/iai.50.3.695-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell T., Head S., Petric M., Cohen A., Lingwood C. Globotriosyl ceramide is specifically recognized by the Escherichia coli verocytotoxin 2. Biochem Biophys Res Commun. 1988 Apr 29;152(2):674–679. doi: 10.1016/s0006-291x(88)80091-3. [DOI] [PubMed] [Google Scholar]
- Young P. R. Enhancement of immunoblot staining using a mixed chromogenic substrate. J Immunol Methods. 1989 Jul 26;121(2):295–296. doi: 10.1016/0022-1759(89)90174-9. [DOI] [PubMed] [Google Scholar]




