Abstract
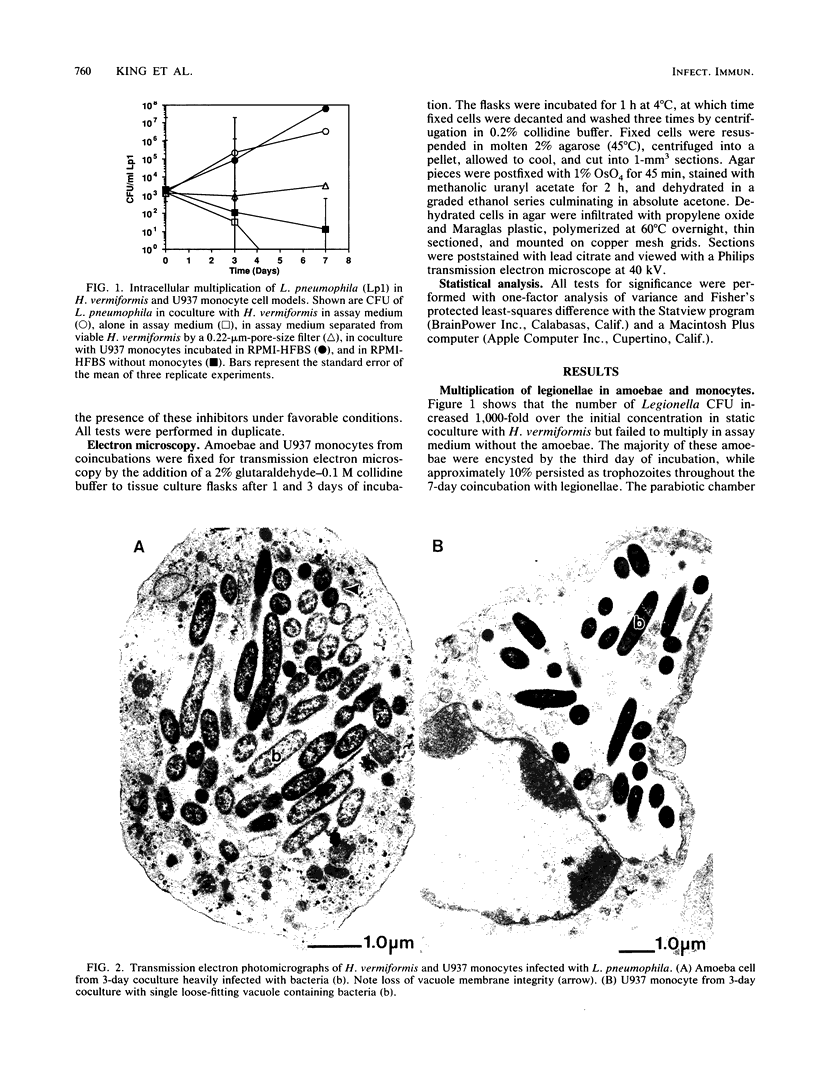
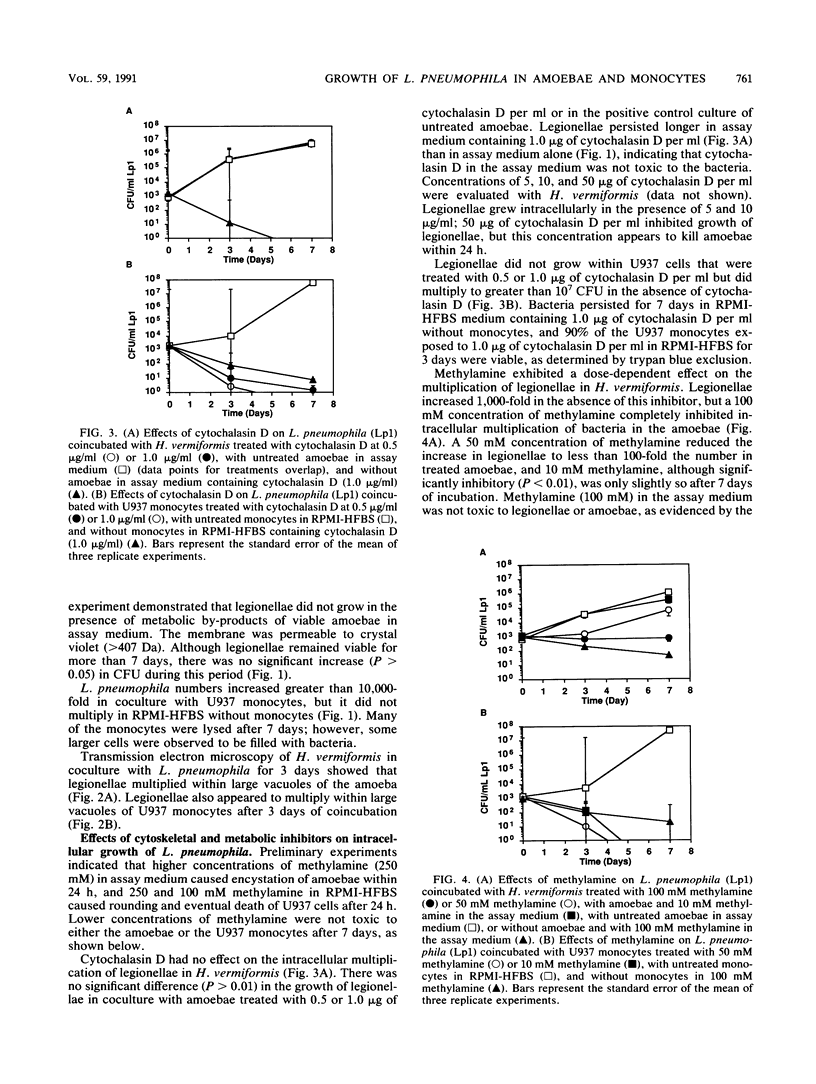
A cloned and axenically cultured strain of Hartmannella vermiformis was used as a model to study intracellular multiplication of Legionella pneumophila in amoebae. The growth of L. pneumophilia in both H. vermiformis and a human monocyte-like cell line (U937) was investigated with cytoskeletal and metabolic inhibitors. L. pneumophila replicated only intracellularly in these cellular models, and electron microscopy showed ultrastructural similarities in the initial phase of multiplication. Treatment of amoebae with an inhibitor of microfilament-dependent phagocytosis (cytochalasin D, 0.5 or 1.0 micrograms/ml) did not inhibit intracellular growth of L. pneumophila; however, intracellular multiplication was inhibited by treatment of U937 monocytes with the same concentrations of cytochalasin D. Methylamine (10 to 100 mM), an inhibitor of adsorptive pinocytosis, inhibited the replication of L. pneumophila in amoebae in a dose-dependent manner. All doses of methylamine tested (10 to 50 mM) inhibited growth of L. pneumophila in U937 monocytes. Cytochalasin D and methylamine had no effect on the multiplication of L. pneumophila in culture medium or on the viability of amoebae or U937 monocytes. Intracellular replication of L. pneumophila in H. vermiformis may be accomplished by a cytochalasin D-independent mechanism, such as adsorptive pinocytosis. In contrast, both cytochalasin D- and methylamine-sensitive mechanisms may be essential for the intracellular multiplication of L. pneumophila in U937 monocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Davies P., De Petris S. Role of contractile microfilaments in macrophage movement and endocytosis. Nat New Biol. 1971 Aug 4;232(31):153–155. doi: 10.1038/newbio232153a0. [DOI] [PubMed] [Google Scholar]
- Anand C. M., Skinner A. R., Malic A., Kurtz J. B. Interaction of L. pneumophilia and a free living amoeba (Acanthamoeba palestinensis). J Hyg (Lond) 1983 Oct;91(2):167–178. doi: 10.1017/s0022172400060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman R. F., Fields B. S., Sanden G. N., Volmer L., Meier A., Spika J. S. Association of shower use with Legionnaires' disease. Possible role of amoebae. JAMA. 1990 Jun 6;263(21):2924–2926. [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dournon E., Bibb W. F., Rajagopalan P., Desplaces N., McKinney R. M. Monoclonal antibody reactivity as a virulence marker for Legionella pneumophila serogroup 1 strains. J Infect Dis. 1988 Mar;157(3):496–501. doi: 10.1093/infdis/157.3.496. [DOI] [PubMed] [Google Scholar]
- Elliott J. A., Winn W. C., Jr Treatment of alveolar macrophages with cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infect Immun. 1986 Jan;51(1):31–36. doi: 10.1128/iai.51.1.31-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Barbaree J. M., Sanden G. N., Morrill W. E. Virulence of a Legionella anisa strain associated with Pontiac fever: an evaluation using protozoan, cell culture, and guinea pig models. Infect Immun. 1990 Sep;58(9):3139–3142. doi: 10.1128/iai.58.9.3139-3142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Barbaree J. M., Shotts E. B., Jr, Feeley J. C., Morrill W. E., Sanden G. N., Dykstra M. J. Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun. 1986 Sep;53(3):553–559. doi: 10.1128/iai.53.3.553-559.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. S., Nerad T. A., Sawyer T. K., King C. H., Barbaree J. M., Martin W. T., Morrill W. E., Sanden G. N. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J Protozool. 1990 Nov-Dec;37(6):581–583. doi: 10.1111/j.1550-7408.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Fields B. S., Shotts E. B., Jr, Feeley J. C., Gorman G. W., Martin W. T. Proliferation of Legionella pneumophila as an intracellular parasite of the ciliated protozoan Tetrahymena pyriformis. Appl Environ Microbiol. 1984 Mar;47(3):467–471. doi: 10.1128/aem.47.3.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Henke M., Seidel K. M. Association between Legionella pneumophila and amoebae in water. Isr J Med Sci. 1986 Sep;22(9):690–695. [PubMed] [Google Scholar]
- Horwitz M. A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987 Nov 1;166(5):1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984 Jan;36(1):27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Keogh E. A. Analysis of the effect of amines on inhibition of receptor-mediated and fluid-phase pinocytosis in rabbit alveolar macrophages. Cell. 1981 Jun;24(3):925–932. doi: 10.1016/0092-8674(81)90118-5. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Meyer R. D. Legionella infections: a review of five years of research. Rev Infect Dis. 1983 Mar-Apr;5(2):258–278. doi: 10.1093/clinids/5.2.258. [DOI] [PubMed] [Google Scholar]
- Nash T. W., Libby D. M., Horwitz M. A. Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Invest. 1984 Sep;74(3):771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome A. L., Baker R. L., Miller R. D., Arnold R. R. Interactions between Naegleria fowleri and Legionella pneumophila. Infect Immun. 1985 Nov;50(2):449–452. doi: 10.1128/iai.50.2.449-452.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham L. J., Rodgers F. G. Adhesion, penetration and intracellular replication of Legionella pneumophila: an in vitro model of pathogenesis. J Gen Microbiol. 1985 Apr;131(4):697–706. doi: 10.1099/00221287-131-4-697. [DOI] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Jiwa A. H., Engleberg N. C., Eisenstein B. I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988 Aug;5(2):87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- Prain C. J., Pearce J. H. Ultrastructural studies on the intracellular fate of Chlamydia psittaci (strain guinea pig inclusion conjunctivitis) and Chlamydia trachomatis (strain lymphogranuloma venereum 434): modulation of intracellular events and relationship with endocytic mechanism. J Gen Microbiol. 1989 Jul;135(7):2107–2123. doi: 10.1099/00221287-135-7-2107. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I., Guerrant R. L., Sperelakis N. Entamoeba histolytica: impedance measurements and cytotoxicity in the presence of bepridil, verapamil, and cytochalasin D. Exp Parasitol. 1985 Aug;60(1):63–72. doi: 10.1016/s0014-4894(85)80023-0. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I., Murphy C. F., Salata R. A., Guerrant R. L., Hewlett E. L. N-Acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. I. Partial purification and relation to amoebic virulence in vitro. J Infect Dis. 1985 May;151(5):804–815. doi: 10.1093/infdis/151.5.804. [DOI] [PubMed] [Google Scholar]
- Rowbotham T. J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980 Dec;33(12):1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockem W., Hoffmann H. U., Gruber B. Dynamics of the cytoskeleton in Amoeba proteus. I. Redistribution of microinjected fluorescein-labeled actin during locomotion, immobilization and phagocytosis. Cell Tissue Res. 1983;232(1):79–96. doi: 10.1007/BF00222375. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Effect of methylamine and monodansylcadaverine on the susceptibility of McCoy cells to Chlamydia trachomatis infection. Infect Immun. 1983 May;40(2):534–541. doi: 10.1128/iai.40.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshigawara K., Kannagi R., Noro N., Masuda T. Possible involvement of transglutaminase in endocytosis and antigen presentation. Microbiol Immunol. 1985;29(8):737–750. doi: 10.1111/j.1348-0421.1985.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Tyndall R. L., Domingue E. L. Cocultivation of Legionella pneumophila and free-living amoebae. Appl Environ Microbiol. 1982 Oct;44(4):954–959. doi: 10.1128/aem.44.4.954-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadowsky R. M., Butler L. J., Cook M. K., Verma S. M., Paul M. A., Fields B. S., Keleti G., Sykora J. L., Yee R. B. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl Environ Microbiol. 1988 Nov;54(11):2677–2682. doi: 10.1128/aem.54.11.2677-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J Gen Microbiol. 1984 Jul;130(7):1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- Wilkinson H. W., Reingold A. L., Brake B. J., McGiboney D. L., Gorman G. W., Broome C. V. Reactivity of serum from patients with suspected legionellosis against 29 antigens of legionellaceae and Legionella-like organisms by indirect immunofluorescence assay. J Infect Dis. 1983 Jan;147(1):23–31. doi: 10.1093/infdis/147.1.23. [DOI] [PubMed] [Google Scholar]
- Winn W. C., Jr, Myerowitz R. L. The pathology of the Legionella pneumonias. A review of 74 cases and the literature. Hum Pathol. 1981 May;12(5):401–422. doi: 10.1016/s0046-8177(81)80021-4. [DOI] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]