Abstract
Microglia, the tissue macrophages of the central nervous system (CNS), intimately interact with neurons physically and through soluble factors that can affect microglial activation state and neuronal survival and physiology. We report here a new mechanism of interaction between these cells, provided by the formation of gap junctions composed of connexin (Cx) 36. Among eight Cxs tested, expression of Cx36 mRNA and protein was found in microglial cultures prepared from human and mouse, and Cx45 mRNA was found in mouse microglial cultures. Electrophysiological measurements found coupling between one-third of human or mouse microglial pairs that averaged below 30 pico-Siemens and displayed electrical properties consistent with Cx36 gap junctions. Importantly, similar frequency of low-strength electrical coupling was also obtained between microglia and neurons in cocultures prepared from neocortical or hippocampal rodent tissue. Lucifer yellow dye coupling between neurons and microglia was observed in 4% of pairs tested, consistent with the low strength and incidence of electrical coupling. Cx36 expression level and/or the degree of coupling between microglia did not significantly change in the presence of activating agents, including lipopolysaccharide, granulocyte-macrophage colony-stimulating factor, interferon-γ, and tumor necrosis factor-α, except for some reduction of Cx36 protein when exposed to the latter two agents. Our findings that intercellular coupling occurs between neuronal and microglial populations through Cx36 gap junctions have potentially important implications for normal neural physiology and microglial responses in neuronopathology in the mammalian CNS.
Keywords: intercellular communication, dye coupling, electrical coupling, connexin43, activated microglia
Microglia are relatives of the hematopoietic lineage of cells and an important arm of the immune system within the CNS. Present throughout the parenchyma, microglia reside in several cytoarchitectonic positions, including positions intimately adjacent to neuronal cell bodies and in longitudinal arrays in white matter. These close physical arrangements might contribute to the fact that microglia are sensitive and rapid indicators of CNS pathology. Neuronal compromise can lead to myriad microglial changes, such as up-regulation of immunoregulatory molecules, release of neuroactive compounds, and enhanced motility and phagocytotic activity, some of which are evident within hours of the insult (Streit et al., 1999; Nelson et al., 2002). The mechanisms by which neurons might directly initiate these microglial responses are not fully understood but could include soluble ligand stimulation of fractalkine and purinergic receptors found on microglia (Cotter et al., 2002; Inoue, 2002). The degree of microglial ‘‘activation’’ has in turn been implicated as a determinant of neuronal death vs. survival and of neurite regeneration following neuronal injury (Streit et al., 1999; Batchelor et al., 2002; Schwarz, 2003). Various agents released by microglia that may act on neurons include nitric oxide, superoxide radical, prostanoids, cytokines, neurotrophins, and other neurotoxic and trophic compounds (Aloisi, 2001; Hanisch, 2002). Thus, a clear understanding of the mechanisms by which neurons and microglia interact is of considerable clinical interest. In addition, there is growing recognition that neuronal-microglial interactions are important in CNS development (Cuadros and Navascues, 1998; Marin-Teva et al., 2004).
One means of rapid intercellular signal transmission is through gap junctions present in apposing membranes of neighboring cells. These channels allow the bidirectional exchange of ions and small molecules (up to 900 Da) between the cytosols of coupled cells. Gap junction channels are formed by two hemichannels (connexons) each composed of six subunits, known as connexins (Cxs). These channels are typically formed between cells of the same type and involve relatively cell-specific connexins. Within the nervous system, gap junctions have been found between neurons, astrocytes, oligodendrocytes, and leptomeningeal cells and formed by connexins, including Cx26, Cx30, Cx32, Cx36, Cx43, Cx45, and Cx47 (Dermietzel et al., 2000; Nagy and Rash, 2000; Rozental et al., 2000; Bennett and Zukin, 2004; Nagy et al., 2004). Possible microglial connexins have been less well investigated. Cx43 expression was reported in microglia activated by interferon-γ (IFNγ) plus lipopolysaccharide (LPS) or tumor necrosis factor-α (TNF-γ; Eugenin et al., 2001) but was undetectable in microglia cocultured with astrocytes, which expressed Cx43 (Roach et al., 2002; Faustmann et al., 2003). After our initial studies on Cx36 reported in abstract form (Scemes et al., 2000; Chang et al., 2000), another group found Cx36 mRNA and immunocytochemical evidence of Cx36 protein in rat microglial cultures (Parenti et al., 2002).
We report here that cultured mouse and human microglial cells express Cx36 mRNA and protein and that coupling between these microglia is consistent with properties of channels formed of Cx36. In addition, mRNA for Cx45 is detectable in mouse but not human microglia. Given previous studies on Cx43 expression upon activation of microglia, we further investigated the effects of treatment with activating factors and did not find significant changes in Cx36 expression levels or microglial coupling. Verification of functional Cx36 protein in microglia was of particular interest, because, aside from possible expression in immature oligodendrocytes (Parenti et al., 2002), Cx36 within the CNS has been thought to be largely limited to neurons (Condorelli et al., 1998; Sohl et al., 1998; Nagy et al., 2004). This raised the important possibility that gap junctions might form between microglia and neurons, and we show for the first time evidence that functional Cx36 channels do form between these two cell types.
MATERIALS AND METHODS
Cell Culture Preparations
Purified cultures of murine microglia were prepared as previously described (Dobrenis, 1998) from neocortex or hippocampus of 2-day postnatal C57Bl/6J mice. Briefly, selected brain tissue was stripped of meninges and superficial vasculature and chopped into finer pieces, and tissue fragments were put through three consecutive rounds of digestion with 0.1% trypsin (Invitrogen-Life Technologies, La Jolla, CA) in the presence of 0.05% DNase I (Sigma, St. Louis, MO) at 37°C with shaking (75 strokes/min). Dissociated cells released during mincing and the first round of digestion were discarded to deplete the preparation of peripheral blood cells and nonparenchymal macrophages. Cells from the next two rounds were collected, and remaining tissue was further dissociated by trituration in the presence of DNase I. After centrifugation through 4% bovine serum albumin to remove debris, cells were plated at high density to generate mixed glial cell-type cultures. Cultures were maintained in ‘‘OAM,’’ which consists of basal medium Eagle’s (BME), glucose (6 g/liter), glutamine (1 g/liter), and 15% fetal bovine serum (FBS). After 2 weeks or more in vitro, loosely adherent microglia from overconfluent cultures were selectively recovered by gently tapping culture vessels for 15 sec and added to new tissue culture plates for 20 min to limit attachment to microglia. The new vessels were then repeatedly washed to remove any remaining unattached nonmicroglial cells. With these methods, microglial cultures are essentially pure (> 99%) as assessed by cell-type specific markers (F4/80 antibody, MAC-1 antibody, and DiI-acetylated LDL binding and uptake; Dobrenis, 1998). Purified microglia were maintained in OAM supplemented with 10 ng/ml recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF; catalog No. 415-ml; R&D, Minneapolis, MN).
Human fetal microglial cell cultures were prepared as described previously (Lee et al., 1992). Collection of human tissue was approved by the Albert Einstein College of Medicine (AECOM) Institutional Review Board. Fetal brain tissue was obtained through the AECOM Human Fetal Tissue Repository at the time of elective termination of pregnancy from normal women. Briefly, cerebral tissues were freed from meninges, minced, and digested in HBSS containing 0.05% trypsin and DNase for 45 min at 37°C by gentle shaking. After addition of 10% fetal calf serum (FCS), the digest was passed through 230- and 130-μm nylon meshes, washed twice, and resuspended in DMEM containing 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, Grand Island, NY). Cells were seeded at 4 × 107/75 cm2 tissue culture flask and kept at 37°C in a 5% CO2 humidified incubator. The cells were washed and fed with fresh medium on day 7. On day 14, flasks were gently tapped, and floating microglia were harvested, counted, and reseeded at 1–2 × 106 cells/10-cm-diameter plate. Microglial cultures were washed twice with fresh media after 1 hr. Purity of cultures is greater than 99% as verified by immunocytochemistry using antibodies to CD68 (EBM-11; Dako, Carpinteria, CA) to identify microglia (Lee et al., 1992). Both the mouse- and the human-derived cultures used contained microglia that displayed primarily round or flattened amoeboid-like phenotypes. For activation studies, LPS (catalog No. L-2880; Escherichia coli serotype 055:B5) and recombinant mouse TNF-α (catalog No. T-7539) and IFNγ (catalog No. I-4777) were obtained from Sigma.
Long-term dissociated cultures of neurons were prepared from embryonic day 15 mouse (C57Bl/6J) neocortex or hippocampus or from embryonic day 17/18 rat (Sprague-Dawley) hippocampus. Tissue was isolated as described for mouse microglia; digested in 0.05% trypsin, 0.05% DNase (4 min, 37°C); and mechanically dissociated through a 60-mesh filter screen. Cells were plated on poly-D-lysine-coated Assistent-brand glass coverslips at 4–5 × 104 cells/cm2 in DMEM supplemented with B27 (Invitrogen-Life Technologies), 6 g/liter glucose, 110 mg/ml sodium pyruvate, and 10% FBS. In the first feeding, cultures were switched to Neurobasal medium (Invitrogen-Life Technologies) supplemented with B27, glucose (6 gm/liter), 4 mM glutamine, and 2.5% FBS and maintained without antibiotics. The use of animals and methods of euthanasia were approved by IACUC, the Albert Einstein College of Medicine committee on animal research.
Cocultures of Neurons and Microglial Cells
For electrophysiology and dye-coupling studies, isolated microglia were added to neuronal cultures to achieve a density equivalent to 10–20% confluence. To facilitate live microglial identification, microglia cultures were labeled in advance by incubation with DiI (10 μg/ml, 3 hr; Molecular Probes, Eugene, OR), a lipophilic fluorescent dye that inserts into the plasmalemma. Medium with some floating microglia was removed, cultures were washed, and medium was centrifuged to return collected microglia to the original cultures in fresh medium. Cultures were maintained for ≥1 week with additional feeding prior to cell transfer, further removing any free DiI. Then, floating labeled microglia from overconfluent cultures were harvested, centrifuged, and resuspended in neuronal culture medium and added to neuronal cultures of 7–103 days in vitro age. Cocultures were maintained ≥1 day before performing experiments. Microglia remained strongly labeled with DiI and were identified by using epifluorescence microscopy.
RT-PCR
Total RNA was extracted from confluent microglial cultures using Trizol reagent (Gibco) and then treated with RNase-free DNase I (Boehringer Mannheim and Roche) to eliminate contamination with genomic DNA. Reverse transcription (RT) was performed with 2 μg RNA using random hexamer primers. Thirty-five cycles of PCR were then performed on samples containing first-strand cDNA with the sense- and antisense-specific primers (Table I) for the mouse and human Cx26, Cx30, Cx32, Cx36, Cx37, Cx40, Cx43, and Cx45 (all of which, except for human Cx30, previously validated by Srinivas et al., 1999; Urban et al., 1999; Dermeitzel et al., 2000; Rozental et al., 2001; Suadicani et al., 2004), using a PTC-100 Thermocycler (M.J. Research Inc.) by denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 30 sec each. The last cycle was followed by a final extension cycle at 72°C for 8 min and a soak cycle at 4°C. Reaction products were analyzed by electrophoresis on 2.0% agarose gels. For semiquantitative RT-PCR, primers for ribosomal 18S and competimers (Ambion, Austin, TX) at 1:9 ratio were added to the samples and used as an invariant endogenous control against which the products from the gene of interest were normalized. Images of gels were taken on the Kodak EDAS 120 system (Invitrogen) and whole bands quantified in Scion NIH Image software.
TABLE I.
Primer Pairs Used for PCR to Detect Connexins*
| Connexin | Sense primer | Antisense primer | Size (bp) |
|---|---|---|---|
| mCx26 | TTCCCCATCTCTCACATCC | GCAGAATGCAAATTCCAGAC | 409 |
| hCx26 | AATACAGACTGGATGTACCACC | AAAATTGGAGGCTGAAGG | 300 |
| M&hCx30 | CTACAGACATGAAACTGCCC | CCTCGAAATGAAGCAGTCC | 262 |
| mCx32 | GAAGAGGTAAAGAGACACAAGG | ACGTTGAGGATAATGCAGATG | 266 |
| hCx32 | AAGGTTACTGGGAGTGTG | CTGCTCCAACTTATCTGC | 351 |
| m&hCx36 | GAGCAAACGAGAAGATAAGAAG | TGGATGATGTAGAAGCGG | 195 |
| mCx37 | GGCTGGACCATGGAGCCGGT | CCATAACGAACCTGGATGAAAC | 421 |
| hCx37 | GCAAGTTGTTCTTGAACAC | GTCCTGTTTATTTCAGAAGC | 379 |
| mCx40 | TTGTCACTGTGGTAGCCCTGAGG | TTTGGCAAGTCACGGCAGGG | 311 |
| hCx40 | GGGCTGGAAGAAGATCAGAC | CCATAACGAACCTGGATGAAC | 267 |
| mCx43 | TACCACGCCACCACTGGC | AATCTCCAGGTCATCAGG | 407 |
| hCx43 | GGATTGTCCTTAAGTCCCTG | CACAAGTCCATTGACACCTG | 100 |
| mCx45 | AAAGAGCAGAGCCAACCAAA | GTCCCAAACCCTAAGTGAAGC | 313 |
| hCx45 | CAGTCACCAAAACAACCC | CATCCCCTGATTTGCTAC | 101 |
m, mouse; h, human; bp, base pairs.
Immunoprecipitation and Western Blots
Confluent cultures of mouse and human microglial cells grown in 60- or 100-mm dishes were washed twice in PBS, and 150 μl of lysate buffer (150 mM NaCl, 25 mM Tris-HCl, 5 mM EDTA, 1% NP40, 0.25 mM deoxycholate, and 1 mM PMSF at pH 7.5) containing freshly prepared 1% protease inhibitor cocktail (Sigma, catalog No. P2714) was added to each dish. Cell lysates (2.0–3.0 μg/μl total protein) were incubated overnight at 4°C with 5 μl rabbit polyclonal antibodies specific for Cx36 (Zymed, South San Francisco, CA; catalog No. 51-600) and with 20 μl immobilized protein G (Pierce, Rockford, IL). After several washes, immunocomplexes were eluted from the immobilized protein G with 50 mM Tris-HCl buffer containing 2% sodium dodecyl sulfate (SDS), 10% glycerol, and dithiothreitol (DDT). Eluted samples were separated by electrophoresis in a 10% SDS-polyacrylamide gel (Bio-Rad, Hercules, CA). After electrophoretic transfer to a nitrocellulose membrane (Scheicher & Schuell, Keene, NH), the membranes were incubated overnight at 4°C with BLOTTO (5% dry nonfat milk in PBS) solution and then incubated with anti-Cx36 antibodies (1:500; Zymed; catalog No. 51-6300) at room temperature for 1 hr. After several washes in PBS containing 0.05% Tween-20, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (1:1,000; Vector, Burlingame, CA). Detection was performed on X-ray film (Kodak) after incubation of the membranes with enhanced chemiluminescence (ECL) reagents (Amersham, Arlington Heights, IL). A parental neuroblastoma cell line (N2A) and a stable clone of Cx36 N2A transfectants were used as negative and positive controls for expression of Cx36, respectively.
In assays with growth factors and cytokines, microglial cultures were directly harvested and centrifuged (1,000 rpm, 2 min). Cells were resuspended in PBS (pH 7.4) and centrifuged and the pellets extracted with 300 μl lysate buffer plus 1% protease inhibitor cocktail (as described above) for 45 min on ice. The resulting lysate was microcentrifuged (10,000 rpm, 10 min, 4°C) and the supernatant collected and centrifuged to obtain a final supernatant for analysis. Equivalent amounts of protein for each extract were loaded onto gels. Mouse monoclonal antibodies used here for Western blotting were anti-Cx35/36 (MAB3045; Chemicon, Temecula, CA), anti-Cx43 at 1:500 dilution (catalog No. 35-5000; Zymed) and anti-GAPDH at 1:5,000 (clone 6C5; Research Diagnostics, Minneapolis, MN). For semiquantititative protein analysis, entire bands were imaged in Western blots and quantified in Scion NIH Image. Mean values for each Cx band were divided by the values from GAPDH bands serving as invariant internal controls.
Electrical Coupling
Junctional conductance was characterized by using the dual whole-cell voltage clamp technique (see Srinivas et al., 1999). Microglial cell pairs and neuronal-microglial pairs were voltage clamped at holding potentials of 0 mV, and command ramps from –110 mV to +110 mV or from –100 mV to +100 mV were presented to one cell in Pclamp 6 software (Axon Instruments). Junctional currents (Ij) were recorded in the unstepped cell; junctional conductance (gj) was calculated as –Ij/V (Spray et al., 1981). Patch pipettes were filled with 140 mM CsCl, 10 mM EGTA, and 5 mM Mg2ATP (pH 7.25). Cells were bathed in a solution containing 140 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM BaCl2, 2 mM CsCl, 1 mM MgCl2, and 5 mM HEPES (pH 7.2). Microgliamicroglial coupling was examined in purified cultures at subconfluent density. Neuronal-microglial pairs were examined in cocultures in which microglia were recognized by using epi-fluorescence microscopy to detect DiI labeling (see above under Cocultures of neurons and microglial cells). Neurons were recognized by their large, semirounded perikarya and large nuclei with prominent nucleoli and characteristic tapering dendrites under phase-contrast microscopy. Then neuronal identity was further confirmed by the presence of spontaneous synaptic currents and robust inward currents. Recordings were performed from microglia adjacent to neuronal perikarya or proximal neurites.
Dye Coupling
To assess dye coupling between neuron-microglial pairs, we elected to inject only neurons because of the significantly larger neuronal volume relative to microglia. Because of the size differential, loading of microglia could yield dye concentrations in coupled neurons insufficient to detect in time and thus lead to false negatives. Lucifer yellow (5% w/v in 150 mM LiCl) or Alexa Fluor 488 (1 mM; Molecular Probes, Eugene, OR) was iontophoretically injected, with a brief overcompensation of the capacitance control on a WPI electrometer, into neuron cell bodies until a strong fluorescent signal was obtained in perikarya and proximal processes. At subsequent 1-min intervals, injected cells were examined on a Nikon Diaphot microscope equipped with a xenon arc lamp and photographed with a Nikon Coolpix 950 camera.
RESULTS
Connexin Expression in Mouse and Human Microglial Cells
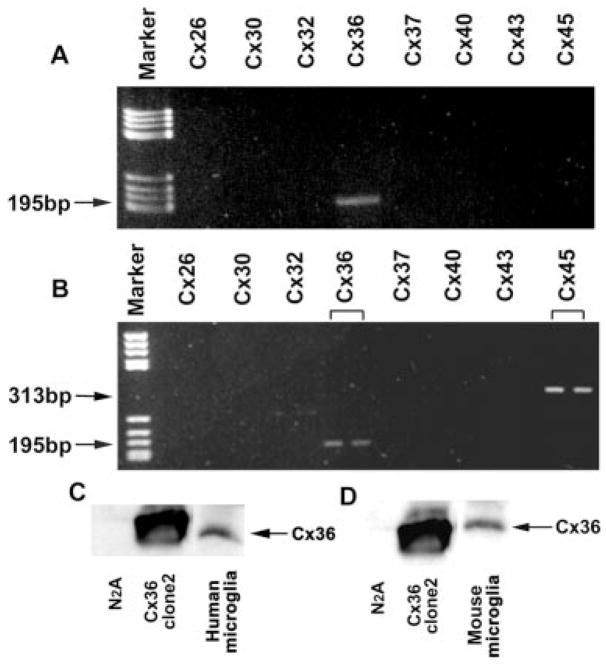
Primary human and mouse neocortical microglia were examined for the presence of mRNA species corresponding to eight connexins: Cx26, Cx30, Cx32, Cx36, Cx37, Cx40, Cx43, and Cx45. As shown in Figure 1A,B, both mouse and human microglia expressed Cx36 mRNA and mouse microglia also expressed Cx45 mRNA. The lack of detectable mRNA for Cx32 and Cx43, highly expressed in astrocytes and oligodendrocytes, argued that detected mRNA was not attributable to trace contamination by these cell types. Presence of Cx36 protein was also detected by Western blot on purified mouse and human microglia cultures (Fig. 1C,D). In initial Western blot analyses of whole-cell homogenates using rabbit antibodies to Cx36, bands were barely detectable (not shown), suggesting low levels of Cx36 expression in microglia. Subsequently, to facilitate detection and further verify presence of Cx36 protein, cell homogenates were first immunoprecipitated with rabbit anti-Cx36 antibody to enrich the protein and then Western blotting of the concentrated samples was performed with a second rabbit antibody (see Materials and Methods), resulting in prominent bands (Fig. 1C,D).
Fig. 1.
Analysis of connexin mRNA and protein expression in purified microglia. Agarose gel electrophoresis of PCR with Cx-specific primers on RT-PCR product from human (A) and mouse (B) microglial cultures. Lanes corresponding to Cx26, Cx30, Cx32, Cx36, Cx37, Cx40, Cx43, and Cx45 specific reaction samples are labeled. Arrows indicate bands consistent with the expected amplicons for human and mouse Cx36 (195 bp) and mouse Cx45 (313 bp). Similar results were obtained for three separate preparations of mouse and human microglia. Western blot analysis of immunoprecipitated proteins showing Cx36 expression in human (C) and mouse (D) microglia. In both cases, Cx36 is present in microglial immunoprecipitated extracts (last lane), and negative (parental, nontransfected N2A cells; first lane) and positive (N2A clone stably transfected with Cx36) controls prepared in the same manner are shown.
Antibodies to Cx36 were also verified to be suitable for immunocytochemical staining using N2A cells expressing Cx36-EGFP. However, at best, attempts at staining of microglia for Cx36 by immunofluorescence [indirect as well as three and four-step procedures (Molecular Probes; catalog No. A-11053)] in purified cultures and in coculture with neurons produced very low levels of detection above background (data not shown). It was not possible to obtain unequivocal evidence of microglial gap junctional plaques by this method, which was not surprising in light of the small number of channels involved as revealed by the experiments described below.
Coupling Between Microglia
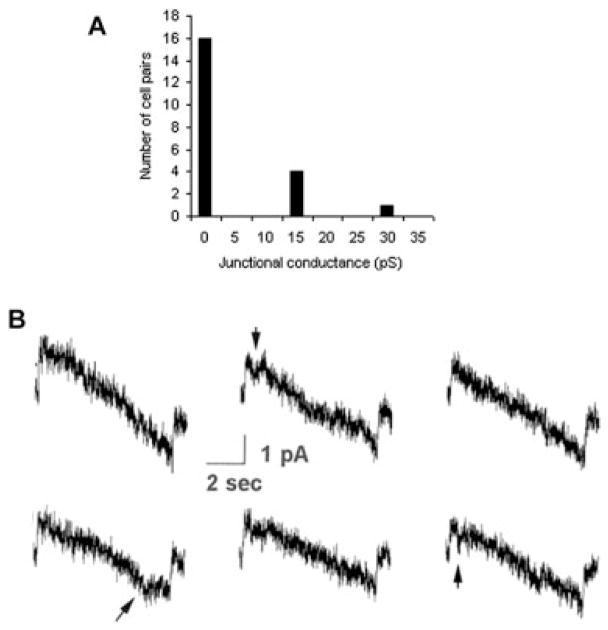
Approximately one-third of mouse microglial cell pairs (7 of 23) showed evidence of electrical coupling in purified cultures from neocortex. Dual whole-cell voltage clamp recordings measured low junctional conductance between microglia, averaging below 30 pico-Siemens (pS; Fig. 2A). Similar results were obtained from pairs of human microglia (Fig. 2B). Electrophysiological recordings revealed small junctional currents, with gj values typically 10–15 pS. Infrequent transitions showed similar change in conductance. In addition, gj was only weakly sensitive to transjunctional voltage, reflected by a lack of transition at high ±Vj (Fig. 2B). These channel properties of very small unitary conductance and very low voltage sensitivity are characteristic of those previously shown for Cx36 junctions (Srinivas et al., 1999; Teubner et al., 2000).
Fig. 2.
Electrophysiology shows functional coupling between pairs of microglia consistent with the properties of Cx36 channels. A: Summary of junctional conductance (gj) measurements obtained in 23 pairs of mouse neocortical microglial cells. Seven of twenty-three pairs were measurably coupled; although gj was generally quite low, it exceeded 5 nS in two cell pairs (not illustrated). B: Transjunctional currents recorded between pairs of human microglial cells in response to voltage ramps ±100 mV. Junctional currents were small, corresponding to junctional conductances of 10–15 pS. In addition, transitions were occasionally seen, corresponding to similar change in conductance. Junctional conductance was only very poorly voltage dependent, as indicated by the lack of transition at high positive or negative Vj values. Arrows indicate Cx36-like channel activity.
Coupling Between Microglia and Neurons
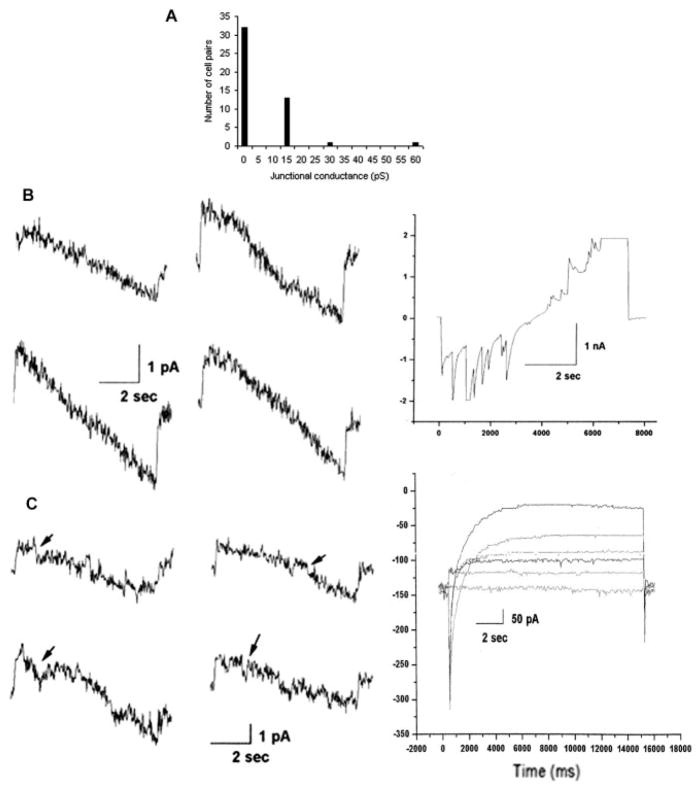
Gap junctional communication was examined in cocultures of microglia and neurons from the neocortex and from the hippocampus. Electrophysiological recordings were performed on fluorescently tagged microglia in contact with neurons. The latter were confirmed to display characteristic neuronal properties, including spontaneous synaptic currents (Fig. 3B, right) and strong inward currents (Fig. 3C, right). Input resistance measured at a holding potential of 0 mV was in excess of 200 MOhms. Among the 47 microglial-neuronal pairs analyzed, heterocellular junctional coupling was found to be present in 15 cases, with junctional conductance on the order of 15 pS for most of the coupled cell pairs (Fig. 3A). Heterocellular coupling was found between mouse cortical microglia and rat hippocampal neurons (1 of 8 pairs), mouse hippocampal neurons and microglia (8 of 14), and mouse cortical neurons and microglia (6 of 25). As with the homocellular channels, these heterocellular junctional channels showed small unitary conductance and very low voltage sensitivity, again consistent with the characteristics of Cx36 channels (see Fig. 3B, left, and C, left). There was no indication of electrical rectification; junctional current in response to voltage ramps from +100 mV to –100 mV was generally linear (Fig. 3B, left, and C, left), and little voltage gating was seen in response to high negative and high positive voltages (e.g., Fig. 3B, left).
Fig. 3.
Frequency and electrophysiological properties of neuronal-microglial gap junctional coupling. A: In total 47 neuron-microglial pairs were examined and 15 showed coupling. B,C: Two examples of coupling between pairs of neurons and microglial cells. In both examples, voltage ramps ±100 mV were applied to neurons, and junctional currents were recorded in microglia as shown at left. For the pair shown in B, currents corresponded to junctional conductances of 10–20 pS, with few openings or closures. In C, from a different cell pair, initial currents corresponded to junctional conductances of 15–20 pS, with occasional transitions of similar conductance (arrows). Note absence of appreciable voltage sensitivity of gating of these channels, which is a characteristic feature of Cx36 channels. B: Right panel shows spontaneous activity revealed in neuronal current recording during the voltage clamp. C: Right panel shows inward and outward currents evoked in neuron held at –80 mV in response to voltage steps from –60 to +60 mV in 20-mV increments.
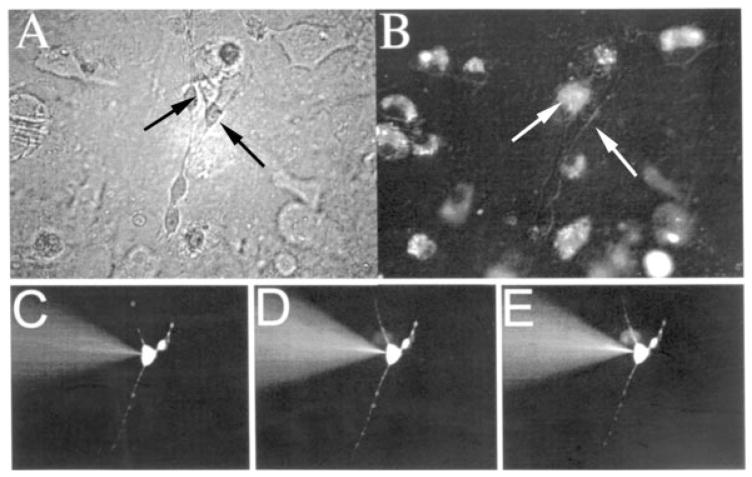
Further support for heterocellular coupling was obtained from dye transfer assays in cocultures of mouse hippocampal microglia and neurons. Using Lucifer yellow and Alexa Fluor 488 microinjections into neurons, the incidence of dye coupling with microglia was 3 of 70 (Fig. 4) and 2 of 52, respectively. These results are in agreement with the presence of relatively few channels as indicated by the electrophysiological recordings.
Fig. 4.
Dye transfer between mouse hippocampal neurons and microglia. A: Phase-contrast photograph of cocultured hippocampal neurons (e.g., right arrow) and microglia (e.g., left arrow). B: Epifluorescence photomicrograph of same field as A, revealing DiI-labeled microglia. C–E: Epi-fluorescence photomicrographs showing gradual Lucifer yellow transfer from a neuron (C; corresponding to right arrow in A,B) to a microglia cell (D,E; corresponding to left arrow in A,B).
Connexin Expression and Coupling Between Microglia Exposed to Extrinsic Agents
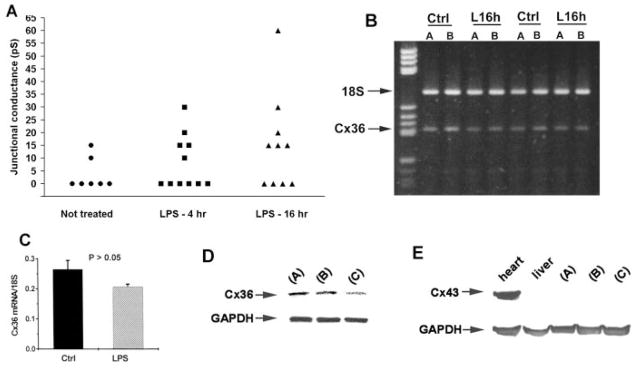
Human microglial cultures were challenged for 4 and 16 hr with LPS (10 ng/ml), a commonly used bacterial-derived agent that stimulates activation. This treatment did not lead to significant changes in electrical coupling or in Cx36 mRNA expression levels. Similar to untreated microglia, junctional conductance between coupled pairs remained low, generally 15 to 30 pS, though there was a trend toward higher gj and more frequent coupling (Fig. 5A). By semiquantitative RT-PCR, no statistically significant difference was found for Cx36 mRNA levels between cultures treated for 16 hr. vs. parallel nontreated cultures (Fig. 5B,C).
Fig. 5.
Evaluation of connexin expression and coupling in microglial cultures treated with activating agents/growth factors. A: Junctional conductance measurements in pairs of untreated human microglia and in pairs after 4 or 16 hr of exposure to LPS. In untreated microglia, 2 of 7 pairs were electrically coupled; after 4 hr of treatment, 5 of 11 were coupled; and, after 16 hr of treatment, 5 of 9 pairs were coupled. In all cases, junctional conductance was low, generally 10–30 pS, indicating the presence of only one to three junctional channels. No statistically significant difference was found either between LPS treatment period and no treatment in the proportion of cells coupled (Fisher’s exact test, two-sided; P > 0.05) or in the mean conductance of coupled cells (Student’s t-test, unpaired, two-sided; P > 0.05). B: Electrophoresis of RT-PCR for Cx36 along with 18S ribosomal RNA to normalize shows no apparent difference between human microglia not treated (ctrl) and treated for 16 hr with LPS (L16h). C: Semiquantification of RT-PCR results verifies no statistically significant difference (Student’s t-test) between treatments. D: Western blot assessment of Cx36 protein in mouse neocortical microglial cultures in basal medium only (A), or with addition of GM-CSF (B), or with IFNγ plus TNF-α (C) for 24 hr. E: Western blot assessment for Cx43 protein in extracts from the same cultures described in D. Heart and liver extracts were run as positive and negative controls, respectively. Note that Cx43 protein was undetectable in all culture extracts. The experiment was repeated, and again no Cx43 was detected in cultures (not shown).
Cx36 protein levels in mouse neocortical microglial cultures were assessed after 24 hr in 10 ng/ml GM-CSF, or 1 ng/ml IFNγ plus 1 ng/ml TNF-γ, or basal culture medium alone (Fig. 5D). Optical density measurement on Western blots from two experiments gave Cx36/GAPDH band ratio values of 1.01 and 1.01 for control, 1.11 and 0.95 for GM-CSF, and 0.63 and 0.69 for IFNγ plus TNF-α conditions. A statistically significant difference was present between IFNγ +TNF-α and control or GM-CSF conditions but not between control and GM-CSF (ANOVA, P < 0.05; Tukey-Kramer post-hoc test, P < 0.05). Expression of Cx43 protein, previously reported to be upregulated with the latter combination in rat microglia (Eugenin et al., 2001), was undetectable in all three conditions (Fig. 5E).
DISCUSSION
In this study, we found that both mouse and human microglia in vitro express Cx36 mRNA and protein, and mouse microglia also express Cx45 mRNA. Electrophysiological results indicated the presence of gap junction channels between mouse microglia with low unitary conductance (< 20 pS) and very low voltage sensitivity, properties that distinguish channels formed by Cx36 from all other connexins (Srinivas et al., 1999; Teubner et al., 2000). Coupled cells contained an approximate maximum of five open channels, suggesting that relatively small junctional plaques were present. This likely explains the inability to clearly detect gap junction plaques by immunocytochemistry in our study, a problem also encountered with small Cx36 junctional plaques between some neuronal subtypes (see, e.g., Meier et al., 2002). Both the incidence and the strength of coupling that we observed were consistent with the low levels of Cx36 detected in Western blots. Most importantly, we found in cocultures that microglia could also form functional gap junctions with neurons, providing a new mechanism for microglial-neuronal interactions potentially relevant to neuronal physiology and microglial responses in neuronopathology.
The identification of Cx36 protein expression in several mammalian species, in human and mouse microglia in this study, and immunocytochemical evidence in cultures of rat microglia (Parenti et al., 2002), supports the likelihood that it plays an important role in this cell type. Another group attributed dye coupling between activated microglia primarily to junctions composed of Cx43. However, a low incidence of dye coupling between microglia isolated from Cx43 knockout mice was also noted (Eugenin et al., 2001), compatible with the low coupling frequency that we obtained. Future experiments employing cells from available Cx36 knockout mice harboring reporter genes (Deans et al., 2001; Degen et al., 2004), and potentially from Cx36/Cx43 double-knockout mice generated by cross-breeding, would be useful in further verifying the components responsible for junctional coupling between microglia. The LacZ reporter present might further assist in identifying individual cells that would otherwise express Cx36. Although we also detected low amounts of Cx45 at the mRNA level in mouse microglia, we found no biophysical evidence for functional Cx45 gap junction channels, which exhibit strong voltage sensitivity and larger unitary conductances (see Moreno et al., 1995) than we obtained here. Our results do not exclude the possibility that Cx45 protein expression and gap junction formation might occur under extrinsic conditions not tested here, and this deserves further study in that Cx45 may be expressed by some neurons (Condorelli et al., 2003; Maxeiner et al., 2003).
It was of interest to investigate whether factors known to activate microglia might increase Cx36 levels or coupling, insofar as reactive microglia show up-regulated expression of numerous macrophage-like properties (Flaris et al., 1993; Streit et al., 1999; Nelson et al., 2002; Guillemin and Brew, 2004) and of Cx43 (Eugenin et al., 2001). However, we found no significant evidence of such increases under the conditions tested, which included treatment of mouse microglia with a combination of IFNγ and TNF-γ. We found that this condition also did not elicit detectable Cx43 protein expression, although it did increase levels in studies with rat microglia cultures (Eugenin et al., 2001). It is possible that this discrepancy arises from differences between animal species used or the precise method of preparation of microglia or culture maintenance conditions between the two studies, in that even serum lots and routine basal medium components can affect microglial phenotype (Gebicke-Harter et al., 1989; Tanaka et al., 1998). Two other groups also reported no detectable Cx43 expression in rat microglia, including activated-like cells, when cocultured with astrocytes (Rouach et al., 2002; Faustmann et al., 2003). Cx43 expression has been found in numerous other hematopoietically derived macrophages and other immune system cells (Rosendaal et al., 1994; Eugenin et al., 2001, 2003; Oveido-Orta et al., 2004; Bodi et al., 2004) but apparently was absent from F4/80-positive macrophages in bone marrow (Rosendaal et al., 1994). Additional studies will be necessary to resolve Cx expression patterns in microglia fully. Nevertheless, the evidence of Cx36 expression in microglia, which as yet has not been found in other macrophages, contributes to the view that microglia are relatively unique, displaying some properties (Giulian and Baker, 1986; Norenberg et al., 1994; Guilian et al., 1995; Wilms et al., 1999) that distinguish these cells from other macrophages of myelomonocytic origin.
The finding that neurons and microglia can couple through gap junctions has potential significance to neuropathology. It is well known that microglia are rapid and highly sensitive responders to neuronal compromise, and the extent of microglial activation is thought to be a key determinant of neuronal survival resulting from a differential balance of microglial release of neurotoxic and neurotrophic factors (Streit et al., 1999; Hanisch, 2002; Inoue, 2002; Schwarz, 2003). Neuronal-microglial coupling may play a role in initiating sensitive microglial responses and/or subsequent modulation of neuronal physiology. For example, elevation of basal intracellular calcium in coupled microglia from neuronal insult could lead to or contribute to an activated state that produces release of nitric oxide, cytokines, and chemokines (Hoffmann et al., 2003). Although our findings clearly demonstrate the capacity for neuronal-microglial coupling, in vivo studies are needed in which neuronal and microglial Cx36 expression levels and frequency and strength of coupling may be different from the case in vitro. Furthermore, comparative studies employing Cx36 knockout mice (Deans et al., 2001; Degen et al., 2004) could prove useful in assessing the functional significance of such coupling.
Studies in vivo should also resolve how microglial state of activation affects Cx expression and gap junctional coupling. Our results do not suggest that activation leads to increases, and other studies on Cx43 and microglial coupling demonstrated that only very specific combinations of activating agents could elicit an increase (Eugenin et al., 2001). It may be worth considering another perspective, that coupling might be highest in resting ramified microglia. Although extrinsic agents have been tested to promote activation, the procedure of culturing microglia itself produces some degree of activation, and generation of genuine ‘‘resting’’ microglia is not readily attained in vitro (Dobrenis, 1998; Sievers et al., 1998; Tanaka et al., 1998). Therefore, the studies to date cannot be considered to have adequately evaluated resting microglia. Indeed, resting cells would arguably have a greater possibility of establishing stable gap junctions than reactive microglia, which are highly migratory (Rieske et al., 1989; Chen et al., 2000; Nelson et al., 2002). Compatible with the hypothesis is our observation of reduced expression of Cx36 protein upon exposure to TNF-α and IFNγ. Furthermore, in the normal brain, in regions such as the neocortex or hippocampus, microglia are intimately wrapped over neuronal cell bodies and proximal dendrites (Rio Hortega, 1932; Peters et al., 1991), providing opportunities for extensive junctional communication.
Gap junctions are found largely between cells of the same type, and it has been argued that glia and neurons remain segregated in this pathway of communication (see Rash et al., 2000; Nagy et al., 2004). However, contrary examples, such as evidence for functional gap junction coupling between neurons and astrocytes in dissociated cell cultures and slices from rat brain (Froes et al., 1999; Alvarez-Maubecin et al., 2000) and in human fetal cell cultures (Rozental et al., 2001), have been reported. Our study with microglia now provides important evidence of a different type of heterocellular coupling involving neurons. Continued investigations of Cx36 expression in microglia and the functional consequences of coupling with neurons are warranted and will add to this new insight on neuronal-microglial interactions.
Acknowledgments
The authors thank D. Litvinenko for technical assistance and AECOMs HTFR for access to human tissue. This work was supported in part by grants from the NIH (NS42807 and MH65495 to D.C.S., NS42152 to R.R., MH55477 to S.C.L., and NS41023 to E.S.), the Millenium Institute for Tissue Bioengineering (CNPq; to R.R.), the Christopher Reeve Paralysis Foundation (SB1-9802; to E.S.), and the Epilepsy Foundation (to R.R.).
References
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Alvarez-Maubecin V, Garcia-Hernandez F, Williams JT, Van Bockstaele JV. Functional coupling between neurons and glia. J Neurosci. 2000;20:4091–4098. doi: 10.1523/JNEUROSCI.20-11-04091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Porritt MJ, Martinello P, Parish CL, Liberatore GT, Donnan GA, Howells DW. Macrophages and microglia produce local trophic gradients that stimulate axonal sprouting toward but not beyond the wound edge. Mol Cell Neurosci. 2002;21:436–453. doi: 10.1006/mcne.2002.1185. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Bodi E, Hurtado SP, Carvalho MA, Borojevic R, Carvalho AC. Gap junctions in hematopoietic stroma control proliferation and differentiation of blood cell precursors. An Acad Bras Cienc. 2004;76:743–756. doi: 10.1590/s0001-37652004000400009. [DOI] [PubMed] [Google Scholar]
- Chang HY, Scemes E, Srinivas M, Lee SC, Spray DC. Cultured human microglia express the neuronal gap junction protein, connexin36. Soc Neurosci Abstr. 2000;26:1640. [Google Scholar]
- Chen A, Kumar SM, Sahley CL, Muller KJ. Nitric oxide influences injury-induced microglial migration and accumulation in the leech CNS. J Neurosci. 2000;20:1036–1043. doi: 10.1523/JNEUROSCI.20-03-01036.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli DF, Parenti R, Spinella F, Trovato, Salinaro A, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Trovato-Salinaro A, Mudo G, Mirone MB, Belluardo N. Cellular expression of connexins in the rat brain: neuronal localization, effects of kainate-induced seizures and expression in apoptotic neuronal cells. Eur J Neurosci. 2003;18:1807–1827. doi: 10.1046/j.1460-9568.2003.02910.x. [DOI] [PubMed] [Google Scholar]
- Cotter R, Williams C, Ryan L, Erichsen D, Lopez A, Peng H, Zheng J. Fractalkine (CX3CL1) and brain inflammation: Implications for HIV-1-associated dementia. J Neurovirol. 2002;8:585–598. doi: 10.1080/13550280290100950. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Prog Neurobiol. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Selilitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Degen J, Meier C, Van der Giessen RS, Sohl G, Petrasch-Parwez E, Urschel S, Dermietzel R, Schilling K, De Zeeuw CI, Willecke K. Expression pattern of LacZ reporter gene representing connexin36 in transgenic mice. J Comp Neurol. 2004;473:511–525. doi: 10.1002/cne.20085. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MVL, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Dobrenis K. Microglia in cell culture and transplantation therapy for central nervous system disease. Methods. 1998;16:320–344. doi: 10.1006/meth.1998.0688. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Eckardt D, Theis M, Willecke K, Bennett MVL, Saez JC. Microglia at brain stab wounds express connexin43 and in vitro form functional gap junctions after treatment with interferon-γ and tumor necrosis factor-γ. Proc Natl Acad Sci U S A. 2001;98:4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Branes MC, Berman JW, Saez JC. TNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J Immunonol. 2003;170:1320–1328. doi: 10.4049/jimmunol.170.3.1320. [DOI] [PubMed] [Google Scholar]
- Faustmann PM, Haase CG, Romberg S, Hinkerohe D, Szlachta D, Smikalla D, Krause D, Dermietzel R. Microglia activation influences dye coupling and Cx43 expression of the astrocytic network. Glia. 2003;42:101–108. doi: 10.1002/glia.10141. [DOI] [PubMed] [Google Scholar]
- Flaris NA, Densmore TL, Molleston MC, Hickey WF. Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia. 1993;7:34–40. doi: 10.1002/glia.440070108. [DOI] [PubMed] [Google Scholar]
- Froes MM, Correia AHP, Garcia-Abreu J, Spray DC, Campos de Carvalho AC, Moura Neto V. Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proc Natl Acad Sci U S A. 1999;96:7541–7546. doi: 10.1073/pnas.96.13.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebicke-Harter PJ, Bauer J, Schobert A, Northoff H. Lipopolysac-charide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. J Neurosci. 1989;9:183–194. doi: 10.1523/JNEUROSCI.09-01-00183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from the developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick JB. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J Neurosci. 1995;15:7712–7726. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Hanisch U-K. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Kann O, Ohlemeyer C, Hanisch UK, Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. J Neurosci. 2003;23:4410–4419. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K. Microglial activation by purines and pyrimidines. Glia. 2002;40:156–163. doi: 10.1002/glia.10150. [DOI] [PubMed] [Google Scholar]
- Lee SC, Liu W, Brosnan CF, Dickson DW. Characterization of primary human fetal dissociated central nervous system cultures with an emphasis on microglia. Lab Invest. 1992;67:465–476. [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Maxiener S, Kruger O, Schilling K, Traub O, Urschel S, Willecke K. Spatiotemporal transcription of connexin 45 during brain development results in neuronal expression in adult mice. Neuroscience. 2003;119:689–700. doi: 10.1016/s0306-4522(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Meier C, Petrasch-Parwez E, Habbes H-W, Teubner B, Guldenagel M, Degen J, Sohl G, Willecke K, Dermietzel R. Immunohistochemical detection of the neuronal connexin36 in the mouse central nervous system in comparison to connexin 36-deficient tissues. Histochem Cell Biol. 2002;117:461–471. doi: 10.1007/s00418-002-0417-z. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Laing JG, Beyer EC, Spray DC. Properties of gap junction channels formed of connexin 45 endogenously expressed in human hepatoma (SKHep1) cells. Am J Physiol. 1995;268:C356–C365. doi: 10.1152/ajpcell.1995.268.2.C356. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Ann Med. 2002;34:491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Illes P, Gebicke-Harter PJ. Sodium channel in isolated human brain macrophages (microglia) Glia. 1994;10:165–172. doi: 10.1002/glia.440100303. [DOI] [PubMed] [Google Scholar]
- Oveido-Orta E, Howard Evans W. Gap junctions and connexin-mediated communication in the immune system. Biochim Biophys Acta. 2004;1662:102–112. doi: 10.1016/j.bbamem.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Parenti R, Campisi A, Vanella A, Cicirata F. Immunocytochemical and RT-PCR analysis of connexin36 in cultures of mammalian glial cells. Arch Ital Biol. 2002;140:101–108. [PubMed] [Google Scholar]
- Peters A, Palay SL, deF Webster H. The fine structure of the nervous system. New York: Oxford University Press; 1991. pp. 273–311. [Google Scholar]
- Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2000;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieske E, Graeber MB, Tetzlaff W, Czlonkowska A, Streit WJ, Kreutzberg GW. Microglia and microglia-derived brain macrophages in culture: generation from axotomized rat facial nuclei, identification and characterization in vitro. Brain Res. 1989;492:1–14. doi: 10.1016/0006-8993(89)90883-4. [DOI] [PubMed] [Google Scholar]
- Rio-Hortega PD. Microglia. In: Penfield W, editor. Cytology and cellular pathology of the nervous system. New York: Hafner Publishing Co.; 1932. pp. 483–534. [Google Scholar]
- Rosendaal M, Green CR, Rahman A, Morgan D. Up-regulation of the connexin43+ gap junction network in haemopoietic tissue before the growth of stem cells. J Cell Sci. 1994;107:29–37. doi: 10.1242/jcs.107.1.29. [DOI] [PubMed] [Google Scholar]
- Rouach N, Calvo CF, Glowinski J, Giaume C. Brain macrophages inhibit gap junctional communication and dowregulate connexin 43 expression in cultured astrocytes. Eur J Neurosci. 2002;15:403–407. doi: 10.1046/j.0953-816x.2001.01868.x. [DOI] [PubMed] [Google Scholar]
- Rozental R, Giaume C, Spray DC. Gap junctions in the nervous system. Brain Res Rev. 2000;32:11–15. doi: 10.1016/s0165-0173(99)00095-8. [DOI] [PubMed] [Google Scholar]
- Rozental R, Andrade-Rozental AF, Zheng X, Urban M, Spray DC, Chiu FC. Gap junction-mediated bi-directional signaling between human fetal hippocampal neurons and astrocytes. Dev Neurosci. 2001;23:420–431. doi: 10.1159/000048729. [DOI] [PubMed] [Google Scholar]
- Scemes E, Dobrenis K, Rozental R, Chang HY, Spray DC. Neurons and microglia interact through gap junction channels composed of connexin36. Soc Neurosci Abstr. 2000;26:1640. [Google Scholar]
- Schwarz M. Macrophages and microglia in central nervous system injury: Are they helpful or harmful? J Cereb Blood Flow Metab. 2003;23:385–394. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- Sievers J, Parwaresch R, Wottge H-U. Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: morphology. Glia. 1994;12:245–258. doi: 10.1002/glia.440120402. [DOI] [PubMed] [Google Scholar]
- Sohl G, Degen J, Teubner B, Willecke K. The murine gap junction gene connexin 36 is highly expressed in mouse retina and regulated during brain development. FEBS Lett. 1998;428:27–31. doi: 10.1016/s0014-5793(98)00479-7. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77:77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin 36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Flores CE, Urban-Maldonado M, Beelitz M, Scemes E. Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation. Glia. 2004;48:217–229. doi: 10.1002/glia.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Toku K, Matsuda S, Sudo S, Fujita H, Sakanaka M, Maeda N. Induction of resting microglia in culture medium devoid of glycine and serine. Glia. 1998;24:198–215. [PubMed] [Google Scholar]
- Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, De Zeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J Membrane Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M, Rozental R, Spray DC. A simple RT-PCR-based strategy for screening connexin identity. Braz J Med Biol Res. 1999;32:1029–1037. doi: 10.1590/s0100-879x1999000800014. [DOI] [PubMed] [Google Scholar]
- Wilms H, Wollmer MA, Sievers J. In vitro-staining specificity of the antibody 5-D-4 for microglia but not for monocytes and macrophages indicates that microglia are a unique subgroup of the myelomonocytic lineage. J Neuroimmun. 1999;98:89–95. doi: 10.1016/s0165-5728(99)00066-1. [DOI] [PubMed] [Google Scholar]