Abstract
The anterior pituitary gland (adenohypophysis) comprises anterior and intermediate lobes (the pars distalis and pars intermedia) arising from placodal ectoderm at the anterior neural ridge. Signaling molecules including SHH, FGF, WNT, BMP and Notch are involved in regulating primordial pituitary proliferation and lineage determination. However, morphogenic events and molecular mechanisms governing anterior and intermediate lobe specification are not clear. Pituitary expression of proopiomelanocortin (POMC), the common precursor for adrenocorticotropin (ACTH) of pars distalis corticotropes and α-melanocyte-stimulating hormone (α-MSH) of pars intermedia melanotropes, provides a unique marker for anterior and intermediate lobe morphogenesis. We performed time-lapse confocal microscopy lineage tracing in live zebrafish embryos expressing GFP driven by the pomc promoter and show distinct migration pathways of POMC cells destined to the anterior and intermediate lobes. Using morpholino oligonucleotides, we show that hypomorphic FGF3 down-regulation induces specific defects of pars intermedia POMC cells while pomc, growth hormone and prolactin expression remain intact in the pars distalis. This lineage-specific process is independent of the FGF3 effect on early pituitary specifying transcription factors as indicated by normal Lim3 and Pit1 expression in hypomorphic FGF3 morphants. These findings suggest that the FGF3 signal, in addition to its previously described role of regulating progenitor proliferation and survival, delineates the melanotrope and corticotrope lineage boundary, contributing to establishment of the pituitary pars distalis and pars intermedia.
Keywords: pituitary, proopiomelanocortin, zebrafish, fibroblast growth factors
INTRODUCTION
The adenohypophysis (anterior pituitary), comprising the pars distalis and pars intermedia (anterior and intermediate lobe), is derived from the ectodermal midline structure located immediately anterior to the neural plate, the anterior neural ridge (ANR), while the neurohypophysis (pars nervosa or posterior lobe) arises from the ventral diencephalic neuro-ectodermal infundibulum (Treier and Rosenfeld, 1996). In mammals, development of the anterior pituitary starts from the formation of Rathke’s pouch (RP), a midline dorsal invagination of oral cavity toward the presumptive hypothalamus and infundibulum. In response to multiple developmental signals, primordial cells of the ventromedial RP progress to form the anterior lobe containing corticotropes producing adrenocorticotropin (ACTH), thyrotropes producing thyrotropin (TSH), somatotropes producing growth hormone (GH), lactotropes producing prolactin (PRL), and gonadotropes producing the gonadotropins (LH, FSH). The dorsal RP region eventually becomes the intermediate lobe, containing melanotropes producing α-melanocyte-stimulating hormone (α-MSH).
In zebrafish, pituitary patterning and terminal differentiation initiate while progenitor cells are still organized in a horseshoe-like pattern as a placode within the ANR (Glasgow et al., 1997; Herzog et al., 2003; Kawamura et al., 2002; Liu et al., 2003; Sbrogna et al., 2003). The posteriorly apposed anterior-end of the neural plate forms the hypothalamus and infundibulum. During morphogenesis, adenophypophyseal primordia migrate caudally under the ventral forebrain and establish dorso-ventral connections within the infundibulum and neurohypophysis. Hormone-producing cells are eventually organized into three distinct domains along the anterior-posterior axis with corticotropes and lactotropes localizing in the rostral pars distalis (RPD), somatotropes, thyrotropes and gonadotropes in the proximal pars distalis (PPD), and melanotropes in the pars intermedia (PI) (Herzog et al., 2003).
Anterior lobe corticotropes and intermediate lobe-derived cells share the same proopiomelanocortin (POMC) prohormone with distinct posttranslational derivatives. Corticotropes secret ACTH acting on the adrenal cortex to stimulate cortisol synthesis and secretion. α-MSH is a potent melanotropin involved in inflammatory modulation, however the physiologic role of the pars intermedia is largely unexplained (Melmed, 2003). During human embryonic development, the pars distalis and pars intermedia show fully differentiated POMC cells at 12 weeks of gestation. Later, pars intermedia cells may migrate into the anterior lobe, or remain at the original anatomical site but give rise in later adult life to hyperplastic “basophil invasion” into the neurohypophysis (Horvath and Kovacs, 2003). The pars intermedia may play a role in the histogenesis of silent “corticotrope” adenomas that exhibit histologic, immunocytochemical and ultrastructural features similar to those of ACTH secreting corticotrope adenomas, but without excess ACTH secretion (Horvath and Kovacs, 2003; Scheithauer et al., 2000). In the murine pituitary, POMC cells differentiate in the ventromedial zone of the pars distalis on Day E12.5, and in the pars intermedia on Day E14.5 (Japon et al., 1994). As pituitary progenitors leave their niche to populate the anterior and intermediate lobes, corticotropes and melanotropes may specify and differentiate together, but afterwards localize into different lobes. Alternatively, POMC cell differentiation in the pars intermedia may occur subsequently to posterior migration of the pituitary anlage.
Early in organogenesis, several homeodomain transcription factors and dorsal-ventral signals of FGFs, BMPs, SHH and Wnts regulate pituitary cell fate determination, specification and proliferation (Burgess et al., 2002; Dasen and Rosenfeld, 1999; Raetzman et al., 2002; Scully and Rosenfeld, 2002; Ward et al., 2005). Ventral diencephalic FGF signaling opposes BMP signaling, promotes progenitor proliferation and prevents their exit from the cell cycle while maintaining a high level of LIM homeobox gene Lhx3 expression (Ericson et al., 1998; Ohuchi et al., 2000; Treier et al., 1998). Wnt signaling affects pituitary proliferation (Brinkmeier et al., 2003; Kioussi et al., 2002) and targets pituitary β-catenin, which interacts directly with the paired-like homeobox protein Prop1 to activate the POU-homeodomain transcription factor Pit1, a lineage-determining factor for somatotropes, lactotropes and thyrotropes, while repressing Hesx1 that favors cellular pluripotency (Kioussi et al., 2002; Olson et al., 2006). Pit1 lineage commitment also requires sustained endogenous Notch signaling, which activates Prop1 expression and prevents progenitor conversion to early-arising lineages such as corticotropes via its down-stream DNA-binding protein Rbp-J and transcriptional repressor Hes1 (Raetzman et al., 2006; Zhu et al., 2006). In the absence of Hes1 or Rbp-J activity, anterior lobe progenitors take on the corticotrope cell fate at the expense of Pit1+ lineages with up-regulation of the lineage-restricted T-box transcription factor Tpit/Tbx19 and basic helix-loop-helix (bHLH) factor NeuroD1, both required for POMC cell terminal differentiation (Drouin et al., 1998; Kita et al., 2007; Lamolet et al., 2004; Lamolet et al., 2001; Liu et al., 2001; Pulichino et al., 2003b; Suh et al., 2002; Tremblay et al., 1998; Zhu et al., 2006). On the other hand, Hes1 is required for melanotrope specification and dorsal RP progression into the intermediate lobe, a function likely independent of Notch signaling (Kita et al., 2007; Raetzman et al., 2007; Zhu et al., 2006). In the Prop1 deficient mouse pituitary, abundant POMC+ precursors are expressed throughout the expanded intermediate as well as anterior lobe. However, α-MSH is only expressed in intermediate lobe regions with direct infundibular contact, suggesting that ventral hypothalamic signals are required for hormone-secreting intermediate lobe cell differentiation (Ward et al., 2005). It remains unclear how POMC progenitors are fated to distinct lineages of the anterior versus intermediate lobes.
Hypothalamic FGF expression coincides with all stages of pituitary development promoting progenitor proliferation and survival (De Moerlooze et al., 2000; Ericson et al., 1998; Herzog et al., 2004; Norlin et al., 2000; Ohuchi et al., 2000; Treier et al., 1998). Targeted gene-knockout mice deficient in FGF10 (Ohuchi et al., 2000) or its high affinity receptor, FGF receptor 2 IIIb (FGFR2IIIb), exhibit initial RP formation with subsequent extensive apoptosis by e10 (De Moerlooze et al., 2000). Zebrafish ventral diencephalic FGF3, which also predominantly binds to the FGF receptor 2 IIIb isoform (Fgfr2IIIB)(Kiefer et al., 1996), plays a role similar to mouse FGF10 in progenitor proliferation and survival during initial pituitary commitment and patterning (Herzog et al., 2004). Furthermore, gain of function genetic studies and ex vivo organ culture experiments indicate the essential function of the dorso-ventral FGF gradient during adenohypophyseal patterning. FGF, integrating with BMP signaling, controls the onset of corticotrope terminal differentiation by regulating progenitor proliferation (Ericson et al., 1998; Norlin et al., 2000; Treier et al., 1998). However, mechanisms for FGF contribution to lineage-specific effect on intermediate lobe melanotropes are still elusive. We postulate that while FGF signal attenuation is essential for POMC precursor progression into anterior lobe corticotropes(Ericson et al., 1998; Norlin et al., 2000; Treier et al., 1998), sustained FGF signaling may be required for melanotrope terminal differentiation. Using in vivo lineage tracing by time-lapse confocal microscopy combined with morpholino oligonucleotide-mediated gene inactivation, we report the function of FGF3 during zebrafish POMC cell ontogeny, defining a genetic and developmental boundary between two distinct pituitary POMC lineages in a gene-dose-dependent fashion.
MATERIALS AND METHODS
Whole-mount RNA in situ hybridization, TUNEL assay, bromodeoxyuridine (BrdU) immunostain
Zebrafish POMC, PRL, GH, Pit1 and Lim3 antisense mRNA were generated as described (Liu et al., 2006). RNA in situ hybridizations of zebrafish whole-mount embryos were performed according to established protocols (Liu et al., 2003).
For detection of apoptosis, 1–2 cell stage POMC-GFP transgenic embryos were injected with either 0.4 ng/embryo of standard control MO or FGF3 MO then cultured in routine medium. At 36 hpf, the embryos were fixed overnight in 4% paraformaldehyde and rehydrated in PBS/methanol series (50%, 70%, 95% and 100%) followed by incubation in 100% acetone (−20 °C) for 10 minutes and three rinses in PBS containing 0.1% Tween-20. Cell death was then detected using a in situ Cell Death Detection Kit, TMR Red (Roche) at manufacture’s instructions.
For BrdU staining, embryos at 10-somite stage were placed in 10 mM BrdU (Sigma) in E3 medium and kept in dark at 28 °C until 36 hpf. Then embryos were fixed in 4% PFA for 2 hours at room temperature, and incubated in 1 N HCl for 1 hour. After washing in PBT, the embryos were incubated with 5% goat serum in PBT for 1 hour, then over night at 4 °C in 1:100 mouse anti-BrdU monoclonal antibody (Chemicon). The embryos were then washed in PBT followed by incubation over night at 4 °C in 1:100 Cy2 conjugated donkey anti-mouse antibody (Jackson Immunoresearch) and visualized under a fluorescein isothiocyanate (FITC) filter on a Zeiss microscope (Zeiss Axioplan-2).
Morpholino antisense microinjection
FGF3 and standard control morpholino oligonucleotides were purchased from Gene Tools, LLC (Philomath, Oregon). FGF3 MO sequence: 5’-CATTGTGGCATGGCGGGATGTCGGC (Maroon et al., 2002). For injection, morpholinos were diluted in 1 × Danieu’s buffer to a concentration of 1 mM. Each one- to two-cell stage embryo was injected with 1 nl at different doses of 0.4, 0.8 and 1.2 ng. For control group, 1.2 ng of standard control MO was injected to each embryo.
Fluorescent microscopy and confocal microscopy
Transgenic embryos were examined at various developmental stages under a fluorescein isothiocyanate (FITC) filter on a Zeiss microscope (Zeiss Axioplan-2). Live embryo images were generated with an Axiocam video system (Zeiss). For time-lapse confocal microscopy studies, 18-somite stage embryos were dechorionated and embedded in 0.8% low melting point agarose with 0.05% 3-aminobenzoic acid ethyl ester in E3 medium. To prevent drying, the embedded embryo was covered within a home made holding chamber maintained at 28 °C. The images were captured every 30 minutes for 20–24 hours using a Leica TCS SP confocal microscope (Leica Microsystems, Germany). GFP was detected at a spectral range from 507 to 550 nm and RFP from 585 to 690 nm.
Maintenance of zebrafish and SU5402 treatment
Embryos were obtained from natural spawning of wild type (AB) or homozygous POMC-GFP adult zebrafish and maintained as described (Kimmel et al., 1995; Liu et al., 2003). Embryo staging was carried out according to Kimmel (Kimmel et al., 1995). SU5402 (gift from Dr. Bo Zhang, Beijin University, China) was dissolved in DMSO at a stock concentration of 2 mM, diluted in E3 medium and added to live embryos at 22–26 hpf. After treatment, the embryos were washed and transferred to fresh E3 medium.
RESULTS
Temporally and spatially distinct ontogeny of POMC cells destined to the pars distalis and pars intermedia
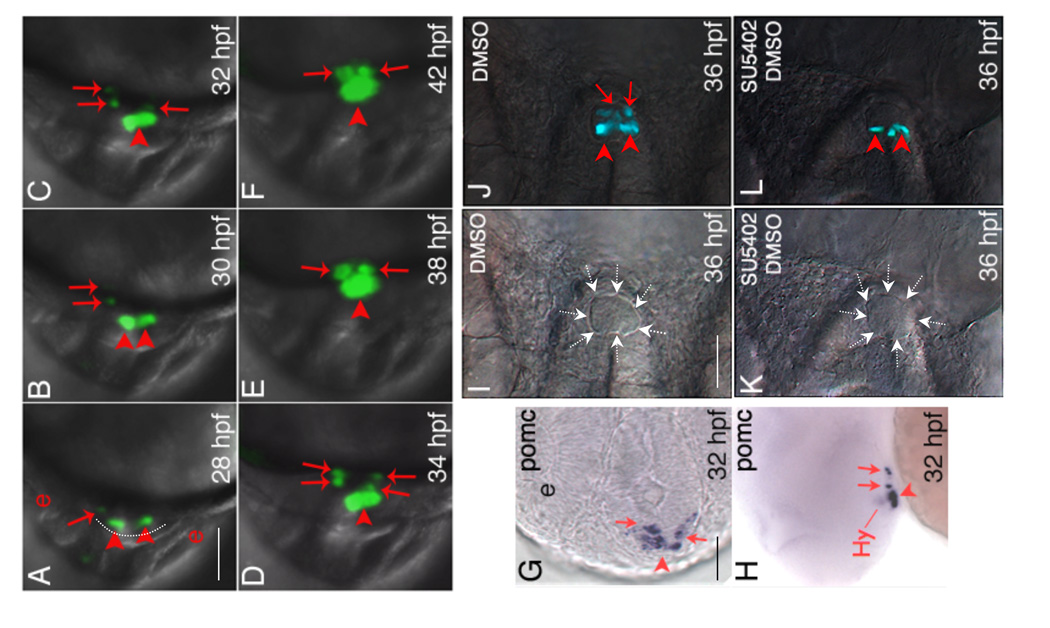
Little is known about how POMC cells are destined to become either anterior lobe corticotropes versus intermediate lobe melanotropes. It is also unclear whether POMC cells in the PD and PI specify and differentiate independently, or they migrate later into distinct adenohypophyseal regions. To investigate in vivo pituitary POMC lineage ontogeny, we generated germline transgenic zebrafish (POMC-GFP) expressing green fluorescent protein (GFP) driven by a 1006-bp POMC gene promoter corresponding to exon 1 and the first 22 bp of exon 2. Transgenic POMC-GFP expression recapitulates the pattern of endogenous pituitary Pomc expression from embryonic through adult stages (Liu et al., 2003). We carried out lineage tracing in live POMC-GFP transgenic embryos using time-lapse confocal microscopy analysis. The recorded video is depicted in Supplemental Information 1. At 28 hour-post-fertilization (hpf), POMC-GFP cells were detected within the most anterior neural ridge (ANR) area, bilateral to the midline adenohypophyseal placode (Supplemental Information 1 and Fig. 1A arrowheads). As the embryo develops, POMC cells also start to appear as asymmetric longitudinal groups from the lateral-posterior surface of the ANR, first on the left (Supplemental Information 1 and Fig. 1A–B arrows) and then on the right side of the placode (Supplemental Information 1 and Fig. 1C arrows). As a result of medial-posterior migration, POMC cells within the most anterior neural ridge are eventually destined to the RPD whereas the lateral-posterior POMC cells are located in the PI (Supplemental Information 1 and Fig. 1A–F). Taken together, the results demonstrate that zebrafish POMC cells in the RPD and the PI arise with distinct temporal and spatial patterns within the ANR, following lineage-specific migratory pathways before reaching a definitive pituitary destination.
Figure 1.
Temporally and spatially distinct POMC cell ontogeny in zebrafish pituitary. (A–F) In vivo time-lapse imaging of POMC-GFP expressing-cells in the pituitary anlage visualized using a Leica TCS SP confocal microscope from 28 to 42 hpf. Developmental stages are indicated at bottom right corner. POMC-GFP expressing cells are detected within the anterior most and lateral-posterior surface of ANR at 28 hpf (A), followed by asymmetric bilateral-posterior POMC cell differentiation and medio-posterior migration into the rostral pars distalis (RPD) and pars intermedia (PI). (G and H) in situ hybridization at 32 hpf using a POMC probe. Note the endogenous pituitary pomc expression pattern is similar to that of POMC-GFP transgene. (I–L) Treatment of POMC-GFP transgenic embryo with 10 µM SU5402 or DMSO between 22–26 hpf leads to diminished POMC-GFP cells in PI by 36 hpf visualized under a fluorescein isothiocyanate (FITC) filter on a Zeiss microscope. I and K, DIC images showing pituitary anlage outlined by dashed arrows. J and L, POMC-GFP fluorescent images superimposed onto DIC images. A–F and I–L: ventral view; G: ventral-frontal view; H: ventrolateral view (top, dorsal; bottom, ventral). All: left, anterior; right, posterior. Arrowhead: anterior-most or RPD POMC cells; arrow: lateral-posterior or PI POMC cells; Hy: hypothalamic POMC expressing cells. The anterior-most neural ridge surface is traced as a reference point in (A). e, eye. Scale bars, 50 µm.
Differential sensitivity of PI and PD POMC cells to loss of FGF3 signaling
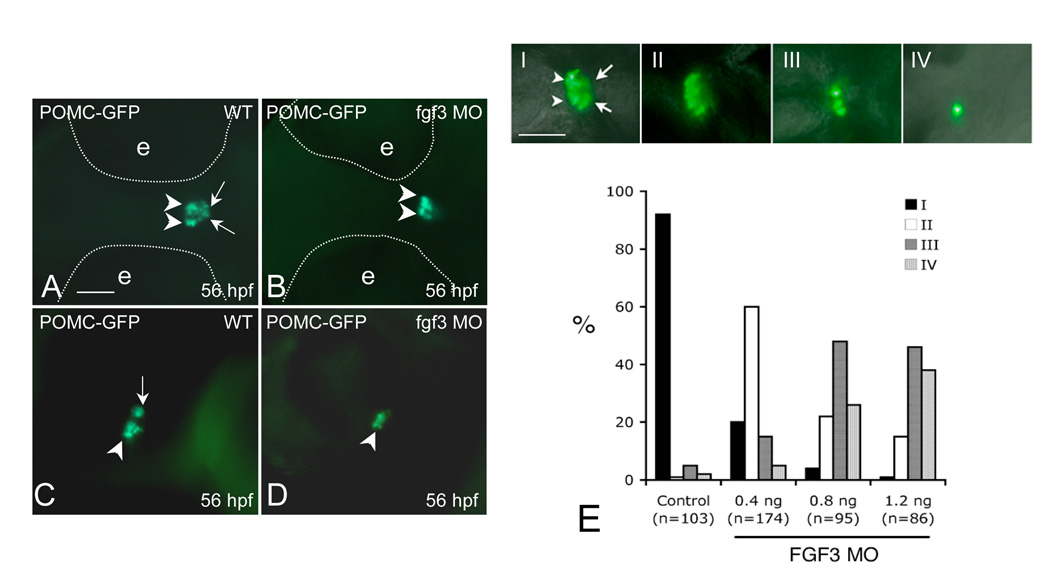
During zebrafish forebrain development, diencephalic FGF3 signaling is required for early specification of the adenohypophyseal anlage, which is in close contact with the presumptive infundibulum (Herzog et al., 2004). From early to late somite stages, FGF3 expression within the dorsal telencephalon and ventral diencephalon coincides with pituitary development from early specification to terminal differentiation (Walshe and Mason, 2003). Receptor binding experiments indicate that zebrafish FGF3 predominantly binds the FGF receptor 2 IIIb isoform (Fgfr2IIIB) (Kiefer et al., 1996). Starting from early somatogenesis, zebrafish Fgfr2 is diffusely expressed in the central nervous system including ventral telencephalon, anterior/ventral diencephalon, midbrain and hindbrain. This expression pattern is essentially unchanged until at least 48 hpf (Tonou-Fujimori et al., 2002). Due to further cell migration during brain development, anterior-most adenohypophyseal progenitor cells within the ANR (giving rise to PD) are subsequently distanced from the diencephalons while lateral-posterior cells (giving rise to PI) continue to be in close contact with the infundibular FGF3 signal (Herzog et al., 2004). This is similar to development patterns of the mouse embryonic pituitary: as pluoripotent progenitors leave their niche from the dorsal-most RP and grow ventromedially away from the ventral hypothalamus to populate the anterior lobe (or PD), cells proliferating into the dorsal RP to form the intermediate lobe (or PI) have continuous direct infundibular contact. Gain of function studies suggest that FGF signal attenuation in anterior lobe POMC precursors is required for corticotrope terminal differentiation. However, these studies did not address FGF effects on intermediate lobe melanotrope ontogeny (Ericson et al., 1998; Norlin et al., 2000; Treier et al., 1998). We postulate that different FGF3 requirements of the anterior-most and lateral-posterior ANR pituitary precursors may contribute to establishing distinct POMC cell lineages in zebrafish RPD and PI. The potential role of FGF3 in RPD and PI POMC cell specification and differentiation may have been masked in Fgf3 null mutants due to early adenohypophyseal progenitor apoptosis (Herzog et al., 2004). To test this hypothesis, we treated POMC-GFP transgenic embryos with the FGF receptor inhibitor SU5402 between 22 to 26 hpf to transiently block FGF signaling. At 36 hpf, SU5402-treated embryos show diminished PI POMC-GFP cells while adenohypophyseal morphology appears normal (Fig.1 I–L). Overall, 105 of 132 embryos treated with SU5402 presented with diminished PI POMC-GFP cells, while 10 of 87 embryos treated with vehicle show abnormal POMC-GFP phenotype (data not shown). Furthermore, we performed antisense morpholino oligonucleotide (MOs) mediated gene knockdown to investigate FGF3 effects on POMC cell ontogeny in distinct parts of the adenohypophysis. One to two cell stage POMC-GFP transgenic embryos were injected with FGF3 MOs, which have been previously established to specifically inhibit FGF3 protein translation and cause hypomorphic reductions in FGF3 activity (Maroon et al., 2002). At 24 hpf, embryos (morphants) injected with 0.4 ng/embryo FGF3 MO exhibit mild craniofacial and posterior axis defects but normal POMC-GFP expression within the anterior-most ANR (data not shown). By 56 hpf, FGF3 morphants show either diminished PI POMC-GFP expression with intact RPD POMC-GFP expression, or diminished POMC-GFP in both RPD and PI (Fig. 2A–D and E, top panels). For further quantification, FGF3 morphants were classified into four groups according to the severity of POMC-GFP defects (Fig. 2E). When injected with 0.4 ng/embryo, 60% of FGF3 morphants show diminished POMC-GFP expression only in PI. Further dose increase of FGF3 MO injection to 1.2 ng/embryo leads to further POMC-GFP suppression in both RPD and PI (Fig. 2E). These results suggest that FGF3 exhibits a dose-dependent effect on distinct POMC lineages with PI POMC cells being more susceptible to altered FGF3 signals than those of the RPD.
Figure 2.
FGF3 dose-dependent regulation of POMC expression in the RPD and PI of zebrafish pituitary. 1–2 cell stage POMC-GFP transgenic embryos were injected with vehicle (A and C) or 0.4 ng/embryo of FGF3 MO (B and D) and then cultured in routine medium. At 56 hpf, POMC-GFP expression patterns were analyzed using fluorescent microscopy. For quantification, POMC-GFP transgenic embryos injected with 1 nL of FGF3 MO at increasing concentrations or 1.2 ng/embryo standard control MO were characterized under fluorescent microscopy and devided into 4 subgroups (E, I–IV) according to pituitary POMC-GFP phenotypes: Phenotype I, normal POMC-GFP expression in both the rostral pars distalis (arrowheads) and pars intermedia (arrow) ; Phenotype II, loss of POMC-GFP expression in the pars intermedia; Phenotype III, loss of POMC-GFP expression in the pars intermedia and partial loss in the rostral pars distalis (< 50% loss in pars distalis compared to I); Phenotype IV, loss of POMC-GFP expression in pars intermedia and rostral pars distalis (> 50% loss in pars distalis compared to I). Frequencies (%) of each phenotype corresponding to injections of standard control MO (1.2 ng) or FGF3 MO (0.4 ng, 0.8 ng or 1.2 ng) are analyzed and shown in (E). Data are collected from two independent experiments. n, number of analyzed embryos. A–D: ventral view. left, anterior; right, posterior. Scale bar, 50 µm.
Low dose FGF3 morphants with diminished POMC cells in the pars intermedia display intact Pit-1, Gh and Prl expression
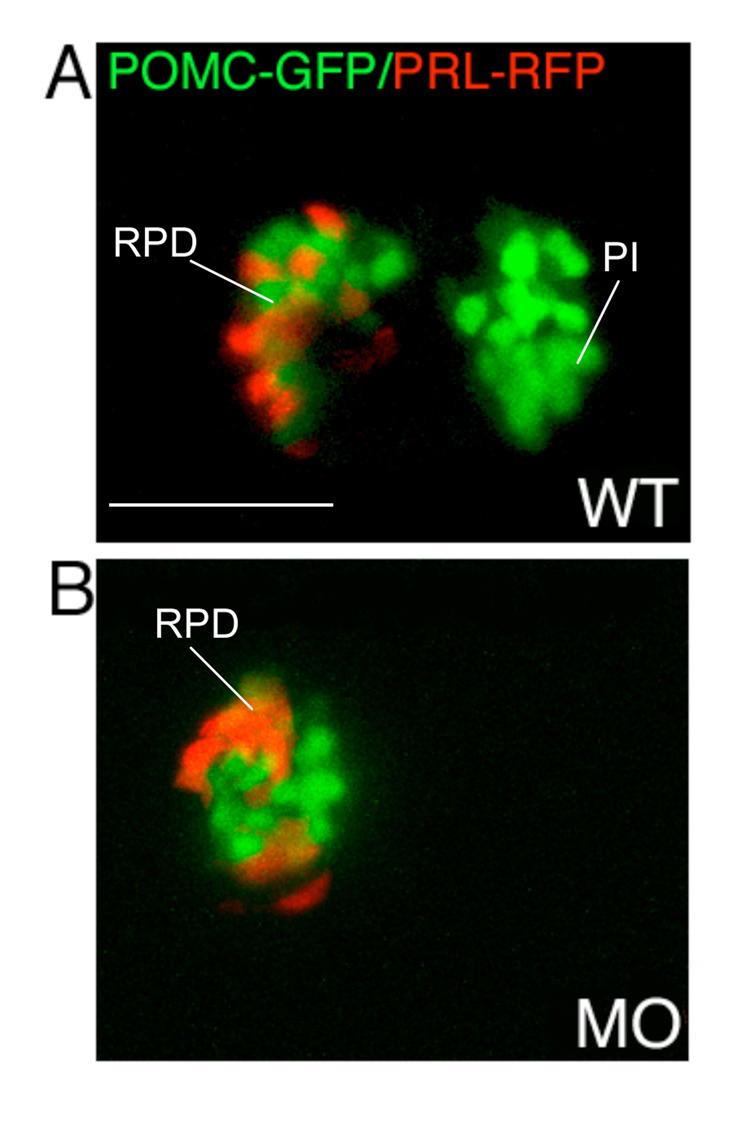
To further determine whether diminished POMC cells in response to hypomorphic FGF3 gene knockdown represent lineage specific or general suppression involving other pituitary cell types, we carried out whole mount in situ analyses of lineage specific hormone expression targeting Pit1-dependent lactotropes and somatotropes (Fig. 3). Low dose FGF3 morphants with RPD POMC-GFP expression, but absent PI expression exhibit intact Pit1, Prl, and Gh expression at 56 hpf (Fig. 3E–G), indicating that other cell lineages in the pars distalis are not affected by low dose FGF3 knock down. This was further confirmed in vivo by injecting 0.4 ng/embryo of FGF3 morpholino into germline double transgenic zebrafish embryos expressing GFP targeted to POMC cells and RFP targeted to lactotropes by the Prl promotor (Liu et al., 2006). The hypomorphic FGF3 attenuation leads to down-regulation of PI POMC-GFP without affecting POMC-GFP and PRL-RFP in the RPD (Fig. 4). Therefore, the zebrafish FGF3 signal selectively regulates POMC expression in the RPD and PI, independently of lactotrope and somatotrope lineages.
Figure 3.
Expression of Prl, Gh, and Pit-1 in FGF3 morphants are intact. In situ hybridization at 56 hpf using probes indicated in the lower right corner of each panel. (A–D) wild type embryos. (E–H) Low dose FGF3 morphants with diminished POMC-GFP expression in the pars intermedia. (I–L) High dose FGF3 morphants with diminished POMC-GFP expression in the pars intermedia and rostral pars distalis. Ventral view of head, anterior to the left. Arrowheads, RPD. Arrow, PI. P, pituitary; Hy, hypothalamic POMC cells. Scale bar, 50 µm.
Figure 4.
Lineage specific suppression of PI POMC cells in FGF3 morphants. 1–2 cell stage POMC-GFP/PRL-RFP double transgenic embryos were either injected with 0.4 ng of FGF3 MO (B) or vehicle (A) and then cultured in routine medium. At 56 hpf, pituitary POMC-GFP/PRL-RFP expression patterns were analyzed in live embryos using confocal microscopy. In FGF3 morphant, POMC-GFP expressing cells are detected within the rostral pars distalis but not in the pars intermedia, whereas PRL-RFP expression is intact. Ventral pituitary view under high magnification (63x), anterior to the left. WT, wild type; MO, FGF3 morphant. RPD, rostral pars distalis; PI, pars intermedia. Scale bar, 50 µm.
Further suppression of FGF3 activity with higher MO doses causes decreased Pit1, Prl and Gh expression, in addition to diminished POMC cells in both RPD and PI (Fig. 3I–K), a phenotype consistent with that of the Fgf3 null mutation affecting all pituitary lineages due to failed progression of progenitors beyond the initial anlage (Herzog et al., 2004). It is, however, unclear whether higher doses of FGF3 MO down-regulate RPD POMC expression independently of effects on general pituitary progenitors.
Selectively diminished POMC cells in the pars intermedia of low dose FGF3 morphants are not due to post-differentiation disruption
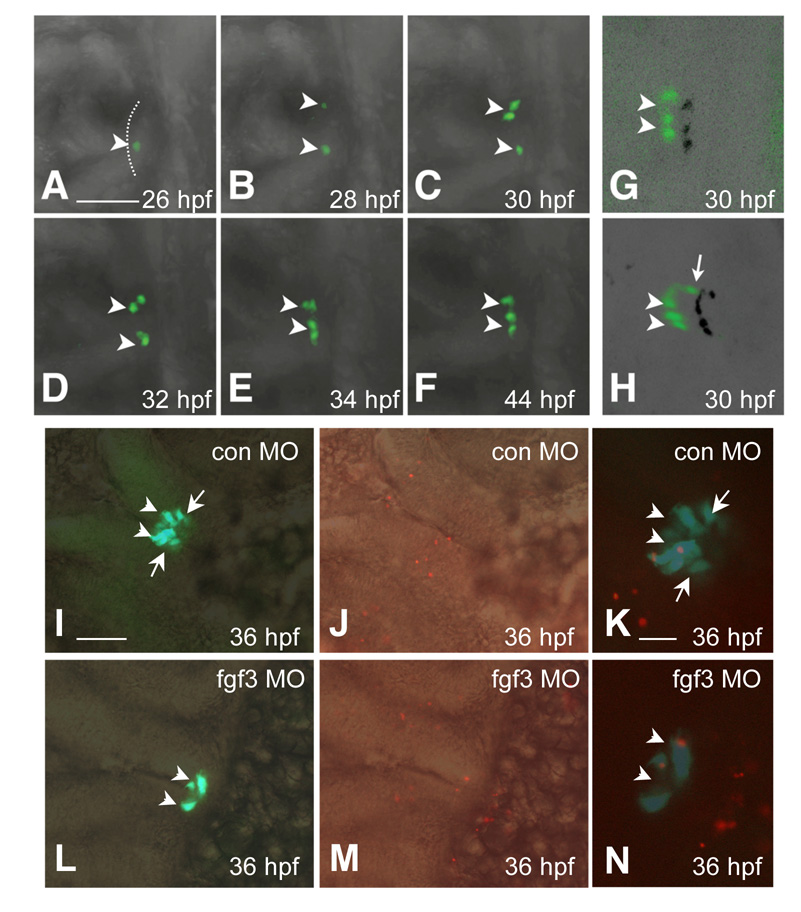
Defective expression of PI POMC with low dose FGF3 morphants may indicate disruption of patterning, lineage specification, terminal differentiation, or post-differentiation events involving proliferation and/or survival. To date, lineage-specific transcription factors required for POMC cell differentiation such as Tpit/Tbx19 and NeuroD1 have not been identified in the zebrafish pituitary. Pituitary terminal differentiation, as evidenced by lineage specific hormone expression, starts while progenitor cells are still organized as a placode within the ANR (Herzog et al., 2003; Liu et al., 2003; Sbrogna et al., 2003). To monitor in vivo effects of FGF3 MO on RPD and PI POMC cell ontogeny, we performed time-lapse confocal microscopic tracing of GFP positive cells in germline POMC-GFP transgenic embryos injected with 0.4 ng/embryo FGF3 MO. Initially at 26 hpf, cells within the anterior-most surface of the ANR exhibit intact GFP expression while undergoing lateral-medial migration (Fig. 5A–F, arrowhead). During the subsequent 18 hours, however, GFP cell initiation was not detected within the lateral-posterior ANR area, as the anterior-most cells continue to move medio-posterior into the head (compare Fig. 1A–F and Fig. 5A–F, G and H, Supplemental Information 1 and 2).
Figure 5.
Loss of POMC cells in the pars intermedia of FGF3 morphants is associated with absent lineage induction. Developmental stages are indicated at bottom right corner. (A–F) In vivo time-lapse imaging of POMC-GFP expressing-cells in the pituitary anlage of an FGF3 morphant from 26 to 44 hpf. POMC-GFP expressing cells are detected within the anterior-most ANR surface but not the lateral-posterior areas during the entire process. (G and H) 3-D confocal microscopy image of POMC-GFP expressing-cells with shadow projections (black signals). The 3-D image further confirms lack of lateral-posterior POMC cells in the FGF3 morphant (G) compared with wild type embryos (H) at 30 hpf. (I–N) POMC-GFP cell apoptosis is unaffected in hypomorphic FGF3 morphants. DIC images of pituitary ventral views superimposed with fluorescent images of POMC-GFP (I and L), or TMR Red labeling of apoptotic cells in red (J and M). Fluorescent images of POMC-GFP superimposed with TMR Red cell labeling in red show occasional pituitary apoptotic cells in embryos injected with standard control MO (K) or FGF3 MO (N). Scale bar (A–J, L, M), 50 µm. K and N, 20 µm. All ventral-frontal view; left, anterior; arrowhead: anterior-most POMC cells in ANR; arrow: lateral-posterior POMC cells. The anterior-most neural ridge surface is traced as a reference point in (A).
To further investigate mechanisms for diminished PI POMC-GFP, whole mount TUNEL assay was performed to detect apoptotic cells. At 36 hpf, few pituitary cells undergo apoptosis and no detectable differences were observed in embryos with intact POMC-GFP (from control MO injection) or diminished PI POMC-GFP (from 0.4 ng/embryo FGF3 MO injection) (Fig. 5 I–N). Similarly, as pituitary cells replicate slowly after initial specification(Chesnokova et al., 2007), BrdU incorporation rate was undistinguishable in both control and FGF3 morphants (Supplemental Information 3). These results suggest that hypomorphic FGF3 signaling disruption leads to failed onset of POMC cells within the lateral-posterior region of ANR that were destined to the PI, rather than post-differentiation disruption.
Hypomorphic FGF3 morphants with diminished PI POMC-GFP exhibit intact Lim3 expression
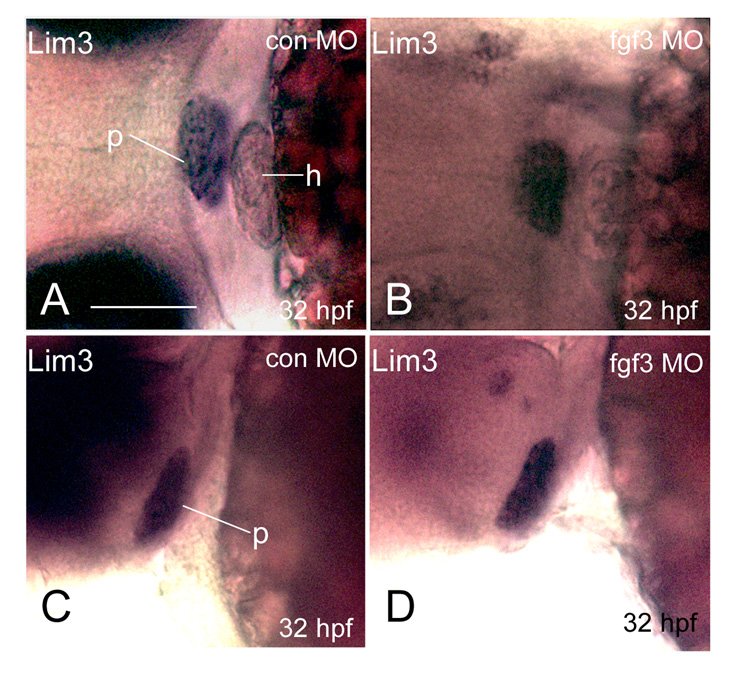
As FGF is required for maintaining adequate levels of Lim3/Lhx3 expression essential for pituitary progenitor proliferation and survival (Ericson et al., 1998; Ohuchi et al., 2000; Treier et al., 1998), the diminished PI POMC-GFP expression in FGF3 morphants may result from suppressed Lim3/Lhx3 expression leading to loss of POMC progenitors. We therefore performed whole mount mRNA in situ hybridization analysis of Lim3/Lhx3 expression. In FGF3 morphants with diminished lateral-posterior POMC-GFP only (phenotype II in Fig. 2E), pituitary Lim3 expression remains intact at 32 hpf (Fig. 6), suggesting that hypomorphic FGF3 reduction selectively suppresses PI POMC cells via Lim3 independent mechanisms. Increasing the FGF3 MO injection dose to 1.2 ng/embryo eventually leads to significant loss of Lim3 expression, similar to that of fgf3 null mutants ((Herzog et al., 2004) and data not shown). As Lim3 is one of the earliest pituitary specifying transcription factors, intact lim3 expression at 32 hpf suggest preserved progenitor cells in hypomorphic FGF3 morphants. Therefore, higher FGF3 levels are required for PI POMC cell specification and/or differentiation than early specification of the general pituitary progenitors.
Figure 6.
Hypomorphic FGF3 morphants exhibit intact Lim3 expression at 32 hpf. 1–2 cell stage POMC-GFP transgenic embryos were injected with either 0.4 ng/embryo standard control MO (E and G) or FGF3 MO (F and H) and then cultured in routine medium. At 32 hpf, Lim3 in situ hybridization was performed. E and F: frontal view; left, top. G, H: lateral view; left, anterior; right, posterior. p, pituitary; h, heart. Scale bar, 50 µm.
DISCUSSION
Pars distalis (PD) and pars intermedia (PI) POMC cells are closely associated as indicated by their common POMC prohormone expression that is a hallmark of corticotrope and melanotrope lineage terminal differentiation. The current study depicts in vivo temporal and spatial pathways of POMC lineage ontogeny within the zebrafish pars distalis and pars intermedia. Importantly, the distinct lineage boundary is clearly established while the pituitary placode is still organized within the ANR, and is well maintained throughout organogenesis.
While analysis of FGF or FGF receptor null embryos generated by gene targeting or chemical mutagenesis have revealed important FGF functions for pituitary progenitor specification, proliferation and survival, abundant embryonic pituitary apoptosis prevents further study of FGF roles in pituitary lineage specification and differentiation (De Moerlooze et al., 2000; Herzog et al., 2004; Ohuchi et al., 2000). Gain of function studies suggest a biphasic dependence of anterior lobe POMC cell differentiation on FGF signaling: an early phase which requires the FGF signal to establish a corticotrope progenitor state, and a late phase which requires attenuation of FGF activity as anterior lobe cells grow beyond ventral hypothalamic signaling (Ericson et al., 1998; Norlin et al., 2000; Treier et al., 1998). However, it remains to be determined whether FGF signaling is required throughout the specification and differentiation of intermediate lobe POMC cells, which have a closer infundibular contact than cells of the anterior lobe.
In the current study, morpholino antisense-mediated FGF3 gene knockdown in zebrafish causes hypomorphically reduced FGF3 activity, which is inadequate for pars intermedia POMC cell ontogeny, but sufficient for maintaining early pituitary progenitors as indicated by intact Lim3 and Pit1 expression. Our study reveals a dose-dependent FGF3 effect on distinct POMC lineages with PI POMC cells being more susceptible to reduced FGF3 signals than those of the RPD. The relative FGF3-indepence of RPD POMC-GFP expression is consistent with previous findings in murine pituitary where progression to definitive corticotropes occurs in absence of FGF signaling (Ericson et al., 1998).
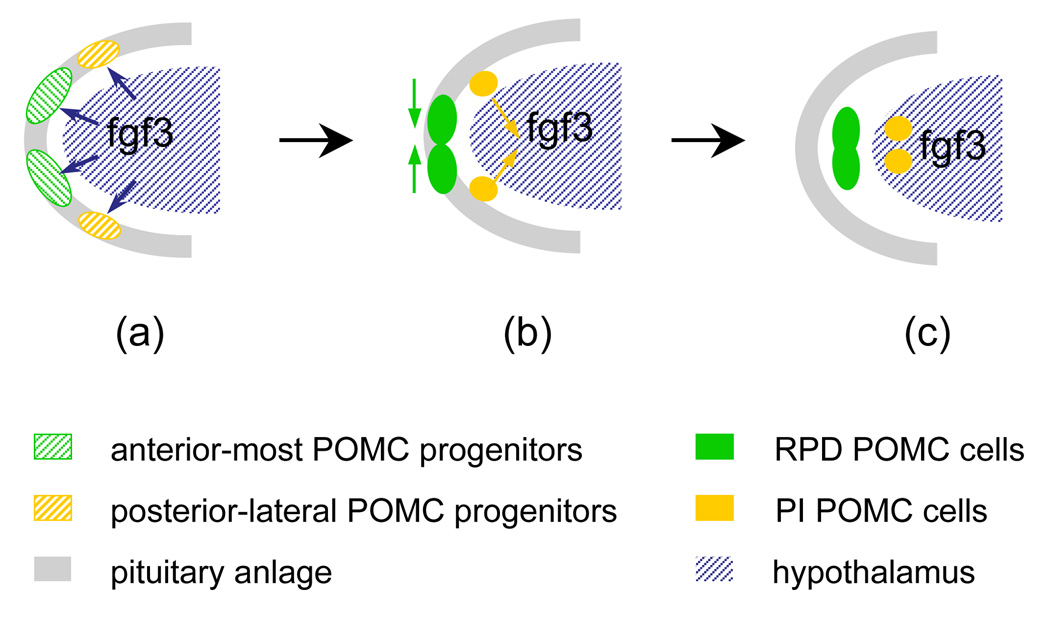
Integrating the previously published results and our current study, we propose a model of POMC cell migrating pathways in relation to diencephalic FGF3 expression during pituitary ontogeny. We propose a requirement for the FGF3 signal in establishment of distinct POMC cell lineages in RPD and PI (Fig. 7). During early stages of pituitary specification, the zebrafish adenohypophyseal anlage within the ANR is equally exposured to diencephalic FGF3 signals, ensuring overall pituitary anlage proliferation and survival (Herzog et al., 2004). Diencephalic FGF signals are required for establishing the POMC lineage progenitor state (Ericson et al., 1998; Herzog et al., 2004; Norlin et al., 2000; Treier et al., 1998). During further development, anterior-most POMC progenitor cells within the ANR (giving rise to RPD) undergo predominantly ventromedial migration and are therefore distanced from the dorsal-posterior migrating diencephalon. Lateral-posterior POMC cells (giving rise to PI) continue to be in closer association with the diencephalic (presumptive infundibular) FGF3 signal via rapid medial-ventral-posterior migration ((Herzog et al., 2004), Fig.1, 2, supplemental information 1 and 2). As a result, FGF signal attenuation allows PD corticotrope differentiation, while sustained FGF signaling is required for lateral-posterior POMC cell progression into PI melanotropes.
Figure 7.
Model depicting the role of FGF3 in regulating pituitary proopiomelanocortin lineage. At early stages of pituitary specification, the zebrafish adenohypophyseal anlage within the ANR experience equal exposure to diencephalic FGF3 signals, ensuring overall pituitary anlage proliferation and survival. Diencephalic FGF signals are required for establishing the POMC lineage progenitor state (a). During further development, anterior-most POMC progenitor cells within the ANR (giving rise to RPD) undergo predominantly ventromedial migration and are therefore distanced from the dorsal-posterior migrating diencephalon. Lateral-posterior POMC cells (giving rise to PI) continue to be in closer association with diencephalic (presumptive infundibular) FGF3 signal via rapid medial-ventral-posterior migration (b). As a result, FGF signal attenuation allows POMC precursor progression into PD corticotropes, similar to murine corticotrope development. Sustained FGF signaling in lateral-posterior POMC cells is required for PI melanotrope terminal differentiation (c). Different FGF3 requirements of anterior-most and lateral-posterior ANR POMC precursors contribute to establishment of distinct POMC RPD and PI cell lineages.
Several possible mechanisms may underline selective PI POMC cell suppression in response to FGF3 hypomorphic down-regulation. Due to the lack of zebrafish POMC lineage-specific makers other than POMC itself, it is still uncertain whether RPD and PI POMC cells share equivalent or distinct progenitors prior to terminal differentiation. Our data support distinct RPD and PI POMC progenitor populations as indicated by the temporally and spatially distinguishable onset of POMC cells with the ANR, although precursor cells may derive from each other. FGF3 may promote both RPD and PI POMC progenitor expansion. While RPD cells are distanced from the ventral hypothalamus and differentiate into POMC+ cells, PI progenitors may require a sustained FGF3 signal for further proliferation before emerging as definitive melanotropes. Alternatively, FGF3 may only be required for PI melanotrope terminal differentiation. Ultimately, different susceptibility to FGF3 signaling delineates two POMC lineages into distinct anterior and intermediate lobe destinations. However, it remains to be determined whether the FGF3 signal acts directly or indirectly on POMC cells.
Molecular and cellular mechanisms underlying FGF3 differential regulation of POMC lineages are beyond the scope of this manuscript. Several transcription factors and soluble factors that control pituitary POMC cell development have been identified (Bousquet et al., 2000; Chesnokova and Melmed, 2002; Lamolet et al., 2004; Lamolet et al., 2001; Melmed, 2001; Poulin et al., 1997; Pulichino et al., 2003a; Pulichino et al., 2003b). However, few studies have associated transcriptional factors with signaling molecules during specification, patterning and differentiation of hormone producing cells (Kioussi et al., 2002; Olson et al., 2006; Raetzman et al., 2004). During vertebrate axis formation, FGF signaling regulates anterior-posterior patterning by targeting the downstream mesoderm T-box transcription factors Spadetail/tbx16 (Spt) and Brachyury (Ntl for no tail in zebrafish) both essential for posterior structural (spinal cord, somites) development (Amaya et al., 1993; Griffin et al., 1998; Isaacs et al., 1994; Schulte-Merker and Smith, 1995). Spt and Ntl also act upstream of FGF via a positive auto-regulatory loop, interacting with other signaling molecule(s) to form complex genetic networks governing the posterior mesoderm formation (Griffin and Kimelman, 2003). In the ectodermal adenohypophysis, Tpit/Tbx19 is a T box transcription factor closely related to Brachyury and specifically expressed in POMC cells. The Tpit/Tbx19 is required for POMC lineage differentiation but not for lineage commitment (Lamolet et al., 2001; Liu et al., 2001; Pulichino et al., 2003b). Recent studies indicate that sustained endogenous Notch signaling suppress early corticotrope differentiation, whereas Nortch target gene Hes1 is required for intermediate lobe and melanotrope specification (Kita et al., 2007; Raetzman et al., 2007; Zhu et al., 2006). In neuronal and pancreatic cells, FGF and Notch signaling interact to integrate cell growth and terminal differentiation (Akai et al., 2005; Faux et al., 2001; Norgaard et al., 2003). It will be interesting to determine whether Tpit/Tbx19 and Notch/Hes signals are involved in selective FGF signaling on POMC lineages.
Supplementary Material
Acknowledgments
This work was supported by NIH grants KO8 DK 064806 (N.L.), CA75979 (S.M.), RR13227 (S.L.), and the Doris Factor Molecular Endocrinology Laboratory.
Abbreviations
- GFP
green fluorescent protein
- POMC
pro- opiomelanocortin
- MO
morpholino
- FGF
fibroblast growth factors
- RPD
rostral pars distalis
- PPD
proximal pars distalis
- PI
pars intermedia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akai J, Halley PA, Storey KG. FGF-dependent Notch signaling maintains the spinal cord stem zone. Genes Dev. 2005;19:2877–2887. doi: 10.1101/gad.357705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. doi: 10.1242/dev.118.2.477. [DOI] [PubMed] [Google Scholar]
- Bousquet C, Zatelli MC, Melmed S. Direct regulation of pituitary proopiomelanocortin by STAT3 provides a novel mechanism for immuno-neuroendocrine interfacing. J Clin Invest. 2000;106:1417–1425. doi: 10.1172/JCI11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Potok MA, Cha KB, Gridley T, Stifani S, Meeldijk J, Clevers H, Camper SA. TCF and Groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17:2152–2161. doi: 10.1210/me.2003-0225. [DOI] [PubMed] [Google Scholar]
- Burgess R, Lunyak V, Rosenfeld M. Signaling and transcriptional control of pituitary development. Current Opinion in Genetics & Development. 2002;12:534–539. doi: 10.1016/s0959-437x(02)00337-4. [DOI] [PubMed] [Google Scholar]
- Chesnokova V, Melmed S. Minireview: Neuro-immuno-endocrine modulation of the hypothalamic-pituitary-adrenal (HPA) axis by gp130 signaling molecules. Endocrinology. 2002;143:1571–1574. doi: 10.1210/endo.143.5.8861. [DOI] [PubMed] [Google Scholar]
- Chesnokova V, Zonis S, Rubinek T, Yu R, Ben-Shlomo A, Kovacs K, Wawrowsky K, Melmed S. Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res. 2007;67:10564–10572. doi: 10.1158/0008-5472.CAN-07-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, Rosenfeld MG. Signaling mechanisms in pituitary morphogenesis and cell fate determination. Curr Opin Cell Biol. 1999;11:669–677. doi: 10.1016/s0955-0674(99)00034-4. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Drouin J, Lamolet B, Lamonerie T, Lanctot C, Tremblay JJ. The PTX family of homeodomain transcription factors during pituitary developments. Mol Cell Endocrinol. 1998;140:31–36. doi: 10.1016/s0303-7207(98)00026-4. [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- Faux CH, Turnley AM, Epa R, Cappai R, Bartlett PF. Interactions between fibroblast growth factors and Notch regulate neuronal differentiation. J Neurosci. 2001;21:5587–5596. doi: 10.1523/JNEUROSCI.21-15-05587.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow E, Karavanov AA, Dawid IB. Neuronal and neuroendocrine expression of lim3, a LIM class homeobox gene, is altered in mutant zebrafish with axial signaling defects. Dev Biol. 1997;192:405–419. doi: 10.1006/dbio.1997.8761. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development. 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kimelman D. Interplay between FGF, one-eyed pinhead, and T-box transcription factors during zebrafish posterior development. Dev Biol. 2003;264:456–466. doi: 10.1016/j.ydbio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Herzog W, Sonntag C, von der Hardt S, Roehl HH, Varga ZM, Hammerschmidt M. Fgf3 signaling from the ventral diencephalon is required for early specification and subsequent survival of the zebrafish adenohypophysis. Development. 2004;131:3681–3692. doi: 10.1242/dev.01235. [DOI] [PubMed] [Google Scholar]
- Herzog W, Zeng X, Lele Z, Sonntag C, Ting JW, Chang CY, Hammerschmidt M. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Developmental Biology. 2003;254:36–49. doi: 10.1016/s0012-1606(02)00124-0. [DOI] [PubMed] [Google Scholar]
- Horvath E, Kovacs K. Lost and found: The pars intermedia of the human pituitary and its role in the histogenesis of silent "corticotroph" adenomas. In: Gaillard R, editor. The ACTH axis. Kluwer Academic Publishers; 2003. [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. Embo J. 1994;13:4469–4481. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Kouki T, Kawahara G, Kikuyama S. Hypophyseal development in vertebrates from amphibians to mammals. Gen Comp Endocrinol. 2002;126:130–135. doi: 10.1006/gcen.2002.7784. [DOI] [PubMed] [Google Scholar]
- Kiefer P, Mathieu M, Mason I, Dickson C. Secretion and mitogenic activity of zebrafish FGF3 reveal intermediate properties relative to mouse and Xenopus homologues. Oncogene. 1996;12:1503–1511. [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin ⤍ Pitx2 pathway mediating cell-typespecific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification and posterior lobe formation in the pituitary development. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0039. [DOI] [PubMed] [Google Scholar]
- Lamolet B, Poulin G, Chu K, Guillemot F, Tsai MJ, Drouin J. Tpit-independent function of NeuroD1(BETA2) in pituitary corticotroph differentiation. Mol Endocrinol. 2004;18:995–1003. doi: 10.1210/me.2003-0127. [DOI] [PubMed] [Google Scholar]
- Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin C, Gleiberman A, Ohgi KA, Herman T, Huang HP, Tsai MJ, Rosenfeld MG. Tbx19, a tissue-selective regulator of POMC gene expression. Proc Natl Acad Sci U S A. 2001;98:8674–8679. doi: 10.1073/pnas.141234898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NA, Huang H, Yang Z, Herzog W, Hammerschmidt M, Lin S, Melmed S. Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Molecular Endocrinology. 2003;17:959–966. doi: 10.1210/me.2002-0392. [DOI] [PubMed] [Google Scholar]
- Liu NA, Liu Q, Wawrowsky K, Yang Z, Lin S, Melmed S. Prolactin receptor signaling mediates the osmotic response of embryonic zebrafish lactotrophs. Mol Endocrinol. 2006;20:871–880. doi: 10.1210/me.2005-0403. [DOI] [PubMed] [Google Scholar]
- Maroon H, Walshe J, Mahmood R, Kiefer P, Dickson C, Mason I. Fgf3 and Fgf8 are required together for formation of the otic placode and vesicle. Development. 2002;129:2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- Melmed S. Series introduction. The immuno-neuroendocrine interface. J Clin Invest. 2001;108:1563–1566. doi: 10.1172/JCI14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest. 2003;112:1603–1618. doi: 10.1172/JCI20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgaard GA, Jensen JN, Jensen J. FGF10 signaling maintains the pancreatic progenitor cell state revealing a novel role of Notch in organ development. Dev Biol. 2003;264:323–338. doi: 10.1016/j.ydbio.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Norlin S, Nordstrom U, Edlund T. Fibroblast growth factor signaling is required for the proliferation and patterning of progenitor cells in the developing anterior pituitary. Mechanisms of Development. 2000;96:175–182. doi: 10.1016/s0925-4773(00)00393-2. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochemical & Biophysical Research Communications. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Poulin G, Turgeon B, Drouin J. NeuroD1/beta2 contributes to cell-specific transcription of the proopiomelanocortin gene. Mol Cell Biol. 1997;17:6673–6682. doi: 10.1128/mcb.17.11.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulichino AM, Vallette-Kasic S, Couture C, Gauthier Y, Brue T, David M, Malpuech G, Deal C, Van Vliet G, De Vroede M, Riepe FG, Partsch CJ, Sippell WG, Berberoglu M, Atasay B, Drouin J. Human and mouse TPIT gene mutations cause early onset pituitary ACTH deficiency. Genes & Development. 2003a;17:711–716. doi: 10.1101/gad.1065603. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. Tpit determines alternate fates during pituitary cell differentiation. Genes & Development. 2003b;17:738–747. doi: 10.1101/gad.1065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Developmental Biology. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Ward R, Camper SA. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–4239. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006 doi: 10.1210/me.2005-0394. [DOI] [PubMed] [Google Scholar]
- Sbrogna JL, Barresi MJ, Karlstrom RO. Multiple roles for Hedgehog signaling in zebrafish pituitary development. Developmental Biology. 2003;254:19–35. doi: 10.1016/s0012-1606(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Scheithauer BW, Jaap AJ, Horvath E, Kovacs K, Lloyd RV, Meyer FB, Laws ER, Jr, Young WF., Jr Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery. 2000;47:723–729. doi: 10.1097/00006123-200009000-00039. discussion 729–30. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Smith JC. Mesoderm formation in response to Brachyury requires FGF signalling. Curr Biol. 1995;5:62–67. doi: 10.1016/s0960-9822(95)00017-0. [DOI] [PubMed] [Google Scholar]
- Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–337. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- Tonou-Fujimori N, Takahashi M, Onodera H, Kikuta H, Koshida S, Takeda H, Yamasu K. Expression of the FGF receptor 2 gene (fgfr2) during embryogenesis in the zebrafish Danio rerio. Mech Dev. 2002;119 Suppl 1:S173–S178. doi: 10.1016/s0925-4773(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treier M, Rosenfeld MG. The hypothalamic-pituitary axis: co-development of two organs. Current Opinion in Cell Biology. 1996;8:833–843. doi: 10.1016/s0955-0674(96)80085-8. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Lanctot C, Drouin J. The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol. 1998;12:428–441. doi: 10.1210/mend.12.3.0073. [DOI] [PubMed] [Google Scholar]
- Walshe J, Mason I. Unique and combinatorial functions of Fgf3 and Fgf8 during zebrafish forebrain development. Development. 2003;130:4337–4349. doi: 10.1242/dev.00660. [DOI] [PubMed] [Google Scholar]
- Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of PROP1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20:2739–2753. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.