Abstract
Identification of progenitor/stem cell populations that differentiate specifically towards superficial zone articular chondrocytes is an unmet challenge for cartilage tissue engineering. Using fluorescence activated cell sorting (FACS) analysis we found a characteristic pattern of “side population” (SP) stem cells identified by the Hoechst33342 dye. We established micromass cultures from this population of cells and tested their chondrogeneic potential. Control (untreated) cultures were minimally stained for alcian blue- a marker of chondrogenesis. However, with BMP-7 treatment, alcian blue staining was increased. Superficial zone protein- a specific marker for articular cartilage superficial zone chondrocytes- increased with BMP-7 and/or TGF-β1 treatment in SP micromass cultures. Our results demonstrate the presence of stem/progenitor cells in the SP fraction isolated from the surface zone of bovine cartilage and have the ability to specifically differentiate towards the superficial zone articular chondrocyte.
Keywords: BMP7, TGFβ1, Superficial zone protein, Articular Cartilage, Stem/Progenitor cells, Side population
Introduction
Articular cartilage has little capacity for regeneration and repair. The reason for this low repair potential is unknown, but the lack of blood supply, low cell mobility due to surrounding matrix, and a limited number of progenitor cells could be contributing factors. It is becoming increasingly apparent that articular cartilage growth is achieved by appositional growth from the articular surface[1], suggesting the existence of chondroprogenitor cells[2; 3]. Identification of progenitor/stem cell populations specific for the articular chondrocyte phenotype is an unmet priority for cartilage tissue engineering.
The side population (SP) cells identified by Goodell and others function as stem cells in the hematopoietic system[4]. This population has been named “Side Population” based on a characteristic pattern by flow cytometry analysis to discriminate SP cells based on the differential efflux of Hoechst 33342 dye by a multi-drug-like transporter[5]. The differential efflux of Hoechst dye by multi-drug transporter is a characteristic common to all adult stem cells. Recent research revealed the SP cells in various tissues[6; 7; 8; 9; 10; 11; 12]. We therefore examined the hypothesis that SP cells are present in articular cartilage surface with chondrogenic stem/progenitor cell properties.
Superficial zone protein (SZP) is a ~345 kDa proteoglycan that is specifically synthesized by chondrocytes located in the superficial zone of bovine articular cartilage11. SZP also appears to be specifically synthesized and secreted by articular cartilage superficial zone chondrocytes, but not chondrocytes from mid- and deep zones12. The majority of the SZP synthesized by chondrocyte culture is secreted into the media, suggesting that most of it is deposited in the synovial fluid in vivo. The secreted SZP is thus a marker for superficial zone of articular cartilage.
The aim of this study was to isolate progenitor/stem cells for superficial zone chondrocytes in the articular cartilage surface.
Materials and methods
Harvest of bovine articular cartilage and isolation of the cells
Surface zone of bovine articular cartilage was obtained from the calf knee joint as described previously in our lab by Khalafi et al [13]. The bovine articular cartilage was divided into three zones (surface, middle and deep). The total thickness of articular cartilage was about 5mm. The surface zone of approximately 100μm thickness was obtained by dermatome. After washing with medium, they were minced and digested with 0.2% collagenase P [Roche Molecular Biochemicals] in DMEM/F12 containing 3% fetal bovine serum (FBS) for 3 hours at 37°C. The superficial zone, the middle and deep zones were obtained by using a custom device.
The gap interval was 1.25mm. The deep zone consists of the first 1.25mm close to subchondral bone. Next 1.25mm was designated as middle zone. The middle and deep zone cartilage was digested with collagenase (0.2%) for 6 hours. The dispersed cells were filtered through a 70μm cell strainer to minimize cell aggregates and washed with DMEM/F12 containing 10% FBS and penicillin and streptomycin twice. The cells were placed in same medium for 1 hour before counting the cells.
Hoechst 33342 staining
Isolated cells were plated in monolayer on 100 mm culture dish at 4×106 in DMEM/F12 containing 10% FBS and 1% penicillin/streptomycin [Gibco]. After 5 days monolayer culture, they were released by trypsin treatment, counted, and resuspended in prewarmed Hanks’ Balanced Salt Solution (HBSS) containing 2% FBS, 10mM HEPES buffer (HBSS+) at a density of 1×106 cells/ml. 5μg/ml Hoechst 33342 [Sigma Chemical Co] was added and incubated for 60 min at 37°C. When the verapamil was used, the cells were stained in presence of 50μM verapamil [Sigma Chemical Co]. After that, cells were washed once excess ice-cold HBSS+ and pelleted through a serum cushion. The cells were resuspended in ice-cold HBSS+ containing 2μg/ml propidium iodide (PI). After Hoechst staining, the cells were maintained at 4°C before FACS analysis.
Flow cytometry analysis
Analysis and cell sorting were performed on a MoFlo cell sorter [DakoCytomation] using a Coherent Enterprise laser [Coherent] for excitation of Hoechst 33342 and propidium iodide. The Hoechst dye was excited at 350 nm and Hoechst emission was measured using a 450/65 BP filter (Hoechst blue) and a 670/30 EFLP (Hoechst red) optical filter [Chroma Technology Corp]. A 510 DCLP (510 nm long pass dichroic mirror) was used to separate the emission wavelengths. PI fluorescence was measured through a 580/30 BP (having been excited at 488nm). Hoechst “blue” measured through the 450 BP filter, represents the standard analysis wavelength for Hoechst 33342 DNA content analysis. Cells positive for PI were seen on a plot of PI vs FSC (forward scatter) and excluded. The addition of PI did not affect the Hoechst staining profile, but permitted exclusion of dead cells. Both Hoechst blue and red fluorescence were performed on a linear scale. The gating on forward and side scatter was not stringent; only erythrocytes and debris were excluded. The side population sorting gates were established as follows. A live gate was defined on the flow cytometry using a two-dimensional profile with PI vs FSC. We established PI negative populations as a live gate and apply this gate to following operations. After collecting 105 events within this live gate, the Hoechst blue vs red profile was displayed. The SP cells were able to be defined. A new gate was established on this profile. The sorted cells were cultured to amplify for 7 days in monolayer culture on 100mm dish. And also, main population (non-SP, NSP) was sorted at the same time as a control.
Cell culture
Cell culture was performed using micromass culture technique. Briefly, 10μl (containing 105 cells) of a concentrated cell suspension (1×107/ml) were plated onto the center of each well of a twelve-well culture plate and the cells were allowed to attach at 37°C for 1 hour. Chondrogenic medium (DMEM/F12 supplemented with 1% FBS, 50μg/ml ascorbate phosphate [Sigma Chemical Co] 1% penicillin/streptomycin [Gibco], and 50mg/ml ITS+Premix [Becton-Dickinson; a final concentration of 6.25μg/ml bovine insulin, 6.25μg/ml transferrin, 6.25ng/ml selenous acid, 1.25mg/ml bovine serum albumin, and 5.35μg/ml linoleic acid]was gently overlaid from the periphery so as not to detach the cell nodules, and cultures were maintained in chondrogenic medium for 7 days prior to analysis. The day of plating in micromass culture was designated as day 0. Starting on day 0, recombinant human Bone Morphogenetic Protein (BMP)-4, BMP-7, and transforming growth factor β1 (TGFβ1) [R&D Systems] was added to the each well at a final concentration of 300ng/ml for BMPs, 10 ng/ml for TGF-β1. The chondrogenic medium was changed at day 4 and the BMPs and TGF-β1 were re-added during change of culture medium.
Alcian blue staining
After 7 days, the micromass cultures were rinsed twice with PBS, fixed with 10% neutral buffered formalin (pH7.4) including 1% CPC (Cetylyridinium Chrolide) for 15 minutes at room temperature, washed with PBS, and covered with alcian blue (pH 1.0) (1% alcian blue 8 GS [Sigma Chemical Co] in 0.1M HCl). After 1 hour staining, cultures were washed extensively with distilled water and photographed. To quantify alcian blue intensity, cultures were rinsed with 50mM Tris-HCl (pH7.4), incubated with 300μl 4M Guanidine-HCl, 50mM Tris-HCl, 0.1% Chaps, pH7.4 for 2 hours at room temperature. Optical density of the extracted dye was evaluated at 595nm in a microplate reader [Bio-Rad].
Immunoblotting
For immunoblotting, the proteins secreted in media were electrophoretically resolved by SDS-PAGE and transferred onto PVDF membrane. The blots were then washed once in sterilized water, blocked with 5% non-fat dry milk in TBST for 1 hour at room temperature, and incubated with a mouse anti-bovine SZP antibody (generous gift of T. Schmid) diluted 1:5000 in blocking buffer at 4°C over night. The blots were then washed three times, 20 minutes each, in TBST. The blots were then incubated with a 1:3000 dilution of secondary antibody conjugated to horseradish peroxdase (goat anti-mouse IgG antibody) for 1 hour at room temperature in 1% non-fat dry milk in TBST. After washing three times, 20 minutes each, in TBST, the immunoblots were then processed for detection using the chemiluminescence kit. The immunoblots were exposed to X-ray film for 60 seconds.
Histology and Immunohistochemistry
After 7 days, the micromass cultures were fixed with 10% neutral buffered formalin, peeled off the wells, embedded in paraffin, and sectioned at 7 μm. For histologic evaluation, sections were deparaffinized and stained with toluidine blue.
For immunohistochemical analysis, after deparaffinization, nonspecific binding was blocked with 3% goat or horse serum albumin. The slides were incubated over night at 4°C with a rabbit anti-bovine type II collagen antibody [Chemicon International], diluted 1: 150 in normal blocking serum or mouse anti-bovine SZP antibody diluted 1:1000 in normal blocking serum. Slides were then incubated for 60 minutes at room temperature with biotinylated goat anti-rabbit IgG or goat anti-mouse IgG diluted in normal blocking serum. The antibody-biotin conjugates were detected with a streptavidin–biotin–horseradish peroxidase complex (vectastain elite ABC kit) [Vector] applied for 3 minutes at room temperature, using diaminobenzidine [Vector] as a substrate. Negative controls were sections of micromass cultures incubated with normal rabbit IgG instead of primary antibody.
Results and Discussion
Flow cytometry analysis
The superficial zone of bovine articular cartilage was digested in collagenase-P for 3 hours and the released cells were cultured as monolayer on 100mm culture dish. The cells were analyzed by flow cytometry and we identified a population which effluxed Hoechst 33342 dye. This fraction is designated as Side Population (SP) cells. (fig 1-a). The proportion of the SP population relative to total number of cells was about 0.1%. The SP population was not distinguished when surface zone cells were treated with verapamil, an inhibitor of multi-drug resistance activity (fig 1-b). It is noteworthy that SP cells were not observed in the middle and deep zone chondrocytes (fig 1-c, d). Articular cartilage SP cells existed only in the superficial zone of articular cartilage.
Fig 1. Flow cytometry analysis.
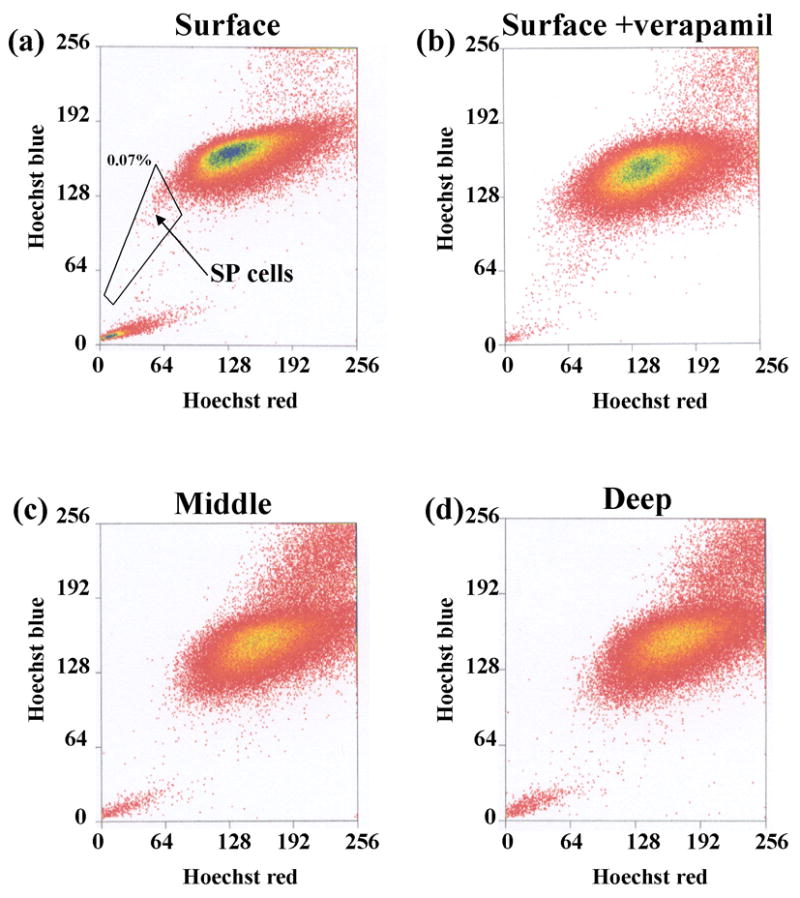
The distribution of chondrocytes by flow cytometry from the surface, middle and deep zone. (a) surface zone: we identified the side population cells. (b) addition of verapamil prevented the fractionation of SP cells. (c),(d) The middle zone and deep zone were devoid of SP cells.
Alcian blue staining for micromass
We proposed the hypothesis that the SP cells with progenitor properties may be enriched in the surface zone of articular cartilage. Therefore, we investigated chondrogenesis in vitro using a micromass culture technique. The cells were isolated from surface zone of articular cartilage and cultured in monolayer for 5 days (primary). Next, SP cells were derived by sorting the primary cells by FACS and then expanding cells in culture for 7 days. The main population of non-SP cells were designated NSP.
In untreated control group, all micromass cultures derived from the surface zone were minimally stained (fig 2-a, b and c). However, with BMP-4 and BMP-7 or TGF-β1 treatment, the staining by alcian blue was increased. Micromass cultures using middle or deep zone chondrocytes, cultures were stained strong by alcian blue without morphogen treatment (data not shown). When we compared SP with NSP, the SP cells absorbance was dramatically higher after BMP treatment (fig 2-b). Thus, SP cells appear to be enriched in stem/progenitor cells for chondrogenesis by BMP-7.
Fig 2. Alcian blue staining of the masses and intensity.
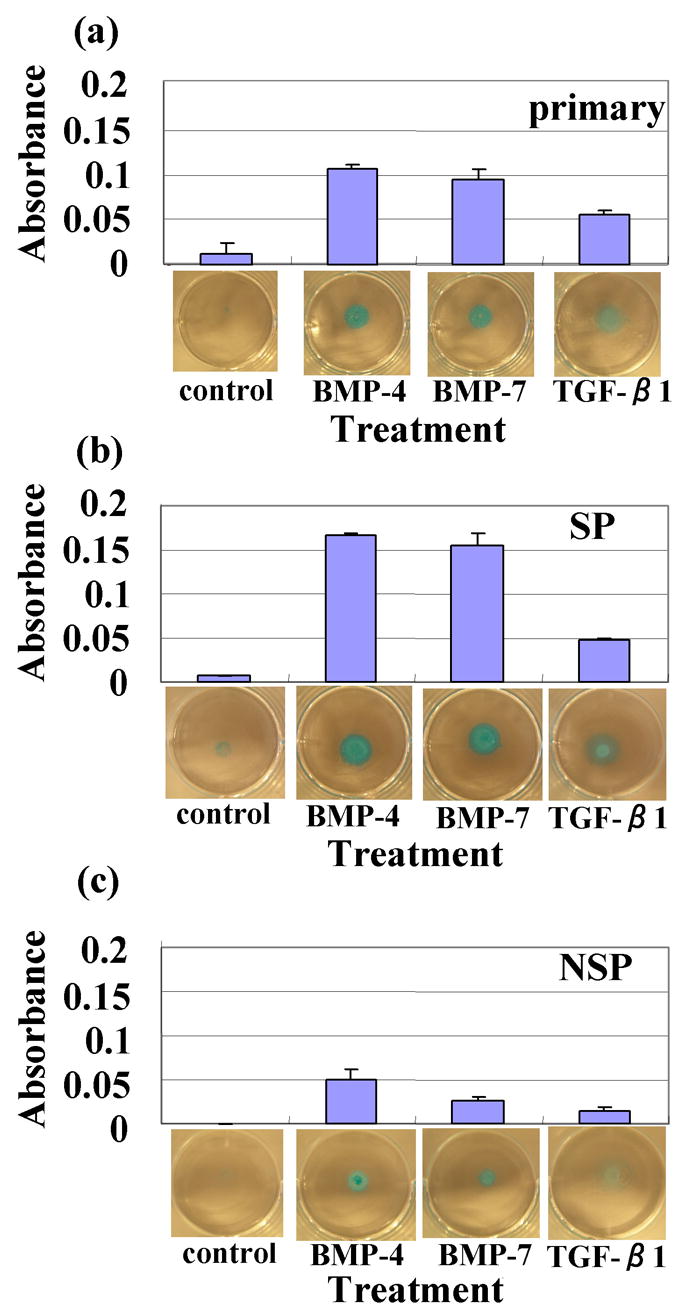
(a) primary (5 days culture of monolayer) (b) SP (were derived by sorting the primary cells and then expanding the culture for 7 days); The absorbance was dramatically higher with BMPs treatment. (c) The non-SP (NSP) cells were cultured similarly.
Immunoblotting
We examined SZP expression which is a unique marker for superficial zone of articular cartilage (11). We examined the SZP expression from culture media. The SZP expression of all samples was dramatically increased with TGF-β1 treatment. For primary micromass cultures, the SZP was the most increased of all samples. When we compared SP with NSP about SZP expression, SP was stronger than NSP clearly. It suggested that SZP expressing cells were enriched in SP fraction of superficial zone. And also, the expression of SZP was increased by BMP-7 treatment (fig 3).
Fig 3. Immunoblotting for SZP.
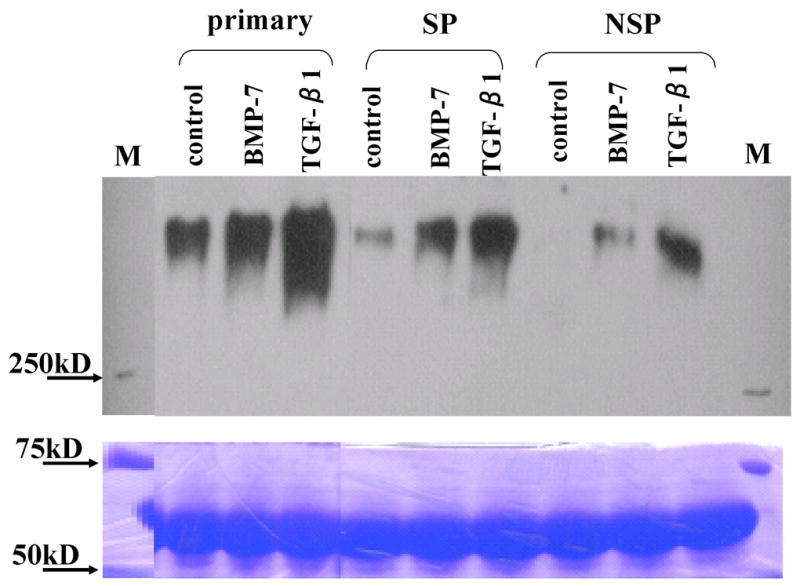
SZP expression was dramatically increased with BMP-7 and TGF-β1 treatment in primary, SP and non-SP cells.
Histology and Immunohistochemistry
Histological sections of the micromass cultures were made for immunohistochemistry of SZP and collagen type 2. Treatment of SP cells with BMP-7 resulted in robust chondrogenesis compared to TGF-β1 (Fig 4-D, G). SP cells were responsive to BMP-7 and produced extracellular matrix for cartilage. While SP micromass cultures with TGF-β1 treatment was almost same thickness as the primary micromass cultures and the cell shape was flat (fig 4-G). With TGF-β1 treatment, the SP cells secreted SZP.
Fig 4. Histology and Immunohistochemistry.
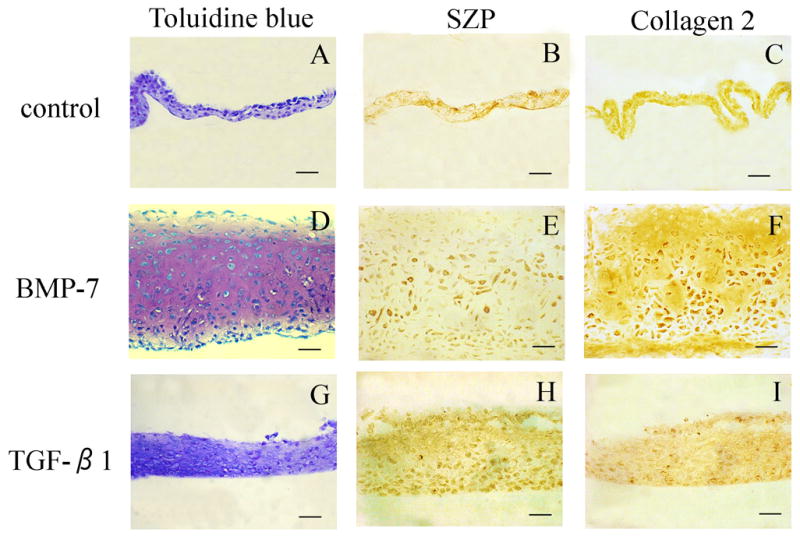
The SP masses were stained with toluidine blue. In controls, there was no metachromasia with toluidine blue (A). The SP masses with BMP-7 treatment was thicker than with TGF-β1 treatment masses, and many round shape cells like a chondrocyte were observed (D and G).
For SZP expression, the SP masses with TGF-β1 treatment was stronger than BMP-7 treatment (E, H). However, for collagen type 2 expression, there is no difference with BMP-7 and TGF-β1 treatment (F, I). Bar is 50μm.
SZP and collagen type 2 expression were examined by immunohistochemistry (Fig 4-B, C, E, F, H and I). All SP micromass cultures were positive for both collagen type 2 and SZP. In SP cells treated with TGF-β1, SZP expression was pronounced compared to BMP-7 treatment (Fig 4-E, H). On the other hand, BMP-7 treatment increased collagen type 2 staining.
There is a growing realization of the presence of stem/progenitor cells in a variety of tissues. In this investigation, we set out to determine the utility of Hoechst dye33342 in the identification of stem/progenitor cells in the articular cartilage. The efflux of the dye by stem/progenitor cells has permitted the isolation of a “Side Population” (SP) of cells with stem cell properties4. It is noteworthy that in the surface zone of articular cartilage, there are cells with stem/progenitor characteristics for both surface and middle zone chondrocytes. Biochemical characterization revealed that SP cells can express collagen type 2 (middle zone) and superficial zone protein (SZP) (surface zone chondrocytes). The differential response of SZP to BMP-7 and TGF-β1 is interesting. SZP expression is stimulated by TGF-β1 whereas collagen type 2 expression is increased by BMP-7. These progenitor cells for articular chondrogenesis are present only in the surface zone but not in middle and deep zones of articular cartilage. In conclusion, the SP cells from cartilage are enriched in progenitors for chondrogenesis with superficial zone protein (surface layer) and collagen type 2 (middle zone) expression in micromass cultures.
Acknowledgments
The authors would like to thank D. Raunschweig for technical support in FACS analysis and cell sorting. We thank our colleagues for helpful comments.
Financial support: This study was funded by NIH, the Lawrence Ellison Chair Endowment and The Musculoskeletal Transplant Foundation
Footnotes
Disclaimer: There are no conflicts of interest for the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203:469–79. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 2.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117:889–97. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 3.Martin JM, Smith M, Al-Rubeai M. Cryopreservation and in vitro expansion of chondroprogenitor cells isolated from the superficial zone of articular cartilage. Biotechnol Prog. 2005;21:168–77. doi: 10.1021/bp049821o. [DOI] [PubMed] [Google Scholar]
- 4.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20:11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 6.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–6. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschmann-Jax C, Foster AE, Wulf GG, Goodell MA, Brenner MK. A distinct “side population” of cells in human tumor cells: implications for tumor biology and therapy. Cell Cycle. 2005;4:203–5. [PubMed] [Google Scholar]
- 8.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–33. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–34. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poliakova L, Pirone A, Farese A, MacVittie T, Farney A. Presence of nonhematopoietic side population cells in the adult human and nonhuman primate pancreas. Transplant Proc. 2004;36:1166–8. doi: 10.1016/j.transproceed.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144–52. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 12.Darling EM, Hu JC, Athanasiou KA. Zonal and topographical differences in articular cartilage gene expression. J Orthop Res. 2004;22:1182–7. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Khalafi A, Schmid TM, Neu C, Reddi AH. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]


