Abstract
Neurotoxicity is a frequent accompaniment of cancer chemotherapy, and held by many oncologists to be the major dose-limiting side effect. It appears in many forms, but attracted attention during the past decade primarily because of complaints by patients of impaired cognitive function they have labeled as “chemobrain”. Neuropsychological testing confirmed the validity of these complaints and has generated a substantial literature examining different aspects of cognitive impairment in various clinical populations undergoing a variety of treatments. Cognitive impairment is far from the only manifestation of neurotoxicity induced by chemotherapy, however. It alters sensory function and motor function as well. A critical need for patients is a suite of methods that will enable clinicians to trace the onset and progression of neurotoxicity so as to guide and balance decisions about the course of chemotherapy. This commentary describes some of the potential methods and encourages neurotoxicologists to enlist their unique skills in the service of these needs.
Introduction
Neurotoxicity is a serious, almost overwhelming problem in cancer chemotherapy. Some of its manifestations are listed in Table 1. It has yet to draw much attention from neurotoxicologists, whose main efforts have been applied to issues of public health arising from environmental exposures. Such neglect is no longer warranted; it probably arose from the view that cancer treatment side effects are inexorable accompaniments to a life-threatening disease. And, in fact, until relatively recently, only the more severe neurotoxic side effects of treatment aroused concerns by oncologists. This view has been tempered by newer research demonstrating that these severe effects are only the culmination of a progression that begins much earlier in treatment and that falls into the dominion of neurotoxicology.
Table 1.
Examples of cancer therapies and severe neurotoxic outcomes
| AGENT | DIAGNOSIS | NEUROTOXICITY EXAMPLES |
|---|---|---|
| l-asparaginase | Lymphocytic anemia | Depression, confusion |
| Busulfan | Bone marrow transplant | Seizures (children) |
| Cisplatin (intra-arterial) | Brain tumor | Peripheral neuropathy |
| Ifosfamide | Solid tumors (children) | Confusion, irritability |
| Interleukin-2 | Renal cancer | Disorientation, confusion |
| Nitrogen mustards | Bone marrow transplant | Hallucinations, tremor |
| Methotrexate | Child leukemia | Personality changes |
| Paclitaxel (taxol) | Breast cancer | Confusion, neuropathy |
| Carmustine (BCNU) | Gliomas | Leukoencephalopathy |
| Vincristine | Hodgkin’s disease | Peripheral neuropathy |
| Cranial irradiation (children) | Acute lymphoblastic leukemia (ALL) | Somnolence, advanced cognitive impairment |
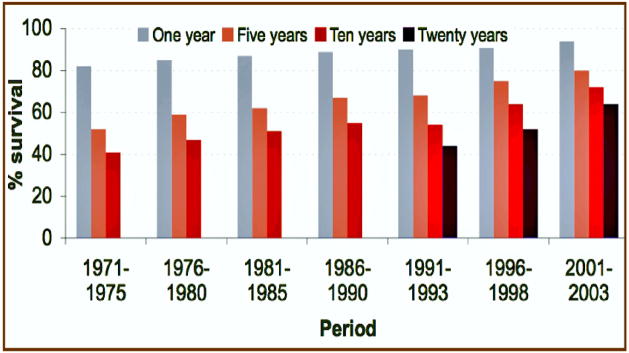
One major reason for the increased attention to neurotoxicity on the part of oncologists is the change in cancer patient survival during the past 30 years. Many patients now survive for decades after diagnosis and treatments that often incur disabling neurotoxic effects (Figure 1). Wefel et al. (2004) posed the implications in this way: “Cancer is becoming a chronic illness…The number of long-term cancer survivors will continue to increase…current understanding of the cognitive and neurobehavioral effects of these treatments is extremely limited…it is imperative that future investigations use well designed longitudinal methodologies that will assist in defining the relative risks and benefits…” Moreover, with newer treatment methods and chemicals, it has become increasingly apparent that not only is neurotoxicity the major dose-limiting side effect, but that it is appearing in forms not seen earlier (Schiff and Wen, 2006). With 70 million cancer survivors estimated world-wide in 2020, the limitations imposed by neurotoxicity on treatment choices and quality of life should engage the efforts of scientists equipped to study its features in detail.
Figure 1.
Cancer survival 1971–2003. Over this period, the number of survivors has increased markedly. By 2020, the estimated number of world-wide survivors will exceed 70,000,000.
Concerns such as those expressed above are highlighted by recent research. Dietrich et al. (2006) reported that three chemotherapeutic agents, carmustine (BCNU), cisplatin, and cytosine arabinoside (cytarabine) increased cell death and decreased cell division in the CNS of treated mice at doses lower than those required to kill tumor cells. As they noted, “Our studies have multiple implications for future strategies of cancer treatment…it seems that [doses of] chemotherapeutic agents sufficient to harm cancer cells may also damage many cell populations of the CNS…It is also possible, however, that our results actually understate the extent of damage that occurs in association with chemotherapy.”
Neurotoxicology is a broadly-encompassing discipline. Its practitioners range across many related disciplines. No methodological or conceptual barriers impede their studying the neurotoxic manifestations of chemotherapy by the same methods proven so fruitful, for example, in studying environmental chemicals. No one of us, though, is equipped to discourse knowledgeably about all of its facets. In this commentary I have restricted myself to offering examples of how capably our methods and approaches are equipped to assist in balancing the benefits and drawbacks of current clinical practices, and the assessment methods that may lead to improvments in patient welfare. I have neither addressed mechanistic research, such as that of Dietrich et al. (2006) nor the promise of animal models. These warrant separate surveys. My aim is to encourage the practitioners of neurotoxicology to apply their tools and talents along two paths. First, to improve the lives of cancer patients by providing reliable and sensitive techniques for tracing the onset and progression of neurotoxicity. Second, to apply their methods for studying, predicting, and intervening in the neurotoxic process to cancer chemotherapy. It is more than a worthwhile translational challenge. It is a vocation that calls on us both ethically and humanistically to respond.
Chemobrain Initiatives
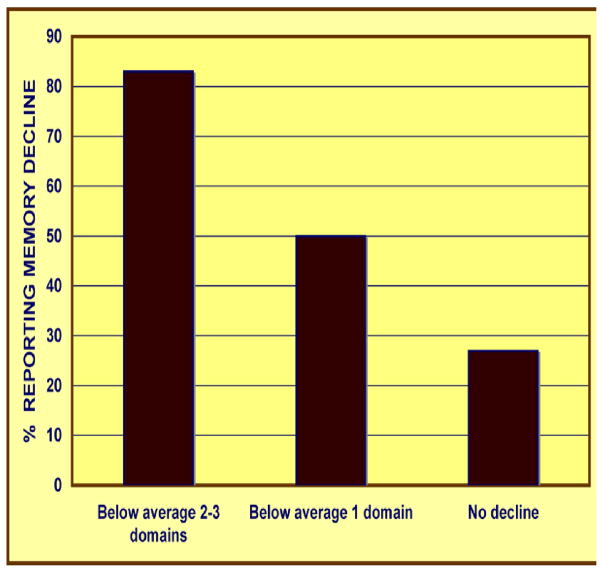
The data that compelled concern about subtle and emergent neurotoxicity were rooted in subjective complaints. Clinicians expect reports of anxiety, fatigue, pain, and depression in patients undergoing the rigors of chemotherapy. But patients also reported memory lapses, inability to concentrate, short attention span, periods of confusion, and other indices of impaired neurobehavioral function. These are complaints not readily connected to the usual clinical markers. They also tended to persist after the cessation of therapy, however, an indication that these functional deficits were not simply a product of acute side effects during the course of treatment. Patients began to describe their state as “chemobrain” or “chemofog.” Figure 2, based on Hurria et al. (2006), is an example depicting subjective assessments of memory decline in patients following a course of treatment for breast cancer.
Figure 2.
Subjective reports of memory difficulties in patients undergoing chemotherapy for breast cancer (based on data from Hurria et al., 2006).
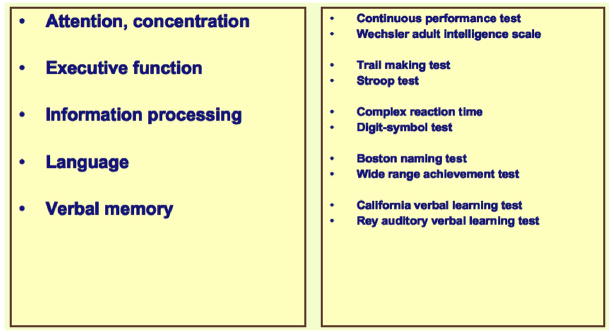
Once these complaints claimed the attention and skills of neuropsychologists, their validity was confirmed (e.g., Castellon et al., 2004). The entries in Figure 3 were extracted from the current literature. Several pertinent reviews are available (e.g., Vardy et al., 2007; Taillibert et al., 2007; Ahles and Saykin, 2007). Cognitive impairment following chemotherapy is now benefitting from the close scrutiny of experts in a variety of specialties. A workshop held in 2003 in Banff, Canada (Tannock et al., 2004) and reviewed later by Ahles and Saykin (2007) offered the following recommendations for continued progress:
Figure 3.
Neuropsychological tests used to investigate cognitive impairments in cancer patients undergoing chemotherapy.
* Conduct longitudinal studies that include pre- and post-treatment neuropsychological assessments that enable the evaluation of acute and long-term cognitive changes, and that include appropriate comparison groups such as cancer patients not treated with chemotherapy and/or matched healthy controls.
* Design studies that examine factors that increase the risk of cognitive changes (for example, genetic factors) and define the mechanism(s) that underlies chemotherapy-induced cognitive changes.
* Use advanced imaging techniques such as magnetic resonance imaging (MRI) and positron emission tomography (PET) to define structural and functional changes in the brain associated with chemotherapy.
* Develop animal models that will aid in identifying the mechanism(s) of chemotherapy-induced cognitive changes.
* Identify the neuropsychological tests that are the most sensitive to the cognitive side effects of chemotherapy and develop new tests that are more closely related to cognitive functioning in the real world.
* Develop and evaluate medication and cognitive rehabilitation interventions.
These recommendations are consistent with those that would follow an appraisal of the data by neurotoxicologists. They are too narrow in scope, however, to reflect the multitude of questions that we would then seek to answer. They also, regrettably, suffer from a lack of familiarity with the literature of neurotoxicology; calls for initiatives such as animal models and sensitive neuropsychological tests are several decades out of date. In the sections that follow, I describe the varieties of neurotoxicity encountered in the course of chemotherapy, and some of the methods used in neurotoxic assessment to study them. Anyone familiar with neurotoxicology will quickly see how easily they blend into the needs of oncologists and patients who wish for an optimal balance of clinical effectiveness and maintainence of neurobehavioral function. Some of the methods discussed are feasible only in research settings where specialzed equipment is available. This is a domain in which neurotoxicologists can contribute fundamental findings for translation into to the clinical setting. For example, clinical oncology lacks information about the relationships among dose, latency, and severity for the different varieties of neurotoxic phenomena.
In addition to a detailed scientific appraisal of chemotherapy-induced neurotoxicity, sketched only in outline here, our discipline can also offer assessment methods that are suitable for settings where specialized facilities and personnel are not readily available. Some are described below. The following sections, then, are written with two aims. First, to prompt more basic research on the neurotoxic mechanisms of chemotherapeutic agents and on the measurement of their functional effects. Second, to encourage neurotoxicologists to collaborate with oncologists on efforts to follow the onset and progression of adverse neurobehavioral effects in patients. The findings would enable treatment decisions to more finely weigh the treatment choices and quality of life.
This survey primarily addresses neurotoxicity in adults. Pediatric populations differ in many respects. Not only may they be more vulnerable because of the sensitivity of the developing nervous system, but also because they have to carry the burden over a longer proportion of the lifespan. They deserve a separate survey exploring their unique status.
Scope of neurxotoxic assessment
Cognitive endpoints
It is fair to say that the predominant emphasis in neurotoxic assessment has always been on cognitive function. From the reliance on IQ as an index of lead’s effects on neurobehavioral development to the role of memory tests in evaluating the role of amyloid beta in models of Alzheimer’s disease, cognitive measures continue to occupy the foremost position in such assessments. The chemobrain literature underscores the abundance of techniques for evaluating cognition, a richness of possibilities that is almost overwhelming. Castellon et al. (2004) deemed it necessary to include eight different tests in their study of breast cancer survivors treated with adjuvant chemotherapy and tamoxifen. Wefel et al. (2004) used thirteen tests in their study of similar patients. Both could have chosen, in fact, from a vast catalog of test instruments, most of which have found their way into neurotoxicology. As noted by Slikker et al. (2000), over 50 years of solid scientific investigations by cognitive researchers have established “very reproducible, sensitive, and quantitative approaches to assess memory, learning, and attention functions in both animals and humans.”
The most immediate situation faced in most clinical settings is not whether to carry out an extensive research study following the recommendations of the Banff workshop but to trace the progression of neurotoxicity, if present, and to help the oncologist and patient to decide on the course of therapy. Although the neuropsychological tests listed in Figure 3 are suitable for documenting the chemobrain phenomenon, and for exploring the enduring effects of particular regimens, they have limitations for most clinical settings. First, a single time point for evaluation is inconsistent with the clinical needs. One problem is how to guard against practice effects if testing is performed, say, every three months. Alternate versions of some tests are available, but this is a generic problem.
Furthermore, in many settings, the time taken for cognitive evaluation is limited, so that a compact but comprehensive test battery is more suitable than the typical, largely paper and pencil collection of tests that may require at least 60 minutes to administer, often requiring a trained neuropsychologist. In addition, the responses on paper and pencil tasks have to be scored and transcribed, leading to transcription errors and delays in analysis when decisions about the next stage of treatment are awaited. One solution is to adopt computerized test batteries whose elements can change from one occasion to the next.
Computerized test batteries incorporating cognitive assessments are widely used in neurotoxicology. A high proportion of them have been assembled to serve a specific investigative purpose, but too few provide data on their reliability, validity, and predictive ability. Choosing as their model the NES3 battery, White et al. (2003) compared its results with those yielded by an experienced neuropsychologist who administered a battery of established paper-and-pencil tests. The results indicated that an appropriate computerized battery could be used to at least label patients as cognitively impaired or unimpaired. Slikker et al. (2000) also discuss the properties and usefulness of computerized test batteries as well as the broader issues permeating cognitive testing.
One flaw characteristic of most such batteries is that they are not designed with alternate forms for repeated testing. At least three, however, might meet the requirements for chemotherapy monitoring. The CogState battery includes tests of psychomotor function, attention, learning, memory, problem solving/strategy use and delayed recall. They are presented on tablet computers using a touchscreen for responses (Collie et al., 2007). The CANTAB (Fray and Robbins, 1996) consists of a suite of computerized tests, now numbering 22, that embrace a variety of cognitive functions: visual memory, executive function, working memory, semantic and verbal memory, attention, decision making, and response control (designed to assess behaviors such as impulsivity). Most of the tests are explicity designed to be independent of language and culture. The various functional categories are represented by more than one test, so that alternate forms are available for repeated testing. It has been used in neurotoxicology to study the effects of lead on executive function in children with a particular dopamine receptor polymorphism (Froehlich et al., 2007). It has also been used to study a variety of neurobehavioral disorders such as schizophrenia (Jazbec et al., 2007) and has been adapted for nonhuman primates (Spinelli et al., 2004). In a number of respects, the CANTAB resembles the adaptation of operant methods to humans (Chelonis et al., 2000).
The BARS (Behavioral Assessment and Research System) battery, described by Anger in Slikker et al. (2000), is specifically designed for the detection of neurotoxicity in populations with limited education or literacy (Rohlman et al., 2003). It too can be used for repeated assessments. The question to be resolved for each case is the depth of assessment sought versus the time and resources devoted to it.
Sensory endpoints
Cognitive dysfunction, because it is so often detectable in social exchanges, possesses an immediacy for patients that is not always as evident in subtle disturbances of sensory function. Some computerized test batteries include crude sensory and motor components, but these are not useful for detecting subtle functional changes of which patients may be unaware.
Vision
The visual system is a frequent target of the agents used for chemotherapy, as noted by Schmid et al. (2006): “Many ophthalmic complications have been reported for these new cytotoxic chemotherapeutics, some of which are reversible if detected early enough… At first, many of these ocular toxicities are hardly detected…However, these side effects may turn out to be irreversible by the time the symptoms are recognized.” Detection at an incipient stage of toxicity is a critical matter. It is an issue that has led neurotoxicologists to recognize and study such effects in many settings. For example, Iregren et al. (2002) sought to uncover impaired hue discrimination in workers exposed to organic solvents by using the Lanthony D-15 color chips, an extension of the lengthier Farnsworth Munsell 100 hue test. It has not been used up to now to assess subtle color vision deficits in chemotherapy patients although psychophysical assessments in patients treated with tamoxifen indicate that such losses occur (Eisner and Incognito, 2006).
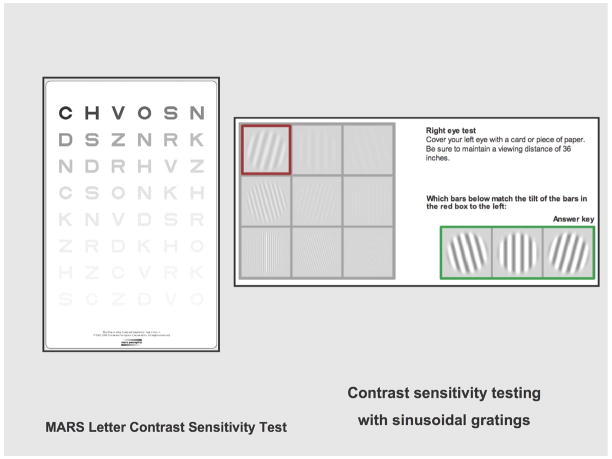
Another aspect of visual dysfunction studied by neurotoxicologists that so far has been neglected despite patient complaints is blurred vision, which is quantitatively assessed as deficits in contrast sensitivity. It is not reflected by performance on the usual Snellen eye chart, which presents black block letters on a white background, while our usual views of the world are marked by gradations in brightness and contours from which we have to extract information. Impaired spatial contrast sensitivity has appeared as a result of exposure to methylmercury (Burbacher et al., 2005, in monkeys; LeBel et al., 1998, in humans); acrylamide (Lynch et al., 1992, in monkeys); and volatile organic solvents (Schreiber et al., 2002, in humans). Altered contrast sensitiivity is also seen in Parkinson’s disease and multiple sclerosis. Studies probing the mechanisms of contrast sensitivity require electronic instrumentation, as in Hayes and Merigan (2007), but portable charts based on contrast can be used as a guide to possible deficits that could be subjected to further analysis (Figure 4). The Pelli-Robson and Mars tests use a single large letter size with contrast varying across groups of letters (Haymes et al., 2006). The Pelli-Robson chart uses letters (6 per line), arranged in groups whose contrast varies from high to low. The Mars test is similar. A more elaborate test, the Functional Acuity Contrast Test uses sine-wave gratings, the standard for vision research, mounted on a chart. It was used by Schreiber et al. (2002).
Figure 4.
Tests for visual contrast sensitivity. Left: Mars Test Chart; contrast diminishes left to right, up to down. Right: online sample from Functional Acuity Contrast Test, (Stereo Optical Co., Chicago, IL).
Hearing
Ototoxicity is a recognized problem in chemotherapy. The auditory system is vulnerable to agents such as cisplatin, which is used to treat a variety of cancers. A core problem with ototoxicity in chemotherapy is that the National Cancer Institute’s reporting system, Common Terminology Criteria for Adverse Events, or CTCAE does not consider high-frequency hearing loss (above say, 8,000 Hz). In many settings, including the workplace, and characteristic of many drugs including those used to treat cancer, such losses are the first indication of auditory system damage. The frequencies important for vocal communication are significantly lower, so that ordinary patient interviews, such as the Hearing Handicap Inventory in the Elderly, from the Surgeon General. Conventional audiograms will fail to detect the early signs of hearing loss because they typically do not assay frequencies above 4,000 Hz. This is a special concern for children, because diminished hearing predicts diminished school performance (Knight et al., 2007).
Many chemicals and drugs are ototoxic, and much of what we’ve learned in humans has come from workplace exposures. The bulk of this literature is based mostly on conventional audiograms except for organic solvents (Fechter and Pouyatos, 2005; Chang et al., 2006; Odkvist et al., 1992). Fuente and McPherson (2006), reviewing the effects of organic solvents, emphasize that tests beyond conventional audiometry such as otoacoustic emissions (OAEs) and evoked potentials (e.g., brainstem auditory evoked responses, or BAERs) make it possible to detect auditory damage at an early stage. Such a conclusion, based on incipient workplace neurotoxicity rather than the often parlous status of treated patients, is a message for both neurotoxicologists and oncologists. Much more needs to be understood about the process at the structural, biochemical, and psychophysical level. It would also be quite useful to incorporate auditory tests into a battery without requiring the resources of an audiology clinic. A compact portable system, such as the Ear Scan (Beckett et al., 2000), which can provide frequencies up to 8,000 Hz would be a desirable adjunct to conventional test batteries.
Somatosensory function
Peripheral neuropathy is an enduring problem in cancer patients. The vinca alkaloids, platinum compounds, and the taxanes, all widely-used for a variety of malignancies such as ovarian and breast cancer, and for hematological cancers, are notable for inducing damage to peripheral nerves. Cavaletti et al. (2003) observed that, “Chemotherapy-induced peripheral neurotoxicity (CIPN) is a common and potentially disabling side effect of….anticancer agents…it might represent the dose-limiting side effect of the treatment, thus impairing patients’ quality of life and the effectiveness of treatment.” Indeed, the incidence may be as high as 100% according to Postma et al. (2005), who described a questionnaire devised to elicit patients’ symptoms and functional limitations related to CIPN for the European Organization for Research and Treatment of Cancer (EORTC). It is meant as a supplement to the Total Neuropathy Scale (TNS) used by neurologists (Cavaletti et al., 2007), which does not include quality of life ratings. The TNS is a 5-point scale that includes ratings for sensory symptoms, motor symptoms, autonomic symptoms, pin and vibration sensitivity, and strength, plus electrophysiological measures. It is not designed specifically as a means for detecting incipient dysfunction.
In addition to rating scales, neurologists and many investigators may rely on a variety of instruments to assay somesthetic sensitivity. Tuning forks are often used (Wampler et al., 2005) but their amplitude cannot be controlled. One electronic device, used in many studies (e.g., Duke et al., 2007), is the The biothesiometer, which uses a rheostat attached to a vibrating probe; as the voltage is increased, the amplitude of the vibration also increases. Similar devices are also on the market. They suffer from a fundamental defect: the degree to which they deform the skin is not controlled, but depends upon the pressure exerted by the tester (Maurissen and Weiss, 1980). Cutaneous electrical stimulation and thermal stimuli are also used in sensory testing (e.g., Savic et al., 2007). These two modalities transmit information from their receptors via small, unmyelinated nerve fibers. Vibrotactile stimuli are transmitted via large, myelinated fibers.
The mechanisms by which chemotherapeutic agents exert these effects require the same kind of elegant analysis applied by LoPachin et al. (2007) to acrylamide and similar chemicals. Although the commercial instruments described above may be useful in the clinic, this level of molecular analysis demands meticulous control of stimulus conditions if the aim of the research is accurate determination of somatosensory sensitivity. It requires precise instrumentation of the kind used by Rice and Gilbert (1995) in monkeys and Maurissen and Chrzan (1989) in humans. Additional examples of the level of instrumentation required can be found in Bensmaia et al. (2005) and Tommerdahl et al. (2005). In addition, appropriate psychophysical methods must be used in testing to avoid deceptive findings (Maurissen, 1985).
Olfaction, taste
The chemical senses are also susceptible to neurotoxic chemotherapeutic agents. In fact, taste and smell alterations are among the most common side effects accompanying chemotherapy (Ravasco, 2005). Moreover, loss of and alterations in taste qualities can also induce food aversions, which then create inadequate energy intake (Hutton et al., 2007). Bernhardson et al. (2007) used interviews to ascertain the mode, intensity, and duration of alterations in taste and smell in chemotherapy patients, and found changes in the sensory qualities of food especially daunting. The Chemosensory Questionnaire devised and tested by Goldberg et al. (2005) was devised to meet psychometric standards for validity and reliabiity and should prove useful in tracking patient responses during a course of chemotherapy. It might be even more useful to correlate such responses with taste sensitivity measures derived from quantitative psychophysical methods applied to the five recognized taste qualities of sweet, sour, bitter, salty and umami. As noted by Chandrashekar et al. (2006), these taste qualities are coded by distinct cell types expressing unique receptors that are tuned to detect each of the five basic tastes. Although taste sensitivity has not attracted much attention from neurotoxicologists, Gobba (2003) pointed out that enough is already known about the effects of some envionmental exposures to encourage it as a neurotoxic endpoint.
Olfaction has attracted somewhat more attention in neurotoxicology, and a number of studies have examined olfactory deficits associated with toxic exposures. In an experimental study of olfactory thresholds induced by exposure to volatile organic solvents, Mergler and Beauvais (1992) detected a significant rise in thresholds for identifying methylethyl carbinol following a 7-hour solvent exposure. A more relevant question arises with chronic exposure. Antunes et al. (2007) measured olfactory function in welders employed as workers on the San Francisco/Oakland Bay Bridge for durations ranging from 6 to 28 months. They measured olfactory function with the University of Pennsylvania Smell Identification Test (UPSIT; Sensonics, Inc., Haddon Heights, NJ). The UPSIT determines the ability of the subject to identify 40 microencapsulated odorants. Performance is impaired in patients suffering from neurodegenerative disorders, particularly Parkinson’s disease (e.g., Ahlskog, 2007) but also Alzheimer’s disease (e.g., Djordjevic et al.., 2007). These authors, in addition to the UPSIT, also employed measured smell detection thresholds for phenyl ethyl alcohol, basing them on a staircase procedure, a psychophysical method that converges on these values in steps, approaching the detection limit with concentrations both above and below this final empirical value. Such data are more useful than smell identification scores for linking to mechanistic studies.
Motor Function
Despite the recognition by clinicians that patients may suffer from muscle weakness and coordination problems, surprisingly little research has been conducted on motor endpoints in adults. Pediatric populations have received the bulk of attention, but with relatively crude tools rather than the quantitative instruments that provide a basis for early detection of diminished capacity. For example, Reinders-Messelink et al. (2001) analyzed handwriting as a reflection of motor skills in children undergoing treatment for acute lymphoblastic leukemia. Hartman et al. (2007) assessed muscle strength in various pediatric malignancies treated by chemotherapy with a hand-held dynamometer and ankle dorsiflexion with a goniometer. For motor performance they relied on the Movement Assessment Battery for Children (movement-ABC). Inventories similar to the M-ABC have also been developed (Rosenblum, 2006).
For conditions other than cancer chemotherapy, such as stroke and PD, the literature offers numerous questionnaires and rating scales with clear potential for adult cancer patients. Most, however, tend to be qualitative guides to quality of life criteria rather than quantitative assessments, and less useful as instruments for detecting emerging dysfunction. Vannozzi et al. (2007) provide a review of how, for elderly subjects, the two approaches can be reconciled by applying data mining technology to interweave both approches. Kaufman et al. (2006) provide an example of how the results of a simple clinical tool, the Tinetti Assessment Tool for assessing a patient’s gait and balance, can be amplified by computerized dynamic posturography. Neither the clinical nor instrumented approach has been exploited for monitoring patients undergoing chemotherapy.
The volume of publications covering motor function assessment extends over many scientific disciplines. Given the need for such assessments in evaluating the neurotoxic outcomes of chemotherapy, it is surprising that they have largely been overlooked. In fact, much of that literature, some published in engineering journals, has even been overlooked by neurotoxicologists, perhaps due to the technical resources required to implement them (see Newland, 1988). Even without them, however, the neurotoxicology literature contains numerous publications from which cancer researchers can draw. Examples can be found in the following areas:
Pesticides: Rohlman et al. 2007
Mercury: Ellingsen et al. 2001; Letz et al. 2000
Methylmercury: Yokoo et al. 2003
Lead: Bleecker et al. 2007; Dorsey et al. 2006
Manganese: Bowler et al. 2006; Zoni et al. 2007; Hochberg et al. 1996
They range from simple techniques that can be embedded in a test battery to those that require complex instrumentation.
Other functional endpoints
A comprehensive evaluation of patient function would also include subjective measures of mood and fatigue. I have not included these, focusing instead on measures that require some degree of instrumentation. Subjective indices can easily be incorporated into computerized test batteries.
A Role for Neurotoxicologists?
This commentary has been written to challenge those scientists whose research in neurotoxicology has mainly been directed to questions about environmental agents. Chemicals used to treat cancer may superficially seem to be members of a distinctly different classification, and frank disease and toxicity may seem beyond the ken of those whose primary concerns have been embedded in threats to public health and their prevention. But the victims of cancer and the efficacy of their treatment are a major problem in public health as well. I commend neurotoxicologists to direct their skills to this problem because it will bring its own rewards.
The questions are provocative and intriguing
They can amplify brain-behavior relationships
They link directly to disease
The questions embrace the entire lifespan
Patients are living longer after treatment
Childhood cancer treatment is effective
The questions call upon a broad range of methods, specialties and biological levels
The solutions offer direct tangible rewards
They can improve the quality of life of cancer patients
They offer direct connections with environmental issues
Neurobehavioral function is a sensitive predictor of patient survival
Acknowledgments
Preparation supported in part by NIEHS grants ES013247 and ES015509 and Center grant ES01247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlskog JE. Beating a dead horse: Dopamine and parkinson disease. Neurology. 2007;69:1701–1711. doi: 10.1212/01.wnl.0000296942.14309.4a. [DOI] [PubMed] [Google Scholar]
- Antunes MB, Bowler R, Doty RL. San Francisco/Oakland bay bridge welder study: Olfactory function. Neurology. 2007;69:1278–1284. doi: 10.1212/01.wnl.0000276988.50742.5e. [DOI] [PubMed] [Google Scholar]
- Beckett WS, Chamberlain D, Hallman E, May J, Hwang SA, Gomez M, Eberly S, Cox C, Stark A. Hearing conservation for farmers: Source apportionment of occupational and environmental factors contributing to hearing loss. J Occup Environ Med. 2000;42:806–813. doi: 10.1097/00043764-200008000-00008. [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ, Leung YY, Hsiao SS, Johnson KO. Vibratory adaptation of cutaneous mechanoreceptive afferents. J Neurophysiol. 2005;94:3023–3036. doi: 10.1152/jn.00002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardson BM, Tishelman C, Rutqvist LE. Chemosensory changes experienced by patients undergoing cancer chemotherapy: A qualitative interview study. J Pain Symptom Manage. 2007;34:403–412. doi: 10.1016/j.jpainsymman.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Bleecker ML, Ford DP, Vaughan CG, Walsh KS, Lindgren KN. The association of lead exposure and motor performance mediated by cerebral white matter change. Neurotoxicology. 2007;28:318–323. doi: 10.1016/j.neuro.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Grant KS, Mayfield DB, Gilbert SG, Rice DC. Prenatal methylmercury exposure affects spatial vision in adult monkeys. Toxicol Appl Pharmacol. 2005;208:21–28. doi: 10.1016/j.taap.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, Parma G, Lissoni A, Fei F, Cundari S, Zanna C. Grading of chemotherapy-induced peripheral neurotoxicity using the total neuropathy scale. Neurology. 2003;61:1297–1300. doi: 10.1212/01.wnl.0000092015.03923.19. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Frigeni B, Lanzani F, Piatti M, Rota S, Briani C, Zara G, Plasmati R, Pastorelli F, Caraceni A, Pace A, Manicone M, Lissoni A, Colombo N, Bianchi G, et al. The total neuropathy score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: Comparison with the national cancer institute-common toxicity scale. J Peripher Nerv Syst. 2007;12:210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- Chang SJ, Chen CJ, Lien CH, Sung FC. Hearing loss in workers exposed to toluene and noise. Environ Health Perspect. 2006;114:1283–1286. doi: 10.1289/ehp.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelonis JJ, Daniels-Shaw JL, Blake DJ, Paule MG. Developmental aspects of delayed matching-to-sample task performance in children. Neurotoxicol Teratol. 2000 Sep-Oct;22:683–694. doi: 10.1016/s0892-0362(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Collie A, Darekar A, Weissgerber G, Toh MK, Snyder PJ, Maruff P, Huggins JP. Cognitive testing in early-phase clinical trials: Development of a rapid computerized test battery and application in a simulated phase I study. Contemp Clin Trials. 2007;28:391–400. doi: 10.1016/j.cct.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and alzheimer’s disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Dorsey CD, Lee BK, Bolla KI, Weaver VM, Lee SS, Lee GS, Todd AC, Shi W, Schwartz BS. Comparison of patella lead with blood lead and tibia lead and their associations with neurobehavioral test scores. J Occup Environ Med. 2006;48:489–496. doi: 10.1097/01.jom.0000199678.86629.3b. [DOI] [PubMed] [Google Scholar]
- Duke J, McEvoy M, Sibbritt D, Guest M, Smith W, Attia J. Vibrotactile threshold measurement for detecting peripheral neuropathy: Defining variability and a normal range for clinical and research use. Diabetologia. 2007;50:2305–2312. doi: 10.1007/s00125-007-0813-y. [DOI] [PubMed] [Google Scholar]
- Eisner A, Incognito LJ. The color appearance of stimuli detected via short-wavelength-sensitive cones for breast cancer survivors using tamoxifen. Vision Res. 2006;46:1816–1822. doi: 10.1016/j.visres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Bast-Pettersen R, Efskind J, Thomassen Y. Neuropsychological effects of low mercury vapor exposure in chloralkali workers. Neurotoxicology. 2001;22:249–258. doi: 10.1016/s0161-813x(01)00012-2. [DOI] [PubMed] [Google Scholar]
- Fechter LD, Pouyatos B. Ototoxicity. Environ Health Perspect. 2005;113:A443–4. doi: 10.1289/ehp.113-a443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW. CANTAB battery: Proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18:499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Dietrich KN, Cory-Slechta DA, Wang N, Kahn RS. Interactive effects of a DRD4 polymorphism, lead, and sex on executive functions in children. Biol Psychiatry. 2007;62:243–249. doi: 10.1016/j.biopsych.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Fuente A, McPherson B. Organic solvents and hearing loss: The challenge for audiology. Int J Audiol. 2006;45:367–381. doi: 10.1080/14992020600753205. [DOI] [PubMed] [Google Scholar]
- Gobba F. Occupational exposure to chemicals and sensory organs: a neglected research field. Neurotoxicology. 2003 Aug;24(4–5):675–91. doi: 10.1016/S0161-813X(03)00038-X. [DOI] [PubMed] [Google Scholar]
- Goldberg AN, Shea JA, Deems DA, Doty RL. A ChemoSensory questionnaire for patients treated for cancer of the head and neck. Laryngoscope. 2005;115:2077–2086. doi: 10.1097/01.mlg.0000187394.12264.d6. [DOI] [PubMed] [Google Scholar]
- Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer. 2007 doi: 10.1002/pbc.21325. [DOI] [PubMed] [Google Scholar]
- Hayes RD, Merigan WH. Mechanisms of sensitivity loss due to visual cortex lesions in humans and macaques. Cereb Cortex. 2007;17:1117–1128. doi: 10.1093/cercor/bhl021. [DOI] [PubMed] [Google Scholar]
- Haymes SA, Roberts KF, Cruess AF, Nicolela MT, LeBlanc RP, Ramsey MS, Chauhan BC, Artes PH. The letter contrast sensitivity test: Clinical evaluation of a new design. Invest Ophthalmol Vis Sci. 2006;47:2739–2745. doi: 10.1167/iovs.05-1419. [DOI] [PubMed] [Google Scholar]
- Hochberg F, Miller G, Valenzuela R, McNelis S, Crump KS, Covington T, Valdivia G, Hochberg B, Trustman JW. Late motor deficits of chilean manganese miners: A blinded control study. Neurology. 1996;47:788–795. doi: 10.1212/wnl.47.3.788. [DOI] [PubMed] [Google Scholar]
- Hurria A, Goldfarb S, Rosen C, Holland J, Zuckerman E, Lachs MS, Witmer M, van Gorp WG, Fornier M, D’Andrea G, Moasser M, Dang C, Van Poznak C, Robson M, Currie VE, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient’s perspective. Breast Cancer Res Treat. 2006;98:343–348. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]
- Hutton JL, Baracos VE, Wismer WV. Chemosensory dysfunction is a primary factor in the evolution of declining nutritional status and quality of life in patients with advanced cancer. J Pain Symptom Manage. 2007;33:156–165. doi: 10.1016/j.jpainsymman.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Iregren A, Andersson M, Nylen P. Color vision and occupational chemical exposures: I an overview of tests and effects. Neurotoxicology. 2002;23:719–733. doi: 10.1016/S0161-813X(02)00088-8. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Pantelis C, Robbins T, Weickert T, Weinberger DR, Goldberg TE. Intra-dimensional/extra-dimensional set-shifting performance in schizophrenia:impact of distractors. Schizophr Res. 2007 Jan;89:339–349. doi: 10.1016/j.schres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Kaufman KR, Brey RH, Chou LS, Rabatin A, Brown AW, Basford JR. Comparison of subjective and objective measurements of balance disorders following traumatic brain injury. Med Eng Phys. 2006;28:234–239. doi: 10.1016/j.medengphy.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: Use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, Dolbec J. Neurotoxic effects of low-level methylmercury contamination in the amazonian basin. Environ Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- Letz R, Gerr F, Cragle D, Green RC, Watkins J, Fidler AT. Residual neurologic deficits 30 years after occupational exposure to elemental mercury. Neurotoxicology. 2000;21:459–474. [PubMed] [Google Scholar]
- LoPachin RM, Gavin T, Geohagen BC, Das S. Neurotoxic mechanisms of electrophilic type-2 alkenes: Soft soft interactions described by quantum mechanical parameters. Toxicol Sci. 2007;98:561–570. doi: 10.1093/toxsci/kfm127. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, 3rd, Silveira LC, Perry VH, Merigan WH. Visual effects of damage to P ganglion cells in macaques. Vis Neurosci. 1992;8:575–583. doi: 10.1017/s0952523800005678. [DOI] [PubMed] [Google Scholar]
- Maurissen JPJ, Weiss B. Vibration sensitivity as an index of somato-sensory function in monkeys and humans. In: Spencer PS, Schaumberg HH, editors. Experimental and Clinical Neurotoxicology. Williams and Wilkins; 1980. [Google Scholar]
- Maurissen JP. Psychophysical testing in human populations exposed to neurotoxicants. Neurobehav Toxicol Teratol. 1985;7:309–317. [PubMed] [Google Scholar]
- Maurissen JP, Chrzan GJ. One-year reliability of vibration sensitivity thresholds in human beings. J Neurol Sci. 1989;90:325–334. doi: 10.1016/0022-510x(89)90119-6. [DOI] [PubMed] [Google Scholar]
- Mergler D, Beauvais B. Olfactory threshold shift following controlled 7-hour exposure to toluene and/or xylene. Neurotoxicology. 1992;13:211–215. [PubMed] [Google Scholar]
- Newland MC. Quantification of motor function in toxicology. Toxicol Lett. 1988;43:295–319. doi: 10.1016/0378-4274(88)90035-5. [DOI] [PubMed] [Google Scholar]
- Odkvist LM, Moller C, Thuomas KA. Otoneurologic disturbances caused by solvent pollution. Otolaryngol Head Neck Surg. 1992;106:687–692. doi: 10.1177/019459989210600612. [DOI] [PubMed] [Google Scholar]
- Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lanteri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R EORTC Quality of Life Group. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Ravasco P. Aspects of taste and compliance in patients with cancer. Eur J Oncol Nurs. 2005;9(Suppl 2):S84–91. doi: 10.1016/j.ejon.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Reinders-Messelink HA, Schoemaker MM, Snijders TA, Goeken LN, Bokkerink JP, Kamps WA. Analysis of handwriting of children during treatment for acute lymphoblastic leukemia. Med Pediatr Oncol. 2001;37:393–399. doi: 10.1002/mpo.1216. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Effects of developmental methylmercury exposure or lifetime lead exposure on vibration sensitivity function in monkeys. Toxicol Appl Pharmacol. 1995;134:161–169. doi: 10.1006/taap.1995.1180. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Gimenes LS, Eckerman DA, Kang SK, Farahat FM, Anger WK. Development of the behavioral assessment and research system (BARS) to detect and characterize neurotoxicity in humans. Neurotoxicology. 2003;24:523–531. doi: 10.1016/s0161-813x(03)00023-8. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Lasarev M, Anger WK, Scherer J, Stupfel J, McCauley L. Neurobehavioral performance of adult and adolescent agricultural workers. Neurotoxicology. 2007;28:374–380. doi: 10.1016/j.neuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Rosenblum S. The development and standardization of the children activity scales (ChAS-P/T) for the early identification of children with developmental coordination disorders. Child Care Health Dev. 2006;32:619–632. doi: 10.1111/j.1365-2214.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- Savic G, Bergstrom EM, Davey NJ, Ellaway PH, Frankel HL, Jamous A, Nicotra A. Quantitative sensory tests (perceptual thresholds) in patients with spinal cord injury. J Rehabil Res Dev. 2007;44:77–82. doi: 10.1682/jrrd.2005.08.0137. [DOI] [PubMed] [Google Scholar]
- Schiff D, Wen P. Central nervous system toxicity from cancer therapies. Hematol Oncol Clin North Am. 2006;20:1377–1398. doi: 10.1016/j.hoc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51:19–40. doi: 10.1016/j.survophthal.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Schreiber JS, Hudnell HK, Geller AM, House DE, Aldous KM, Force MS, Langguth K, Prohonic EJ, Parker JC. Apartment residents’ and day care workers’ exposures to tetrachloroethylene and deficits in visual contrast sensitivity. Environ Health Perspect. 2002;110:655–664. doi: 10.1289/ehp.02110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Jr, Beck BD, Cory-Slechta DA, Paule MG, Anger WK, Bellinger D. Cognitive tests: Interpretation for neurotoxicity? (workshop summary) Toxicol Sci. 2000;58:222–234. doi: 10.1093/toxsci/58.2.222. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, Pryce CR. Performance of the marmoset monkey on computerized tasks of attention and working memory. Brain Res Cogn Brain Res. 2004;19:123–137. doi: 10.1016/j.cogbrainres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Taillibert S, Voillery D, Bernard-Marty C. Chemobrain: Is systemic chemotherapy neurotoxic? Curr Opin Oncol. 2007;19:623–627. doi: 10.1097/CCO.0b013e3282f0e224. [DOI] [PubMed] [Google Scholar]
- Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: Report of a workshop. J Clin Oncol. 2004;22:2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Hester KD, Felix ER, Hollins M, Favorov OV, Quibrera PM, Whitsel BL. Human vibrotactile frequency discriminative capacity after adaptation to 25 hz or 200 hz stimulation. Brain Res. 2005;1057:1–9. doi: 10.1016/j.brainres.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Vannozzi G, Cereatti A, Mazza C, Benvenuti F, Della Croce U. Extraction of information on elder motor ability from clinical and biomechanical data through data mining. Comput Methods Programs Biomed. 2007;88:85–94. doi: 10.1016/j.cmpb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- Wampler MA, Hamolsky D, Hamel K, Melisko M, Topp KS. Case report: Painful peripheral neuropathy following treatment with docetaxel for breast cancer. Clin J Oncol Nurs. 2005;9:189–193. doi: 10.1188/05.CJON.189-193. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Kayl AE, Meyers CA. Neuropsychological dysfunction associated with cancer and cancer therapies: A conceptual review of an emerging target. Br J Cancer. 2004;90:1691–1696. doi: 10.1038/sj.bjc.6601772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, James KE, Vasterling JJ, Letz R, Marans K, Delaney R, Krengel M, Rose F, Kraemer HC. Neuropsychological screening for cognitive impairment using computer-assisted tasks. Assessment. 2003;10:86–101. doi: 10.1177/1073191102250185. [DOI] [PubMed] [Google Scholar]
- Yokoo EM, Valente JG, Grattan L, Schmidt SL, Platt I, Silbergeld EK. Low level methylmercury exposure affects neuropsychological function in adults. Environ Health. 2003;2:8. doi: 10.1186/1476-069X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoni S, Albini E, Lucchini R. Neuropsychological testing for the assessment of manganese neurotoxicity: A review and a proposal. Am J Ind Med. 2007;50:812–830. doi: 10.1002/ajim.20518. [DOI] [PubMed] [Google Scholar]