Abstract
The role of estrogen exposure in breast cancer risk is well-documented, and both estrogen synthesis and actions through the estrogen receptor (ER) have been targeted by therapies to control hormone-dependent breast cancer. The discovery of a second ER form and its therapeutic implications sparked great interest. Both the original ERα and the more recently identified ERβ subtypes bind and respond similarly to many physiological and pharmacological ligands. However, differences in phytoestrogen binding have been noted, and subtype-specific ligands have been developed. Cell-based assays show that ERβ and its variants are generally less active on gene transcription than ERα, and may influence ERα activity; however, both gene- and cell-specific responses occur, and nongenomic activities are less well explored. Specific ligands, and methods to disrupt or eliminate receptor subtype expression in animal and cell models, demonstrate that the ERs have both overlapping and distinct biological functions. Overall, in cell-based studies, ERα appears to play a predominant role in cell proliferation, and ERβ is suggested to be antiproliferative.
The potential for distinct populations of breast tumors to be identified based on ER subtype expression, and to exhibit distinct clinical behaviors, is of greatest interest. Several studies suggest that the majority of ER-positive tumors contain both subtypes, but that some tumors contain only ERβ and may have distinct clinical behaviors and responses. Expression of ERβ together with ERα favors positive responses to endocrine therapy in most studies, and additional studies to determine if the addition of ERβ to ERα as a tumor marker is of clinical benefit are warranted. In contrast, the positive association between ERβ and HER2 expression in high-grade ERα-negative breast cancer does not favor positive responses to endocrine therapy. Expression of ERβ in specific clinical subpopulations, and the potential for therapies targeting ERβ specifically, is discussed.
Introduction
The steroid hormone 17β-estradiol (E2) plays an important role in the development and growth of the mammary gland during puberty, pregnancy, and lactation, as well as cell proliferation under both physiological and pathophysiological states [1]. Increased time of E2 exposure, including early menarche and later menopause, is associated with increased risk of breast cancer, and may contribute to tumor growth [2, 3]. E2 treatment stimulates breast cancer cell proliferation in vitro, and the growth of human tumor cell xenografts in nude mice [1]. The ability of E2 to modulate gene transcription is well-documented, although additional biological activities such as cytoplasmic signaling have recently been described [1].
E2 exerts its biological responses by binding to two estrogen receptor (ER) subtypes, ERα and ERβ. For many years the presence of immunopositive ERα, alone or with expression of the E2-stimulated progesterone receptor (PR), was used as a criterion for treatment of patients with adjuvant antiestrogen therapy such as tamoxifen (TAM) or ICI 182,780 (fulvestrant/faslodex) [2–5], or recently, aromatase inhibitors that prevent E2 biosynthesis [6]. ERα is present in 40–70% of breast tumors; with ER+/PR+ tumors accounting for approximately 30–50% and ER+/PR-representing approximately 10–20% of all breast tumors [2–4]. Although data on aromatase inhibitor therapy is still accumulating, only 40–80% of patients with ERα-positive (ER+) tumors respond to adjuvant antiestrogen treatment with longer time to recurrence (disease-free or relapse-free survival time), and the majority of patients eventually acquire resistance to such therapies [7, 8]. Consequently, additional markers to predict clinical responses are still needed and sought. The absence of PR has been one predictor for poor responses to TAM [7, 8]. Because the PR gene is ER-regulated, its expression has been interpreted to indicate a functioning ER and, therefore, an E2-responsive tumor. Alternative growth factor-sensitive pathways, identified by the presence of the EGF receptor family member HER2, are also associated with poor response to endocrine therapy and may cause decreased PR expression [8–10]. The discovery in the mid 1990’s that there are two subtypes of ER, with different expression profiles in normal and malignant tissues, opened the door to the possibility that ER+ breast tumors might be even more heterogeneous than originally supposed [1]. The first ER identified and the first to determine breast tumor ER status was named ERα, and the more recently isolated receptor named ERβ. As tools to identify and measure ERβ have become available, its potential role in breast tumor formation and response to endocrine therapy is of considerable interest and investigation, and is the focus of this review.
2. Structure of ERα and ERβ
Both ERα (595 aa) and ERβ (530 aa) are members of the nuclear receptor superfamily. Although they are encoded by separate genes on different chromosomes, they have similar modular protein structures with considerable homology (Fig. 1). The DNA-binding (DBD) regions have 96% homology and bind most estrogen response elements (EREs) identically. However, because other ERα and ERβ regions differentially associate with tethered transcription factors such as Sp1 or AP-1 to modulate gene transcription, ER subtype-specific gene regulation can occur [1, 11]. Genes regulated in this manner include cyclin D1, through CREB and AP-1 sites, and IGF-1 through the AP-1/Jun/Fos complex [12, 13]. The ligand-binding (LBD) regions have approximately 54% homology, and bind natural estrogens and several selective estrogen receptor modulators (SERMS) such as TAM with similar affinity [14]. However, phytoestrogens bind preferentially to ERβ, and SERMS that activate or inhibit only one ER subtype have been synthesized and allow independent control of subtype activity [14,15]. Binding of E2 to either ER reorganizes the LBD structure, and allows binding of coactivator proteins such as steroid coactivator-1 (SRC-1) or SRC-3, also called Amplified in Breast Cancer-1 (AIB1) [1]. These proteins, alone or with coregulators such as CBP, have histone-modifying activity that influences chromatin accessibility to the transcription machinery. In contrast, binding of antagonists like TAM to ERα increases association of corepressor proteins NCOR and SMRT that actively repress gene transcription. ERβ also binds corepressors in the absence of ligand [16]. Consequently, alteration in the levels or ratio of coactivators to corepressors may alter tissue or tumor responses to SERMS and differentially affect ERα and ERβ [16, 17].
Fig. 1.
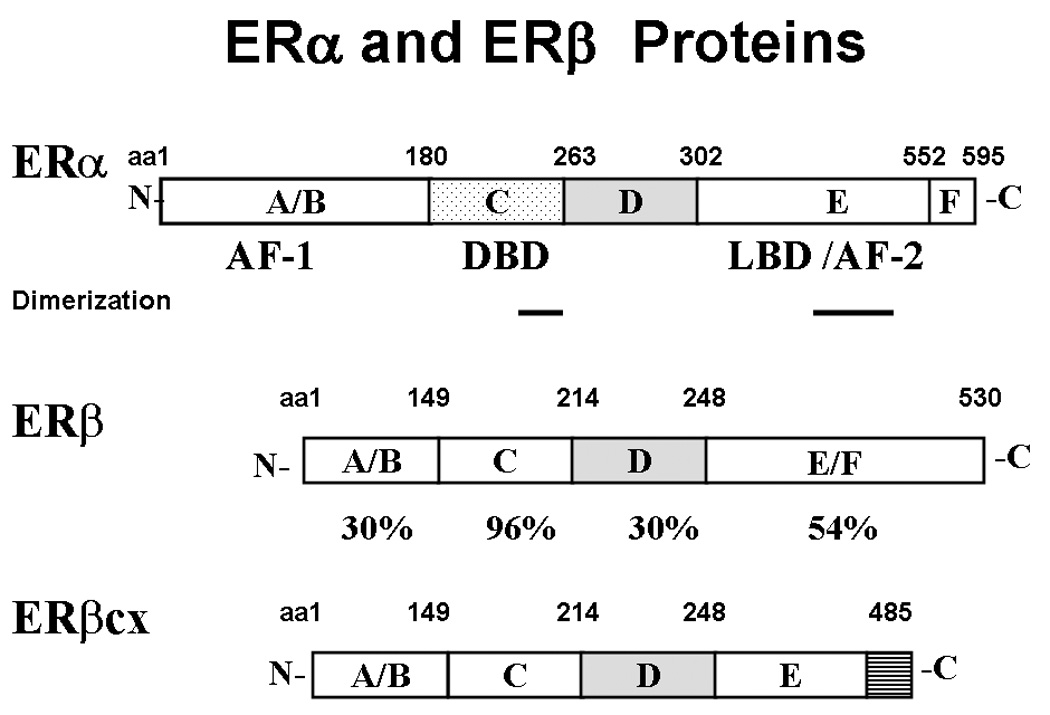
Structures of the two ER subtype proteins, and the ERβ variant ERβcx. The amino acid positions for each structural motif are shown above the proteins, and the percent amino acid homology between the two subtypes is shown below ERβ. ERβcx has 100% homology with ERβ, except for replacement of the last 61 amino acids by 26 novel amino acids, shown by the striped bar.
The human ERα and ERβ genes have been isolated and each contains eight exons [1, 5]. For both genes, alternative mRNA splicing, in which one or more coding exons are deleted, results in proteins with altered functional domains and either dominant-negative, dominant-positive, or null biological activity [reviewed in 5]. All ERα variants in breast cancer occur along with full-length ER, and are generally present at low amounts; their clinical relevance is unproven. ERβcx, also known as ERβ2, is a splice variant with potential clinical relevance. This variant is generated by replacing the last 61 amino acids of ERβ with 26 novel amino acids (Fig. 1). The resulting translated protein does not respond to ligand, exhibits lost or reduced DNA binding abilities, and has dominant-negative activity against ERα [18]. Although it has been reported to be present at high levels and may correspond with an ER+/PR- phenotype in some breast cancers, the clinical significance or causal effect for any phenotype is unproven [18].
3. ERα and ERβ Activity – Homodimers and Heterodimers
a. Transcriptional Activity of ERβ versus ERα
ERα and ERβ are often coexpressed, and could contribute to physiological responses as either homodimers or heterodimers [19]. As suggested by their structures, the ERs have both common and divergent activity. ERα alone has greater transcriptional activity than ERβ on ERE reporters or on chromatin in cell-based assays [20–22]. In ERα the N-terminal ligand-independent activation function AF-1, and the C-terminal ligand-dependent AF-2, act independently and cooperatively to stimulate ERE-reporter activity [21]. In contrast, the isolated AF-1 of ERβ cannot activate transcription, and AF-2 has higher activity than the entire receptor, suggesting a repressive role for AF-1 [23].
AF-1 domains of each receptor play a critical role in their activity, but ligands determine biological responses [23–26]. SERMS, phytoestrogens, derivatives, and metabolites exhibit ER isoform specificity in binding and activation in different cell and promoter contexts [20–25]. For example, TAM stimulates ERα, but not ERβ, through the N-terminus to activate EREs and complex promoters in uterine cells, thus contributing to agonist activity of TAM in that tissue [20, 21]. In breast cancer cells E2-bound ERα stimulates the proliferative gene cyclin D1, but ERβ only activates this promoter in response to SERMS such as TAM or raloxifene [13, 26]. Thus, the complement of ER subtypes could influence biological responses, and the ratio between the two expressed proteins would be critical in defining the overall response.
Microarray analysis of endogenous gene expression illustrates both common and divergent genes regulated by ERα versus ERβ in the reproductive tissues of mice treated with isoform-specific ligands [27, 28], or in bone from ERα (ERKO) and ERβ (BERKO) gene-disrupted mice [29]. Effects of ERβ-E2 on gene expression were generally lower than ERα-E2 [29]. In breast cancer cells, both ERs stimulate some cell cycle regulation genes such as pS2, TGFα, and p21Cip1, but only ERα stimulates c-myc [30]. ERβ in the absence of ligand regulates expression of many genes modulated by ERα plus E2, whereas ERβ plus E2 modulates some genes not regulated by ERα-E2 [31–33]. Because context plays a major role in ER responses, it is critical to evaluate the role of ERβ in model systems expressing both ER subtypes.
b. Influence of ERβ on ERα Actions: Novel Activity of Heterodimers
ERα and ERβ preferentially form functional heterodimers that bind DNA with an affinity similar to that of ERα homodimers, and greater than that of ERβ homodimers [34]. Many cell-based studies suggest that ERβ acts as a negative modulator of ERα action. When ERα and ERβ are co-transfected into ER-negative (ER-) cells, ERβ inhibits ERα transcriptional activity and decreases the sensitivity of the cells to E2 [1, 35]. In HeLa cells transfected with ERα or ERβ, ERβ inhibits cyclin D1 gene activation by E2, suggesting that ERβ may suppress proliferation [36].
Importantly, ERβ can influence endogenous ERα activity in ER+ breast cancer cells. Overexpression of ERβ or ERβcx decreases ERα transcriptional activity and E2 stimulation of endogenous genes such as VEGF or PR [18, 37, 38]. Chromatin immunoprecipitation assays show that ERβ alters ERα-mediated recruitment of c-Fos and c-Jun and decreases ERα recruitment to E2-responsive promoters such as PR [39]. ERβ also increases the E2-induced degradation of ERα, and lowers overall ERα mRNA and protein levels in MCF7 cells, thus indirectly influencing ERα responses [39, 40]. Interestingly, female mice in which the ERβ gene has been disrupted do not show large differences in expression of ERα in many tissues, including the mammary gland [41].
4. Crosstalk with Growth Factors and “Nongenomic” E2 Responses
Crosstalk between growth factor and ER pathways may contribute to the pathology of breast cancer, and growth factors can activate ERs independently of hormone [42]. For example, EGF stimulates ERα activity and coactivator binding via MAPK phosphorylation of Ser118, and ERβ through MAPK phosphorylation of Ser60 [42, 43]. Overexpression of the EGFR family member HER2 or constitutive activation of the PI3 kinase pathway also activates ERα in MCF7 cells [44, 45].
ERs reciprocally impact growth factor and other signaling pathways through rapid “nongenomic” effects occurring independently of transcription and translation. These E2-activated responses are thought to be mediated through a membrane-associated population of classical ERs. ERα and ERβ are detectable in membrane fractions of breast cancer cells [46] and by immunohistochemistry (IHC) in breast tumor samples within and outside the nucleus [47]. In ER+ MCF7 cells, E2 increases ERα association with c-Src, Shc, and the IGF-1 receptor, ERα-dependent PI3 kinase activation, and proliferation [48–50]. Both ER subtypes can signal via nongenomic pathways, but cell context, including levels of ER expression or stoichiometry between signaling molecules, may be crucial. In ER- cells, introduction of ERα or ERβ permits E2 activation of G proteins and MAPK [42]. E2 stimulates MAPK and Akt and is anti-apoptotic in ERα-transfected HeLa cells, but activates p38 and apoptosis in ERβ-transfected cells [51].
Crosstalk between ER and growth factor pathways may provide alternative growth pathways in breast tumors treated with SERMS. For example, HER2 expression correlates with decreased responsiveness to TAM, but perhaps not to aromatase inhibitors [52–56]. Inhibition of both E2 and growth factor signaling in breast cancer cells is more effective in suppressing growth than either strategy alone [54, 55]. Clinical trials directed towards joint therapies have begun investigating this potential [10, 56].
5. ERα and ERβ Expression and Activities
The two ER subtypes have both overlapping and distinct expression patterns, and mammary gland development in animal models requires ER signaling. Although a consensus has now been reached regarding the characteristics of the mammary gland phenotype in ERKO mice, some phenotypic differences exist amongst BERKO mouse models that were developed and housed in different laboratories [1, 19, 41]. In mice lacking ERα or both ERs, both sexes exhibit complete infertility. Female BERKO mice exhibit a range of deficiencies from total infertility to fewer pregnancies and smaller litter size. Most male BERKO mice maintain normal fertility, but one group has reported sterility in male and female BERKO mice [19, 41]. ERKO mice undergo normal pre-pubescent development of mammary glands, but not the extensive expansion and branching of ducts to infiltrate the entire fat pad observed in WT mice. In BERKO mice, this development is not impaired, nor is lactation [19]. However, one group has observed decreased terminal differentiation, adhesion molecule expression, and increased proliferation marker expression in the alveoli of lactating BERKO mice [57].
Human mammary tissue expresses both ER subtypes [58, 59]. It is estimated that only 7–10% of the epithelial cells in normal human breast express ERα, whereas 80–85% of cells express ERβ; only ERα expression fluctuates during the menstrual cycle [58, 59]. Surprisingly, both ERα and ERβ are seen primarily in non-proliferating cells [60, 61]. It has been hypothesized that dysregulated ER signaling may lead to abnormal cellular proliferation and survival, thus impacting development and progression of breast cancer. In breast tumors, ERα expression increases several-fold compared to normal tissue, with 75% of the cells expressing high levels of ERα in low-grade ductal carcinoma in situ (DCIS) and 30% of the cells expressing low levels of ERα in high-grade DCIS [5, 62]. DCIS lesions have reduced ERβ expression compared with normal epithelium, with high-grade DCIS showing the most significant reduction [61]. Invasive breast carcinomas tend to have lower levels of ERα and ERβ than DCIS, but approximately two-thirds of the tumors are still positive by IHC [63]. Interestingly, hypermethylation of one of two ERβ promoters was observed in cell lines and tumors, and correlated with decreased ERβ expression [64].
To date, it appears that ERα expression is increased and ERβ expression is decreased in early breast cancers, whereas expression of both receptors declines in more invasive cancers [61–63]. This correlates with the loss of ERβ preferentially in other cancers compared to normal tissue, and led to the hypothesis that ERβ is a tumor suppressor [65, 66]. Some investigators have proposed that the ratio between the two subtypes is most important in determining the character of ER signaling [62]. In addition, ligand binding may influence ER protein stability. In breast cancer cells, ERα and ERβ protein levels are decreased by E2 but increased by TAM [67]. Faslodex treatment results in ERα degradation, but ERβ stabilization [68]. Thus, specific SERM treatments may result in altered ERα:ERβ ratios.
6. Potential Role of ERα/ERβ Heterodimers in Biological Responses and Breast Cancer Proliferation
Given that ERβ inhibits ERα transcriptional activity, it was hypothesized that ERβ is antiproliferative. In support of this, abnormal epithelial growth, expression of the proliferation marker Ki67, and age-related cystic breast disease were observed in one model of BERKO mice [57, 69]. However, these results have not been replicated in other BERKO mouse models, and normal mammary gland histology was observed in a recently developed model of old BERKO females [41]. Microarray analysis showed that ERβ modulates expression of many ERα-regulated genes in ER+ breast cancer cells, including TGFβ and class 3 semaphorins, which are involved in cell proliferation [40]. ERβ overexpression in ERα+ MCF7 and T47D breast cancer cells also inhibits ERα regulation of a subset of genes involved in DNA replication, cell-cycle regulation, and proliferation [32, 33]. Furthermore, in ER+ breast tumor samples there was a significant inverse correlation between ERβ transcripts and several cell cycle and DNA replication genes including CDC2, CKS2, and CDC6, suggesting that ERα/ERβ heterodimers negatively affect breast cancer proliferation [32].
This ability of ERβ to transcriptionally inhibit proliferative gene expression in breast cancer cells was confirmed by RT-PCR, in vitro cell proliferation assays, and in vivo xenografts. Overexpression of ERβ in ERα+ MCF7 and T47D breast cancer cells inhibits cell proliferation in response to E2 [33, 40, 70, 71], in part by increasing expression of antiproliferative genes (p21Cip1 and p27 Kip1) and decreasing expression of proliferative and antiapoptotic genes (c-myc, cyclin A, and cyclin D1), thus inducing G2 cell cycle arrest [40, 70]. ERβ overexpression also inhibits tumor establishment and growth as well as E2-induced tumor formation in vivo in mouse xenografts of MCF7 and T47D cells [70–72]. Decreased microvessel density and angiogenesis and expression of the proangiogenic factors VEGF and PDGFβ is also observed [72]. ERβ or ERβcx expression in MCF7 cells decreases the percentage of cells in the S phase of the cell cycle [37, 71], as well as the number of colonies that grow in soft agar [37].
Although ERβ reduces E2-stimulated proliferation in ERα+ breast cancer cells, it can have the opposite effect in more invasive ER- breast cancer cells. Introduction of ERβ into ER-MDA-MB-435 and MDA-MB-231 breast cancer cells increases cell proliferation in an E2-independent manner, cell invasiveness, and metastasis [73]. In contrast, introduction of ERα into ER- breast cancer cells generally decreases their proliferation in an E2-dependent manner, and their invasiveness and migration by both ligand-dependent and ligand-independent mechanisms [74, 75]. Thus, ERβ actions on proliferation and the potential value of ERβ-specific ligands may depend on cellular context and ERα status.
Coexpression of ERβ with ERα does not confer TAM resistance in cell lines, and appears to favor antiproliferative responses to TAM [31, 76, 77]. ERβ overexpression in MCF7 cells enhances TAM suppression of cell growth [76]. A recent study showed that ERβ overexpression in MCF7 cells influences TAM action on 75% of the genes regulated by ERα, including reversal of TAM stimulation of YWHAZ/14-3-3z and LOC441453, two genes whose high expression significantly correlates with disease recurrence in patients with ER+ tumors treated with TAM [31]. Thus, cellular levels of ERα and ERβ can determine sensitivity to E2 and SERMs, and increased ERβ expression favors TAM suppressive responses in cell models.
7. Coexpression of ERβ with ERα and Other Potential Markers in Human Tumors
Coexpression studies of ERα and ERβ have been performed at the mRNA level using semiquantitative or quantitative RT-PCR and at the protein level using primarily IHC. RT-PCR fails to take into account translational control or turnover of protein, and has the potential to measure ER expression in contaminating cell types such as normal epithelial and stromal vascular cells [78, 79]. The region of the mRNA targeted by primers may also influence which variants are detected. Several investigators have reported that ERβ mRNA levels often do not correlate with ERβ protein in breast tumor tissues [80–84], and confusion with ERβ status in tumors may result from this method. If fact, in one report only 54% of ERβ mRNA-positive tumors correlated with ERβ protein levels [84]. Another method to measure functional ER protein, by ligand-binding assay with dextran-coated charcoal, seems to correlate well with ERα, but not necessarily with ERβ in tumors expressing only one ER subtype based on IHC [81]. Although both ERs bind E2 identically, lower levels of ERβ versus ERα in tumors, and lower levels of ERβ in tumors versus normal tissue, may mean that ERβ protein is under the detection level of the ligand-binding assay in tumors. Thus, detecting ERβ protein by IHC appears to be the preferred method to assess ERβ status, providing both a measure of potentially functional protein and localization to tumors. Because it is protein that should influence tumor phenotypes and therapeutic responses, we will confine our discussion to studies that measured ER proteins.
Recently, reliable antibodies detecting either N- (such as Ab288/14C8) or C-terminal (such as GC17/385P) epitopes of ERβ have been developed [79]. N-terminal antibodies recognize both full-length ERβ and its splice variants (Fig. 1), whereas ERβ C-terminal antibodies specifically detect the wild type or variant forms of ERβ [79] and allow distinctions to be made between full-length ERβ and its variants. Both antibody types have been used; however, sample preparation and integrity as well as specific staining protocols may influence outcomes [79]. With variation in the choice of ERβ antibody and tissue fixation protocols, there is not yet firm agreement on the appropriate cutoff value for determining ERβ positivity [78, 82].
Even with these caveats, a picture of ERβ expression in breast cancer is emerging. Coexpression of both ERα and ERβ protein within the same cell is more common in breast tumor versus normal tissue, and although ERα protein levels increase with tumorigenesis, ERβ protein levels generally decrease [85]. Combined analysis from several studies showed that approximately 55% of all breast tumors were positive for both ERs, while single positive and double negative classifications each accounted for 13–16% of the tumors [81, 85]. In general, expression of ERβ is low in these tumors [63, 85]. In one instance ERβ was observed in over 40% of the ERα- samples analyzed [86], and ERβ variants such as ERβcx have been noted in up to 60% of ERα- samples [87]. Thus, there may be subpopulations of ERβ+ tumors, with and without ERα, with potentially different phenotypes.
PR is frequently used as an indicator of ER activity and is strongly correlated with ERα expression [81, 85]. Studies examining correlations between ERβ and PR have reported either no correlation [88, 89], a weak positive correlation [81, 90], or an inverse correlation [91]. One confounding variable for interpreting these studies is that there are two populations of tumors - one containing ERβ only, and another expressing both ERα and ERβ, which may have different phenotypes. Within this context, one study specifically analyzed significant numbers of tumors expressing ERβ only compared to those that express both ERs [81]. In this study, tumors that were ERα+/ERβ+ or ERα+/ERβ− had a strong correlation with PR expression, whereas ERα− /ERβ+, i.e., ERβ only tumors, had no correlation with PR [81]. Thus, the number of tumors included in a study expressing ERβ only could influence the overall correlation with PR. ER expression may also correlate with expression of coregulatory proteins such as SRC-1, AIB1, and NCoR [92–94], and may impact therapeutic response. For example, high SRC-1 expression is associated positively with endocrine therapy resistance and inversely with ERβ expression [93, 94]. However, there is no simple correlation for all coregulators, and changes in the overall ratio of coactivators to corepressors, or specific coactivators, may be most important [85].
HER2 overexpression is a poor prognostic indicator in breast cancer and has been implicated in TAM resistance [8–10]. In general, HER2 gene amplification and protein overexpression are inversely correlated with expression of ERα, or with ERα and ERβ together [63, 90, 94–97]. Some ER+ tumors have been shown to have HER2 gene amplification, and patients treated with TAM whose tumors are HER2+/ER+ have poorer disease-free survival (DFS) and overall survival (OS) than those without HER2 [10, 96]. In contrast, there is a positive association between ERβ and HER2 expression in high-grade ERα-breast cancer [98]. In invasive ductal carcinoma, HER2 overexpression correlates inversely with ERα levels, but positively with ERβ [90]. Thus, ERα−/ERβ+ tumors tend to express HER2 but not PR, whereas ERα+/ERβ+, like ERα+/ERβ− tumors, tend to express PR but not HER2. ER+ (ERα+) breast tumors are most often thought to resemble luminal epithelial breast cells, whereas ER− (ERα−) tumors have gene expression levels resembling myoepithelial cells [79]. It is of interest to note that ERβ is the only ER expressed in breast myoepithelial cells, and thus ERα−/ERβ+ tumors might arise from a different cancer cell population than those expressing ERα. However, the reason for such different phenotypes at this time relies only on speculation, and requires additional information.
8. Clinical Correlations Between ERβ Expression, Proliferation, and Invasiveness
Whereas ERα expression increases during breast tumorigenesis, ERβ decreases [61, 85, 99]. ERβ protein expression decreases significantly from normal breast tissue through ductal hyperplasia and DCIS to invasive cancer [99], and is reduced in proliferative preinvasive mammary tumors [61]. Increased ERβ promoter methylation, indicative of lower gene expression, was noted in premalignant lesions as well as in two-thirds of invasive breast cancer, and corresponds with poor clinical prognosis [64].
In general, ERα protein expression correlates with low tumor grade and negative lymph node status, and ERα+ tumors are associated with better DFS and OS [63, 81, 82]. ERα+ tumors are usually less invasive and have a more favorable prognosis [100]. However, the correlation between ERβ expression and invasiveness is less clear. Although numerous researchers found no significant correlation between ERβ protein expression and tumor grade [81, 82, 88, 90, 95, 97, 98, 101–104], several groups showed that ERβ expression significantly correlates with low tumor grade [63, 80, 105] or in one case higher histological grade [89]. In these studies, all ERβ-containing tumors were analyzed together and included ERα+/ERβ+ (the majority) as well as ERα−/ERβ+ tumors.
Other clinical studies have investigated the role of ERβ in breast cancer proliferation. ERβ protein expression in breast cancer epithelium is associated with elevated levels of the proliferation markers Ki67 and Cyclin A, whereas ERα is associated with decreased levels [98, 106]. Ki67 and Cyclin A were expressed at the highest levels in ERα−/ERβ+ tumors, and ERβ protein expression significantly correlated with Ki67 staining in ERα− tumors [82, 87, 106], suggesting that ERβ may play a role in the proliferation of ERα− breast tumors. In contrast, one group reported an inverse correlation between ERβ protein and Ki67, especially in high grade DCIS [61]. Other studies suggested that ERβ and Ki67 expression are not associated in ERα+/ERβ+ tumors [81, 102], but ERα− tumors had higher Ki67, with or without ERβ [81]. These investigators propose that ERα status could be most critical in determining some, but not all, prognostic factors [81]. Thus, there is some evidence supporting potentially separate roles of ERβ in ERα+/ERβ+ (antiproliferative) versus ERα−/ ERβ+ (proliferative) tumors, but additional data focusing on these groups needs to be obtained. A recent study concentrating exclusively on 216 ERα−/ERβ+ tumors defined by IHC and ligand-binding assays noted that the latter group of tumors also expressed markers such as CK5/6 or CK14 of the basal epithelial phenotype, and that ERβ is widely expressed in basal myoepithelium and luminal epithelium in normal breast [87]. Although proliferation and basal phenotype markers are associated with poor survival, the ERα−/ERβ+ patients in this study had no differences in clinical outcome (relapse-free survival-time to progression or OS) that correlated with either high versus low levels of ERβ, or high versus low markers of proliferation or markers of the basal phenotype. The authors postulate the wide variety of treatments the cohort received may have influenced this result [87].
Although several studies have reported no significant association between ERβ expression and metastasis based on axillary lymph node status [82, 88, 89, 97, 104, 107], a few showed that ERβ protein expression correlates with negative axillary lymph node status [63, 93, 102, 105]. In one study, a gradual reduction in, but not a complete loss of, ERβ expression was observed during the transition from normal and pre-invasive lesions to invasive cancers, where ERβ was lost in 21% of cases. If ERβ was in the primary tumor, it persisted in metastasis [103].
Clinical correlations between expression of the ERβ variant ERβcx/β2 and breast tumor invasiveness have also been explored in a few studies. ERβcx protein levels, which in general are more highly expressed in breast cancer tissue versus normal tissue, significantly increase from normal glands to DCIS and invasive cancer [80, 108]. Since ERβ and ERβcx are still frequently expressed in ERα- invasive breast tumors [79, 87, 99, 109], they may be potential therapeutic targets in these cancers. A few studies have shown that ERβcx protein expression does not correlate with tumor size, histological grade, or lymph node status [18, 83, 110]. However, Sugiura et al. reported that ERβcx protein significantly correlates with ERα expression and low histological grade [105], and decreased ERβcx protein expression was correlated with venous invasion of cancer cells in one study [18]. Thus, the relationship between ERβcx and clinicopathological factors requires further investigation.
9. Clinical Correlations Between ERβ Expression and Response to Endocrine Therapy
Numerous clinical studies have shown that ERα expression predicts a greater likelihood of response to TAM therapy and is associated with increased survival in patients treated with adjuvant TAM [2, 7, 82], but only about 40–80% of patients with ERα+ tumors initially respond to TAM, and many of these patients eventually become resistant [7–10]. A recent review notes that nine of ten retrospective studies support the idea that increased expression of ERβ protein in ERα+/ERβ+ breast tumors is associated with higher likelihood of response to endocrine therapy [79]. We found fifteen studies investigating ERβ expression and response to endocrine therapy. Of these, thirteen examined ERβ protein expression using primarily IHC [82, 86, 87, 93, 94, 97, 104, 105, 107, 111–114]. Only three of these studies [82, 97, 107] separate ERβ tumor populations based on ERα status, although the majority of tumors typically contain both subtypes. Both ERβ N- and C-terminal antibodies are utilized in these studies. Although more recent studies tend to use the more specific C-terminal antibody, there has been no significant correlation to date between the type of antibody used and prognosis.
Of the thirteen studies examining ERβ protein expression (Table 1), one reported no significant correlation between ERβ levels and the response to TAM therapy in ERα+ only tumors [111]. Another group looked exclusively at ERα− tumors from patients treated with hormonal therapy, chemotherapy, or radiotherapy, either alone or in combination [87]. They observed no difference in relapse-free survival (RFS) or OS between patients whose tumors express high versus low ERβ levels, although they did not compare this to survival in patients with ERβ- tumors [87]. However, ten out of thirteen studies suggest that increased ERβ expression predicts a more favorable response to endocrine therapy as well as better disease outcome. These studies look at either ERα+ only tumors, or a mixed cohort containing primarily ERα+ but also ERα− tumors. Iwase et al. have reported that patients with ERβ+ tumors tend to have a better response to endocrine therapy than those with ERβ− tumors [112]. In breast cancer patients treated with adjuvant TAM, high ERβ expression significantly correlates with increased overall [86, 107] and disease-free survival [107], no disease progression [113], or no relapse within five years [93, 114]. In patients treated with chemotherapy as well as TAM, ERβ expression also significantly correlates with increased OS [97, 105] and DFS [94, 97, 104, 105]. Higher ERβ expression is observed more frequently in TAM-sensitive breast tumors than in TAM-resistant tumors [113], and lower ERβ is associated with TAM resistance [107, 113, 114]. One study analyzing 138 postmenopausal patients with invasive cancer observed a trend toward worse outcome in ERβ+ patients treated with TAM, although it was not statistically significant and only seventeen ERβ− tumors were used in this comparison. ERα status was not defined for the ERβ− cohort, but a trend for worse OS was seen in a mixed cohort of ERα+ and ERα− tumors as well as in ERα+ only tumors [82]. Thus, out of a total of 1,463 breast cancer patients from thirteen clinical studies, high ERβ expression is associated with a better response to TAM therapy and increased patient survival in 1,079 patients, compared to a trend towards a worse response in only 138 patients.
Table 1.
Summary of studies evaluating ERβ and ERβcx protein expression and response to endocrine therapy in breast cancer
| Author | Patients | ERβ Detection, Antibody |
ERα Status | Treatment, Setting | Clinical Outcome |
|---|---|---|---|---|---|
| Mann et al. [84] | 118 | IHC, N | ERα+, − mixed | TAM, Adj | ERβ+ correlated with increased OS |
| Omoto et al. [102] | 88 | IHC, C | ERα+, − mixed | TAM + Chemo, Adj | ERβ+ correlated with increased DFS |
| Murphy et al. [111] | 27 | IHC, N | ERα+ only | TAM, Adj, node - | Higher ERβ correlated with no disease progression |
| Iwase et al. [110] | 77 | IHC, C | ERα+, − mixed | TAM or other Endo therapies, Rec/LABC, NS | ERβ+ trended towards better response to endo therapy |
| Esslimani-Sahla et al. [112] | 50 | IHC, N | ERα+ only | TAM, Adj | Higher ERβ correlated with no relapse within 5 yrs |
| Hopp et al. [105] | 186 | WB, N | ERα+, − mixed | TAM, Adj | Higher ERβ correlated with increased DFS and OS |
| Fleming et al. [91] | 52 | IHC, C | ERα+, − mixed | TAM, Adj | ERβ+ correlated with no relapse within 5 yrs |
| Myers et al. [92] | 150 | IHC, C | ERα+, − mixed | TAM + Chemo, No distant metastases, NS | ERβ+ correlated with increased DFS |
| Nakopoulou et al. [95] | 181 (T) | IHC, C | ERα+, − mixed | TAM + Chemo, Adj | ERβ+ correlated with increased DFS and OS |
| 117 | IHC, C | ERα+ only | TAM + Chemo, Adj | ERβ+ correlated with increased DFS and OS | |
| 61 | IHC, C | ERα− only | TAM + Chemo, Adj | ERβ+ correlated with less favorable DFS and OS | |
| O'Neill et al. [80] | 138 (T) | IHC, C | ERα+, − mixed | TAM, Adj | ERβ+ trended towards worse OS |
| 91 | IHC, C | ERα+ only | TAM, Adj | ERβ+ trended towards worse OS | |
| Miller et al. [109] | 36 | IHC, C | ERα+ only | TAM, NeoAdj | No correlation in response to TAM |
| Skliris et al. [85] | 210 | IHC, C | ERα− only | Hormonal, Chemo, or rad alone or combined, some Meta, NS | No difference in RFS or OS between ERβ levels |
| Sugiura et al. [103] | 150 | IHC, C | ERα+, − mixed | TAM +/− Chemo, Adj | ERβ+ correlated with increased OS and DFS |
| Total = | 1463 |
| Author | Patients | ERβcx Detection, Antibody |
ERα Status | Treatment | Clinical Outcome |
|---|---|---|---|---|---|
| Esslimani-Sahla et al. [112] | 50 | IHC, C | ERα+ only | TAM, Adj | No correlation in response to TAM |
| Palmieri et al. [108] | 23 | WB, C | ERα+, − mixed | Endo therapy, NeoAdj | ERβcx+ correlated with a longer survival rate |
| Saji et al. [18] | 18 | IHC, C | ERα+ only | TAM, NeoAdj | ERβcx+ correlated with poor response to TAM |
| Miller et al. [109] | 36 | IHC, C | ERα+ only | TAM, NeoAdj | No correlation in response to TAM |
| Skliris et al. [85] | 199 | IHC, C | ERα− only | Hormonal, Chemo, or rad alone or combined, some Meta, NS | No difference in RFS or OS between ERβcx levels |
| Sugiura et al. [103] | 150 | IHC, C | ERα+, − mixed | TAM +/− Chemo, Adj | ERβcx+ correlated with increased OS |
| Vinayagam et al. [81] | 141 (T) | IHC, C | ERα+, − mixed | TAM or other endo, Adj | Higher ERβcx correlated with increased RFS |
| 98 | IHC, C | ERα+ only | TAM or other endo, Adj | No correlation between ERβcx levels and survival | |
| Total = | 617 |
Patients: T, total
ERβcx: also known as ERβ2, is a splice variant of full-length ERβ
Antibodies: N, detects an N-terminal epitope of ERβ and ERβcx; C, detects a specific C-terminal epitope of full-length ERβ or ERβcx
Detection: IHC, immunohistochemistry; WB, western blot
Treatment: TAM, tamoxifen; endo, endocrine; chemo, chemotherapy; rad, radiotherapy; Adj, adjuvant; NeoAdj, neoadjuvant; NS, treatment setting not specified; REC, recurrent; LABC, locally advanced breast cancer; Meta, metastatic
Outcome: OS, overall survival; DFS, disease-free survival; RFS, relapse-free survival
Thus, the value of ERβ in predicting response to endocrine therapy may vary with ERα coexpression. The majority of studies in Table 1 indicate that patients with ERα+/ERβ+ tumors respond better to adjuvant endocrine therapy, suggesting that measurement of ERβ status along with ERα may allow for better prediction of response. One group found that patients with ERα+/ERβ+ tumors have increased DFS and OS after chemotherapy and TAM, patients with ERα−/ERβ+ tumors have a less favorable prognosis, and those with ERα−/ERβ− tumors have the worst prognosis [97]. In another study of ER+ patients receiving no adjuvant therapy, patients with ERα+/ERβ+ tumors (n=45) had improved DFS, but patients with ERα− /ERβ+ tumors (n=7) had a significantly worse prognosis [107]. Thus, coexpression of ERα and ERβ, or ERα, ERβ, and PR, may have favorable implications for TAM therapy. Expression of ERβ alone may predict a worse outcome, but significant numbers of patients have yet to be analyzed.
The role of ERβcx has also been investigated in seven studies [18, 83, 87, 105, 110, 111, 114] analyzing protein expression levels (Table 1). Two observed no significant correlation between ERβcx protein expression and response to TAM [111, 114], and a third showed no difference in RFS or OS between patients with ERα− only tumors expressing high versus low ERβcx levels [87]. However, three studies have utilized ERβcx-specific C-terminal antibodies to show that ERβcx expression significantly correlates with increased OS [105], increased RFS [83], and a longer survival rate [110] in a total of 314 patients treated with adjuvant or neoadjuvant endocrine therapy. These three studies used both ERα+/ERβ− and ERα−/ERβ+ tumors together in their analyses. One of the studies also found no correlation between ERβcx protein and outcome when only ERα+ tumors were considered [83]. In contrast, another group reported that ERβcx protein expression correlates with poor responses to TAM treatment, especially in tumors with low PR expression [18]. However, these investigators evaluated core needle biopsies from only eighteen tumors [18]. Thus, although some groups have found ERβcx expression to be beneficial, additional investigations of a possible role of ERβcx expression in TAM therapy are required. Because this protein can form heterodimers with both ERα and ERβ, it might act in opposition to both receptor subtypes, and the overall ER expression (ERα, ERβ, ERβcx) and their ratio might control the therapeutic response.
10. Clinical Correlations Between ERβ Expression and Patient Population
Several groups have examined ER subtype expression in specific patient populations. In general, ERα expression is higher in tumors of older patients, and older or postmenopausal women are more likely to have ER+ tumors than younger women [88, 115, 116]. However, several studies failed to find significant correlations between ERβ expression and patient age [88, 103, 104, 107]. Although one group found no association between ERβ and menopausal status [97], two different studies found that ERβ protein expression significantly correlates with premenopausal status [63, 91].
In general, there is a higher proportion of ER- breast tumors among African American, Hispanic, and Indian women than Caucasian women [116, 117]. Although no studies to date have examined differences in ERβ protein expression with regards to ethnicity, two studies showed that ERβ mRNA levels are significantly decreased in ERα+ breast tumors from African American women (n=18) and from Taiwan-raised East Asian women (n=49), although there is no significant change in ERα mRNA [118, 119]. In contrast, ERβ mRNA levels are either unchanged or increased in ERα− tumors from African American women (n=6) [118]. However, these are small studies and the results need to be confirmed with additional patients and IHC, particularly as ERβ protein does not correlate with mRNA in several studies [80–84]. If apparent mRNA trends are verified at the protein level, it would suggest that ER+ tumors in different ethnic groups could have different clinical phenotypes and responses to endocrine therapy.
However, although limited data exists, studies to date indicate that African American and Caucasian women respond similarly to endocrine therapy, both in the adjuvant and metastatic setting, when they are diagnosed at a comparable disease stage [120–122]. Thus, the mRNA studies [118, 119] do not correlate with overall clinical data, reinforcing the importance of examining protein expression for both ER subtypes. Although African American women in the United States have a higher age-adjusted breast cancer mortality rate, this could be due to more advanced cancer stage at diagnosis, differences in tumor biology that include cell type of origin, sociodemograhic issues, treatment differences, and the presence of comorbid illnesses [120]. One study showed that African American and Caucasian women with both lymph node-negative and lymph node-positive tumors benefit to a similar extent from the addition of systemic adjuvant TAM therapy to surgery [121], and others showed that African American and Caucasian women with ER+ breast cancer experience a similar reduction in contralateral breast cancer following adjuvant TAM treatment [122]. However, both studies included trials with disproportionately more Caucasian participants than African Americans [121, 122], and tumors were not analyzed according to both ER subtypes. A more recent study demonstrates that five years of letrozole treatment following five years of TAM in postmenopausal females with early stage breast cancer significantly improves DFS in Caucasian women but not in minorities [123]. It is important to note, however, that this study included 4,708 Caucasian women but only 352 minority women, about half of which were African American [123]. Furthermore, the results did not distinguish between minority subgroups, and the minority patients had significantly poorer compliance with the study’s protocols than Caucasians [123]. Thus, additional clinical studies including larger numbers of defined groups of minority women, and similar compliance rates, will be required to clarify any racial differences that may exist in response to endocrine therapy.
11. Summary and Future Considerations
The current published data on tumors and patient profiles shows that the majority of ER+ breast tumors contain both ERα and ERβ, and that a small population of tumors contains only ERβ. Data from cell and xenograft studies suggest that ERβ modifies the responses of ERα in breast cancer cells, and is generally antiproliferative. This may be because ERβ is less active transcriptionally and constrains ERα activity through heterodimers, and/or because the heterodimers or ERβ homodimers may have distinct beneficial activities. Because ERβ mRNA levels do not always correspond to protein levels, and protein is the gold standard for evaluation of biological relevance, ERβ measurement by IHC appears to be most valuable. Because ERβ protein measurement does not correspond to ER measurement by ligand-binding assays, and there are significant populations of tumors that express one or both ER subtypes, ERβ is not simply a surrogate for measuring ERα. At this point, measuring total ERβ protein (with N-terminal antibodies) generally gives similar results and correlates with measuring distinct full-length ERβ1 (with C-terminal antibodies).
In the majority of clinical studies, ERβ expression indicates a favorable response to adjuvant TAM therapy, and patients with ERα+/ERβ+ tumors appear to respond at least as well as or better to endocrine therapy than patients with ERα+/ERβ− tumors. Thus, ERβ has emerged as an important potential prognostic marker for predicting response to endocrine therapy. Although some groups have suggested that expression of the variant ERβcx is also beneficial, its role is not clear-cut and requires further investigation. This protein could act in opposition to both beneficial and harmful effects of ERs, and might counteract both ERα and ERβ effects, although this has not been tested directly.
The presence of only ERβ in breast tumors (ERα−/ERβ+ tumors) appears to be associated with a poorer response to endocrine therapy and a poorer clinical outcome than the presence of both ER subtypes. However, it is unknown if this is due to the direct activities of ERβ or the coexpression of other molecules such as HER2 in high grade tumors, or from the development of such tumors from a different type of mammary cancer cell such as myoepithelial cells. Given that combination of the ER subtypes results in novel biological responses, and that crosstalk between E2 and other cellular signaling pathways contributes to proliferation and resistance to endocrine therapy, it is unclear if benefit would be gained from treatment of ERα+/ERβ+ tumors with subtype-specific ligands, or if treatment of ERβ only tumors with ERβ− specific antagonists would be successful or more successful compared to current aromatase inhibitor therapies that prevent activation of both ERα and ERβ. However, the potential to treat ERα−/ERβ+ tumors with specific ERβ antagonists might allow for fewer unwanted effects on ERα-containing tissues, and could be combined with additional therapies based on other tumor signaling molecules, such as those targeting HER2. Measurement of ERα, ERβ, PR and additional molecules such as HER2 and the EGFR in breast tumors could provide important additional information for predicting therapeutic responses and choices. As the degree of heterogeneity of breast tumors is increasingly appreciated, multiple biomarkers for molecular profiling and tracking therapeutic treatment and outcome should increase the potential for choosing optimal treatments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 2.McGuire WL. Endocrine therapy of breast cancer. Annu Rev Med. 1975;26:353–363. doi: 10.1146/annurev.me.26.020175.002033. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst. 1998;90:814–823. doi: 10.1093/jnci/90.11.814. [DOI] [PubMed] [Google Scholar]
- 4.Osborne CK. Tamoxifen in the treatment of breast cancer. New Engl J Med. 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 5.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 6.Baum M. Current status of aromatase inhibitors in the management of breast cancer and critique of the NCIC MA-17 trial. Cancer Control. 2004;11:217–221. doi: 10.1177/107327480401100402. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, et al. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22:7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 8.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 9.Shupnik MA. Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene. 2004;23:7979–7989. doi: 10.1038/sj.onc.1208076. [DOI] [PubMed] [Google Scholar]
- 10.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–870s. [PubMed] [Google Scholar]
- 11.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 12.Saville B, Wormke M, Wang F, Nyugen T, Enmark E, Kuiper G, et al. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- 13.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 14.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 15.Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor transcription and transactivation: Estrogen receptor alpha and estrogen receptor beta: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res. 2000;2:335–344. doi: 10.1186/bcr78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J Mol Endocrinol. 2004;33:387–410. doi: 10.1677/jme.1.01541. [DOI] [PubMed] [Google Scholar]
- 17.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 18.Saji S, Omoto Y, Shimizu C, Warner M, Hayashi Y, Horiguchi S, et al. Expression of estrogen receptor (ER) (beta)cx protein in ER(alpha)-positive breast cancer: specific correlation with progesterone receptor. Cancer Res. 2002;62:4849–4853. [PubMed] [Google Scholar]
- 19.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 20.McInerney EM, Weis KE, Sun J, Mosselman S, Katzenellenbogen BS. Transcription activation by the human estrogen receptor subtype beta (ER beta) studied with ER beta and ER alpha receptor chimeras. Endocrinology. 1998;139:4513–4522. doi: 10.1210/endo.139.11.6298. [DOI] [PubMed] [Google Scholar]
- 21.Hall JM, McDonnell DP. The estrogen receptor beta isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 22.Cheung E, Schwabish MA, Kraus WL. Chromatin exposes intrinsic differences in the transcriptional activities of estrogen receptors alpha and beta. EMBO J. 2003;22:600–611. doi: 10.1093/emboj/cdg037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi P, Bhagat S, Hilf R, Bambara RA, Muyan M. Differences in the abilities of estrogen receptors to integrate activation functions are critical for subtype-specific transcriptional responses. Mol Endocrinol. 2002;16:1810–1827. doi: 10.1210/me.2001-0323. [DOI] [PubMed] [Google Scholar]
- 24.Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ER alpha) and ER beta in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- 25.Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003;206:13–22. doi: 10.1016/s0303-7207(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 26.Weatherman RV, Clegg NJ, Scanlan TS. Differential SERM activation of the estrogen receptors (ERalpha and ERbeta) at AP-1 sites. Chem Biol. 2001;8:427–436. doi: 10.1016/s1074-5521(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 27.Waters KM, Safe S, Gaido KW. Differential gene expression in response to methoxychlor and estradiol through ERalpha, ERbeta, and AR in reproductive tissues of female mice. Toxicol Sci. 2001;63:47–56. doi: 10.1093/toxsci/63.1.47. [DOI] [PubMed] [Google Scholar]
- 28.Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology. 2003;144:3159–3166. doi: 10.1210/en.2002-0143. [DOI] [PubMed] [Google Scholar]
- 29.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, et al. Estrogen receptor (ER) β reduces ERα-regulated gene transcription, supporting a “ying yang” relationship between ERα and ERβ in mice. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 30.Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F. ERβ inhibits proliferation and invasion of breast cancer cells. Endocrinology. 2001;142:4120–4130. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frasor J, Chang EC, Komm B, Lin CY, Vega VB, Liu ET, et al. Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res. 2006;66:7334–7340. doi: 10.1158/0008-5472.CAN-05-4269. [DOI] [PubMed] [Google Scholar]
- 32.Lin CY, Strom A, Li Kong S, Kietz S, Thomsen JS, Tee JB, et al. Inhibitory effects of estrogen receptor beta on specific hormone-responsive gene expression and association with disease outcome in primary breast cancer. Breast Cancer Res. 2007;9:R25. doi: 10.1186/bcr1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams C, Edvardsson K, Lewandowski SA, Strom A, Gustafsson JA. A genome-wide study of repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2007:1–14. doi: 10.1038/sj.onc.1210712. (PMID: 17700529; Epub ahead of print Aug 13) [DOI] [PubMed] [Google Scholar]
- 34.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen Receptors α and β form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 35.Pettersson K, Delaunay F, Gustafsson JA. Estrogen receptor β acts as a dominant regulator of estrogen signaling. Oncogene. 2000;19:4970–4978. doi: 10.1038/sj.onc.1203828. [DOI] [PubMed] [Google Scholar]
- 36.Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, et al. Opposing action of estrogen receptors α and β on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- 37.Omoto Y, Eguchi H, Yamamoto-Yamaguchi Y, Hayashi S. Estrogen receptor (ER) β1 and ERβcx/β ERα function differently in breast cancer cell line MCF7. Oncogene. 2003;22:5011–5020. doi: 10.1038/sj.onc.1206787. [DOI] [PubMed] [Google Scholar]
- 38.Buteau-Lozano H, Ancelin M, Lardeux B, Milanini J, Perrot-Applanat M. Transcriptional regulation of vascular endothelial growth factor by estradiol and tamoxifen in breast cancer cells: a complex interplay between estrogen receptors α and β. Cancer Res. 2002;62:4977–4984. [PubMed] [Google Scholar]
- 39.Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson JA. Estrogen receptor (ER) β modulates ERα-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol. 2006;20:534–543. doi: 10.1210/me.2005-0140. [DOI] [PubMed] [Google Scholar]
- 40.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor β on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 41.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci USA. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17:309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 43.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 44.Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in human breast cancer cells. Oncogene. 1995;10:2435–2446. [PubMed] [Google Scholar]
- 45.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 46.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and function as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 47.Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, et al. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 49.Lee YR, Park J, Yu HN, Kim JS, Youn HJ, Jung SH. Up-regulation of PI3K/Akt signaling by 17beta-estradiol through activation of estrogen receptor-alpha, but not estrogen receptor-beta, and stimulates cell growth in breast cancer cells. Biochem Biophys Res Commun. 2005;336:1221–1226. doi: 10.1016/j.bbrc.2005.08.256. [DOI] [PubMed] [Google Scholar]
- 50.Migliaccio A, Di Domenico M, Castoria G, Nanayakkara M, Lombardi M, de Falco A, et al. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res. 2005;65:10585–10593. doi: 10.1158/0008-5472.CAN-05-0912. [DOI] [PubMed] [Google Scholar]
- 51.Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, et al. Survival versus apoptotic 17beta-estradiol effect: the role of ER alpha and ER beta activated non-genomic signaling. J Cell Physiol. 2005;203:193–201. doi: 10.1002/jcp.20219. [DOI] [PubMed] [Google Scholar]
- 52.De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and the response to endocrine treatment in advanced breast cancer. Clinical Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 53.Dowsett M, Johnston S, Martin LA, Salter J, Hills M, Detre S, et al. Growth factor signalling and response to endocrine therapy: the Royal Marsden Experience. Endocr Relat Cancer. 2005;12 Suppl 1:S113–S117. doi: 10.1677/erc.1.01044. [DOI] [PubMed] [Google Scholar]
- 54.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 55.Ropero S, Menendez JA, Vazquez-Martin A, Montero S, Cortes-Funes H, Colomer R. Trastuzumab plus tamoxifen: anti-proliferative and molecular interactions in breast carcinoma. Breast Cancer Res Treat. 2004;86:125–137. doi: 10.1023/b:brea.0000032981.20384.c6. [DOI] [PubMed] [Google Scholar]
- 56.Moulder SL, Arteaga CL. A phase I/II trial of trastuzumab and gefitinib in patients with metastatic breast cancer that overexpresses HER2/neu (ErbB-2) Clin Breast Cancer. 2003;4:142–145. doi: 10.3816/cbc.2003.n.020. [DOI] [PubMed] [Google Scholar]
- 57.Forster C, Makela S, Warri A, Kietz S, Becker D, Hultenby K, et al. Involvement of estrogen receptor β in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci U S A. 2002;99:15578–15583. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricketts D, Turnbull L, Ryall G, Bakhshi R, Rawson NS, Gazet JC, et al. Estrogen and progesterone receptors in the normal female breast. Cancer Res. 1991;51:1817–1822. [PubMed] [Google Scholar]
- 59.Markopoulos C, Berger U, Wilson P, Gazet JC, Coombes RC. Oestrogen receptor content of normal breast cells and breast carcinomas throughout the menstrual cycle. Br Med J (Clin Res Ed) 1988;296:1349–1351. doi: 10.1136/bmj.296.6633.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–4991. [PubMed] [Google Scholar]
- 61.Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor β protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–2541. [PubMed] [Google Scholar]
- 62.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor α and β messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58:3197–3201. [PubMed] [Google Scholar]
- 63.Jarvinen TA, Pelto-Huikko M, Holli K, Isola J. Estrogen receptor β is coexpressed with ERα and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am J Pathol. 2000;156:29–35. doi: 10.1016/s0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rody A, Holtrich U, Solbach C, Kourtis K, von Minckwitz G, Engels K, et al. Methylation of estrogen receptor β promoter correlates with loss of ERβ expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr Relat Cancer. 2005;12:903–916. doi: 10.1677/erc.1.01088. [DOI] [PubMed] [Google Scholar]
- 65.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor β in malignant human colon. Cancer Res. 2000;60:245–248. [PubMed] [Google Scholar]
- 66.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERβ expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537–551. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearce ST, Liu H, Jordan VC. Modulation of estrogen receptor alpha function and stability by tamoxifen and a critical amino acid (Asp-538) in helix 12. J Biol Chem. 2003;278:7630–7638. doi: 10.1074/jbc.M211129200. [DOI] [PubMed] [Google Scholar]
- 68.Peekhaus NT, Chang T, Hayes EC, Wilkinson HA, Mitra SW, Schaeffer JM, et al. Distinct effects of the antiestrogen Faslodex on the stability of estrogen receptors-alpha and -beta in the breast cancer cell line MCF-7. J Mol Endocrinol. 2004;32:987–995. doi: 10.1677/jme.0.0320987. [DOI] [PubMed] [Google Scholar]
- 69.Palmieri C, Cheng GJ, Saji S, Zelada-Hedman M, Warri A, Weihua Z, et al. Estrogen receptor beta in breast cancer. Endocr Relat Cancer. 2002;9:1–13. doi: 10.1677/erc.0.0090001. [DOI] [PubMed] [Google Scholar]
- 70.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor β inhibits human breast cancer cell proliferation and tumor formation by causing a G2 Cell Cycle Arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 71.Behrens D, Gill JH, Fichtner I. Loss of tumourigenicity of stably ERβ-transfected MCF-7 breast cancer cells. Mol Cell Endocrinol. 2007;274:19–29. doi: 10.1016/j.mce.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 72.Hartman J, Lindberg K, Morani A, Inzunza J, Strom A, Gustafsson JA. Estrogen receptor β inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 2006;66:11207–11213. doi: 10.1158/0008-5472.CAN-06-0017. [DOI] [PubMed] [Google Scholar]
- 73.Hou YF, Yuan ST, Li HC, Wu J, Lu JS, Liu G, et al. ERβ exerts multiple stimulative effects on human breast carcinoma cells. Oncogene. 2004;23:5799–5806. doi: 10.1038/sj.onc.1207765. [DOI] [PubMed] [Google Scholar]
- 74.Boerner JL, Gibson MA, Fox EM, Posner ED, Parsons SJ, Silva CM, et al. Estrogen negatively regulates epidermal growth factor (EGF)-mediated signal transducer and activator of transcription 5 signaling in human EGF family receptor-overexpressing breast cancer cells. Mol Endocrinol. 2005;19:2660–2670. doi: 10.1210/me.2004-0439. [DOI] [PubMed] [Google Scholar]
- 75.Platet N, Cunat S, Chalbos D, Rochefort H, Garcia M. Unliganded and liganded estrogen receptors protect against cancer invasion via different mechanisms. Mol Endocrinol. 2000;14:999–1009. doi: 10.1210/mend.14.7.0492. [DOI] [PubMed] [Google Scholar]
- 76.Murphy LC, Peng B, Lewis A, Davie JR, Leygue E, Kemp A, et al. Inducible upregulation of oestrogen receptor-β1 affects oestrogen and tamoxifen responsiveness in MCF7 human breast cancer cells. J Mol Endocrinol. 2005;34:553–566. doi: 10.1677/jme.1.01688. [DOI] [PubMed] [Google Scholar]
- 77.Speirs V, Carder PJ, Lane S, Dodwell D, Lansdown MR, Hanby AM. Oestrogen receptor beta: what it means for patients with breast cancer. Lancet Oncology. 2004;5:174–181. doi: 10.1016/S1470-2045(04)01413-5. [DOI] [PubMed] [Google Scholar]
- 78.Skliris GP, Parkes AT, Limer JL, Burdall SE, Carder PJ, Speirs V. Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol. 2002;197:155–162. doi: 10.1002/path.1077. [DOI] [PubMed] [Google Scholar]
- 79.Murphy LC, Watson PH. Is oestrogen receptor-β a predictor of endocrine therapy responsiveness in human breast cancer? Endocr Relat Cancer. 2006;13:327–334. doi: 10.1677/erc.1.01141. [DOI] [PubMed] [Google Scholar]
- 80.Omoto Y, Kobayashi S, Inoue S, Ogawa S, Toyama T, Yamashita H, et al. Evaluation of oestrogen receptor β wild-type and variant protein expression, and relationship with clinicopathological factors in breast cancers. Eur J Cancer. 2002;38:380–386. doi: 10.1016/s0959-8049(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 81.Fuqua SA, Schiff R, Parra I, Moore JT, Mohsin SK, Osborne CK, et al. Estrogen receptor β protein in human breast cancer: correlation with clinical tumor parameters. Cancer Res. 2003;63:2434–2439. [PMC free article] [PubMed] [Google Scholar]
- 82.O’Neill PA, Davies MP, Shaaban AM, Innes H, Torevell A, Sibson DR, et al. Wild-type oestrogen receptor beta (ERβ1) mRNA and protein expression in Tamoxifen-treated post-menopausal breast cancers. Br J Cancer. 2004;91:1694–1702. doi: 10.1038/sj.bjc.6602183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vinayagam R, Sibson DR, Holcombe C, Aachi V, Davies MP. Association of oestrogen receptor beta 2 (ER beta 2/ER beta cx) with outcome of adjuvant endocrine treatment for primary breast cancer- a retrospective study. BMC Cancer. 2007;7:131. doi: 10.1186/1471-2407-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balfe P, McCann A, McGoldrick A, McAllister K, Kennedy M, Dervan P, et al. Estrogen receptor alpha and beta profiling in human breast cancer. Eur J Surg Oncol. 2004;30:469–474. doi: 10.1016/j.ejso.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Murphy L, Cherlet T, Lewis A, Banu Y, Watson P. New insights into estrogen receptor function in human breast cancer. Ann Med. 2003;35:614–631. doi: 10.1080/07853890310014579. [DOI] [PubMed] [Google Scholar]
- 86.Mann S, Laucirica R, Carlson N, Younes PS, Ali N, Younes A, et al. Estrogen receptor beta expression in invasive breast cancer. Hum Pathol. 2001;32:113–118. doi: 10.1053/hupa.2001.21506. [DOI] [PubMed] [Google Scholar]
- 87.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-β in oestrogen receptor-α negative human breast tumors. Br J Cancer. 2006;95:616–626. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jarzabek K, Koda M, Kozlowski L, Mittre H, Sulkowski S, Kottler ML, et al. Distinct mRNA, protein expression patterns and distribution of oestrogen receptors α and β in human primary breast cancer: correlation with proliferation marker Ki-67 and clinicopathological factors. Eur J Cancer. 2005;41:2924–2934. doi: 10.1016/j.ejca.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 89.Miyoshi Y, Taguchi T, Gustafsson JA, Noguchi S. Clinicopathological characteristics of estrogen receptor-beta-positive human breast cancers. Jpn J Cancer Res. 2001;92:1057–1061. doi: 10.1111/j.1349-7006.2001.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Umekita Y, Souda M, Ohi Y, Sagara Y, Rai Y, Takahama T, et al. Expression of wild-type estrogen receptor β protein in human breast cancer: specific correlation with HER2/neu overexpression. Pathol Int. 2006;56:423–427. doi: 10.1111/j.1440-1827.2006.01983.x. [DOI] [PubMed] [Google Scholar]
- 91.Wen XF, Shen Z, Shen ZZ, Nguyen M, Shao ZM. The expression of ERβ protein correlates with vascular endothelial growth factor and its prognostic significance in human breast cancer. Oncol Rep. 2002;9:937–944. [PubMed] [Google Scholar]
- 92.Hudelist G, Czerwenka K, Kubista E, Marton E, Pischinger K, Singer CF. Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res Treat. 2003;78:193–204. doi: 10.1023/a:1022930710850. [DOI] [PubMed] [Google Scholar]
- 93.Fleming FJ, Hill AD, McDermott EW, O’Higgins NJ, Young LS. Differential recruitment of coregulator proteins steroid receptor co-activator-1 (SRC-1) and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by β-estradiol and 4-hydroxytamoxifen in human breast cancer. J Clin Endocrinol Metab. 2004;89:375–383. doi: 10.1210/jc.2003-031048. [DOI] [PubMed] [Google Scholar]
- 94.Myers E, Fleming FJ, Crotty TB, Kelly G, McDermott EW, O’Higgins NJ, et al. Inverse relationship between ER-beta and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer. 2004;91:1687–1693. doi: 10.1038/sj.bjc.6602156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rody A, Diallo R, Poremba C, Speich R, Wuelfing P, Kissler S, et al. Estrogen receptor α and β, progesterone receptor, pS2 and HER-2/neu expression delineate different subgroups in ductal carcinoma in situ of the breast. Oncol Rep. 2004;12:695–699. [PubMed] [Google Scholar]
- 96.Ciocca DR, Gago FE, Fanelli MA, Calderwood SK. Co-expression of steroid receptors (estrogen receptor alpha and/or progesterone receptors) and Her-2/neu: Clinical implications. J Steroid Biochem Mol Biol. 2006;102:32–40. doi: 10.1016/j.jsbmb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 97.Nakopoulou L, Lazaris AC, Panayotopoulou EG, Giannopoulou I, Givalos N, Markaki S, et al. The favourable prognostic value of oestrogen receptor beta immunohistochemical expression in breast cancer. J Clin Pathol. 2004;57:523–528. doi: 10.1136/jcp.2003.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choi Y, Pinto M. Estrogen receptor β in breast cancer: associations between ERβ, hormonal receptors, and other prognostic biomarkers. Appl Immunohistochem Mol Morphol. 2005;13:19–24. doi: 10.1097/00129039-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 99.Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, et al. Declining estrogen receptor-β expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27:1502–1512. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 100.Platet N, Cathiard AM, Gleizes M, Garcia M. Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Crit Rev Oncol Hematol. 2004;51:55–67. doi: 10.1016/j.critrevonc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 101.Shaw JA, Udokang K, Mosquera JM, Chauhan H, Jones JL, Walker RA. Oestrogen receptors α and β differ in normal human breast and breast carcinomas. J Pathol. 2002;198:450–457. doi: 10.1002/path.1230. [DOI] [PubMed] [Google Scholar]
- 102.Koda M, Sulkowski S, Kanczuga-Koda L, Surmacz E, Sulkowska M. Expression of ERα, ERβ and Ki-67 in primary tumors and lymph node metastases in breast cancer. Oncol Rep. 2004;11:753–759. [PubMed] [Google Scholar]
- 103.Skliris GP, Munot K, Bell SM, Carder PJ, Lane S, Horgan K, et al. Reduced expression of oestrogen receptor β in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol. 2003;201:213–220. doi: 10.1002/path.1436. [DOI] [PubMed] [Google Scholar]
- 104.Omoto Y, Inoue S, Ogawa S, Toyama T, Yamashita H, Muramatsu M, et al. Clinical value of the wild-type estrogen receptor beta expression in breast cancer. Cancer Lett. 2001;163:207–212. doi: 10.1016/s0304-3835(00)00680-7. [DOI] [PubMed] [Google Scholar]
- 105.Sugiura H, Toyama T, Hara Y, Zhang Z, Kobayashi S, Fujii Y, et al. Expression of Estrogen Receptor β Wild-type and its variant ERβcx/β2 is correlated with better prognosis in breast cancer. Jpn J Clin Oncol. 2007 doi: 10.1093/jjco/hym114. (PMID: 17932113; Epub ahead of print Oct 11) [DOI] [PubMed] [Google Scholar]
- 106.Jensen EV, Cheng G, Palmieri C, Saji S, Makela S, Van Noorden S, et al. Estrogen receptors and proliferation markers in primary and recurrent breast cancer. Proc Natl Acad Sci U S A. 2001;98:15197–15202. doi: 10.1073/pnas.211556298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SA. Low levels of estrogen receptor β protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res. 2004;10:7490–7499. doi: 10.1158/1078-0432.CCR-04-1114. [DOI] [PubMed] [Google Scholar]
- 108.Esslimani-Sahla M, Kramar A, Simony-Lafontaine J, Warner M, Gustafsson JA, Rochefort H. Increased estrogen receptor βcx expression during mammary carcinogenesis. Clin Cancer Res. 2005;11:3170–3174. doi: 10.1158/1078-0432.CCR-04-2298. [DOI] [PubMed] [Google Scholar]
- 109.Poola I, Fuqua SA, De Witty RL, Abraham J, Marshallack JJ, Liu A. Estrogen receptor α-negative breast cancer tissues express significant levels of estrogen-independent transcription factors, ERβ1 and ERβ5: potential molecular targets for chemoprevention. Clin Cancer Res. 2005;11:7579–7585. doi: 10.1158/1078-0432.CCR-05-0728. [DOI] [PubMed] [Google Scholar]
- 110.Palmieri C, Lam EW, Mansi J, MacDonald C, Shousha S, Madden P, et al. The expression of ERβcx in human breast cancer and the relationship to endocrine therapy and survival. Clin Cancer Res. 2004;10:2421–2428. doi: 10.1158/1078-0432.ccr-03-0215. [DOI] [PubMed] [Google Scholar]
- 111.Miller WR, Anderson TJ, Dixon JM, Saunders PT. Oestrogen receptor β and neoadjuvant therapy with tamoxifen: prediction of response and effects of treatment. Br J Cancer. 2006;94:1333–1338. doi: 10.1038/sj.bjc.6603082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Iwase H, Zhang Z, Omoto Y, Sugiura H, Yamashita H, Toyama T, et al. Clinical significance of the expression of estrogen receptors alpha and beta for endocrine therapy of breast cancer. Cancer Chemother Pharmacol. 2003;52 Suppl 1:S34–S38. doi: 10.1007/s00280-003-0592-1. [DOI] [PubMed] [Google Scholar]
- 113.Murphy LC, Leygue E, Niu Y, Snell L, Ho SM, Watson PH. Relationship of coregulator and oestrogen receptor isoform expression to de novo tamoxifen resistance in human breast cancer. Br J Cancer. 2002;87:1411–1416. doi: 10.1038/sj.bjc.6600654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Esslimani-Sahla M, Simony-Lafontaine J, Kramar A, Lavaill R, Mollevi C, Warner M, et al. Estrogen receptor beta (ER beta) level but not its ER beta cx variant helps to predict tamoxifen resistance in breast cancer. Clin Cancer Res. 2004;10:5769–5776. doi: 10.1158/1078-0432.CCR-04-0389. [DOI] [PubMed] [Google Scholar]
- 115.Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 116.Kumar VL, Srivastava A, Singhal R, Kumar V. Immunoreactive estrogen receptor in breast tumor and adjacent tumor: association with clinicopathological characteristics in Indian population. J Surg Oncol. 2005;89:251–255. doi: 10.1002/jso.20211. [DOI] [PubMed] [Google Scholar]
- 117.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. doi: 10.1093/jnci/86.9.705. [DOI] [PubMed] [Google Scholar]
- 118.Poola I, Clarke R, DeWitty R, Leffall LD. Functionally active estrogen receptor isoform profiles in the breast tumors of African American women are different from the profiles in breast tumors of Caucasian women. Cancer. 2002;94:615–623. doi: 10.1002/cncr.10274. [DOI] [PubMed] [Google Scholar]
- 119.Hsiao WC, Cho WC, Lin PW, Lin SL, Lee WY, Young KC. Quantitative profile of estrogen receptor variants/isoforms in Taiwanese women with breast cancer. Eur J Surg Oncol. 2006;32:492–497. doi: 10.1016/j.ejso.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 120.Moormeier J. Breast cancer in black women. Ann Intern Med. 1996;15:897–905. doi: 10.7326/0003-4819-124-10-199605150-00007. [DOI] [PubMed] [Google Scholar]
- 121.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001;30:36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 122.McCaskill-Stevens W, Wilson J, Bryant J, Mamounas E, Garvey L, James J, et al. Contralateral breast cancer and thromboembolic events in African American women treated with tamoxifen. J Natl Cancer Inst. 2004;96:1762–1769. doi: 10.1093/jnci/djh321. [DOI] [PubMed] [Google Scholar]
- 123.Moy B, Tu D, Pater JL, Ingle JN, Shepherd LE, Whelan TJ, et al. Clinical outcomes of ethnic minority women in MA.17: a trial of letrozole after 5 years of tamoxifen in postmenopausal women with early stage breast cancer. Ann Oncol. 2006;17:1637–1643. doi: 10.1093/annonc/mdl177. [DOI] [PubMed] [Google Scholar]


