Abstract
In order to generate a zebrafish model of β cell regeneration, we have expressed an E.coli gene called nfsB in the β cells of embryonic zebrafish. This bacterial gene encodes a nitroreductase (NTR) enzyme, which can convert prodrugs such as metronidazole (Met) to cytotoxins. By fusing nfsB to mCherry, we can simultaneously render β cells susceptible to prodrug and visualize Met dependent cell ablation. We show that the neighboring α and δ cells are unaffected by prodrug treatment and that ablation is β cell specific. Following drug removal and 36hrs of recovery, β cells regenerate. Using ptf1a morphants, it is clear that this β cell recovery occurs independently of the presence of the exocrine pancreas. Also, by using photoconvertible Kaede to cell lineage trace and BrdU incorporation to label proliferation, we investigate mechanisms for β regeneration. Therefore, we have developed a unique resource for the study of β cell regeneration in a living vertebrate organism, which will provide the opportunity to conduct large-scale screens for pharmacological and genetic modifiers of β cell regeneration.
Introduction
Zebrafish embryos develop morphologically simple pancreata and when compared to mammalian systems, there is a high degree of conservation of both expression and function of genes involved in pancreas development (Argenton et al., 1999; Biemar et al., 2001; Lin et al., 2004; Yee et al., 2001; Zecchin et al., 2004). Utilizing transgenics allows the detailed observation of interactions between pancreatic tissues in living zebrafish embryos (Godinho et al., 2005; Huang et al., 2001; Wan et al., 2006). For instance, using lines transgenic for either gut:eGFP (Field et al., 2003) or ptf1a:eGFP (Godinho et al., 2005) permits the visualization of ventrally derived exocrine cells migrating toward the dorsal pancreas, enveloping the endocrine cells (or principal islet) and forming the first rudimentary pancreas by 44hrs post fertilization (hpf). The cellular composition and structure of the zebrafish principal islet is reminiscent of mammalian adult islets consisting of a central core of β cells and a peripheral mantle of α cells (Argenton et al., 1999). Thus, the molecular pathways, as well as the cellular components and physiology, are conserved between the zebrafish and mammalian model systems. For these reasons the zebrafish has increasingly been used to study the development and diseases of the pancreas. For instance, it has been shown recently that pancreatic ductal adenocarcinoma can be induced in the zebrafish by recapitulating the same signal transduction errors that occur commonly in pancreas cancer in humans (Park and Leach personal communication).
Juvenile diabetes (diabetes type I) is a debilitating disease caused by the chronic loss of the insulin producing β cells of the pancreas. Research into a cure for type I diabetes is centered on islet cell replacement. Considerable success in alleviating the symptoms of juvenile diabetes has been achieved using human islet transplantation; however, the availability of donors is very limiting and the need for continued immunosuppression with its risks and side effects has limited the clinical utility of this approach (Rood et al., 2006). Expanding β cells in tissue culture or inducing proliferation following transplantation could be a potent method to alleviate the paucity of donor tissue available. Another approach to recovering glucose homeostasis would be to induce endogenous regeneration of β cells. Type 1 diabetes in humans and Non Obese Diabetic (NOD) mice is caused by an autoimmune T cell dependent destruction of β cells. In long standing type 1 diabetes in humans, there are occasional β cells scattered in the pancreas along with continued apoptosis of β cells (Meier et al., 2005). Similarly, β cell mass can be restored to cure type 1 diabetes in NOD mice when subjected to a complex treatment protocol to restrain autoimmunity (Chong et al., 2006). Together these results suggest some capacity for regeneration of endogenous β cells exists in patients with type 1 diabetes. It is hoped that elucidating the factors controlling β cell regeneration, therefore, will be useful in providing therapeutic solutions to diseases such as diabetes.
We have turned to the embryonic zebrafish to generate a model of β cell regeneration, as the small size and biology make this organism ideal for screening for both genetic and chemical modifiers of the regeneration process. To create such a model, first an inducible system to cause β cell ablation is required. Injection of the alkylating agent, streptozotocin into mammals causes apoptosis of the β cells (O’Brien et al., 1996); however, addition of the drug to the water of embryonic zebrafish has no detectable effect on β cell mass as judged by in situ hybridization or ins:eGFP transgene expression (data not shown). Another way to have temporal control of β cell ablation is to drive expression of a prodrug converting enzyme to the insulin producing cells using transgenesis. Under the action of the exogenous enzyme in the β cell, the prodrug is converted to a cytotoxin and causes cell death. By incorporating the E.coli gene nfsB into transgenes, mice have been created that express the enzyme nitroreductase (NTR). Exogenous NTR converts added prodrug CB1954 to a cytotoxin, leading to cell death (Bridgewater et al., 1995; Drabek et al., 1997). This system has had limited use in cell ablation in the mouse, but luminal cells of the mammary gland (Clark et al., 1997; Cui et al., 1999), and stem cells within the developing prostate (Wang et al., 2004) have been successfully ablated. The restricted use of this system might be due to the diffusible cytotoxic metabolites of CB1954, which causes bystander effects (Helsby et al., 2004).
In this report we have used metronidazole (Met) as a substrate for NTR-mediated cell ablation for the first time within a live vertebrate. By expressing NTR in β cells in zebrafish embryos we demonstrate prodrug dependent cell ablation, without affecting neighboring cells. In so doing, we have generated a model in which to study β cell regeneration. We also demonstrated the utility of using photo-convertible Kaede in transgenic zebrafish to trace cell lineage and investigate mechanisms of β cell recovery.
Results
Metronidazole induces apoptosis in a NTR dependent manner in embryos
To test the capability of NTR to elicit drug dependent cell ablation, the bacterial nfsB gene was cloned from E.coli genomic DNA into the plasmid T2KXIGΔIN (Kawakami lab), replacing the eGFP marker. With this new construct EF1α:nfsB (Fig. 1A), nfsB is transcribed under the control of the ubiquitous EF1α promoter. This plasmid was co-injected with Tol2 transposase mRNA into single cell embryos, which created embryos with widespread expression of NTR, albeit in a mosaic fashion. In initial experiments these transient transgenic embryos were incubated from 56hpf to 96hpf in 7.5mM or 10mM of Met. By 80hpf there was a prodrug and gene dependent effect on the embryos as assessed by gross pathological changes including edema, lack of heart beat and massive necrosis in the somatic musculature. When used alone, neither the gene nfsB nor the prodrug was toxic to zebrafish embryos.
Fig. 1.
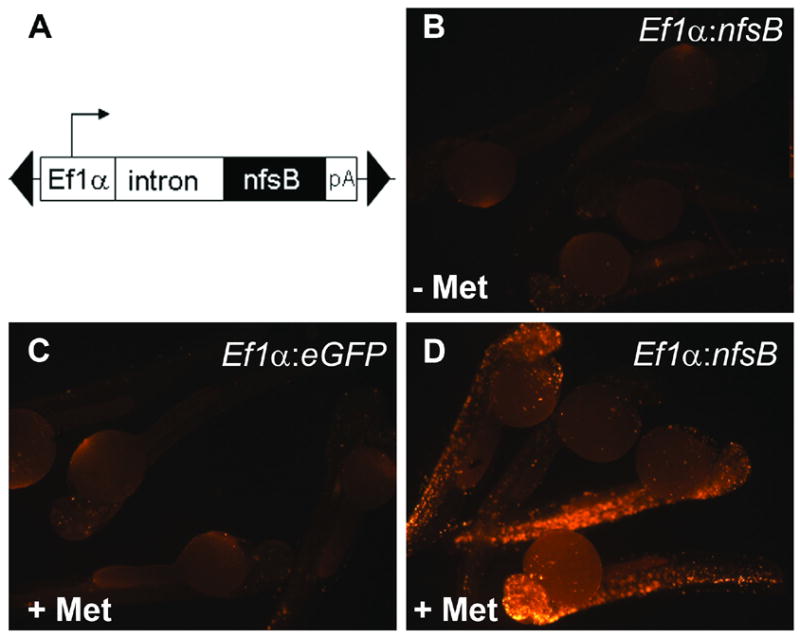
Met induces apoptosis in a NTR dependent manner. (A) Schematic of construct used to drive ubiquitous expression of NTR. Construct includes two arms from the Tol2 transposable element (black triangles), the EF1α promoter and intronic sequence from the rabbit β-globin gene (Kawakami, 2004). EF1α drives ubiquitous expression of NTR. Embryos injected at 1 cell stage and incubated with or without Met were fixed at 48hpf and assayed for apoptosis using fluorescent TUNEL. (B) Negative control, mosaic for Ef1α:nfsB with no prodrug treatment. (C) Negative control, mosaic for Ef1α:eGFP incubated in Met (6 to 48hpf). (D) Mosaic embryos for Ef1α:nfsB, incubated in Met. (6 to 48hpf). Results demonstrate increased apoptosis when NTR is expressed in presence of Met.
To test how the apparent tissue damage occurred, similar EF1α:nfsB transient transgenic embryos were incubated in Met from 6 to 48hpf and then tested for the presence of apoptotic cell death. Two negative controls were used; 1) embryos similarly injected with EF1α:nfsB not incubated in drug, and 2) embryos injected with EF1α:eGFP (T2KXIGΔIN) and incubated in drug. At 48hpf all embryos were fixed and assayed for apoptosis using TUNEL. Negative control embryos displayed a small amount of apoptosis; however, embryos injected with the EF1α:nfsB plasmid and incubated in Met displayed widespread and significantly elevated levels of apoptosis (Figs. 1B–D). It was concluded that nfsB encoded NTR can cause drug dependent apoptosis in cells of developing zebrafish embryos.
Insulin promoter reliably drives expression to the β cells of the developing zebrafish
In order to cause drug dependent ablation of the β cells, NTR needs to be specifically expressed in these cells. The earliest known marker of β cells in the developing zebrafish is the preproinsulin (ins) gene and the ins promoter is known to confer expression in the nascent endocrine pancreas (Huang et al., 2001). A 995bp fragment upstream of the ATG of the ins gene was amplified by PCR and cloned into the plasmid T2KXIGΔIN, replacing the existing promoter sequence (EF1α) (Fig. 2A). Utilizing transposon mediated DNA integration, lines of transgenic fish were established and characterized in detail for expression of eGFP. In ins:eGFP transgenic lines, made using this construct, the earliest time point in embryogenesis we could detect fluorescence was around 20hpf just prior to the β cells coalescing as the principal islet forms. Fluorescence was maintained through development and the ins:eGFP was localized to the pancreas in both ins:eGFP larvae (Figs. 2B–F) and adult fish (Figs. 2G–L). To verify that the fluorescence was localized specifically to β cells, we performed immunofluorescent staining for insulin on ins:eGFP larvae,10 days post fertilization (dpf). By confocal microscopy, we saw that only the insulin expressing cells were also fluorescent for GFP (Fig. 2F). In adult ins:eGFP fish, the fluorescence could still be observed transcutaneously (Fig. 2G). Dissection, sectioning, and immunofluorescent imaging demonstrated that β cells in adult pancreas reside in three kinds of aggregations: a large principal islet, secondary islets and very small accumulations of β cells (Fig. 2H). Co-staining for both insulin and eGFP, confirmed that the ins:eGFP transgene was reliably labeling β cells (Fig. 2L). Altogether, these data indicate that the insulin promoter in the context of the Tol2 transposable element provides an accurate label of β cells in live zebrafish throughout their life span.
Fig. 2.
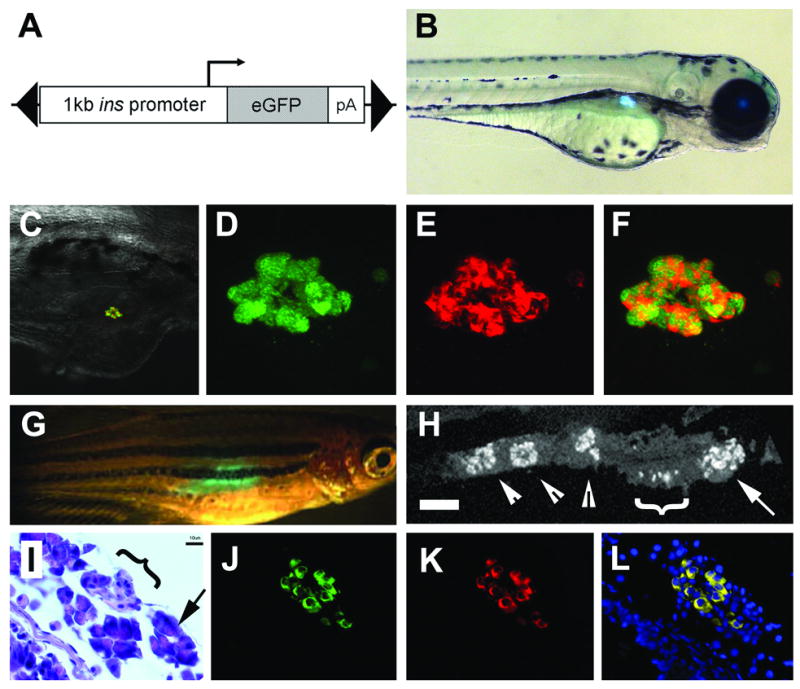
Insulin promoter reliably drives expression to the β cells. (A) Schematic of construct used including the 1kb promoter region of preproinsulin, and gene encoding eGFP, all within a Tol2 transposable element (black triangles). (B) Fluorescent image of a 3 day old ins:eGFP embryo showing expression within the islet of the pancreas. (C) Rendered composite of confocal images of a 10 day old ins:eGFP embryo following immunofluorescence to detect insulin, with overlaid 20x bright field. (D) Close up images of eGFP fluorescence and (E) immunofluorescence to detect Insulin and (F) merged image demonstrating co-localization of eGFP with Insulin. (G) 3 month ins-eGFP adult fish showing transcutaneous fluorescence. (H) Section through dissected pancreas showing immunofluorescence to detect Insulin (scale bar = 100μM). The principal islet can be seen in the anterior head of the pancreas (arrow). More posterior are small aggregatons of β cells (white bracket) and three secondary islets (arrowheads). (I) High magnification, the morphology of the smaller islets can be seen (black Targeted ablation of β cells bracket) surrounded by acinar tissue (arrow), by H&E staining (scale bar =10μM). Immunofluorescence detection for eGFP in (J), insulin in (K). (I) Merged image demonstrate co-localization in adult tissues (Hoescht staining for nuclei in blue). In (B to H) anterior is to the right and posterior to the left.
Expression of NTR via the insulin promoter leads to β cell loss
In order to test the ability of NTR to render β cells susceptible to drug dependent ablation, two new constructs were made (Figs. 3A and 3B); ins:mCherry drives expression of mCherry to the β cells (Fig. 3C) and ins:nfsB-mCherry drives expression of a fusion protein, mCherry-NTR, to the β cells (Fig. 3D). Ins:nfsB-mCherry F1 transgenic embryos were used to study the dose response of Met in ablating β cells. In initial studies, 56hpf embryos were incubated in a range of Met concentrations (1mM to 10mM). After 24hrs of incubation in prodrug, only a concentration of 10mM resulted in complete loss of fluorescence. The relatively high concentration of Met needed to ablate β cells most likely reflects the low affinity of prodrugs toward NTR (Johansson et al., 2003). Another explanation is low prodrug penetration, as suggested by the low octanol to water partition coefficient for Met (Mahfouz and Hassan, 2001). This concentration (10mM) was used in all later studies. To further assess the effects of Met, individual 24hpf embryos were kept in wells of a 24 well plate and incubated in 10mM of the prodrug and examined over time using an epi-fluorescence microscope. Addition of the prodrug for 24hrs (i.e. until 48hpf) had no effect on the fluorescence in ins:mCherry embryos (n=8), demonstrating that there were no apparent off target effects of this drug on β cell survival (Fig. 3C). Similarly, ins:nfsB-mCherry embryos incubated for an identical time without prodrug, displayed the expected fluorescence in the β cells (Fig. 3D, n=30). However, sibling embryos incubated in the prodrug for 24 hrs, showed no detectable fluorescence (Fig. 3E, n=31). Identical results where obtained by starting drug treatments at later developmental time points, namely 36 hrs or 56 hrs and incubating again for 24hrs, until 60hpf or 80hpf respectively (n=23 and n=46 respectively, data not shown and Fig. 3K). These results confirmed that expressing NTR via the insulin promoter causes a prodrug dependent loss of fluorescence in the pancreatic β cells.
Fig. 3.
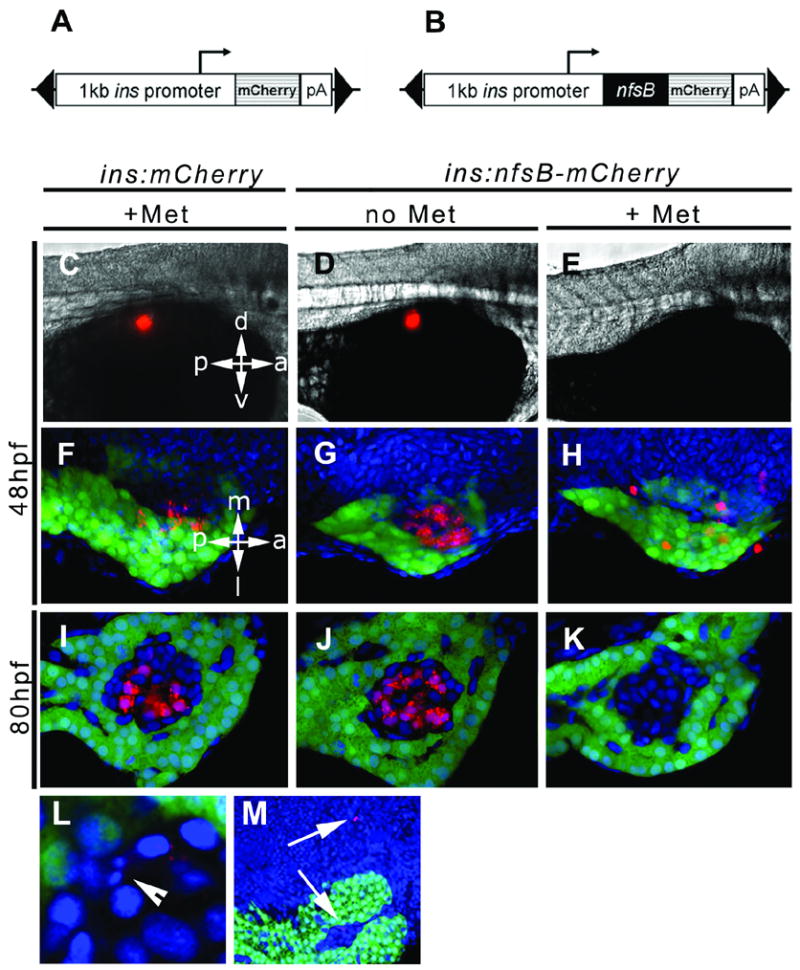
The ins:nsfB-mCherry transgene leads to prodrug dependent loss of β cells. Schematic of constructs used to drive β cell expression of mCherry in (A) and the fusion protein NTR-mCherry in (B). The expression cassettes were cloned inside of a Tol2 transposable element (black triangles). In the experiment shown here, two groups of controls were used, ins:mCherry embryos incubated in Met (+Met, C, F, I) and ins:nfsB-mCherry without prodrug (no Met, D, G, J). Experimental groups consisted of ins:nfsB-mCherry embryos incubated for 24 hrs in Met (+Met, E, H, K). Two separate time points were chosen for addition of prodrug as shown, incubation starting at 24hpf and finished by 48hpf (48hpf, C to H) and starting at 56hpf and finishing at 80hpf (80hpf, I to K). Embryos were photographed by either epi-fluorescence in (C) to (E), or by confocal microscopy on isolated pancreata plus adjacent endoderm in (F) to (M). Red fluorescence indicated presence of mCherry (C, F, I) or NTR-mCherry fusion protein (D, E, G, H, J, K, L and M). eGFP marks the exocrine pancreas with green fluorescence and nuclei are stained blue (Hoescht). (L) Example of fragmented nucleus indicative of apoptosis (arrowhead) and cell debris inside and outside of islet in (M) (arrows). Orientation as indicated, d = dorsal, v = ventral, a = anterior, p = posterior, m = medial and l = lateral.
Confocal microscopy was used to visualize the extent of cell ablation in greater detail. By crossing the insulin transgenic lines with a ptf1a:eGFP line (Godinho et al., 2005), we could visualize the cells of the islet as marked by the boundary with the surrounding green fluorescent exocrine cells. Two separate developmental stages were examined for the ability to undergo cell ablation; embryos were exposed to Met for 24hrs beginning at either 24 or 56hpf. At the time of harvest (48 or 80hpf), embryos were fixed and then dissected to remove the gut and pancreatic endoderm. Two control groups of embryos were used in these experiments, ins:mCherry embryos incubated in prodrug for 24hrs (Figs. 3F, I) and ins:nfsB-mCherry kept without prodrug addition (Figs.3G, J). In all controls the β cells were detected by red fluorescence of mCherry. The fluorescence was diffuse throughout the cells and also punctate in appearance, which could be due to the exogenous protein being maintained as inclusion bodies within the cytoplasm. As seen in whole embryos using the epi-fluorescence microscope, following 24hr of prodrug treatment, few cells appear red within the region of the islet. By increasing the strength of the laser, weakly red fluorescent cells could be seen within the pancreas of treated embryos at 48hpf (Fig. 3H). These cells do not possess the punctate distribution of fluorescence seen in control embryos and many cells no longer appear within the region of islet, or indeed the pancreas. When embryos were examined at the later time point of 80hpf under maintained laser strength, fluorescent cells were not detected in the islet (Fig. 3K).
Detailed inspection of the cells in the islet revealed the presence of weakly red cells containing fragmented nuclei (Fig. 3L). This morphology is indicative of cells undergoing apoptosis. Cells presenting this appearance were rarely detected. Although streptozotocin also kills β cells in the mouse by inducing apoptosis, cells undergoing apoptosis are hard to detect as the process is rapid and there are a relatively small number of β cells (O’Brien et al., 1996). In addition to occasional apoptotic cells, other evidence of cellular degradation was detected following prodrug exposure. Red fluorescence can also be detected in debris smaller than cells. These particles are often located outside of the pancreas (Fig. 3M) and following 24hrs of prodrug treatment, were frequently distributed across the surface of the yolk (data not shown). Together these data strongly suggest that there is a drug dependent loss of β cells expressing the NTR protein.
Ablation of β cells does not affect neighboring cell populations
In order to validate the specificity of NTR-induced cell death, we looked at other cells within the developing pancreas for signs of abnormality. Using the ptf1a:eGFP transgene as a marker of nascent exocrine pancreas, we saw no difference in the localization, polarization or length of the pancreas as a whole, whether the β cells were ablated from 24–48 hrs (Fig. 3H) or 56–80 hrs (Fig. 3K). Expression of the trypsin gene is an indicator of differentiated exocrine tissue and the development of acinar tissue. Using in situ hybridization to detect trypsin transcripts, we similarly observed no difference between treated and untreated sibling embryos (data not shown). Taken together these results suggest that β cell ablation has no detectable effect on exocrine formation, migration or differentiation.
Next we looked at markers of other endocrine pancreatic cell types. Ins:nfsB-mCherry embryos were raised until 56hpf, then incubated in Met for 24hrs until 80hpf. Untreated sibling embryos were kept as a control. At 80hpf all these embryos were fixed and examined by either in situ hybridization or immunofluorescence to detect endocrine markers. At 80hpf insulin expression is robust and limited to the islet as shown in controls (n= 20 example in Fig. 4A). Following Met treatment, no ins transcripts were detected in 23 out of 24 embryos by in situ hybridization (Fig. 4D); lending further evidence that the β cells have been ablated. As in mammalian systems, the zebrafish pancreas contains Glucagon expressing α cells at the islet periphery (Argenton et al., 1999). There is no difference in the localization or number of these cells between controls (n=18, Fig. 4B) and prodrug treated embryos (n=20, Fig. 4E). Unlike α cells, the Somatostatin producing δ cells are intercalated with β cells within the core of the islet (Argenton et al., 1999). Immunofluorescent staining for Somatostatin demonstrated that δ cells were present in comparable numbers in both prodrug treated (n=10, Fig. 4F) and untreated embryos (n=10, Fig. 4C). These results indicate that prodrug induced ablation of β cells has no detectable effect on closely associated cell neighbors within or immediately outside the islet.
Fig. 4.
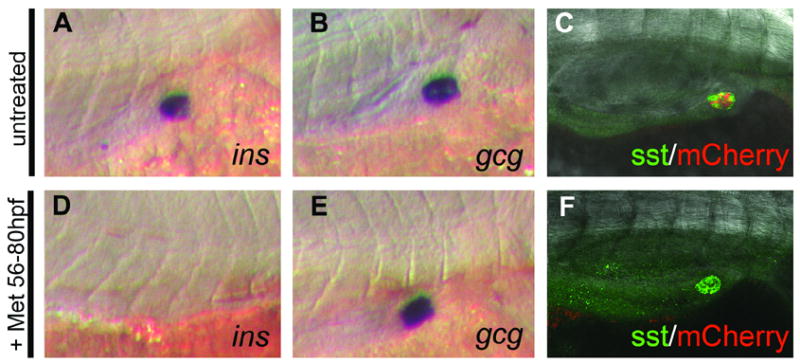
Expression of insulin but not glucagon or Somatostatin, is lost following prodrug dependent β cell ablation. (A – C) Marker analysis on ins:nfsB-mCherry untreated control embryos or embryos treated with Met from 56hpf to 80hpf (D to F). In situ hybridization with riboprobes to detect the following transcripts: insulin (ins) (A, D); glucagon (gcg) (B, E). Immunofluorescence detection of Somatostatin protein, using confocal microscopy, (C, F). Presence of the NTR-mCherry fusion protein can be seen in the control embryo (C) as red fluorescence, but is lost following ablation (F).
β cell numbers recover following ablation
In order to determine whether the ins:nfsB-mCherry transgenic fish might serve as a model of β regeneration, we assayed if β cell number recovers over time following prodrug treatment. An ins:nfsB-mCherry embryo at 24hpf is shown in Fig. 5B; and as seen previously, expression of the mCherry fusion protein could clearly be seen by epi-fluorescence microscopy. Treatment with Met for 24hrs until 48hpf, lead to a loss of insulin positive cells (n=31, Fig. 5F), whereas untreated controls showed no effect (n=15, Fig. 5C). After prodrug removal and a further incubation of 36hrs, fluorescence could again be detected in the pancreatic field in all embryos tested (31/31, Fig. 5G). By repeating these experiments in ptf1a:eGFP; ins:nsfB-mCherry double transgenic embryos and observing the islet following recovery, we could clearly see red fluorescent cells within the islet (Figs. 5K and 5L). These cells exhibited the same kind of punctate distribution of fluorescence observed in control embryos. There was still a reduction in the absolute number of cells that were positive for red fluorescence, indicating that regeneration was not completely compensating for the loss of ablated β cells at this timepoint (c.f. Figs. 5I, L). We subsequently maintained both prodrug treated and untreated embryos for longer periods of recovery. At 8 dpf we re-examined the embryos for fluorescence in the pancreas. As shown (Fig. 5J, M) there was only a small residual reduction in the number of fluorescent cells in embryos that had been previously treated in drug (n=12, controls n=3). We maintained these larvae which in due time grew into fertile adults demonstrating that neither β cell ablation nor Met treatment for 24hrs in the larvae have any long term deleterious affects on viability.
Fig. 5.
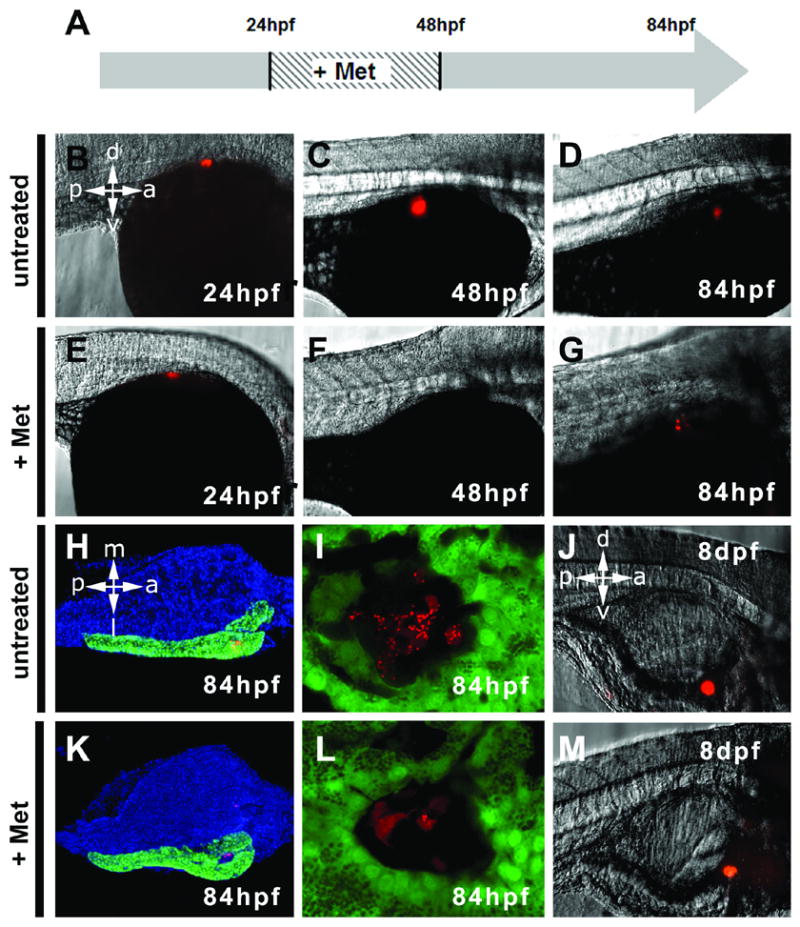
β cell recovery following ablation. (A) Schematic timeline of prodrug treatment. (B – D) Untreated ins:nfsB-mCherry embryos at stages indicated. Ins:nfsB-mCherry embryos before prodrug incubation (E), following 24hrs of ablation with prodrug (F) and following 36hrs of recovery in absence of prodrug (G). Return of β cells is indicated by the reoccurrence of NTR- mCherry. Dissected pancreas and adjacent endoderm from a untreated ins:nfsB-mCherry, 84hpf embryo (H) and treated and recovered 84hpf embryo (K). Nuclei are blue due to Hoescht counter staining. Images generated from ins:nfsB-mCherry; ptf1a:eGFP transgenics, hence exocrine pancreas appears green. (H, K) In comparison to controls, treated pancreata look morphologically normal. Close up images of an islet following treatment and recovery in (L), show presence of β cells with a normal appearance but in a reduced number compared to untreated controls (I). (J) Untreated ins:nfsB-mCherry in a fry, 8 days post fertilization (dpf), and following prodrug treatment described above (M).
β cell recovery is independent of the exocrine pancreas
In mammals, several studies have raised the possibility that differentiated exocrine cells might serve as a source for β cell regeneration (Hao et al., 2006; Lardon et al., 2004). To test the role of the exocrine pancreas in zebrafish β cell recovery, we performed prodrug dependent cell ablation in embryos lacking exocrine pancreas. To do this, single cell ins:nfsB-mCherry embryos were injected with a ptf1a antisense morpholino to prevent exocrine pancreas development (Lin et al., 2004; Zecchin et al., 2004). These embryos were cultured until 56hpf and then incubated in Met as before. The β cell fluorescence is extinguished within the pancreas following 24hrs of treatment, both in morphant embryos without exocrine pancreas (n= 24, Fig. 6F) and in non-morpholino controls (n= 18, Fig. 6B). Following 36hrs of prodrug removal, an equivalent amount of β cell recovery can be observed compared to non-morphant controls which possess exocrine pancreas. To verify that exocrine pancreas formation was completely inhibited by the morpholino injections, we assayed for Carboxypeptidase (CPA - a marker of differentiated acinar cells of the exocrine pancreas) by immunofluorescence. CPA immunoreactivity was absent in all ins:nfsB-mCherry, ptf1a morphants examined (n=16, example shown in Fig. 6H), while CPA was present in all non-morphant controls (n=16, Fig. 6D). This result demonstrates that β cell recovery is independent of the exocrine pancreas. Hence, no cell type or extracellular factor from the exocrine pancreas is required for β cell recovery in this system.
Fig. 6.
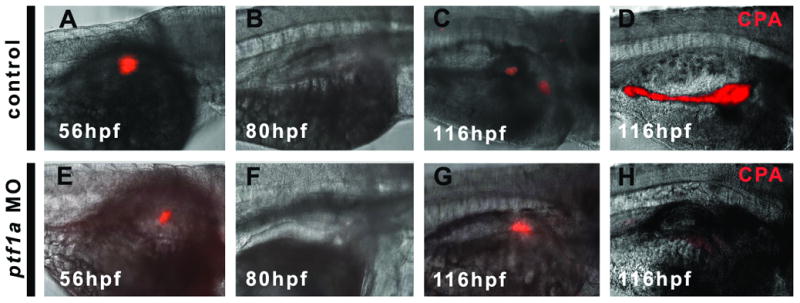
β cell recovery is independent of the exocrine pancreas. (A, E) Ins:nfsB-mCherry embryos before prodrug incubation. (B,F) Ablation following 24hrs of prodrug incubation. (C, D, G, H) Following 36hrs of prodrug removal, recovery can be detected. Control embryos (A – D), show normal exocrine pancreas as demonstrated by CPA immunofluorescent detection (pseudo color image D). Ptf1a morphant embryos shown in E to H lack an exocrine pancreas as demonstrated by absence of CPA (H), but still recover β cells (G).
Mechanism of β cell recovery
One possible explanation for our observed results is that non-insulin expressing progenitors escape cell ablation as they are not producing NTR; and that later in development, concurrent with prodrug removal, these cells differentiate to generate insulin positive β cells. To define a temporal window during which β cell progenitors normally differentiate, we generated a stable transgenic line of zebrafish where a fluorescent protein called Kaede is expressed in β cells. The green fluorescent Kaede photoconverts to red when exposed to UV light (Ando et al., 2002; Sato et al., 2006). The time at which the embryo is exposed to UV light determines when the red fluorescent marker is formed. Following removal of UV light, the photoconverted red Kaede persists as red fluorescent protein, while newly translated Kaede will be green. The red fluorescence is durable and inherited by daughter cells, allowing this utility of temporal labeling via photoconversion to be applied to cell lineage tracing experiments.
Following exposure of ins:kaede embryos to UV light, the β cells fluoresce red (represented in Fig. 7B and seen in 7D). New β cells created from the division of other β cells inherit pre-converted red fluorescent protein and also produce their own green fluorescent Kaede protein (red and green cells are represented as yellow in Fig. 7B and seen in the islet in 7H). Alternatively, a newly formed β cell originating from the differentiation of a non-β cell will inherit no red fluorescent protein at all, but will produce green fluorescent protein (green in Fig. 7B). We photoconverted 6 F1 embryos at 48hpf and looked for the appearance of green cells at 120hpf by confocal microscopy. In two embryos we observed distinct green fluorescent cells at the islet periphery (Fig. 7H). This result indicated that new β cells can mature during the time we are seeing β cell recovery. This maturation of β cells is obviously variable but represents a possible mechanism by which new β cells can be formed between 48 and 120hpf. This result also demonstrates that non-β cells continue to contribute to the β cell pool, even after exocrine/endocrine cell clustering in the zebrafish.
Fig. 7.
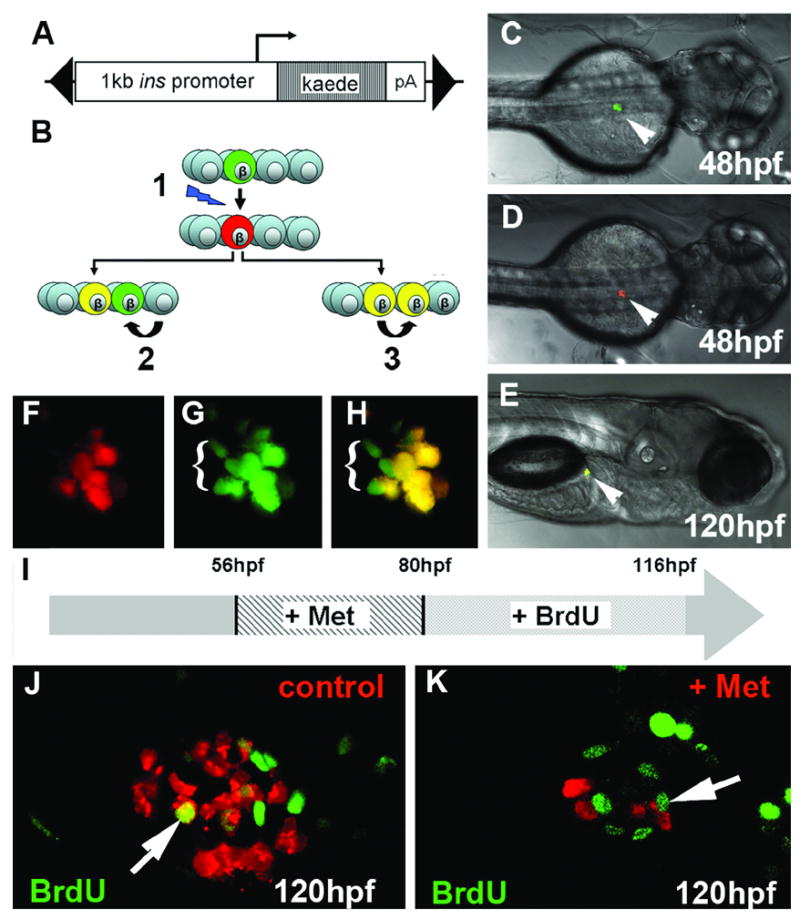
Following ablation, both continued maturation and proliferation may contribute to recovery in β cell numbers. (A) Construct used to generate ins:Kaede transgenics, made within a transposable element (black triangles). (B) Model of Kaede labeling, a green β cell is photoconverted at 48hpf (1). Subsequent differentiation of progenitors gives rise to β cells that possess only green Kaede (2) whereas division of β cells already present during photoconversion inherit red Kaede, produce green kaede and hence appears yellow (3). A live ins:Kaede F1 embryo viewed before (C) and after photoconversion (D). The β cells of the islet can be detected (arrowhead). (E) Later in development, at 120hpf, fluorescence in both channels is apparent and via confocal, it is clear that there are less cells fluorescing red in (F) than green in (G). (H) In a merged image β cells that fluoresce only green can be detected indicating that these cells have arisen from non-β cells. (I) Schematic of the experiment to test for proliferation following β cell ablation in ins:nfsB-mCherry embryos. (J, K) 1μM confocal sections through islet showing BrdU labelling (green nuclei) and β cells (red ). In both controls (J) and islets recovering from β cell ablation (K), proliferation of β cells can be detected as double labeled cells (arrows).
Recovery of β cell mass following prodrug dependent ablation could also occur from proliferation. To test this hypothesis, ins:nfsB-mCherry 56hpf embryos were first treated in prodrug for 24hrs, then washed into BrdU solution and left to recover for 36hrs until 118hpf. Following detection of BrdU incorporation, pancreata were dissected and visualized via confocal microscopy. In dissected pancreata from ins:nfsB-mCherry embryos, BrdU positive β cells can clearly be detected. This observation was made for both embryos recovering from prodrug treatment (n=6, Fig. 7K) and in untreated controls (n=4, Fig. 7J). These results indicate that proliferation of either β cells or their precursors is occurring during the time in which we observe β cell recovery. Whether there is an increase in proliferation that compensates for β cell loss will require detailed further investigation.
In summary we have presented a new method to ablate β cells and by combining micro-dissection with confocal microscopy can monitor in detail cell behavior following perturbation. By expressing and photoconverting the protein Kaede in the β cells, we have demonstrated the ability to map in time when cell differentiation occurs. Together these techniques provide valuable new tools to investigate β cell biology in the zebrafish.
Discussion
Experiments designed to elucidate the process of regulative development or regeneration often focus on the removal of a tissue and the observation of tissue recovery. The most remarkable examples include the amputation and complete recovery of amphibian limbs (Kintner and Brockes, 1984; Wallace et al., 1981). Such classical ablation studies have contributed immensely toward our understanding of cell interactions and their involvement in tissue patterning. For instance, studies in C.elegans have revealed the range of developmental effects resulting from cell ablation, such as, cell autonomy, induction, lineage regulation (Sulston and Horvitz, 1977), and provision of supporting (Kimble and White, 1981) and repressive factors (Seydoux et al., 1990). Each of these classical ablation methods has some inherent caveats. For example, microsurgical techniques can perturb tensional integrity (Ingber, 1993) and dynamic reciprocal interactions (Bissell et al., 1982) that contribute heavily toward proper gene regulation and cell behavior; while laser ablation can leave debris that can affect downstream interactions, and is often limited by the numbers of cells that can be ablated at one time (Yang et al., 2004). Finally, it is often impossible to be certain which cells are being removed without specific molecular markers. Chemical ablation using pharmaceutical agents that target specific cell types can avoid these problems, allowing investigation into the mechanisms and components of regeneration. Examples include the use of (2-morpholinobutyl)-4-thiophenol which causes melanocytotoxicity in zebrafish (Yang and Johnson, 2006) and streptozotocin which causes apoptosis in mammalian β cells (O’Brien et al., 1996).
In this report we describe a prodrug dependent system in the zebrafish that allows for spatial and temporal control of cell ablation, with minimal disturbance to the tissue environment. We used a 1kB fragment from the insulin promoter to drive the expression of NTR to the β cells, and demonstrated β cell ablation following prodrug exposure. Examination of molecular markers showed that damage was limited to the insulin positive cells with no detectable injury to the surrounding tissues. After 24hrs of prodrug treatment, we saw a loss of ins transcript; however, there was variability in the localization of mCherry fluorescence. Residual fluorescent elements were often detected throughout the embryo and occasionally within the pancreas. These elements might represent weakly expressing cells, dead cells or cell debris. Continuing work includes trying to establish whether this movement of fluorescent particles out of the islet is coincident with the action of an early immune response by cells such as macrophages. We were only able to make a limited association of cell death markers with ablation, most likely due to the speed of death and the removal of cells from the islet. To further investigate the method of cell death, we are currently developing transgenic reporters, which in conjunction with confocal microscopy are designed to analyze mitochondrial and nuclear morphology. It is hoped this will allow a more thorough investigation of β cell ablation in our zebrafish model and if the mechanism of cell death is reminiscent of that seen in type I diabetes (Steer et al., 2006).
By following β cell ablation and 36hrs of recovery in the absence of prodrug, we observed a steady increase in fluorescence within the islet. During this same stage of development we observed cell proliferation, as marked by BrdU incorporation, and the differentiation of non-β cells to β cells, as marked by cell lineage tracing using photo-convertible Kaede. We have, therefore, shown that two potential mechanisms exist for the observed replacement of the ablated β cells, namely; differentiation of non-β cells and proliferation (reviewed by (Gu et al., 2003). Photoconversion of Kaede has been used to lineage trace neurons (Sato et al., 2006), but this is the first example of using changes in Kaede fluorescence to determine the timing of cellular differentiation during embryogenesis. The ins:kaede transgenic fish will, therefore, provide a useful tool in assaying the kinetics of β cell differentiation throughout the zebrafish lifespan.
The observation that β cell numbers recover implies the existence of a mechanism for sensing and maintaining β cell mass. In other words the β cells en masse are behaving as a robust system (Nijhout, 2002) and are resistant to perturbation. For the observed regeneration to occur, the decrease in β cell number or a recruitment signal must first be detected by the responding cells. This detection may take the form of assessing changes in cellular contact, changes in autocrine signaling or indeed a systemic change caused by loss of β cells. Muscle mass in mammals is controlled in part by the amounts of a negative regulator called myostatin that circulates through the blood (Lee, 2004; Magee et al., 2006); and there is evidence that this negative regulator inhibits the action of the growth factor IGF-1 (Shyu et al., 2005). It is possible that such a negative regulator is also released by β cells either locally or throughout the embryo to regulate β cell proliferation.
Taking advantage of the small size of zebrafish larvae, it is hoped the ins:nfsBmCherry transgenic fish can be used to identify genetic or pharmacological modifiers of β cell recovery. Such studies could be in the form of mutagenesis or chemical screens and assaying the degree or speed of β cell recovery following ablation. Any candidate gene product or pharmacological agent shown to influence β cell regeneration could then be validated in a recently developed mouse model where β cell ablation can be easily induced in the adult (Pedro Herrera, personal communication).
While the pancreas has been the focus of intense study for many years, we are still learning of new cell types that occupy the islet (Wierup et al., 2004) and how the endocrine and exocrine compartments may interact during zebrafish pancreas development. Our studies suggest that a resident insulin negative population can contribute to β cell regeneration. We have established that the exocrine pancreas is not essential for the regeneration of new β cells, but the identity of the progenitor population remains to be clarified. A stem cell niche consists of enclosed progenitors capable of sensing requirements to proliferate (Fuchs et al., 2004). We have shown that pancreatic cells do proliferate and differentiate quickly in response to perturbation, suggesting the embryonic zebrafish islet might contain stem cell niches. Variation seen in the Kaede experiments indicating that β cells can differentiate between 80 and 116hpf could be explained by small fluctuations in the careful balance that maintains these niches.
Recently, an unbiased screen using ENU mutagenesis has identified several classes of mutations that affect islet morphogenesis, although no mutations that only affect the insulin positive cells were found (Kim et al., 2006). Morpholino analyses have also implicated non-canonical Wnt/Frz interactions in organizing the clustering behavior of the islet, but a careful study of the individual cell populations showed that this mechanism is likely to be indirect (Kim et al., 2005). The ins:nfsB-mCherry zebrafish lines provide not only a model system to study β cell regeneration, but also an alternative strategy to manipulate cell interactions during pancreas development. Using this method, we have shown that the insulin positive population is dispensable for both exocrine cell migration and the maintenance of the islet as a discrete cluster.
The method of prodrug dependent ablation demonstrated in this report has been previously shown to be effective in a number of tissues in the mouse (Clark et al., 1997; Drabek et al., 1997; Wang et al., 2004) and this NTR/Met method is not restricted to β cells in the zebrafish. Recent work has also demonstrated effective ablation of liver and heart cells (D.Stainier personal communication) and the creation of a UAS:nfsB-mCherry line has allowed several recently established Gal4 driver lines to be utilized for ablation of floor plate, notochord and muscle cells (J.Davison personal communication). It is therefore likely that prodrug dependent cell ablation in zebrafish will play an important role in our understanding of system requirements for the control of cell behavior, and ultimately for the treatment of diseases.
Experimental Procedures
Cloning of transgenic constructs
The E.coli gene nfsB was amplified by PCR from bacterial genomic DNA using the primers shown below. Coding regions are capitalized with restriction sites underlined and endonucleases shown in parentheses.
Forward primer (Nco I) 5′ ccATGGATATCATTTCTGTCGCCTTA 3′
Reverse primer (Cla I) 5′ atcgaTTACACTTCGGTTAAGGTGATGT 3′
Reverse primer removing stop codon (Cla I) 5′
atcgatCACTTCGGTTAAGGTGATGTTT 3′
NfsB was cloned into T2KXIGΔIN(Kawakami, 2004) as a Nco/Cla fragment, placing nfsB downstream of the Ef1α promoter. 995bp immediately upstream from the ATG of preproinsulin (ins) (ENSDARG00000035350) was amplified by PCR from zebrafish genomic DNA using the primers below.
Forward primer (BamH I) 5′ ggatccatttaacttcagcccacagtct 3′
Reverse primer (Nco I) 5′ CACACTGCCATggtcacact
EGFP and mCherry were amplified by PCR with primers containing Cla restriction sites in both primers. PCR products were designed to make inframe fusions on C terminus of NTR (minus stop codon). Constructs were assembled from these components into BamHI/ClaI cut T2KXIGΔIN vector backbone(Kawakami, 2004) (Figs. 3A, B). Backbone includes a SV40 poly adenylation site and flanks the insert (promoter, genes and polyA) with the Tol2 transposon arms (500bp left and 500bp right). The Kaede open reading frame was amplified from pKaede-S1 (CoralHue™, MBL®) using Forward primer (Nco I) 5′ ccATGGTGAGTCTGATTAAACC and reverse primer (Cla I) atcgATTACTTGACGTTGTCCGGCAATCC. Kaede was then cloned downstream of the insulin promoter as shown in Fig. 7a. These constructs were used to make transgenic fish (Kawakami, 2004) and F2 progeny from incrossed F1 fish were used in drug ablation studies.
Drug dependent cell ablation
Metronidazole (Met, M3761, Sigma®), was used due to its efficacy over CB1954 in ablating β cells. Met was dissolved in E3 media (Westerfield, 1995) by vigorous agitation. Embryos were incubated at 28°C in the dark. Ins:nfsB-mCherry F1 transgenic embryos were used to study the dose response of Met in ablating β cells. In initial studies, 56hpf embryos were incubated in a range of Met concentrations (1mM to 10mM). After 24hrs of incubation in prodrug, only a concentration of 10mM resulted in complete loss of fluorescence. A concentration of 10mM was used in all later studies and had no deleterious affects on the larvae (n.b. this concentration is highly toxic to adult fish). Ptf1a morphants were created by as previously reported (Lin et al., 2004).
In situ hybridization and Immunofluorescence
Whole-mount in situ hybridization of zebrafish embryos was performed using digoxigenin labeled antisense riboprobes (Thisse, 1998). Probes against zebrafish insulin, glucagon and pdx1 have been described previously(Biemar et al., 2001; Wallace and Pack, 2003). For whole-mount immunofluorescence, embryos were treated as per (Lin et al., 2004) and incubated overnight in 10% goat serum with 1:100 guinea pig anti-insulin , rabbit anti-carboxypeptidase A (Linco Research) and rabbit anti-somatostatin (Dako), washed briefly, and then incubated overnight in 10% goat serum with 1:100 Cy2- and Cy3-conjugated secondary anti-guinea pig or anti-rabbit antibodies. For immunofluorescence on adult fish sections, tissues were fixed in 10% formalin, embedded in paraffin and 5μM sections cut and processed as per standard procedures (Cell signaling technology) followed by double labeling using rabbit polyclonal anti-GFP (Chemicon international; 1: 1000): goat anti rabbit Cy3 (Jackson ImmunoResearch; 1:300) and guinea pig polyclonal anti-insulin (Linco Research Inc; 1:1000): goat anti guinea pig Cy2 (Jackson ImmunoResearch; 1:300).
Whole Mount TUNEL assay
Fixed and methanol permeabilized embryos were rehydrated and treated for 15 minutes with 1μg/ml proteinase K, followed several washes in PBS 0.1% Tween and then a 1 hr incubation at 37°C in a cell death detection reagent (in situ cell death Detection Kit-TMR Red, Roche Diagnostics). Washed embryos were visualized for fluorescence.
BrdU staining
Embryos were placed in 10mM solution of BrdU (Sigma) in E3 and kept in dark at 28° C for 36 hrs. Then embryos were fixed in 4% PFA for 2 hrs at room temperature, washed and incubated in 1 N HCl for 1hr. The washed embryos were blocked using 5% goat serum for 1 hr and incubated over night at 4°C in 1:100 mouse anti-BrdU monoclonal antibody (Chemicon). After washing, embryos were incubated over night at 4°C in 1:100 Cy2 conjugated donkey anti-mouse antibody (Jackson Immunoresearch) and dissected pancreata visualized via confocal microscopy.
Image acquisition
Kaede protein was photoconverted by 30 second exposure of widefield UV using a DAPI filter (λ=375nm). Widefield and confocal sections were acquired using an Axiovert 200M microscope coupled to the Zeiss LSM 5 Pascal system. A Plan-Apochromat 10X/0.45 lens for widefield or Plan-NeoFluar 40X/1.3 Oil DIC objective for confocal sections were used. Blue, green and red channels were excited using a UV, Argon and He/Ne laser, and emissions were detected using BP 420–480, BP 505–530 and LP 560 filters, respectively, and controlled through the Multi-track mode on Zeiss AIM software. All rendering and quantitation used the AIM software. Fixed pancreata were dissected using pulled glass needles on a bed of agarose and mounted on a cover slip for imaging.
Acknowledgments
This work was supported by a pilot project from Johns Hopkins Dept of Surgery (MJP), and by NIH grants DK61215 and DK56211 (SDL). HP is supported by NIH NCRR T32 grant 07002. The authors thank Koichi Kawakami for the T2KXIG IN plasmid and Yangseon Park for expert technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:12651–6. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenton F, Zecchin E, Bortolussi M. Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mech Dev. 1999;87:217–21. doi: 10.1016/s0925-4773(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bridgewater JA, Springer CJ, Knox RJ, Minton NP, Michael NP, Collins MK. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur J Cancer. 1995;31A:2362–70. doi: 10.1016/0959-8049(95)00436-x. [DOI] [PubMed] [Google Scholar]
- Chong AS, Shen J, Tao J, Yin D, Kuznetsov A, Hara M, Philipson LH. Reversal of diabetes in non-obese diabetic mice without spleen cell-derived beta cell regeneration. Science. 2006;311:1774–5. doi: 10.1126/science.1123510. [DOI] [PubMed] [Google Scholar]
- Clark AJ, Iwobi M, Cui W, Crompton M, Harold G, Hobbs S, Kamalati T, Knox R, Neil C, Yull F, Gusterson B. Selective cell ablation in transgenic mice expression E. coli nitroreductase. Gene Ther. 1997;4:101–10. doi: 10.1038/sj.gt.3300367. [DOI] [PubMed] [Google Scholar]
- Cui W, Gusterson B, Clark AJ. Nitroreductase-mediated cell ablation is very rapid and mediated by a p53-independent apoptotic pathway. Gene Ther. 1999;6:764–70. doi: 10.1038/sj.gt.3300873. [DOI] [PubMed] [Google Scholar]
- Drabek D, Guy J, Craig R, Grosveld F. The expression of bacterial nitroreductase in transgenic mice results in specific cell killing by the prodrug CB1954. Gene Ther. 1997;4:93–100. doi: 10.1038/sj.gt.3300366. [DOI] [PubMed] [Google Scholar]
- Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wong RO. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–79. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med. 2006;12:310–6. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- Helsby NA, Ferry DM, Patterson AV, Pullen SM, Wilson WR. 2-Amino metabolites are key mediators of CB 1954 and SN 23862 bystander effects in nitroreductase GDEPT. Br J Cancer. 2004;90:1084–92. doi: 10.1038/sj.bjc.6601612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Vogel SS, Liu N, Melton DA, Lin S. Analysis of pancreatic development in living transgenic zebrafish embryos. Mol Cell Endocrinol. 2001;177:117–24. doi: 10.1016/s0303-7207(01)00408-7. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104 ( Pt 3):613–27. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Johansson E, Parkinson GN, Denny WA, Neidle S. Studies on the nitroreductase prodrug-activating system. Crystal structures of complexes with the inhibitor dicoumarol and dinitrobenzamide prodrugs and of the enzyme active form. J Med Chem. 2003;46:4009–20. doi: 10.1021/jm030843b. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Transgenesis and Gene Trap Methods in Zebrafish by Using the Tol2 Transposable Element. Methods in Cell Biology. 2004;77:201–224. doi: 10.1016/s0091-679x(04)77011-9. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Schleiffarth JR, Jessurun J, Sumanas S, Petryk A, Lin S, Ekker SC. Wnt5 signaling in vertebrate pancreas development. BMC Biol. 2005;3:23. doi: 10.1186/1741-7007-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Sumanas S, Palencia-Desai S, Dong Y, Chen JN, Lin S. Genetic analysis of early endocrine pancreas formation in zebrafish. Mol Endocrinol. 2006;20:194–203. doi: 10.1210/me.2005-0189. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81:208–19. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating limb regeneration. Nature. 1984;308:67–9. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Lardon J, Huyens N, Rooman I, Bouwens L. Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Arch. 2004;444:61–5. doi: 10.1007/s00428-003-0930-z. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- Lin JW, Biankin AV, Horb ME, Ghosh B, Prasad NB, Yee NS, Pack MA, Leach SD. Differential requirement for ptf1a in endocrine and exocrine lineages of developing zebrafish pancreas. Dev Biol. 2004;274:491–503. doi: 10.1016/j.ydbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Magee TR, Artaza JN, Ferrini MG, Vernet D, Zuniga FI, Cantini L, Reisz-Porszasz S, Rajfer J, Gonzalez-Cadavid NF. Myostatin short interfering hairpin RNA gene transfer increases skeletal muscle mass. J Gene Med. 2006 doi: 10.1002/jgm.946. [DOI] [PubMed] [Google Scholar]
- Mahfouz NM, Hassan MA. Synthesis, chemical and enzymatic hydrolysis, and bioavailability evaluation in rabbits of metronidazole amino acid ester prodrugs with enhanced water solubility. J Pharm Pharmacol. 2001;53:841–8. doi: 10.1211/0022357011776199. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–8. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- Nijhout HF. The nature of robustness in development. Bioessays. 2002;24:553–63. doi: 10.1002/bies.10093. [DOI] [PubMed] [Google Scholar]
- O’Brien BA, Harmon BV, Cameron DP, Allan DJ. Beta-cell apoptosis is responsible for the development of IDDM in the multiple low-dose streptozotocin model. J Pathol. 1996;178:176–81. doi: 10.1002/(SICI)1096-9896(199602)178:2<176::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Rood PP, Bottino R, Balamurugan AN, Fan Y, Cooper DK, Trucco M. Facilitating physiologic self-regeneration: a step beyond islet cell replacement. Pharm Res. 2006;23:227–42. doi: 10.1007/s11095-005-9095-6. [DOI] [PubMed] [Google Scholar]
- Sato T, Takahoko M, Okamoto H. HuC:Kaede, a useful tool to label neural morphologies in networks in vivo. Genesis. 2006;44:136–42. doi: 10.1002/gene.20196. [DOI] [PubMed] [Google Scholar]
- Seydoux G, Schedl T, Greenwald I. Cell-cell interactions prevent a potential inductive interaction between soma and germline in C. elegans. Cell. 1990;61:939–51. doi: 10.1016/0092-8674(90)90060-r. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Ko WH, Yang WS, Wang BW, Kuan P. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res. 2005;68:405–14. doi: 10.1016/j.cardiores.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006;3:e17. doi: 10.1371/journal.pmed.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Horvitz HR. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977;56:110–56. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Zebrafish Science Monitor. Eugene: University of Oregon Press; 1998. High resolution whole-mount in situ hybridization; p. 5. [Google Scholar]
- Wallace H, Watson A, Egar M. Regeneration of subnormally innervated axolotl arms. J Embryol Exp Morphol. 1981;62:1–11. [PubMed] [Google Scholar]
- Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255:12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Wan H, Korzh S, Li Z, Mudumana SP, Korzh V, Jiang YJ, Lin S, Gong Z. Analyses of pancreas development by generation of gfp transgenic zebrafish using an exocrine pancreas-specific elastaseA gene promoter. Exp Cell Res. 2006;312:1526–39. doi: 10.1016/j.yexcr.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Wang XD, Shou J, Wong P, French DM, Gao WQ. Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J Biol Chem. 2004;279:24733–44. doi: 10.1074/jbc.M401602200. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish book. University of Oregon Press; Eugene, OR: 1995. p. 10.4. [Google Scholar]
- Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52:301–10. doi: 10.1177/002215540405200301. [DOI] [PubMed] [Google Scholar]
- Yang CT, Johnson SL. Small molecule-induced ablation and subsequent regeneration of larval zebrafish melanocytes. Development. 2006 doi: 10.1242/dev.02533. [DOI] [PubMed] [Google Scholar]
- Yang CT, Sengelmann RD, Johnson SL. Larval melanocyte regeneration following laser ablation in zebrafish. J Invest Dermatol. 2004;123:924–9. doi: 10.1111/j.0022-202X.2004.23475.x. [DOI] [PubMed] [Google Scholar]
- Yee NS, Yusuff S, Pack M. Zebrafish pdx1 morphant displays defects in pancreas development and digestive organ chirality, and potentially identifies a multipotent pancreas progenitor cell. Genesis. 2001;30:137–40. doi: 10.1002/gene.1049. [DOI] [PubMed] [Google Scholar]
- Zecchin E, Mavropoulos A, Devos N, Filippi A, Tiso N, Meyer D, Peers B, Bortolussi M, Argenton F. Evolutionary conserved role of ptf1a in the specification of exocrine pancreatic fates. Dev Biol. 2004;268:174–84. doi: 10.1016/j.ydbio.2003.12.016. [DOI] [PubMed] [Google Scholar]


