Abstract
Background
Management of advanced head and neck carcinoma is a challenging proposition. Presently concomitant chemo-irradiation has become the standard of care in such patients. Many chemotherapeutic drugs have shown radio-sensitising effects when used concomitantly along with radiation. The present study was carried out with the objective of assessing the feasibility and efficacy of low dose gemcitabine as radiosensitizer when used during radical radiotherapeutic management of patients with locally advanced head and neck carcinomas.
Patients and methods
From November 2000 to March 2003, Eighty histopathologically proven cases of squamous cell head and neck carcinoma were included in this trial, 40 patients were randomly assigned to receive radiotherapy alone and 40 patients to receive gemcitabine along with radiotherapy.
Results
All patients were assessable for toxicity and response. Severe mucositis (WHO level 5 reactions were observed in 67% patients in the CT/RT group vs 16% patients in the RT only group. No severe hematological toxicity was seen. The rates of complete and partial responses were 42.5% & 57.5% respectively for RT only and 62.5% &37.5%, respectively for CT/RT group. There was no significantdifference in the response rates at the end of treatment but disease free survival at three years was better in the CT/RT group (63.3% vs 20%). Nine of the 17 patients with complete response in the radiation only group developed relapse while no relapses were seen in CT/RT group.
Conclusion
In the present study the combination of gemcitabine and radiotherapy has not shown any statistical difference in locoregional control but survival advantage was seen as compared to radiotherapy alone. At the same time more mucosal and skin toxicity was encountered when Gemcitabine is given concurrently with radiation.
Introduction
Globally, cancer is a growing problem. From 2000 AD – 2025 AD a significant rise in the number of cases is anticipated worldwide. At present, with a world population of 6 billion, about 10 million cases are diagnosed and 6 million deaths occur annually. This figure will rise to 20 million by 2020 AD from a world population of 12 billion, with 12 million deaths. Of these 20 million cases of cancer, 70% will occur in developing countries.1
The head and neck malignancies constitute 5% of all the cancers world-wide with relatively low incidences in Western Europe and the USA, whereas the high incidence regions include South-East Asian countries, parts of Africa and South America.2,3,4 The majority of these patients i.e. about 70–80% are diagnosed with locally advanced disease with lymph node involvement in upto 30–50%.5,6 Because of high incidence of advanced disease at presentation and local failure rates of 50–60%, the management of head and neck carcinomas is a challenging proposition.7
Historically, early stage cancers of the head and neck have been treated with surgery or radiotherapy with curative intent and produce similar cure rates. The treatment of patients with local surgery, or radiotherapy, or both is associated with poor long term survival because of local recurrence (60%) and development of distant metastasis (10%) in locally advanced tumors.8
Though more aggressive radiotherapy schedules like hyperfractionated or accelerated radiotherapy, high LET radiation, hyperbaric oxygen, hyperthermia and “concomitant boost” strategy indicate a slight increase in locoregional control, yet to date these strategies have failed to demonstrate a clinically meaningful survival advantage.9,10
Therefore, for unresectable head and neck cancers, combined chemotherapy and radiotherapy has become the standard of care as chemoradiotherapy has been found to be superior to radiotherapy alone9.
The use of halogenated pyrimidines as an adjunct to radiotherapy began in the 1960s which included 5-chloro-, 5-bromo-, and 5-iododeoxyuridine; 5-bromo-, and 5-iodo-deoxycytidine; and trifluoromethyl deoxyuridine. Radiosenstizing nucleoside that is currently under most active preclinical and clinical investigation is difluorodeoxycytidine (gemcitabine). It is characterized by relatively low toxicity and a broad spectrum of activity. It results in potent radiosensitization of colon, pancreas, breast, and head and neck cancer at noncytotoxic concentrations.11–15 In 1997, Eisbruch et al.16 reported the results of a phase I study evaluating low-dose gemcitabine concurrent with radiation. They found high tumor control rate at a dose of 300 mg/m2/week, although excessive mucosal toxicity led them to reduce the dose. We carried out a preliminary study with 200 mg/m2/week. Encouraging response rate was observed (75% complete response). All the patients developed grade III mucositis and one patient died during treatment, and because of this the study was terminated. Based on these data, we decided to use a third of the initial dose used by Eisbruch et al. (100 mg/ m2 /week) in this study.
The present study was carried out with the objective of assessing the feasibility and efficacy of low dose gemcitabine as radiosensitizer when used during radical radiotherapeutic management of patients with locally advanced head and neck carcinomas.
Patients and methods
The study was conducted in the Department of Radiotherapy, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak, India. The patients who had locally advanced (T3, T4, any N, M0) previously untreated histopathologically proven squamous cell carcinoma of head and neck were taken up for the study. Patients were included in the study if they had unresectable disease or refused surgery. Eligibility criteria included Karnofsky performance status score ≤=70%, adequate liver function tests, bone marrow reserve (hemoglobin>10 g%, leukocyte count>4000/dl, platelet count>100 000/dl) and renal function (serum creatinine ≤=1.5 md/dl and blood urea≤= 45 mg/dl). The pretreatment evaluation of all the patients included complete history, thorough general physical examination and detailed local examination. The investigations done in all patients were haemoglobin, total leucocyte count (TLC), differential leucocyte count (DLC), blood urea, serum creatinine, SGOT/SGPT, serum alkaline phosphatase, X-ray chest, X-ray soft tissue neck, and CT scan with IV contrast. Approval of the Institutional Review Board was taken before undertaking this trial. All the patients signed an informed consent form approved by the institutional review board.
Treatment Protocol
A prospective, randomized trial was conducted in the Department of Radiotherapy, Post Graduate Institute of Sciences, Rohtak, India, from November 2000 to March 2003. The patients were divided into two groups. Forty patients each were randomly assigned to the two groups, group I patients received only conventional radiotherapy, and group II patients received radiotherapy along with weekly Gemcitabine.
Radiation therapy
In both the groups, external beam radiation was delivered on telecobalt unit and was individualized according to the site and extent of the disease. Radiotherapy was delivered once daily, 5 days a week as a single 2 Gy fraction to a total dose of 64 Gy. Radiation was administered on telecobalt unit and was individualized according to the site and extent of the disease. The shrinking field technique was used and the spinal cord was excluded from the radiation field after a dose of 44 Gy.
Chemotherapy
Gemcitabine was administered intravenously over 30 minutes once weekly, 1–2 h before radiation, for 6 consecutive weeks at a dose of 100 mg/m2.
Toxicity Evaluation
During the therapy, each patient was evaluated weekly for acute reactions. The acute reactions due to chemotherapy and the radiotherapy that were specifically observed were- hematological (Hb, TLC, platelets), skin reactions, oral mucosal reactions, nausea and vomiting, and weight loss. The treatment related toxicities were graded according to the WHO Criteria.
Response criteria
The assessment of tumor response was done 4 weeks after the completion of treatment based on the WHO Criteria. Disappearance of all known disease was taken as complete response and partial response (PR) was defined as 50% or more decrease in total tumor load of the lesions that have been measured to determine the effect of therapy by two observations not less than 4 weeks apart.
Follow-Up
All the patients were followed up fortnightly for one month and then monthly during the first year, two monthly during the second year and 3 monthly thereafter. At each follow-up each patient was examined for radiation and chemotherapy induced reactions on skin, mucosa and tumor response. The response of the tumor was assessed based on the WHO Criteria.
Statistical analysis
The outcome variables were defined as severity of oral mucositis, skin reactions, hematological toxicity, weight loss and nausea-vomiting during a planned period of radiation therapy. To assess the homogeneity of treatment groups with respect to certain characteristics and to test for treatment differences, the Fisher's exact test was used. Treatment related toxicities were assessed using the Mann-Whitney test. The statistical analysis was performed using the SPSS software.
Results
Patient population
From November 2000 to March 2003, eighty histopathologically proven cases of squamous cell head and neck carcinoma were included in this trial, 40 patients were randomly assigned to receive radiotherapy alone and 40 patients to receive gemcitabine along with radiotherapy. There was good balance in the prognostic factors, including performance status, tumor and nodal stages, and histology, between the two groups (Tables 1 and 2).
Table 1.
| Patient Characteristics | RT | CT/RT |
| Age, years | ||
| Median | 50 | 51.5 |
| Range | 28–72 | 30–69 |
| Sex | ||
| Male | 37 (92.5) | 38(95) |
| Female | 3(7.5) | 2(5) |
Table 2.
| Tumor Characteristics Primary site |
RT | CT/RT |
| Oral cavity | 3 | 4 |
| Oropharynx | 30 | 30 |
| Hypopharynx | 6 | 5 |
| Larynx | 1 | 1 |
| Stage | ||
| III | 18 | 20 |
| IV | 22 | 20 |
| Tumor size | ||
| T3 | 36 | 37 |
| T4 | 4 | 3 |
| Nodal status | ||
| N0 | 6 | 7 |
| N1 | 16 | 16 |
| N2 | 19 | 14 |
| N3 | 0 | 3 |
| Histology | ||
| WDSCC | 5 | 5 |
| MDSCC | 31 | 32 |
| Not Specified | 4 | 3 |
WDSCC-well differentiated squamous cell carcinoma, MDSCC- moderately differentiated squamous cell carcinoma.
All patients had squamous cell carcinoma and most tumors (91.25%) were well or moderately differentiated. Thirty eight werestaged as III (18 patients RT group and 20 patients CT/RT group) and forty two were in stage IV (22 patients in RT group and 20 patients in CT/RT group). Oropharynx was the most commonly involved primary site (60 patients, 75% each in RT and CT/RT group). None of the patients had previously received either radiotherapy or chemotherapy.
Toxicity
All 80 patients were assessable for toxicity. Mucositis and nausea/vomiting were the most common acute side-effects. The treatment had to be interrupted in 3 patients in the RT group and 4 patients in the CT/RT group because of severe mucositis. These patients were given split treatment.
Toxicities During Treatment
Haematological
The haemoglobin level of the patients in the RT group showed grade-I toxicity in about 47.5% of patients at the end of treatment and about 7.5% showed Grade-II toxicity, while in the CT/RT group they were in 80% and 20% of patients (p<0.05), respectively. The leukocyte and platelet counts remained within normal limits during the treatment schedule in both the groups.
Skin Reactions
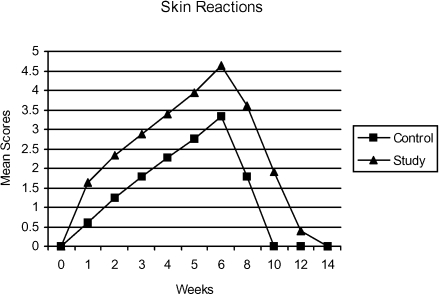
The pattern of appearance of skin reactions during the treatment schedule in both the RT and the CT/RT group is shown in figure 1. There was early onset of skin reactions in the CT/RT arm and the severity was also more.
Figure 1.
Mean scores for cutaneous reactions (0=Nil, 1=Thershold erythema, 2=Erythema grade I, 3=Erythema and desquamation, 4=desquamation, grade 2, 5=Desquamation, blistering, 6=Moist exudation, grade 3) observed during radiation therapy and recovery period.
At the completion of treatment 7.5 % patients developed level 5 and 2.5% patients level 6 reactions and the corresponding figures for CT/RT arm are 50% and 7.5% (p<0.05). In the RT and the CT/RT group one patient each developed level-6 skin reactions at the beginning of 6th week treatment and thus further course of treatment was withheld for a period of 2 weeks, after which the scheduled treatment was completed with a gap- correction.
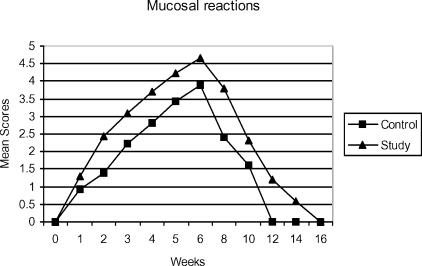
Mucosal reactions
The appearance of mucosal reactions during the treatment in both the groups is shown in figure 2. Level 5 reactions were seen in 17.5% patients in the RT arm at the end of the treatment and the corresponding figures for CT/RT arm are 67.5% (p<0.05). During the 5th week in the CT/RT group two patients developed level-6 mucosal reactions due to which treatment had to be interrupted and further course of treatment was resumed after a gap of 2 weeks.
Figure 2.
Mean scores for mucositis (0=No reaction, 1=Threshold erythema, 2=Definite erythema, 3=Patchy mucositis (less than half of field), 4=Patchy mucositis (more than half of field), 5=Confluent mucositis, 6= Confluent mucositis with bleeding) observed during radiation therapy and recovery period.
Nausea/Vomiting
In both the RT and CT/RT groups only mild nausea and transient vomiting, which did not require medication were observed.
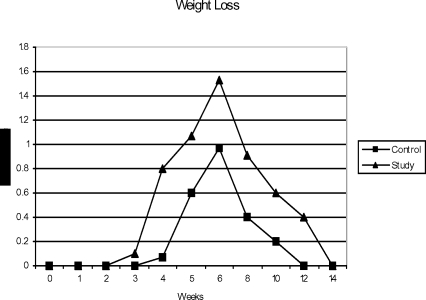
Weight Loss
Prominent weight loss was seen towards the later half of treatment in both the groups (figure 3). At the end of treatment, in the RT group 87.5% of the patients developed weight loss of which 85.7% had grade-I and 14.3% had Grade-2 weight loss, while in the CT/RT group all the patients suffered weight loss of which 47.5% patients had grade-I and 52.5% had grade-2 weight loss. Thus, there was significant loss of weight in the CT/RT group (p<0.05) as compared to the RT group during the later half of treatment which seems to be due to poor intake because of the debilitating oral mucosal reactions.
Figure 3.
Mean scores for weight loss observed during radiation therapy and recovery period.
Response to treatment
The complete response of primary tumor with T3 stage in RT group was seen in 52.5% and partial response in 47.5% of the patients (table 3). The corresponding figures in the CT/RT group were 82.5% and 17.5% respectively. The complete response seen in patients with T4 tumor in RT group was 32.5% and partial response was 67.5% while in the CT/RT group it is 50% complete response and 50% partial response. The over all complete response seen for the RT group patients with both the sites, primary and nodal, combined was 43%, and corresponding figures in the CT/RT group was 63% (p =.1953)
Table 3.
Response to treatment
| RT (n=40) |
CT/RT (n=40) |
|||
| CR | PR | CR | PR | |
| Primary | ||||
| T3 | 19 | 17 | 31 | 6 |
| T4 | 1 | 3 | 2 | 1 |
| Nodal | ||||
| N1 | 10 | 6 | 14 | 2 |
| N2 | 11 | 8 | 9 | 5 |
| N3 | 0 | 0 | 2 | 1 |
RT-radiotherapy only, CT/RT-radiotherapy plus weekly gemcitabine, CR complete response, PR-partial response.
Outcome
The median follow-up time was 9 months (range 6–52) in the RT group and 11 months in the CT/RT group (range 5–51). There was no significantdifference in the response rates at the end of treatment but the disease free survival were better in the CT/RT group. At three years follow up DFS was 20% in the RT group and 63.3% in the CT/RT group. Local control was good, none of the 19 patients with complete response developed relapse in the CT/RT group. Seven of the 13 patients with complete response in the radiation only group relapsed (3 at primary site, 3 at nodal and 1 distant) (table 4).
Table 4.
Relapse
| RT (n=30) |
CT/RT (n=30) |
|
| Primary | 3 | 0 |
| Nodal | 3 | 0 |
| Distant | 1 | 0 |
Discussion
The present study was conducted to assess the feasibility and efficacy of low dose gemcitabine as radiosensitizer in the management of locally advanced head and neck cancers. This study was also meant to determine the local control rate, toxicities and complications if any in the patients receiving gemcitabine. All the patients were stage III or IV and considered unresectable. This present study is only partly similar to only a few study reported so far in the world literature.
A trial, by Benasso et al., used the combination of cisplatin, gemcitabine and radiation therapy. In this study, the incidence and severity of toxicity led the authors to stop accrual after 14 patients were treated; the chemotherapy regimen used was based on typical ‘systemic’ doses of both cisplatin and gemcitabine, explaining the development of severe hematological toxicity and mucositis in >80% of patients. Despite the unacceptable toxicity profile, this combination demonstrated high activity and good local control; however, only 21% of patients received the planned dose of gemcitabine. This study though not similar to the present study supports the use of gemcitabine in HNSCC as a radiation sensitizer ratherthan as a cytotoxic agent.
Another phase I study of concomitant chemoradiotherapy with paclitaxel, fluorouracil, gemcitabine, and twice-daily radiation conducted by Milano et al18 demonstrated good clinical outcome in the advanced head and neck cancers, with complete response of 59%, 5-year locoregional control >60%, and 5-year cause-specific survival >40%. This promising clinical outcome is at the expense of a large percentage of patients experiencing grade 3–4 toxicity. Only 79% completed their course of chemoradiotherapy, and, of these, 35% underwent chemotherapy dose reductions because of dose-related events. Better results of this study are due to the concomitant use of multiple potent chemotherapeutic drugs as compared to single gemcitabine used in the present study.
Severe acute mucositis is the most frequent toxicity observed in most of the studies using concomitant chemoradiation for head and neck cancer patients. The present study also indicates the same.) In the chemoradiation group, WHO level-4 mucosal reactions were seen in 33% and level-5 in 67% of the patients. Treatment had to be interrupted in two of the patients who developed level-6 mucosal reactions.
In another phase-II trial, Wildfang et al19 conducted a study on 26 locally advanced and progressive head and neck, and thyroid carcinoma patients to evaluate the toxicity and efficacy of a combination of radiotherapy and low-dose gemcitabine (200 mg/m2 i/v weekly). The patents were treated with 2–10 cycles of gemcitabine in combination with radiotherapy (mean 42Gy, range 18–67Gy), 14 untreated and 12 patients pre-irradiated. All the patients were evaluated for toxicity and most common were mucositis and erythema. In 8 patients, toxicities more than grade-2 (6 patients mucositis gr-3, one patient skin gr-3, one patient leukopenia gr-3) were observed. 20/26 patients were evaluated for response: 3 CR (15%; first line 3, pre-irradiated 0), 9 PR (45%; first line 4, pre-irradiated 5), 6 no change (30%), 2 progressive disease (10%). In untreated patients the response rate is 70% vs 50% in pre-irradiated patients. The results of this study are not fully comparable to the present study as this study included pre-irradiated patients also and all the patients did not receive the same number of cycles of Inj. Gemcitabine.
Huilgol et al20 conducted a phase-I study to investigate the toxicity and activity of concurrent gemcitabine and radical radiotherapy, in squamous cell carcinoma of head and neck, oesophagus, and cervix. Fifteen treatment naive and histopathologically proven squamous cell carcinoma of head and neck, oesophagus, and cervix were given Inj gemcitabine 200 mg in a 30 minute intravenous infusion once a week along with conventional radical radiotherapy (60 Gy/ 30F with 5F/wk schedule). Out of the 7 head and neck patients enrolled, 6 patients had complete response with an overall survival of 15.6 months. All the patients developed grade-III or IV mucositis at various points of time. Only, one patient could complete the treatment of 5 cycles of gemcitabine as planned whereas 3 patients developed severe mucositis as early as after two weeks 3 of 7 patients received amifostine once grade-III or IV mucositis appeared early in the course of radiation.
Most studies using combined schedules of chemoradiation report a high, sometimes unacceptable, systemic toxicity, particularly hematological toxicity, such as febrile neutropenia and sepsis. Such severe unacceptable toxicity was not observed in the present study) The most important theoretical advantage of using ‘low’ dose gemcitabine is maintaining a high response rate and radiosensitization with low systemic toxicity. In this study the haemoglobin level of the patients in the radiation group showed grade II toxicity in only 7%, while in the chemoradiation 20% of patients showed grade II toxicity, not requiring any active intervention.
In our trial WHO level-5 and level- 6 cutaneous reactions were observed in 6.7% and 3.3% patients in the radiation only group, the corresponding figures in chemoradiation group 50% and 6.7%. In the trial by Eisbruch et al,16 it is seen that when 150 mg/m2 of gemcitabine was given weekly along with conventional radiotherapy, the acute skin reactions were seen in all patients and 25% patients developed RTOG grade-2 toxicity and 67% developed grade-3 toxicity and 8% developed grade-4 toxicity at the end of treatment. The reactions observed with 50 mg/m2 of gemcitabine were grade-I-17%, Grade-II-67% and Grade-III- 17% of patients. In our study the gemcitabine dose was in between the two doses used above and so, were the grades of skin toxicities which are comparable. The patients of study group not only developed skin reactions more rapidly but in larger number and more severe grade too. However, these were manageable.
In the present study complete response at both the sites was seen in 65% patients in the chemoradiation group vs 20% in the radiation only group. CR at either site was observed in 31% study group patients vs 50% in the RT group. 3.5% vs 26.7% patients showed partial response at both sites and 0% vs 3.3% showed progressive disease at both sites in the study and the RT group, respectively. The overall response in study group is comparable with the available literature.35
Conclusion
The concomitant use of gemcitabine and radiation has not shown any difference in the locoregional control at the end of treatment but better disease free survival was observed at three years follow up, as compared to radiation alone. However, combined administration produced more toxicities and required parenteral nutritional support in all the patients, especially near the completion of treatment. This necessitated great attention and care. However, these reactions were manageable.
Acknowledgement
The authors are thankful to Dr Manoj Kumar (Lecturer, Statistics, Department of Preventive and Social Medicine, Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak, India) for his contributions during the preparation of the manuscript.
References
- 1.Vokes EE. Head and neck cancer. In: Fauci AS, Braunwald E, Isselbacher KJ, Wilson JD, Maltin JB, Kasper DL, et al., editors. Harrison's Principles of Internal Medicine. New York: Mc Graw Hill; 1998. pp. 549–552. [Google Scholar]
- 2.Mohanti BK, Bahadur S, Lal P, Gairola M, Rath GK. Cancers of the head and neck. In: Rath GK, Mohanti BK, editors. Textbook of Radiation Oncology, Principles and Practice. New Delhi: B.I. Churchill Livingstone; 2000. pp. 131–199. [Google Scholar]
- 3.Robson MC, Phillips LG. Head and neck: Over view. In: Moosa AR, Schimpff SC, Robson MC, editors. Comprehensive Text Book of Oncology. Baltimore: Williams & Wilkins; 1991. pp. 1125–1207. [Google Scholar]
- 4.Tobias JS. Cancer of the head and neck. BMJ. 1994;308:961–966. doi: 10.1136/bmj.308.6934.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Sarraf M. Head and neck cancer: Chemotherapy concepts. Semin Oncol. 1988;15:70–85. [PubMed] [Google Scholar]
- 6.Stupp R, Weichselbaum RR, Vokes EE. Combined modality therapy of head and neck cancer. Semin Oncol. 1994;21:349–358. [PubMed] [Google Scholar]
- 7.Ervin TJ, Clark JR, Weichselbaum PR. An analysis of induction and adjuvant chemotherapy in the multidisciplinary treatment of squamous cell carcinoma of head & neck. J Clin Oncol. 1987;5:10–20. doi: 10.1200/JCO.1987.5.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, Forastiere AA. Chemotherapy of head and neck Cancers. In: Haskell CM, Berek JS, editors. Cancer Treatment. 5th ed. USA: W.B. Saunders Company; 2001. pp. 1030–1042. [Google Scholar]
- 9.Vokes EE, Haraf DJ, Kies MS. The use of concurrent chemotherapy and radiotherapy for locoregionally advanced head & neck cancer. Semin Oncol. 2000;27(4) Suppl 8:34–38. [PubMed] [Google Scholar]
- 10.Kumar A, Das BP, Hooda HS, Kaushal V, Manocha KK. Continuous hyperfractionated accelerated radiotherapy in head and neck carcinoma. Ind J Radiol Imag. 1992;2:197–201. [Google Scholar]
- 11.Shewach DS, Hahn TM, Chang E. Metabolism of 2′,2′difluoro-2′-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Res. 1994;54:3218–3223. [PubMed] [Google Scholar]
- 12.Shewach DS, Lawrence TS. Radiosensitization of human solid tumour cell lines with gemcitabine. Semin Oncol. 1996;23(Suppl. 10):65–71. [PubMed] [Google Scholar]
- 13.Lawrence TS, Chang EY, Hahn TM. Radiosensitization of pancreatic cells by 2′,2′-di fluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence TS, Eisbruch A, Schewach DS. gemcitabine-mediated radiosensitization. Semin Oncol. 1997;24(Suppl 7):24. [PubMed] [Google Scholar]
- 15.Rockwell S, Grindey GB. Effect of 2′,2-difluoro deoxycytidine on the viability and radiosensitivity of EMT6 Cells in vitro. Oncol Res. 1992;4:151–155. [PubMed] [Google Scholar]
- 16.Eisbruch A, Shewach DS, Bradford CR. Radiation concurrent with gemcitabine for locally advanced head and neck cancer: a phase —I trial and intracellular drug incorporation study. J Clin Oncol. 2001 Feb 1;19(3):792–799. doi: 10.1200/JCO.2001.19.3.792. [DOI] [PubMed] [Google Scholar]
- 17.Benasso M, Merlano M, Sanguineti G, et al. Gemcitabine, cisplatin, and radiation in advanced unresectable squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2001;24:618–622. doi: 10.1097/00000421-200112000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Milano MT, Haraf DJ, Stenson KM, Witt ME, Eng C, Mittal BB, Argiris A, Pelzer H, Kozloff MF, Vokes EE. Phase I Study of Concomitant Chemoradiotherapy with Paclitaxel, Fluorouracil, Gemcitabine, and Twice-Daily Radiation in Patients with Poor-Prognosis Cancer of the Head and Neck. Clinical Cancer Research. 2004;10:4922–4932. doi: 10.1158/1078-0432.CCR-03-0634. [DOI] [PubMed] [Google Scholar]
- 19.Wildfang I, Raub M, Guner SA. Low dose gemcitabine with radiotherapy in advanced head and neck and thyroid cancer, a new radiosensitizing approach. J Can Res and Clin Oncol. 2000;126(1):R40. [Google Scholar]
- 20.Huilgol NG, Roy S, Shah D, Chatterjee N. Gemcitabine once a week with Radical Radiation-A phase I trial. J Clin Radioth & Onco. 2002;2(2):30–33. [Google Scholar]