Summary
Courtship song is a critical component of male courtship behavior in Drosophila, making the female more receptive to copulation and communicating species-specific information [1–6]. Sex mosaic studies have shown that the sex of certain regions of the central nervous system (CNS) is critical to song production [7]. Our examination of one of these regions, the mesothoracic ganglion (Msg), revealed the coexpression of two sex-determination genes, fruitless (fru) and doublesex (dsx). Because both genes are involved in creating a sexually dimorphic CNS [8, 9] and are necessary for song production [10–13], we investigated the individual contributions of fru and dsx to the specification of a male CNS and song production. We show a novel requirement for dsx in specifying a sexually dimorphic population of fru-expressing neurons in the Msg. Moreover, by using females constitutively expressing the male-specific isoforms of fru (FruM), we show a critical requirement for the male isoform of dsx (DsxM), alongside FruM, in the specification of courtship song. Therefore, although FruM expression is sufficient for the performance of many male-specific behaviors [14], we have shown that without DsxM, the determination of a male-specific CNS and thus a full complement of male behaviors are not realized.
Keywords: SYSNEURO
Results and Discussion
Courtship behavior in Drosophila melanogaster consists of a sequence of behaviors performed by males to interest females in copulation. The male orients to the female, follows her, taps her abdomen with his foreleg, sings a species-specific courtship song, licks her genitals, attempts copulation, and finally copulates [15, 16]. Sex mosaic studies have shown that the sex of the central nervous system (CNS) is critical to the performance of these behaviors, suggesting that sex determination in the CNS is required for male sexual behavior in flies [7, 15, 17–21]. In particular, one sex-determination gene, fruitless (fru), is a key regulator of many steps in the courtship ritual [10, 12–14, 22].
Transcripts derived from the fru P1 promoter are spliced in females by the sex-specific splice factor Transformer (Tra) in conjunction with the non-sex-specific Transformer-2 (Tra-2), introducing a premature stop codon into female P1 transcripts. In males, a default splice occurs, giving rise to a class of male-specific fru isoforms (FruM proteins) [10, 13, 22–25] that are expressed in the CNS and peripheral nervous system (PNS) [23, 25–28] in regions associated with male-specific behaviors.
The constitutive expression of FruM isoforms in females triggers many male-specific courtship behaviors [14]. However, these females perform subnormal amounts of courtship and do not attempt copulation [14], suggesting that fru alone cannot specify all male courtship behaviors.
We examined the role of doublesex (dsx), another sex-determination gene, in the specification of male sexual behavior. dsx transcripts also undergo sex-specific splicing by Tra, producing male- and female-specific isoforms: DsxM and DsxF, respectively [29, 30]. dsx is responsible for somatic sexual differentiation [15, 31–33] and aspects of sex-specific development in the CNS [8]. dsx is also expressed in the CNS and is necessary for wild-type courtship song in males [11, 34]. Recently, dsx was shown to act alongside fru in the differentiation of male-specific neurons in the abdominal ganglion [25]; however, few other studies have examined the relative contributions of both fru and dsx in specifying a male-specific CNS and regulating male sexual behavior. Therefore, this study examined the individual contributions of both genes in specifying courtship song.
FruM Is Not Sufficient for Courtship Song
Courtship song in Drosophila melanogaster is male-specific and is critical to stimulating the female [4, 6]. It consists of a humming sound called sine song, and a rhythmically patterned pulse song, which together stimulate the female to mate, reducing the time to copulation [4, 6]. Pulse song also communicates species-specific information, allowing females to recognize conspecific males [1–3].
FruM mutant males lack pulse song [10, 12, 13], and constitutive FruM expression in the CNS of fruM and fruΔtra females induces the performance of many steps of the male courtship ritual [14], suggesting an important role for FruM in specifying courtship song. To determine the contribution of FruM in the specification of courtship song, we analyzed song production in females of genotype fruM and fruΔtra.
Song analysis was based on 29 fruM and fruΔtra females because most fruM and fruΔtra females did not perform sufficient courtship behavior or song for analysis (total n = 61; Table S1 in the Supplemental Data available online). In accordance with [14], the wing-extension indices (WEIs) of these 29 fruM and fruΔtra females were not significantly different from wild-type and control fruM and fruΔtra males; however, we found a significant decrease in the song index (SI) (the percentage of time spent singing during wing extension) (p < 0.05) (Table S1). Also, the fruM and fruΔtra females' pulse song was highly aberrant (Figure 1). The number of pulse trains per minute (PTPM), mean pulses per train (MPPT) and interpulse interval (IPI) were all significantly lower compared to wild-type and control males (Table S1). Most striking, however, was the complete absence of sine song in these females (n = 29). Although the fruM and fruΔtra females were capable of wild-type wing extension, they spent significantly less time singing during courtship and produced song of poor quality. Thus, FruM expression alone cannot specify wild-type song production.
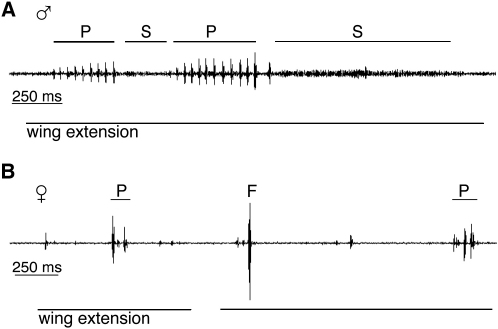
Figure 1.
Representative Song Traces of fruΔtra Males and Females
Representative song traces from a 5–7-day-old fruΔtra/Df(3R)fru4-40 male (A) and a fruΔtra/Df(3R)fru4-40 female (B) produced in the presence of a single wild-type Canton S (CS) virgin female. Each song trace represents a fraction of a 5 min recording for a given individual. Wing extension during the recording is indicated below the song trace. Bouts of pulse song (“P”) and sine song (“S”) are recorded above the song trace. “F” indicates a noise caused by the CS target female falling.
DsxM Is Required to Specify Courtship Song
To dissect the individual contributions of both FruM and Dsx to the specification of courtship song, we analyzed males lacking FruM and Dsx (genotype fru3,In(3R)dsx23/fru3,Df(3R)dsx15). These double mutants had a courtship index (CI) of 0 toward females (n = 11) and no song. We have shown that FruM expression in females is not sufficient for courtship song. Likewise, the expression of DsxM in females is also not sufficient for song [35]. Thus neither fru nor dsx alone can specify courtship song. In fact, only the presence of both FruM and DsxM, as in transformer (tra) mutant females, renders females capable of wild-type courtship song [36, 37], a finding we confirm. Together, these results demonstrate a previously unrecognized requirement for DsxM, in conjunction with FruM, in specifying courtship song.
Dsx and FruM Colocalize in the CNS
Studies with male-female mosaics have shown that in gynandromorphs with a male head, the ventral thoracic ganglia of the adult CNS (including the mesothoracic ganglion [Msg]) must also be male for courtship song [7]. This suggests that the neural foci of courtship song are located in the ventral thoracic ganglia and that the sex of this region is critical to song. fru and dsx are both expressed in neurons located in this region, and mutations in both genes cause song defects [10–13, 23, 34]. In the abdominal ganglion (Abg) of the CNS, FruM and Dsx were shown to colocalize in a proportion of neurons and play critical roles in the development of male-specific clusters of serotonergic neurons [25]. Therefore, we asked whether FruM and Dsx were also coexpressed in the thoracic ganglia, and whether they act in parallel (if expressed in different neurons) or in concert (if expressed in the same neurons) to determine the neuronal substrate for courtship song in the CNS.
We determined that Dsx and FruM colocalize in the Msg of the CNS (Figure 2B). Colocalization occurred in a subset of Dsx-expressing neurons (TN1 cluster; nomenclature as per [34]) from the pupal stage onward. The number of neurons coexpressing Dsx and FruM in 2-day-old male pupae was 17.4 ± 1.7 per hemisegment (mean ± standard deviation [SD]; n = 10), and was not significantly different in 5-day-old adults (p < 0.05). Colocalization occurred in a further two subsets of Dsx-expressing neurons in the posterior brain, pC1 and pC2 (nomenclature as per [34]), in addition to previously reported colocalization in the Abg [25] (Figure S1). Given the critical importance of the sex of the ventral ganglia (including the Msg) to song production, the colocalization of FruM and Dsx in this region suggests that sexually dimorphic developmental mechanisms might be operating in the Msg, contributing to the sex-specific nature of courtship song production.
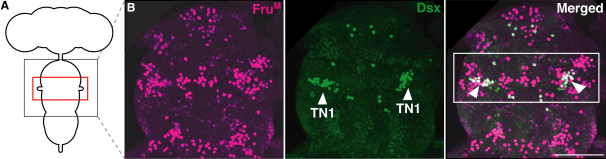
Figure 2.
Colocalization of FruM and Dsx in the Mesothoracic Ganglion
(A) Schematic representation of the CNS. The large black box indicates the region shown in the images, and the smaller red box surrounds the region of colocalization, the Msg.
(B) Whole-mount ventral nerve cord (VNC) from 2-day-old pupal wild-type males colabeled with anti-FruM and anti-Dsx. The colocalization of FruM and Dsx in a subset of neurons in the TN1 cluster (arrowheads) of dsx-expressing neurons in the Msg of the VNC (white box) is shown. A ventral view is shown, with the anterior at the top. For the complete coexpression in the CNS, see Figure S1. The scale bar represents 50 μm.
The Neuromuscular Junctions of the Direct Flight Muscles Are Not Sexually Dimorphic
Electrophysiological studies show that the activity of seven of the direct flight muscles (DFMs) is directly related to the beating of the wing during song [38]. These seven DFMs are the basalar muscles B1–B4, the anterior muscles of the first and third axillaries AX1a and AX3a, and the sternobasalar muscle SB (nomenclature as per [38]). The axonal morphology and cell-body location of the motor neurons innervating six of these DFMs (mnDFMs) has also been reported [39]. The cell bodies of these six mnDFMs lie in the ventral thoracic ganglia, five having cell bodies in the Msg [39]. We therefore investigated whether male-specific song production could be attributed to fru- and/or dsx-regulated sexually dimorphic characteristics of these motor neurons.
First, we asked whether any of the mnDFMs were fru or dsx expressing. By using fruGAL4, a GAL4 driver expressing in all fru neurons [28], we determined that only mnB3/B4 (a single motor neuron innervating both B3 and B4 [39]) was fruGAL4 positive, and thus is a fru neuron (Figures 3A and 3B). This neuron was fruGAL4 positive in both males and females, and the innervation was not obviously sexually dimorphic. However, because some dsx-expressing neurons in the Msg are not fru expressing, we examined the axonal morphology of all mnDFMs to eliminate the possibility of sex-specific DFM innervation.
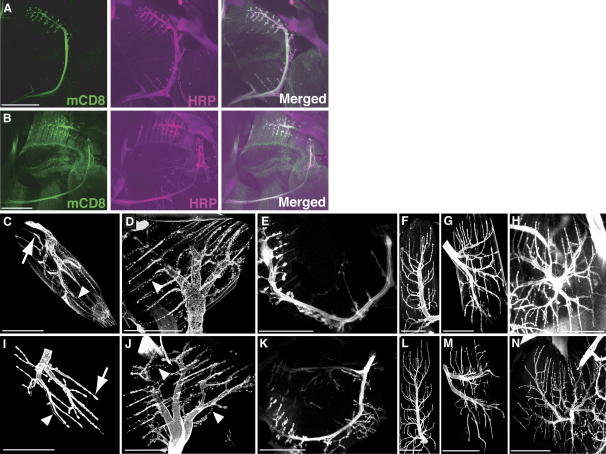
Figure 3.
Motor Neurons Innervating the Direct Flight Muscles
(A and B) fruGAL4 generates expression in a motor neuron innervating the direct flight muscles B3 and B4 (mnB3/B4). fruGAL4 was used to drive expression of the mouse lymphocyte (transmembrane) marker CD8 and green fluorescent fusion protein (UAS-mCD8::GFP) and was detected by anti-mCD8 labeling (mCD8; green). Anti-horseradish peroxidase (Anti-HRP) conjugated to Cy3 (HRP; purple) was used to reveal the neuronal projections of mnB3/B4 (purple) [48]. fruGAL4 drives expression in mnB3/B4 in both 5–7-day-old males (A) and females (B).
(C–N) Axonal morphology of the motor neurons innervating the mnDFMs. The anti-HRP::Cy3 conjugate was used to reveal the neuronal projections [48]. Type I terminals (arrows) and type II terminals (arrowheads) are shown. Both types of synaptic terminals are present on all DFMs. The following axonal projections of mnDFMs from 5-day-old wild-type Canton S males were used: mnB1 (C), mnB2 (D), mnB3/B4 (E), mnAX1a (F), mnAX3a (G), and mnSB (H). The following axonal projections of mnDFMs from 5-day-old wild-type Canton S females were used: mnB1 (I), mnB2 (J), mnB3/B4 (K), mnAX1a (L), mnAX3a (M), and mnSB (N).
The DFM nomenclature is as per [38]. Scale bars represent 50 μm.
The axonal morphology and expression of common neurotransmitters at the neuromuscular junction (NMJ) of all seven DFMs were examined, and no obvious differences between the sexes were found (Figures 3C–3N). Type I and type II synaptic terminals were present on all mnDFMs, where type I terminals expressed glutamate and type II terminals expressed octopamine, in accordance with previous reports of neurotransmitter expression at the adult NMJ [40]. Moreover, no obvious differences in either axonal morphology or common neurotransmitters were observed in either fru or dsx mutant males (data not shown; genotypes are in the Supplemental Experimental Procedures). Therefore, the sexually dimorphic production of song is not likely to be a result of an obvious dimorphism in the neuronal morphology of the mnDFMs or in the neurotransmitter expression at the NMJs. Where might the critical difference(s) then lie?
The Mesothoracic Ganglion Is Sexually Dimorphic
Kimura et al. [9] showed that FruM expression prevented reaper-mediated programmed cell death [41, 42] in a cluster of cells, resulting in more neurons in this cluster in males. DsxM, on the other hand, prolongs neuroblast divisions in the Abg of males [8], again resulting in more neurons in males. Thus, sexual dimorphisms might be present in regions in which Dsx and FruM colocalize, as suggested by the ability of FruM and Dsx to generate sexually dimorphic neuronal populations [8, 9, 25]. Given that our investigation found no obvious sex-specific dimorphisms in the mnDFMs, the dimorphism might lie in a population of interneurons. Therefore, the Msg was examined so that it could be determined whether a sexually dimorphic population of neurons was present.
By using fruGAL4, which expresses in both males and females [28], to drive a GAL4-responsive UAS-LacZ.NZ reporter, we quantified the number of β-Gal-positive neurons in males and females (Figure 4A). The number of β-Gal-positive neurons was significantly higher in males, with 136.4 ± 3.3 cells per hemisegment (n = 10) versus 111.6 ± 3.1 cells per hemisegment in females. Previously, Lee et al. [23] reported a sexual dimorphism in the number of neurons expressing fru P1 transcripts in the Msg. Together, these results suggest that a sexually dimorphic population of neurons is present in the Msg; therefore, we examined the individual contributions of FruM and Dsx in the creation of this difference in fruGAL4-positive neuron number in the Msg.
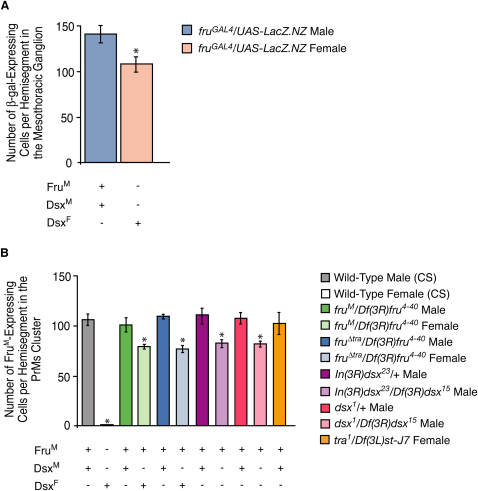
Figure 4.
Sexually Dimorphic Neuron Numbers in the Mesothoracic Ganglion
(A) Mean number of nuclei expressing β-Gal per hemisegment (±SD) in the Msg of the CNS in 5–7-day-old adult males and females. The mean number of nuclei is calculated from 10 hemisegments. fruGAL4 drives the expression of β-Gal in both males and females. “∗” indicates a significant difference (p < 0.05).
(B) Mean number of nuclei expressing FruM per hemisegment (±SD) in the PrMs cluster of FruM-expressing neurons (nomenclature as per [23]) in 5–7-day-old adult flies. The mean number of nuclei per hemisegment is calculated from 10 hemisegments per genotype. “∗” indicates a significant decrease from wild-type and control males (p < 0.05). The presence or absence of FruM, DsxM, and DsxF is noted below each histogram bar.
FruM Expression Alone Cannot Create a Sexually Dimorphic Msg
We found a sexually dimorphic number of fruGAL4-expressing neurons in the Msg, a region of the CNS central to song production [7] and in which FruM and Dsx colocalize. To determine the individual contributions of dsx and fru in the creation of this sexually dimorphic number of neurons, we examined fruM and fruΔtra females to see if FruM expression alone abolishes the observed difference in neuronal number in the Msg between the sexes. We found that the number of FruM-expressing neurons in the Msg of these females was significantly reduced in comparison to wild-type and control males (Figure 4B; Table S2). Furthermore, this decrease in FruM-expressing neurons was comparable to the difference in neuron number observed in the Msg of fruGAL4 males and females driving the UAS-LacZ.NZ reporter.
Our results demonstrate that the difference in neuronal populations of males and females in the Msg lies in a subpopulation of FruM-expressing neurons, and that FruM expression alone cannot eliminate this difference. Thus FruM expression cannot, by itself, dictate the creation of the sexually dimorphic population of neurons in the Msg. We therefore asked whether Dsx, which colocalizes with FruM in the Msg, plays a role in the specification of this sexually dimorphic population of neurons, helping to determine the full complement of FruM neurons.
DsxM Is Required for Wild-Type FruM Expression
dsx affects the sex-specific development of other regions of the CNS [8, 25]. To determine whether dsx contributes to creating the sex-specific population of neurons in the Msg, we tabulated the number of FruM-expressing neurons in the Msg of dsx null and dsx heterozygote control males. We found that dsx mutant males had significantly fewer FruM-expressing neurons in the Msg than did wild-type and control males, demonstrating that Dsx is indeed required to obtain a full complement of FruM-expressing neurons (Figure 4B; Table S2). Because fruM and fruΔtra females (who express the female-specific isoform of dsx, DsxF) do not have a full complement of FruM-expressing neurons in the Msg, we have demonstrated a critical role for DsxM in the creation of a sexually dimorphic Msg. In fact, only when both FruM and DsxM are present, as in tra mutant females, can a full complement of FruM-expressing neurons in the Msg be obtained (Figure 4B). Thus, we have demonstrated a previously unrecognized requirement for DsxM in the specification of a population of FruM-expressing neurons in the Msg.
DsxM prolongs the division of neuroblasts in the Abg of males, resulting in more neurons in the male Abg [8]. Also in the Abg, DsxM plays a critical role alongside FruM in the differentiation of a male-specific serotonergic population of neurons [25]. Our findings suggest that DsxM operates in a similar manner in the Msg and the posterior brain (E.J.R. and S.F.G., unpublished data) to create sexually dimorphic neuronal numbers. These differences in neuronal populations suggest a common developmental theme in colocalization regions, where DsxM generates a sexually dimorphic population of neurons, which is exploited by FruM to fashion a male-specific behavioral neural network [25] (cf. [9, 15]).
It is not clear why the absence of a sexually dimorphic population of FruM-expressing neurons in the Msg is associated with striking defects in courtship song because our results suggest that this population of FruM-expressing neurons does not directly innervate the DFMs. We propose that the FruM-expressing neurons form at least part of a male-specific neural network responsible for controlling the production of courtship song.
Thus, although FruM expression can specify many male-specific behaviors, we show that without DsxM, the determination of a complete male-specific CNS, and therefore a full complement of male behaviors, is not realized. This additional gene function is critical to understanding complex sex-specific phenotypes compared to previous interpretations of function, where fru has been described as the only gene needed for a “genetic switch” to male sexual behavior in Drosophila. Significantly, it adds to the growing evidence that fru and dsx are both necessary for a complete male courtship repertoire, in both neural and nonneural tissues [11, 25, 34, 43–47].
Acknowledgments
We are grateful to Adriana Villella, John Rieffel, and Jeff Hall (Brandeis University) for generously providing, and assisting in the use of, LifeSongX software. We thank Gyunghee Lee for the generous gift of Dsx antibody, Barry Dickson for fruΔtra and fruM flies, and Paul Schedl for dsx1 flies. We thank Megan Neville, Tony Dornan, Jeff Hall, and Michelle Arbeitman for comments on the manuscript. E.J.R., J.-C.B., and S.F.G. were supported by grants from the Wellcome Trust.
Published online: August 23, 2007
Footnotes
Experimental Procedures, one figure, and two tables are available at http://www.current-biology.com/cgi/content/full/17/17/1473/DC1/.
Supplemental Data
References
- 1.Kyriacou C.P., Hall J.C. The function of courtship song rhythms in Drosophila. Anim. Behav. 1982;30:794–801. doi: 10.1006/anbe.1998.0976. [DOI] [PubMed] [Google Scholar]
- 2.Kyriacou C.P., Hall J.C. Learning and memory mutants impair acoustic priming of mating behavior in Drosophila. Nature. 1984;314:171–173. doi: 10.1038/308062a0. [DOI] [PubMed] [Google Scholar]
- 3.Kyriacou C.P., Hall J.C. Interspecific genetic control of courtship song production and reception in Drosophila. Science. 1986;232:494–497. doi: 10.1126/science.3083506. [DOI] [PubMed] [Google Scholar]
- 4.Griffith L.C., Verselis L.M., Aitken K.M., Kyriacou C.P., Danho W., Greenspan R.J. Inhibition of calcium/calmodulin-dependent protein kinase in Drosophila disrupts behavioral plasticity. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- 5.Ritchie M.G., Halsey E.J., Gleason J.M. Drosophila song as a species-specific mating signal and the behavioural importance of Kyriacou & Hall cycles in D. melanogaster song. Anim. Behav. 1999;58:649–657. doi: 10.1006/anbe.1999.1167. [DOI] [PubMed] [Google Scholar]
- 6.Rybak F., Aubin T., Moulin B., Jallon J.M. Acoustic communication in Drosophila melanogaster courtship: Are pulse- and sine-song frequencies important for courtship success? Can. J. Zool. 2002;80:987–996. [Google Scholar]
- 7.von Schilcher F., Hall J.C. Neural topography of courtship song in sex mosaics of Drosophila melanogaster. J. Comp. Physiol. [A] 1979;129:85–95. [Google Scholar]
- 8.Taylor B.J., Truman J.W. Commitment of abdominal neuroblasts in Drosophila to a male or female fate is dependent on genes of the sex-determining hierarchy. Development. 1992;114:625–642. doi: 10.1242/dev.114.3.625. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K., Ote M., Tazawa T., Yamamoto D. fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- 10.Ryner L.C., Goodwin S.F., Castrillon D.H., Anand A., Villella A., Baker B.S., Hall J.C., Taylor B.J., Wasserman S.A. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 11.Villella A., Hall J.C. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics. 1996;143:331–344. doi: 10.1093/genetics/143.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villella A., Gailey D.A., Berwald B., Ohshima S., Barnes P.T., Hall J.C. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin S.F., Taylor B.J., Villella A., Foss M., Ryner L.C., Baker B.S., Hall J.C. Aberrant splicing and altered spatial expression patterns in fruitless mutants of Drosophila melanogaster. Genetics. 2000;154:725–745. doi: 10.1093/genetics/154.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demir E., Dickson B.J. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Billeter J.C., Rideout E.J., Dornan A.J., Goodwin S.F. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr. Biol. 2006;16:R766–R776. doi: 10.1016/j.cub.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Villella A., Hall J.C. Neurogenetics of Courtship and Mating in Drosophila. Adv. Genet. 2007 doi: 10.1016/S0065-2660(08)00603-2. in press. [DOI] [PubMed] [Google Scholar]
- 17.Hall J.C. Control of male reproductive behavior by the central nervous system of Drosophila: Dissection of a courtship pathway by genetic mosaics. Genetics. 1979;92:437–457. doi: 10.1093/genetics/92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Dell K.M., Armstrong J.D., Yang M.Y., Kaiser K. Functional dissection of the Drosophila mushroom bodies by selective feminization of genetically defined subcompartments. Neuron. 1995;15:55–61. doi: 10.1016/0896-6273(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 19.Ferveur J.F., Stortkuhl K.F., Stocker R.F., Greenspan R.J. Genetic feminization of brain structures and changed sexual orientation in male Drosophila. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- 20.Ferveur J.F., Greenspan R.J. Courtship behavior of brain mosaics in Drosophila. J. Neurogenet. 1998;12:205–226. doi: 10.3109/01677069809108559. [DOI] [PubMed] [Google Scholar]
- 21.Kido A., Ito K. Mushroom bodies are not required for courtship behavior by normal and sexually mosaic Drosophila. J. Neurobiol. 2002;52:302–311. doi: 10.1002/neu.10100. [DOI] [PubMed] [Google Scholar]
- 22.Ito H., Fujitani K., Usui K., Shimizu-Nishikawa K., Tanaka S., Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. USA. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee G., Foss M., Goodwin S.F., Carlo T., Taylor B.J., Hall J.C. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J. Neurobiol. 2000;43:404–426. doi: 10.1002/1097-4695(20000615)43:4<404::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 24.Usui-Aoki K., Ito H., Ui-Tei K., Takahashi K., Lukacsovich T., Awano W., Nakata H., Piao Z.F., Nilsson E.E., Tomida J., Yamamoto D. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat. Cell Biol. 2000;2:500–506. doi: 10.1038/35019537. [DOI] [PubMed] [Google Scholar]
- 25.Billeter J.C., Villella A., Allendorfer J.B., Dornan A.J., Richardson M., Gailey D.A., Goodwin S.F. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr. Biol. 2006;16:1063–1076. doi: 10.1016/j.cub.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 26.Billeter J.C., Goodwin S.F. Characterization of Drosophila fruitless-Gal4 transgenes reveals expression in male-specific fruitless neurons and innervation of male reproductive structures. J. Comp. Neurol. 2004;475:270–287. doi: 10.1002/cne.20177. [DOI] [PubMed] [Google Scholar]
- 27.Manoli D.S., Foss M., Villella A., Taylor B.J., Hall J.C., Baker B.S. Male-specific fruitless specifies the neural substrates of Drosophila courtship behavior. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 28.Stockinger P., Kvitsiani D., Rotkopf S., Tirian L., Dickson B.J. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Nagoshi R.N., McKeown M., Burtis K.C., Belote J.M., Baker B.S. The control of alternative splicing at genes regulating sexual differentiation in D. melanogaster. Cell. 1988;53:229–236. doi: 10.1016/0092-8674(88)90384-4. [DOI] [PubMed] [Google Scholar]
- 30.Hoshijima K., Inoue K., Higuchi I., Sakamoto H., Shimura Y. Control of doublesex alternative splicing by transformer and transformer-2 in Drosophila. Science. 1991;252:833–836. doi: 10.1126/science.1902987. [DOI] [PubMed] [Google Scholar]
- 31.Steinmann-Zwicky M., Amrein H., Nothiger R. Genetic control of sex determination in Drosophila. Adv. Genet. 1990;27:189–237. doi: 10.1016/s0065-2660(08)60026-7. [DOI] [PubMed] [Google Scholar]
- 32.Cline T.W., Meyer B.J. Vive la difference: Males vs females in flies vs worms. Annu. Rev. Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- 33.Christiansen A.E., Keisman E.L., Ahmad S.M., Baker B.S. Sex comes in from the cold: The integration of sex and pattern. Trends Genet. 2002;18:510–516. doi: 10.1016/s0168-9525(02)02769-5. [DOI] [PubMed] [Google Scholar]
- 34.Lee G., Hall J.C., Park J.H. doublesex gene expression in the central nervous system of Drosophila melanogaster. J. Neurogenet. 2002;16:229–248. doi: 10.1080/01677060216292. [DOI] [PubMed] [Google Scholar]
- 35.Taylor B.J., Villella A., Ryner L.C., Baker B.S., Hall J.C. Behavioral and neurobiological implications of sex-determining factors in Drosophila. Dev. Genet. 1994;15:275–296. doi: 10.1002/dvg.1020150309. [DOI] [PubMed] [Google Scholar]
- 36.Kyriacou C.P., Hall J.C. Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the male's courtship song. Proc. Natl. Acad. Sci. USA. 1980;77:6729–6733. doi: 10.1073/pnas.77.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernstein A.S., Neumann E.K., Hall J.C. Temporal analysis of tone pulses within the courtship songs of two sibling Drosophila species, their interspecific hybrid, and behavioral mutants of D. melanogaster (Diptera: Drosophilidae) J. Insect Behav. 1992;5:15–36. [Google Scholar]
- 38.Ewing A.W. The neuromuscular basis of courtship song in Drosophila: The role of the direct and axillary wing muscles. J. Comp. Physiol. [A] 1979;130:87–93. [Google Scholar]
- 39.Trimarchi J.R., Schneiderman A.M. The motor neurons innervating the direct flight muscles of Drosophila melanogaster are morphologically specialized. J. Comp. Neurol. 1994;340:427–443. doi: 10.1002/cne.903400311. [DOI] [PubMed] [Google Scholar]
- 40.Rivlin P.K., St Clair R.M., Vilinsky I., Deitcher D.L. Morphology and molecular organization of the adult neuromuscular junction of Drosophila. J. Comp. Neurol. 2004;468:596–613. doi: 10.1002/cne.10977. [DOI] [PubMed] [Google Scholar]
- 41.White K., Grether M.E., Abrams J.M., Young L., Farrell K., Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 42.White K., Tahaoglu E., Steller H. Cell killing by the Drosophila gene reaper. Science. 1996;271:805–807. doi: 10.1126/science.271.5250.805. [DOI] [PubMed] [Google Scholar]
- 43.Waterbury J.A., Jackson L.L., Schedl P. Analysis of the doublesex female protein in Drosophila melanogaster: role on sexual differentiation and behavior and dependence on intersex. Genetics. 1999;152:1653–1667. doi: 10.1093/genetics/152.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dauwalder B., Tsujimoto S., Moss J., Mattox W. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev. 2002;16:2879–2892. doi: 10.1101/gad.1010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujii S., Amrein H. Genes expressed in the Drosophila head reveal a role for fat cells in sex-specific physiology. EMBO J. 2002;21:5353–5363. doi: 10.1093/emboj/cdf556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bray S., Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 47.Lazareva A.A., Roman G., Mattox W., Hardin P.E., Dauwalder B. A role for the adult fat body in Drosophila male courtship behavior. PLoS Genet. 2007;3:e16. doi: 10.1371/journal.pgen.0030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jan L.Y., Jan Y.N. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc. Natl. Acad. Sci. USA. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.