Abstract
Myogenic Akt signaling coordinates blood vessel recruitment with normal tissue growth. Here, we investigated the role of Follistatin-like 1 (Fstl1) in the regulation of endothelial cell function and blood vessel growth in muscle. Transgenic Akt1 overexpression in skeletal muscle led to myofiber growth that was coupled to an increase in muscle capillary density. Myogenic Akt signaling or ischemic hind limb surgery led to the induction of Fstl1 in muscle and increased circulating levels of Fstl1. Intramuscular administration of an adenoviral vector expressing Fstl1 (Ad-Fstl1) accelerated flow recovery and increased capillary density in the ischemic hind limbs of wild-type mice, and this was associated with an increase in endothelial nitric oxide synthase (eNOS) phosphorylation at residue Ser-1179. In cultured endothelial cells, Ad-Fstl1 stimulated migration and differentiation into network structures and inhibited apoptosis under conditions of serum deprivation. These cell responses were associated with the activating phosphorylation of Akt and eNOS. Conversely, transduction with dominant-negative Akt or LY294002 blocked Fstl1-stimulated eNOS phosphorylation and inhibited Fstl1-stimulated cellular responses. Treatment with the eNOS inhibitor NG-nitro-l-arginine methyl ester also reduced endothelial cell migration and differentiation induced by Ad-Fstl1. The stimulatory effect of Ad-Fstl1 on ischemic limb reperfusion was abolished in mice lacking eNOS. These data indicate that Fstl1 is a secreted muscle protein or myokine that can function to promote endothelial cell function and stimulates revascularization in response to ischemic insult through its ability to activate Akt-eNOS signaling.
Skeletal muscle hypertrophy is associated with blood vessel recruitment, such that capillary density is either maintained or increased during muscle growth (1-4). A number of studies have shown that the serine-threonine protein kinase Akt1 plays a key role in regulation of cellular hypertrophy and organ size (5). The expression of constitutively active Akt1 in skeletal muscle stimulates muscle hypertrophy both in vitro and in vivo (6-9). Adenovirus-mediated transduction of constitutively active Akt1 also promotes the induction of the vascular endothelial growth factor (VEGF),3 and this is accompanied by an increase in limb perfusion and capillary density in muscles where Akt1 is overexpressed (6). Constitutive activation of Akt1 in the heart also promotes myocardial angiogenesis, which is partly mediated by the induction of VEGF (10, 11). Collectively, these data suggest that the Akt-VEGF signaling axis is a critical regulator of blood vessel recruitment during tissue growth. However, the process of angiogenesis is regulated by complex molecular mechanisms involving the participation of multiple factors (12). Thus, we sought to identify novel factors secreted from skeletal muscle that coordinate blood vessel recruitment with myofiber growth.
Follistatin-like 1 (Fstl1), also referred to as TSC36, is an extracellular glycoprotein that, despite limited homology, has been grouped into the follistatin family of proteins (13). Fstl1 is poorly understood with regard to its functional significance. Transduction of cancer cell lines with Fstl1 has been shown to result in suppression of growth and invasiveness (14, 15). Recently, we demonstrated that Fstl1 is up-regulated in myocardium in cardiac-specific Akt1 transgenic (TG) mice and that Fstl1 functions as a cardioprotective molecule with anti-apoptotic actions in cardiac myocytes (16). To date, however, the secretion of Fstl1 by skeletal muscle has not been investigated, and no functional analysis of Fstl1 has been performed in the setting of vascular disease. In the present study, we tested whether Fstl1 is secreted from skeletal muscle and whether it participates in blood vessel recruitment that is associated with muscle ischemia or myogenic Akt1 signaling. We also investigated whether Fstl1 affects cellular behavior and modulates intracellular signaling in cultured endothelial cells. Our observations indicate that Fstl1 is a secreted factor in myogenic cells that favors ischemia-induced revascularization through activation of Akt-eNOS-dependent signaling within endothelial cells.
EXPERIMENTAL PROCEDURES
Materials—Phospho-Akt (Ser-473), phospho-eNOS (Ser-1177), phospho-p42/44 extracellular signal-regulated kinase (ERK) (Thr-202/Tyr-204), phospho-GSK-3β (Ser-9), Akt, and ERK antibodies were purchased from Cell Signaling Technology. eNOS and GSK-3 antibodies was from Santa Cruz Biotechnology, tubulin antibody was from Oncogene, and Fstl1 antibody was obtained from R&D Systems. Anti-human Fstl1 antibody was obtained from Abcam. LY294002 was obtained from Calbiochem, and NG-nitro-l-arginine methyl ester (l-NAME) was obtained from Sigma.
Muscle-specific Akt1 Transgenic Mice—The generation of skeletal muscle-specific inducible myrAkt1 TG mice was described previously (9). Briefly, tetracycline-responsive element constitutively active myrAkt1 (TRE-myrAkt1) TG mouse line was crossed with 1256 [3Emut] MCK-rtTA lines that express reverse tetracycline transactivator from 1256 [3Emut] MCK promoter to generate double-transgenic mice (DTG). To activate Akt1 transgene, DTGs were administered doxycycline (0.5 mg/ml) in their drinking water. Single 1256 [3Emut] MCK-reverse tetracycline transactivator TG littermates were used as controls and treated with doxycycline in the same way as DTG mice.
Microarray Analysis—Total RNA from gastrocnemius muscles of DTG mice (2 weeks after Akt1 induction) and control reverse tetracycline transactivator TG littermates was analyzed by Affymetrix GeneChip Mouse Expression Set 430 microarrays. Among transcripts that are up-regulated by Akt1 activation, we selected transcripts that have full-length open reading frame cDNAs available in the National Center for Biotechnology Information website. Amino acid sequences were examined for signal sequences by using Signal IP software. Amino acid sequences were also analyzed with SOSUI signal beta version software to predict proteins lacking transmembrane domain.
Mouse Model of Revascularization—Male wild-type and eNOS-deficient (eNOS-KO) mice (Jackson Laboratory) in a C57/BL6 background were used for this study. Study protocols were approved by the Institutional Animal Care and Use Committee in Boston University. Mice, at the ages of 10 weeks, were subjected to unilateral hind limb surgery under anesthesia with sodium pentobarbital (50 mg/kg, intraperitoneally). In this model, the entire left femoral artery and vein were excised surgically (17, 18). In some experiments, the 2 × 108 plaque-forming units (pfu) of adenoviral constructs encoding Fstl1 (Ad-Fstl1) or expressing β-galactosidase (Ad-β-gal), as a control, were injected into five different sites of adductor muscle in the ischemic limb 3 days prior to the ischemic hind limb as previously described (17, 18). Hind limb blood flow was measured using a laser Doppler blood flow analyzer (Moor LDI, Moor Instruments) immediately before surgery and on postoperative days 3, 7, and 14. Hind limb blood flow was expressed as the ratio of left (ischemic) to right (non-ischemic) laser Doppler blood flow. In some experiments, Ad-Fstl1 or Ad-β-gal was injected into gastrocnemius muscle of wild-type mice (2 × 108 pfu each). Following sacrifice, capillary density within gastrocnemius or thigh adductor muscle was quantified by histological analysis (17, 18). Muscle samples were imbedded in OCT compound (Miles, Elkhart, IN) and snap-frozen in liquid nitrogen. Tissue slices (5 μm in thickness) were stained with anti-CD31 (PECAM-1, BD Biosciences) antibodies. Fifteen randomly chosen microscopic fields from three different sections in each tissue block were examined for the presence of CD31-positive capillary endothelial cells. Capillary density was expressed as the number of CD31-positive cells per muscle fiber or per high power field.
Cell Culture, Adenoviral Infection, and Western Blot Analysis—Human umbilical vein endothelium cells (HUVECs) were cultured in endothelial cell growth medium-2 (Lonza) (19). HUVECs were infected with adenoviral constructs encoding mouse Fstl1 (Ad-Fstl1) (16), or Ad-β-gal at a multiplicity of infection (m.o.i.) of 10 for 8 h and placed in endothelial cell basal medium-2 (Lonza) without serum for the indicated lengths of time. In some experiments, HUVECs were treated with LY294002 (10 μm), l-NAME (1 mg/ml), or vehicle along with transduction with Ad-Fstl1 or Ad-β-gal. In some experiments, HUVECs were infected with adenoviral constructs encoding dominant-negative Akt1 (Ad-dnAkt) with a hemagglutinin (HA) tag (19, 20) or Ad-β-gal at an m.o.i. of 10 together with Ad-Fstl1 or Ad-β-gal. C2C12 mouse myoblasts (American Type Culture Collection) were maintained in growth medium (Dulbecco's modified Eagle's medium supplemented with 20% fetal bovine serum) and shifted to differentiation medium (Dulbecco's modified Eagle's medium supplemented with 2% heat-inactivated horse serum) for 4 days to induce differentiation (17). C2C12 myocytes were infected with Ad-Fstl1, or Ad-β-gal at an m.o.i. of 250 for 16 h followed by incubation with serum-free Dulbecco's modified Eagle's medium for 24 h. Cell and tissue lysates or culture media were resolved by SDS-PAGE. The membranes were immunoblotted with the indicated antibodies at a 1:1000 dilution followed by the secondary antibody conjugated with horseradish peroxidase at a 1:5000 dilution. An ECL Western blotting Detection kit (Amersham Biosciences) was used for detection. Relative phosphorylation or protein levels were quantified by using the ImageJ program. Immunoblots were normalized to total loaded protein.
Determination of Fstl1 mRNA—Total RNA was prepared by Qiagen using the manufacturer's suggested protocol, and cDNA was produced using ThermoScript RT-PCR Systems (Invitrogen). Quantitative real-time PCR (QRT-PCR) was performed on an iCycler iQ Real-Time PCR Detection System (Bio-Rad) using SYBR Green I as a double-stranded DNA-specific dye as described previously (17). Primers were: 5′-AACAGCCATCAACATCACCACTTAT-3′ and 5′-TTTCCAGTCAGCGTTCTCATCA-3′ for mouse Fstl1, 5′-TCACCACCATGGAGAAGGC-3′ and 5′-GCTAAGCAGTTGGTGGTGCA-3′ for mouse glyceraldehyde-3-phosphate dehydrogenase.
Migration Assay—Migratory activity was measured using a modified Boyden chamber assay (19). Serum-deprived cells were trypsinized and resuspended in endothelial cell basal medium-2 in the absence of serum. Cell suspensions (250 μl, 2.0 × 104 cells/well) were added to the Transwell insert (8.0-μm pore size, BD Biosciences). After 18 h, migrated cells on the lower surface of the membrane were fixed and stained with Giemsa stain solution, and eight random microscopic fields per well were quantified.
Differentiation Assay—The formation of network structures by HUVECs on growth factor-reduced Matrigel (BD Biosciences) was performed as previously described (19). Twenty-four-well culture plates were coated with Matrigel according to the manufacturer's instructions. HUVECs were seeded on coated plates at 5 × 104 cells/well in serum-free endothelial cell basal medium-2 and incubated at 37 °C for 18 h. Network formation was observe using an inverted phase contrast microscope (Nikon). Images were captured with a video graphic system (DEI-750 CE digital output camera, Optronics). The degree of differentiation into vascular-like structures was quantified by measuring the network areas in three randomly chosen fields from each well using the ImageJ program.
Analysis of Apoptotic Activity—Cells were transduced with adenoviral constructs for 8 h followed by incubation with serum-free endothelial cell basal medium-2 for 48 h. Nucleosome fragmentation was assessed by enzyme-linked immunosorbent assay using Cell Death Detection kit (Roche Applied Science). Cell viability was also measured by MTS reagent using the CellTiter 96 AQueous kit (Promega) (21). TUNEL staining was performed using the In Situ Cell Death detection kit (Roche) (22). TUNEL-positive cells were counted in five randomly selected microscopic fields. Each experiment was repeated four times.
Statistical Analysis—All data are expressed as means ± S.D. or S.E. as indicated in the figure legends. Differences were analyzed by Student's unpaired t test or analysis of variance for multiple comparisons. A level of p < 0.05 was accepted as statistically significant.
RESULTS
Akt1 Overexpression in Skeletal Muscle Increases Capillary Vessel Formation and Up-regulates Fstl1 Expression—Initially we examined the consequences of Akt1 transgene expression on blood vessel formation in skeletal muscle employing skeletal muscle-specific Akt1 TG mice (9). Mice were treated with doxycycline for 2 weeks to activate Akt1 transgene, resulting in an increase of ∼40% in gastrocnemius muscle mass weight (9). At this time point, capillary density in gastrocnemius muscle was assessed by staining with an endothelial marker CD31. Fig. 1A shows representative photographs of tissues stained with CD31. Quantitative analysis revealed that the capillary density per high power field was significantly increased in mice following 2 weeks of Akt1 activation compared with control mice (Fig. 1A). Similarly, the number of CD31-positive cells per muscle fiber was significantly higher in Akt1-TG mice than in control mice (0.84 ± 0.07 in control mice and 2.45 ± 0.19 in Akt1-TG mice).
FIGURE 1.
Transgenic Akt activation increases capillary density and Fstl1 expression in gastrocnemius muscle. A, increased capillary vessels in gastrocnemius muscles in muscle-specific Akt-TG mice following 2 weeks of Akt1 activation. Immunostaining of gastrocnemius tissues of control (n = 5) and Akt-TG (n = 5) mice was performed with anti-CD31 monoclonal antibody. Capillary density was expressed as the number of capillaries per high power field. Results are shown as the mean ± S.E. *, p < 0.01 versus control mice. B, up-regulation of Fstl1 expression in gastrocnemius muscle and serum in muscle-specific Akt-TG mice after Akt1 activation for 2 weeks. Fstl1 expression was determined by QRT-PCR and Western blot analyses (n = 5). Fstl1 mRNA levels were expressed relative to levels of glyceraldehyde-3-phosphate dehydrogenase mRNA. Results are expressed relative to control. Relative protein levels of Fstl1 were quantified (n = 4-5) by using ImageJ. Results are shown as the mean ± S.E. *, p < 0.01 versus control. C, Fstl1 is secreted from C2C12 myotube cultures. C2C12 cells were transduced with adenoviral vectors expressing Fstl1 (Ad-Fstl1), or β-galactosidase (Ad-β-gal) for 16 h followed by 24 h of incubation in serum-free media. Fstl1 protein levels were determined in media, and cell lysates were determined by Western blot analysis. Representative blots are shown from three independent experiments.
Microarray analysis of expressed transcripts was performed, and transcripts up-regulated by myogenic Akt signaling were evaluated for the presence of a signal peptide and the absence of a transmembrane domain in their open reading frames using Signal IP and SOSUI software, respectively. The Fstl1 transcript was of interest, because it was up-regulated by a factor of 1.9 after Akt1 transgene activation by microarray analysis, and it is predicted to encode a secreted protein. We next confirmed the effect of transgenic Akt1 activation on Fstl1 expression in skeletal muscle. Fstl1 mRNA levels were up-regulated in gastrocnemius muscle by a factor of 5.3 following 2 weeks of Akt1 activation, as determined by QRT-PCR analysis (Fig. 1B). Fstl1 protein levels were also increased in gastrocnemius muscle by Akt1 transgene induction as assessed by Western blot analysis (Fig. 1B). Two immunoreactive bands of Fstl1 (37- and 46-kDa proteins) were detected in mouse skeletal muscle. Quantitative analysis of the 37-kDa band indicated the 4.1 ± 0.5-fold increase in Fstl1 protein. Fstl1 protein was also detected in mouse serum, and serum Fstl1 levels were markedly increased by a factor of 3.4 ± 0.4 at 2 weeks after Akt transgene activation in skeletal muscle (Fig. 1B).
To examine whether Fstl1 is secreted from cultured muscle cells, differentiated C2C12 cells were treated with adenoviral vectors expressing Fstl1 (Ad-Fstl1) or β-galactosidase (Ad-β-gal). Fstl1 protein was detected in both the cell pellet lysate and media of control cells treated with Ad-β-gal (Fig. 1C). Ad-Fstl1 treatment increased Fstl1 protein levels in both the cell lysate (2.7 ± 0.1-fold increase) and media (2.0 ± 0.3-fold increase) (Fig. 1C). Collectively, these data suggest that Fstl1 is up-regulated by Akt signaling and secreted from skeletal muscle.
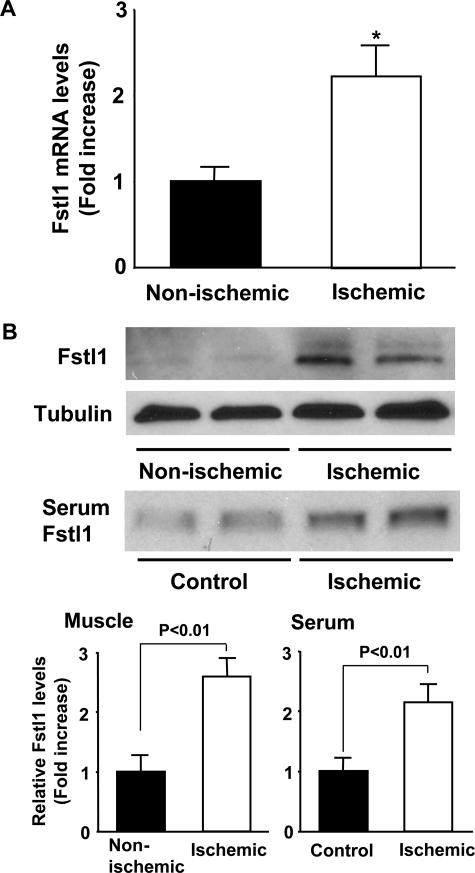
Muscle Ischemia Up-regulates Fstl1 Expression—To further characterize the regulation of Fstl1, expression was determined in ischemic adductor muscle following femoral artery excision. Fstl1 mRNA levels were 2.3-fold higher in ischemic muscles than in non-ischemic muscles at 7 days after ischemic surgery as measured by QRT-PCR analysis (Fig. 2A). Fstl1 protein levels in ischemic muscles were also increased by a factor of 2.6 ± 0.3 as assessed by Western blot analysis (Fig. 2B). Furthermore, hind limb ischemia increased Fstl1 levels in serum by a factor of 2.1 ± 0.3 at 2 weeks following ischemic surgery (Fig. 2B).
FIGURE 2.
Elevated Fstl1 levels in ischemic muscle and serum after hind limb ischemic surgery. A, Fstl1 expression in non-ischemic or ischemic adductor muscles was measured by QRT-PCR (n = 5) at day 7 after femoral artery resection. Fstl1 transcript levels were expressed relative to levels of glyceraldehyde-3-phosphate dehydrogenase mRNA. Results are expressed relative to control. B, Fstl1 expression in non-ischemic (n = 4) and ischemic skeletal muscle (n = 4) was measured by Western blot analyses on the postoperative day 14. Serum was collected from control (n = 4) or mice subjected to hind limb ischemic surgery (n = 4), and Fstl1 levels were determined by Western blot analyses. Relative protein levels of Fstl1 were quantified (n = 4) by using ImageJ. Results are shown as the mean ± S.E. *, p < 0.05 versus non-ischemic.
Fstl1 Promotes Revascularization in Response to Ischemia in Vivo—To test whether Fstl1 can modulate revascularization under conditions of ischemia in vivo, we employed C57BL/6 wild-type mice that underwent unilateral femoral artery resection. This model of vascular insufficiency has been used to evaluate the in vivo angiogenic actions of growth factors, including VEGF (23, 24). Adenoviral vectors expressing Fstl1 (Ad-Fstl1) or Ad-β-gal (control) were injected intramuscularly into the adductor muscle 3 days before surgery. Fstl1 protein levels in ischemic muscle were significantly elevated by a factor of 6.9 ± 0.5 at 6 days after injection of Ad-Fstl1 (Fig. 3A). Ad-Fstl1-treated mice showed a significant increase in flow recovery at 3, 7, and 14 days after ischemic surgery as determined by laser Doppler blood flow analysis (Fig. 3B). To investigate the extent of revascularization at the microcirculatory level, capillary density was measured in histological sections harvested from the ischemic muscles. Quantitative analysis revealed that the capillary density was significantly increased in Ad-Fstl1-treated mice compared with control mice on postoperative day 14 (Fig. 3C).
FIGURE 3.
Fstl1 promotes perfusion recovery and capillary vessel formation of ischemic limbs in mice in vivo. Adenoviral vectors expressing Fstl1 (Ad-Fstl1), or β-galactosidase (Ad-β-gal, control) were injected into five sites in adductor muscle of wild-type mice (2 × 108 pfu each) at 3 days prior to ischemic surgery. A, Fstl1 expression in ischemic muscle at 6 days after injection of Ad-Fstl1 or Ad-β-gal. Fstl1 protein expression was determined by Western blot analysis. Representative blots are shown from five independent experiments. B, quantitative analysis of the ischemic/non-ischemic laser Doppler blood flow ratio in wild-type mice treated with Ad-Fstl1 (n = 8) and Ad-β-gal (n = 8). Results are shown as the mean ± S.D. *, p < 0.05 versus control mice. **, p < 0.01 versus control mice. C, quantitative analysis of capillary density in ischemic muscles of wild-type mice treated with Ad-Fstl1 (n = 5) and Ad-β-gal (n = 5) on postoperative day 14. Immunostaining of ischemic tissues was performed with anti-CD31 monoclonal antibody. Capillary density was expressed as the number of capillaries per muscle fiber. Results are shown as the mean ± S.E. *, p < 0.01 versus control mice. D, quantitative analysis of capillary density in non-ischemic gastrocnemius muscles of wild-type mice treated with Ad-Fstl1 (n = 4) and Ad-β-gal (n = 4) at day 7 after injection (2 × 108 pfu each). Immunostaining of gastrocnemius muscle tissues was performed with anti-CD31 monoclonal antibody. Capillary density was expressed as the number of capillaries per muscle fiber. Results are shown as the mean ± S.E.
To investigate whether Fstl1 can stimulate blood vessel growth in non-ischemic muscle, the gastrocnemius muscle of wild-type mice was injected with Ad-Fstl1 or Ad-β-gal. No significant differences in the capillary density of non-ischemic muscles were observed between Ad-Fstl1-treated and control mice (Fig. 3D). Collectively, these data indicate that Fstl1 will enhance revascularization in ischemic tissue, but it is not sufficient to activate an angiogenic response in normal tissue.
Fstl1 Promotes Endothelial Cell Function and Survival in Vitro—To examine whether Fstl1 can directly act on endothelial cells, HUVECs were transduced with Ad-Fstl1 or Ad-β-gal and plated on a Matrigel matrix. Fstl1 protein expression was readily detected in both cell lysate (4.6 ± 0.3-fold increase) and media (3.5 ± 0.4-fold increase) from HUVECs treated with Ad-Fstl1, whereas endogenous levels of Fstl1 expression were low (Fig. 4A). Quantitative analyses of endothelial cell network area revealed that treatment with Ad-Fstl1 significantly promoted the formation of network structures relative to control cultures treated with Ad-β-gal (Fig. 4B). To test whether Ad-Fstl1 influences endothelial cell migration, a modified Boyden chamber assay was performed. Ad-Fstl1 treatment significantly stimulated HUVEC migration in this assay (Fig. 4B).
FIGURE 4.
Fstl1 promotes endothelial cell migration and differentiation into vascular-like structures. A, expression of Fstl1 protein in media and cell lysates from HUVECs. HUVECs were transduced with Ad-Fstl1 and Ad-β-gal for 8 h followed by 24 h of incubation in serum-free media. Fstl1 protein levels were determined in media, and cell lysates were determined from HUVECs by Western blot analysis. Representative blots are shown from four independent experiments. B, endothelial cell network formation in response to Fstl1. After 24 h of serum deprivation and transduction with Ad-Fstl1 and Ad-β-gal, HUVECs were seeded on Matrigel-coated culture dishes. Representative cultures are shown (upper panel). Quantitative analyses of network formation are shown (bottom panel). C, migratory activities of HUVECs following treatment with Fstl1. A modified Boyden chamber assay was performed using HUVECs transduced with Ad-Fstl1 and Ad-β-gal. Results are shown as the mean ± S.E. (n = 7-8). Results are expressed relative to the values compared with control. *, p < 0.01 versus Ad-β-gal.
To evaluate the role of Fstl1 in endothelial apoptosis, HUVECs were treated with Ad-Fstl1 or Ad-β-gal followed by 48 h of incubation in serum-free media. Fstl1 markedly suppressed the extent of nucleosome fragmentation as determined by enzyme-linked immunosorbent assay (Fig. 5A). Ad-Fstl1 also reduced HUVEC death caused by serum deprivation as assessed by an MTS-based assay (Fig. 5B). To corroborate these findings, TUNEL-positive cells were analyzed in the HUVEC cultures. As shown in the Fig. 5C, treatment with Ad-Fstl1 diminished the frequency of TUNEL-positive cells under serum-deprived conditions.
FIGURE 5.
Fstl1 protects endothelial cells from apoptosis. HUVECs were transduced with Ad-Fstl1 and Ad-β-gal for 8 h followed by incubation with serum-free media for 48 h. A, inhibitory effect of Fstl1 on nucleosome fragmentation of HUVECs. Nucleosome fragmentation was assessed by enzyme-linked immunosorbent assay. Results are expressed relative to the values compared with control. B, inhibition of HUVEC death by Fstl1 assessed by a quantitative MTS-based assay. C, the frequency of TUNEL-positive HUVECs is reduced after treatment with Fstl1. Representative photomicrographs of TUNEL-positive HUVECs are shown (upper panels). Quantitative analyses of the frequency of TUNEL-positive HUVECs are shown (bottom panel). Apoptotic nuclei were identified by TUNEL staining (green), and total nuclei were identified by 4′,6-diamidino-2-phenylindole counterstaining (blue). Results are shown as the mean ± S.E. (n = 8-10). *, p < 0.01 versus Ad-β-gal.
Fstl1 Stimulates the Phosphorylation of Akt and eNOS—Akt has been shown to be a key mediator of growth factor-dependent angiogenic and survival signals in endothelial cells (20, 25). Therefore, to test whether Fstl1 influences Akt signaling in endothelial cells, the activating phosphorylation of Akt at Ser-473 was assessed by Western blot analysis. Treatment of HUVECs with Ad-Fstl1 enhanced the phosphorylation of Akt by a factor of 4.2 ± 0.3 (Fig. 6A). Because Akt can phosphorylate eNOS at Ser-1179 (26, 27), eNOS phosphorylation was also examined in these cultures. Ad-Fstl1 stimulation resulted in a 3.0 ± 0.3-fold increase in eNOS phosphorylation at Ser-1179 (Fig. 6A). Consistent with an increase in Akt signaling, a 2.0 ± 0.1-fold increase in GSK-3β phosphorylation at Ser-9, a downstream target of Akt signaling in endothelial cells (28), was seen under these conditions (Fig. 6A). In contrast, Ad-Fstl1 had no effect on the phosphorylation of ERK at Thr-202/Tyr-204 (1.3 ± 0.1-fold) (Fig. 6A). To examine the role of Akt in the regulation of eNOS phosphorylation by Fstl1, HUVECs were infected with a HA-tagged dominant-negative Akt (Ad-dnAkt) or Ad-β-gal. Transduction with Ad-dnAkt reduced Fstl1-induced Akt and eNOS phosphorylation (1.0 ± 0.2 in Ad-β-gal, 3.5 ± 0.3 in Ad-Fstl1, 0.9 ± 0.1 in Ad-β-gal+Ad-dnAkt, 1.2 ± 0.2 in Ad-Fstl1+Ad-dnAkt) (Fig. 6B). These data indicate that Akt mediates eNOS phosphorylation downstream from Fstl1.
FIGURE 6.
Fstl1-stimulated endothelial cell responses are dependent on Akt signaling. A, Fstl1-stimulated signaling in endothelial cells. HUVECs were transduced with Ad-Fstl1 and Ad-β-gal for 8 h followed by 24 h of incubation with serum-free media. Changes in the phosphorylation of eNOS (P-eNOS), Akt (P-Akt), GSK (P-GSK), and ERK (P-ERK) following Ad-Fstl1 treatment were determined by Western blot analysis. Representative blots are shown. Relative phosphorylation levels of eNOS and Akt were quantified (n = 6) by using ImageJ. Immunoblots were normalized to total loaded protein. B, role of Akt in regulation of Fstl1-induced signaling. HUVECs were infected with adenoviral constructs encoding dominant-negative Akt1 (Ad-dnAkt) or Ad-β-gal at an m.o.i. of 10 along with Ad-Fstl1 or Ad-β-gal at an m.o.i. of 10 for 8 h, followed by serum deprivation for 24 h. Phosphorylation of eNOS (P-eNOS) and Akt (P-Akt) were determined by Western blot analysis. Representative blots are shown from four independent experiments. C and D, contribution of Akt to Fstl1-mediated cellular responses. HUVECs were transduced with Ad-dnAkt or Ad-β-gal along with Ad-Fstl1 or Ad-β-gal for 8 h. After 24 h of serum deprivation, Matrigel (C) or modified Boyden chamber assays (D) were performed. E, involvement of Akt in Fstl1-induced endothelial cell survival. After transduction with Ad-dnAkt or Ad-β-gal along with Ad-Fstl1 or Ad-β-gal for 8 h, cells were incubated in serum-free media. Nucleosome fragmentation was assessed by enzyme-linked immunosorbent assay. Results are shown as the mean ± S.E. (n = 6-8). Results are expressed relative to the values compared with control. *, p < 0.01.
Role of eNOS Signaling in Fstl1-stimulated Revascularization—To test whether the activation of Akt signaling is required for Fstl1-stimulated differentiation, migration, and survival, HUVECs were infected with Ad-dnAkt or Ad-β-gal, and endothelial cell function and survival was assessed. Transduction with Ad-dnAkt blocked Ad-Fstl1-induced network formation by HUVECs plated on Matrigel (Fig. 6C). Ad-Fstl1-stimulated endothelial cell migration was also diminished by transduction with Ad-dnAkt, whereas Ad-dnAkt had no effect on basal migration (Fig. 6D). Furthermore, transduction with Ad-dnAkt reversed the inhibitory effects of Ad-Fstl1 on the degree of nucleosome fragmentation (Fig. 6E). These results indicate that Akt signaling is required for Fstl1-induced endothelial cell differentiation, migration, and survival.
Akt is activated by many growth factors through the phosphatidylinositol-3 kinase (PI3K)-dependent pathway (25). To investigate whether PI3K participates in Fstl1-induced signaling, HUVECs were treated with PI3K inhibitor LY294002. Treatment with LY294002 abolished Ad-Fstl1-stimulated phosphorylation of Akt and eNOS in HUVECs (Fig. 7A). Ad-Fstl1-stimulated network formation and migration of HUVECs were also blocked by treatment with LY294002 (Fig. 7, B and C). These data indicate that PI3K is essential for endothelial cell responses to Fstl1 and that PI3K functions upstream from the Akt-eNOS regulatory axis in Fstl1-stimulated cells.
FIGURE 7.
PI3K and eNOS signaling is involved in Fstl1-induced endothelial cell responses. A, effect of LY294002 on Fstl1-induced phosphorylation of eNOS and Akt. HUVECs were treated with LY294002 (10 μm) or vehicle following transduction with Ad-Fstl1 or Ad-β-gal. After 24 h serum deprivation, phosphorylation of eNOS (P-eNOS), and Akt (P-Akt) were determined by Western blot analysis. Representative blots are shown. Relative phosphorylation levels of eNOS and Akt were quantified (n = 4) by using ImageJ. Immunoblots were normalized to total loaded protein. B and C, contribution of PI3K to Fstl1-mediated endothelial differentiation and migration. HUVECs were treated with LY294002 (10 μm), l-NAME (1 mg/ml), or vehicle along with Ad-Fstl1 or Ad-β-gal for 8 h. After 24-h serum-starvation, Matrigel (B) or modified Boyden chamber assays (C) were performed. Results are shown as the mean ± S.E. (n = 5-8). Results are expressed relative to the values compared with control. *, p < 0.01.
To test the role of eNOS in the cellular responses to Fstl1, HUVECs were treated with the NOS inhibitor l-NAME. l-NAME treatment significantly reduced Ad-Fstl1-induced endothelial cell differentiation into network structures and migration (Fig. 7, B and C), indicating that Fstl1 promotes endothelial cell function in an eNOS-dependent manner.
To analyze the potential role of eNOS activation in Fstl1-mediated regulation of revascularization in vivo, the phosphorylation status of eNOS and Akt in ischemic muscles of C57/BL6 mice was assessed by Western blot analysis at day 6 after intramuscular injection of Ad-Fstl1 and Ad-β-gal. Ad-Fstl1 treatment stimulated eNOS phosphorylation at serine residue 1177 in ischemic adductor muscle by a factor of 3.3 ± 0.3 without affecting total eNOS protein levels (Fig. 8A). Akt phosphorylation at Ser-473 in ischemic muscles was also stimulated 1.9 ± 0.1-fold by Ad-Fstl1 treatment (Fig. 8A).
FIGURE 8.
Fstl1 stimulates ischemia-induced revascularization through an eNOS-dependent mechanism. A, phosphorylation of eNOS and Akt in ischemic muscle tissues of wild-type and eNOS-KO mice at 6 days after transduction with Ad-Fstl1 or Ad-β-gal. Ad-Fstl1 or Ad-β-gal (control) was injected into five sites in adductor muscle of wild-type and eNOS-KO mice (2 × 108 pfu each), 3 days before ischemic surgery. Phosphorylation of eNOS (P-eNOS) and Akt (P-Akt), total eNOS, total Akt, and Fstl1 levels were analyzed by Western blotting. Representative blots are shown from four independent experiments. B, quantitative analysis of the ischemic/non-ischemic laser Doppler blood flow ratio in eNOS-KO mice treated with Ad-Fstl1 (n = 7) and Ad-β-gal (n = 7). Results are shown as the mean ± S.D. N.S., not significant.
To assess the contribution of eNOS signaling to the stimulatory actions of Fstl1 on ischemia-driven revascularization in vivo, we intramuscularly injected Ad-Fstl1 or Ad-β-gal into eNOS-knock-out (eNOS-KO) mice 3 days before induction of ischemia. At 6 days after Ad-Fstl1 infection, Fstl1 protein levels in ischemic muscles of eNOS-KO mice increased by a factor of 7.3 ± 0.6 that was similar to a 7.1 ± 0.4-fold increase in wild-type C57/BL6 mice (Fig. 8A). Fstl1 protein levels did not differ between Ad-β-gal-treated wild-type and eNOS-KO mice (1.0 ± 0.1-fold versus 0.8 ± 0.1-fold). Akt phosphorylation in ischemic muscles of eNOS-KO mice was stimulated by Ad-Fstl1 treatment to a similar extent (1.8 ± 0.1-fold) compared with that of wild-type mice (1.9 ± 0.1-fold) (Fig. 8A). However, in contrast to wild-type mice (Fig. 3B), treatment with Ad-Fstl1 did not promote flow recovery in ischemic hind limbs in eNOS-KO mice (Fig. 8B). Thus the stimulatory action of Fstl1 on revascularization in vivo is dependent on eNOS.
DISCUSSION
The present study shows for the first time that Fstl1 plays a role in promoting endothelial cell function and revascularization under conditions of ischemic stress. Fstl1 expression in muscle was up-regulated by Akt1 transgene activation during muscle hypertrophy and by ischemic injury. Fstl1 overexpression was shown to enhance endothelial cell differentiation and migration and diminish endothelial cell apoptosis. Administration of Fstl1 improved revascularization in ischemic limbs of wild-type mice.
It is well documented that Akt-eNOS signaling participates in regulation of endothelial cell function and blood vessel growth under conditions of ischemic stress (20, 25, 29). We provide evidence that the stimulation of revascularization of ischemic tissue by Fstl1 is dependent on its ability to activate Akt-eNOS signaling in endothelial cells. Fstl1 stimulated the activating phosphorylation of Akt and eNOS, whereas transduction with dominant-negative Akt reduced Fstl1-stimulated endothelial cell differentiation, migration, survival, and eNOS phosphorylation. Inhibitors of PI3K or eNOS also blocked the increase in endothelial differentiation and migration caused by Fstl1. The consequences of Fstl1 overexpression on revascularization in ischemic muscle was associated with increased eNOS phosphorylation at Ser-1177, and the vascular actions of Fstl1 were abolished in eNOS-KO mice. Collectively, these observations suggest that the Fstl1-Akt-eNOS regulatory signaling axis functions to stimulate vascular cell function under ischemic conditions, thereby promoting revascularization.
It has been proposed that skeletal muscle secretes factors, referred to as “myokines,” that influence the behavior of neighboring or remote cells (30). Several lines of evidence suggest that Fstl1 can be designated as a myokine that acts on vascular endothelial cells. Both Akt transgene-induced myofiber hypertrophy and ischemic hind limb surgery led to an increase in tissue-resident and serum levels of Fstl1. Furthermore, Fstl1 is secreted into the media by cultured skeletal muscle cells, and it can directly act on endothelial cell signaling pathways that promote function and survival.
Tissue ischemia will lead to the up-regulation of multiple growth factors that function to coordinate the repair of the vascular network (12). It is also recognized that skeletal muscle hypertrophy is coupled to angiogenesis, through molecular mechanisms that are independent of tissue hypoxia (6). VEGF, a strong stimulator of angiogenesis, is up-regulated during myofiber hypertrophy by myogenic Akt signaling. The overexpression of VEGF will stimulate angiogenesis in muscle under normoxic conditions leading to the development of a disorganized vascular complex (31). In contrast to VEGF, Fstl1 overexpression accelerates revascularization in ischemic muscle, but does not stimulate vessel growth in normoxic muscle. Thus, it appears that Fstl1 does not function as an “angiogenic factor” per se, but it has salutary effects on the endothelium under conditions of stress and thereby promotes the revascularization process in response to chronic tissue ischemia. Thus, we hypothesize that the up-regulation and secretion of Fstl1 by skeletal muscle, under conditions of hypertrophic growth or ischemic stress, will contribute to revascularization through its ability to promote endothelial cell function.
In agreement with the current study, we recently reported that Fstl1 is up-regulated during cardiac hypertrophy (16). In this study, Fstl1 was shown to activate Akt signaling in cardiac myocytes and inhibit apoptosis. Thus, Fstl1 can function as a survival factor for both cardiac myocytes and endothelial cells via the activation of Akt signaling. Fstl1 overexpression has also been shown to protect the heart from ischemia-reperfusion injury in mice. Treatments aimed at increasing angiogenesis represent a promising strategy for treatment of ischemic limb and heart diseases (32). Thus, Fstl1 could be considered a potential therapeutic agent for ischemic diseases based upon its ability to directly stimulate blood vessel formation and inhibit the death of cardiovascular cells.
Other members of the follistatin family function to regulate transforming growth factor-β superfamily proteins through their ability to function as binding partners (33). Activin A has been shown to suppress endothelial cell growth and attenuate angiogenesis by a chorioallantoic membrane assay (34), and follistatin is reported to promote angiogenesis through its ability to bind to activin (35). However, it remains to be determined whether Fstl1 binds to members of transforming growth factor-β superfamily in a manner that is similar to follistatin (36). In this regard Fstl1 exhibits little amino acid sequence homology with follistatin (7%), and our in vitro data show that overexpression of Fstl1 results in enhanced Akt signaling in cultured endothelial cells under conditions of serum deprivation. Thus, it is unlikely that the actions of Fstl1 on endothelial cell signaling and phenotype are mediated by its ability to modulate the function of a second secreted protein. Attempts are currently in progress to identify the Fstl1 receptor in endothelial cells.
In conclusion, our data demonstrate that Fstl1 is a myokine that activates Akt-eNOS signaling in endothelial cells. Overexpression of Fstl1 stimulates ischemia-induced revascularization in mice through activation of eNOS. Because the dysregulated eNOS signaling is linked to endothelial dysfunction, impaired neovascularization, and atherogenesis (37-39), strategies to increase Fstl1-eNOS signaling could be a useful treatment for vascular complications.
This work was supported, in whole or in part, by National Institutes of Health Grants HL77774, HL86785, AG15052, and HL81587 (to K. W.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: VEGF, vascular endothelial growth factor; HUVEC, human umbilical vein endothelium cell; Fstl1, Follistatin-like 1; eNOS, endothelial nitric-oxide synthase; PI3K, phosphatidylinositol-3 kinase; eNOS-KO, eNOS-knockout; TG, transgenic; DTG, double transgenic; ERK, extracellular signal-regulated kinase; l-NAME, NG-nitro-l-arginine methyl ester; pfu, plaque-forming unit; m.o.i., multiplicity of infection; dnAkt, dominant-negative Akt1; HA, hemagglutinin; RT, reverse transcription; QRT-PCR, quantitative real-time PCR; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; Ad-β-gal, adenoviral construct encoding or expressing β-galactosidase.
References
- 1.Degens, H., Veerkamp, J. H., van Moerkerk, H. T. B., Turek, Z., Hoofd, L. J. C., and Binkhorst, R. A. (1993) Int. J. Biochem. 25 1141-1148 [DOI] [PubMed] [Google Scholar]
- 2.Degens, H., Turek, Z., Hoofd, L. J. C., Van't Hof, M. A., and Binkhorst, R. A. (1992) J. Anat. 180 455-463 [PMC free article] [PubMed] [Google Scholar]
- 3.Ingjer, F. (1979) Eur. J. Appl. Physiol. Occup. Physiol. 40 197-209 [DOI] [PubMed] [Google Scholar]
- 4.Kano, Y., Shimegi, S., Masuda, K., Ohmori, H., and Katsuta, S. (1997) Eur. J. Appl. Physiol. Occup. Physiol. 75 97-101 [DOI] [PubMed] [Google Scholar]
- 5.Shiojima, I., and Walsh, K. (2006) Genes Dev. 20 3347-3365 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi, A., Kureishi, Y., Yang, J., Luo, Z., Guo, K., Mukhopadhyay, D., Ivashchenko, Y., Branellec, D., and Walsh, K. (2002) Mol. Cell. Biol. 22 4803-4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai, K. M., Gonzalez, M., Poueymirou, W. T., Kline, W. O., Na, E., Zlotchenko, E., Stitt, T. N., Economides, A. N., Yancopoulos, G. D., and Glass, D. J. (2004) Mol. Cell. Biol. 24 9295-9304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodine, S. C., Stitt, T. N., Gonzalez, M., Kline, W. O., Stover, G. L., Bauerlein, R., Zlotchenko, E., Scrimgeour, A., Lawrence, J. C., Glass, D. J., and Yancopoulos, G. D. (2001) Nat. Cell Biol. 3 1014-1019 [DOI] [PubMed] [Google Scholar]
- 9.Izumiya, Y., Hopkins, T., Morris, C., Sato, K., Zeng, L., Viereck, J., Hamilton, J. A., Ouchi, N., LeBrasseur, N. K., and Walsh, K. (2008) Cell Metab. 7 159-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiojima, I., Sato, K., Izumiya, Y., Schiekofer, S., Ito, M., Liao, R., Colucci, W., and Walsh, K. (2005) J. Clin. Invest. 115 2108-2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izumiya, Y., Shiojima, I., Sato, K., Sawyer, D. B., Colucci, W. S., and Walsh, K. (2006) Hypertension 47 887-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmeliet, P. (2005) Nature 438 932-936 [DOI] [PubMed] [Google Scholar]
- 13.Shibanuma, M., Mashimo, J., Mita, A., Kuroki, T., and Nose, K. (1993) Eur. J. Biochem. 217 13-19 [DOI] [PubMed] [Google Scholar]
- 14.Sumitomo, K., Kurisaki, A., Yamakawa, N., Tsuchida, K., Shimizu, E., Sone, S., and Sugino, H. (2000) Cancer Lett. 155 37-46 [DOI] [PubMed] [Google Scholar]
- 15.Johnston, I. M., Spence, H. J., Winnie, J. N., McGarry, L., Vass, J. K., Meagher, L., Stapleton, G., and Ozanne, B. W. (2000) Oncogene 19 5348-5358 [DOI] [PubMed] [Google Scholar]
- 16.Oshima, Y., Ouchi, N., Sato, K., Izumiya, Y., Pimentel, D., and Walsh, K. (2008) Circulation 117 3099-3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouchi, N., Shibata, R., and Walsh, K. (2005) Circ. Res. 96 838-846 [DOI] [PubMed] [Google Scholar]
- 18.Shibata, R., Ouchi, N., Kihara, S., Sato, K., Funahashi, T., and Walsh, K. (2004) J. Biol. Chem. 279 28670-28674 [DOI] [PubMed] [Google Scholar]
- 19.Ouchi, N., Kobayashi, H., Kihara, S., Kumada, M., Sato, K., Inoue, T., Funahashi, T., and Walsh, K. (2004) J. Biol. Chem. 279 1304-1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujio, Y., and Walsh, K. (1999) J. Biol. Chem. 274 16349-16354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi, H., Ouchi, N., Kihara, S., Walsh, K., Kumada, M., Abe, Y., Funahashi, T., and Matsuzawa, Y. (2004) Circ. Res. 94 e27-e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata, R., Sato, K., Pimentel, D. R., Takemura, Y., Kihara, S., Ohashi, K., Funahashi, T., Ouchi, N., and Walsh, K. (2005) Nat. Med. 11 1096-1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couffinhal, T., Silver, M., Kearney, M., Sullivan, A., Witzenbichler, B., Magner, M., Annex, B., Peters, K., and Isner, J. M. (1999) Circulation 99 3188-3198 [DOI] [PubMed] [Google Scholar]
- 24.Rivard, A., Silver, M., Chen, D., Kearney, M., Magner, M., Annex, B., Peters, K., and Isner, J. M. (1999) Am. J. Pathol. 154 355-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiojima, I., and Walsh, K. (2002) Circ. Res. 90 1243-1250 [DOI] [PubMed] [Google Scholar]
- 26.Fulton, D., Gratton, J. P., McCabe, T. J., Fontana, J., Fujio, Y., Walsh, K., Franke, T. F., Papapetropoulos, A., and Sessa, W. C. (1999) Nature 399 597-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimmeler, S., Fleming, I., Fisslthaler, B., Hermann, C., Busse, R., and Zeiher, A. Z. (1999) Nature 399 601-605 [DOI] [PubMed] [Google Scholar]
- 28.Kim, H.-S., Skurk, C., Thomas, S. R., Bialik, A., Suhara, T., Kureishi, Y., Birnbaum, M., Keaney, J. F., Jr., and Walsh, K. (2002) J. Biol. Chem. 277 41888-41896 [DOI] [PubMed] [Google Scholar]
- 29.Kureishi, Y., Luo, Z., Shiojima, I., Bialik, A., Fulton, D., Lefer, D. J., Sessa, W. C., and Walsh, K. (2000) Nat. Med. 6 1004-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen, B. K., Akerstrom, T. C., Nielsen, A. R., and Fischer, C. P. (2007) J. Appl. Physiol. 103 1093-1098 [DOI] [PubMed] [Google Scholar]
- 31.Lee, R. J., Springer, M. L., Blanco-Bose, W. E., Shaw, R., Ursell, P. C., and Blau, H. M. (2000) Circulation 102 898-901 [DOI] [PubMed] [Google Scholar]
- 32.Vale, P. R., Isner, J. M., and Rosenfield, K. (2001) J. Interv. Cardiol. 14 511-528 [DOI] [PubMed] [Google Scholar]
- 33.Balemans, W., and Van Hul, W. (2002) Dev. Biol. 250 231-250 [PubMed] [Google Scholar]
- 34.Breit, S., Ashman, K., Wilting, J., Rossler, J., Hatzi, E., Fotsis, T., and Schweigerer, L. (2000) Cancer Res. 60 4596-4601 [PubMed] [Google Scholar]
- 35.Kozian, D. H., Ziche, M., and Augustin, H. G. (1997) Lab. Invest. 76 267-276 [PubMed] [Google Scholar]
- 36.Mashimo, J., Maniwa, R., Sugino, H., and Nose, K. (1997) Cancer Lett. 113 213-219 [DOI] [PubMed] [Google Scholar]
- 37.Kawashima, S., and Yokoyama, M. (2004) Arterioscler. Thromb. Vasc. Biol. 24 998-1005 [DOI] [PubMed] [Google Scholar]
- 38.Forstermann, U., and Munzel, T. (2006) Circulation 113 1708-1714 [DOI] [PubMed] [Google Scholar]
- 39.Murohara, T., Asahara, T., Silver, M., Bauters, C., Masuda, H., Kalka, C., Kearney, M., Chen, D., Symes, J. F., Fishman, M. C., Huang, P. L., and Isner, J. M. (1998) J. Clin. Invest. 101 2567-2578 [DOI] [PMC free article] [PubMed] [Google Scholar]