Abstract
ApoA-I contains a tandem array of amphipathic helices with varying lipid affinity, which are critical in its ability to bind and remove lipids from cells by the ABCA1 transporter. In this study, the effect of asymmetry in the lipid affinity of amphipathic helices in a bihelical apoA-I mimetic peptide, 37pA, on lipid efflux by the ABCA1 transporter was examined. Seven peptide variants of 37pA were produced by substituting a varying number of hydrophobic amino acids for alanine on either one or both helices. The 5A peptide with five alanine substitutions in the second helix had decreased helical content compared with 37pA (5A, 12 ± 1% helicity; 37pA, 28 ± 2% helicity) and showed less self-association but, similar to the parent peptide, was able to readily solubilize phospholipid vesicles. Furthermore, 5A, unlike the parent peptide 37pA, was not hemolytic (37pA, 27 ± 2% RBC lysis, 2 h, 18 μm). Finally, the 5A peptide stimulated cholesterol and phospholipid efflux by the ABCA1 transporter with higher specificity (ABCA1-transfected versus untransfected cells) than 37pA (5A, 9.7 ± 0.77%, 18 h, 18 μm versus 1.5 ± 0.27%, 18 h, 18 μm (p < 0.0001); 37pA, 7.4 ± 0.85%, 18 h, 18μm versus 5.8 ± 0.20%, 18 h, 18μm (p = 0.03)). In summary, we describe a novel bihelical peptide with asymmetry in the lipid affinity of its helices and properties similar to apoA-I in terms of specificity for cholesterol efflux by the ABCA1 transporter and low cytotoxicity.
ApoA-I, the predominant protein constituent of HDL5 (1), can promote the efflux of excess cholesterol from cells by the ABCA1 transporter (2), which is one of the main antiatherogenic functions of HDL (3). ApoA-I contains a tandem array of amphipathic helical domains (4), but the amino and carboxyl terminal helices have the highest lipid affinity and are critical for the physical and biological properties of apoA-I (5–7). When synthesized as individual peptides, helices 1 and 2 and helices 9 and 10 exhibit good detergent-like properties, as assessed by dimyristoylphosphatidylcholine (DMPC) vesicle solubilization (5, 7, 8). The lipid affinity and detergent properties of the amphipathic helices of apoA-I are also important in how it facilitates cholesterol efflux from cells. In particular, the high lipid affinity COOH-terminal helix of apoA-I is essential to lipid efflux by the ABCA1 transporter (1, 6, 8, 9, 11, 12). Recently, it has been proposed that the ABCA1 transporter promotes lipid efflux from cells by creating a lipid microdomain that is solubilized and removed by apoA-I by a microsolubilization process dependent upon the detergent-like properties of its amphipathic helices (13–17).
ApoA-I and other apolipoproteins (18), including some relatively short peptides (13, 17, 19, 20), can promote cholesterol efflux from cells if they contain an amphipathic helix. ApoA-I mimetic peptides, containing amphipathic helices, are currently being investigated as an alternative to apoA-I for acute HDL therapy of patients with acute coronary syndromes (21, 22). One of these peptides, 37pA, is a dimer of the original 18A (Ac-18A-NH2) peptide, sometimes referred to as 2F due to two phenylalanine residues at positions 6 and 18 (15). We have previously shown that the 37pA peptide containing amphipathic helices with relatively high hydrophobic moment and high lipid affinity (15) removes cholesterol and phospholipid from HeLa cells transfected with the ABCA1 transporter (13). A significant fraction of lipid efflux by 37pA was, however, nonspecific, since cholesterol and phospholipid efflux was also observed from untransfected HeLa cells not expressing the transporter. The 37pA peptide was also found to be partially cytotoxic (13). In contrast, lipid-free apoA-I is almost completely dependent on ABCA1 for lipid efflux and showed no evidence of cytotoxicity (13).
Although the central low lipid affinity helices of apoA-I cannot promote lipid efflux by themselves, they have been shown to work cooperatively with the terminal high lipid affinity helices of apoA-I in promoting lipid efflux from cells and in the formation of HDL (6, 23). The relative unique ability of apoA-I to readily associate and dissociate with lipid, crucial for its role in HDL metabolism, has also been shown to be due to the presence of a mixture of low and high lipid affinity amphipathic helices (9, 24). Synthetic peptides based on apoA-I that contain one of the terminal high lipid affinity helices of apoA-I linked with one of the central helices, have been shown to stimulate lipid efflux by the ABCA1 transporter (17). A cluster of negative charged amino acids on the polar surface of these peptides spanning the two helices was found to be necessary for these peptides to be active in cholesterol efflux, but no consistent relationship was found between the lipid affinity of these peptides (17) and apoE-based peptides (25) on their ability to promote lipid efflux. Unlike the 37pA peptide (13), none of the bihelical peptides based on apoA-I (17) or apoE (25) showed cholesterol efflux independent of the ABCA1 transporter; nor were they described to be cytotoxic.
In this study, we examine the effect of asymmetry in the lipid affinity (i.e. combination of helices with low and high lipid affinity) of helices on modified bihelical 37pA peptides on their physical, structural, and biological properties. The lipid affinity of the helices of these peptides was reduced by substituting amino acids on the hydrophobic face of the parent 37pA peptide with alanine. We found that peptides, with one high lipid affinity helix paired with a low lipid affinity helix, showed not only the greatest specificity for cholesterol and phospholipid efflux by the ABCA1 transporter but also the lowest cytoxicity. In contrast, cholesterol efflux by peptides containing two high lipid affinity helices was independent of the ABCA1 transporter and cytotoxic. Peptides containing two low lipid affinity helices were not cytotoxic but were inactive in cholesterol efflux. These results are relevant in understanding the mechanism for the interaction of native exchangeable apolipoproteins with the ABCA1 transporter and in the design of therapeutic amphipathic helical peptides for the treatment of cardiovascular disease (22).
EXPERIMENTAL PROCEDURES
Peptide Synthesis and Calculations—All peptides were synthesized by a solid-phase procedure, using an Fmoc (N-(9-fluorenyl)methoxycarbonyl)/N,N′-diisopropylocarbodiimide/1-hydroxybenzotriazole protocol on a Biosearch 9600 peptide synthesizer and were purified to greater than 99% purity by reverse-phase HPLC on an Aquapore RP-300 column, as assessed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (Bruker UltraFlex). Retention time of peptides was determined after injection of 1 mg of purified peptide at 24 °C on a C-18 reverse-phase HPLC column and elution with a 25–85% gradient of acetonitrile, containing 0.1% trifluoroacetic acid. The hydrophobic moment of the peptides was calculated, using the combined consensus hydrophobicity scale (available on the World Wide Web). The mean hydrophobic moment was calculated as the vectorial sum of all the hydrophobicity indices, divided by the number of residues.
Solubilization of Phospholipid Vesicles by Peptides—Multilamellar vesicles of DMPC were produced by sonication (5). Peptides were incubated and continuously mixed at 24 °C with vesicles (1 mg/ml) in PBS, containing 8% NaBr, with the peptides at a final peptide concentration of ∼71 μm for 50 min, and absorbance was monitored at 430 nm every 5 s in a PerkinElmer Life Sciences plate reader with temperature control. Change in turbidity of samples, expressed as change in the initial rate constant (k) over time, was calculated for all peptides by a nonlinear curve fit for two-phase exponential decay and expressed as -fold change relative to H2O.
The ability of the peptides to solubilize phospholipid vesicles produced from a mixture of lipids commonly found in cell membranes (bovine liver phosphatidylcholine (55%, w/v), porcine brain spingomyelin (12%), egg lysophosphatidylcholine (5%), bovine liver phosphatidylethanolamine (10%), porcine brain phosphatidylserine (8%), bovine liver phosphatidylinositol (10%)) was tested as previously described at 37 °C with a final peptide concentration of 71 μm (12).
Fluorescence Assays—Tryptophan fluorescence from peptides (18 μm) in 20 mm NaHCO3, 0.15 m NaCl and from peptides complexed with DMPC (5A·DMPC; 1:15 molar ratio) was monitored in the presence and absence of guanidinium at 24 °C (26). Fluorescence was measured in a Shimatzu RF150 spectrophotometer with an excitation wavelength of 291 nm and emission wavelength ranging from 300 to 400 nm. Peptides and DMPC were complexed by co-lyophilization after first being dissolved in glacial acetic acid. The resultant lyophilized cakes were reconstituted with 20 mm NaHCO3, 0.15 m NaCl and heated to 50 °C in a water bath for 3 min and then allowed to cool at room temperature for 3 min for a total of three cycles.
Emission spectra of 5A and 37pA peptides before and after reconstitution with DMPC (1:15 molar ratio) were recorded following quenching with increasing acrylamide concentration (0–1 m) at 24 °C. The results are shown according to the Stern-Volmer equation (9).
The fluorescence spectra (400–600 nm) of peptides (20 μm) dissolved in PBS in the presence of 8-anilino-1-naphthalenesulfonic acid (ANS) at 250 μm at 24 °C was also monitored at an excitation wavelength of 395 nm (9).
Circular Dichroism—Free peptides or peptides (100 μg/ml) associated with DMPC (1:15 molar ratio) were dissolved in 10 mm phosphate buffer and analyzed by circular dichroism spectroscopy at 24 °C. The far UV circular dichroism spectra were obtained on a Jasco 7-715 spectropolarimeter at room temperature between 250 and 185 nm. The instrument response time was 0.5 s, with a bandwidth of 1 nm and a scanning speed of 50 nm/min. Three separate dilutions of a single preparation of each peptide or complex were analyzed and subjected to four accumulations each. The raw data in millidegrees was converted to mean residual ellipticity, using a mean residual weight of 121.1 Helical estimates were determined from the mean residual ellipticity at 222 nm (27). None of the tested solutions were turbid; therefore, no adjustment for light scattering was made.
Size Analysis of Peptide Complexes—Analysis of the number of peptide monomers present per peptide complex was performed by mixing a 20-fold molar excess of the cross-linker dimethylsuberimidate (Pierce) to the peptides at 24 °C in PBS, followed by analysis on a 10–20% Tris-Tricine SDS-polyacrylamide gel (28). Peptides were also analyzed by gel filtration on a Superose G75 column. Low molecular weight gel filtration standards were used as calibrators (GE Healthcare).
Red Cell Hemolysis Assay—Washed red blood cells were obtained from EDTA-anticoagulated blood and suspended in PBS at approximately a 4% hematocrit. Cell toxicity of peptides was tested by incubating red blood cells for 2 h at 37 °C with the indicated concentration of the peptides, followed by centrifugation at 3000 × g for 5 min and measurement of the absorbance of the supernatant at 540 nm. Complete lysis was determined after the addition of 1% Triton X-100.
Lipid Efflux Assay—HDL (d = 1.063–1.021 g/ml) was isolated from human serum by density gradient ultracentrifugation (29). Cholesterol and phospholipid efflux was performed as described previously (30), using a stably transfected HeLa cell line expressing the human ABCA1 transporter (18). HeLa cells transfected with a hygromycin-resistant control plasmid were used as the control cell line. Cholesterol efflux was examined at 37 °C in cells labeled with [3H]cholesterol for 48 h, washed, and challenged with either apoA-I or the free peptides at the concentrations indicated for 18 h. Percentage efflux was calculated by subtracting the radioactive counts in the blank medium (α-minimal essential medium with 10 μg/ml bovine serum albumin) from the radioactive counts in the presence of an acceptor and then dividing the result by the sum of the radioactive counts in the medium plus the cell fraction. Cholesterol efflux after 4 h at 37 °C to the indicated acceptors at 80 μg/ml was also examined in THP-1 cells before and after stimulation with 4 μm TO901317 and before and after a 10-min fixation with 3% paraformaldehyde (13).
For phospholipid efflux, cells were labeled with [methyl-3H]choline chloride for 24 h, washed, and challenged for 18 h with the indicated acceptors in α-minimal essential medium with 10 μg/ml bovine serum albumin at 37 °C. The percentage efflux was calculated in the same way as for cholesterol efflux, except that the radioactive counts in the medium were obtained following extraction with hexane/isopropyl alcohol (3:2).
Statistical Analyses—Phospholipid vesicle solubilization by peptides was examined as change in turbidity and expressed as -fold change in rate constant relative to H2O calculated by a nonlinear curve fit for two-phase exponential decay. Student's t test was used to compare means, and a two-sided p value <0.05 was considered significant.
RESULTS
The peptides synthesized for this study and their physical characteristics are listed in Table 1. The peptides are all variants of the 37pA peptide, which is a bihelical peptide containing two 18A amphipathic helices with relatively high lipid affinity linked with a proline (15). The 37pA peptide was altered by reducing its lipid affinity by substituting hydrophobic amino acids on its hydrophobic face with alanine, which is only partially hydrophobic (4). In peptides designated 1A–5A, 1–5 alanine substitutions were made in the second COOH-terminal helix. For the 5A-2 peptide, three alanine substitutions were made in the first NH2-terminal helix and two alanine substitutions in the second COOH-terminal helix to produce a peptide predicted to contain two moderate lipid affinity helices. For the 10A peptide, five alanine substitutions were made in both helices to produce a peptide predicted to contain two amphipathic helices with relatively low lipid affinity.
TABLE 1.
Characteristics and sequences of peptides
Rate of solubilization (k) was calculated from a nonlinear curve fitted to a two-phase exponential decay for all peptides.
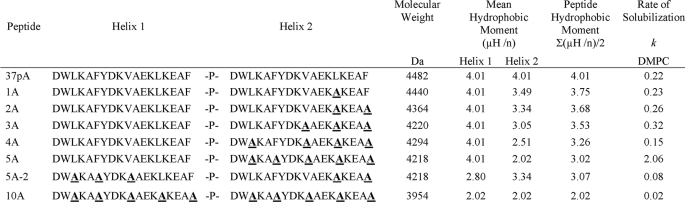
Fig. 1A shows that after each successive alanine substitution, the modified 37pA peptides when dissolved in acetonitrile had reduced lipid affinity, as assessed by their reduced retention time on a reverse-phase HPLC. A nearly linear relationship was observed between the calculated mean hydrophobic moment of the peptides and their retention time. The parent peptide, 37pA, and the 5A and 5A-2 peptides were also tested for their ability to bind ANS (Fig. 1B), a fluorescent lipophilic dye, which shows enhanced fluorescence when in a hydrophobic environment (31). In this experiment, the peptides were dissolved in an aqueous buffer, and the 37pA peptide showed much higher ability to bind and shield ANS compared with the 5A peptide. The emission spectra for the 5A-2 peptide overlapped the spectra for free ANS, indicating that the 5A-2 peptide was ineffective in binding ANS.
FIGURE 1.
Peptide retention time and ANS fluorescence. A, retention time on reverse-phase HPLC is shown versus average hydrophobic moment for each peptide. Retention time was calculated by injecting 1 mg of purified peptide at 24 °C on a C-18 reverse-phase HPLC column and elution with a 25–85% gradient of acetonitrile containing 0.1% trifluoroacetic acid. The hydrophobic moment was calculated as the average of the mean hydrophobic moment for the two helices. B, fluorescence spectra at 24 °C for ANS dissolved in PBS in the absence of any peptide (solid line) or in the presence of the 5A (dotted line) or 37pA peptide (dashed line). A.U., absorbance units.
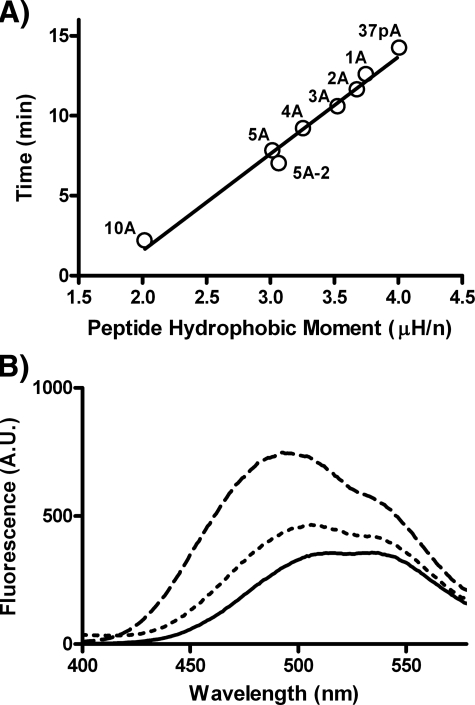
The ability of the peptides to act as detergents was assessed in Fig. 2 by monitoring the kinetics of the initial rate of solubilization of DMPC vesicles. The 5A peptide was most effective in solubilizing DMPC vesicles, as determined by the rate constant for the solubilization (Table 1). Consistent with its shorter retention time on HPLC and reduced ANS binding, the 5A-2 peptide was less effective than 5A. As expected, the 10A peptide containing two relatively low lipid affinity helices was the least effective (Fig. 2A). Peptides 1A–4A with 1–4 alanine substitutions in the second helix were intermediate in their ability to solubilize DMPC vesicles compared with 37pA and the 5A peptide (Fig. 2A). Similar results were obtained when examining the solubilization of phospholipid vesicles made with a mixture of natural lipids commonly found in cell membranes (Fig. 2B) (9). In this case, however, the 37pA peptide was the most effective, but the 5A peptide still showed considerable activity, whereas the 5A-2 peptide, as before, was ineffective (Fig. 2B).
FIGURE 2.
Peptide solubilization of phospholipid vesicles. A, relative absorbance of DMPC vesicles incubated with the indicated peptides (71 μm) was measured at 430 nm every 5 s for 50 min at 24 °C. B, relative absorbance of vesicles made with a mixture of phospholipids, as described under “Experimental Procedures,” was measured at 430 nm every 5 s for ∼8 min at 37 °C.
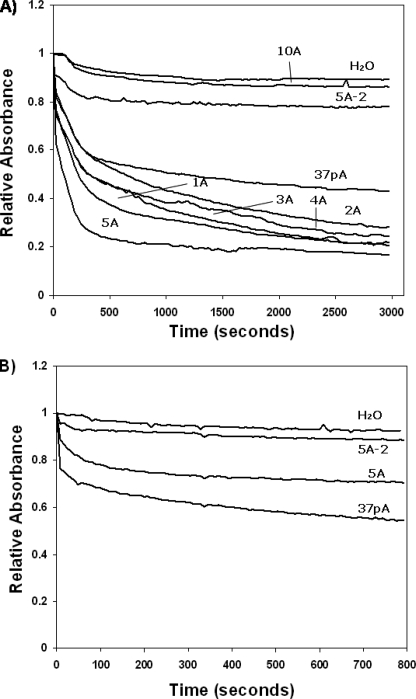
It has been previously shown that the detergent-like properties of amphipathic helical peptides, as well as their biological properties, can be affected by their ability to self-associate (32), which was examined in Fig. 3A by gel filtration. The 5A-2 peptide eluted as a single nearly symmetric peak near the retention volume of the column, consistent with its predicted molecular mass of 4482 Da. In contrast, both the parent 37pA peptide and the 5A peptide eluted with an apparent molecular mass of 65 and 27 kDa, respectively, which is much larger than their monomer molecular weights (Table 1). In addition, their elution peaks were asymmetric with a skewed right tail consistent with a concentration-dependent dissociation of a peptide complex during gel filtration. In Fig. 3B, the peptides dissolved in an aqueous buffer were cross-linked with dimethylsuberimidate and analyzed by SDS-PAGE. A similar ladder of cross-linked peptide complexes was observed for the 37pA and 5A peptides, with predominant bands at locations consistent with 4–5 peptide monomers/complex, although fainter bands of higher aggregates could also be observed. Interestingly, 5A-2 appeared to contain a much smaller number of peptides/complex. Consistent with its gel filtration profile, it appears to be primarily a monomer (Fig. 3B).
FIGURE 3.
Size analysis of peptides. A, gel filtration analysis of 37pA (diamond), 5A (open circle), and 5A-2 (open square) on a Superose G75 30/300 fast protein liquid chromatography column at 24 °C. The filled arrow indicates void volume, and the open arrow indicates retention volume of the column. Absorbance at 280 nm was recorded for each peptide (1 mg) with a flow rate of 0.5 ml/min. B, SDS-PAGE analysis (10–20% gradient Tris-Tricine gel) of lipid-free 37pA (lanes 2 and 5), 5A (lanes 3 and 6), and 5A-2 (lanes 4 and 7) before (lanes 2–4) and after (lanes 5–7) cross-linking with excess dimethylsuberimidate at 24 °C.
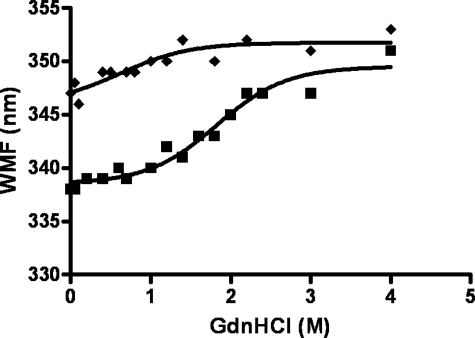
The self-association properties of the 37pA and 5A peptides were further examined by monitoring the fluorescence of Trp, which undergoes a blue shift in its peak emission wavelength from ∼351 nm for a highly solvent-exposed Trp residue to about 333 nm for a Trp that is shielded by either protein or lipid interactions (33). Each bihelical peptide in Table 1 contains two Trp residues/helix. On a helical wheel plot, each Trp is situated at the interface between the hydrophobic and hydrophilic part of the helix (15). In Fig. 4, the stability of the peptide complexes was determined by monitoring the emission wavelength of maximum fluorescence (WMF) of Trp in the presence of increasing concentrations of guanidine HCl. A clear red shift in WMF was observed for 37pA from ∼339 nm when dissolved in an aqueous buffer to 346 nm when the peptide complex was completely denatured by guanidine. By contrast, the WMF of the 5A peptide in aqueous buffer of 347 nm indicates higher solvent exposure for its Trp residues compared with 37pA. Consistent with this observation, the 5A peptide showed less of a red shift when denatured and required less guanidine to achieve complete denaturation.
FIGURE 4.
Guanidinium denaturation of peptides. The WMF of the 5A (diamonds) and 37pA (squares) peptides were determined at 24 °C in the presence of increasing concentrations of guanidinium (0–4 m). Each peptide at 18 μm in 20 mm NaHCO3-0.15 m NaCl was monitored for fluorescence at an excitation wavelength of 291 nm and emission wavelength from 300 to 400 nm.
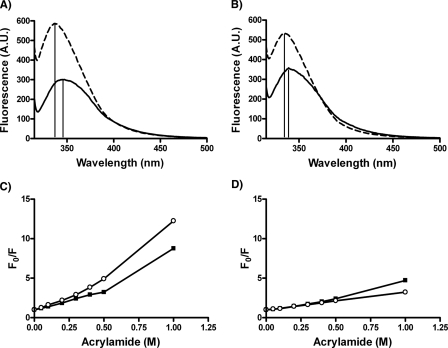
The complete Trp emission spectra are shown for the 37pA and 5A peptides before and after reconstitution with DMPC in Fig. 5, A and B. Both peptides showed enhanced fluorescence, which shifted to lower wavelengths when reconstituted with lipids, suggesting greater shielding of the Trp in a less polar environment. The 37pA peptide, however, showed a greater change, consistent with its greater lipid affinity (Fig. 1). Furthermore, quenching peptide fluorescence with acrylamide showed that the Trp residues in lipid-free 5A were more solvent-exposed than was 37pA (Fig. 5C), which is consistent with the denaturation experiments (Fig. 4). When the two peptides were complexed to DMPC, the Trp residues for both peptides were overall less susceptible to quenching and showed a similar response (Fig. 5D).
FIGURE 5.
Tryptophan fluorescence analysis of peptides. Tryptophan fluorescence spectra for the 37pA (A) and 5A peptide (B) were recorded in the absence of any lipid (solid line) and after reconstitution with DMPC (dashed line). The vertical lines mark the wavelength of maximum fluorescence. Stern-Volmer plots of 5A (circles) and 37pA (squares) in the lipid-free state (C) and after DMPC reconstitution (D). Emission spectra (300–400 nm) were recorded at an excitation wavelength of 291 nm for each peptide (18 μm) in the presence of increasing acrylamide concentrations (0–1 m) at 24 °C. F0 is the fluorescence with no acrylamide, and F the fluorescence at a given acrylamide concentration. A.U., absorbance units.
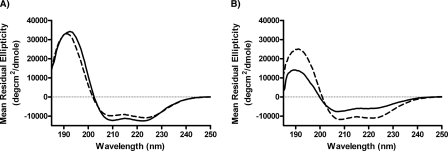
In order to further characterize the structure of the peptides, far UV circular dichroism spectroscopy was performed (Fig. 6). The circular dichroism spectra for both the free 37pA and 5A peptides showed a characteristic shape that is consistent with α-helical content, with minima at 208 and 222 nm and a maximum around 191 nm. Judging by mean residual ellipticity at 222 nm, 37pA was more helical than the 5A peptide. Interestingly, the 5A peptide exhibited an atypical reduced intensity at 222 nm relative to 208 nm (ratio of 222/208 nm less than 0.85) (Fig. 6B). This suggests that the limited helicity adopted by the lipid-free 5A is most likely a nonideal 3-10 helix that does not have the same hydrogen bonding pattern as an ideal helix, exemplified by 37pA. Upon lipid association with DMPC, which results in discoidal shape structures, as determined by electron microscopy (data not shown), 37pA underwent a modest increase in helicity from 28 ± 2% to 34 ± 1%, which is similar to the previously reported results (34) (Fig. 6A). The 5A peptide underwent a considerably larger increase in helicity from a base line of 12 ± 1% in the lipid-free state to 29 ± 1% helicity after lipid reconstitution, which is comparable with lipid-bound 37pA. Furthermore, the resulting helix formed by 5A when reconstituted with phospholipid exhibited properties of a more ideal helix, based on the normalization of the 222/208 nm ratio. Thus, it appears that the 5A peptide undergoes a more substantial conformational rearrangement upon binding to lipid than does 37pA, but after association with lipid the two peptides are similar in structure.
FIGURE 6.
Circular dichroism analysis of peptides. Mean residual ellipticity for 37pA (A) and 5A (B) is shown for the free peptides (solid line) and the DMPC-reconstituted peptides (dashed line) at the indicated wavelengths. Each peptide preparation was examined by three dilutions, and the results shown are the mean of four spectra examined at 24 °C.
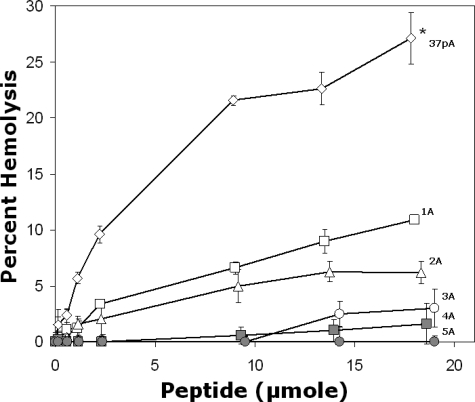
Consistent with previous observations on the release of the intracellular enzyme LDH (13), the 37pA peptide was found to be cytotoxic, as determined by its ability to lyse red blood cells (Fig. 7). At the maximum dose, it lysed almost 30% of red blood cells in 2 h. Interestingly, with increasing alanine substitutions, the modified 37pA peptides became less hemolytic, although they were superior to the 37pA peptide in solubilizing DMPC vesicles (Fig. 2A). The 5A peptide, like free apoA-I (data not shown), showed no evidence for hemolysis. In addition, the 5A-2 and 10A peptides were also nonhemolytic (data not shown).
FIGURE 7.
Red cell hemolysis by peptides. Peptides at the indicated concentrations were incubated with red blood cells for 2 h at 37 °C followed by centrifugation. The supernatant was measured at an optical density of 540 nm. Results are expressed as the mean of triplicates ± S.D. *, pairwise comparison of hemolysis for the highest concentration of each peptide was significantly reduced compared with 37pA (p values <0.006). Open diamond, 37pA; open square, 1A; open triangle, 2A; open circle, 3A; gray diamond, 4A; gray circle, 5A; gray triangle, 5A-2; gray diamond, 10A.
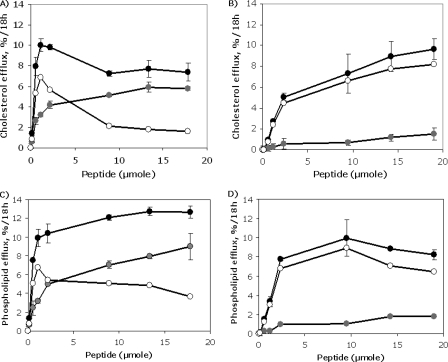
In Fig. 8, the effect of alanine substitutions on the ability of the modified peptides to promote lipid efflux was examined. The peptides were incubated with HeLa cells stably transfected with the human ABCA1 transporter or with wild type HeLa cells, which have very low levels of endogenous ABCA1 (13, 18). ABCA1-dependent efflux was the difference between the results for the control HeLa cell line and the ABCA1-transfected HeLa cell line (13, 18). The 37pA peptide was able to promote cholesterol efflux, but it was relatively nonspecific, since the cholesterol efflux from the control cell line (at the maximum dose tested) was only slightly lower than from the ABCA1-transfected cells (5.8 ± 0.20% versus 7.4 ± 0.85%, respectively, at 18 μm; p = 0.03) (Fig. 8A). In contrast, the 5A peptide caused only a minimal amount of cholesterol efflux from the control cells but was able to promote a significant amount of cholesterol efflux from the ABCA1-transfected cells (1.5 ± 0.27% versus 9.7 ± 0.77%, respectively, at 18 μm, p < 0.0001) (Fig. 8B).
FIGURE 8.
Lipid efflux by 5A and 37pA from ABCA1-transfected Cells. Shown is cholesterol efflux by ABCA1 HeLa cells (black circles) and control HeLa cells (gray circles) after 18 h at 37 °C to 37pA (A) and 5A (B) by increasing concentration of peptides. Shown is phospholipid efflux by ABCA1 HeLa cells (black circles) and control HeLa cells (gray circles) after 18 h at 37 °C to 37pA (C) and 5A (D) by increasing concentration of peptides. The contribution of ABCA1 (open circles) to cholesterol and phospholipid efflux was calculated by subtracting the results from the control cell line. Results represent the mean of triplicates ± S.D.
Similar results were observed when the 37pA and 5A peptide were examined for phospholipid efflux (Fig. 8, C and D). The 5A peptide primarily removed phospholipid from only the ABCA1-transfected cell line, whereas the amount of phospholipid efflux from the control cell line by the 37pA peptide was ∼50–75% of the level observed from the ABCA1-transfected cell line. Although the 5A peptide was more specific for promoting lipid efflux by the ABCA1 transporter, it appeared to require a higher concentration than 37pA for maximum lipid efflux; thus, it has a higher apparent Km than 37pA for lipid efflux.
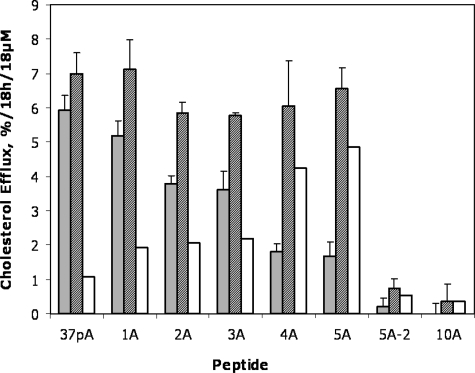
Fig. 9 shows the results for cholesterol efflux from both the control cells and ABCA1-transfected cells for all peptides. A clear trend was observed in peptides 1A–5A for increased specificity for ABCA1-dependent cholesterol efflux after each alanine substitution in the second helix. Interestingly, there was no significant difference in cholesterol efflux from the ABCA1-transfected cells for the 1A–5A peptides, but there was a marked decrease in cholesterol efflux from the control cells with the alanine substitutions, thus indicating increased specificity for cholesterol efflux by ABCA1 (2A, p = 0.001; 3A, p = 0.004; 4A, 5A, 5A-2, and 10A, p ≤ 0.0001) for the pairwise comparison with 37pA for each peptide (Fig. 9). Unlike the 5A peptide, which contains one helix with high lipid affinity paired with a low lipid affinity helix (Table 1), the 5A-2 peptide with two helices with moderate lipid affinity did not promote appreciable amounts of cholesterol efflux from either cell line. The 10A peptide with five alanine substitutions in both helices was also ineffective for either cell line.
FIGURE 9.
Lipid efflux by peptides from ABCA1-transfected cells. Cholesterol efflux by ABCA1 HeLa cells (striped bars) and control HeLa cells (gray bars) to alanine-substituted peptides examined at 37 °C. The contribution of ABCA1 (open bars) to cholesterol efflux was calculated by subtracting the results from the control cell line. Results represent the mean of triplicates ± S.D. *, p = 0.001; **, p = 0.004; ***, p ≤ 0.0001 for the pairwise comparison with 37pA.
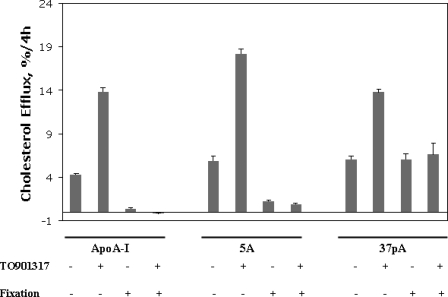
The specificity for cholesterol efflux by the ABCA1 transporter for the 37pA and 5A peptides was compared with apoA-I in the THP-1 macrophage cell line (Fig. 10). THP-I cells normally only express a relatively modest amount of the ABCA1 transporter, but the transporter is significantly induced after stimulation with the LXR agonist TO901317 (35, 36). Both peptides and apoA-I showed a similar level of cholesterol efflux from untreated cells and a similar -fold increase in cholesterol efflux after stimulation with TO901317. As expected, fixation of the cells with paraformaldehyde completely inhibited cholesterol efflux by apoA-I from either the treated or nontreated cells (Fig. 10). Similar results were observed with 5A after fixation of cells, although there was still a small amount of residual cholesterol efflux after fixation (Fig. 10). In contrast, fixation of cells only decreased cholesterol efflux by ∼50% for the 37pA peptide, indicating that 37pA, unlike 5A, can remove considerable amounts of cholesterol from cells by a nonspecific detergent-like extraction process from even fixed cells.
FIGURE 10.
Lipid efflux by peptides from THP-I cells. Cholesterol efflux from activated (4 μm TO901317) and nonactivated THP-1 cells before and after fixation in 3% paraformaldehyde. Cells were incubated with 80 μg/ml either apoA-I, 5A, or the 37pA peptide for 4 h at 37°C. Results represent the mean of triplicates ± S.D.
DISCUSSION
The main finding of this study is that asymmetry in the lipid affinity of bihelical amphipathic peptides has significant and complex effects on both their physical and biological properties. When 37pA and the Ala-substituted peptides were dissolved in a hydrophobic solvent like acetonitrile, which was used for the determination of retention time on a reverse-phase column (Fig. 1), they would be expected to exist as free monomers. Under these conditions, their calculated mean hydrophobic moment closely correlated with their measured lipid affinity, as assessed by their retention time. In aqueous buffers, however, amphipathic peptides are known to self-associate and form large multimeric complexes (32, 37), as was observed in this study (Figs. 3 and 4). Thermodynamically, amphipathic peptides are predicted to form such complexes in aqueous buffers to conceal the hydrophobic regions of their helices (4). Compared with 37pA, which has two identical high lipid affinity helices, the 5A peptide, however, appeared to form a less stable and smaller peptide complex, as determined by gel filtration (Fig. 3A) and guanidinium denaturation studies (Figs. 4 and 5). This is most likely a consequence of the increased polarity of the second helix of 5A due to the Ala substitutions. The Ala substitutions also resulted in a peptide with reduced helical content (Fig. 6). When reconstituted with phospholipids, however, the 5A showed a similar helical content to 37pA (Fig. 6) and a similar resistance to Trp quenching by acrylamide (Fig. 5, C and D), indicating that the presence of a single high affinity lipid helix is sufficient for a bihelical peptide to associate and tightly integrate into lipids.
We also found a complex relationship between the calculated mean hydrophobic moment of the peptides and their ability to solubilize phospholipid vesicles (Fig. 2). In the case of DMPC vesicles, the 5A peptide was unexpectedly better than the parent 37pA peptide. Reducing the lipid affinity of both helices, as was done for the 5A-2 and 10A peptides, completely blocked the ability of these peptides to solubilize DMPC vesicles. This again suggests that only a single high affinity helix is necessary for the detergent-like property of these peptides. One potential explanation for why the 5A peptide was superior to the 37pA peptide in solubilizing DMPC vesicles is that the higher mean hydrophobic moment of the 37pA peptide, which enables it to form a more stable multimeric complex in aqueous buffers (Figs. 3, 4, 5), may reduce its ability to interact with DMPC vesicles. In other words, the strong protein-protein interaction of the multimeric peptide complex limits the initial interaction of the peptide with lipid. This is consistent with an observed increase in stability and helical content of this peptide and has previously been observed to also occur for the phenylalanine-substituted 18A peptides (38).
In contrast, the 37pA peptide was slightly better than the 5A peptide in solubilizing phospholipid vesicles produced with a mixture of phospholipids (Fig. 2B), which were more readily solubilized by these peptides than DMPC vesicles (Fig. 2). There are several differences between the DMPC vesicles and the mixed phospholipid vesicles that can potentially explain the observed differences in the two studies. First, the mixed phospholipid vesicles contained charged phospholipids, unlike the vesicles made with DMPC. It may be that the 37pA peptide, perhaps because of its greater ability to form an amphipathic helix, may have a more favorable charge distribution for interacting with the ionic head groups on the charged phospholipids, as has been previously suggested for other proteins (39). Another possibility is that the mixed phospholipid vesicles may have phospholipid packing defects and or lipid microdomains, which have been previously proposed to be sites of interaction of amphipathic peptides with phospholipid vesicles (9, 39). Again, the increased helicity of the 37pA peptide may enable it to better interact with such sites. These data point out that the interaction of these amphipathic peptides with lipid membranes is very dependent upon the chemical and physical properties of the membranes.
A potentially important consideration in the design and use of therapeutic amphipathic peptides is their cytotoxicity. Some amphipathic peptides are known to be cytotoxic, because of their ability to deeply insert into membranes and/or to self-aggregate in membranes and perturb the normal bilayer lipid organization (40, 41). The insertion of an amphipathic peptide into a lipid bilayer, resulting in cell lysis, is known, for example, to be dependent upon the breadth of its hydrophobic face, the overall hydrophobicity of the amino acid residues in the hydrophobic face, and the cross-sectional shape of the helix (42). 37pA was found in this study to cause membrane damage to red blood cells, whereas 5A, like apoA-I, caused no significant hemolysis (Fig. 7), despite the fact that 5A was more effective in solubilizing DMPC vesicles (Fig. 2A). The substitution of hydrophobic amino acids for alanine in other peptides has previously been shown to prevent the deep penetration of peptides into phospholipid bilayers (43). Although the first helix of 5A has high lipid affinity, covalently coupling it to a low lipid affinity helix appears to impede the overall insertion of the peptide into cell membranes. At least in the aqueous solution, the 37pA peptide was also found to more readily self-associate than 5A (Figs. 3 and 4). If it also does so when embedded in the lipids of cell membranes, this could also account for its increased hemolytic ability, as has been described for other amphipathic peptides (40, 41). Overall, these results suggest that the central low lipid affinity helices of apoA-I may serve a similar role in limiting a deeper interaction of apoA-I with membranes and thus preventing membrane damage when it interacts with cells.
Another important consideration in the design and use of therapeutic amphipathic peptides is their specificity for promoting cholesterol efflux by the ABCA1 transporter. Recently, a multistep process has been proposed for how ABCA1 promotes lipid efflux from cells (12). The first step involves a protein-protein interaction between apolipoproteins as well as amphipathic peptides (44) with the ABCA1 transporter. This low capacity interaction may facilitate the initial binding of lipid-free apolipoproteins to cells (12) and stabilizes the transporter against degradation (45). This has also been described to occur for the 37pA peptide (46). The increased level of ABCA1 then promotes the formation of surface protrusions in the plasma membrane, similar to the membrane structures reported to be formed by other ABC transporters (47). These exovesiculated lipid domains have been proposed to form in response to the membrane strain caused by the transfer of lipid by the ABCA1 transporter to the extracellular leaflet of the plasma membrane (12). The high curvature of the lipid domains created by ABCA1 then promotes the higher capacity binding of apolipoproteins, which ultimately solubilize the phospholipids and cholesterol in these exovesiculated lipid structures to form nascent HDL particles. The therapeutic use of nonselective amphipathic peptides, such as 37pA, for removing excess cholesterol is potentially problematic for two reasons. First, non-ABCA1-dependent cholesterol efflux by peptides with high lipid affinity appears to be associated with cytotoxicity (Fig. 7). Second, in vivo, these peptides may initially become saturated with lipids removed nonselectively from nontarget before interacting with their intended target cells, namely cholesterol-loaded macrophages in atherosclerotic plaque, which express ABCA1 (22). For these reasons, the 5A peptide may have an advantage over the 37pA and other non-selective peptides as a therapeutic agent. It is important to note, however, that amphipathic peptides appear to have many other beneficial antioxidative, anti-inflammatory, and provasodilatory effects besides their effect in promoting cholesterol efflux (48, 49).
The ability of 37pA to remove cholesterol independent of the ABCA1 transporter from even fixed cells (Fig. 10) is most likely a consequence of its relatively high lipid affinity. Based on its calculated hydrophobic moment, as well as its experimentally derived surface monolayer exclusion pressure, 37pA has higher lipid affinity than any of the individual helices of apoA-I, including the high lipid affinity terminal helices (5, 7, 8). Due to its high lipid affinity, it may interact with sites on cell membranes besides the lipid microdomains created by ABCA1. This is consistent with previous studies showing that it competes with the same cellular binding site as apoA-I, but it also binds to additional sites on cells that do not bind apoA-I (10, 13). This perhaps may explain the complex dose-response relationship observed for the 37pA peptide for lipid efflux (Fig. 8). At relatively low doses, it appeared to be more effective than 5A in removing lipid by ABCA1, but at higher doses it also caused non-ABCA1-dependent lipid efflux (Fig. 8).
In contrast to 37pA, apoA-I, and 5A, which both contain high and low lipid affinity helices, appear to be only able to remove cellular cholesterol from the lipid microdomains formed by ABCA1. The COOH-terminal helix of 5A, in fact, has a hydrophobic moment similar to that of the central helices of apoA-I. Perhaps because of the altered lipid packing in the lipid domains created by ABCA1, 5A, like apoA-I (12), can insert into these sites, whereas the other parts of the membrane are not accessible to it, unlike 37pA. The low affinity helix of 5A, because of its potential increased surface exposure when in a multimeric peptide complex (Fig. 4), may also promote lipid efflux by facilitating the initial interaction of the peptide with the lipid microdomains created by ABCA1. Once this interaction occurs, however, at least one high lipid affinity helix is necessary for extracting lipid, as evidence by the inability of the 5A-2 and 10A peptides to promote lipid efflux by ABCA1 (Fig. 9). This may be analogous to the two-domain structural model for the interaction of apoA-I with lipids. The more solvent-exposed COOH-terminal helix of apoA-I is believed to initially interact with lipid on cell membranes, and then the rest of the protein, which is in a helix bundle configuration, undergoes a conformational change so that the remaining amphipathic helices of apoA-I unfold and cooperatively bind and efflux lipid (6).
Besides the lipid affinity of the amphipathic helices of apolipoproteins and peptides, a cluster of negative charge residues on the polar face of helices has also been described as necessary for cholesterol efflux by the ABCA1 transporter (17). The 37pA peptide shares a similar structural motif of negatively charged residues that spans both helices (15, 17), thus possibly enabling it to interact with the ABCA1 transporter for lipid efflux. All of the modified 37pA peptides used in this study share the same motif, because all alanine substitutions were made on the hydrophobic face only. This motif on the polar face of the peptides may be necessary but not sufficient for specific lipid efflux by the ABCA1 transporter, because both of the peptides inactive in cholesterol efflux as well as the cytotoxic and nonspecific lipid efflux peptides contain this motif. It appears, therefore, that a multisite interaction of both the polar and nonpolar faces of the helices of apolipoproteins and their peptide mimics with the ABCA1 transporter and/or the lipid microdomain created by ABCA1 is necessary for lipid efflux to occur.
Acknowledgments
We thank Maureen Sampson for expert help in making the figures.
This work was supported, in whole or in part, by intramural funds from the National Institutes of Health, NHLBI. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HDL, high density lipoprotein; DMPC, dimyristoylphosphatidylcholine; HPLC, high pressure liquid chromatography; ANS, 8-anilino-1-naphthalenesulfonic acid; PBS, phosphate-buffered saline; Tricine, N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine; WMF, wavelength of maximum fluorescence.
References
- 1.Panagotopulos, S. E., Witting, S. R., Horace, E. M., Hui, D. Y., Maiorano, J. N., and Davidson, W. S. (2002) J. Biol. Chem. 277 39477–39484 [DOI] [PubMed] [Google Scholar]
- 2.Nofer, J. R., and Remaley, A. T. (2005) Cell Mol. Life Sci. 62 2150–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueda, M., Sethi, A. A., Freeman, L. A., Vaisman, B. L., Amar, M., Shamburek, R., and Remaley, A. T. (2005) J. Clin. Ligand Assay 4 216–232 [Google Scholar]
- 4.Phoenix, D. A., Harris, F., Daman, O. A., and Wallace, J. (2002) Curr. Protein Pept. Sci. 3 201–221 [DOI] [PubMed] [Google Scholar]
- 5.Mishra, V. K., Palgunachari, M. N., Datta, G., Phillips, M. C., Lund-Katz, S., Adeyeye, S. O., Segrest, J. P., and Anantharamaiah, G. M. (1998) Biochemistry 37 10313–10324 [DOI] [PubMed] [Google Scholar]
- 6.Saito, H., Lund-Katz, S., and Phillips, M. C. (2004) Prog. Lipid Res. 43 350–380 [DOI] [PubMed] [Google Scholar]
- 7.Palgunachari, M. N., Mishra, V. K., Lund-Katz, S., Phillips, M. C., Adeyeye, S. O., Alluri, S., Anantharamaiah, G. M., and Segrest, J. P. (1996) Arterioscler. Thromb. Vasc. Biol. 16 328–338 [DOI] [PubMed] [Google Scholar]
- 8.Gillotte, K. L., Zaiou, M., Lund-Katz, S., Anantharamaiah, G. M., Holvoet, P., Dhoest, A., Palgunachari, M. N., Segrest, J. P., Weisgraber, K. H., Rothblat, G. H., and Phillips, M. C. (1999) J. Biol. Chem. 274 2021–2028 [DOI] [PubMed] [Google Scholar]
- 9.Saito, H., Dhanasekaran, P., Nguyen, D., Holvoet, P., Lund-Katz, S., and Phillips, M. C. (2003) J. Biol. Chem. 278 23227–23232 [DOI] [PubMed] [Google Scholar]
- 10.Mendez, A. J., Anantharamaiah, G. M., Segrest, J. P., and Oram, J. F. (1994) J. Clin. Invest. 94 1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vedhachalam, C., Ghering, A. B., Davidson, W. S., Lund-Katz, S., Rothblat, G. H., and Phillips, M. C. (2007) Arterioscler. Thromb. Vasc. Biol. 27 1603–1609 [DOI] [PubMed] [Google Scholar]
- 12.Vedhachalam, C., Duong, P. T., Nickel, M., Nguyen, D., Dhanasekaran, P., Saito, H., Rothblat, G. H., Lund-Katz, S., and Phillips, M. C. (2007) J. Biol. Chem. [DOI] [PubMed]
- 13.Remaley, A. T., Thomas, F., Stonik, J. A., Demosky, S. J., Bark, S. E., Neufeld, E. B., Bocharov, A. V., Vishnyakova, T. G., Patterson, A. P., Eggerman, T. L., Santamarina-Fojo, S., and Brewer, H. B. (2003) J. Lipid Res. 44 828–836 [DOI] [PubMed] [Google Scholar]
- 14.Segrest, J. P., Jones, M. K., De Loof, H., Brouillette, C. G., Venkatachalapathi, Y. V., and Anantharamaiah, G. M. (1992) J. Lipid Res. 33 141–166 [PubMed] [Google Scholar]
- 15.Anantharamaiah, G. M., Jones, J. L., Brouillette, C. G., Schmidt, C. F., Chung, B. H., Hughes, T. A., Bhown, A. S., and Segrest, J. P. (1985) J. Biol. Chem. 260 10248–10255 [PubMed] [Google Scholar]
- 16.Navab, M., Anantharamaiah, G. M., Hama, S., Garber, D. W., Chaddha, M., Hough, G., Lallone, R., and Fogelman, A. M. (2002) Circulation 105 290–292 [DOI] [PubMed] [Google Scholar]
- 17.Natarajan, P., Forte, T. M., Chu, B., Phillips, M. C., Oram, J. F., and Bielicki, J. K. (2004) J. Biol. Chem. 279 24044–24052 [DOI] [PubMed] [Google Scholar]
- 18.Remaley, A. T., Stonik, J. A., Demosky, S. J., Neufeld, E. B., Bocharov, A. V., Vishnyakova, T. G., Eggerman, T. L., Patterson, A. P., Duverger, N. J., Santamarina-Fojo, S., and Brewer, H. B., Jr. (2001) Biochem. Biophys. Res. Commun. 280 818–823 [DOI] [PubMed] [Google Scholar]
- 19.Bielicki, J. K., and Oda, M. N. (2002) Biochemistry 41 2089–2096 [DOI] [PubMed] [Google Scholar]
- 20.Yancey, P. G., Bielicki, J. K., Johnson, W. J., Lund-Katz, S., Palgunachari, M. N., Anantharamaiah, G. M., Segrest, J. P., Phillips, M. C., and Rothblat, G. H. (1995) Biochemistry 34 7955–7965 [DOI] [PubMed] [Google Scholar]
- 21.Nicholls, S. J., Tuzcu, E. M., Sipahi, I., Schoenhagen, P., Crowe, T., Kapadia, S., and Nissen, S. E. (2006) J. Am. Coll. Cardiol. 47 992–997 [DOI] [PubMed] [Google Scholar]
- 22.Sethi, A. A., Amar, M., Shamburek, R. D., and Remaley, A. T. (2007) Curr. Opin. Investig. Drugs 8 201–212 [PubMed] [Google Scholar]
- 23.Tanaka, M., Saito, H., Dhanasekaran, P., Wehrli, S., Handa, T., Lund-Katz, S., and Phillips, M. C. (2005) Biochemistry 44 10689–10695 [DOI] [PubMed] [Google Scholar]
- 24.Pownall, H. J., and Ehnholm, C. (2006) Curr. Opin. Lipidol. 17 209–213 [DOI] [PubMed] [Google Scholar]
- 25.Vedhachalam, C., Narayanaswami, V., Neto, N., Forte, T. M., Phillips, M. C., Lund-Katz, S., and Bielicki, J. K. (2007) Biochemistry 46 2583–2593 [DOI] [PubMed] [Google Scholar]
- 26.Vivian, J. T., and Callis, P. R. (2001) Biophys. J. 80 2093–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen, Y. H., Yang, J. T., and Martinez, H. M. (1972) Biochemistry 11 4120–4131 [DOI] [PubMed] [Google Scholar]
- 28.Calabresi, L., Vecchio, G., Longhi, R., Gianazza, E., Palm, G., Wadensten, H., Hammarstrom, A., Olsson, A., Karlstrom, A., and Sejlitz, T. (1994) J. Biol. Chem. 269 32168–32174 [PubMed] [Google Scholar]
- 29.Schumaker, V. N., and Puppione, D. L. (1986) Methods Enzymol. 128 155–169 [DOI] [PubMed] [Google Scholar]
- 30.Remaley, A. T., Schumacher, U. K., Stonik, J. A., Farsi, B. D., Nazih, H., and Brewer, H. B., Jr. (1997) Arterioscler. Thromb. Vasc. Biol. 17 1813–1821 [DOI] [PubMed] [Google Scholar]
- 31.Stryer, L. (1965) J. Mol. Biol. 13 482–495 [DOI] [PubMed] [Google Scholar]
- 32.Datta, G., Chaddha, M., Hama, S., Navab, M., Fogelman, A. M., Garber, D. W., Mishra, V. K., Epand, R. M., Epand, R. F., Lund-Katz, S., Phillips, M. C., Segrest, J. P., and Anantharamaiah, G. M. (2001) J. Lipid Res. 42 1096–1104 [PubMed] [Google Scholar]
- 33.Davidson, W. S., Arnvig-McGuire, K., Kennedy, A., Kosman, J., Hazlett, T. L., and Jonas, A. (1999) Biochemistry 38 14387–14395 [DOI] [PubMed] [Google Scholar]
- 34.Gazzara, J. A., Phillips, M. C., Lund-Katz, S., Palgunachari, M. N., Segrest, J. P., Anantharamaiah, G. M., and Snow, J. W. (1997) J. Lipid Res. 38 2134–2146 [PubMed] [Google Scholar]
- 35.Venkateswaran, A., Laffitte, B. A., Joseph, S. B., Mak, P. A., Wilpitz, D. C., Edwards, P. A., and Tontonoz, P. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costet, P., Luo, Y., Wang, N., and Tall, A. R. (2000) J. Biol. Chem. 275 28240–28245 [DOI] [PubMed] [Google Scholar]
- 37.Mishra, V. K., Palgunachari, M. N., Lund-Katz, S., Phillips, M. C., Segrest, J. P., and Anantharamaiah, G. M. (1995) J. Biol. Chem. 270 1602–1611 [DOI] [PubMed] [Google Scholar]
- 38.Dashti, N., Datta, G., Manchekar, M., Chaddha, M., and Anantharamaiah, G. M. (2004) J. Lipid Res. 45 1919–1928 [DOI] [PubMed] [Google Scholar]
- 39.Sugar, I. P., Mizuno, N. K., and Brockman, H. L. (2005) Biophys. J. 89 3997–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Handattu, S. P., Garber, D. W., Horn, D. C., Hughes, D. W., Berno, B., Bain, A. D., Mishra, V. K., Palgunachari, M. N., Datta, G., Anantharamaiah, G. M., and Epand, R. M. (2007) J. Biol. Chem. 282 1980–1988 [DOI] [PubMed] [Google Scholar]
- 41.Datta, G., Epand, R. F., Epand, R. M., Chaddha, M., Kirksey, M. A., Garber, D. W., Lund-Katz, S., Phillips, M. C., Hama, S., Navab, M., Fogelman, A. M., Palgunachari, M. N., Segrest, J. P., and Anantharamaiah, G. M. (2004) J. Biol. Chem. 279 26509–26517 [DOI] [PubMed] [Google Scholar]
- 42.Boggs, J. M., Jo, E., Polozov, I. V., Epand, R. F., Anantharamaiah, G. M., Blazyk, J., and Epand, R. M. (2001) Biochim. Biophys. Acta 1511 28–41 [DOI] [PubMed] [Google Scholar]
- 43.Victor, K., Jacob, J., and Cafiso, D. S. (1999) Biochemistry 38 12527–12536 [DOI] [PubMed] [Google Scholar]
- 44.Mukhamedova, N., Fu, Y., Bukrinsky, M., Remaley, A. T., and Sviridov, D. (2007) Biochemistry 46 9388–9398 [DOI] [PubMed] [Google Scholar]
- 45.Oram, J. F., and Yokoyama, S. (1996) J. Lipid Res. 37 2473–2491 [PubMed] [Google Scholar]
- 46.Arakawa, R., Hayashi, M., Remaley, A. T., Brewer, B. H., Yamauchi, Y., and Yokoyama, S. (2004) J. Biol. Chem. 279 6217–6220 [DOI] [PubMed] [Google Scholar]
- 47.Borst, P., Zelcer, N., and van Helvoort, A. (2000) Biochim. Biophys. Acta 1486 128–144 [DOI] [PubMed] [Google Scholar]
- 48.Navab, M., Anantharamaiah, G. M., Reddy, S. T., and Fogelman, A. M. (2006) Nat. Clin. Pract. Cardiovasc. Med. 3 540–547 [DOI] [PubMed] [Google Scholar]
- 49.Navab, M., Anantharamaiah, G. M., Reddy, S. T., Van Lenten, B. J., Ansell, B. J., and Fogelman, A. M. (2006) Nat. Clin. Pract. Endocrinol. Metab. 2 504–511 [DOI] [PubMed] [Google Scholar]