Abstract
Interaction between apoptotic cells and phagocytes through phosphatidylserine recognition structures (PSRS) results in the production of transforming growth factor-β (TGF-β) which has been shown to play pivotal roles in the anti-inflammatory and anti-immunogenic responses to apoptotic cell clearance. Using 3T3-TβRII and RAWTβRII cells in which a truncated dominant negative TGF-β eceptor II was stably transfected in order to avoid autofeedback induction of TGFβ, we investigate the mechanisms by which TGF-β as produced through PSRS engagement. We show, in the present study, that TGF-β was regulated at both transcriptional and translational steps. P38 MAPK, ERK and JNK were involved in TGF-β transcription whereas translation required activation of Rho GTPase, PI-3 kinase, Akt and mTOR with subsequent phosphorylation of translation initiation factor eIF4E. Strikingly, these induction pathways for TGF-β production were different from those initiated in the same cells responding to LPS or PMA.
Keywords: TGF-β, apoptosis, efferocytosis, phosphatidylserine, MAPKs, Rho activation
Introduction
Deletion of apoptotic cells in vivo involves recognition of surface changes on the cells leading to their ingestion by unique phagocytic mechanisms – a process that we have termed efferocytosis (1–5). Accompanying this recognition, whether or not the apoptotic cell is actually engulfed by the phagocyte, is the generation of anti-inflammatory mediators (6–9) and the establishment of a generally anti-inflammatory and anti-immunogenic local environment. We have earlier suggested that TGF-β is a major mediator of this response and that a number of secondary anti-inflammatory effects result from the autocrine/paracrine actions of the active TGF-β produced.
The TGF-β-family comprises more than 60 structurally related growth and differentiation factors that play important roles in regulation of numerous physiological processes, including cell proliferation, differentiation, apoptosis, early embryonic development, and extracellular matrix protein synthesis (10–14). TGF-β exerts its effects through a heteromeric receptor complex consisting of type I and II transmembrane serine/threonine kinase receptors (15). The most well defined signaling pathways of TGF-β are through Smad family members and the MAP kinase family (11, 16, 17). In mammals, there are three isoforms of TGF-β (TGF-β1, β2 and β3), which are structurally identical and have similar bioactivities. TGF-β may be released as a result of apoptotic cell interaction with inflammatory cells, such as macrophages, but also by structural cells such as airway epithelial cells, endothelial cells and fibroblasts.
During apoptosis, a number of changes occur on the plasma membrane that contribute to recognition of these dying cells by potential phagocytes. One of these is phosphatidylserine (PS), normally restricted to the inner membrane leaflet, but exposed on the outer leaflet as a consequence of loss of membrane phospholipid asymmetry during apoptosis (18, 19). There is considerable evidence to support a major role for recognition of this PS in the production of TGF-β and the anti-inflammatory effects of apoptotic cells (6, 8, 9, 20–22). Thus, in our previous studies, we have demonstrated that interaction of phagocytes with apoptotic cells through PS results in production of the active TGF-β both in vitro and in vivo (6, 8, 9). Its anti-inflammatory effect and TGF-β-dependency has been shown in the inflamed lung instilled with apoptotic Jurkat cells (8) and anti-immunogenic effects likewise following PS administration during an adaptive immune response (22).
The objective of this study was to determine the signal pathways involved in the generation of TGF-β by apoptotic cell stimulation. Unfortunately, the receptor(s) that recognizes PS (PS recognition structures, PSRS) that is responsible for this effect is unknown. A number of recent papers have described candidate PS receptors (23–27) but only one of these (28) was studied for its potential induction of TGFβ. In addition, a number of bridge molecules that bind apoptotic cell PS and link this to various phagocyte receptors have been described (29–33). At this point it is not clear which of these are involved in TGFβ synthesis and release. Accordingly, the experiments herein used whole apoptotic cells as stimulus. In addition, to avoid the complexity of assay systems that may be confounded by the presence of both apoptotic as well as responder cells we have included studies with an IgM monoclonal antibody (mAb217) created earlier by immunizing with macrophages that were active in PS-recognition (34). This antibody was used originally to identify a candidate PS receptor that was originally termed PSR (34) but is now known not to serve this function (35, 36) but rather to belong to the Jumonji family of proteins (JMJD6) and to act as a histone demethylase (37)). We now interpret this original identification as due to non-specific binding of the antibody in the phage display. Nonetheless, this Mab217 antibody binds to, and activates macrophages and other potential phagocytes, mimicking exactly the effects of PS exposed on apoptotic cells in contributing to uptake, and the generation of anti-inflammatory mediators including TGF-β (7, 22, 38). Accordingly, the studies reported here have used intact apoptotic cells and mAb217 to stimulate macrophages and fibroblasts for TGF-β production. Including the antibody stimulation allows us to bypass any confusion that may result from contribution of signaling molecules from the apoptotic cells themselves as well as to provide a coordinated stimulus where needed to avoid the issue of the time it takes individual apoptotic cells and responding cells to interact. TGFβ production may be regulated at many steps, including transcription, translation, secretion and especially activation in the extracellular environment. It is likely that apoptotic cell stimulation can alter each of these steps. However, in this study we have focused on only the first two. The results suggest regulation of both transcription and translation by pathways that differ substantially from those used by other stimuli of TGF-β synthesis.
Materials and methods
Antibodies and Reagents
TGF-β1 was from R&D Systems. Lipopolysaccharide (LPS, Escherichia coli 0111:B4) was from List Biological Lab. SB 203580, PD 98059, JNK inhibitor II, wortmannin, LY 294002, rapamycin, and Protease Inhibitor Cocktail Set I were from Calbiochem-Novabiochem. Actinomycin D and cycloheximide were from Sigma Chemical Co. Gene Specific Relative RT-PCR kit was from Ambion Inc. Advantage RT-for-PCR kit was from BD Biosciences. Lipofectamine Plus reagent was from GibcoBRL. Rho Activation Assay kit and recombinant C3 transferase were from Cytoskeleton. Phospho-ERK (E-4), ERK-2 (K 23), p38 (c-20), TGF-β (V)-G, phospho-eIF4E and total eIF4E antibodies were from Santa Cruz Biotech. Anti-p38 MAPK phospho-specific antibody, Phospho-SAPK/JNK (Thr183/Tyr185), phospho-Akt, β-actin, phospho-mTOR and mTOR antibodies were from Cell Signaling. Generation of monoclonal IgM antibody 217 and its IgM control was described by Fadok et al (34). Induction of apoptotic Jurkat cells and characterization of apoptotic and control cells were described previously (7).
Stable transfection of truncated TGF-β receptor II
Stable cell lines of 3T3TβRII, 3T3V, RAWTβRII and RAWV cells were made by transfecting truncated TGF-β receptor II or empty vector. Briefly, pcDNA3.1 plasmids with or without MYC-tagged truncated TGF-β receptor II sequence were transfected into 3T3-L1 and RAW 264 cells using Lipofectamine Plus reagent according to the manufacturer's instructions. Seventy-two hours after transfection, the cells were incubated in the fresh medium containing 500 µg/ml G418 for 4 weeks. Cell colonies resistant to G418 were isolated and screened by limited dilution.
Cell Culture and ELISA Assay
The cells (5.0 × 105 cells/well) were plated in each well of a 24-well tissue culture plate and incubated overnight in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, L-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 µg/ml) under a humidified 10% (3T3 cells) or 5% (RAW cells) CO2 atmosphere at 37°C before stimulation for 18 h in the serum free DMEM. Total TGF-β in cell culture supernatant was quantitated by enzyme-linked immunosorbent assay (ELISA) according to the instructions of the manufacturer (ELISA Tech, Denver, Colorado).
Transient Cell Transfection and Reporter Gene Assays
TGF-β-responsive luciferase reporter pSBE-Luc (SBE, Smad Binding Elements) was transfected into 3T3TβRII and 3T3V cells using Lipofectamine Plus reagent according to the manufacturer's instructions. pSV-β-galactosidase vector (Promega) was co-transfected as internal control to measure differences in transfection efficiency. Luciferase and β-galactosidase activities were measured 18 h after TGF-β stimulation using Luciferase Assay System (Promega) and Galacto-Light (Tropix), respectively. Dominant negative RhoA N19 and constitutively active RhoA V14 (kindly provided by Dr. Jiancheng Hu) and empty vector were transiently transfected into 3T3TβRII cells using Lipofectamine Plus reagent. After 48 h, the cells were stimulated with mAb 217 for 30 min, and total cell lysates were analyzed using immunoblotting for phospho-eIF4E and phospho-Akt.
Relative Quantitative Reverse Transcription Polymerase Chain Reaction of TGF-β Messenger RNA
Total RNA was isolated from cultured cells using TRIzol reagent (GIBCO BRL). The concentration and purity of RNA were evaluated by spectrophotometry at 260 and 280 nm. Reverse transcription (RT) was carried out for 60 min at 42°C with 1 µg total RNA using Advantage RT-for-PCR kit (BD Biosciences). TGF-β mRNA level was determined using Relative Quantitative RT-PCR kit (Ambion Inc.). The cDNA was denatured for 5 min at 94°C, and the amplification was achieved in a temperature cycler (Perkin Elmer GeneAmp PCR System 2400) by 24 cycles of temperature (94°C for 30 s, 57°C for 30 s, and 72°C for 30 s) followed by a 7-min final extension at 72°C. A total of 5 µl of each PCR sample was loaded on a 1.5% agarose gel stained with ethidium bromide. The relative fluorescence of TGF-β vs. 18 S rRNA was analyzed by densitometry.
RhoA Activation Assay
3T3TβRII (5 × 106 cells) cells were plated in 10-cm tissue culture dishes. Clarified cell lysates from 12 h serum-starved cells were used for Rho activity assay according to the manufacturer’s instructions (Cytoskeleton). To confirm equal loading of proteins from each sample, 40 µl of total cell lysate from each sample was blotted with β-actin antibody before binding to GST tagged Rhotekin RBD protein.
Immunoblotting Analysis
Immunoblotting analysis was carried out as described previously with some modification (39). Briefly, cells (3.0 × 105 cells/well) were plated in each well of a 12-well tissue culture plate and incubated in serum free medium overnight before use. Following stimulation, the cells were lysed in lysis buffer (20 mM HEPES (pH7.4), 150 mM NaCl, 1 mM DTT, 0.5% Triton X-100 and 1 X Protease Inhibitor Cocktail Set I), resolved on 10% SDS-PAGE, and blotted to nitrocellulose membranes. The membranes were probed with primary antibodies at 4°C overnight and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech) according to the manufacturer's instructions. Equal loading of proteins in each lane was confirmed by Ponceau S staining or re-probed with corresponding antibodies against the native proteins or β-actin antibody (17).
Statistical analysis
All data are presented as means ± SEM from three or more separate experiments. The means were analyzed using ANOVA for multiple comparisons. When ANOVA indicated significance, the Tukey-Kramer honestly significant difference test for all pairs was used to compare groups. All data were analyzed using JMP (version 5; SAS Institute, Inc., Cary, NC) Statistical Software for the Macintosh.
Results
Effect of apoptotic cells or mAb 217 on TGF-β production
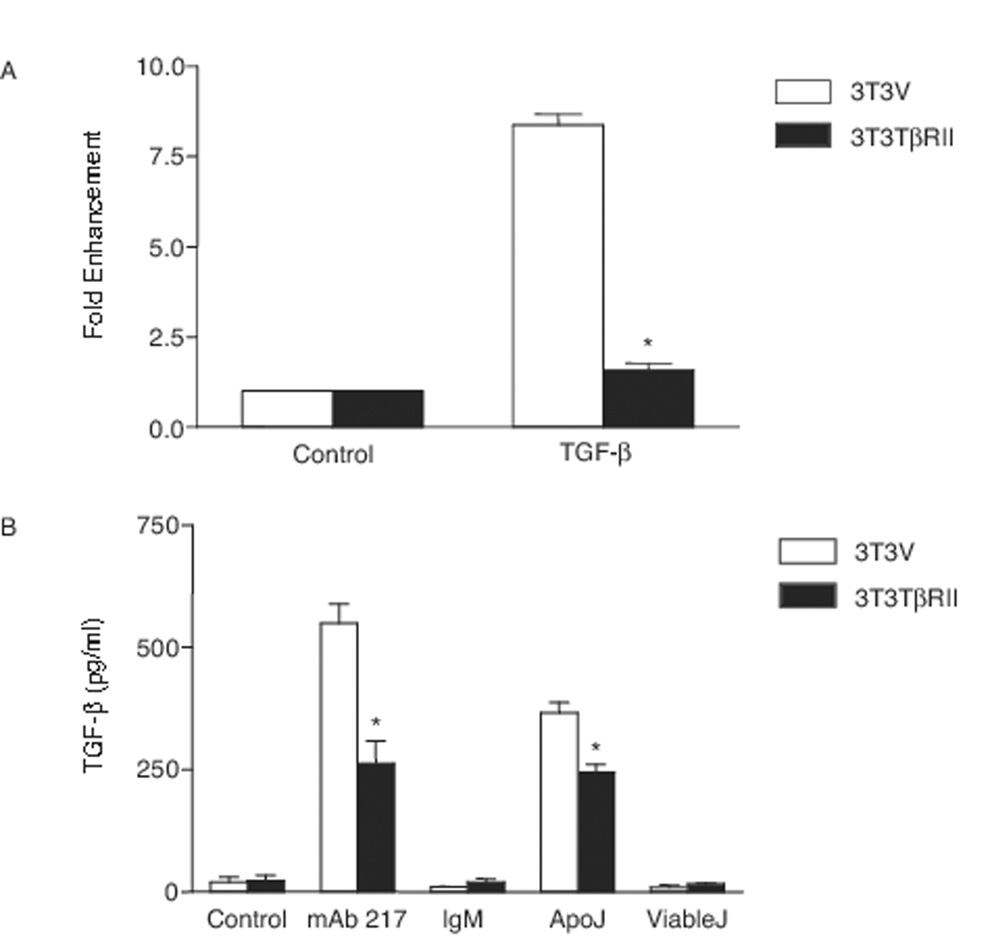
To pursue the mechanisms by which apoptotic cells or mAb 217 induced TGF-β production in a manner that avoided auto-stimulation by TGF-β itself, we made stable cell lines that were unresponsive to TGF-β by transfecting truncated TGF-β receptor II (or empty vector as control) constructs into 3T3-L1 cells and RAW 264 cells (9) to block the paracrine and autocrine effects from TGF-β (40, 41). Blockade of TGF-β signaling was verified using TGF-β-responsive luciferase reporter SBE-luc (SBE, Smad Binding Elements) assay. As shown in Figure 1A, 3T3TβRII cells with truncated TGF-β receptor II did not respond to TGF-β stimulation. Moreover, incubation of these cells with apoptotic Jurkat cells or mAb 217 resulted in reduced amounts of TGF-β in the conditioned medium (Fig. 1B) relative to similarly-treated 3T3V cells, supporting the supposition that in the normal circumstance, the TGF-β that was induced by apoptotic cells could indeed provide positive autostimulation feedback with enhanced overall production of the mediator.
FIGURE 1. Apoptotic cells or mAb 217 induces TGF-β production in 3T3-L1 cells.
A, 3T3-L1 cells stably transfected with truncated TGF-β receptor II (3T3TβRII) or empty vector (3T3V) were transiently co-transfected with SBE-luc and pSV-β galactosidase constructs. After 48 h, the cells were incubated in the presence of TGF-β (10 ng/ml) for 18 h. The luciferase assays, which were normalized to β-galactosidase, are expressed as fold enhancement. *, significantly different from 3T3V cells stimulated with TGF-β. B, 3T3V and 3T3TβRII cells were cultured in the presence of mAb 217 (100 µg), isotype IgM control (100 µg), apoptotic Jurkat cells (ApoJ) or viable Jurkat cells (ViableJ) for 18 h. Total TGF-β in the conditioned medium was analyzed by ELISA. *, significantly different from 3T3V cells.
Effect of apoptotic cells or mAb 217 on TGF-β mRNA expression
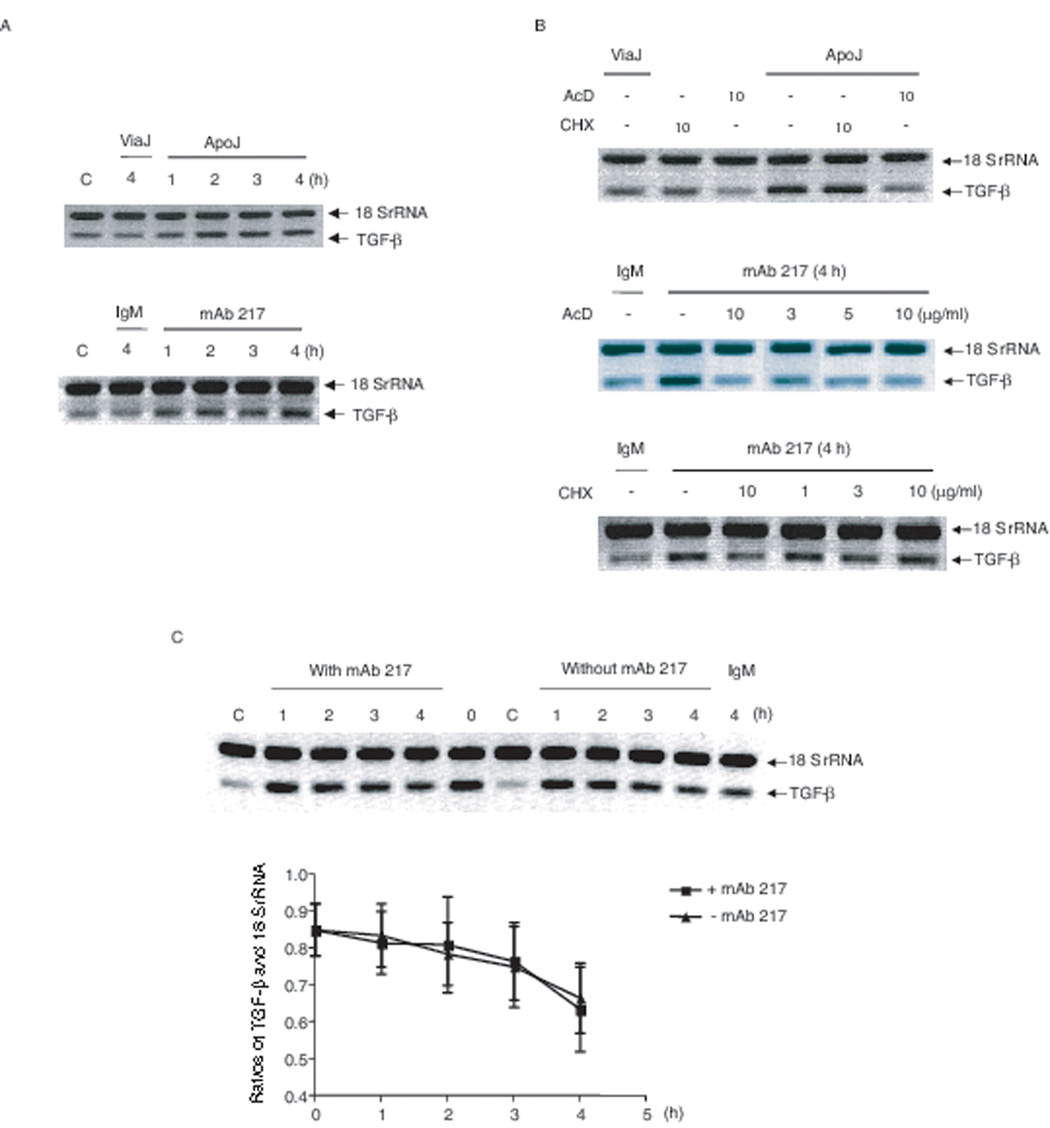
As previously described and as shown in Figure 1, interaction of non-professional, or professional phagocytes with apoptotic cells (or stimulation with mAb 217) induces the production of TGF-β (6, 9, 34). In the present study, apoptotic cells or mAb 217 were each shown to induce TGF-β mRNA expression; detectable at 1 h and reaching a plateau from 2 h in 3T3TβRII cells (Fig. 2A). As expected, TGF-β mRNA expression was significantly inhibited by actinomycin D; however, not by the protein synthesis inhibitor, cycloheximide suggesting that new protein synthesis was not required for the induction of TGF-β transcription (Fig. 2B). To rule out the possibility that the increase in TGF-β mRNA was caused by enhancement of TGF-β message stability, 3T3TβRII cells were first treated with PMA (100 nM) overnight to increase the steady state TGF-β mRNA level, and then washed and treated with actinomycin D (10 µg/ml) in the absence or presence of mAb 217. The remaining TGF-β mRNA level after actinomycin D treatment was measured using Relative Quantitative RT-PCR. As shown in Figure 2C, mAb 217 did not affect TGF-β mRNA stability. These findings suggest that the upregulation is at the level of transcription.
FIGURE 2. Effect of apoptotic Jurkat cells or mAb 217 on TGF-β mRNA levels.
A, 3T3TβRII cells were cultured in the presence of viable Jurkat cells, apoptotic cells, isotype IgM (100 µg/ml) or mAb 217 (100 µg/ml) for the time indicated; B, 3T3TβRII cells were pre-treated with the indicated concentrations of actinomycin D (ActD) or cycloheximide (CHX) for 1 h, before stimulation for 4 h; C, 3T3TβRII cells were cultured in the presence of PMA (100 nM) overnight to increase the steady state TGF-β mRNA level, and then the cells were incubated with actinomycin D (10 µg/ml) in the presence or absence of mAb 217 (100 µg/ml) for the time indicated. TGF-β mRNA levels were analyzed by Relative Quantitative RT-PCR.
Requirement for MAP kinases in apoptotic cell-induced transcriptional upregulation of TGF-β production
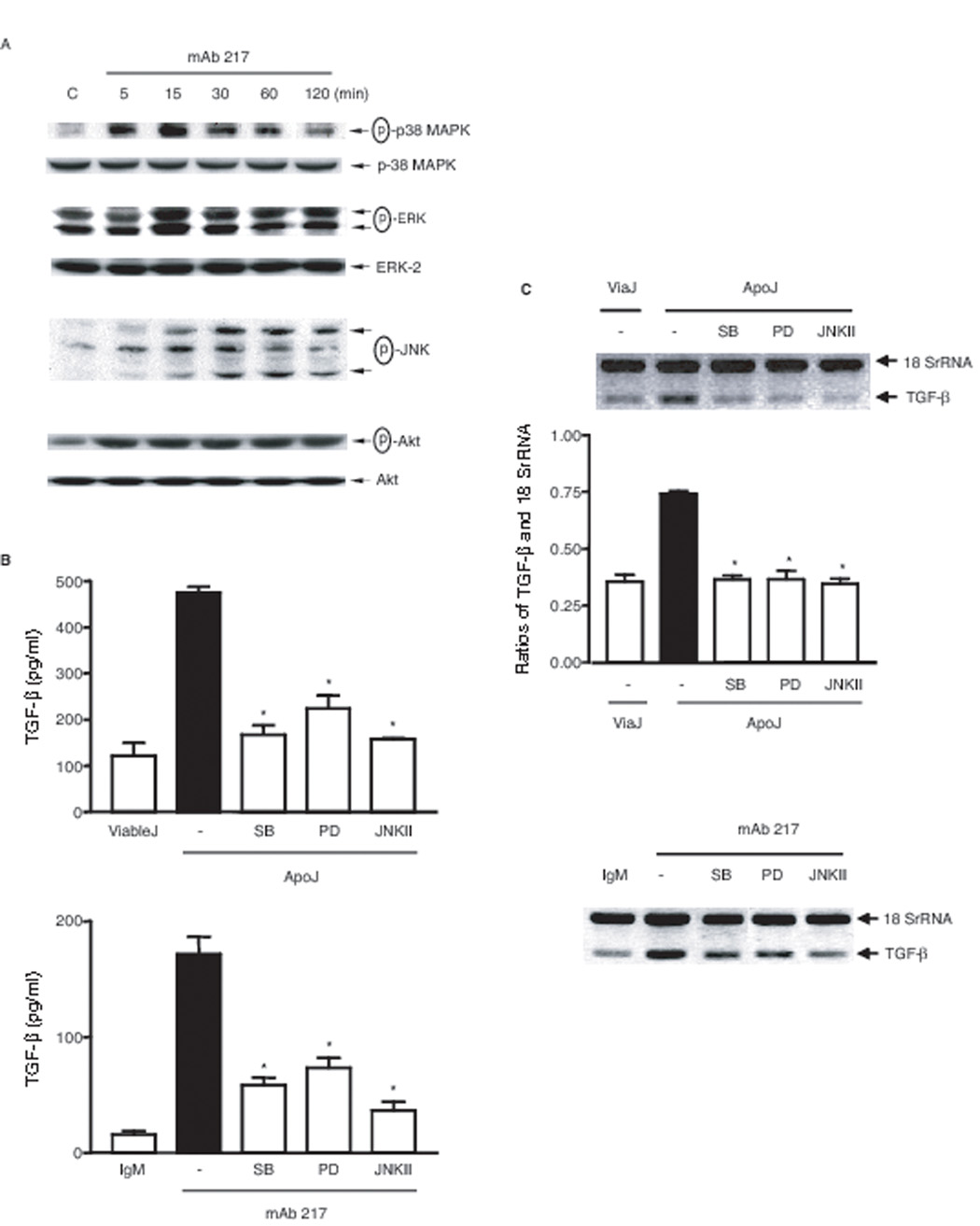
When 3T3TβRII cells were stimulated with apoptotic Jurkat cells (data not shown) or 217 mAb, phosphorylation of p38 MAPK, ERK and JNK were shown to be increased with time, reaching a maximum at 15 min for p38 MAPK and ERK, and 30 min for JNK (Fig. 3A). To examine involvement of these MAP kinases in the TGF-β production, 3T3TβRII cells were pretreated with SB 203580, a specific p38 MAPK inhibitor, PD 98059, a specific MEK-1 inhibitor, and JNK inhibitor II for one hour, and then incubated with apoptotic Jurkat cells or mAb 217 for 18 hours. As shown in Figure 3B and 3C both TGF-β protein and enhanced mRNA production was reduced by all three inhibitors at concentrations shown to be effective in previous work and without toxicity (17, 42). These findings suggest that MAPKs might play important roles in apoptotic cell-induced TGF-β transcription.
FIGURE 3. Roles of MAP kinases in apoptotic cell or mAb 217-induced TGF-β production.
A, 3T3TβRII cells were stimulated with mAb 217 (100 µg/ml) for the time indicated, total cell lysates were immunoblotted for phospho-p38 MAPK, phospho-ERK and phospho-JNK. B, 3T3TβRII cells were pre-treated with SB 203580 (10 µM), PD 98059 (30 βM) or JNK inhibitor II (30 βM) for 1 h and then stimulated with apoptotic Jurkat cells (upper panel) or mAb 217 (lower panel) for 18 h. Total TGF-β in the conditioned medium was measured by ELISA. *, significantly different from apoJ or mAb 217 alone. C, 3T3TβRII cells were pre-treated with SB 203580 (10 µM), PD 98059 (30 µM) or JNK inhibitor II (30 µM) for 1 h and then stimulated with apoptotic cells (upper panel) or mAb 217 (lower panel) for 4 h. TGF-β mRNA level was analyzed by Relative Quantitative RT-PCR. Ratios of TGF-β and 18 SrRNA were measured using densitometric analysis. *, significantly different from apoJ alone.
Involvement of RhoA activation in apoptotic cell-induced TGF-β translation
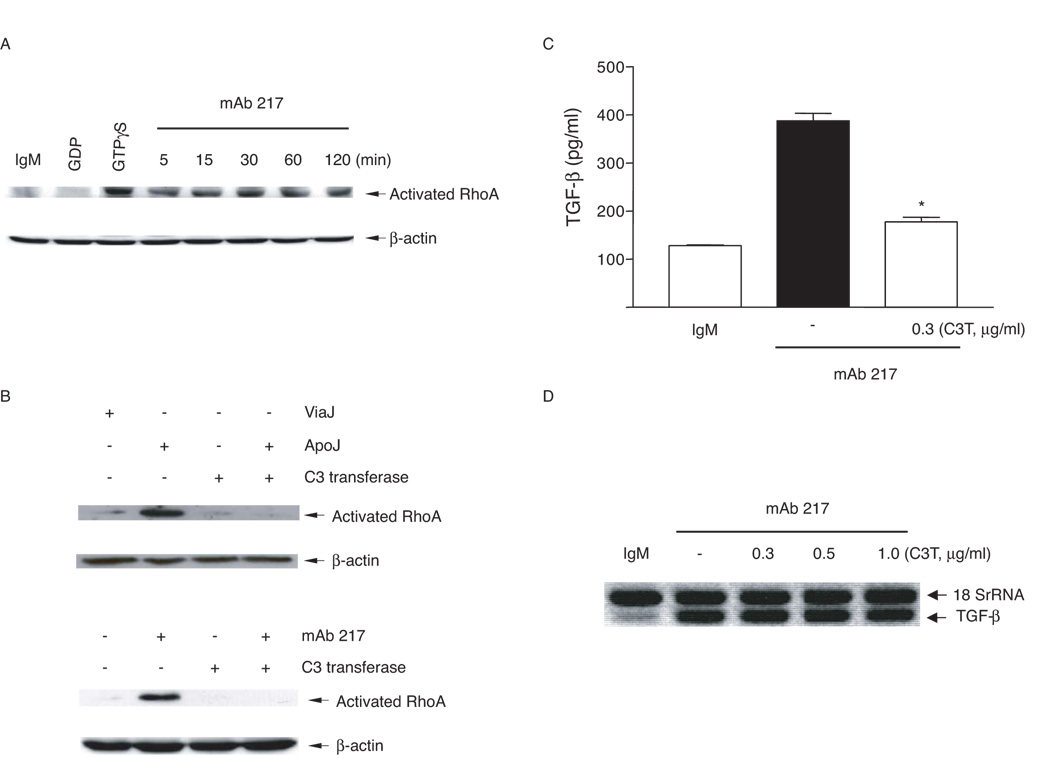
Small GTP-binding proteins of the Rho family have been found to play an important role in efferocytosis of apoptotic cells (4, 43–46). Shown in Fig 4A and B, is the activation of RhoA in 3T3TβRII cells after exposure to apoptotic Jurkat cells or mAb 217. There was no change in the total levels of Rho in the cells over the time course of the experiments (data not shown). The Rho activation was completely inhibited by C3 transferase (Fig. 4B). Consistent with a role for Rho in synthesis of TGF-β, C3 transferase suppressed its production (Fig. 4C). However, blockade of Rho activation did not affect TGF-β mRNA expression (Fig. 4D), suggesting that Rho acts at a post-transcriptional step.
FIGURE 4. Requirement for RhoA in apoptotic cell or mAb 217-induced TGF-β production.
A, 3T3TβRII cells were stimulated with mAb 217 (100 µg/ml) for the time indicated and the levels of activated Rho determined (see methods); B, 3T3TβRII cells were pre-incubated with C3 transferase (0.3 µg/ml) for 48 h and then stimulated with apoptotic Jurkat cells or mAb 217 for 30 min. C, 3T3TβRII cells were pre-incubated with C3 transferase (0.3 µg/ml) for 48 h and then stimulated with mAb 217 for 18 h, TGF-β in the conditioned medium was measured by ELISA. *, significantly different from mAb 217 alone. D, 3T3TβRII cells were pre-incubated with the indicated concentrations of C3 transferase for 48 h and then stimulated with mAb 217 for 4 h, TGF-β mRNA levels were analyzed by Relative Quantitative RT-PCR.
Effect of apoptotic cells on TGF-β translation through RhoA/PI-3K/Akt/mTOR/eIF4E
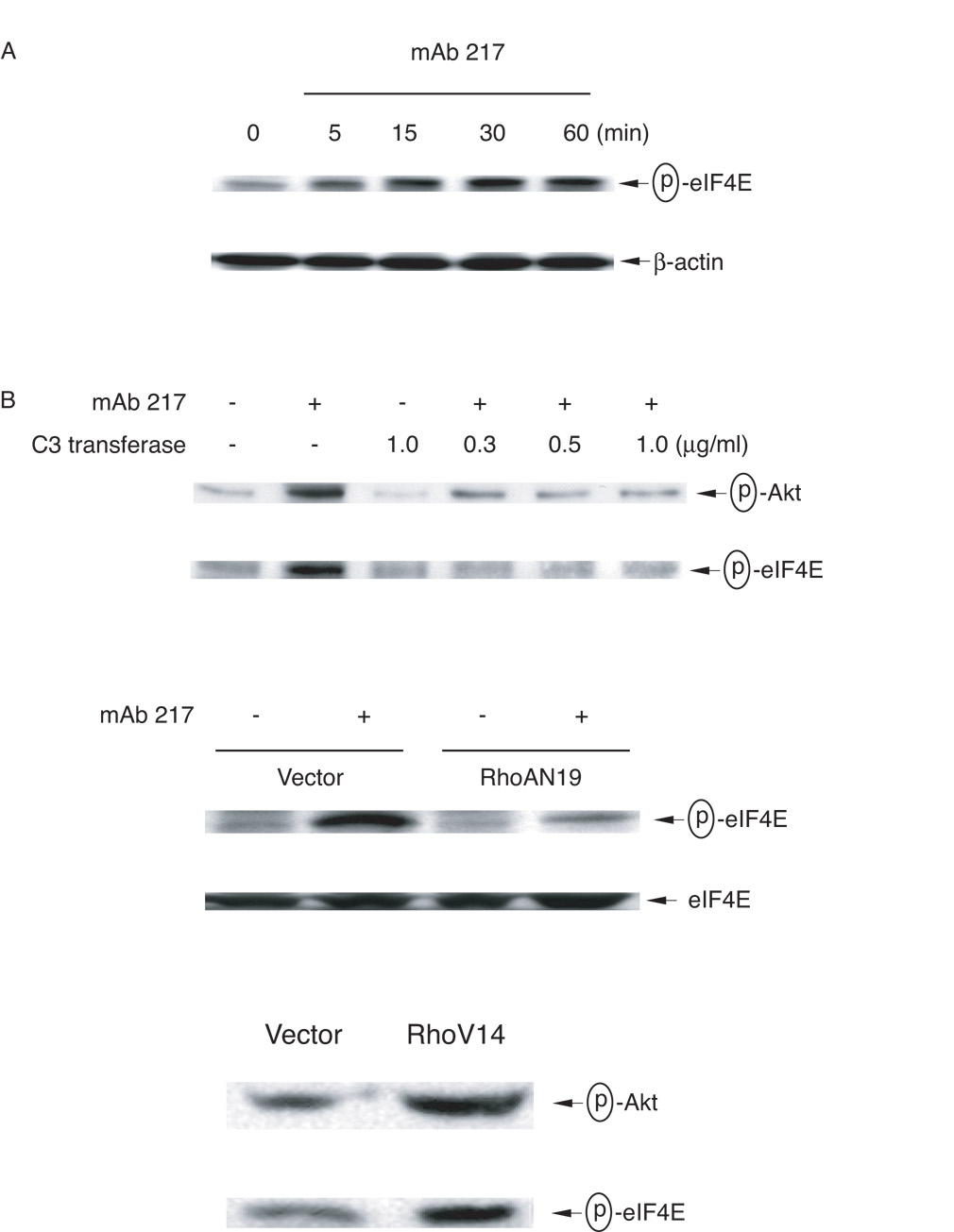
To examine the mechanisms by which translational regulation of TGF-β occurred, 3T3TβRII cells were stimulated with mAb 217, and phosphorylation of translation initiator factor eIF4E was determined. As shown in Figure 5A, phosphorylated eIF4E became detectable at 5 min and reached maximum at 30 min after stimulation. Importantly, the phosphorylation was inhibited by C3 transferase, and this was further confirmed by overexpression of the dominant negative RhoAN19. Moreover, overexpression of the constitutively active RhoAV14 increased phosphorylation of eIF4E, supporting a requirement of RhoA for TGF-β protein translation (Fig. 5B).
FIGURE 5. Requirement for Rho activation in mAb 217-induced Akt and eIF4E phosphorylation.
A, 3T3TβRII cells were stimulated with mAb 217 (100 µg/ml) for the time indicated and total cell lysates immunoblotted for phospho-eIF4E. B, 3T3TβRII cells were pre-incubated with the indicated concentrations of C3 transferase for 48 h and then stimulated with mAb 217 for 30 min (upper panel). 3T3TβRII cells were transiently transfected with empty vector, dominant negative RhoA N19 or constitutively active RhoA V14. After 48 h, the cells were incubated in the presence or absence of mAb 217 for 30 min (lower panel). Total cell lysates were immunoblotted for phospho-Akt or phospho-eIF4E.
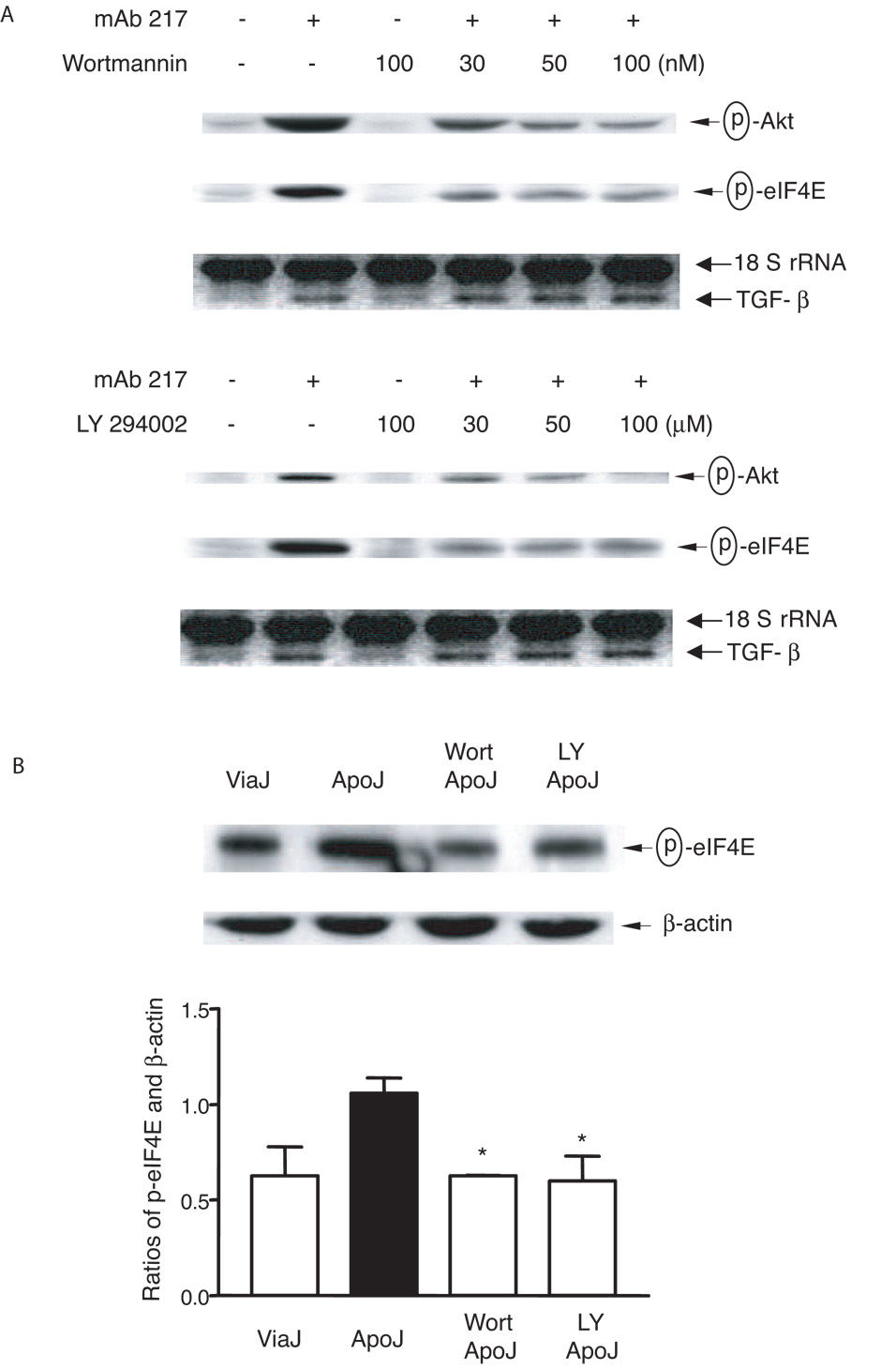
It has been reported that PI-3K, Akt and mTOR can act upstream of eIF4E (reviewed in Ref. (47)). In addition to activation of MAPKs, apoptotic cell or mAb 217 each stimulate phosphorylation of Akt (time course not shown) and, as depicted in Fig 5B, this was inhibited by C3 transferase. Accordingly, when 3T3TβRII cells were treated with the PI-3 Kinase inhibitors wortmannin or LY 294002 for one hour before stimulation, phosphorylation of Akt and eIF4E were both inhibited (Fig. 6A and B). Moreover, constitutively active RhoAV14 increased phosphorylation of Akt and eIF4E (Fig. 5B lower panel).
FIGURE 6. PI3 kinase and Akt activation are downstream of RhoA.
A, 3T3TβRII cells were pre-treated with the indicated concentrations of wortmannin (upper panel) or LY 294002 (lower panel) for 1 h and then stimulated with mAb 217 (100 µg/ml) for 30 min (immunoblotting) or 4 h (TGF-β mRNA). Phospho-Akt and phospho-eIF4E were examined by immunoblotting, and TGF-β mRNA levels were analyzed using Relative Quantitative RT-PCR. B, 3T3TβRII cells were pre-treated with wortmannin (Wort, 100 nM) or LY 294002 (LY, 100 µM) for 1 h and then stimulated with viable or apoptotic Jurkat cells for 30 min. Total cell lysates were immunoblotted for phospho-eIF4E. Ratios of p-eIF4E and β-actin were measured densitometrically. *, significantly different from apoJ alone.
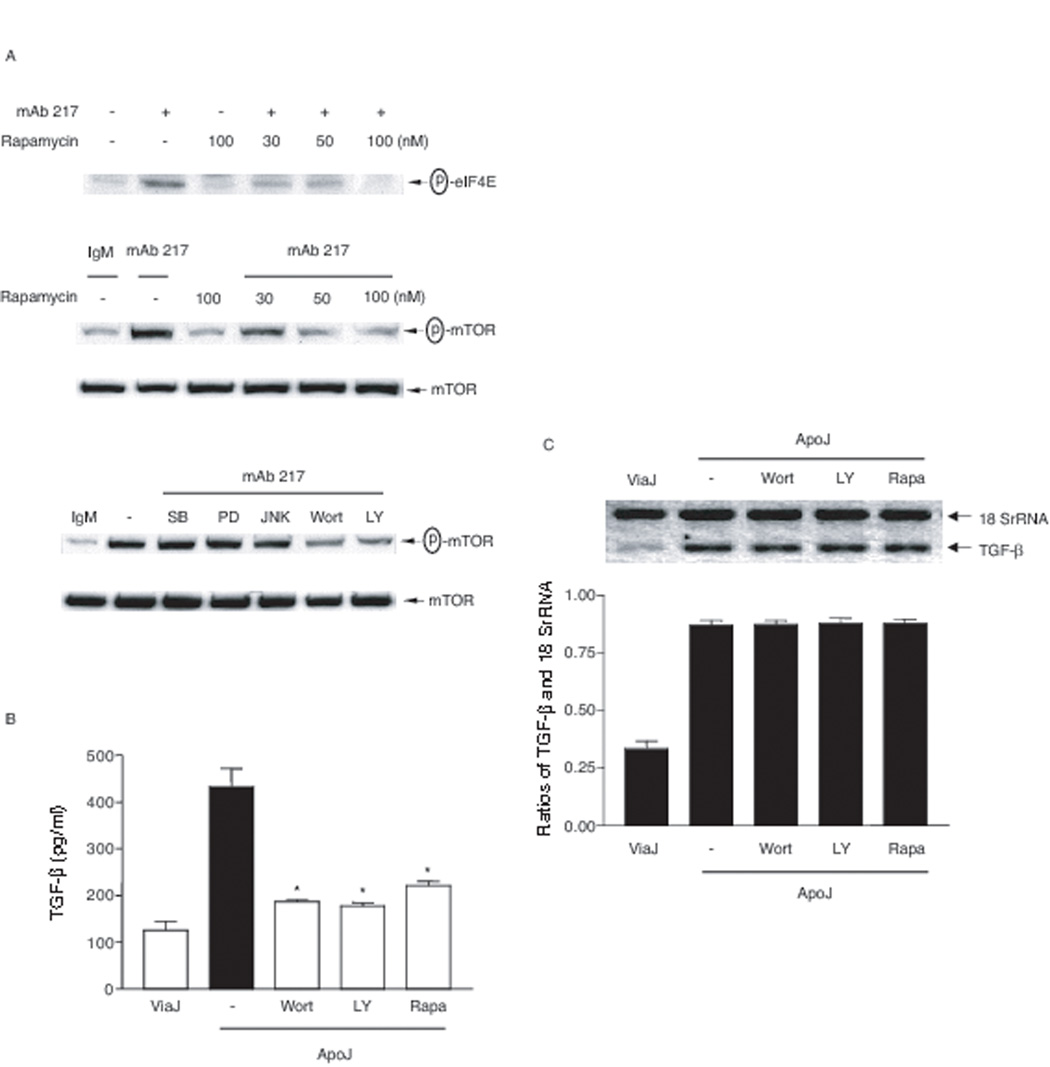
It has been shown that mTOR is an important mediator downstream of PI-3K/Akt for eIF4E phosphorylation (47, 48). Consistent with these findings, rapamycin inhibited mAb 217-induced mTOR phosphorylation as expected but also blocked phosphorylation of eIF4E (Fig. 7A). By contrast, mTOR phosphorylation was not altered by the MAP kinase inhibitors SB 203580, PD 98059 or JNK inhibitor II (Fig. 7A). A role for the PI-3K/Akt/mTOR pathway in TGFβ translation was supported by finding that the PI-3K and mTOR inhibitors were able to block the production of TGF-β protein but had no effect on levels of mRNA (Figs 7B and C). Collectively, these findings suggest that apoptotic cells regulate TGF-β translation through activation of RhoA/PI-3K/Akt/mTOR/eIF4E.
FIGURE 7. RhoA/PI-3K/mTOR pathways are involved in TGF-β protein production.
A, 3T3TβRII cells were pre-treated with indicated concentrations of rapamycin overnight, SB 203580 (SB, 10 µM), PD 98059 (PD, 50 µM), JNK inhibitor II (JNKII, 50 µM), wortmannin (Wort, 100 nM) or LY 294002 (LY, 100 µM) for 1 h and then stimulated with mAb 217 (100 µg/ml) for 30 min. Total cell lysates were immunoblotted for phospho-mTOR and phospho-eIF4E. B, 3T3TβRII cells were pre-treated with rapamycin (100 nM) overnight, wortmannin (100 nM) or LY 294002 (50 µM) for 1 h and then stimulated with apoptotic Jurkat cells for 18 h. Total TGF-β in the conditioned medium was analyzed by ELISA. *, significantly different from apoJ alone. C, 3T3TβRII cells were pre-treated with rapamycin (Rapa, 100 nM) overnight, wortmannin (100 nM) or LY 294002 (100 µM) for 1 h and then stimulated with apoptotic Jurkat cells for 4 h. TGF-β mRNA levels were analyzed using Relative Quantitative RT-PCR. Ratios of TGF-β and 18 SrRNA were measured densitometrically.
Apoptotic cells or mAb 217 regulate TGF-β production via unique signal pathways
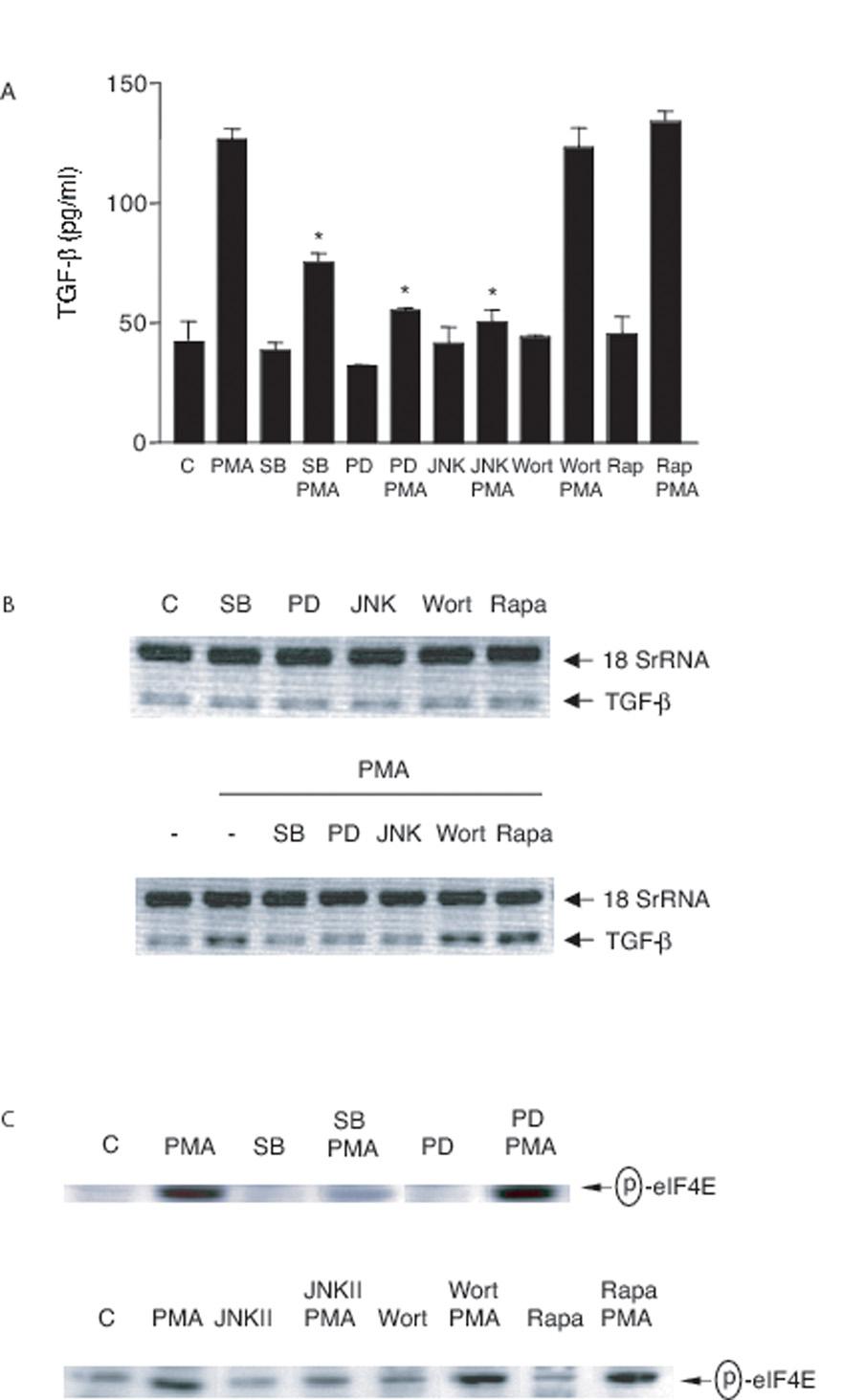
PMA and LPS are both able to stimulate TGF-β production (7, 49, 50). As shown in Fig 8A, PMA was found to induce increased levels of TGF-β protein in our system in a fashion that was inhibited by blockade of the MAP kinases but not by wortmannin or rapamycin. As expected, the MAP kinase inhibitors also prevented the upregulation of TGF-β mRNA in response to PMA (Fig 8B) but in this case, no effect of wortmannin or rapamycin was seen. PMA has been reported to regulate protein translation through p38 MAPK-mediated eIF4E phosphorylation (51–53) and as shown in Fig 8C, both SB 203580 and JNK inhibitor II did block PMA-induced eIF4E phosphorylation. It appears, therefore, that p38 MAPK, ERK and JNK are involved in both TGF-β transcription and possibly translation induced by PMA but that the PI-3K and mTOR pathways are not required for this stimulus.
FIGURE 8. Effect of inhibitors on PMA-induced TGF-β production.
A, 3T3TβRII cells were pre-treated with rapamycin (Rapa, 100 nM) overnight, SB 203580 (SB, 10 µM), PD 98059 (PD, 30 µM), JNK inhibitor II (JNKII, 30 µM) or wortmannin (Wort, 100 nM) for 1 h and then stimulated with PMA (100 nM) for 18 h. Total TGF-β in the conditioned medium was analyzed by ELISA. *, significantly different from PMA alone. B, 3T3TβRII cells were pre-treated with rapamycin (Rapa, 100 nM) overnight, SB 203580 (SB, 10 µM), PD 98059 (PD, 50 µM), JNK inhibitor II (JNKII, 50 µM) or wortmannin (Wort, 100 nM) for 1 h and then stimulated with PMA (100 nM) for 4 h. TGF-β mRNA levels were analyzed using Relative Quantitative RT-PCR. C, 3T3TβRII cells were pre-treated with rapamycin (Rapa, 100 nM) overnight, SB 203580 (SB, 10 µM), PD 98059 (PD, 50 µM), JNK inhibitor II (JNKII, 50 µM) or wortmannin (Wort, 100 nM) for 1 h and then stimulated with PMA (100 nM) for 30 min. Total cell lysates were immunoblotted for phospho-eIF4E.
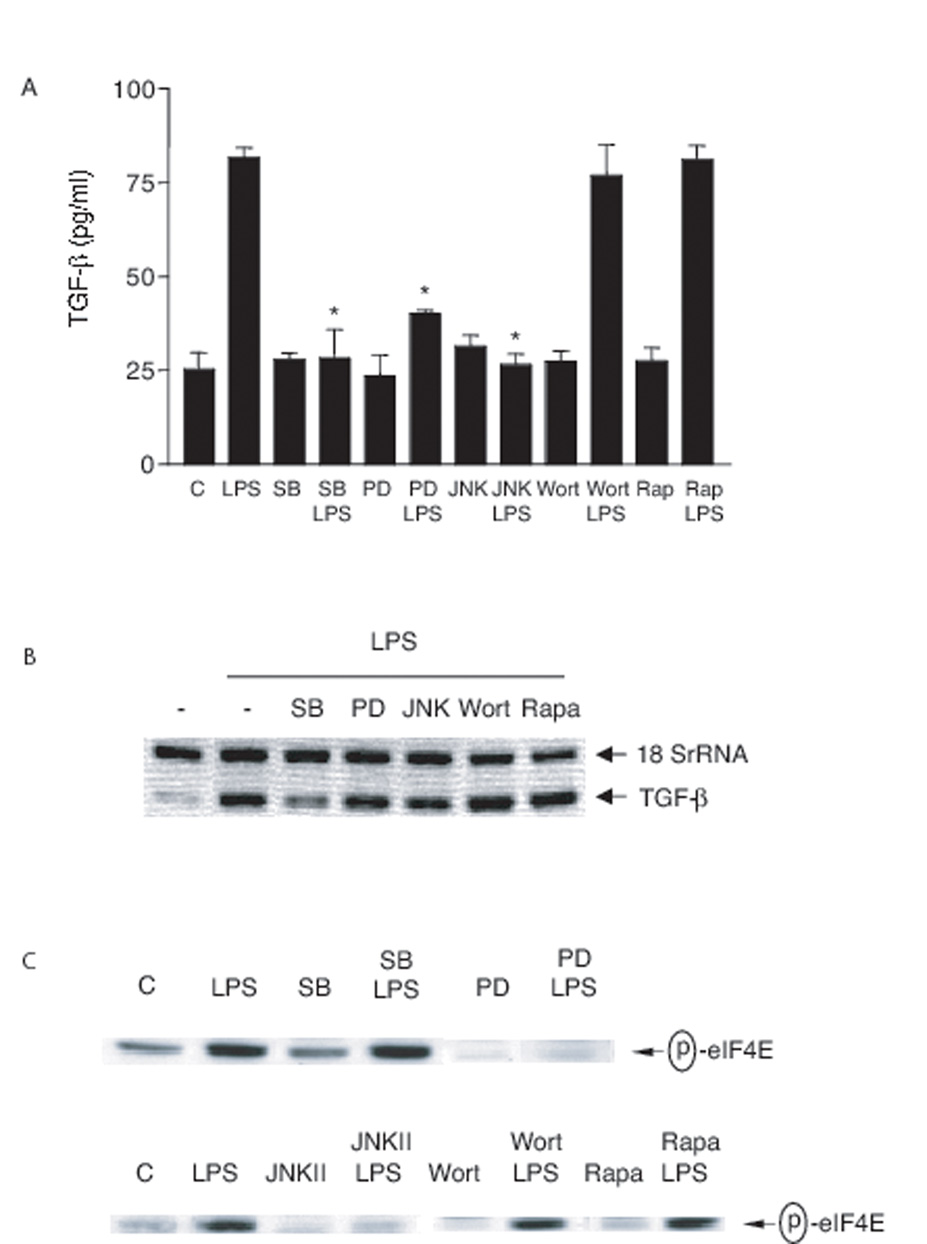
Similar to PMA stimulation, LPS-induced TGF-β protein production was inhibited by SB 203580, PD 98059, JNK inhibitor II but not by wortmannin or rapamycin (Fig. 9A). However, LPS-induced TGF-β mRNA expression was only substantially blocked by the p38 MAPK inhibitor, SB 203580 (Fig. 9B). Surprisingly, LPS-induced eIF4E phosphorylation was inhibited by PD 98059 (MEK inhibitor) and JNK inhibitor II but not by SB 203580, wortmannin or rapamycin (Fig 9C). These findings suggest a) TGF-β production in response to different stimuli is differently regulated (Table 1); b) apoptotic cells or mAb 217 induce TGF-β production through unique signaling pathways, by which p38 MAPK, ERK and JNK are involved in transcription, and RhoA/PI-3K/Akt/mTOR/eIF4E are involved in translation (Fig. 10).
FIGURE 9. Effect of inhibitors on LPS-induced TGF-β production.
A, RAWTβRII cells were pre-treated with rapamycin (Rapa, 100 nM) overnight, SB 203580 (SB, 10 µM), PD 98059 (PD, 30 µM), JNK inhibitor II (JNKII, 30 µM) or wortmannin (Wort, 100 nM) for 1 h and then stimulated with LPS (100 ng/ml) for 18 h. Total TGF-β in the conditioned medium was analyzed by ELISA. *, significantly different from LPS alone. B, RAWTβRII cells were pre-treated with rapamycin (Rapa, 100 nM) overnight, SB 203580 (SB, 10 µM), PD 98059 (PD, 50 µM), JNK inhibitor II (JNKII, 50 µM) or wortmannin (Wort, 100 nM) for 1 h and then stimulated with LPS (100 ng/ml) for 4 h. TGF-β mRNA levels were analyzed using Relative Quantitative RT-PCR. C, RAWTβRII cells were pre-treated with rapamycin (Rapa, 100 nM) overnight, SB 203580 (SB, 10 µM), PD 98059 (PD, 50 µM), JNK inhibitor II (JNKII, 50 µM) or wortmannin (Wort, 100 nM) for 1 h and then stimulated with LPS (100 ng/ml) for 30 min. Total cell lysates were immunoblotted for phospho-eIF4E.
Table 1.
Comparison of kinases and signal pathways involved in TGF-β production.
| p38 MAPK | ERK | JNK | Rho/PI3K/mTOR/eIF4E | |
|---|---|---|---|---|
| ApoJ or mAb 217 |
Transcription | Transcription | Transcription | Translation |
| PMA | Transcription Translation |
Transcription | Transcription Translation |
Not involved |
| LPS | Transcription | Translation | Translation | Not involved |
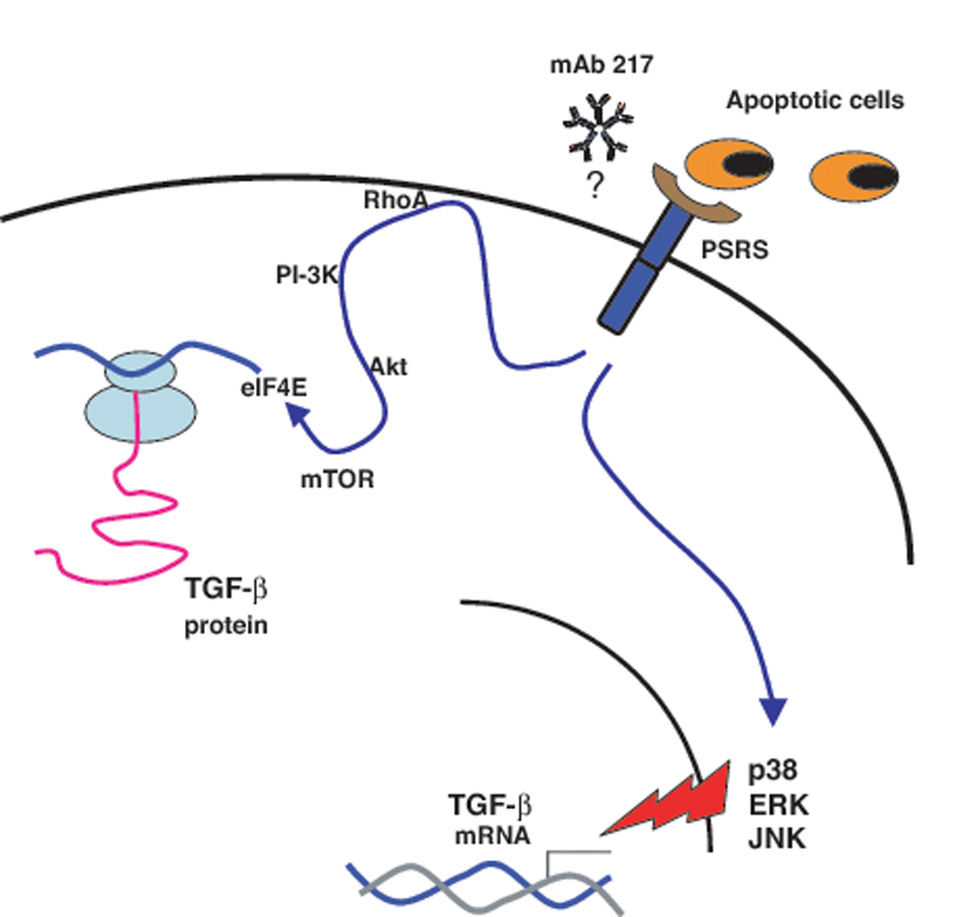
FIGURE 10. Summary of the mechanisms by which apoptotic cells or mAb217 induce TGF-β production.
Apoptotic cells or mAb217 up-regulate TGF-β mRNA expression through p38 MAPK, ERK and JNK, and enhance protein translation through and enhance protein translation through RhoA/PI3K/Akt/mTOR/eIF4E signaling pathway. PSRS – Phosphatidylserine recognition structures.
Discussion
The ability of apoptotic cells to signal for their quiet, non-inflammatory and non-immunogenic removal in vivo is critical for normal tissue homeostasis and for resolution of inflammation. Just as there is considerable redundancy in the recognition and removal receptors and pathways for apoptotic cells, it seems likely that the anti-inflammatory effects are also mediated by various separate mechanisms. One of these is the selective stimulation by apoptotic cells of the anti-inflammatory and anti-immunogenic mediator, TGFβ. Thus, in previous studies (8, 22) we have shown that the ability of PS-exposing apoptotic cells or PS liposomes to block ongoing pulmonary inflammation or adaptive immunity was significantly dependant on the production of active TGFβ.
To explore the signaling mechanisms by which apoptotic cells induce TGF-β production it was first necessary to rule out the autocrine and paracrine stimulating effects of TGF-β itself. Accordingly, we set up a cell culture system using cells stably transfected with the dominant negative, truncated TGF-β receptor II. These were shown not to respond to added TGF-β and, as expected, stimulation with apoptotic cells showed lower overall levels of TGF-β produced. This observation supports the presence of autocrine/paracrine effects of TGF-β in enhancing the amounts of the molecule normally generated by apoptotic cells. The monoclonal antibody 217, which was raised against PS recognizing macrophages, binds to, and activates cells in a fashion that mimics exactly the effects of apoptotic cells in terms of efferocytosis and the generation of anti-inflammatory mediators (7, 22, 38). Unfortunately, however, attempts by us and a number of other laboratories have so far been unsuccessful in clearly identifying the antigen(s) with which it interacts. It was used herein as an adjunct to stimulation with apoptotic cells to initiate TGF-β production without the complexities of adding whole cells into the system. In all cases the stimulating effects of mAb217 and intact apoptotic cells were qualitatively identical, although as expected, not always in quantity or exact time course.
We demonstrated that apoptotic cells or mAb 217 increased total cellular levels of TGF-β mRNA without affecting its stability. Furthermore, the upregulated TGF-β transcription was inhibited by SB 203580, PD 98059 and JNK inhibitor II suggesting the involvement of all three MAP kinases (p38 MAPK, ERK and JNK). Notably, each individual inhibitor almost completely suppressed protein or message expression. Presumably, TGF-β mRNA level is not proportionally related to TGF-β protein, and vice versa (54). TGF-β contains predicted AP-1, Egr-1, and SP-1 binding sites in its promoter regions (41, 55–58). The AP-1-type proteins mediating this induction may act at one or more AP-1 sites in the two promoters of TGF-β gene and three putative, overlapping AP-1 binding sites about 200 base pairs downstream of TGF-β (52, 59, 60). Consistent with these, we have observed that blockade of mAb 217-induced ERK and JNK phosphorylation inhibited phospho-c-jun, although, surprisingly, SB 203580 increased mAb 217-induced phospho-c-jun (data not shown). The role of p38 MAPK in regulation of gene expression was not well understood, although transcriptional factors such as activating transcription factor-2, SRF accessory protein-1 and CCAAT-enhancer binding protein-β are known as substrates of p38 MAPK (61). It is important to note that Otsuka et al (62, 63) have earlier reported that PS-liposomes induce TGFβ production with a requirement for ERK and PI3Kinase. However, they did not address possible selective effects on transcription and translation.
The Rho family of small GTPases, including Rho, Rac and Cdc42, are involved in regulation of a variety of cellular functions such as cell migration and adhesion, proliferation, vesicle trafficking, bacterial ingestion and inflammation (64–67). Interaction of apoptotic cells initiates a delayed activation of Rho in responding cells and this is known to negatively regulate subsequent efferocytosis ((4, 46, 68, 69) and Gardai et al unpublished). Accordingly we then investigated the possible involvement of Rho in apoptotic cell-induced TGF-β production. Inactivation of RhoA by C3 transferase inhibited apoptotic cell or mAb 217-induced TGF-β protein production, but did not affected TGF-β mRNA expression. These findings suggested that posttranscriptional regulation of TGF-β generation is another important control point in regulating the activity of this anti-inflammatory mediator. Further exploration of the downstream effectors leading to TGF-β translation revealed that Rho inhibition resulted in lower levels of Akt and eIF4E phosphorylation.
The mammalian target of rapamycin (mTOR) is a central regulator of translation and cell proliferation (70–72). Two major substrates for mTOR are the serine/threonine kinase p70S6K and the 4E-binding protein 4EBP-1. Phosphorylation of 4EBP-1 by mTOR results in release of the cap-binding protein translation initiation factor, eukaryotic initiation factor (eIF4E) (47, 73), which is inactive when bound to hypo-phosphorylated 4EBP-1. Moreover, eIF4E activity is also regulated by phosphorylation and enhances translation rates of cap-containing mRNAs (74), which include TGF-β (54, 72, 75–77). The upstream regulator of mTOR in this circumstance appears to be PI3-kinase/Akt (48, 72). PI3-kinase has been reported to be upstream of Rac and Rho (78, 79). On the other hand, RhoA also has been shown to prevent myoblast death by inducing the PI3-K/Akt pathway (80). In the present study, we suggest that PI3-kinase is a downstream signal mediator from Rho, which then leads to apoptotic cell-induced TGF-β translation through Akt/mTOR/eIF4E. TGF-β itself is known to activate Rho (81–84), however, this was avoided by use of the 3T3TβRII cells or RAWTβRII cells (Fig. 9). Positive or negative involvement of RhoA in TGF-β production has been reported (85, 86). Our findings suggest that the mechanisms leading to TGF-β regulation might be cell type and stimulus dependent.
MAP kinases (p38 MAPK, ERK and JNK) have been shown to regulate cytokine production at both transcription and translation (17, 87–92). Intriguingly, when we examined a potential role for p38 MAPK, ERK or JNK in induction of TGF-β to non-apoptotic cell stimuli PMA or LPS, we did indeed find evidence of their involvement in its translational regulation via eIF4E (51, 53). However, apoptotic cell-induced TGF-β translation appears to be regulated independent of p38 MAPK, ERK and JNK. Thus, in the present study, we compared apoptotic cells or mAb 217 to PMA and LPS for stimulation of TGF-β production. Although all three stimuli activated p38 MAPK, ERK and JNK, the outcome of the same kinase activation was entirely different (Table 1).
These experiments have begun to address the signal pathways involved in enhanced transcription and translation of TGFβ in response to apoptotic cells. Other points of regulation likely include secretion and extracellular activation of the molecule. Of note, an earlier study in vivo (8) suggested that the administration of apoptotic cells to an ongoing inflammatory site induced an immediate release of TGFβ that was not dependent on protein synthesis, suggesting that indeed apoptotic cell recognition might enhance TGFβ liberation from the cell. There are many potential mechanisms by which latent TGFβ may be activated, but at this point, a possible role for the interaction of the apoptotic cell in this process has not been addressed. These two steps in the process of achieving functional TGFβ are areas for important future research. It will also be critical to sort out the contribution of the various PS-recognizing receptors and bridge molecules involved in its production, release and activation, just as it has now become equally important to sort out their various contributions to the uptake and clearance process.
In summary, these studies show that apoptotic cells up-regulate TGF-β mRNA expression through p38 MAPK, ERK and JNK, and enhance protein translation through a newly defined PSRS/RhoA/PI-3K/Akt/mTOR/eiF4E signaling pathway (Fig. 10). The present findings demonstrate that the mechanisms leading to apoptotic cell-induced TGF-β production can be distinguished from other stimuli.
Acknowledgments
This Study was supported by National Institute of Health Grants HL81151, GM61031, AI058228 and HL34303.
Abbreviations
- TGF-β
transforming growth factor-β
- PS
phosphatidylserine
- PSRS
phosphatidylserine recognition structures
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- JNK
c-jun N-terminal kinase
- MEK
MAPK/ERK kinase
- mTOR
mammalian target of rapamycin
- 4EBP-1
4E-binding protein-1
- eIF4E
eukaryotic initiation factor 4E
References
- 1.deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays in Biochemistry. 2003;39:105–117. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- 2.Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: a perspective. J Leukoc Biol. 2006;79:896–903. doi: 10.1189/jlb.1005550. [DOI] [PubMed] [Google Scholar]
- 3.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Morimoto K, Janssen WJ, Fessler MB, McPhillips KA, Borges VM, Bowler RP, Xiao YQ, Kench JA, Henson PM, Vandivier RW. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J Immunol. 2006;176:7657–7665. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 5.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 6.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 8.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald PP, Fadok VA, Bratton D, Henson PM. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-beta in macrophages that have ingested apoptotic cells. J Immunol. 1999;163:6164–6172. [PubMed] [Google Scholar]
- 10.Chin BY, Petrache I, Choi AM, Choi ME. Transforming growth factor beta1 rescues serum deprivation-induced apoptosis via the mitogen-activated protein kinase (MAPK) pathway in macrophages. J Biol Chem. 1999;274:11362–11368. doi: 10.1074/jbc.274.16.11362. [DOI] [PubMed] [Google Scholar]
- 11.Dennler S, Goumans MJ, ten Dijke P. Transforming growth factor beta signal transduction. J Leukoc Biol. 2002;71:731–740. [PubMed] [Google Scholar]
- 12.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 13.Hartsough MT, Mulder KM. Transforming growth factor-beta signaling in epithelial cells. Pharmacol Ther. 1997;75:21–41. doi: 10.1016/s0163-7258(97)00020-x. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Hutter D, Liu Y, Wang X, Sheikh MS, Chan AM, Holbrook NJ. Transforming growth factor-beta 1 suppresses serum deprivation-induced death of A549 cells through differential effects on c-Jun and JNK activities. J Biol Chem. 2000;275:18234–18242. doi: 10.1074/jbc.M909431199. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 16.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 17.Xiao YQ, Malcolm K, Worthen S, Gardai S, Schiemann WP, Fadok VA, Bratton DL, Henson PM. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem. 2002;277:14884–14893. doi: 10.1074/jbc.M111718200. [DOI] [PubMed] [Google Scholar]
- 18.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 19.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 21.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok VA. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 23.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 24.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi N, Karisola P, Pena-Cruz V, Dorfman DM, Jinushi M, Umetsu SE, Butte MJ, Nagumo H, Chernova I, Zhu B, Sharpe AH, Ito S, Dranoff G, Kaplan GG, Casasnovas JM, Umetsu DT, Dekruyff RH, Freeman GJ. TIM-1 and TIM-4 Glycoproteins Bind Phosphatidylserine and Mediate Uptake of Apoptotic Cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santiago C, Ballesteros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casasnovas JM. Structures of T Cell Immunoglobulin Mucin Protein 4 Show a Metal-Ion-Dependent Ligand Binding Site where Phosphatidylserine Binds. Immunity. 2007;27:941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savill J, Gregory C. Apoptotic PS to Phagocyte TIM-4: Eat Me. Immunity. 2007;27:830–832. doi: 10.1016/j.immuni.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, Park RW, Kim IS. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 29.Akakura S, Singh S, Spataro M, Akakura R, Kim JI, Albert ML, Birge RB. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res. 2004;292:403–416. doi: 10.1016/j.yexcr.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramanian K, Chandra J, Schroit AJ. Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of beta2-glycoprotein I in macrophage recognition. J Biol Chem. 1997;272:31113–31117. doi: 10.1074/jbc.272.49.31113. [DOI] [PubMed] [Google Scholar]
- 31.Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp Eye Res. 2005;81:581–591. doi: 10.1016/j.exer.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 33.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem (Tokyo) 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- 34.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 35.Bose J, Gruber AD, Helming L, Schiebe S, Wegener I, Hafner M, Beales M, Kontgen F, Lengeling A. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J Biol. 2004;3:15. doi: 10.1186/jbiol10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell JE, Cvetanovic M, Tibrewal N, Patel V, Colamonici R, Li MO, Flavell RA, Levine JS, Birge RB, Ucker DS. The presumptive phosphatidylserine receptor is dispensable for innate anti-inflammatory recognition and clearance of apoptotic cells. J Biol Chem. 2006;281:5718–5725. doi: 10.1074/jbc.M509775200. [DOI] [PubMed] [Google Scholar]
- 37.Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318:444–447. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frasch SC, Nick JA, Fadok VA, Bratton DL, Worthen GS, Henson PM. p38 mitogen-activated protein kinase-dependent and -independent intracellular signal transduction pathways leading to apoptosis in human neutrophils. J Biol Chem. 1998;273:8389–8397. doi: 10.1074/jbc.273.14.8389. [DOI] [PubMed] [Google Scholar]
- 40.Van Obberghen-Schilling E, Roche NS, Flanders KC, Sporn MB, Roberts AB. Transforming growth factor beta 1 positively regulates its own expression in normal and transformed cells. J Biol Chem. 1988;263:7741–7746. [PubMed] [Google Scholar]
- 41.Kim SJ, Jeang KT, Glick AB, Sporn MB, Roberts AB. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J Biol Chem. 1989;264:7041–7045. [PubMed] [Google Scholar]
- 42.Xiao YQ, Minami K, Mue S, Ohuchi K. Pharmacological analysis of protein kinases responsible for chemotaxis of rat peritoneal neutrophils. Eur J Pharmacol. 1998;360:195–204. doi: 10.1016/s0014-2999(98)00681-5. [DOI] [PubMed] [Google Scholar]
- 43.Henson PM. Engulfment: ingestion and migration with Rac, Rho and TRIO. Curr Biol. 2005;15:R29–R30. doi: 10.1016/j.cub.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem. 2006;281:8836–8842. doi: 10.1074/jbc.M510972200. [DOI] [PubMed] [Google Scholar]
- 45.Niedergang F, Chavrier P. Regulation of phagocytosis by Rho GTPases. Curr Top Microbiol Immunol. 2005;291:43–60. doi: 10.1007/3-540-27511-8_4. [DOI] [PubMed] [Google Scholar]
- 46.Tosello-Trampont AC, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J Biol Chem. 2003;278:49911–49919. doi: 10.1074/jbc.M306079200. [DOI] [PubMed] [Google Scholar]
- 47.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E--from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 48.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 49.Batuman OA, Ferrero A, Cupp C, Jimenez SA, Khalili K. Differential regulation of transforming growth factor beta-1 gene expression by glucocorticoids in human T and glial cells. J Immunol. 1995;155:4397–4405. [PubMed] [Google Scholar]
- 50.Jun CD, Choi BM, Lee SY, Kang SS, Kim HM, Chung HT. Nitric oxide inhibits the expression of protein kinase C delta gene in the murine peritoneal macrophages. Biochem Biophys Res Commun. 1994;204:105–111. doi: 10.1006/bbrc.1994.2432. [DOI] [PubMed] [Google Scholar]
- 51.Rolli-Derkinderen M, Machavoine F, Baraban JM, Grolleau A, Beretta L, Dy M. ERK and p38 inhibit the expression of 4E-BP1 repressor of translation through induction of Egr-1. J Biol Chem. 2003;278:18859–18867. doi: 10.1074/jbc.M211696200. [DOI] [PubMed] [Google Scholar]
- 52.Scotto L, Vaduva PI, Wager RE, Assoian RK. Type beta 1 transforming growth factor gene expression. A corrected mRNA structure reveals a downstream phorbol ester responsive element in human cells. J Biol Chem. 1990;265:2203–2208. [PubMed] [Google Scholar]
- 53.Wang X, Flynn A, Waskiewicz AJ, Webb BL, Vries RG, Baines IA, Cooper JA, Proud CG. The phosphorylation of eukaryotic initiation factor eIF4E in response to phorbol esters, cell stresses, and cytokines is mediated by distinct MAP kinase pathways. J Biol Chem. 1998;273:9373–9377. doi: 10.1074/jbc.273.16.9373. [DOI] [PubMed] [Google Scholar]
- 54.Kim SJ, Park K, Koeller D, Kim KY, Wakefield LM, Sporn MB, Roberts AB. Post-transcriptional regulation of the human transforming growth factorbeta 1 gene. J Biol Chem. 1992;267:13702–13707. [PubMed] [Google Scholar]
- 55.Kim SJ, Denhez F, Kim KY, Holt JT, Sporn MB, Roberts AB. Activation of the second promoter of the transforming growth factor-beta 1 gene by transforming growth factor-beta 1 and phorbol ester occurs through the same target sequences. J Biol Chem. 1989;264:19373–19378. [PubMed] [Google Scholar]
- 56.Kim SJ, Glick A, Sporn MB, Roberts AB. Characterization of the promoter region of the human transforming growth factor-beta 1 gene. J Biol Chem. 1989;264:402–408. [PubMed] [Google Scholar]
- 57.McCaffrey TA, Fu C, Du B, Eksinar S, Kent KC, Bush H, Jr, Kreiger K, Rosengart T, Cybulsky MI, Silverman ES, Collins T. High-level expression of Egr-1 and Egr-1-inducible genes in mouse and human atherosclerosis. J Clin Invest. 2000;105:653–662. doi: 10.1172/JCI8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoo YD, Chiou CJ, Choi KS, Yi Y, Michelson S, Kim S, Hayward GS, Kim SJ. The IE2 regulatory protein of human cytomegalovirus induces expression of the human transforming growth factor beta1 gene through an Egr-1 binding site. J Virol. 1996;70:7062–7070. doi: 10.1128/jvi.70.10.7062-7070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, Karin M, Roberts AB. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990;10:1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts AB, Kim SJ, Kondaiah P, Jakowlew SB, Denhez F, Glick AB, Geiser AG, Watanabe S, Noma T, Lechleider R, et al. Transcriptional control of expression of the TGF-betas. Ann N Y Acad Sci. 1990;593:43–50. doi: 10.1111/j.1749-6632.1990.tb16098.x. [DOI] [PubMed] [Google Scholar]
- 61.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 62.Otsuka M, Negishi Y, Aramaki Y. Involvement of phosphatidylinositol-3-kinase and ERK pathways in the production of TGF-beta1 by macrophages treated with liposomes composed of phosphatidylserine. FEBS Lett. 2007;581:325–330. doi: 10.1016/j.febslet.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 63.Otsuka M, Tsuchiya S, Aramaki Y. Involvement of ERK, a MAP kinase, in the production of TGF-beta by macrophages treated with liposomes composed of phosphatidylserine. Biochem Biophys Res Commun. 2004;324:1400–1405. doi: 10.1016/j.bbrc.2004.09.198. [DOI] [PubMed] [Google Scholar]
- 64.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 65.Grimsley CM, Kinchen JM, Tosello-Trampont AC, Brugnera E, Haney LB, Lu M, Chen Q, Klingele D, Hengartner MO, Ravichandran KS. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem. 2004;279:6087–6097. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 66.Kraynack NC, Corey DA, Elmer HL, Kelley TJ. Mechanisms of NOS2 regulation by Rho GTPase signaling in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L604–L611. doi: 10.1152/ajplung.00459.2001. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;261:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- 68.Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- 69.Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci U S A. 2006;103:12825–12830. doi: 10.1073/pnas.0605331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halayko AJ, Kartha S, Stelmack GL, McConville J, Tam J, Camoretti-Mercado B, Forsythe SM, Hershenson MB, Solway J. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol. 2004;31:266–275. doi: 10.1165/rcmb.2003-0272OC. [DOI] [PubMed] [Google Scholar]
- 71.Mateo-Lozano S, Tirado OM, Notario V. Rapamycin induces the fusion-type independent downregulation of the EWS/FLI-1 proteins and inhibits Ewing's sarcoma cell proliferation. Oncogene. 2003;22:9282–9287. doi: 10.1038/sj.onc.1207081. [DOI] [PubMed] [Google Scholar]
- 72.Rosenwald IB. The role of translation in neoplastic transformation from a pathologist's point of view. Oncogene. 2004;23:3230–3247. doi: 10.1038/sj.onc.1207552. [DOI] [PubMed] [Google Scholar]
- 73.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 74.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 75.Rajasekhar VK, Holland EC. Postgenomic global analysis of translational control induced by oncogenic signaling. Oncogene. 2004;23:3248–3264. doi: 10.1038/sj.onc.1207546. [DOI] [PubMed] [Google Scholar]
- 76.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–486. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 77.van der Velden AW, Thomas AA. The role of the 5' untranslated region of an mRNA in translation regulation during development. Int J Biochem Cell Biol. 1999;31:87–106. doi: 10.1016/s1357-2725(98)00134-4. [DOI] [PubMed] [Google Scholar]
- 78.Ridley AJ. Rho proteins, PI 3-kinases, and monocyte/macrophage motility. FEBS Lett. 2001;498:168–171. doi: 10.1016/s0014-5793(01)02481-4. [DOI] [PubMed] [Google Scholar]
- 79.Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207–4223. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reuveny M, Heller H, Bengal E. RhoA controls myoblast survival by inducing the phosphatidylinositol 3-kinase-Akt signaling pathway. FEBS Lett. 2004;569:129–134. doi: 10.1016/j.febslet.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 81.Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CA, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2003;284:F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- 83.Murata T, Arii S, Mori A, Imamura M. Therapeutic significance of Y-27632, a Rho-kinase inhibitor, on the established liver fibrosis. J Surg Res. 2003;114:64–71. doi: 10.1016/s0022-4804(03)00202-6. [DOI] [PubMed] [Google Scholar]
- 84.Shen X, Li J, Hu PP, Waddell D, Zhang J, Wang XF. The activity of guanine exchange factor NET1 is essential for transforming growth factor-beta-mediated stress fiber formation. J Biol Chem. 2001;276:15362–15368. doi: 10.1074/jbc.M009534200. [DOI] [PubMed] [Google Scholar]
- 85.Baccante G, Mincione G, Di Marcantonio MC, Piccirelli A, Cuccurullo F, Porreca E. Pravastatin up-regulates transforming growth factor-beta1 in THP-1 human macrophages: effect on scavenger receptor class A expression. Biochem Biophys Res Commun. 2004;314:704–710. doi: 10.1016/j.bbrc.2003.12.150. [DOI] [PubMed] [Google Scholar]
- 86.Sakata R, Ueno T, Nakamura T, Ueno H, Sata M. Mechanical stretch induces TGF-beta synthesis in hepatic stellate cells. Eur J Clin Invest. 2004;34:129–136. doi: 10.1111/j.1365-2362.2004.01302.x. [DOI] [PubMed] [Google Scholar]
- 87.Huttunen P, Hyypia T, Vihinen P, Nissinen L, Heino J. Echovirus 1 infection induces both stress- and growth-activated mitogen-activated protein kinase pathways and regulates the transcription of cellular immediate-early genes. Virology. 1998;250:85–93. doi: 10.1006/viro.1998.9343. [DOI] [PubMed] [Google Scholar]
- 88.Kaminska B. MAPK signalling pathways as molecular targets for anti-nflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 89.Shin SY, Lee JH, Min B, Lee YH. The translation inhibitor anisomycin induces Elk-1-mediated transcriptional activation of egr-1 through multiple mitogen-activated protein kinase pathways. Exp Mol Med. 2006;38:677–685. doi: 10.1038/emm.2006.80. [DOI] [PubMed] [Google Scholar]
- 90.Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stressactivated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu CY, Hsieh HL, Jou MJ, Yang CM. Involvement of p42/p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem. 2004;90:1477–1488. doi: 10.1111/j.1471-4159.2004.02682.x. [DOI] [PubMed] [Google Scholar]
- 92.Xiao YQ, Someya K, Morita H, Takahashi K, Ohuchi K. Involvement of p38 MAPK and ERK/MAPK pathways in staurosporine-induced production of macrophage inflammatory protein-2 in rat peritoneal neutrophils. Biochim Biophys Acta. 1999;1450:155–163. doi: 10.1016/s0167-4889(99)00042-7. [DOI] [PubMed] [Google Scholar]