Abstract
Purpose:
To investigate the IFOX regimen (gefitinib, 5-fluorouracil [5-FU], leucovorin and oxaliplatin) as first-line therapy in patients with metastatic colorectal cancer.
Experimental Design:
Eligible patients had stage IV colorectal adenocarcinoma, and had not received prior chemotherapy for metastatic disease. Each cycle consisted of 14 days. Cycle 1 consisted of FOLFOX-4 (oxaliplatin, leucovorin, and 5-FU). All subsequent cycles consisted of FOLFOX-4 with gefitinib at 500 mg PO daily throughout the 14 days cycle.
Results:
Forty-five patients were enrolled and are assessable for toxicity. Forty-three patients are assessable for response. Thirty-one of the 43 patients (72%), had either a complete or partial response by RECIST criteria. Median overall survival was 20.5 months. Median time to progression was 9.3 months. Commonly encountered grade 3/4 toxicities included diarrhea in 67% of patients and neutropenia in 60%. Grade 2 acneiform skin rash typical of gefitinib occurred in 60% of patients.
Conclusions:
IFOX is an active first-line regimen in patients with metastatic colorectal adenocarcinoma, demonstrating higher response rates but also increased toxicities compared with FOLFOX-4 alone in a similar patient population.
Keywords: EGFR, gefitinib, 5-fluorouracil, oxaliplatin, colorectal cancer
INTRODUCTION
Over the last 10 years, incremental gains in response rates and median survival for patients with metastatic colorectal cancer (CRC) have been achieved with the introduction of active new chemotherapeutic and biologically targeted agents. Combinations of irinotecan or oxaliplatin with 5-FU and leucovorin for first-line therapy of metastatic CRC have demonstrated improved response rates and median survival times over 5-FU and leucovorin alone (1, 2). In addition, infusional 5-FU has replaced bolus 5-FU as a platform for combination chemotherapy based on decreased toxicity and improved efficacy (3, 4). In the initial study investigating the combination of oxaliplatin, 5-FU, and leucovorin (FOLFOX-4) for first-line therapy of metastatic CRC, there was a 50% objective response rate compared to 22% with infusional 5-FU/leucovorin (LV5FU2) alone (2). The FOLFOX-4 regimen was also associated with a longer time to progression (9.0 months vs. 6.2 months) and a trend towards improved overall survival. Although a direct comparison of FOLFOX-4 and IFL (irinotecan, 5-FU, and leucovorin) in previously untreated patients demonstrated that FOLFOX-4 was superior, a subsequent comparison of FOLFOX-6 and FOLFIRI (folinic acid, 5-FU, and irinotecan) showed no significant difference in efficacy (5, 6). This confirmed that both irinotecan and oxaliplatin are active agents against colorectal cancer, and selection of an appropriate regimen could focus on their different toxicity profiles.
The effort to further improve the efficacy and tolerability of treatment for metastatic colorectal cancer has led to the discovery of new agents targeting cell signaling molecules such as epidermal growth factor receptor (EGFR). EGFR expression has been demonstrated in 60-80% of CRC (7, 8), and has been associated with decreased survival (9). Preclinical studies inhibiting EGFR with either antibodies or small molecules demonstrated a dose-dependent inhibition of tumor cell growth (10-14) and sensitization of tumor cells to chemotherapy (15-17).
A phase III clinical trial has validated both of these concepts with the demonstration that the anti-EGFR antibody, cetuximab, in combination with irinotecan produced a 22% response rate in patients refractory to irinotecan-based chemotherapy, while cetuximab alone produced a 10% response rate (18). Cetuximab has also been reported to improve response rate and progression free survival when added to FOLFIRI in the first-line treatment of patients with metastatic CRC (19).
Gefitinib (Iressa™, ZD1839), a small molecule inhibitor of the tyrosine kinase domain of EGFR, has been extensively studied in patients with tumors of epithelial origin, such as lung and head and neck cancers, but studies in patients with CRC are limited (20-25). We recently reported a phase II study with gefitinib in combination with FOLFOX-4 for pretreated patients with metastatic CRC that demonstrated a high response rate (33%), further supporting the chemosensitizing role of EGFR inhibition (26). We now report on a phase II study evaluating the efficacy of the IFOX regimen for the first-line treatment of patients with metastatic CRC.
PATIENTS AND METHODS
Patients
Patients were considered eligible for this study if they were older than 18 years of age and had histologically confirmed metastatic colorectal adenocarcinoma. Patients had not received prior chemotherapy for this disease, with the exception of 5-fluorouracil for adjuvant therapy greater than 6 months prior to enrollment. Other criteria for eligibility included measurable disease by RECIST criteria, no prior exposure to oxaliplatin or EGFR inhibitors, an ECOG performance status ≤ 2, adequate blood counts (neutrophils ≥ 1500/mm3 and platelets ≥ 100,000/mm3), renal function within normal limits, total bilirubin ≤1.5 mg/dL, and transaminases ≤ 2.5 times the upper limit of normal. Confirmation of tumor EGFR status was not required for inclusion in this study and there was no determination made of EGFR status prior to treatment initiation. All patients signed an informed consent form approved by the Stanford University Committee for the Protection of Human Subjects.
Treatment
The first cycle of treatment was FOLFOX-4 chemotherapy alone at dosages previously published (27). This was done to obtain more experience with the acute toxicities of IFOX compared to FOLFOX-4 alone. On day 1, patients received oxaliplatin 85 mg/m2 intravenously concurrent with leucovorin 200 mg/m2 intravenously over 2 hours. Then, 5-FU 400 mg/m2 was given by intravenous bolus injection followed by 5-FU 600 mg/m2 given by continuous intravenous infusion over 22 hours. On day 2, leucovorin, bolus 5-FU and infusional 5-FU were delivered at identical doses as day 1. Pretreatment with a 5-hydroxytryptamine-3-receptor antagonist and dexamethasone was given prior to oxaliplatin.
In the second and subsequent cycles of treatment, patients received the IFOX regimen. Each cycle lasted 14 days. Thus, beginning with cycle 2 and for each subsequent cycle, gefitinib 500 mg PO daily was administered continuously.
All toxicities were graded according to the NCI Common Toxicity Criteria (CTC) version 2.0 except for neurotoxicity. Grade 1 neurotoxicity was defined as paresthesias or dysesthesias of short duration that resolved and did not interfere with function, grade 2 as symptoms that interfered with function but not activities of daily living (ADL), grade 3 as symptoms with pain or impairment interfering with ADL, and grade 4 as any paresthesias or dysesthesias that were disabling or life-threatening. Retreatment at the start of each cycle required adequate hematologic function (ANC ≥ 1500/mm3 and platelets ≥ 100,000/mm3) and resolution of all toxicities to ≤ CTC grade 2.
During treatment, dose modifications for dermatitis, diarrhea and myelosuppression were made. The first episode of dermatitis ≥ CTC grade 3 resulted in a reduction of gefitinib to 250 mg PO daily and the second episode led to a discontinuation of gefitinib. Diarrhea ≥ CTC grade 3, refractory to oral anti-diarrheal medication, resulted in a reduction in the 5-FU bolus and infusion by 20%, with a second episode leading to a reduction in gefitinib to 250 mg PO daily, a third episode resulting in a reduction of oxaliplatin by 20%, and a fourth episode resulting in withdrawal of the patient from study. For myelosuppression, a nadir ANC ≤500/mm3 or a nadir platelet ≤ 50,000/mm3, resulted in a 20% reduction of oxaliplatin, with second, third and fourth episodes resulting in a reduction of 5-FU bolus and infusion by 20%, further reduction in oxaliplatin by 20%, and a final reduction in 5-FU and oxaliplatin at investigator discretion, respectively. Daily gefitinib was continued if initiation of chemotherapy for the next cycle of treatment was delayed due to myelosuppression. No change was made in oxaliplatin dose for grade 1 neurotoxicity; however the oxaliplatin dose was reduced to 65 mg/m2 for grade 2 neurotoxicity that persisted between cycles. Grade 3 symptoms led to an oxaliplatin dose reduction to 65 mg/m2 and 40 mg/m2, for the first and second episodes respectively, and if the symptoms persisted between cycles oxaliplatin was stopped. Any grade 4 symptoms led to immediate discontinuation of oxaliplatin. Finally, pharyngo-laryngeal dysesthesias that lasted more than 7 days led to an increase in oxaliplatin infusion time to 6 hours.
Treatment was continued until development of progressive disease or unacceptable toxicity, withdrawal of patient consent, completion of protocol, or decision to perform surgical resection of disease.
Evaluation
Baseline tumor measurements by computed tomography were obtained within 28 days prior to starting study treatment. Physical examination, including medical history, laboratory studies and assessment of performance status, were conducted at the beginning of each two-week cycle. Patients were asked to keep a diary of daily gefitinib ingestion and record their experience of nausea and diarrhea.
Tumor response was evaluated every 8 weeks by computed tomography imaging and tumor measurement performed using RECIST criteria (28). A response was defined as a reduction of ≥ 30% in the sum of the longest diameters of all measured lesions, confirmed on a subsequent scan performed at least 4 weeks after the initial scan documenting the reduction.
Immunohistochemical Staining for EGFR Expression
Formalin-fixed, paraffin-embedded tissue was retrieved and four micron sections were cut, placed on slides, deparaffinized in xylene and hydrated. Sections were stained using the DAKO EGFR detection system (Carpinteria, CA, USA) and a DAKO automated staining machine. Antigen retrieval was carried out by proteinase K digestion. Endogenous peroxidase was suppressed by incubation with 3% H2O2. Positive and negative controls were run in parallel. Immunostaining was scored semiquantitiatively by one of the authors (E.S.) using a two-tiered scale for percentage of lesional cells stained (>50% called positive or <50% called negative for EGFR).
Statistical Analysis
The primary endpoint of the study was to determine the objective response rate for patients with metastatic CRC treated with this study regimen. Secondary endpoints included determination of the safety profile, median time to progression, and overall survival.
Time to progression was defined as the interval of time from enrollment on this study to the first evidence of progressive disease by RECIST criteria. If patients went off study prior to progression either due to toxicity, surgical resection or radiofrequency ablation of residual disease, the first progression date after withdrawal from the study was recorded. The overall survival time was calculated as the interval of time from enrollment until the date of death from any cause or until the date of the last follow-up, at which time the data was censored. Both the time to progression and overall survival times were estimated by the Kaplan-Meier method.
To calculate the proposed sample size, we used a baseline response rate of 40% seen with FOLFOX-4 in the first-line setting as our null hypothesis. In order to detect a 20% improvement in response rate (40% versus 60%) with the proposed regimen, with an alpha and beta of 0.10, we estimated accrual to be 46 patients in a two-stage design (29).
RESULTS
Baseline Characteristics
Between May 2002 and September 2004, forty-five patients were enrolled on this study and are evaluable for toxicity. Two patients are not evaluable for response, either due to withdrawal from study prior to completing one cycle of therapy (one patient) or due to misdiagnosis of metastatic disease (one patient). The baseline characteristics of the 45 assessable patients are shown in Table 1. Forty-four of 45 patients had an ECOG performance status of 1 or better, with 37 demonstrating ECOG PS 0.
Table 1.
Baseline Characteristics of Patients
| Parameter | No. of Patients | % (n = 45) | |
|---|---|---|---|
| Age (years) | |||
| Median | 57 | ||
| Range | 29-79 | ||
| Sex | |||
| Male | 24 | 53 | |
| Female | 21 | 47 | |
| Race | |||
| White | 35 | 78 | |
| Asian | 7 | 16 | |
| Hispanic | 2 | 4 | |
| African-American | 1 | 2 | |
| Performance Status (ECOG) | |||
| 0 | 37 | 82 | |
| 1 | 7 | 16 | |
| 2 | 1 | 2 | |
Treatment Administration
The total number of cycles administered was 372, with a median of 9 (range, 1-16), and a mean of 8 cycles per patient. Median duration of follow-up is 36 months for surviving patients.
Efficacy
The primary efficacy endpoint for this study was objective response rate. Of the 43 patients assessable for response, one patient (2%) experienced a complete response, 30 patients (70%) experienced a partial response, for an overall remission rate of 72% (Table 2). Only 3 patients (7%) had evidence of progressive disease at the first assessment time point of 2 months.
Table 2.
Antitumor Response Rates to IFOX Therapy
| Response | All evaluable patients (n = 43) |
|---|---|
| Complete Remission | 1 (2%) |
| Partial Remission | 30 (70%) |
| Stable Disease | 8 (19%) |
| Progressive Disease | 3 (7%) |
| Early Death | 1 (2%) |
The secondary efficacy endpoints were time to progression (TTP) and overall survival (OS). For the 43 evaluable patients, including the 14 patients who discontinued IFOX to receive surgical resection or radiofrequency ablation of residual metastases, median time to disease progression was 9.3 months (95% CI, 6.4 to 20.0 months). IFOX was stopped in 14 patients (33%) with either stable or responding disease in order to attempt definitive treatment with surgical resection or radiofrequency ablation of residual metastases (Table 3). There was no peri-operative mortality in patients going on to hepatic resections, although fatty changes were found in the livers of some patients. One patient experienced a complete response by RECIST and was taken off study after 10 cycles. Fifteen patients (35%) were taken off study treatment for progressive disease, and 12 patients (28%) were taken off due to toxicity. The median OS for all evaluable patients was 20.5 months (95% CI, 14.0 to 28.0 months) (Figure 1). The median OS for responders and non-responders was 25.5 months (95% CI, 20.0 to 33.2 months) and 12.4 months (95% CI, 13.0 to 23.7) (Figure 2).
Table 3.
Events Leading to Discontinuation of Study Treatment
| Event | No. of Patients (%) |
|---|---|
| Surgery/RFA | 14 (33%) |
| Progressive Disease | 15 (35%) |
| Toxicity | 12 (28%) |
| Completion of Protocol | 1 (2%) |
| Early Death | 1 (2%) |
Figure 1.
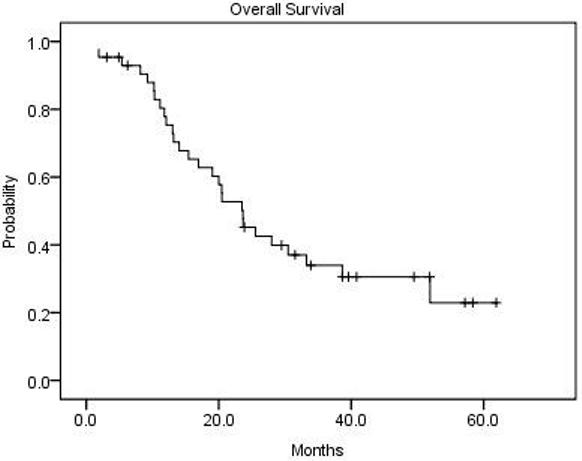
Overall survival for the 43 assessable patients (median 20.5 months).
Figure 2.
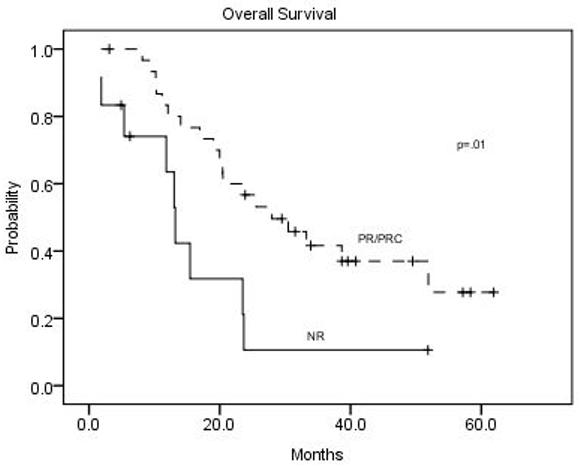
Overall survival for responders vs. non-responders among the 43 assessable patients.
Toxicities
The toxicities of IFOX were evaluated as a secondary endpoint of this study. Toxicity data with the IFOX regimen has been previously described in the Phase I study using this regimen as well as a Phase II study with this regimen in previously treated patients with metastatic colorectal cancer (25, 30). This study provides further information on toxicities encountered with the IFOX regimen (Table 4). Of the 45 assessable patients for toxicity, 67% of patients experienced grade 3 or 4 diarrhea at some point in the treatment course. Neutropenia was the second most common grade 3 or 4 toxicity, occurring in 60% of patients. Four patients (9%) experienced fever and neutropenia. Additional toxicities included grade 2 dermatitis or dry skin attributable to gefitinib in 27 patients (60%), grade 3 hypokalemia in 15 patients (33%), grade 3 nausea/vomiting in 12 patients (27%), grade 3 fatigue in 10 patients (22%), and grade 2 peripheral neuropathy attributable to oxaliplatin in 7 patients (16%). One patient (2%) had early death from sepsis after 3 cycles of therapy.
Table 4.
Grade 3 or 4 Adverse Events Related to Treatment
| Adverse Event | All Patients (n = 45) |
|
|---|---|---|
| No. | % | |
| Diarrhea | 30 | 67 |
| Neutropenia | 27 | 60 |
| Hypokalemia | 15 | 33 |
| Nausea/vomiting | 12 | 27 |
| Dehydration | 10 | 22 |
| Fatigue | 10 | 22 |
| Anorexia | 7 | 16 |
| Infection | 3 | 7 |
| Thrombosis | 3 | 7 |
| Hyponatremia | 3 | 7 |
| Ileus | 3 | 7 |
| Mucositis | 2 | 4 |
| Renal insufficiency | 2 | 4 |
| Hand and Foot Syndrome | 1 | 2 |
| Syncope | 1 | 2 |
| Anemia | 1 | 2 |
A total of 25 patients (56%) underwent at least one dose reduction of oxaliplatin (Table 5) with the most common reasons being grade 3 anorexia (28%) and grade 3 or 4 neutropenia (24%). Thirty patients (67%) underwent a dose reduction of 5-FU due to grade 3 diarrhea (87%), grade 3 or 4 neutropenia (10%), or grade 3 mucositis (3%). Eleven patients (24%) underwent a dose reduction of gefitinib due to grade 3 diarrhea (73%), grade 2 dermatitis or dry skin (18%), or pulmonary infiltrates (9%).
Table 5.
Frequency and Cause of Drug Dose Reductions
| Agent | No. Requiring Reduction (%) |
Most common reasons for dose reduction | ||||
|---|---|---|---|---|---|---|
| Oxaliplatin | 25 (56%) | Anorexia (28%) |
Neutropenia (24%) |
Fatigue (12%) |
Diarrhea (8%) |
Neuropathy (8%) |
| 5-FU | 30 (67%) | Diarrhea (87%) |
Neutropenia (10%) |
Mucositis (3%) |
||
| Gefitinib | 11 (24%) | Diarrhea (73%) |
Dermatitis (18%) |
|||
EGFR Expression
A total of 41 tumor samples were available for EGFR expression analysis. Twenty-eight patients (68%) were EGFR positive by IHC and 13 (32%) negative. The remission rate in patients with EGFR positive CRC was 70% and in patients with EGFR negative CRC was 75%. EGFR expression did not correlate significantly with response or survival (Figure 3).
Figure 3.
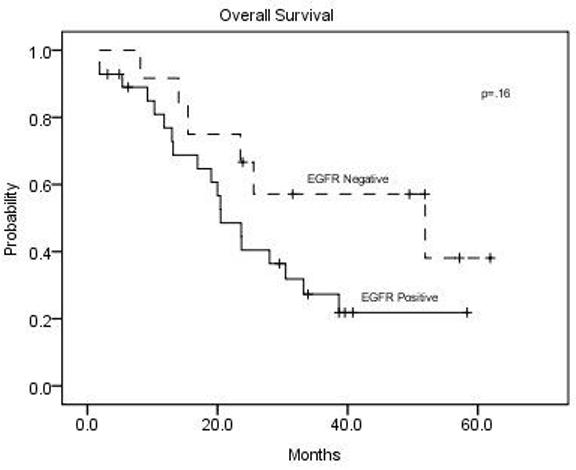
Overall survival according to tumor EGFR expression status for the 41 patients with known EGFR expression by immunohistochemical assay.
DISCUSSION
The recent advent of several new agents for the treatment of metastatic CRC has markedly enhanced the therapeutic armamentarium for this disease. Oxaliplatin in combination with infusional 5-FU in the FOLFOX-4 regimen has been shown to be effective in achieving an improved response and time to progression over LV5FU and IFL in the first-line setting (2, 5). The monoclonal antibodies cetuximab, targeting EGFR, and bevacizumab, targeting vascular endothelial growth factor, have also demonstrated therapeutic efficacy in CRC, and many studies to optimize their utilization in combination with chemotherapies are underway.
The high level of EGFR expression in CRC specimens has sparked great interest in using this target to develop more directed and specific therapies. To date, positive results with EGFR inhibition in CRC have only been reported for the monoclonal antibody cetuximab in combination with irinotecan-based regimens utilizing bolus 5-FU and FOLFIRI (18, 19). The combination of EGFR inhibition with FOLFOX-4 is currently being investigated in a randomized phase III trial of FOLFOX chemotherapy plus and minus cetuximab. However, while cetuximab and gefitinib target the same cellular pathway, there is very limited data on small molecule inhibitors of EGFR in combination with chemotherapy in CRC.
Despite preclinical evidence for chemosensitization, four major randomized trials have shown no benefit for the addition of gefitinib or erlotinib added to standard chemotherapy for non-small cell lung cancer (21, 22). Our data suggests that colorectal cancers differ substantially from non-small cell lung cancers in the ability of EGFR inhibitors to enhance the effects of chemotherapy. The response rate achieved in this study is higher than reported results with FOLFOX-4 alone in a similar setting. While acknowledging that a direct comparison of the two response rates is not possible, the high response rate seen with IFOX suggests that gefitinib exerts a chemosensitizing effect in CRC. This explanation is consistent with our prior IFOX experience with CRC patients who were receiving second-line therapy (30), as well as the results from two phase III trials showing that cetuximab enhances the antitumor efficacy of irinotecan (18,19).
Two previous studies have demonstrated inconsistent results when combining gefitinib at a dose of 250 mg/day with FOLFOX as first line therapy (31, 32). Zampino et al reported gefitinib combined with FOLFOX-6 showed response rates similar to that seen in our study (30). However, this was not confirmed by Cascinu et al. using gefitinib combined with FOLFOX-4 (32). Our study used a higher gefitinib dose of 500 mg/day, which may have added to the efficacy. The question of the efficacy of gefitinib or other oral EGFR inhibitors combined with chemotherapy in CRC will ultimately only be answered by randomized Phase III trials.
The median overall survival in this study was 20.5 months. When time to progression in our study population is calculated, regardless of any subsequent therapy they may have received after discontinuation from the study treatment, the result is 9.3 months.
As would be expected with combination therapy, certain toxicities were significantly increased over FOLFOX alone, as reported in multiple previous studies. Adverse events known to be increased by gefitinib from other phase I and II studies include diarrhea and skin changes (either acneiform rash or dry skin). For example, grade 3 diarrhea was experienced in 67% of patients receiving our study treatment compared with 12% reported previously, strongly suggesting an additive toxicity of gefitinib and 5-FU on the lower gastrointestinal tract (2). Grade 3 or 4 neutropenia was also more prevalent compared to historical controls (60% for IFOX vs. 42% for FOLFOX alone).
This investigation showed no correlation between EGFR expression and response or survival. The limitations of sensitivity for detecting EGFR expression by the IHC assay may have obscured any effect of such expression on outcomes. The trend for increased survival in the EGFR negative patients in Figure 3, although not statistically significant, is consistent with prior studies, which show an adverse prognosis for EGFR expression in CRC (7-9). Previous studies have shown variation in EGFR detection depending on the type of fixative use as well as the duration of storage (33).
In conclusion, this Phase II study demonstrated that EGFR tyrosine kinase inhibition with gefitinib may enhance the anti-tumor efficacy of FOLFOX-4 chemotherapy in patients with previously untreated metastatic CRC, but also increases toxicity. This study further adds to the growing body of evidence that targeting the EGFR pathway can sensitize some colorectal cancers to cytotoxic drugs.
ACKNOWLEDGEMENTS
We thank our colleagues who referred their patients for this study. We give special thanks to the outstanding nurses and other research and administrative staff of the Stanford University General Clinical Research Center, without whom this work would not have been possible.
Supported by NIH grants R01 CA 52168 (B. I. S.) and M01 RR 00070 (General Clinical Research Center, Stanford University School of Medicine), the Cancer Therapy Evaluation Program of the U. S. National Cancer Institute, Astrazeneca Corporation, and Sanofi-Synthelabo. Drs. Sikic and Fisher have received honoraria as consultants for Astrazeneca Corporation.
Footnotes
STATEMENT OF CLINICAL RELEVANCE
This phase II study reports a relatively high (72%) remission rate and duration of survival (10.5 months) in patients with metastatic colorectal cancer treated first-line with the IFOX regimen (5-fluorouracil, leucovorin, oxaliplatin, and the small molecule EGFR inhibitor gefitinib). These results have therapeutic implications in colorectal cancer, since most of the focus on EGFR inhibition in this disease has been with monoclonal antibodies rather than small molecule EGFR tyrosine kinase inhibitors (TKI's). Potential future trials testing this approach might compare one of the anti-EGFR monoclonal antibodies versus a TKI against the same target, with both arms receiving standard chemotherapy. Alternatively, standard first-line chemotherapy and bevacizumab could be compared to the same combination with the addition of an anti-EGFR TKI. Future directions for translational research combining chemotherapy with anti-EGFR agents should enmphasize identification of molecular determinants of response other than EGFR expression, since this has not been a useful predictive marker for colorectal cancers. The results with IFOX in colorectal cancer are in contrast to several trials in non-small cell lung cancers, which showed no benefit for the addition of EGFR inhibitors to first-line chemotherapy.
References
- 1.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–14. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 2.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 3.Lokich JJ, Ahlgren JD, Gullo JJ, Philips JA, Fryer JG. A prospective randomized comparison of continuous infusion fluorouracil with a conventional bolus schedule in metastatic colorectal carcinoma: a Mid-Atlantic Oncology Program Study. J Clin Oncol. 1989;7:425–32. doi: 10.1200/JCO.1989.7.4.425. [DOI] [PubMed] [Google Scholar]
- 4.Falcone A, Cianci C, Pfanner E, et al. Continuous-infusion 5-fluorouracil in metastatic colorectal cancer patients pretreated with bolus 5-fluorouracil: clinical evidence of incomplete cross-resistance. Ann Oncol. 1994;5:291. doi: 10.1093/oxfordjournals.annonc.a058813. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 7.Messa C, Russo F, Caruso MG, Di Leo A. EGF, TGF-alpha, and EGF-R in human colorectal adenocarcinoma. Acta Oncol. 1998;37:285–9. doi: 10.1080/028418698429595. [DOI] [PubMed] [Google Scholar]
- 8.Porebska I, Harlozinska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol. 2000;21:105–15. doi: 10.1159/000030116. [DOI] [PubMed] [Google Scholar]
- 9.Mayer A, Takimoto M, Fritz E, Schellander G, Kofler K, Ludwig H. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71:2454–60. doi: 10.1002/1097-0142(19930415)71:8<2454::aid-cncr2820710805>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1:511–29. [PubMed] [Google Scholar]
- 11.Masui H, Kawamoto T, Sato JD, Wolf B, Sato G, Mendelsohn J. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1984;44:1002–7. [PubMed] [Google Scholar]
- 12.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG, Jakobovits A. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res. 1999;59:1236–43. [PubMed] [Google Scholar]
- 13.Pollack VA, Savage DM, Baker DA, et al. Inhibition of epidermal growth factor receptor-associated tyrosine phosphorylation in human carcinomas with CP-358,774: dynamics of receptor inhibition in situ and antitumor effects in athymic mice. J Pharmacol Exp Ther. 1999;291:739–48. [PubMed] [Google Scholar]
- 14.Lichtner RB, Menrad A, Sommer A, Klar U, Schneider MR. Signaling-inactive epidermal growth factor receptor/ligand complexes in intact carcinoma cells by quinazoline tyrosine kinase inhibitors. Cancer Res. 2001;61:5790–5. [PubMed] [Google Scholar]
- 15.Ciardiello F, Caputo R, Bianco R, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–63. [PubMed] [Google Scholar]
- 16.Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885–92. [PubMed] [Google Scholar]
- 17.Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8:994–1003. [PubMed] [Google Scholar]
- 18.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Mowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial. (Abstract No. 4000).Proc. ASCO. 2007 [Google Scholar]
- 20.Herbst RS, Maddox AM, Rothenberg ML, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002;20:3815–25. doi: 10.1200/JCO.2002.03.038. [DOI] [PubMed] [Google Scholar]
- 21.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. Jama. 2003;290:2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 22.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004;22:777–84. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22:785–94. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 24.Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–7. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 25.Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmaco-dynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002;20:4292–302. doi: 10.1200/JCO.2002.03.100. [DOI] [PubMed] [Google Scholar]
- 26.Kuo T, Cho CD, Halsey J, et al. Phase II study of gefitinib, fluorouracil, leucovorin, and oxaliplatin therapy in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:5613–9. doi: 10.1200/JCO.2005.08.359. [DOI] [PubMed] [Google Scholar]
- 27.Andre T, Bensmaine MA, Louvet C, et al. Multicenter phase II study of bimonthly high-dose leucovorin, fluorouracil infusion, and oxaliplatin for metastatic colorectal cancer resistant to the same leucovorin and fluorouracil regimen. J Clin Oncol. 1999;17:3560–8. doi: 10.1200/JCO.1999.17.11.3560. [DOI] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 30.Cho CD, Fisher GA, Halsey J, Sikic BI. Phase I study of gefitinib, oxaliplatin, 5-fluorouracil, and leucovorin (IFOX) in patients with advanced solid malignancies. Invest New Drugs. 2006;24:117–23. doi: 10.1007/s10637-006-2032-7. [DOI] [PubMed] [Google Scholar]
- 31.Zampino MG, Magni E, Massacesi C, et al. First clinical experience of orally active epidermal growth factor receptor inhibitor combined with simplified FOLFOX6 as first-line treatment for metastatic colorectal cancer. Cancer. 2007;110:752–8. doi: 10.1002/cncr.22851. [DOI] [PubMed] [Google Scholar]
- 32.Cascinu S, Berardi R, Salvagni S, et al. A combination of gefitinib and FOLFOX-4 as first-line treatment in advanced colorectal cancer patients. A GISCAD multicentre phase II study including a biological analysis of EGFR overexpression, amplification and NF-kB activation. Br J Cancer. 2008;98:71–6. doi: 10.1038/sj.bjc.6604121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkins D, Reiffen KA, Tegtmeier CL, Winther H, Bonato MS, Storkel S. Immunohistochemical detection of EGFR in paraffin-embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J Histochem Cytochem. 2004;52:893–901. doi: 10.1369/jhc.3A6195.2004. [DOI] [PubMed] [Google Scholar]


