Abstract
Background & Aims
CXCL12 and CXCR4 signaling plays critical roles in development, homeostasis, and tumor metastasis. Previously we have shown that epigenetic silencing of CXCL12 in colorectal and mammary carcinoma promotes metastasis. Anoikis is an essential process of colonic epithelial turnover and limits the metastatic progression of carcinoma. We sought to determine the role for anoikis in limiting tumor metastasis upon re-expression of CXCL12 in human colorectal carcinoma cells.
Methods
Tumor formation and metastasis of colonic carcinoma cells was monitored using in vivo bioluminescence imaging. Anoikis was defined using caspase-3/7, focal adhesion kinase (FAK) and p130Cas cleavage, DNA fragmentation, and cell survival assays. Phosphorylation of ERK1/2 was monitored by immunoblot and immunohistochemistry and activity inhibited using U0126.
Results
Constitutive expression of CXCL12 in human colorectal carcinoma cells reduced orthotopic tumor formation and inhibited metastasis in SCID mice. Further, CXCL12 expression induced apoptosis specifically in non-adherent colorectal carcinoma cells. Apoptotic cell death was preceded by hypophosphorylation and cleavage of FAK and p130Cas leading to increased cellular detachment in culture and was dependent upon alterations in the extracellular matrix. Similar to in vivo colonic epithelium, CXCL12-induced anoikis of carcinoma cells was dependent upon basal ERK1/2 activation.
Conclusions
These data significantly expand the current paradigm of chemokine signaling in carcinogenesis by showing that endogenous CXCL12, in marked contrast to exogenous ligand, inhibits tumor metastasis through increased anoikis. Altered ERK1/2 signaling provides a mechanism for the dichotomy between the physiologic and pathophysiologic roles of CXCL12-CXCR4 signaling in the intestinal epithelium.
The intestinal epithelium undergoes constant and dynamic turnover. Intestinal epithelial stem cells are born in the base of the crypt and undergo a rapid migration and differentiation process up the crypt-villus axis. Culmination of this process at the villus tip in the small intestine or crypt face in the large bowel results in breakdown of the extracellular matrix (ECM) and programmed cell death of the enterocyte within three to five days.1 This process of cell death due the lack of ECM contacts, termed anoikis, plays critical roles in regulating cell numbers and innate host defense in the intestinal epithelium.2,3 Resistance to anoikis is critical for systemic spread of carcinoma cells, as they must leave the adherent environment of the primary tumor and transit the blood or lymph to reach a metastatic destination.4 Although critical to gut physiology, host defense, and inhibition of tumor cell metastasis, the molecular mechanisms regulating anoikis have yet to be fully identified.
The homeostatic chemokine receptor pair CXCL12 and CXCR4 are widely expressed throughout the body.5 CXCL12, formerly known as stromal cell-derived factor-1 (SDF-1), is a 7.8 kDa CXC chemokine.6 CXCL12 was originally described as a growth factor for developing B cells, however its chemotactic and apoptotic properties were soon after described for T cells and neuronal cells.5,7–9 Genetic ablation of CXCL12 or CXCR4 results in embryonic lethality.10–12 Previous studies by our group have established roles for CXCL12 and CXCR4 in key processes in mucosal immunity and homeostasis including endothelial tube formation in vitro13 and restitutive migration characteristic of mucosal wound healing.14,15 The role of CXCL12-CXCR4 in maintaining mucosal integrity together with the numerous developmental processes that require these molecules, establish it as a critical homeostatic signaling axis.
In contrast to the physiologic roles for CXCL12 and CXCR4, recent data also suggest that CXCR4 can mediate the directed migration of cancer cells to metastatic sites of CXCL12 expression.16 Congruent with dysregulation of mucosal homeostatic and metastatic spread during colorectal cancer we have shown disruption of the normal CXCL12-CXCR4 signaling axis specifically in transformed tissue via epigenetic silencing of the chemokine ligand.17,18 Loss of CXCL12 with maintained expression of CXCR4 imparts to metastatic cancer cells a phenotype similar to that of circulating, highly migratory leukocytes and lymphocytes. Consistent with this CXCR4-restricted phenotype metastatic colorectal carcinoma cells are highly resistant anoikis. To address the possibility that silencing of CXCL12 contributed to anoikis-resistance in colorectal carcinoma we re-established the normal autocrine expression of CXCL12 in carcinoma cells. Previously we have shown that re-expression of CXCL12 in colorectal carcinoma cells drastically reduced their anchorage-independent cell growth and metastatic invasion of the liver.17 In the present study we show that CXCL12 expressing human colorectal carcinoma cells exhibit decreased tumor formation and metastasis upon orthotopic xenograft onto the cecal wall of SCID mice. In agreement with decreased disease progression we show that re-expression of CXCL12 in those cells increased cellular anoikis. Analogous to in vivo intestinal epithelial anoikis, we demonstrate accelerated breakdown of the focal adhesion complex, decreased maintenance of extracellular matrix, and tonic extracellular-regulated kinase-1/2 (ERK1/2) signaling, upon CXCL12 re-expression in colorectal carcinoma cells. Taken together with our previous work, these studies suggest that the metastatic potential of colorectal carcinoma cells is increased through dysregulated ERK1/2 signaling upon epigenetic silencing of CXCL12.
Materials and Methods
Human colorectal carcinoma cells and stable cell lines
HT29 (HTB-38) and HCT116 (CCL-247) colorectal carcinoma cells were purchased from the ATCC (Rockville, MD) and maintained as previously described. 14 HT29 cells were transfected with pcDNA3.1 (Invitrogen) encoding either the human CXCL12α mRNA transcript or eGFP as defined previously.17 HCT116 cells were transfected with plasmid encoding firefly luciferase. Stable plasmid integration was selected for using Zeocin, (Invitrogen, Carlsbad, CA) yielding the HCT-luc cell line. These cells were then transfected with the pcDNA 3.1 plasmid encoding either human CXCL12 or eGFP and stable plasmid integration was selected using G418 sulfate (EMD Biosciences).
SCID mouse bioluminescence metastasis assays
Using protocols approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee, HCT-luc-eGFP or HCT-luc-CXCL12 cells suspended in a 50 µl volume of sterile PBS were implanted to the cecal wall (2×106 cells) of eight-week old female SCID mice (cr-Prdkcscid, Charles Rivers, Wilmington, MA). Mice were monitored by bioluminescence imaging for tumor development using the Lumina IVIS-100 In Vivo Imaging System (Xenogen Corp, Alameda, CA) as detailed previously.18 Mice were imaged from 10 seconds with medium binning and an f-stop of 1. Regions of Interest (ROIs) were created and measured as area flux, defined as radiance (photons/second/cm2/steradian) according to the manufacturer’s calibration (Xenogen).
Cell survival assays
Non-adherent cells from indicated cultures and stimulation conditions were gathered, washed and re-plated into 12-well culture dishes and cultured with fresh medium every 48 hours for 1–2 weeks at which point the adherent cells were stained with crystal violet and solublized in 2% (v/v) SDS. Absorbance at 595 nM was assayed as a measure of cell survival (Victor Wallac)
Cell detachment assays
HT29 or HCT116 cells were grow to 100% confluence with fresh media replacement every 48 hours. Growth medium was replaced with serum-free medium and not renewed for the duration of the experiment. At indicated time points, adherent cells were stained with crystal violet and solublized in 2% (v/v) SDS and levels of adherent cells expressed as absorbance 595 nM (Victor Wallac). Where indicated, culture dishes were coated with 10 µg/ml of Laminin or Fibronectin (Sigma, St. Louis), prior to addition of cells.
Extracellular matrix adhesion assays
HT29 cells expressing CXCL12 or eGFP were cultured to 100% confluence at which point media was conditioned for three days. Cells were removed using enzyme-free cell disassociation buffer (Invitrogen). Wild-type (WT) HT29 cells were cultured to ~50% confluence, serum starved overnight and detached from the tissue culture plate in enzyme-free disassociation buffer. WT cells were washed and loaded with Calcein-AM (Molecular Probes Eugene, OR), counted, and applied (5×105) to matrices of previously cultured and subsequently removed CXCL12 or eGFP-expressing-HT29 cells. Cell-free wells were coated with BSA (10 µg/ml) as a background control for adherence. Cells were allowed to adhere for ~1 hour at 37°C in serum-free media at which point wells were vigorously washed three times using PBS. Adherence was assayed by fluorescence spectroscopy using a standard curve of known cell number (Victor Wallac).
In separate experiments, 96-well plates were pre-coated with BSA or Laminin. HCT-L-CXCL12 or HCT-L-eGFP cells were cultured to ~50% confluence and adherent cells removed using enzyme-free cell disassociation buffer. Cells were washed, counted and applied to coated wells. Cells (5×104) were allowed to adhere for ~1 hour in serum free medium at 37°C at which point wells were vigorously washed three times and adherence quantified by luminescence.
Results
Autocrine CXCL12 expression in colorectal carcinoma cells decreased orthotopic primary tumor formation and metastasis
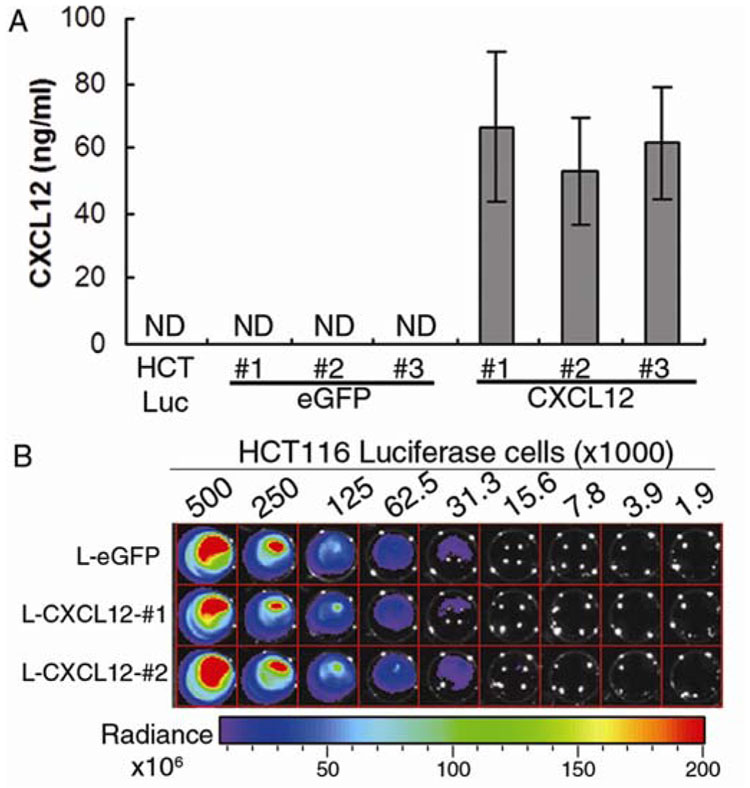
Previously we have shown that CXCL12 and CXCR4 are co-expressed in intestinal epithelial cells and that the ligand, but not the receptor, is silenced in colorectal carcinoma due to promoter hypermethylation. Further, re-expression of CXCL12 drastically reduced liver metastasis upon direct injection of colorectal carcinoma cells into the portal vein.17 In light of these data, we sought to utilize in vivo bioluminescent imaging to characterize both primary tumor formation and subsequent systemic metastasis of colorectal carcinoma cells upon orthotopic engraftment onto the cecal wall. To this end, we created double-stable cell lines expressing luciferase and either CXCL12 (L-CXCL12) or eGFP (L-eGFP) as a control. As we have noted previously for parental HCT116 and other colorectal carcinoma cell lines, CXCL12 was not expressed in the single-stable luciferase line or in the double-stable L-eGFP lines (Figure 1A).17 Secreted CXCL12, which we have previously shown to be functional, is readily detectable in the conditioned medium of L-CXCL12 cells (Figure 1B).17 Several clones of each line were selected and verified for equal expression of luciferase, allowing us to use photon quantification not only as a marker for tumor cell location, but also a comparable measure of cell number between the eGFP and CXCL12-expressing cell lines (Figure 1B).
Figure 1. Expression of CXCL12 in human colorectal carcinoma cells.
(A) CXCL12 or eGFP was stably transfected into a clonal population of HCT116 cells expressing luciferase, creating double-stable lines expressing luciferase and eGFP (L-eGFP) or luciferase and CXCL12 (L-CXCL12). CXCL12 was readily detectable in conditioned medium from L-CXCL12 cells lines. (B) Several double-stable clones of each line were selected and verified for equal expression of luciferase. Heat map indicates luminescence defined as Radiance (photons/second/cm2/steradian) in titrated numbers of cells.
Using in vivo imaging we determined that L-CXCL12 primary tumors were established nearly exclusively at the site of engraftment while L-eGFP control cells were readily detectable throughout the intestinal tract within 5 weeks (Figure 2A). Longitudinal studies across several mice indicated that CXCL12 expression decreased overall tumor expansion and colonization subsequent to engraftment to the cecal wall of SCID mice (Figure 2B). Cell cycle analysis revealed that cellular proliferation in CXCL12 expressing cells was not different from the eGFP control cells (% dividing cells in G2/S phase after 5-days in culture medium was: eGFP = 18.2 ± 3.3; CXCL12 = 22.5 ± 0.9). Autocrine CXCL12 decreased the survival of colorectal carcinoma cells specifically during non-adherent cellular dissemination to ectopic tissues as quantification of ex-vivo images of the intestinal tract and liver defined decreased local (gastric) and systemic (hepatic) organ metastasis following intestinal engraftment of CXCL12 expressing cells as compared to control eGFP cells (Figure 2C and 2D). Similarly, decreased metastasis and colonization of CXCL12-expressing colonic carcinoma cells was obtained following injection of those cells to the portal vein of SCID mice (not shown), in agreement with our previous reports. 17,18 Taken together, these data suggest that autocrine CXCL12 expression inhibits metastasis, a phenomenon which is subverted in in vivo colorectal carcinoma disease progression by epigenetic silencing ofCXCL12. 17
Figure 2. Autocrine CXCL12 expression inhibits primary tumor expansion and metastasis upon orthotopic xenograft.
(A) L-eGFP (n=10) or L-CXCL12 (n=10) cells were orthotopically xenografted to the cecum of SCID mice and tumor growth monitored via bioluminescent imaging. Representative luminescence images of the same mice taken at time of engraftment (T0) and five weeks post-engraftment (5wk), showing tumor growth and metastatic spread of L-eGFP and L-CXCL12 cells. (B) Real-time in vivo imaging values indicated decreased (P≤0.05) overall tumor formation and expansion upon autocrine CXCL12 re-expression in colorectal carcinoma cells. Data presented are the percentage of area flux normalized to the initial T0 values following injection for each mouse. (C) Metastasis was quantified (Area Flux) by ex-vivo imaging of specific internal organs. Gastric metastasis of intestinally engrafted tumors was decreased upon CXCL12 re-expression in colorectal carcinoma cells. (D) Systemic hepatic metastasis was further decreased upon CXCL12 re-expression in colorectal carcinoma cells (P≤0.05).
CXCL12 expression increases apoptosis in non-adherent colorectal carcinoma cells
We next sought to define a mechanism by which autocrine CXCL12 expression in colorectal carcinoma cells inhibited their metastasis and primary tumor formation. Observations in culture indicated that an increase in non-adherent CXCL12-expressing cells, with those cells appearing phenotypically similar with apoptotic cells. In contrast, the vast majority of non-adherent cells in eGFP control cultures remained cellular and phase positive (Figure 3A). To further investigate this phenotype caspase activity was compared between wild-type, CXCL12, and eGFP-expressing cells. Consistent with the gross morphology, caspase-3/7 activity was markedly increased in CXCL12-expressing cells relative to eGFP control cells when adherent and non-adherent cells were pooled (Figure 3B). Moreover, separate clones indicated that increased caspase activity was dose dependent with respect to CXCL12 expression level (Figure 3B). Caspase activity was found to be confined to the non-adherent cell population as CXCL12 expression had no effect on caspase activity of adherent cells. In fact, adherent CXCL12 expressing cells were more resistant to direct caspase activation then eGFP cells upon co-stimulation with TNFα (20 ng/ml) and IFNγ (40 ng/ml) (Figure 3C). A drastic increase in CXCL12-induced caspase-3/7 activity was observed when just the non-adherent cell population was isolated. This increase was again dependent upon CXCL12 expression level (Figure 3D). Consistent with cell morphology and caspase activity, non-adherent CXCL12 expressing cells did not survive nearly as well as eGFP control cells when allowed to re-adhere to fresh culture dishes (Figure 3E). Taken together, these data suggest that constitutive CXCL12 expression induces cell death upon cellular detachment.
Figure 3. CXCL12 expression increases caspase activity and cell death in non-adherent colorectal carcinoma cells.
(A) The adherent phenotype of HT29 cells was similar to that of eGFP control cells, while more CXCL12 expressing cells were noted in the non-adherent fraction (non-adh 100x), from cultures of equal confluence. Further, these non-adherent cells appear apoptotic (non-adh 400x). (B) CXCL12 expression increases caspase-3/7 activity in pooled, adherent and non-adherent colorectal carcinoma cells. (C) CXCL12-induced caspase activity was not observed in adherent colorectal carcinoma cells. Cells stimulated with TNFα and IFNγ are shown as positive control for direct caspase activation. (D) CXCL12-induced caspase activity was restricted to non-adherent cells. Data in panels B–D are representative of three independent experiments completed in triplicate. (E) CXCL12 expression increases non-adherent colorectal carcinoma cell death. Non-adherent cells were gathered and plated in fresh culture plates, stained with crystal violet and solublized as a measure (abs 595nM) of non-adherent cell survival. * indicates statistically significant difference between eGFP and CXCL12 expressing cells (P≤ 0.05, n=3).
Constitutive CXCL12 expression results in focal adhesion breakdown
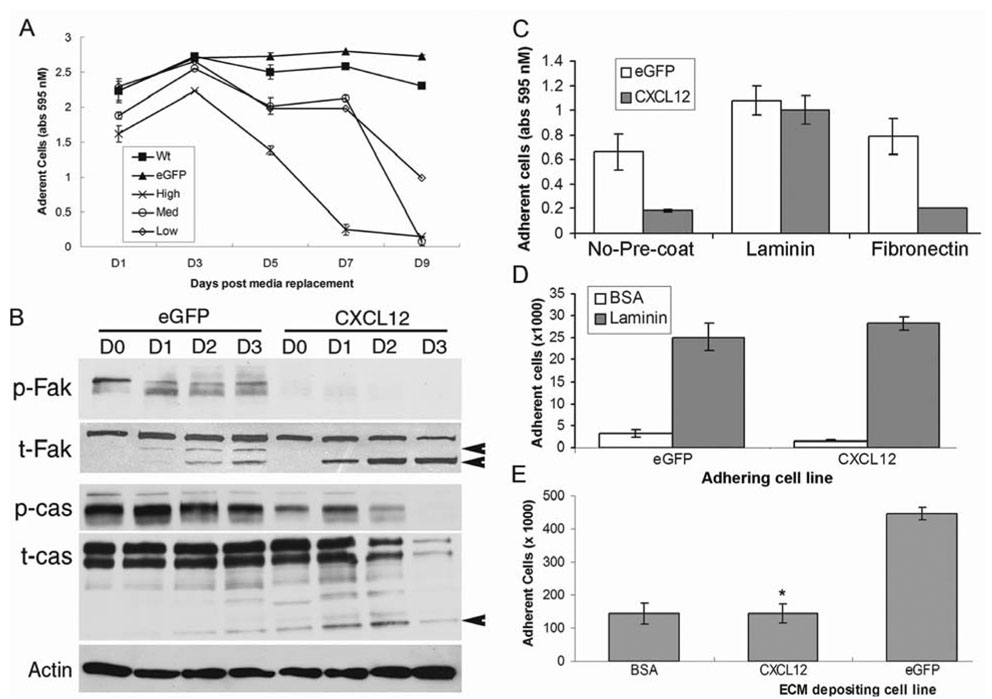
Several molecular mechanisms, in addition to caspase activity, preceding and during the process of epithelial cell anoikis have begun to be identified.19 Recent data suggest that focal adhesion kinase (FAK) and p130Cas (CAS), two proteins critical in maintenance of survival signals emanating from epithelial cell focal adhesions become hypophosphorylated and proteolytically degraded during anoikis.20,21 Concurrent with those processes of anoikis, we showed that CXCL12-expressing colorectal carcinoma cells plated to tissue culture plates exit the adherent compartment at a faster rate than WT or control eGFP cells (Figure 4A). The increased detachment of CXCL12 expressing colorectal carcinoma cells was preceded by FAK and CAS hypophosphorylation and cleavage (Figure 4B).
Figure 4. CXCL12 expression in colorectal carcinoma cells modulates extracellular matrix and increases focal adhesion breakdown.
(A) CXCL12 expression increases colorectal carcinoma cell detachment. Adherent, control (WT and eGFP) or CXCL12 expressing (High, Med, Low) cells were grown to ~100% confluence, stained and solublized at the indicated time points. (B) CXCL12 expression decreases phosphorylation (p-FAK and p-CAS) and accelerates cleavage of total FAK and CAS total protein (t-FAK and t-Cas) in colorectal carcinoma cells (arrowheads indicate defined cleavage products for FAK and CAS). (C) Exogenous laminin rescues CXCL12-induced cell detachment. Cells were cultured in conditioned medium for 8 days and adherent cells were stained and solublized. Where indicated, wells were coated with laminin or fibronectin prior to addition of cells. (D) CXCL12 expression does not affect cellular binding to laminin. CXCL12-expressing and eGFP control cells were allowed to adhere to laminin coated wells for 1 hour and adherent cells assayed. Experiments shown are representative of three independent experiments. (E) CXCL12 expression decreases cellular maintenance of ECM. CXCL12 and eGFP cells were cultured to 100% confluence, released and WT cells deposited onto those matrixes. * indicates a statistically significant difference between eGFP and CXCL12 matrix deposition (P≤0.05, n=3).
Laminin is a major epithelial-derived ECM component of the basement membrane that underlies intestinal epithelial cells.22 Consistent with that notion, adherence was maintained similar to control levels when CXCL12-expressing cells were cultured on laminin coated but not fibronectin coated wells (Figure 4C). These data suggest that constitutive CXCL12 expression may modulate expression of laminin receptors or, alternatively, maintenance of ECM components, leading to increased detachment in conditioned culture (Figure 4A and 4C). We first sought to define potential cellular changes in laminin receptors, such as the various heterodimers of α and β1integrins, following re-expression of CXCL12. To this end, subconfluent CXCL12-expressing and control eGFP cells were disassociated from culture wells and allowed to adhere to laminin coated wells for a short period of time. We found no difference in the ability of CXCL12-expressing cells to adhere to laminin as compared to control cells, suggesting no influence of CXCL12 expression on laminin specific integrin expression (Figure 4D). Next we sought to define CXCL12-mediated alterations in extracellular matrix. Here, confluent monolayers of CXCL12-expressing or eGFP control cells were disassociated from tissue culture wells, and the wild-type parental cell line was allowed to adhere to matrix components deposited by the initial cell monolayers. Matrix deposited from CXCL12-expressing cells supported drastically less WT cell adhesion than matrix deposited by eGFP control cells (Figure 4E). These data suggest that constitutive CXCL12 expression by colorectal carcinoma cells alters the ECM microenvironment, perhaps leading to anoikis-related focal adhesion complex breakdown, and cellular detachment.
CXCL12 expression in colorectal carcinoma cells induces anoikis
By definition anoikis is apoptosis induced by disruption of the interactions between epithelial cells and extracellular matrix.2 Having defined disruption of extracellular matrix contacts we sought to further identify induction of apoptotic processes in CXCL12 expressing colorectal carcinoma cells under non-adherent culture conditions. Previous studies have suggested that anoikis results in activation of both the extrinsic and intrinsic pathways of apoptosis.19 Thus, we sought to define caspase-3/7 activation and DNA fragmentation, two apoptotic events that encompass both apoptotic pathways. When plated over poly-HEMA to prevent adherence, CXCL12-expressing cells demonstrated both increased caspase-3/7 activity and cleaved caspase- 3 and caspase-7 proteins compared to control cells (Figure 5A and 5B). Increased caspase activation was specific to anoikis as treatment with a known caspase activator resulted in similar caspase-3 and caspase-7 cleavage in both CXCL12 and eGFP control cell lines (Figure 5B). Further, upon non-adherent culture on poly-HEMA, CXCL12 expression dose dependently induced increased DNA fragmentation, a critical endpoint to apoptotic cell death (Figure 5C). CXCL12-induced DNA degradation was anoikis specific as no differences in DNA degradation were noted in adherent cells (D0) or upon apoptosis induction with gliotoxin (Figure 5D). These data indicate that CXCL12 expression sensitizes colorectal carcinoma cells to non-adherent apoptotic cell death.
Figure 5. CXCL12 expression increases colorectal carcinoma cell anoikis.
(A) Increased caspase activation in separate HCT116 cell clones expressing CXCL12 (#1, #2, #3) compared eGFP control cells cultured on poly-HEMA. Data are representative of three independent experiments ± SD of triplicate samples. (B) Increased cleavage to active, cleaved caspase-3 and caspase-7 in HT29 cells expressing CXCL12 and cultured on poly-HEMA. Treatment with gliotoxin ensured both cell lines activate caspases equally. (C) Increased percentage of CXCL12-expressing cells contained fragmented DNA after four days on poly-HEMA. Data in B and C are representative of 3–5 independent experiments. (D) DNA fragmentation is increased by CXCL12 expression specifically upon non-adherent culture. No differences in the number of apoptotic (sub G1) cells were noted between CXCL12 and control cells recently removed from adherence (D0) or upon apoptotic induction with gliotoxin. Data are the mean values of three independent experiments completed in duplicate ± SD. * indicates statistical significance between the percentage of sub-G1 CXCL12 expressing cells compared to control cells (P≤0.05).
CXCL12 expression induces anoikis related, sustained basal ERK1/2 phosphorylation
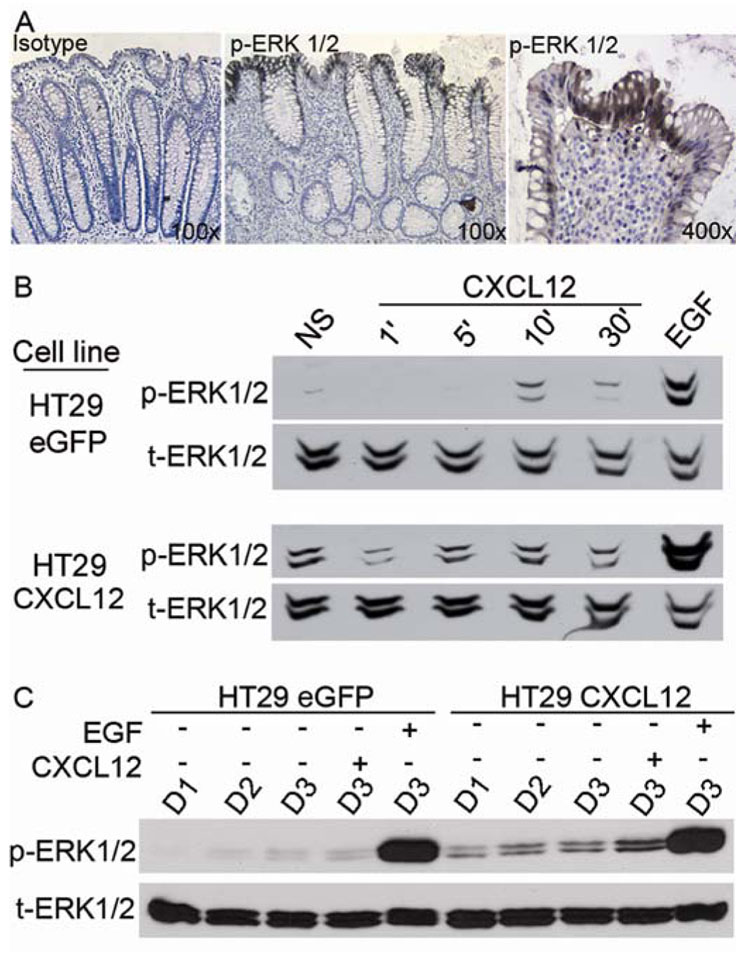
There is an increasing amount of evidence suggesting the involvement of the ERK1/2 pathway in anoikis.23,24 To more specifically define the mechanism by which constitutive CXCL12 induced anoikis, we examined ERK1/2 signaling in colorectal carcinoma cells re-expressing CXCL12. Phosphorylation of ERK1/2 was predominantly localized near and at the surface of the human colonic epithelium (Figure 6A). As immunohistochemistry would not detect transient phosphorylation, our positive immunostaining suggests sustained ERK1/2 signaling in intestinal epithelial cells at the apex of crypt-surface transit. This staining pattern of phosphorylated ERK1/2 is very similar to what has been previously described for cleaved caspase-3 in cells undergoing anoikis.25
Figure 6. CXCL12 expression induces anoikis related, basal ERK1/2 activation.
(A) Consistent with their anoikis status, phosphorylated ERK1/2 in the human colon was confined to the upper crypt and surface epithelium. Fields shown are representative of multiple crypts from two separate patient samples. (B) Low-level, transient ERK1/2 phosphorylation in HT29 control eGFP cells stimulated with exogenous CXCL12 (50 ng/ml) compared to the response of EGF (50 ng/ml) at 30 minutes (upper panels). Low-level ERK1/2 phosphorylation was constitutive in HT29 cells expressing CXCL12. Comparable immunoblot analyses shown were acquired with the same exposure time. (C) Basal ERK1/2 phosphorylation was enhanced by CXCL12 expression over several days (D1–D3) in conditioned culture. Stimulation of three day serum starved cells with CXCL12 does not elicit strong ERK1/2 phosphorylation relative to EGF. Experiments in panel B and C are each representative of three independent experiments.
Thus, we next sought to define the phosphorylation status of ERK1/2 in our CXCL12 re-expressing compared to control controls. As we have previously shown, exogenous stimulation of colorectal carcinoma cells lacking endogenous CXCL12 induced a minimal transient phosphorylation of ERK1/2.14 In contrast, endogenous expression of CXCL12 evoked sustained, low level phosphorylation of ERK1/2 (Figure 6B). Basal ERK1/2 phosphorylation was observed over a number of days when the cells remained in their conditioned medium. Further, transient stimulation of serum-starved CXCL12-expressing cells with exogenous CXCL12 elicited little, if any, ERK1/2 response, as compared to the strong response elicited in response to epidermal growth factor (EGF) under those conditions (Figure 6C). These data illustrate differences between exogenous and endogenous CXCL12 in terms of transient and tonic activation of ERK1/2. They also suggest that activation of ERK1/2 via CXCL12-CXCR4 signaling may be playing roles other than the pro-survival and pro-mitogenic effects of strong ERK1/2 activators such as EGF.
CXCL12-mediated anoikis in colorectal carcinoma cells is dependent upon ERK1/2
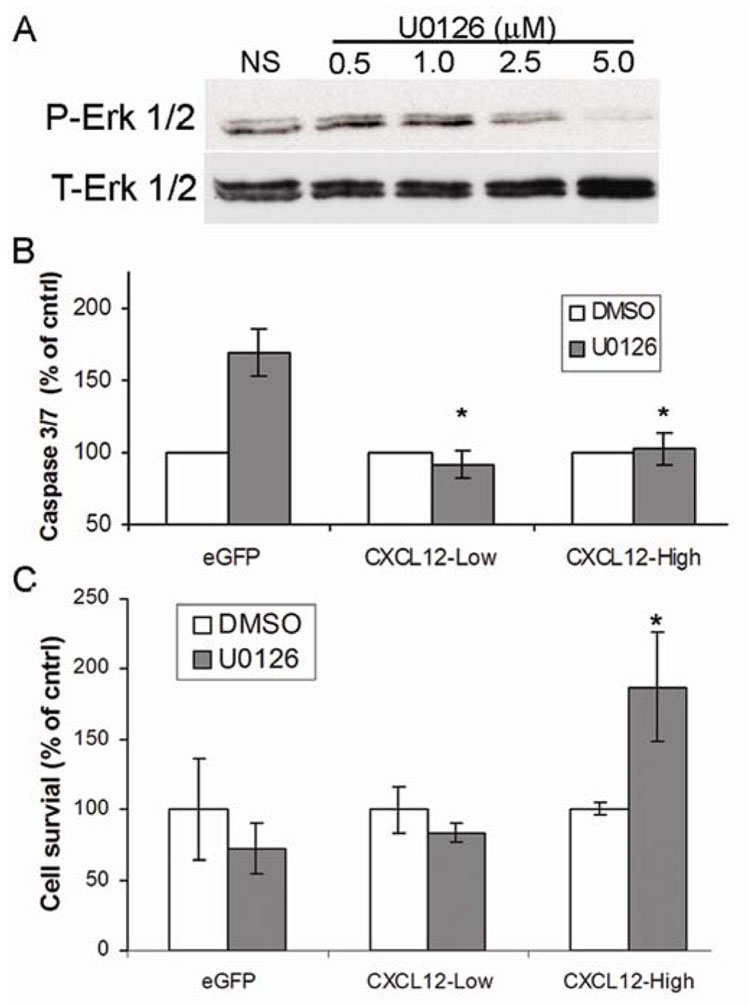
We next sought to define the role of basal ERK1/2 activity in CXCL12-mediated anoikis of colorectal carcinoma cells. To this end, CXCL12-expressing and eGFP control cells were treated with the MEK1 inhibitor U0126 and caspase activity and cell survival were assayed. Phospho-specific immunoblot analysis for ERK1/2 upon treatment with U0126 verified MEK inhibition over a 48 hour time course (Figure 7A). Consistent with the notion that ERK1/2 signaling must be tightly regulated in anoikis-resistant carcinoma cells, treatment with U0126 increased caspase-3/7 activity in control cells (Figure 7B). In contrast, although absolute values for caspase activity were higher in non-adherent CXCL12 expressing cells (Figure 3D) ERK1/2 inhibition did not further increase this activity (Figure 7B). The marked difference in caspase activation between control cells and CXCL12-expressing cells upon MEK inhibition was statistically significant. Further, the role of ERK1/2 in non-adherent colorectal carcinoma cell survival was dependent upon CXCL12 expression level as shown in two separate stable cell lines. As shown in Figure 7C, non-adherent survival of CXCL12 lacking eGFP control cells was decreased upon ERK1/2 inhibition, while similar inhibition significantly increased non-adherent survival of cells expressing high levels of CXCL12 (Figure 7C). Non-adherent survival of cells expressing low levels of CXCL12 was largely unaltered by MEK inhibition (Figure 7C). These data demonstrate the dose relationship between autocrine CXCL12 expression and ERK1/2 during anoikis. Taken together, these results suggest that dysregulation of ERK1/2 signaling, either low level activity or pharmacological inhibition, contributes to anoikis.
Figure 7. CXCL12 induced ERK1/2 activity contributes to colorectal carcinoma cell anoikis.
(A) Two, 24 hour treatments of HT29 cells with the specific MEK inhibitor U0126 (5 µM) efficiently inhibited ERK1/2 phosphorylation. (B) ERK1/2 inhibition does not increase non-adherent caspase activity in CXCL12-expressing colorectal carcinoma cells. CXCL12 expressing or eGFP control HT29 cells were cultured and treated as described in panel A and non-adherent cells were gathered and assayed for caspase-3/7 activity. * indicates statistical difference in caspase activity between eGFP and CXCL12 expressing cells upon ERK1/2 inhibition (P≤0.05). (C) ERK1/2 inhibition increases non-adherent cell survival of CXCL12 expressing colorectal carcinoma cells. * indicates statistical difference between actual survival of CXCL12-high cells treated with or without U0126 (P≤0.05). Data in panel B and C normalized to DMSO control treated cells and are values ± SEM of three independent experiments completed in triplicate.
Discussion
Intestinal epithelial cell anoikis is an essential homeostatic process in maintaining proper cell number and turnover in the gut. Cancer cells resist anoikis during tumor formation and progression. Previously we have shown that CXCL12-CXCR4 are expressed and play important physiological roles in human intestinal epithelial cells.14,15 In concordance with resistance to anoikis and breakdown of mucosal homeostasis we have shown that CXCL12 is epigenetically silenced in colorectal carcinoma.17 These previous studies were the first to define the silencing of CXCL12 in carcinoma and demonstrate its impact on directed carcinoma cell metastasis.17,18 Here we utilize a model of primary carcinoma development that progresses to systemic metastasis upon intestinal xenograft. Coupled with in vivo bioluminescent imaging, the current study clearly indicates that autocrine CXCL12 expression decreases both primary tumor expansion and colonization and inhibits metastasis of colorectal carcinoma cells.
The classic paradigm for chemokines in carcinoma cell metastasis has been that chemokine receptors on carcinoma cells function to draw those cells to metastatic sites of elevated chemokine ligand expression, analogous to leukocytes migrating along chemotactic gradients 26. However, several recent reports have made it clear that CXCL12 stimulation affects cells in ways other than chemotactic responses.7,8,18,27,28 Therefore, it is not well understood how cancer cells utilize this homeostatic signaling axis for pathogenic purposes. By re-establishing autocrine CXCL12 production in transformed carcinoma cells with pathologically silenced expression, this study details anoikis-resistance as a potential mechanism of how carcinoma cells hijack the chemokine system during metastasis. Moreover, this work defines a divergence in tumor cell signaling subsequent to this aberrant epigenetic event.
Using a variety of measures for apoptosis we show that overexpression of CXCL12 induces anoikis in colorectal carcinoma cells. In addition to cell death upon non-adherent culture, autocrine expression of CXCL12 also accelerated cellular detachment. This detachment was coupled with breakdown of the focal adhesion complex and dependent upon alterations in laminin in the extracellular matrix. The coupling of increased cell shedding and sensitivity to cell death following detachment from the substratum thoroughly recapitulates the anoikis process of normal intestinal epithelium. These data suggest that physiologic, endogenous expression of CXCL12 limits tumor metastasis and participates in the homeostatic turnover of intestinal epithelial cells.
CXCL12-induced anoikis, but not cell shedding, was not as strong in HCT116 cells as observed in HT29 cells even though HCT116 stable cells produce more CXCL12.17 These data are supported by prior reports suggesting that mutations in the Ras monomeric G-protein pathway function in anoikis resistance, as HCT116 cells harbor Ras mutations and HT29 cells are Ras wild-type.2,29 Further, differences in mutational status contributing to increased resistance to CXCL12-induced anoikis is evidenced by our work showing CXCL12 re-expression in Ras mutated mammary carcinoma cells does not affect anoikis, but instead increases cellular proliferation.18 Together with previous reports our data support the notion that the Ras, Raf, MEK, ERK pathway is a key regulator of intestinal epithelial and colorectal carcinoma cell anoikis.
Previous reports concluded that ERK1/2 signaling aids in anoikis-resistance, as caspase activity is increased in detached, non-adherent intestinal epithelial cells subsequent to ERK1/2 inhibition. 30 Those data are consistent with what we observed in our eGFP control cells. Thus, lack of this increased caspase activity upon ERK1/2 inhibition in CXCL12 expressing cells extends those findings and suggests tonic ERK1/2 activation also supports anoikis. Accordingly, we show evidence to suggest that ERK1/2 phosphorylation is sustained in the human colonic epithelium, with activation largely restricted to cells in the upper crypt and surface epithelium, a compartment known to be undergoing anoikis.25 It is notable that we and others have observed the most intense staining for CXCL12 in this same upper crypt to surface epithelium compartment.17,31,32
Further, data shown here and supported by our previous studies indicate that exogenous CXCL12 stimulation of colorectal carcinoma cells results in transient ERK1/2 phosphorylation.14 This transient response is in stark contrast to the anoikis-related, constitutive ERK1/2 phosphorylation defined in cells re-expressing autocrine CXCL12. These data may explain why increased caspase activity is not observed when cells are stimulated with exogenous CXCL12 (data not shown). In fact, recent reports suggest that exogenous CXCL12 stimulation can enhance cell survival.33–35 It is tempting to speculate that exogenous stimulation of CXCR4-expressing cells with CXCL12 is analogous to endocrine signaling in vivo. In this case, sensing of ectopic CXCL12 by CXCR4-expressing/CXCL12-null carcinoma cells would enhance their survival and metastasis. This situation is in marked contrast to the system described herein where endogenous, autocrine CXCL12 induces anoikis and inhibits carcinoma disease progression independent of changes in cell growth. Further, autocrine re-expression of CXCL12 resulted in altered ECM and focal adhesion breakdown, leading to cell detachment, and ERK1/2-dependent anoikis, steps consistent with homeostatic cell turnover in the intestinal epithelium. These data present a distinct mechanism linking the physiologic and pathophysiologic roles of the CXCR4-CXCL12 signaling axis.
Supplementary Material
Acknowledgments
Grant Support: These studies were supported in part by grants from the Cancer Center of the Medical College of Wisconsin and the American Cancer Society (IRG-84-004) and the National Institutes of Health (DK062066).
Abbreviations
- ECM
Extracellular matrix
- IEC
intestinal epithelial cell
- FAK
focal adhesion kinase
- ERK
extracellular-regulated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: There are no conflicts of interest to disclose.
References
- 1.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 2.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall PA, Coates PJ, Ansari B, et al. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- 5.Bleul CC, Fuhlbrigge RC, Casasnovas JM, et al. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 7.Colamussi ML, Secchiero P, Gonelli A, et al. Stromal derived factor-1 alpha (SDF-1 alpha) induces CD4+ T cell apoptosis via the functional up-regulation of the Fas (CD95)/Fas ligand (CD95L) pathway. J Leukoc Biol. 2001;69:263–270. [PubMed] [Google Scholar]
- 8.Hesselgesser J, Taub D, Baskar P, et al. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 9.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 11.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 12.Zou YR, Kottmann AH, Kuroda M, et al. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 13.Heidemann J, Ogawa H, Dwinell MB, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 14.Smith JM, Johanesen PA, Wendt MK, et al. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G316–G326. doi: 10.1152/ajpgi.00208.2004. [DOI] [PubMed] [Google Scholar]
- 15.Moyer RA, Wendt MK, Johanesen PA, et al. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest. 2007;87:807–817. doi: 10.1038/labinvest.3700595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 17.Wendt MK, Johanesen PA, Kang-Decker N, et al. Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis. Oncogene. 2006;25:4986–4997. doi: 10.1038/sj.onc.1209505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wendt MK, Cooper AN, Dwinell MB. Epigenetic silencing of CXCL12 increases the metastatic potential of mammary carcinoma cells. Oncogene. 2008;27:1461–1471. doi: 10.1038/sj.onc.1210751. [DOI] [PubMed] [Google Scholar]
- 19.Grossmann J. Molecular mechanisms of "detachment-induced apoptosis--Anoikis". Apoptosis. 2002;7:247–260. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- 20.Wei L, Yang Y, Zhang X, et al. Cleavage of p130Cas in anoikis. J Cell Biochem. 2004;91:325–335. doi: 10.1002/jcb.10760. [DOI] [PubMed] [Google Scholar]
- 21.Grossmann J, Artinger M, Grasso AW, et al. Hierarchical cleavage of focal adhesion kinase by caspases alters signal transduction during apoptosis of intestinal epithelial cells. Gastroenterology. 2001;120:79–88. doi: 10.1053/gast.2001.20879. [DOI] [PubMed] [Google Scholar]
- 22.Beaulieu JF, Vachon PH, Chartrand S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol (Berl) 1991;183:363–369. doi: 10.1007/BF00196837. [DOI] [PubMed] [Google Scholar]
- 23.Rul W, Zugasti O, Roux P, et al. Activation of ERK, controlled by Rac1 and Cdc42 via Akt, is required for anoikis. Ann N Y Acad Sci. 2002;973:145–148. doi: 10.1111/j.1749-6632.2002.tb04624.x. 145–148. [DOI] [PubMed] [Google Scholar]
- 24.Cagnol S, Obberghen-Schilling E, Chambard JC. Prolonged activation of ERK1,2 induces FADD-independent caspase 8 activation and cell death. Apoptosis. 2006;11:337–346. doi: 10.1007/s10495-006-4065-y. [DOI] [PubMed] [Google Scholar]
- 25.Grossmann J, Walther K, Artinger M, et al. Induction of apoptosis before shedding of human intestinal epithelial cells. Am J Gastroenterol. 2002;97:1421–1428. doi: 10.1111/j.1572-0241.2002.05787.x. [DOI] [PubMed] [Google Scholar]
- 26.Moore MA. The role of chemoattraction in cancer metastases. Bioessays. 2001;23:674–676. doi: 10.1002/bies.1095. [DOI] [PubMed] [Google Scholar]
- 27.Ueda Y, Neel NF, Schutyser E, et al. Deletion of the COOH-terminal domain of CXC chemokine receptor 4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells. Cancer Res. 2006;66:5665–5675. doi: 10.1158/0008-5472.CAN-05-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rak J, Mitsuhashi Y, Erdos V, et al. Massive programmed cell death in intestinal epithelial cells induced by three-dimensional growth conditions: suppression by mutant c-H-ras oncogene expression. J Cell Biol. 1995;131:1587–1598. doi: 10.1083/jcb.131.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loza-Coll MA, Perera S, Shi W, et al. A transient increase in the activity of Src-family kinases induced by cell detachment delays anoikis of intestinal epithelial cells. Oncogene. 2005;24:1727–1737. doi: 10.1038/sj.onc.1208379. [DOI] [PubMed] [Google Scholar]
- 31.Agace WW, Amara A, Roberts AI, et al. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr Biol. 2000;10:325–328. doi: 10.1016/s0960-9822(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 32.Brand S, Dambacher J, Beigel F, et al. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res. 2005;310:117–130. doi: 10.1016/j.yexcr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Larsen PH, Hao C, et al. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. %20. [DOI] [PubMed] [Google Scholar]
- 34.Vlahakis SR, Villasis-Keever A, Gomez T, et al. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169:5546–5554. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- 35.Dewan MZ, Ahmed S, Iwasaki Y, et al. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60:273–276. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.