Abstract
Eyeblink classical conditioning (EBC) was observed across a broad developmental period with tasks utilizing two interstimulus intervals (ISIs). In ISI discrimination, two distinct conditioned stimuli (CSs; light and tone) are reinforced with a periocular shock unconditioned stimulus (US) at two different CS-US intervals. Temporal uncertainty is identical in design with the exception that the same CS is presented at both intervals. Developmental changes in conditioning have been reported in each task beyond ages when single-ISI learning is well developed. The present study sought to replicate and extend these previous findings by testing each task at four separate ages. Consistent with previous findings, younger rats (postnatal day – PD - 23 and 30) trained in ISI discrimination showed evidence of enhanced cross-modal influence of the short CS-US pairing upon long CS conditioning relative to older subjects. ISI discrimination training at PD43-47 yielded outcomes similar to those in adults (PD65-71). Cross-modal transfer effects in this task therefore appear to diminish between PD30 and PD43-47. Comparisons of ISI discrimination with temporal uncertainty indicated that cross-modal transfer in ISI discrimination at the youngest ages did not represent complete generalization across CSs. ISI discrimination undergoes a more protracted developmental emergence than single-cue EBC and may be a more sensitive indicator of developmental disorders involving cerebellar dysfunction.
Keywords: Eyeblink conditioning, ISI discrimination, development, cross-modal transfer, rats
Introduction
Appropriate ontogenetic comparisons of learning and memory capabilities require consideration of age-related differences in what is learned in a given situation (c.f., Spear, 1984; Spear & Kucharski, 1984; Spear & Molina, 1987). Young humans and rats appear more likely than adults to encode stimuli in terms of “amodal” properties (e.g., intensity; Kraebel & Spear, 2000; Lewkowicz & Turkewitz, 1980; Mellon, Kraemer, & Spear, 1991; Turkewitz & Mellon, 1989), and therefore may perceive greater similarity between discrete stimuli detected through different sensory systems (e.g., lights, tones). These proposed differences may contribute to enhanced cross-modal transfer in young rats relative to adults (Molina, Hoffman, Serwatka, & Spear, 1991; Spear & Molina). Cross-modal transfer of conditioning is typically demonstrated by (phase 1) training with a discrete CS (e.g., light) that facilitates subsequent (phase 2) conditioning in a similar task using a CS detected through a different sensory modality (e.g., tone; see Kehoe & Holt, 1984). While cross-modal transfer has been documented across multiple species, age ranges, and conditioning procedures (Campolattaro & Freeman, 2006; Duong, Brown, & Stanton, 2007; Frieman & Goyette, 1973; Kehoe & Holt; Molina et al.; Spear and Molina; Thomas, Miller, & Svinicki, 1971), the neurobiological mechanisms underlying ontogenetic changes in this phenomenon are currently unknown.
Eyeblink classical conditioning (EBC) may be amenable to ontogenetic analysis of cross-modal transfer effects. The developmental emergence of EBC is well documented and discrete conditioned stimuli (CS) processed through different sensory modalities (lights and tones) are each capable of promoting robust conditioned responding (CRs; Paczkowski, Ivkovich, & Stanton, 1999; Stanton, Fox, & Carter, 1998; Stanton & Freeman, 2000; Stanton, Freeman & Skelton, 1992). Additionally, the essential neurobiological substrates of EBC for both mature and developing animals have been localized to a discrete cerebellum-brainstem circuit (Christian & Thompson, 2003; Freeman & Nicholson, 2004). Cross-modal transfer has been viewed as an extension of Harlow’s (1949) learning set research (c.f., Kehoe, Horne, & Macrae, 1995) and is thoroughly documented in the Pavlovian literature in studies with adult rabbits trained in the EBC preparation (Holt & Kehoe, 1985; Kehoe & Holt, 1984; Kehoe, Morrow, & Holt, 1984; Kehoe & Napier, 1991; Schreurs & Kehoe, 1987). Cross-modal facilitation of learning in these studies is distinguished from primary stimulus generalization (c.f., Hull, 1939; Pearce, 1987), as robust conditioning is not observed immediately after shifting to the new CS (e.g., tone) following training to criteria with the original CS (e.g., light). This represents a point of departure from some experimental findings in infants, however, which have indicated that some cross-modal transfer effects are primarily a consequence of intersensory equivalence between seemingly distinct CSs (see Spear & Molina, 1987).
One amodal feature shared by CSs detected through different sensory modalities appears to be that of duration. For example, rapid cross-modal transfer of instrumental discriminative responding is observed in adult rats when durations of phase 1 (auditory or visual) and phase 2 (modality not used in phase 1) CSs are equated (Meck & Church, 1982; Roberts, 1982). EBC training involves the production of an adaptively-timed CR sensitive to the duration (or interstimulus interval – ISI) between CS and unconditioned stimulus (US) onset. CRs peak at US onset across a broad range of ISIs and CRs achieve maximum amplitudes at ISIs between approximately 200-500 ms (Ivkovich, Paczkowski, & Stanton, 2000; Kehoe, Graham-Clarke, & Schreurs, 1989; Mauk & Ruiz, 1992; Millenson, Kehoe, & Gormezano, 1977; Smith, 1968). If temporal features of conditioning are encoded similarly across stimuli detected through different sensory modalities in EBC, topographical features of the initial CR may emerge in subsequent conditioning using a CS of a different sensory modality. Indeed, Kehoe and Napier (1991) showed that adult rabbits trained with a 200 ms ISI with one CS (e.g., tone) expressed rapidly-emerging CRs that retained temporal characteristics of initial training (early-peaked CRs) when shifted to training with a CS of a different modality (e.g., light) conditioned at a 400 ms ISI.
Evidence of cross-modal transfer of ISI-specific response features has been demonstrated in our laboratory using a version of the differential conditioning procedure employed in rabbit EBC (Green & Steinmetz, 2005; Kehoe et al., 1989 - Experiment 3; Kehoe, Horne, & Horne, 1993; Mauk & Ruiz, 1992) that we have adapted for rats (Brown, Pagani, & Stanton, 2006). In this “ISI discrimination” task, subjects are trained in a delay EBC procedure using within-sessions presentations of two distinct CSs – one CS (e.g., tone) is conditioned at a short ISI (280 ms) and the other CS (e.g., light) is conditioned at a long ISI (880 ms). When training commences at PD23 or 30, conditioning to the long CS is strongly influenced by the short CS-US pairing, as evidenced by earlier peak latencies, enhanced CR amplitudes, and increased frequency of double-peaked CRs to the long CS relative to controls that do not receive paired short CS-US presentations. However, ISI discrimination performance in adults (PD80+ at the start of training; Brown et al. – Experiment 3) does not demonstrate the magnitude of influence of short ISI conditioning upon performance to the long CS observed in younger subjects. Enhanced cross-modal transfer from the short to long CS in ISI discrimination diminishes – and temporal precision of CRs to the long CS improves - between (approximately) PD30-80.
While substantial transfer of conditioning across sensory modalities is present in younger rats trained in ISI discrimination, it appears that generalization across CSs is incomplete. If young rats (PD23 and 30) generalized completely across the short and long CSs, CR latencies would not be expected to differ between CSs. However, we have found evidence for differential timing between short and long CSs in young rats trained in ISI discrimination (Brown et al., 2006). Dual-ISI conditioning using varying durations of the same CS reinforced at both the short (280 ms) and long (880 ms) CS-US intervals would presumably produce complete transfer of conditioning across ISIs, thus serving as a useful comparison for generalization effects observed with ISI discrimination. Variations of such “temporal uncertainty” training (see Freeman, Nicholson, Muckler, Rabinak, & DiPietro, 2003) typically produce two distinct CRs (particularly during long CS trials) that peak at the temporal locus of the US for each ISI (Choi & Moore, 2003; Hoehler & Leonard, 1976; Kehoe & Joscelyne, 2005; Millenson et al., 1977), though the emergence of robust and well-timed double-peaked CRs is not evident until sometime between PD24-34 in rats (Freeman et al.). The apparent increase in demands on temporal processing in dual-ISI training relative to single-cue EBC (c.f., Kehoe et al., 1989) may contribute to the continued developmental modification of performance in ISI discrimination and temporal uncertainty training beyond ages when conditioning in single-cue EBC is well developed (Ivkovich et al., 2000; Paczkowski et al., 1999; Stanton et al., 1998, 1992).
The present study sought to replicate and extend previous findings of ontogenetic modifications in conditioning beyond the juvenile period in dual-ISI EBC tasks. Interpretation of differences between young and mature rats in previous studies is limited in that age comparisons were made across experiments (e.g., Brown et al., 2006). To enable appropriate, within-experiment comparisons, all ages were trained concurrently in the present study. To our knowledge, this is the first study using the rodent EBC preparation that involves within-experiment comparisons of juveniles (PD23 at the start of training), adolescents (PD30 at the start of training), and adult rats. Additionally, ISI discrimination performance was observed at a previously untested age (PD43-47 at the start of training) intermediate to that of adolescents (PD30) and adults trained in Brown et al. to more precisely identify the approximate age range at which the enhanced cross-modal transfer across distinct CSs that is present in younger rats transitions to the improved discrimination found in adults. A separate set of age-matched subjects was trained concurrently in temporal uncertainty for two primary purposes. First, direct comparison between these tasks presumably provides a relative measure of the degree of transfer across CSs observed in younger subjects trained in ISI discrimination. Generalization across short and long ISI conditioning is essentially complete in temporal uncertainty training since the same CS is presented at both ISIs. Therefore, earlier-timed CRs (to the long CS) in temporal uncertainty relative to ISI discrimination would provide further evidence that the transfer of conditioning across CSs in younger subjects trained in ISI discrimination is not complete. Second, it was of further interest to replicate previous developmental findings reported in temporal uncertainty EBC. We expected significant increases in the frequency of double-peaked CRs generated by adolescents (PD30) relative to juvenile rats (PD23), similar to findings of Freeman et al. (2003).
Materials and Methods
Subjects
The subjects were 171 Long Evans rats (83 female [F], 88 male [M]) derived from 53 litters. Breeders were obtained from Harlan Laboratories (Frederick, MD) and mated overnight at the animal housing colony of the Office of Laboratory Animal Medicine at the University of Delaware. Gestational day (GD) 0 was defined as the day following overnight breeding. Dams were housed in 45 × 24 × 21 cm clear Polypropylene cages in a facility maintained on a 12:12-hr light-dark cycle (lights on at 7:00 am) that operated in accordance with NIH guidelines. The date of birth was designated as PD0 (GD22). On PD3, litters were culled to 8 pups (usually 4 M, 4 F) and received injections of nontoxic ink into their paws for identification. For subjects trained starting at PD30 and beyond, pups were weaned on PD21 and housed in groups of same-sex littermates with a continuous supply of rat chow and water. Subjects trained at PD23 underwent surgery immediately following separation from their mothers at PD21. Following surgery all rats were single-housed for the remainder of the experiment.
Surgery
Subjects were anesthetized with an intraperitoneal injection of a ketamine/xylazine cocktail (87 mg/kg ketamine/13 mg/kg xylazine) at one of the 4 following ages/age ranges: PD21, PD28, PD39-43, or PD60-64. The injection volumes differed as a function of age (PD21 and PD28 = 0.60 to 0.75 ml/kg; PD39-43 and PD60-64 = 0.7 to 1.0 ml/kg). An injection of a dilute buprenex solution (0.06 mg/ml) was delivered subcutaneously 5-10 minutes later. Additional ‘booster’ injections of ketamine/xylazine were delivered as needed. A custom-built electrode (Plastics One, Roanoke, VA) containing both differential electromyograph (EMG) electrodes (implanted in the left upper eyelid – orbicularis oculi - muscle to record eyelid movement) and a bipolar stimulating electrode (placed immediately caudal to the left eye; used to deliver the shock US) was implanted as described previously (Stanton & Freeman, 2000; Stanton et al., 1992). A separate wire connected to the electrode was placed at the back of the neck (under the skin) and served as a ground. Electrode connectors were secured to the skull with dental acrylic and two 15-mm strips of galvanized steel wires (PD21 and 28s) or three screws (PD39-43 and 60-64s) were implanted onto the skull to form an anchor for the dental acrylic. Immediately post-surgery, subjects were housed in cages that were temporarily heated on one side by an electric heating pad (GE model # E12107) placed at the lowest setting.
Apparatus
The conditioning apparatus consisted of sixteen animal chambers (BRS/LVE, Laurel, MD) lined with sound-absorbing foam, as described previously (Stanton & Freeman, 1994; available from JSA Designs, Raleigh, NC). Within each chamber animals were kept in stainless steel wire mesh cages measuring 22 × 22 × 26 cm. Each chamber was equipped with a fan that produced ‘background’ noise (< 60 dB), a house light (15W), and two speakers for delivery of the auditory CS. The present study used a 70 dB, 2.8 kHz tone presented at either 380 or 980 ms as the auditory CS and activation of the house light (against the dark background) for 380 or 980 ms as the visual CS. The US was generated by a constant-current, 60-Hz square wave stimulator (World Precision Instruments, Sarasota, FL). Headstages were connected to wire leads that passed to the peripheral equipment via a commutator (Airflyte, #CAY-675-6). This allowed subjects to move freely about the chamber.
Design and Procedures
Subject assignment and exclusion criteria
Groups were counterbalanced with respect to their experimental chamber and for surgeon. For a given sex within each behavioral condition (ISI discrimination or temporal uncertainty), post-surgery assignment to the training CS modality (tone or light) was further balanced according to body weights and the “puff-test” score. Same-sex littermates in the same behavioral condition were assigned to training with different CS modalities. To reduce the likelihood of “litter effects”, two to four pups (2M, 2F) were typically sampled per litter, with half trained in ISI discrimination and the other half trained in temporal uncertainty. A total of 36 animals were excluded from analyses due to: excessively low unconditioned response (UR) percentages and/or amplitudes (PD21s: 1 F, 1 M; PD28s: 1 M; PD39-43: 2 F, 2 M; PD60-64: 2 F, 6 M), electrode displacement (PD21s: 1 F; PD28s: 1 F, 1 M; PD39-43: 2 M; PD60-64: 1 M), or excessive spontaneous blink rates/noise during the pre-CS baseline period (PD21s: 4 F, 3 M; PD28s: 5 F, 1 M; PD60-64: 1 F, 1 M). Further analyses include the remaining 135 subjects (ISI discrimination [total N = 67], PD21: F, light short CS [4]; F, tone short CS [4]; M, light short CS [2]; M, tone short CS [5]; PD28: F, light short CS [4]; F, tone short CS [3]; M, light short CS [4]; M, tone short CS [5]; PD39-43: F, light short CS [2]; F, tone short CS [4]; M, light short CS [5]; M, tone short CS [5]; PD60-64: F, light short CS [6]; F, tone short CS [6]; M, light short CS [4]; M, tone short CS [4]; Temporal uncertainty [total N = 68], PD21: F, light CS [4]; F, tone CS [5]; M, light CS [5]; M, tone CS [4]; PD28: F, light CS [4]; F, tone CS [3]; M, light CS [4]; M, tone CS [5]; PD39-43: F, light CS [4]; F, tone CS [4]; M, light CS [4]; M, tone CS [4]; PD60-64: F, light CS [5]; F, tone CS [4]; M, light CS [5]; M, tone CS [4]).
Training
Handling, puff testing, and context pre-exposure procedures for PD21, PD28, and PD60-64 subjects followed those outlined in Brown et al. (2006). For PD39-43 subjects, these procedures were similar to those in adults.
One day after puff testing for subjects undergoing surgery on PD21 or PD28 (PD23 and 30) and one day after context pre-exposure for subjects undergoing surgery on PD39-43 and PD60-64 (PD42-46 and PD65-71; these groups will henceforth be termed PD45 and PD70s, respectively), ISI discrimination (Experiment 1a; as described in Brown, Calizo, & Stanton, 2008; Brown et al., 2006) or temporal uncertainty training (Experiment 1b; as described in Brown et al., 2008) commenced (see Figure 1 for an illustration of the conditioning parameters). All 4 ages and both tasks were run concurrently. Subjects were given 10 minutes to acclimate to the chamber before the start of Session 1. Each session consisted of 100 trials: 50 trials of a 380 ms CS, and 50 trials of a 980 ms CS. Both CSs preceded and coterminated with a 2-mA, 100 ms periocular-shock US, producing ISIs of 280 and 880 ms, respectively. In ISI discrimination, the short (280 ms ISI) and long (880 ms ISI) CSs were different modalities (one tone, one light). Temporal uncertainty was identical in design with the exception that the same CS (light or tone) was presented at both ISIs for each subject (CS modality was counterbalanced within each task). Trials were presented in a pseudorandom order (no more than three consecutive trials of the same CS/ISI). The intertrial interval varied randomly around an average of 30 sec (range: 18-42 sec.). Within a block of 10 trials, each CS/ISI was presented 5 times and paired with the US on 4 trials (the 5th trial was CS presented alone). Training consisted of 2 sessions per day for 6 consecutive days (12 total sessions), with sessions starting 5 hours (+/- 30 min) apart within each day. Sessions always began within one hour of the start time from the previous day (typically 9:00 a.m). Immediately following Session 12, a “probe” session (20 trials) was run in which the US reinforcement schedule was changed from 4/5 to 1/5 trials. CS-alone probe trials are sampled for production of “tracings” used to provide a visual representation of CR topography and for assessment of double-peaked CRs. The trial sequence was otherwise identical to that in the regular 100-trial sessions.
Figure 1.
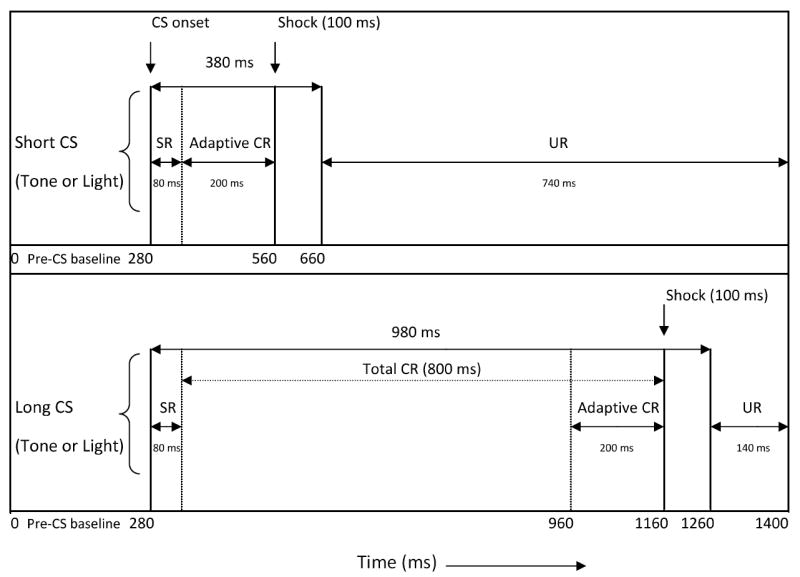
Illustration of stimuli used in the interstimulus interval (ISI) discrimination (Experiment 1a) and temporal uncertainty (Experiment 1b) procedures. All CSs preceded, overlapped, and coterminated with a 100 ms shock US. The CS durations were 380 ms (short CS) and 980 ms (long CS) with ISIs between CS and US onset of 280 and 880 ms, respectively. Adaptive CRs were recorded from the last 200 ms of the CS period prior to US onset. Tasks were identical in design with the exception that the short CS and long CS were of different sensory modalities in ISI discrimination while the same CS was used for both ISIs in temporal uncertainty (Reprinted from Brown et al., 2008).
Data Analysis
Data analysis was similar to that described in previous studies (Brown et al. 2008, 2006). EMG signals were sampled in 3.5 ms bins during the 1400 ms epoch of each trial type. The raw signal was amplified (5K), rectified, and integrated for analysis. Each trial was divided into five time periods: (1) a 280 ms pre-CS baseline period; (2) a startle response (SR; also termed “alpha response”) period reflecting the first 80 ms after CS onset; (3) total conditioned response (CR) period – EMG activity that occurred during either the 200 ms (short-CS trials) or 800 ms (long-CS trials) of CS presentation that preceded US onset; (4) adaptive CR period – EMG activity that occurred during the 200 ms of CS presentation that preceded US onset (for the short CS this is the same as the total CR period); and (5) UR period – EMG activity that occurred from the offset of the US to the end of the trial. The recording was interrupted during the 100 ms US presentation to avoid stimulation artifact. On CS-alone test trials, the CR sampling periods extended to the end of the trial and therefore included the period designated as the “US period” on paired trials.
The threshold for registering an EMG response was set 40 arbitrary units above the average baseline amplitude during the pre-CS period. Trials with excessively high spontaneous blink amplitudes during the pre-CS baseline period were not included in analyses. Subjects exhibiting a high percentage of spontaneous blinks during the pre-CS period (also termed “noise”) were excluded from analyses. Subjects were also excluded if average UR amplitudes fell below 250 units. CR peak amplitude measures counted trials in which CRs were not present as “zero amplitude”, consistent with our previous reports (see Brown et al.,2006). CR percentage and peak amplitude are reported from paired CS-US trials (the pattern of data was virtually identical across CS-US and CS-alone trials). CR topography was presented as composite tracings from CS-alone probe trials following Session 12. Double peak CRs were also analyzed from probe CS-alone trials using the criteria defined in Brown et al. (2006). CR latency (timing) measures were taken from CS-alone trials only from Sessions 4-12 to avoid a high degree of ‘contamination’ of low CR percentages present early in training. Two latency measures are reported here: CR onset latency, the interval between CS onset and initiation of the CR; and CR peak latency, the interval between CS onset and CR peak amplitude.
Statistical analysis
Data were statistically analyzed via analysis of variance (ANOVA) and Newman-Keuls posthoc tests, with significance levels set at p < 0.05. Statistical analyses were performed using Statsoft Statistica software. Short and long CSs were analyzed separately within each task. Tasks were initially analyzed separately (Experiment 1a = ISI discrimination; Experiment 1b = temporal uncertainty) in order to focus on direct age comparisons within each task. ANOVA for each set of analyses involved the between-subjects factors of age (PD23, 30, 45, 70), sex (male, female), and modality (tone, light), and the within-subjects factor of sessions. T-tests were also conducted for CR latency measures (short versus long CS) at each age to assess whether differential responding was present between the short and long CS in each task. Additionally, four separate sets of ANOVAs were run to directly compare tasks at each age.
Results
Behavioral data are reported combined across sex and CS modality since significant interactive effects involving age with these factors occurred infrequently and were inconsistent across measures (CR percentage, peak amplitude, and latency), CSs (short and long), and trial types (CS-US trials and CS-alone trials). In contrast to these infrequent interactive effects involving age with sex or CS modality, the reported effects involving age (alone, or interactive only with sessions) were robust and consistent across CR measures, CSs, and trial types (particularly in ISI discrimination). Consistent main or interactive effects that do not alter conclusions concerning the effects of primary interest are briefly disclosed here. Consistent with previous reports (Brown et al., 2008, 2006), SRs were more robust to the tone CS while conditioning was more robust with light as the CS. CR latencies were also earlier to the light CS. Adult (PD70) females often outperformed their male counterparts, also consistent with our previous findings (Brown et al., 2008).
Experiment 1a: ISI Discrimination
ISI discrimination conditioning differed significantly as a function of age of training. Observation of long CS (880 ms ISI) conditioning revealed elevated CR percentages and peak amplitudes as well as earlier-timed CRs in /juvenile/adolescent subjects (PD23 and PD30) relative to the older groups (PD45 and PD70s). Some age-related effects were present to the short CS (280 ms ISI), though differences across age did not follow the patterns observed in long CS conditioning. Conditioning at PD45 (an age previously untested in our laboratory) closely resembled that observed in PD70 subjects.
Short CS Conditioning
CR Generation (Percentage and Peak Amplitude)
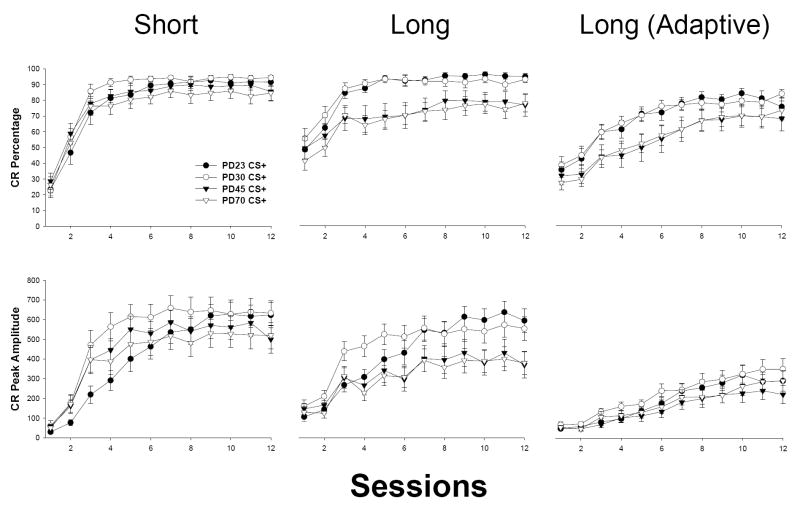
Modest age-related differences emerged in the rate of conditioning to the short CS (see Figure 2, left). Significant effects of sessions [F(11, 561) = 220.687, p < 0.001, CR percentage; F(11, 561) = 127.463, p < 0.001, CR peak amplitude] and Age × Sessions [F(33, 561) = 1.463, p < 0.049, CR percentage; F(33, 561) = 2.429, p < 0.001, CR peak amplitude] were present. There were no significant main effects of age (ps > 0.240). Newman-Keuls post-hoc analyses of the Age × Sessions interaction indicated that CR levels were diminished early in training in PD23s relative to older subjects (PD23 < PD30: Sessions 2-3 [CR percentage], Sessions 3-5 [CR peak amplitude]; PD23 < PD45: Session 2 [CR percentage], Sessions 3-4 [CR peak amplitude]; PD23 < PD70: Sessions 3-4 [CR peak amplitude], ps < 0.05). The only other group contrast that reached significance was between PD30s and PD70s (PD70 < PD30 at Session 4 [CR percentage and peak amplitude], ps < 0.05). In summary, all age groups reached comparable asymptotes of conditioning, although CRs in the youngest age group increased at a slightly slower rate early in training.
Figure 2.
Mean (± SEM) percentage of total CR frequency (top row) and CR peak amplitude (bottom row) from CS+ trials for subjects trained in interstimulus interval (ISI) discrimination (Experiment 1a). The left column represents CRs for the short CS (280 ms CS-US interval); the middle column represents CRs for the long CS (880 ms CS-US interval); the right column represents adaptively-timed CRs (CRs occurring within the last 200 ms prior to US onset) for the long CS. CR peak amplitude is measured in arbitrary units. PD23 = subjects beginning training on postnatal day 23; PD30 = subjects starting training on postnatal day 30; PD45 = subjects beginning training on (approximately) postnatal day 45; PD70 = subjects beginning training on (approximately) postnatal day 70.
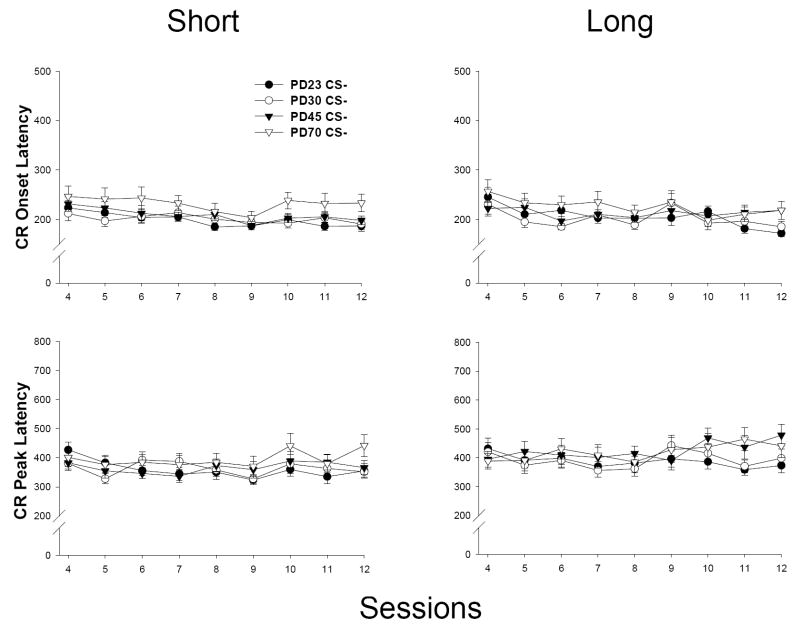
CR Timing (Onset and Peak Latency)
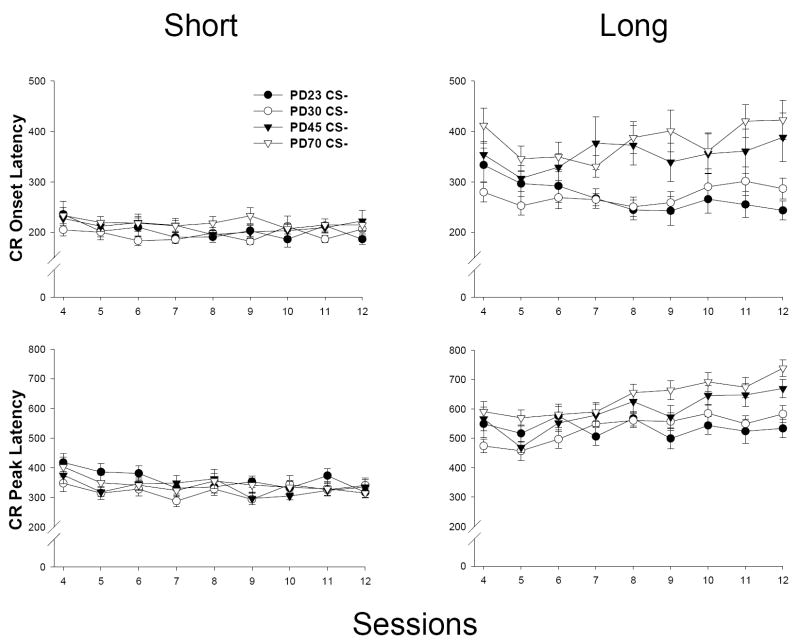
CR timing to the short CS was mildly impacted as a function of age of training (see Figure 3, left). A main effect of sessions was present in both latency measures [F(8, 408) = 2.430, p < 0.015, CR onset latency; F(8, 408) = 6.539, p < 0.001, CR peak latency], indicating a modest decrease in CR latencies as training progressed. The only significant effect involving age was an interaction of Age × Sessions in the CR peak latency measure [F(24, 408) = 1.628, p < 0.033]. However, post-hoc analyses failed to reveal any significant group contrasts (PD30 < PD23 at Session 4 approaching significance, ps < 0.07).
Figure 3.
Mean (± SEM) CR onset latency (in ms; top) and CR peak latency (bottom) from CS-alone trials (Sessions 4-12) for both the short CS (280 ms CS-US interval; left) and the long CS (880 ms CS-US interval; right) in ISI discrimination (Experiment 1a). Note differences in scaling in onset versus peak latency graphs.
Long CS Conditioning
CR Generation (Percentage and Peak Amplitude)
Age-related differences emerged in the rate and asymptote of conditioning to the long CS (see Figure 2, middle). Significant main effects of age [F(3, 51) = 4.845, p < 0.005, CR percentage; CR peak amplitude effect approaching significance – p < 0.095] and sessions [F(11, 561) = 66.244, p < 0.001, CR percentage; F(11, 561) = 67.964, p < 0.001, CR peak amplitude] were present, as was a significant interaction of Age × Sessions [F(33, 561) = 1.511, p < 0.036, CR percentage; F(33, 561) = 2.531, p < 0.001, CR peak amplitude]. Post-hoc analyses of this interaction revealed substantial enhancements in conditioning across most of training in PD23 and 30s relative to PD45 and 70s (PD23 and PD30 > PD45 and PD70: Sessions 3-12 [CR percentage], Sessions 7-12 [CR peak amplitude], ps < 0.05). CR peak amplitudes were significantly lower in PD23s relative to PD30s at Sessions 3-4 (ps < 0.05). There were no significant differences in either measure between PD45 and 70s (ps > 0.09).
CR Timing (Onset and Peak Latency)
CRs to the long CS were differentially timed as a function of age, particularly at later stages of training (see Figure 3, right). Significant main effects of age [F(3, 49) = 4.833, p < 0.006, CR onset latency; F(3, 49) = 5.106, p < 0.004, CR peak latency] and sessions [F(8, 392) = 2.117, p < 0.034, CR onset latency; F(8, 392) = 7.713, p < 0.001, CR peak latency] were present, as was a significant interaction of Age × Sessions [F(24, 392) = 1.782, p < 0.014, CR onset latency; F(24, 392) = 1.690, p < 0.024, CR peak latency]. Post-hoc analyses of this interaction indicated that CRs were significantly earlier to onset in PD23 and 30s compared to PD45 and 70s (PD23 < PD45 at Sessions 7, 8, 11 and 12; PD30 < PD45 at Sessions 7 and 8, Session 12 approaching significance – p < 0.06; PD23 and PD30 < PD70 at Sessions 8, 9, 11 and 12, PD30 only < PD70 at Session 4, ps < 0.05). For the CR peak latency measure, the magnitude of age contrasts was not as robust as those in the onset latency measure. Age differences were present in PD23 and 30s compared to PD70s (PD23 < PD70 at Sessions 9-12; PD30 < PD70 at Session 12, ps < 0.05), though no significant age differences were observed between PD23 and 30s relative to PD45s (PD23 < PD45 approaching significance at Session 12 – p < 0.07; remaining ps > 0.14). There were no significant differences between PD23s and 30s or between PD45s and 70s in either measure (ps > 0.19). Results of t-tests performed on short versus long CS onset and peak latency at each age revealed significant differences (p < 0.01), suggesting that discriminative responding between CSs was present at all ages. This suggests that the short (e.g., tone) and long CSs (e.g., light) in ISI discrimination were not treated as functionally equivalent, even at the youngest ages.
Adaptive CR Measures (long CS)
Adaptively-timed CRs (CRs confined to the 200 ms period prior to US onset) to the long CS did not significantly differ across age, in contrast to effects reported for CRs spanning the entire CS-US interval (see Figure 2, right). The expected main effect of sessions was present [F(11, 561) = 78.205, p < 0.001, adaptive CR percentage; F(11, 561) = 56.066, p < 0.001, adaptive CR peak amplitude], but no statistical effects involving age reached significance (a main effect for age in the adaptive CR percentage measure approached significance – p < 0.093).
Percentage of Double-Peaked CRs and CR Topography
Double-peaked CRs (Table 1) and mean trial tracings (Figure 4) were recorded from CS-alone trials following Session 12. If conditioning to a short CS of one modality (e.g., tone) facilitates conditioning to a long CS from a different modality (e.g., light), a preponderance of double-peaked CRs as well as altered CR topographies (i.e., reflected by early-peaking CRs to long CS trials) may result (see Brown et al., 2006). While the percentage of double-peaked CRs to the long CS was higher in younger subjects relative to older subjects (PD45 and PD70), this difference failed to reach significance (p > 0.290). Double-peaked CRs to the short CS also failed to demonstrate significant differences across age (p > 0.790).
Table 1.
Mean (± SEM) Double Peak Conditioned Response (CR) Percentages (Taken from CS-alone Trials for Short and Long CSs Following Probe Trials After Session 12), Unconditioned Response (UR) Amplitudes (Taken from the 1st Block of Training), and Startle Response (SR) Amplitudes (Taken from Both Short and Long CS Trials From Session 1), and for Experiments 1a (Interstimulus Interval {ISI} Discrimination) and 1b (Temporal Uncertainty - - TeUN)
| Group | Double Peak CR Percentage | Performance Measures | |||
|---|---|---|---|---|---|
| Short CS | Long CS | UR amplitudes | SR amplitude (Short CS) | SR amplitude (long CS) | |
| ISID (PD23) (N = 15) | 34.1 ± 7.2 | 34.7 ± 8.4 | 732.7 ± 44.1 | 2.5 ± 0.7 | 4.9 ± 1.3 |
| ISID (PD30) (N = 16) | 37.5 ± 7.3 | 41.6 ± 8.9 | 675.8 ± 58.4 | 3.5 ± 1.0 | 5.9 ± 2.0 |
| ISID (PD45) (N = 16) | 29.8 ± 6.7 | 29.5 ± 9.1 | 688.2 ± 54.0 | 2.7 ± 1.3 | 4.7 ± 2.5 |
| ISID (PD70) (N = 20) | 30.5 ± 6.5 | 21.6 ± 7.6 | 798.8 ± 42.7 | 3.9 ± 1.5 | 4.6 ± 2.0 |
| TeUN (PD23) (N = 18) | 39.7 ± 6.3 | 53.1 ± 6.2 | 693.4 ± 58.6 | 7.7 ± 3.2 | 6.8 ± 2.1 |
| TeUN (PD30) (N = 16) | 43.2 ± 6.7 | 70.8 ± 7.1 | 621.5 ± 67.0 | 8.7 ± 3.3 | 4.6 ± 1.7 |
| TeUN (PD45) (N = 16) | 41.4 ± 8.6 | 56.5 ± 11.1 | 732.2 ± 44.9 | 5.6 ± 1.8 | 7.4 ± 3.9 |
| TeUN (PD70) (N = 18) | 36.3 ± 8.2 | 47.6 ± 9.8 | 772.8 ± 45.1 | 3.8 ± 1.5 | 7.9 ± 2.9 |
PD23: Postnatal day 23 at the start of training; PD30: Postnatal day 30 at the start of training; PD45: Approximately PD45 at the start of training; PD70: Approximately PD70 at the start of training
Figure 4.
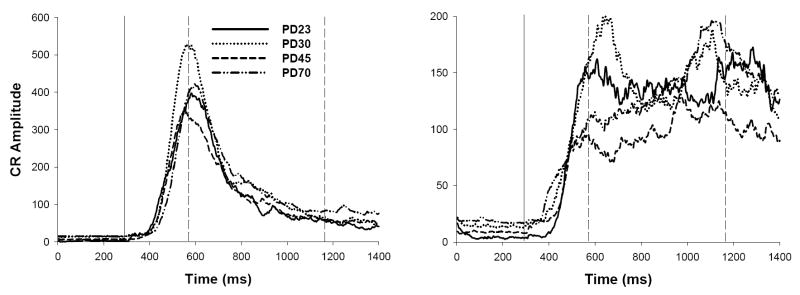
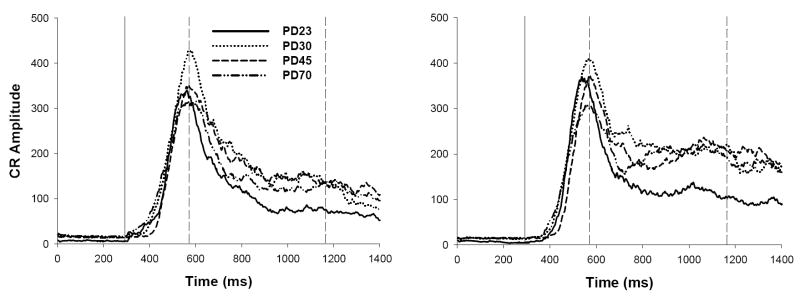
Tracings of integrated electromyography activity averaged across all short- (280 ms CS-US interval; left) and long-CS-alone probe trials (880 ms CS-US interval; right) following Session 12 for ISI discrimination (Experiment 1a). The solid vertical line represents the onset of the CS, and the two dashed vertical lines represent the onset of the short and long US (time expressed in ms). Note differences in scaling across graphs.
Average trial tracings were similar to those observed at similar ages (PD23, 30, and 80+) reported in a previous study (Brown et al., 2006). CRs to the short CS peaked at the onset of the US associated with the short CS at all ages, while CR topography appeared to differ across age in long CS conditioning (see Figure 4). CRs to the long CS for PD23s and 30s displayed two peaks, each occurring near US onset for both ISIs. CRs to the long CS for older subjects, however, displayed one peak occurring closer to US onset for the long CS. CR topography to the long CS in older subjects resembles those typically observed following single-cue EBC training with long CSs (e.g., Smith, 1968), consistent with our previous demonstration that long CS conditioning in ISI discrimination is less influenced by short CS-US training in older subjects relative to the younger subjects (Brown et al.).
Summary of findings
ISI discrimination training revealed ontogenetic differences in the degree of cross-modal influence of conditioning to a short ISI CS of one modality (e.g., tone) upon conditioning to a long ISI CS of a different modality (e.g., light). CRs to the long CS in PD23 and PD30 subjects occurred with greater frequency and were enhanced in amplitude relative to PD45 and PD70 subjects. These age differences are in contrast to that observed in single-cue delay EBC, in which levels of conditioning to a long-ISI CS are similar between adolescent and adult rats (see Tran, Stanton, & Goodlett, 2007). This suggests that age differences reported here are primarily due to enhanced influence of the short CS-US pairing on long CS conditioning, consistent to that reported in ISI discrimination training in Brown et al. (2006). The absence of age-related differences in adaptively-timed CR generation measures (CRs occurring only within the last 200 ms prior to US onset) to the long CS further suggests that the aforementioned age-related effects in long CS conditioning were primarily driven by transfer of ISI-specific response features related to short CS-US conditioning. Additional evidence of enhanced cross-modal influence of short CS-US training upon conditioning to the long CS was demonstrated by earlier latency CRs in PD23 and PD30 rats relative to older subjects (particularly later in training). Conditioning to the long CS in PD45s resembled that of adults (PD70s), suggesting that the degree of cross-modal influence of short CS conditioning on conditioning to the long CS in ISI discrimination diminishes between (approximately) PD30-45.
Experiment 1b: Temporal Uncertainty
Age-related differences in conditioning were less robust in temporal uncertainty training than in ISI discrimination. This lack of a difference seemed to occur because generalization of responding across short- and long-CS trials that declined with age in ISI discrimination failed to decline with age in this task, in which the discrimination was unsolvable.
Short CS Conditioning
CR Generation (Percentage and Peak Amplitude)
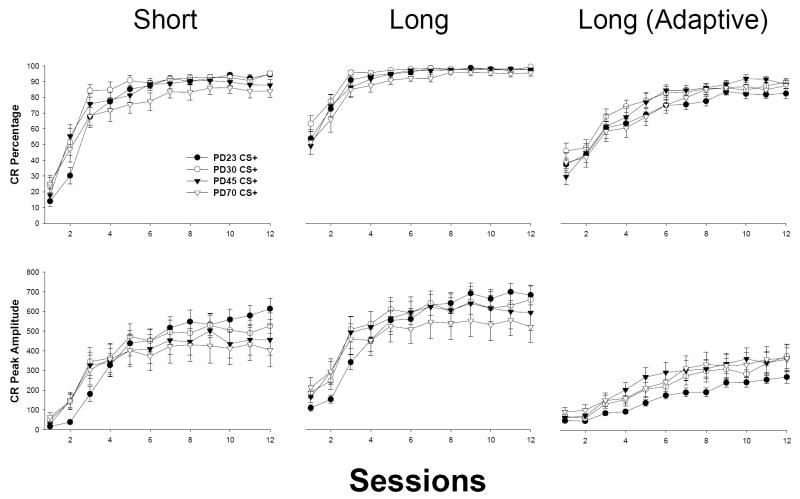
Age-related differences emerged in the rate of conditioning to the short CS in a manner similar to that observed in short CS conditioning in ISI discrimination training (see Figure 5, left). Significant effects of sessions [F(11, 572) = 245.873, p < 0.001, CR percentage; F(11, 572) = 104.078, p < 0.001, CR peak amplitude] and Age × Sessions [F(33, 572) = 2.907, p < 0.001, CR percentage; F(33, 572) = 2.621, p < 0.001, CR peak amplitude] were present. Newman-Keuls post-hoc analyses of the Age × Sessions interaction indicated that CR levels were diminished early in training in PD23s relative to older subjects (PD23 < PD30: Sessions 1-3 [CR percentage], Session 3 [CR peak amplitude]; PD23 < PD45: Session 2 [CR percentage], Session 3 [CR peak amplitude]; PD23 < PD70: Sessions 1-2 [CR percentage], Session 3 [CR peak amplitude], ps < 0.05). Furthermore, CRs were less frequent in adults relative to PD30s early in training (PD30 > PD70 at Sessions 3-5 [CR percentage], ps < 0.05). CR peak amplitudes were enhanced in PD23s relative to PD45s and 70s at the end of training (PD23 > PD45 and PD70 at Session 12, ps < 0.05).
Figure 5.
Mean (± SEM) percentage of total CR frequency (top row) and CR peak amplitude (bottom row) from CS+ trials for subjects trained in temporal uncertainty (Experiment 1b). The left column represents CRs for the short CS (280 ms CS-US interval); the middle column represents CRs for the long CS (880 ms CS-US interval); the right column represents adaptively-timed CRs (CRs occurring within the last 200 ms prior to US onset) for the long CS. CR peak amplitude is measured in arbitrary units.
CR Timing (Onset and Peak Latency)
Similar to results observed in ISI discrimination training, CR timing to the short CS was mildly impacted as a function of age of training (see Figure 6, left). A significant main effect of age was present in the CR onset latency measure [F(3, 52) = 3.050, p < 0.037], as post-hocs indicated that CRs were significantly later to onset in adults (PD70) relative to PD23 and 30s (ps < 0.05). The difference in later-onset CRs in PD70s relative to PD45s approached statistical significance (p < 0.059). This effect appeared to be primarily driven by significantly later-onset CRs early in training in adult males relative to adult females (data not shown). There were no interactive effects of age for CR onset latency, nor were there any effects involving age in the CR peak latency measure (ps > 0.190).
Figure 6.
Mean (± SEM) CR onset latency (in ms; top) and CR peak latency (bottom) from CS-alone trials (Sessions 4-12) for both the short CS (280 ms CS-US interval; left) and the long CS (880 ms CS-US interval; right) in temporal uncertainty (Experiment 1b). Note differences in scaling in onset versus peak latency graphs.
Long CS Conditioning
CR Generation (Percentage and Peak Amplitude)
Age-related differences in conditioning to the long CS followed a similar pattern to that observed in short CS temporal uncertainty conditioning (see Figure 5, middle). Significant effects of sessions [F(11, 572) = 125.950, p < 0.001, CR percentage; F(11, 572) = 112.882, p < 0.001, CR peak amplitude] and Age × Sessions [F(33, 572) = 2.902, p < 0.001, CR peak amplitude; interaction failed to reach significance in the CR percentage measure – p > 0.588] were present. There were no significant main effects of age, though the age main effect approached significance in the CR percentage measure (p < 0.100). Newman-Keuls post-hoc analyses of the Age × Sessions interaction indicated that CR amplitudes were diminished early in training in PD23s relative to older subjects (PD23 < PD30 at Session 3; PD23 < PD45 and PD70 at Sessions 2-3, ps < 0.05). Furthermore, CRs were enhanced in PD23s relative to PD70s at the end of training (PD23 > PD70 at Session 12, p < 0.05; approaching significance at Session 11 – p < 0.069).
CR Timing (Onset and Peak Latency)
There were no significant age-related effects on CR timing to the long CS (ps > 0.120; see Figure 6, right). A main effect of sessions emerged only in the CR onset latency measure [F(8, 116) = 5.160, p < 0.001; CR onset gradually decreased as training progressed]. T-tests revealed no significant differences (p > 0.37) between short and long CS onset latency at any age, indicating – as predicted - that subjects did not effectively discriminate between the onset of short and long ISI trials. Therefore, at CS onset, subjects did appear to be “uncertain” as to when US delivery would occur. However, CR peak latencies did differ significantly across short and long CSs (p < 0.02 for PD23, 30, and 45s; approaching significance – p < 0.06 for adults). Mean CR peak latencies for each CS are as follows: PD23 – 359.3 +/- 15.8 (short CS), 387.3 +/- 16.6 (long CS); PD30 – 363.2 +/- 17.8 (short CS), 392.1 +/- 22.5 (long CS); PD45 – 365.8 +/- 18.7 (short CS), 423.9 +/- 23.6 (long CS); PD70 – 395.1 +/- 26.1 (short CS), 419.3 +/- 33.1 (long CS). This difference may have been related to some decline in later-peaked CRs during short CS trials, possibly resulting from offset of the short CS serving as an inhibitory cue (c.f., Freeman et al., 2003).
Adaptive CR Measures (Long CS)
Contrary to expected outcomes, there were no significant age-related effects in adaptive CR generation measures (ps > 0.158; see Figure 5, right). Only the expected main effect of sessions reached significance [F(11, 572) = 131.964, p < 0.001, adaptive CR percentage; F(11, 572) = 80.464, p < 0.001, adaptive CR peak amplitude].
Percentage of Double-Peaked CRs and CR Topography
Consistent with previous findings (Brown et al., 2008; Choi & Moore, 2003; Freeman et al., 2003; Hoehler & Leonard, 1976; Joscelyne & Kehoe, 2007; Kehoe & Joscelyne, 2005; Millenson et al., 1977), CS-alone trials (particularly in long CS trials) revealed a high percentage of double-peaked CRs (approximately 50% or more) relative to single-cue controls (Brown et al., 2006) and relative to ISI discrimination conditioning (< 50%; see Table 1; Figure 7). Freeman et al. also reported fewer double-peaked CRs in rats trained at PD24-26 relative to PD32-34s. While the number of double-peaked CRs to the long CS in PD23s was less than those in PD30s and there did not appear to be a pronounced second peak corresponding to long US onset in long CS conditioning in PD23s (Figure 7, right), these differences failed to reach significance (p > 0.249). This failure to replicate may stem from inconsistencies across studies in the amount of trials sampled. To achieve greater consistency with analyses performed in Freeman et al. (2003), double peak CR incidence was also analyzed across Sessions 7-12 (from long CS-alone trials). A main effect of sessions was present [F(5, 255) = 4.173, p < 0.002], though significant age-related differences were still not found (p > 0.365). Mean double-peak CR percentages at Sessions 7 and 12 were as follows: PD23 – 56.9 +/- 7.4 (Session 7), 65.5 +/- 6.0 (Session 12); PD30 – 60.0 +/- 9.1 (Session 7), 71.2 +/- 7.5 (Session 12); PD45 – 55.5 +/- 9.6 (Session 7), 60.6 +/- 10.2 (Session 12); PD70 – 45.3 +/- 9.8 (Session 7), 56.2 +/- 9.1 (Session 12). Differences in various training parameters across studies (e.g., auditory CS intensity and frequency; US intensity and duration) may account for these discrepancies.
Figure 7.
Tracings of integrated electromyography activity averaged across all short- (280 ms CS-US interval; left) and long-CS-alone probe trials (880 ms CS-US interval; right) following Session 12 for temporal uncertainty (Experiment 1b).
Summary of findings
Age-related differences in CR generation and CR latency measures were less pronounced in temporal uncertainty relative to ISI discrimination. Consistent with results of short CS conditioning in ISI discrimination (Experiment 1a), conditioning progressed more slowly early in temporal uncertainty training in PD23s relative to older subjects. This delay may have resulted from immaturity of the essential EBC circuitry at this age, as single-cue delay EBC emerges gradually over PD17-24 in rats (Paczkowski et al., 1999; Stanton et al., 1998, 1992). While illustrations of CR topography appear to reflect a diminished second peak in long CS conditioning in PD23s and CR levels were numerically smaller in PD23s relative to older subjects in these measures, the effects did not achieve significance. Adaptively-timed CRs to the long CS presumably indicate the incidence of the 2nd, later-peaked CR in temporal uncertainty conditioning. The absence of age-related effects in this measure therefore suggests that later-peaked CRs were not significantly depressed in PD23s. Furthermore, the percentage and latency of double-peaked CRs did not differ significantly across age. Although similar trends were found, the lack of statistical significance is contrary to findings reported by Freeman et al. (2003). The absence of robust developmental changes in temporal uncertainty conditioning beyond the juvenile period, while unanticipated, suggests that developmental effects reported in ISI discrimination training (enhanced CR generation and early CR latencies to the long CS) are likely task-specific.
Task comparison across age
Long CS conditioning measures (CR amplitude, onset latency, and peak latency) were directly compared across task at each of the 4 ages tested (PD23, 30, 45 and 70). If generalization between short (e.g. tone) and long CSs (e.g. light) in ISI discrimination is complete (“intersensory transfer” – see Spear and Molina, 1987), one would predict equivalence in these measures across task. CR peak amplitudes to the long CS were enhanced in temporal uncertainty conditioning relative to ISI discrimination in PD23, 45, and 70s (ps < 0.05; the difference in PD30s failed to reach significance). Additionally, CR onset and peak latencies were significantly earlier in temporal uncertainty conditioning relative to ISI discrimination at all ages (ps < 0.01). These findings provide further support for our proposal that generalization across CSs observed in ISI discrimination training is not complete.
Performance measures - - startle (SR) and unconditioned response (UR) amplitudes
SR amplitudes are reported from short and long CS trials at Session 1. SRs reflect non-associative eyelid movements to the CS. Equivalent SR levels across groups suggests that differences in CR measures are likely due to differences in associative learning processes and not the result of simple performance deficits (e.g., inability to detect the presence of the CS). UR amplitudes are reported from the first 10 long-CS+ trials from Session 1 to avoid confounding CR-UR summation effects. UR measures provide an additional check on possible performance deficits. Low URs may suggest that a particular group was unable to adequately (1) detect the shock US, and/or (2) produce the desired blink response. As predicted, SR and UR amplitudes did not significantly differ as a function of age (Table 1). Therefore, age differences in CR acquisition likely reflect effects specific to associative learning processes.
General Discussion
The present study replicated and extended developmental findings of Brown et al. (2006), as conditioning with a short CS (e.g., light CS) appeared to have a greater influence upon long CS conditioning (e.g., tone CS) in juveniles and adolescents relative to older rats trained in ISI discrimination. This developmental difference was evidenced by (1) enhanced CR production and amplitude and (2) earlier CR latencies to the long CS in PD23 and 30s relative to PD45 and 70s. Long CS conditioning in PD45s closely resembled that of PD70s (and differed in many respects from PD30s), suggesting that the cross-modal influence of the short CS-US pairing upon long CS conditioning in ISI discrimination diminishes over an even more restricted developmental period than previously demonstrated (Brown et al.). The protracted developmental emergence of well-timed double-peaked CRs in temporal uncertainty conditioning observed in a previous study (Freeman et al., 2003) was not supported statistically, though similar trends were observed. Direct task comparisons as well as comparisons of CR latencies between short and long CSs further suggests that generalization across CSs in younger subjects (PD23 and 30) trained in ISI discrimination was not complete.
Dual-ISI Eyeblink Conditioning in Adult Animals
EBC tasks involving within-subjects training of multiple ISIs have provided empirical support (Coleman & Gormezano, 1971; Kehoe et al., 1989; Mauk & Ruiz, 1992; Millenson et al., 1977) for theories maintaining that the CR is adaptable to CS and/or ISI-specific features of training (see Brandon, Vogel, & Wagner, 2003; Kehoe, 1988, 1990; Moore & Choi, 1997). Consequently, such ‘dual-ISI’ EBC paradigms have been valuable in revealing neurobiological mechanisms associated with adaptive CR timing (Choi & Moore, 2003; Green & Steinmetz, 2005; Medina, Garcia, Nores, Taylor, & Mauk, 2000; Perrett, Ruiz, & Mauk, 1993). The present findings in adults trained in ISI discrimination and temporal uncertainty EBC replicate the primary behavioral effects observed in previous dual-ISI conditioning studies.
ISI discrimination training in the present study (particularly in adults) produced discriminative responding (shorter onset and peak latencies to the short CS relative to the long CS; see Figures 3 and 4), similar to that observed in other dual-ISI EBC tasks using two distinct CSs (Brown et al., 2008, 2006; Green & Steinmetz, 2005; Kehoe et al., 1989, 1993; Mauk & Ruiz, 1992). Whereas ISI discrimination training in adults consistently yielded well-timed, single-peaked CRs, CRs to the long CS in temporal uncertainty conditioning typically displayed 2 peaks near the short and long US during a single trial (particularly during long CS trials; see Figure 7), consistent with previous findings following ‘mixed-ISI’ EBC training (Brown et al., 2008; Choi & Moore, 2003; Freeman et al., 2003; Hoehler & Leonard, 1976; Joscelyne & Kehoe, 2007; Kehoe & Joscelyne, 2005; Millenson et al., 1977). These conditioned response patterns are robust in each task despite differences across the cited studies in the species used (rats or rabbits) and/or in various training conditions (e.g., CS and US intensity and/or duration).
Cross-Modal Transfer in Early Development
Multisensory integration in early development may reflect a de-emphasis on, or insensitivity to, modality-specific information processing, consistent with theories of perceptual learning (Bower, 1989; Gibson, 1969). A propensity for processing different stimuli in terms of shared amodal characteristics (i.e., intensity; duration) may be an adaptive feature of organisms in early stages of development (Bahrick & Lickliter, 2000; Lickliter & Bahrick, 2004; Turkewitz & Melon, 1989). While it is unclear what adaptive advantage the enhanced cross-modal transfer observed in young rats trained in ISI discrimination affords, these results resemble previous developmental findings of cross-modal transfer of various sensory modalities. In these studies, Spear and colleagues (Molina et al., 1991; Spear & Molina, 1987) demonstrated greater cross-modal transfer of conditioning (e.g., olfactory and tactile conditioning) in young rats (approximately PD4-16) relative to older rats, perhaps reflecting a predisposition of younger rats to process discrete stimuli in terms of amodal characteristics (see Kraebel & Spear, 2000; Mellon et al., 1991). The observation of similar effects at much older ages (PD23-30) in the present EBC study suggests that the maturation level of critical task-specific neural circuitry (rather than of the whole organism) may be the factor that is primarily responsible for enhanced cross-modal processing at early developmental stages.
A predisposition for amodal processing may contribute to the cross-modal effects observed in the present study, though at least five separate findings indicate that auditory and visual CSs are not encoded as functionally equivalent in young rats trained in our EBC preparation: (1) PD19-31 rats do not condition to the CS- (less than 20% CRs) in a tone/light discrimination task (Brown et al., 2007; Paczkowski et al., 1999); (2) Short CSs (e.g., tone) presented alone or explicitly unpaired with the US does not lead to appreciable differences in conditioning to the long CS (e.g., light) in juvenile/adolescent rats – only paired short CS-US presentations produce early-timed, enhanced amplitude CRs to the long CS in ISI discrimination training (Brown et al., 2006); (3) PD23-30 rats differentially time the onset of CRs between short (e.g., tone) and long CS (e.g., light) in ISI discrimination (Brown et al., present study); (4) CR onset and peak latencies are earlier to the long CS in temporal uncertainty training relative to ISI discrimination in PD23-30 rats (present study); (5) No immediate transfer of conditioning is evident in PD24 rats (e.g., tone CS) following prior asymptotic training to a CS of a different modality (e.g., light; Duong et al, 2007). Therefore, some alternate mechanism(s) - aside from (or in addition to) perceived similarity between auditory and visual CSs – likely contributes to cross-modal transfer effects observed in the present study.
The common outcome (periocular shock US) associated with the tone and light CSs may be necessary to produce cross-modal transfer in ISI discrimination. Generalization across distinct cues can be enhanced as a result of sharing a common outcome (a phenomenon known as ‘acquired equivalence of cues’; Honey & Hall, 1989; Miller & Dollard, 1941). In an example of such an effect, Molina et al. (1991 – Experiment 5c) demonstrated robust cross-modal facilitation of conditioning in young rats when the same US was used across phases of training but not when the US differed between phases. The enhanced cross-modal transfer of EBC observed in PD23 and 30 rats trained in ISI discrimination may therefore reflect some functional immaturity of neural systems that increases the likelihood of generalization across distinct CSs due to a combination of (1) enhanced amodal processing and (2) a reliance on using common outcomes as a basis for unity. Systematic manipulations of USs and of shared amodal properties (i.e., intensity) across CSs are required to assess the degree to which amodal processing and acquired equivalence effects contribute to the present cross-modal transfer effects.
Neurobiology of Cross-Modal Transfer in EBC
The present findings warrant speculation on the neural basis of cross-modal transfer effects in EBC (see Campolattaro & Freeman, 2006, for neurobiological manipulations in this paradigm). The cerebellum and associated brainstem structures that are essential for EBC (Christian & Thompson, 2003) are perhaps the most logical starting points for investigations into the neural substrates of cross-modal transfer in ISI discrimination. Functional anatomical features of the cerebellar cortex satisfy many conditions that appear to be critical for consideration as a site of essential cross-modal plasticity. For one, the cerebellar cortex is normally involved in the acquisition and maintenance of EBC (Chen, Bao, Lockard, Kim, & Thompson, 1996; Freeman, Barone, & Stanton, 1995; Harvey, Welsh, Yeo, & Romano, 1993; Lavond & Steinmetz, 1989; Lavond, Steinmetz, Yokaitis, & Thompson, 1987; Nolan & Freeman, 2006; Yeo & Hardimann, 1992). The precise role of cerebellar cortical involvement in EBC is unknown, though evidence suggests that it governs features of the CR (e.g., amplitude and timing; Bao, Chen, Kim, & Thompson, 2002; Garcia & Mauk, 1998; Garcia, Steele, & Mauk, 1999; Medina et al., 2000; Nores, Medina, Steele, & Mauk, 2000; Ohyama & Mauk, 2001; Perrett et al., 1993) that transfer across sensory modalities in ISI discrimination training (Brown et al., 2006; present study). Second, the cerebellar cortex receives both auditory and visual sensory input (Brodal, 1967; Glickstein, 1997; Mortimer, 1975; Snider & Stowell, 1944) and is a site of convergence of CS- (via mossy fibers originating in the pontine nuclei that synapse with granule cells) and US-related input (via climbing fibers originating in the inferior olive; Gould, Sears, & Steinmetz, 1993). These are hypothesized to provide the ‘learning’ and ‘teaching’ inputs, respectively, critical for the acquisition and maintenance of EBC (Lewis, LoTurco, & Solomon, 1987; McCormick, Steinmetz, & Thompson, 1985; Steinmetz et al., 1987). Convergence of multi-modal CS input and US input upon a common site (Purkinje cells in the cerebellar cortex) corresponds with the basic organization of a network model (Kehoe, 1988) associated with empirical findings of facilitated cross-modal transfer of conditioning in rabbit EBC (see Kehoe et al., 1995; Kehoe & Napier, 1991). Aspects of cerebellar cortical functional anatomy (e.g., lack of multimodal input separation between granule and Purkinje cells) may therefore contribute to the cross-modal transfer observed in the present study.
To account for developmental differences in the present EBC cross-modal transfer effects, neurobiological mechanisms involved in the relevant cross-modal integration presumably undergo substantial developmental modification beyond PD30. Some Purkinje cell morphological development continues beyond the early juvenile period (Anderson & Flumerfelt, 1985; Berry & Bradley, 1976), though most aspects of Purkinje cell structure (Altman & Bayer, 1997) as well as relevant synaptic connections (Puro & Woodward, 1977a, b) and molecular profiles (Metzger & Kapfhammer, 2003) achieve ‘adult-like’ levels by the 3rd – 4th postnatal weeks. Though these findings do not rule out the possibility of developmental changes in other cerebellar cortical neuronal populations that may contribute to cross-modal transfer effects in EBC, the absence of a clear link between the developmental time course of cerebellar cortical structure and function and developmental cross-modal transfer effects in ISI discrimination training indicates that essential cross-modal plasticity may instead (or additionally) occur upstream and/or downstream (e.g., deep nuclei) of the cerebellar cortex. For instance, the deep nuclei of the cerebellum receives multimodal CS input (c.f., Tracy, Britton, & Steinmetz, 2001) and may modulate relevant multimodal CS information via feedback upon pontine nuclei (see Bao, Chen, & Thompson, 2000; Clark, Gohl, & Lavond, 1997). Developmental changes in cerebello-pontine interactions, as well as in other structures normally involved in EBC (e.g., hippocampus; inferior colliculus; thalamus) may also contribute to cross-modal transfer observed in the present study. Further experimentation is required to identify neural substrates of these developmental changes.
Conclusions
Performance in dual-ISI EBC tasks has been shown to undergo developmental modification beyond the juvenile period (Brown et al., 2006; Freeman et al., 2003; present study), perhaps due to increased difficulty in these tasks relative to single-cue conditioning (see Kehoe et al., 1989). This notion of increased task difficulty is consistent with recent findings from our laboratory suggesting that ISI discrimination and temporal uncertainty EBC display enhanced sensitivity (relative to single-cue tasks) to developmental neurotoxicological exposure associated with cerebellar dysfunction (see Brown et al., 2008). Conditioning of CSs trained at different ISIs may further our understanding of mechanisms of adaptive CR timing in EBC, and ISI discrimination in particular may be useful in characterizing auditory and visual CS pathways in EBC. The enhanced cross-modal processing observed in younger subjects trained in ISI discrimination may have additional implications for the ‘infantile amnesia’ phenomenon. Specifically, if young and mature organisms learn different aspects of identical training situations (i.e., differential processing of amodal and modality-specific features), the failure in adulthood to remember events that occurred early in development may primarily result from developmental differences in the encoding of the relevant information rather than a retrieval failure (c.f., Spear & Molina, 1987). The well-established behavioral, developmental, and neurobiological properties of EBC may make this an ideal task to elucidate mechanisms responsible for developmental cross-modal transfer and infantile amnesia effects.
Acknowledgments
This research was supported by NIH grant 1-R01-AA11945 and NIAAA grant 1-F31-AA16250-01. The authors thank Lyngine Calizo, Huan Bao Duong, Brianne Navetta, Valerie Saxton, and Jesse Sullivan for technical assistance.
References
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- Anderson WA, Flumerfelt BA. Purkinje cell growth beyond the twenty-third postnatal day. Developmental Brain Research. 1985;17:195–200. doi: 10.1016/0165-3806(85)90143-9. [DOI] [PubMed] [Google Scholar]
- Bahrick LE, Lickliter R. Intersensory redundancy guides attentional selectivity and perceptual learning in infancy. Developmental Psychology. 2000;36:190–201. doi: 10.1037//0012-1649.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chen L, Thompson RF. Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behavioral Neuroscience. 2000;114:254–261. doi: 10.1037//0735-7044.114.2.254. [DOI] [PubMed] [Google Scholar]
- Berry M, Bradley P. The growth of dendritic trees of Purkinje cells in the cerebellum of the rat. Brain Research. 1976;112:1–35. doi: 10.1016/0006-8993(76)90331-0. [DOI] [PubMed] [Google Scholar]
- Brandon SE, Vogel EH, Wagner AR. Stimulus representation in SOP: I. Theoretical rationalization and some implications. Behavioural Processes. 2003;62:5–25. doi: 10.1016/s0376-6357(03)00016-0. [DOI] [PubMed] [Google Scholar]
- Bower TGR. The rational infant – learning in infancy. New York: W. H. Freeman and Company; 1989. [Google Scholar]
- Brodal A. Anatomical studies of cerebellar fibre connections with special reference to problems of functional localization. Progress in Brain Research. 1967;25:135–173. doi: 10.1016/S0079-6123(08)60964-4. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Developmental Psychobiology. 2007;49:243–257. doi: 10.1002/dev.20178. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Stanton ME. Dose dependent deficits in dual interstimulus interval classical eyeblink conditioning tasks following neonatal binge alcohol exposure in rats. Alcoholism: Clinical & Experimental Research. 2008;32:277–293. doi: 10.1111/j.1530-0277.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Brown KL, Pagani JH, Stanton ME. The ontogeny of interstimulus interval (ISI) discrimination of the conditioned eyeblink response in rats. Behavioral Neuroscience. 2006;120:1057–1070. doi: 10.1037/0735-7044.120.5.1057. [DOI] [PubMed] [Google Scholar]
- Campolattaro M, Freeman JH. Cerebellar inactivation during cross-modal transfer. Society for Neuroscience Abstracts. 2006:667.2. [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JJ, Thompson RF. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. Journal of Neuroscience. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Moore JW. Cerebellar neuronal activity expresses the complex topography of conditioned eyeblink responses. Behavioral Neuroscience. 2003;117:1211–1219. doi: 10.1037/0735-7044.117.6.1211. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning & Memory. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Clark RE, Gohl EB, Lavond DG. The learning-related activity that develops in the pontine nuclei during classical eyeblink conditioning is dependent on the interpositus nucleus. Learning & Memory. 1997;3:532–544. doi: 10.1101/lm.3.6.532. [DOI] [PubMed] [Google Scholar]
- Coleman SR, Gormezano I. Classical conditioning of the rabbit’s (Oryctolagus cuniculus) nictitating membrane response under symmetrical CS-US interval shifts. Journal of Comparative & Physiological Psychology. 1971;77:447–455. doi: 10.1037/h0031879. [DOI] [PubMed] [Google Scholar]
- Duong HB, Brown KL, Stanton ME. Cross-modal transfer of timing of the classically conditioned eyeblink response in weanling rats. International Society for Developmental Psychobiology Abstracts. 2007:15. [Google Scholar]
- Freeman JH, Barone S, Stanton ME. Disruption of cerebellar maturation by an antimitotic agent impairs the ontogeny of eyeblink conditioning in rats. Journal of Neuroscience. 1995;15:7301–7314. doi: 10.1523/JNEUROSCI.15-11-07301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Nicholson DA. Developmental changes in the neural mechanisms of eyeblink conditioning. Behavioral & Cognitive Neuroscience Reviews. 2004;3:3–13. doi: 10.1177/1534582304265865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Nicholson DA, Muckler AS, Rabinak CA, DiPietro NT. Ontogeny of eyeblink conditioned response timing in rats. Behavioral Neuroscience. 2003;117:283–291. doi: 10.1037/0735-7044.117.2.283. [DOI] [PubMed] [Google Scholar]
- Freeman JH, Spencer CO, Skelton RW, Stanton ME. Ontogeny of eyeblink conditioning in the rat: Effects of US intensity and interstimulus interval on delay conditioning. Psychobiology. 1993;21:233–242. [Google Scholar]
- Frieman J, Goyette CH. Transfer of training across stimulus modality and response class. Journal of Experimental Psychology. 1973;97:235–241. doi: 10.1037/h0033931. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD. Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology. 1998;37:471–480. doi: 10.1016/s0028-3908(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Garcia KS, Steele PM, Mauk MD. Cerebellar cortex lesions prevent acquisition of conditioned eyelid responses. Journal of Neuroscience. 1999;19:10940–10947. doi: 10.1523/JNEUROSCI.19-24-10940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EJ. Principles of perceptual learning and development. New York: Appleton-Century-Crofts; 1969. [Google Scholar]
- Glickstein M. Mossy-fibre sensory input to the cerebellum. Progress in Brain Research. 1997;114:251–259. doi: 10.1016/s0079-6123(08)63368-3. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Sears LL, Steinmetz JE. Possible CS and US pathways for rabbit classical eyelid conditioning: electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behavioral & Neural Biology. 1993;60:172–185. doi: 10.1016/0163-1047(93)90285-p. [DOI] [PubMed] [Google Scholar]
- Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learning & Memory. 2005;12:260–269. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF. The formation of learning sets. Psychological Review. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Welsh JP, Yeo CH, Romano AG. Recoverable and nonrecoverable deficits in conditioned responses after cerebellar cortical lesions. Journal of Neuroscience. 1993;13:1624–1635. doi: 10.1523/JNEUROSCI.13-04-01624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehler FK, Leonard DW. Double responding in classical nictitating membrane conditioning with single-CS dual-ISI training. Pavlovian Journal of Biological Science. 1976;11:180–190. doi: 10.1007/BF03000295. [DOI] [PubMed] [Google Scholar]
- Holt PE, Kehoe EJ. Cross-modal transfer as a function of similarities between training tasks in classical conditioning of the rabbit. Animal Learning & Behavior. 1985;13:51–59. [Google Scholar]
- Honey RC, Hall GH. Acquired equivalence and distinctiveness of cues. Journal of Experimental Psychology: Animal Behavioral Processes. 1989;15:338–346. [PubMed] [Google Scholar]
- Hull CL. The problem of stimulus equivalence in behavior theory. Psychological Review. 1939;46:9–30. [Google Scholar]
- Ivkovich D, Paczkowski CM, Stanton ME. Ontogeny of delay versus trace eyeblink conditioning in the rat. Developmental Psychobiology. 2000;36:148–160. doi: 10.1002/(sici)1098-2302(200003)36:2<148::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Joscelyne A, Kehoe EJ. Time and stimulus specificity in extinction of the conditioned nictitating membrane response in the rabbit (Oryctolagus cuniculus) Behavioral Neuroscience. 2007;121:50–62. doi: 10.1037/0735-7044.121.1.50. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ. A layered network model of associative learning: Learning to learn and configuration. Psychological Review. 1988;95:411–433. doi: 10.1037/0033-295x.95.4.411. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ. Classical conditioning: Fundamental issues for adaptive network models. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: Foundations of adaptive networks. Cambridge, MA: MIT Press; 1990. pp. 389–420. [Google Scholar]
- Kehoe EJ, Graham-Clarke P, Schreurs BG. Temporal patterns of the rabbit’s nictitating membrane response to compound and component stimuli under mixed CS-US intervals. Behavioral Neuroscience. 1989;103:283–295. doi: 10.1037//0735-7044.103.2.283. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Holt PE. Transfer across CS-US intervals and sensory modalities in classical conditioning of the rabbit. Animal Learning & Behavior. 1984;12:122–128. [Google Scholar]
- Kehoe EJ, Horne PS, Horne AJ. Discrimination learning using different CS-US intervals in classical conditioning of the rabbit’s nictitating membrane response. Psychobiology. 1993;21:277–285. [Google Scholar]
- Kehoe EJ, Horne AJ, Macrae M. Learning to learn: real-time features and a connectionist model. Adaptive Behavior. 1995;3:235–271. [Google Scholar]
- Kehoe EJ, Joscelyne A. Temporally specific extinction of conditioned responses in the rabbit (Oryctolagus cuniculus) nictitating membrane preparation. Behavioral Neuroscience. 2005;119:1011–1022. doi: 10.1037/0735-7044.119.4.1011. [DOI] [PubMed] [Google Scholar]
- Kehoe EJ, Morrow LD, Holt PE. General transfer across sensory modalities survives reductions in the original conditioned reflex in the rabbit. Animal Learning & Behavior. 1984;12:129–136. [Google Scholar]
- Kehoe EJ, Napier RM. Temporal specificity in cross-modal transfer of the rabbit nictitating membrane response. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:26–35. [PubMed] [Google Scholar]
- Kraebel KS, Spear NE. Infant rats are more likely than adolescents to orient differentially to amodal (intensity-based) features of single-element and compound stimuli. Developmental Psychobiology. 2000;36:49–66. [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behavioural Brain Research. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE, Yokaitis MH, Thompson RF. Reacquisition of classical conditioning after removal of cerebellar cortex. Experimental Brain Research. 1987;67:569–593. doi: 10.1007/BF00247289. [DOI] [PubMed] [Google Scholar]
- Lewis JL, LoTurco JJ, Solomon PR. Lesions of the middle cerebellar peduncle disrupt acquisition and retention of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1987;101:151–157. doi: 10.1037//0735-7044.101.2.151. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DL, Turkewitz G. Cross-modal equivalence in early infancy: auditory-visual intensity matching. Developmental Psychology. 1980;16:597–607. [Google Scholar]
- Lickliter R, Bahrick LE. Perceptual development and the origins of multisensory responsiveness. In: Calvert GA, Spence C, Stein BE, editors. The handbook of multisensory processes. Cambridge, MA: MIT Press; 2004. pp. 643–654. [Google Scholar]
- Mauk MD, Ruiz BP. Learning-dependent timing of Pavlovian eyelid responses: Differential conditioning using multiple interstimulus intervals. Behavioral Neuroscience. 1992;106:666–681. doi: 10.1037//0735-7044.106.4.666. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Steinmetz JE, Thompson RF. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Research. 1985;359:120–130. doi: 10.1016/0006-8993(85)91419-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. Abstraction of temporal attributes. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:226–243. [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: Testing predictions of a large-scale computer simulation. Journal of Neuroscience. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon RC, Kraemer PJ, Spear NE. Development of intersensory function: age-related differences in stimulus selection of multimodal compounds in rats as revealed by Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:448–464. doi: 10.1037//0097-7403.17.4.448. [DOI] [PubMed] [Google Scholar]
- Metzger F, Kapfhammer JP. Protein kinase C: its role in activity-dependent Purkinje cell dendritic development and plasticity. Cerebellum. 2003;2:206–214. doi: 10.1080/14734220310016150. [DOI] [PubMed] [Google Scholar]
- Millenson JR, Kehoe EJ, Gormezano I. Classical conditioning of the rabbit’s nictitating membrane response under fixed and mixed CS-US intervals. Learning & Motivation. 1977;8:351–366. [Google Scholar]
- Miller NE, Dollard JC. Social Learning and Imitation. New Haven, CT: Yale University Press; 1941. [Google Scholar]
- Molina JC, Hoffman H, Serwatka J, Spear NE. Establishing intermodal equivalence in preweanling and adult rats. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:433–447. doi: 10.1037//0097-7403.17.4.433. [DOI] [PubMed] [Google Scholar]
- Moore JW, Choi JS. Conditioned response timing and integration in the cerebellum. Learning & Memory. 1997;3:116–129. doi: 10.1101/lm.4.1.116. [DOI] [PubMed] [Google Scholar]
- Mortimer JA. Cerebellar responses to teleceptive stimuli in alert monkeys. Brain Research. 1975;83:369–390. doi: 10.1016/0006-8993(75)90831-8. [DOI] [PubMed] [Google Scholar]
- Nolan BC, Freeman JH. Purkinje cell loss by OX7-saporin impairs acquisition and extinction of eyeblink conditioning. Learning & Memory. 2006;13:359–365. doi: 10.1101/lm.168506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nores WL, Medina JF, Steele PM, Mauk MD. Relative contributions of the cerebellar cortex and cerebellar nucleus to eyelid conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning. Vol. 2. Boston: Kluwer Academic Publishers; 2000. pp. 205–229. Animal Models. [Google Scholar]
- Ohyama T, Mauk MD. Latent acquisition of timed responses in cerebellar cortex. Journal of Neuroscience. 2001;21:682–690. doi: 10.1523/JNEUROSCI.21-02-00682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczkowski C, Ivkovich D, Stanton ME. Ontogeny of eyeblink conditioning using a visual conditional stimulus. Developmental Psychobiology. 1999;35:253–263. doi: 10.1002/(sici)1098-2302(199912)35:4<253::aid-dev1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychological Review. 1987;94:61–73. [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro DG, Woodward DJ. Maturation of evoked climbing fiber input to rat cerebellar Purkinje cells: I. Experimental Brain Research. 1977a;28:85–100. doi: 10.1007/BF00237088. [DOI] [PubMed] [Google Scholar]
- Puro DG, Woodward DJ. Maturation of evoked mossy fiber input to rat cerebellar Purkinje cells: II. Experimental Brain Research. 1977b;28:427–441. doi: 10.1007/BF00235721. [DOI] [PubMed] [Google Scholar]
- Roberts S. Cross-modal use of an internal clock. Journal of Experimental Psychology: Animal Behavior Process. 1982;8:2–22. [PubMed] [Google Scholar]
- Schreurs BG, Kehoe EJ. Cross-modal transfer as a function of initial training level in classical conditioning with the rabbit. Animal Learning & Behavior. 1987;15:47–54. [Google Scholar]
- Smith MC. CS-US interval and US intensity in classical conditioning of the rabbit’s nictitating membrane response. Journal of Comparative & Physiological Psychology. 1968;66:679–687. doi: 10.1037/h0026550. [DOI] [PubMed] [Google Scholar]
- Snider RS, Stowell A. Receiving areas of the tactile, auditory, and visual systems in the cerebellum. Journal of Neurophysiology. 1944;7:331–357. [Google Scholar]
- Spear NE. Ecologically determined dispositions control the ontogeny of learning and Memory. In: Kail R, Spear NE, editors. Comparative perspectives on the development of memory. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. pp. 325–358. [Google Scholar]
- Spear NE, Kucharski D. Ontogenetic differences in stimulus selection during Conditioning. In: Kail R, Spear NE, editors. Comparative perspectives on the development of memory. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. pp. 227–252. [Google Scholar]
- Spear NE, Molina JC. The role of sensory modality in the ontogeny of stimulus selection. In: Krasnegor N, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal development: A psychobiological perspective. Orlando, FL: Academic Press; 1987. pp. 83–110. [Google Scholar]
- Stanton ME, Fox GD, Carter CS. Ontogeny of the conditioned eyeblink response in rats: Acquisition or expression? Neuropharmacology. 1998;37:623–632. doi: 10.1016/s0028-3908(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH. Eyeblink conditioning in the developing rat: An animal model of learning in developmental neurotoxicology. Environmental Health Perspectives. 1994;102:131–139. doi: 10.1289/ehp.94102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH. Developmental studies of eyeblink conditioning in the rat. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning. Vol. 2. Boston: Kluwer Academic Publishers; 2000. pp. 17–49. Animal Models. [Google Scholar]
- Stanton ME, Freeman JH, Skelton RW. Eyeblink conditioning in the developing rat. Behavioral Neuroscience. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Miller JT, Svinicki JG. Nonspecific transfer effects of discrimination training in the rat. Journal of Comparative & Physiological Psychology. 1971;74:96–101. [Google Scholar]
- Tracy J, Britton GB, Steinmetz JE. Comparison of single unit responses to tone, light, and compound conditioned stimuli during rabbit classical eyeblink conditioning. Neurobiology of Learning & Memory. 2001;76:253–267. doi: 10.1006/nlme.2001.4024. [DOI] [PubMed] [Google Scholar]
- Tran TD, Stanton ME, Goodlett CR. Binge-like ethanol exposure during the early postnatal period impairs eyeblink conditioning at short and long CS-US intervals in rats. Developmental Psychobiology. 2007;49:589–605. doi: 10.1002/dev.20226. [DOI] [PubMed] [Google Scholar]
- Turkewitz G, Mellon RC. Dynamic organization of intersensory function. Canadian Journal of Psychology. 1989;43:286–301. doi: 10.1037/h0084214. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ. Cerebellar cortex and eyeblink conditioning: a reexamination. Experimental Brain Research. 1992;88:623–638. doi: 10.1007/BF00228191. [DOI] [PubMed] [Google Scholar]