Abstract
We determined the ability of self-complementary adeno-associated virus (scAAV) vectors to deliver and express the pyruvate dehy-drogenase E1α subunit gene (PDHA1) in primary cultures of skin fibroblasts from 3 patients with defined mutations in PHDA1 and 3 healthy subjects. Cells were transduced with scAAV vectors containing the cytomegalovirus promoter-driven enhanced green fluorescent protein (EGFP) reporter gene at a vector:cell ratio of 200. Transgene expression was measured 72 h later. The transduction efficiency of scAAV2 and scAAV6 vectors was 3-to 5-fold higher than that of the other serotypes, which were subsequently used to transduce fibroblasts with wild-type PDHA1 cDNA under the control of the chicken beta-action (CBA) promoter at a vector:cell ratio of 1000. Total PDH-specific activity and E1α protein expression were determined 10 days post-transduction. Both vectors increased E1α expression 40–60% in both control and patient cells, and increased PDH activity in two patient cell lines. We also used dichloroacetate (DCA) to maximally activate PDH through dephosphorylation of E1α. Exposure for 24 h to 5 mM DCA increased PDH activity in non-transduced control (mean 37% increase) and PDH deficient (mean 44% increase) cells. Exposure of transduced patient fibroblasts to DCA increased PDH activity up to 90% of the activity measured in untreated control cells. DCA also increased expression of E1α protein and, to variable extents, that of other components of the PDH complex in both non-transduced and transduced cells. These data suggest that a combined gene delivery and pharmacological approach may hold promise for the treatment of PDH deficiency.
Keywords: Pyruvate dehydrogenase deficiency, AAV vector, Gene therapy, Dichloroacetate
The nuclear-encoded pyruvate dehydrogenase (PDH) multi-enzyme complex plays a central role in cellular energetics. It consists of multiple copies of three different enzymes: the thiamine-dependent pyruvate decarboxylase (E1) is a heterotetrameric (α2β2) protein that irreversibly oxidizes pyruvate to acetyl CoA; dihydrolipoamide acetyl-transferase (E2) forms the structural core of the complex; dihydrolipoamide dehydrogenase (E3) is stably integrated into the complex by an E3-binding protein (E3bp), formally called protein X, that has no known enzymatic function [1]. Rapid regulation of the complex in eukaryotes occurs primarily by reversible phosphorylation of serine residues in the E1α subunit by PDH kinase and PDH phosphatase, in which the unphosphorylated enzyme is catalytically active [2,3].
PDH complex deficiency is one of the more common inborn errors of mitochondrial energy metabolism. Its clinical presentation and course are variable but most patients exhibit progressive neurological degeneration and either persistent or episodic elevation of lactate in blood, cerebrospinal fluid or both [2–4]. By far the majority of biochemically proven cases of PDH deficiency are due to defects in the E1α subunit. Mutations in the X-linked E1α subunit gene (PDHA1) may give rise to diminished enzyme catalytic activity with or without reduction in E1α mRNA and/or protein [5,6]. There are no proven treatments for PDH complex deficiency. Anecdotal studies have reported beneficial effects from the use of high-fat, ketogenic diets, which provide an alternative energy source to carbohydrate [7] and dichloroacetate (DCA), which inhibits PDH kinase and maintains E1α in its unphosphorylated, catalytically active form [8]. Neither intervention has been rigorously evaluated as a treatment for PDH deficiency in a controlled clinical trial. Indeed, recent data [9] from a controlled trial of DCA in children with various forms of congenital lactic acidosis suggest that chronic DCA administration may be particularly effective in the chronic treatment of PDH deficiency.
We have proposed that the PDH complex, specifically the E1α subunit, should be considered an important target for gene therapy of mitochondrial energy failure [10]. As proof of concept, we demonstrated that recombinant adeno-associated virus (AAV) could be used to package gene targeting vectors as single-strand molecules carrying the wild-type PDHA1 cDNA, deliver the vector to cultured mammalian cells [11] and partially restore PDH activity in a primary culture of skin fibroblasts from a patient with E1α deficiency [12]. However the efficiency of transgene expression was low (~25% of cells) due to the single-stranded nature of the recombinant vector genome. Moreover, only one AAV serotype was evaluated (AAV2) and the findings were limited to cells from a single patient.
Therefore, to optimize the delivery and expression of PDHA1, we employed here double-stranded DNA-containing self-complementary AAV (scAAV) vectors that by-pass the requirement of viral second-strand DNA synthesis. We then investigated the relative transduction efficiency of several scAAV-enhanced green fluorescent protein (EGFP) serotype vectors in both normal and E1α-deficient cultured fibroblasts. Finally, we determined the effectiveness of scAAV-mediated gene transfer alone and combined with DCA to restore PDH activity in defective cells.
Materials and methods
Cell culture, treatment and plasmids
Primary cultures of fibroblasts were established from skin biopsies of three healthy subjects and three patients (one male and two females) with PDH E1α deficiency. The patients had the following mutations in the PDHA1 gene: patient 1, c.1163_1166dupAAGT in exon 11; patient 2, c.904C>T in exon 10; and patient 3, c.642G>T in exon 7. Cells were cultured as monolayers in T-flasks in complete Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO). The medium contained 25 mM glucose and was supplemented with 20% fetal bovine serum (Hyclone, Logan, UT), 1% penicillin/streptomycin solution (10,000 U of penicillin and 10 mg/ml of streptomycin) (Sigma, St. Louis, MO), 1% insulin–transferrin solution (0.5 mg/ml), 0.2% fibroblast growth factor solution (1 µg/ml) and 1% l-glutamine solution (0.1 M). Cultures were maintained at 37 °C in a humidified 5% CO2/95% air atmosphere. In some experiments, cells were exposed for the final 24 h of culture to media containing 5 mM DCA (sodium salt; TCI Organics, Portland, OR).
Cells from the human embryonic kidney cell line 293 (HEK 293) were maintained at 37 °C in a 5% CO2 atmosphere in Iscove’s-modified Dulbecco’s medium (IMDM) media supplemented with 10% newborn bovine serum and 1% penicillin/streptomycin solution (10,000 U of penicillin and 10 mg/ml of streptomycin; Sigma, St. Louis, MO). Recombinant AAV-helper plasmid, pAAV2-RC, and adenovirus-helper plasmid, pHelper, were obtained from Stratagene USA (La Jolla, CA). Recombinant AAV packaging plasmids were generously provided, by Dr. R. Jude Samulski (University of North Carolina). A scAAV cloning vector, pdsCBA-EGFP, was a gift from Dr. Xiao Xiao (University of North Carolina).
Construction of the recombinant AAV vector
Standard cloning techniques were used for constructing all recombinant AAV-based plasmids. The University of Florida Powell Gene Therapy Center produced conventional rAAV (ssAAV) serotype 2 vectors for this study and scAAV serotype vectors were packaged in the Division of Cellular and Molecular Therapy. Briefly, virus production included use of the helper/packaging plasmid that supplies all the necessary helper functions as well as rep and cap in trans. The vector plasmid pCBA-PDH-EGFP was cotransfected with pDG, expressing AAV-2 rep and cap genes, into ~70–95% confluent HEK 293 cells by calcium phosphate precipitation. Recombinant AAV was prepared by iodixanol centrifugation and heparin-column purification. Physical titer (genome number) was determined by dot blot. The titer used was 1012 particles/ml for ssAAV-PDH-GFP. For scAAV vectors, each cassette was composed of AAV inverted terminal repeats (ITRs) with a chicken β-actin (CBA) promoter driving the EGFP gene. Human PDHA1 gene insert was derived from plasmid pCBA-PDH as previously described [11,12]. Briefly, pCBA-PDH was digested with NotI and EcoRI to release a 1191-bp fragment and pdsCBA-EGFP was digested with StuI and Hin-dIII. The vector and insert were treated with the Klenow fragment of Escherichia coli DNA polymerase I and then blunt-end ligated. After transformation, the plasmid, pscAAV-CBA-PDH, with the right orientation and ITRs were selected for packaging scAAV vectors.
Recombinant AAV vector-mediated transduction assays
Approximately 2 × 105 cells were plated in each well in 12-well plates and incubated at 37 °C for 12 h. Cells were washed once with serum-free IMDM and then either mock-infected or infected with scAAV1-EGFP using serotypes AAV1–3, 5–7 and 9, at 200 particles per cell. Cells were observed under a Zeiss Axiovert 25 fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) 3 days after infection. The viral transduction efficiency was measured by GFP imaging, using images from three visual fields and was quantitated by ImageJ (NIH, Bethesda, MD).
For ssAAV transduction experiments, cells were coinfected with adenovirus and ssAAV vectors. Cells were grown to approximately 90% confluency in six-well plates (105 cells/well). They were infected with the ssAAV vector at a concentration of 10,000 vector particles per cell (PPC) and with 1 µl of adenovirus/per well (109 particles/ml). Cells were then cultured under normal growth conditions and monitored for GFP expression.
Western immunoblotting
Total protein concentrations were determined using the Bio-Rad protein assay kit. Protein lysates from each cultured cell line were electrophoresed on 10% polyacrylamide–SDS gels. Following transfer to an PVDF membrane (Millipore, Bedford, MA), the membrane was blocked at room temperature (RT) for 1 h with 5% non-fat dry milk in 1× Tris-buffered saline (TBS; 20 mM Tris–HCl, pH 7.5, 150 mM NaCl), and 0.05% Tween 20, then incubated with 6 µg/ml of E1α, E1β, E2 and E2/E3bp mAbs (Mito-Sciences Inc., Eugene, OR) for 1 h at RT. Subsequently, the secondary antibody, a horseradish peroxidase-conjugated bovine anti-mouse IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), was added at a 1:2000 dilution for 1 h at RT. Signals were detected using a chemiluminescence luminol reagent (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) according to the instructions provided by the manufacturer.
PDH activity
PDH complex activity was measured by the rate of 14C formation from [1–14C]-labeled pyruvate, as described [8,13]. Fibroblasts harvested by trypsinization from a confluent T-75 flask were washed in phosphate-buffered saline (PBS) and resuspended in PBS containing the serine protease inhibitors leupeptin and phenylmethylsulfonyl fluoride (PMSF). Cells were incubated for 15 min at 37 °C with DCA. Reactions were halted by addition of a stop solution (25 mM NaF, 25 mM EDTA, 4 mM dithio-threitol, 40% ethanol), and cells were lysed by repeated freezing and thawing. Sixteen aliquots of each fibroblast sample were assayed in test tubes for enzyme activity. Four samples that contained only lysate and water and four samples that also contained thiamine pyrophosphate and coenzyme A were assayed at reaction times of 5 and 10 min. A 14C-labeled pyruvate aliquot of known specific activity was added to the cell lysates and the test tubes were immediately closed with a rubber stopper. The stopper was outfitted with a center well (Kimble-Kontes, Vineland, NJ) that contained 1 cm2 chromatography paper soaked with 100 µl hyamine hydroxide. Reactions were halted by adding stopping buffer. The 14CO2 evolved due to PDH-mediated catalysis and trapped in the paper was determined by liquid scintillation. PDH-specific activity was expressed as nmol 14CO2 produced/min/mg protein. Excess cell lysate was used to determine total protein.
Statistical analysis
Differences between groups were compared using the group-paired Student’s t-test or one-way ANOVA analysis. A p value ≤ 0.01 was considered to be statistically significant.
Results
scAAV vectors promote efficient transduction
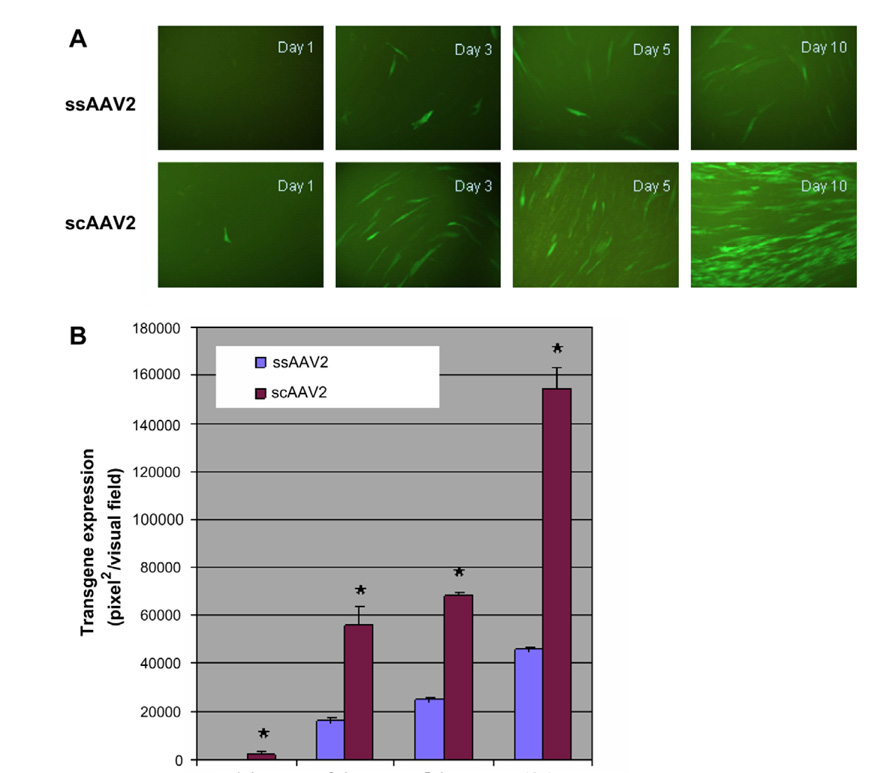
We first compared the effectiveness of transduction of healthy fibroblast cells by scAAV2 and ssAAV2 vectors. On day 1, the scAAV-EGFP vector yielded 10% green fluorescent cells after infection at 10,000 PPC, whereas the yield from ssAAV2-EGFP transduction was less than 1%. By day 10, the scAAV2 vector had yielded 90% green fluorescent cells, while the ssAAV2 vector yielded 30–40% green fluorescent cells after transduction (Fig. 1). Thus, scAAV2 was more effective than ssAAV2 in transducing human fibroblasts in culture.
Fig. 1.
Fluorescence microscopical (A) and quantitative (B) analyses of transduction efficiency of ssAAV and scAAV vectors. The transduction efficiency was evaluated in human fibroblast cells at vector particles per cell (PPC) of 10,000. Transgene expression was evaluated 1 day, 3 days, 5 days and 10 days post-transduction and analyzed using NIH ImageJ. *P < 0.01 (group-paired t-test). Original magnification 200×
scAAV serotype vectors differ in their transduction efficiency
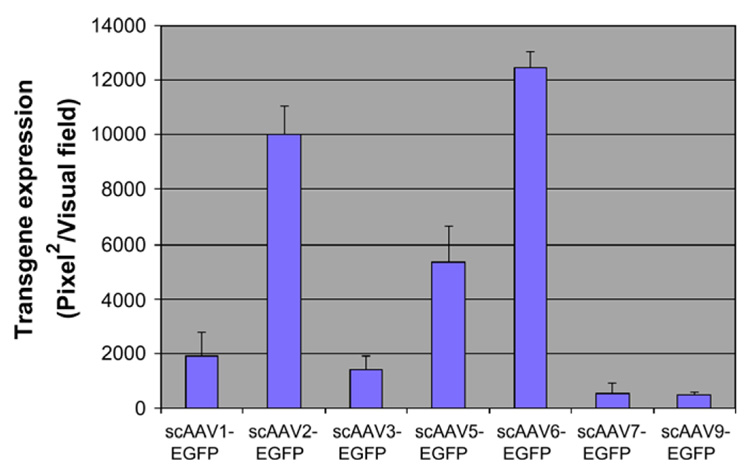
To optimize the delivery and expression of the E1α subunit gene, normal fibroblast cells were transduced with scAAV-EGFP serotypes 1–3, 5–7 and 9. Three days after infection, transgene expression was evaluated under fluorescence microscopy and analyzed using NIH ImageJ. As shown in Fig. 2, the transduction efficiency of scAAV2 and scAAV6 vectors was superior to that of the other serotype vectors and were further selected to deliver the wild-type PDHA1 in a cassette that contained the CBA promoter.
Fig. 2.
Quantitative analyses of transduction efficiency of scAAV-EGFP serotype vectors in human fibroblast cells. Relative transduction efficiencies were evaluated at a vector particle per cell of 200. Transgene expression was evaluated 72 h post-transduction and analyzed using NIH ImageJ.
scAAV-PDHA1 transduction increases E1α protein expression in cultured fibroblasts
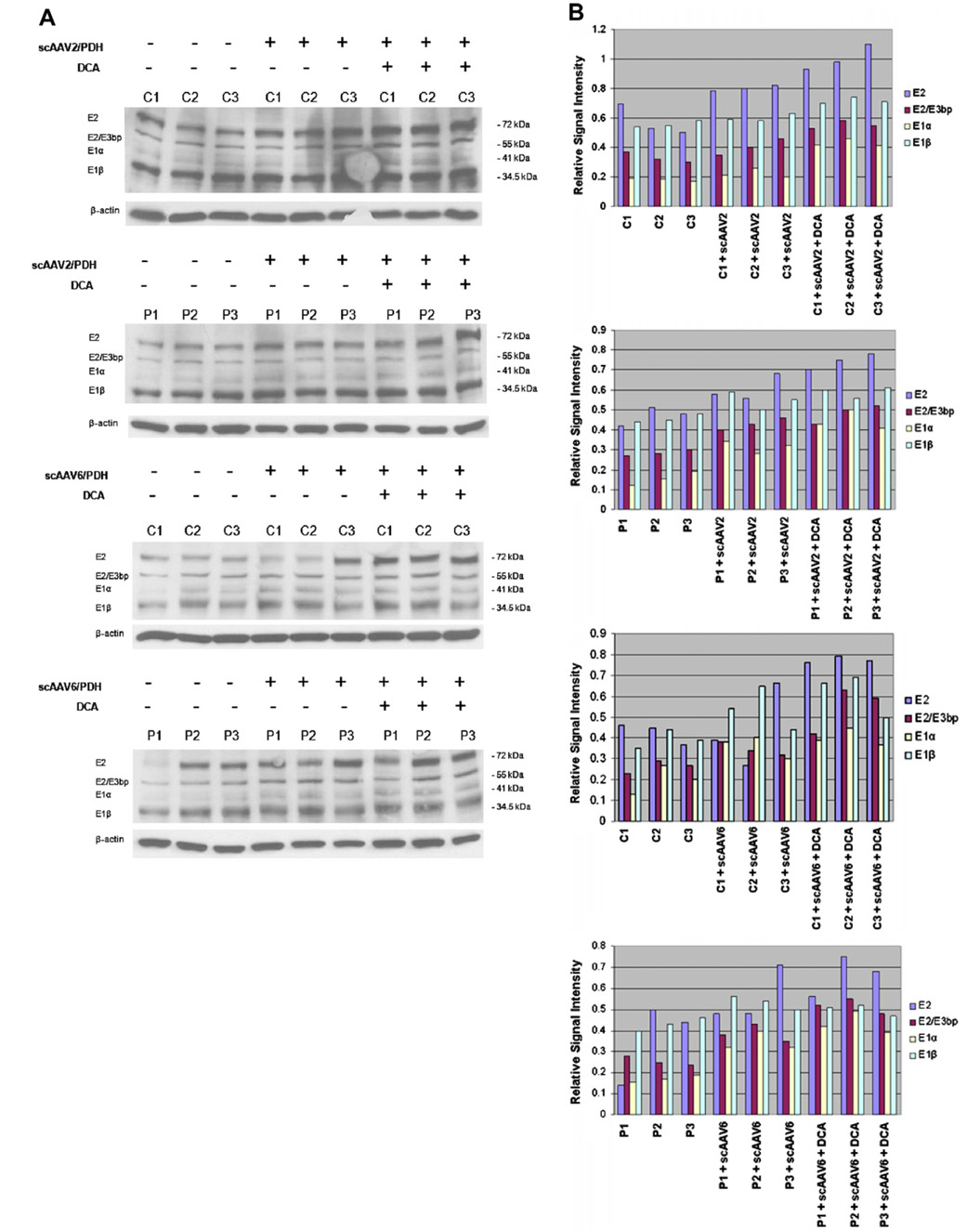
Immunoblot analysis of scAAV-mediated E1α protein expression in control and patient cells was performed by Western blot analysis 10 days after infection at 1000 PPC by scAAV2-PDHA1 or scAAV6-PDHA1 vectors. E1α expression increased 40–60% in patient fibroblasts and ~40% in control fibroblasts, compared to the level of E1α uninfected cells (Fig. 3). Addition of 5 mM DCA to the culture medium for 24 h resulted in a 40–65% increase in E1α expression in cells from both control and PDH deficient subjects (Fig. 3) and had variable effects on the expression of the other components of the PDH complex.
Fig. 3.
Effect of scAAV serotypes 2 and 6, with or without DCA, on expression of PDH complex proteins. (A) Immunoblots showing relative changes in steady-state levels of PDH E1α, E1β, E2 and E2/E3bp after AAV vectors delivery and/or combination of DCA administration. Cell lysates (15 µg) from three healthy subjects (C1, C2 and C3) and three PDH deficiency patients (P1, P2 and P3) were separated on 10% SDS–PAGE gels and immunoblotted with monoclonal Abs against E1α, E1β, E2 and E2/E3bp. (B) Quantitative analysis of immunoblot data. Using ImageJ the pixel densities in each band (E2, E2/E3bp, E1α and E1β) from the gel in (A) were quantitated and the amount of protein was standardized to the amount of β-actin (E2, E2/E3bp, E1α and E1β/β-actin) in each lane, respectively. Data are means of two independent experiments.
Effect of transduction and DCA on PDH activity
Without treatment, the PDH activity in patient cells averaged 51% of that measured in control cells (1.30 vs. 2.55 nmol 14CO2/min/mg; Table 1). Transduction of control cells had only modest effects on PDH activity. In contrast, PDH activity was 48–78% higher in cells from patients 1 and 2 following transduction with either AAV serotype, compared to non-transduced cells. Neither AAV serotype altered enzyme activity in cells from patient 3 who demonstrated only a modest reduction in baseline PDH activity from control cells. Exposure to 5 mM DCA for 24 h increased residual PDH activity in both control (mean 37% increase) and all three PDH deficient (mean 44% increase) cell lines. When transduction of cells was accompanied by subsequent DCA administration, the change in PDH activity was generally greater than the effects produced with either gene vector or drug alone. Cells from patients 1 and 2 demonstrated an 80–91% increase in PDH activity upon combined treatment with an AAV serotype vector and DCA, or 86% of the mean activity found in the three untreated control cell lines (2.20 vs. 2.55 nmol 14CO2/min (mg).
Table 1.
Effect of scAAV serotype vectors 2 and 6, with and without DCA, on PDH activity in fibroblasts
| Cell line | PDH activitya | % of parallel control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| w/o AAV-PDH | w/AAV2-PDH | w/AAV6-PDH | w/DCA | w/DCA+AAV2-PDH | w/DCA+AAV6-PDH | w/AAV2-PDH | w/AAV6-PDH | w/DCA | w/DCA + AAV2-PDH | w/DCA + AAV6-PDH | |
| Control 1 | 2.56 ± 0.64 (4) | 2.78 ± 0.55 (4) | 2.49 ± 0.64 (4) | 3.49 ± 0.53(4) | 3.74 ± 0.62(4) | 3.54 ± 0.47(4) | 9 | −3 | 36 | 46 | 38 |
| Control 2 | 2.78 ± 0.21 (4) | 2.66 ± 0.32 (4) | 2.98 ± 0.21 (4) | 3.67 ± 0.65(4) | 3.89 ± 0.70(4) | 4.01 ± 0.36(4) | −4 | 8 | 32 | 40 | 44 |
| Control 3 | 2.31 ± 0.33 (4) | 2.59 ± 0.13 (4) | 2.81 ± 0.33 (4) | 3.27 ± 0.45(4) | 3.46 ± 0.55(4) | 3.35 ± 0.52(4) | 12 | 22 | 42 | 50 | 45 |
| Patient 1 | 0.56 ± 0.05 (4) | 1.00 ± 0.14 (4) | 0.97 ± 0.05 (4) | 0.81 ± 0.24(4) | 1.07 ± 0.37(4) | 1.04 ± 0.44(4) | 78 | 73 | 44 | 91 | 86 |
| Patient 2 | 1.12 ± 0.35 (4) | 1.66 ± 0.42 (4) | 1.83 ± 0.35 (4) | 1.78 ± 0.41(4) | 2.03 ± 0.17(4) | 2.02 ± 0.22(4) | 48 | 63 | 59 | 81 | 80 |
| Patient 3 | 2.22 ± 0.57 (4) | 2.39 ± 0.39 (4) | 2.37 ± 0.57 (4) | 2.85 ± 0.22(4) | 3.51 ± 0.68(4) | 3.26 ± 0.54(4) | 8 | 7 | 28 | 58 | 47 |
Skin fibroblasts from three controls and three patients with PDH E1α deficiency were cultured with or without scAAV2-PDH/or scAAV6-PDH transduction for 10 days at 1000 vector particles per cell and with or without 5 mM DCA added at day nine for 24 h. Cells were harvested and total PDH activity was determined as described in Materials and methods.
Expressed as nmol 14CO2 formed from 1–14C pyruvate/min/mg protein. Data are means ± SD with number of replicates shown in parentheses. Negative percentages refer to values in transduced cells less than respective non-transduced controls. Abbreviations: AAV, adeno-associated virus; DCA, dichloroacetate; PDH, pyruvate dehydrogenase.
Discussion
These data indicate that scAAV serotype-specific vectors can effectively transduce cultured human fibroblasts with the wild type PDHA1 gene, leading to increased expression and activity of PDH in cell lines from some patients with E1α deficiency. Because of the small number of cell lines studied, we cannot determine whether certain mutations in the PDHA1 gene affect the efficiency of transduction and/or the expression and activity of residual PDH.
scAAV2 and scAAV6 vectors had the greatest transduction efficiency among the several AAV serotypes we evaluated. Moreover, the transduction efficiency of scAAV2 and scAAV6 vectors employed here was far superior to the efficiency achieved with ssAAV vectors Ref. [12] and Fig. 1. Unlike ssAAV vectors, scAAV vectors circumvented the requirement for viral second-strand DNA synthesis and displayed a rapid induction of transgene expression. We observed that an E1α protein of normal size was present in transduced cells. This indicated that the mRNA transcribed from the construct was translated and the resulting polypeptide was imported into the mitochondria and expressed, which resulted in an approximately 40–60% increase in E1α protein in both control and patient cells.
As expected cf. Ref. [8] DCA stimulated PDH activity in control and PDH defective cells, presumably by inhibiting PDH kinase. In addition, it also uniformly increased expression of the E1α protein. Over 20 years ago, short-term exposure of rats to DCA was found to increase the level and duration of total PDH activity in liver, an effect that could not be prevented by inhibitors of protein synthesis [14]. Subsequently it was confirmed that DCA reduced turnover of the E1α protein [15]. The data presented in Table 1, however, suggest that the ability of DCA to increase expression of E1α and, indeed, of other enzyme components of the PDH complex, may be a more general phenomenon than previously thought. This notion is consistent with clinical studies demonstrating that short-term administration of DCA to healthy subjects [16] or to those with-type 2 diabetes [17] has a blood lactate-lowering effect that persists beyond the residence time of the drug in plasma.
It is noteworthy that the combination of transduction with either scAAV2 or scAAV6, followed by exposure of cells to DCA, generally resulted in a greater increase in PDH activity than either treatment alone. This was particularly the case in cells from patient 3, in which the effects of gene and pharmacological delivery were synergistic and resulted in levels of enzyme activity that exceeded those measured in untreated control cells.
It could be argued that increasing the amount of E1α protein alone has no appreciable effect on PDH activity, since the E1α subunit per se has no demonstrable catalytic activity and it is uncertain whether it is the major limiting factor in determining the overall activity of the complex. However it is likely that wild-type E1α competed with mutant E1α proteins for assembly with E1β into an active α2β2 tetramer. Based on the data presented in Fig. 3, it may also be possible that DCA increased the stability of the entire PDH complex, thereby increasing available E1α for dephosphorylation.
Although DCA is capable of altering the expression of some mammalian genes [18,19] there is no evidence to our knowledge that it influences the transcription of any genes associated with the PDH multi-enzyme complex. Thus, the changes in the expression of the protein components may be due exclusively to stabilization of the entire complex. Since the most consistent increase in expression was observed for the E1α subunit, it is intriguing to speculate that the change in its phosphorylation status induced by DCA may provide a molecular signal for the assembly and/or stability of the PDH complex.
In summary, we optimized the in vitro delivery of the PDHA1 gene to human cells by employing scAAV serotype-specific vectors. Two vectors, scAAV2 and scAAV6, demonstrated the highest transduction efficiency and allowed functionally active E1α protein to be expressed. Once we delivered the wild-type PDHA1 gene to cells, we attempted to maximally activate “total” cellular PDH (both wild-type and endogenous enzyme) by adding DCA, to ‘lock’ the E1α subunit in its unphosphorylated, catalytically active, state. This was accomplished, but, in addition, we found that DCA also increased expression of E1α and, to variable extents, of other components of the PDH complex. Taken together, our findings demonstrate the feasibility of a combined gene delivery and pharmacological approach to the treatment of PDH deficiency. Future research will determine the extent to which these findings can be extrapolated in vivo to animal models and, ultimately, to affected humans.
Acknowledgment
We thank Ms. Candace Caputo for editorial assistance. This work was supported in part by the Zachary Foundation and by NIH Grants MO1-000082 and P01 DK-058327.
References
- 1.Smolle M, Prior AE, Brown AE, Cooper A, Byron O, Lindsay JG. A new level of architectural complexity in the human pyruvate dehydrogenase complex. J. Biol. Chem. 2006;281:19772–19780. doi: 10.1074/jbc.M601140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson BH. Lactic academia: disorders of pyruvate carboxylase and pyruvate dehydrogenase. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. eighth ed. New York: McGraw-Hill; 2001. pp. 2275–2296. [Google Scholar]
- 3.Stacpoole PW, Gilbert LR. Pyruvate dehydrogenase complex deficiency. In: Glew RH, Rosenthal MD, editors. Clinical Studies in Medical Biochemistry. third ed. Oxford: Oxford University Press; pp. 77–88. [Google Scholar]
- 4.Debray FG, Lambert M, Chevalier I, Robitaille Y, Decarie JC, Shoubridge EA, Robinson BH, Mitchell GA. Long-term outcome and clinical spectrum of 73 pediatric patients with mitochondrial diseases. Pediatrics. 2007;119:722–733. doi: 10.1542/peds.2006-1866. G.A. [DOI] [PubMed] [Google Scholar]
- 5.Lissens W, De Meirleir L, Seneca S, Liebaers I, Brown GK, Brown RM, Ito M, Naito E, Kuroda Y, Kerr DS, Wexler ID, Patel MS, Robinson BH, Seyda A. Mutations in the X-linked pyruvate dehydrogenase (E1) alpha subunit gene (PDHA1) in patients with a pyruvate dehydrogenase complex deficiency. Hum. Mutat. 2000;15:209–219. doi: 10.1002/(SICI)1098-1004(200003)15:3<209::AID-HUMU1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JM, Levandovskiy V, Mackay N, Tein I, Robinson BH. Deficiency of pyruvate dehydrogenase caused by novel and known mutations in the E1alpha subunit. Am. J. Med. Genet. A. 2004;131:59–66. doi: 10.1002/ajmg.a.30287. [DOI] [PubMed] [Google Scholar]
- 7.Wexler ID, Hemalatha SG, McConnell J, Buist NR, Dahl HH, Berry SA, Cederbaum SD, Patel MS, Kerr DS. Outcome of pyruvate dehydrogenase deficiency treatment with ketogenic diets: studies in patients with identical mutations. Neurology. 1997;49:1655–1661. doi: 10.1212/wnl.49.6.1655. [DOI] [PubMed] [Google Scholar]
- 8.Berendzen K, Theriaque DW, Shuster J, Stacpoole PW. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrian. 2006;6:126–135. doi: 10.1016/j.mito.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O’Brien RG, Perkins LA, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;115:1519–1531. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 10.Stacpoole PW, Owen R, Flotte TR. The pyruvate dehydrogenase complex as a target for gene therapy. Curr. Gene Ther. 2003;3:239–245. doi: 10.2174/1566523034578320. [DOI] [PubMed] [Google Scholar]
- 11.Owen R, IV, Lewin AP, Peel A, Wang J, Guy J, Hauswirth WW, Stacpoole PW, Flotte TR. Recombinant adeno-associated virus vector-based gene transfer for defects in oxidative metabolism. Hum. Gene Ther. 2000;11:2067–2078. doi: 10.1089/104303400750001381. [DOI] [PubMed] [Google Scholar]
- 12.Owen R, IV, Mandel RJ, Ammini CV, Conlon TJ, Kerr DS, Stacpoole PW, Flotte TR. Gene therapy for pyruvate dehydrogenase E1alpha deficiency using recombinant adeno-associated virus 2 (rAAV2) vectors. Mol. Ther. 2002;6:394–399. doi: 10.1006/mthe.2002.0683. [DOI] [PubMed] [Google Scholar]
- 13.Han Z, Gorbatyuk M, Thomas J, Jr, Lewin AS, Srivastava A, Stacpoole PW. Down-regulation of expression of rat pyruvate dehydrogenase E1alpha gene by self-complementary adeno-associated virus-mediated small interfering RNA delivery. Mitochondrion. 2007;7:253–259. doi: 10.1016/j.mito.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans OB, Stacpoole PW. Prolonged hypolactatemia and increased total pyruvate dehydrogenase activity by dichloroacetate. Biochem. Pharmacol. 1982;31:1295–1300. doi: 10.1016/0006-2952(82)90019-3. [DOI] [PubMed] [Google Scholar]
- 15.Morten KJ, Caky M, Matthews PM. Stabilization of the pyruvate dehydrogenase E1α subunit by dichloroacetate. Neurology. 1998;51:1331–1335. doi: 10.1212/wnl.51.5.1331. [DOI] [PubMed] [Google Scholar]
- 16.Curry SH, Chu PI, Baumgartner TG, Stacpoole PW. Plasma concentrations and metabolic effects of intravenous sodium dichloroacetate. Clin. Pharmacol. Ther. 1985;37:89–93. doi: 10.1038/clpt.1985.17. [DOI] [PubMed] [Google Scholar]
- 17.Stacpoole PW, Moore GW, Kornhauser DM. Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. N. Engl. J. Med. 1978;298:526–530. doi: 10.1056/NEJM197803092981002. [DOI] [PubMed] [Google Scholar]
- 18.Thai SF, Allen JW, DeAngelo AB, George MH, Fuscoe JC. Detection of early gene expression changes by differential display in the livers of mice exposed to dichloroacetic acid. Carcinogenesis. 2001;22:1317–1322. doi: 10.1093/carcin/22.8.1317. [DOI] [PubMed] [Google Scholar]
- 19.Ammini CV, Stacpoole PW. Biotransformation, toxicology and pharmacogenomics of dichloroacetate. In: Gribble GW, editor. Natural Production of Organohalogen Compounds. vol. 3/P in the series. Berlin, New York: The Handbook of Environmental Chemistry, Springer-Verlag; 2003. pp. 215–234. [Google Scholar]