Abstract
The vitamin D endocrine system is essential for calcium and bone homeostasis. The precise mode of action and the full spectrum of activities of the vitamin D hormone, 1,25-dihydroxyvitamin D [1,25-(OH)2D], can now be better evaluated by critical analysis of mice with engineered deletion of the vitamin D receptor (VDR). Absence of a functional VDR or the key activating enzyme, 25-OHD-1α-hydroxylase (CYP27B1), in mice creates a bone and growth plate phenotype that mimics humans with the same congenital disease or severe vitamin D deficiency. The intestine is the key target for the VDR because high calcium intake, or selective VDR rescue in the intestine, restores a normal bone and growth plate phenotype.
The VDR is nearly ubiquitously expressed, and almost all cells respond to 1,25-(OH)2D exposure; about 3% of the mouse or human genome is regulated, directly and/or indirectly, by the vitamin D endocrine system, suggesting a more widespread function. VDR-deficient mice, but not vitamin D- or 1α-hydroxylase-deficient mice, and man develop total alopecia, indicating that the function of the VDR and its ligand is not fully overlapping. The immune system of VDR- or vitamin D-deficient mice is grossly normal but shows increased sensitivity to autoimmune diseases such as inflammatory bowel disease or type 1 diabetes after exposure to predisposing factors. VDR-deficient mice do not have a spontaneous increase in cancer but are more prone to oncogene- or chemocarcinogen-induced tumors. They also develop high renin hypertension, cardiac hypertrophy, and increased thrombogenicity. Vitamin D deficiency in humans is associated with increased prevalence of diseases, as predicted by the VDR null phenotype. Prospective vitamin D supplementation studies with multiple noncalcemic endpoints are needed to define the benefits of an optimal vitamin D status.
- I. Introduction
- A. History of the vitamin D endocrine system
- B. Vitamin D metabolism
- C. Vitamin D receptor
- D. VDRE
- E. Corepressors and coactivators
- F. Genomic and nongenomic actions of the vitamin D hormone
- II. VDR-Vitamin D Endocrine System and Bone and Growth Plate
- A. VDR or 1α-hydroxylase inactivation impairs calcium and bone homeostasis
- B. 1,25-(OH)2D-VDR action regulates calcium transport in intestine and kidney
- C. Direct vs. indirect role of 1,25-(OH)2D-VDR action in chondrocytes
- D. Changes in bone metabolism by interference with 1,25-(OH)2D-VDR action
- E. Interaction of 1,25-(OH)2D-VDR pathway with phosphate-regulating hormones
- F. VDR, vitamin D, and tooth development
- G. Conclusion
- III. VDR-Vitamin D Endocrine System and Skin
- A. VDR is essential for hair cycling
- B. CYP27B1 is required for optimal epidermal differentiation
- IV. VDR-Vitamin D Endocrine System and Cell Proliferation and Cancer
- A. Mechanisms responsible for the antineoplastic effects of 1,25-(OH)2D
- B. Lessons from animal models: VDR null mice and cancer
- C. Lessons from epidemiological and intervention studies in humans
- D. Conclusion
- V. VDR-Vitamin D Endocrine System and Immunology
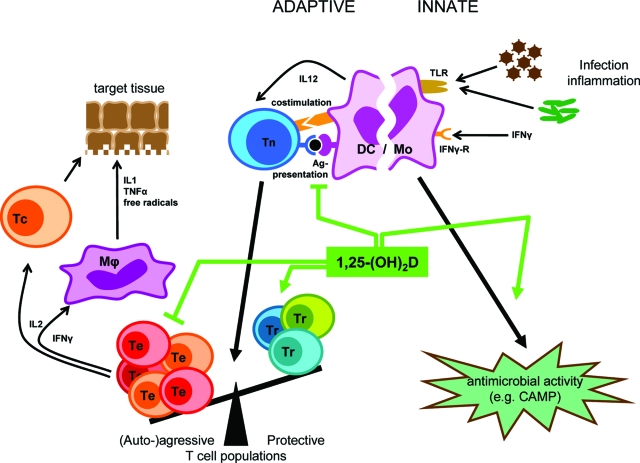
- A. Vitamin D physiology and the immune system
- B. VDR and the immune system
- C. Vitamin D ligand deficiency and the immune system
- D. Discrepancy between loss of VDR and vitamin D deficiency in the immune system
- E. Pharmacological effects of 1,25-(OH)2D and analogs in the immune system
- F. Conclusion
- VI. VDR-Vitamin D Endocrine System and Glucose Homeostasis
- A. Vitamin D deficiency and VDR null mice
- B. Effects of vitamin D metabolites in vitro
- C. Effects of vitamin D metabolites in vivo: clinical implications for type 2 diabetes
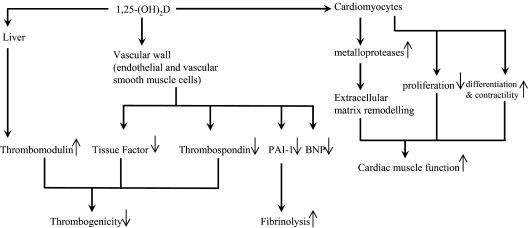
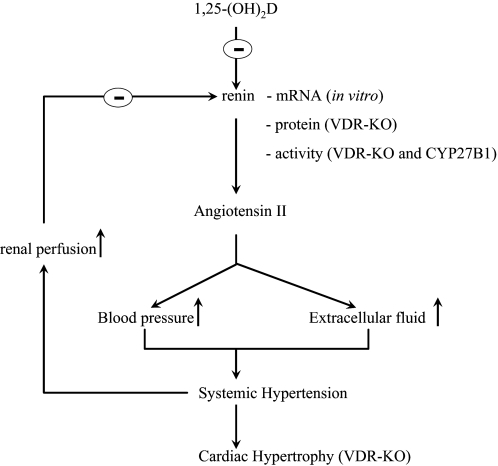
VII. VDR-Vitamin D Endocrine System and Cardiovascular System
VIII. VDR-Vitamin D Endocrine System and Muscle
IX. VDR-Vitamin D Endocrine System and Brain
X. VDR-Vitamin D Endocrine System and Reproduction
XI. VDR-Vitamin D Endocrine System and Adipocytes
XII. General Conclusions and Perspectives
I. Introduction
A. History of the vitamin D endocrine system
The first extensive description of the clinical picture of rickets, including the name, dates from the 17th century in a Ph.D. thesis equivalent presented at the University of Lugdunum Batavorum (Leiden, The Low Countries) in 1645 by Daniel Whistler and, briefly thereafter, by Francis Glisson (in collaboration with G. Bate and A. Regemortel) in London in 1650. In addition, a Dutch physician who also lived briefly in London, Ireland, and Paris published in 1649, in his native language, a description of “tabes pectoralis”, the typical thoracic malformation of rickets (1,2).
The origin of the disease was largely unknown until the discovery of the dual origin of vitamin D at the beginning of the 20th century as a nutritional compound by Mellanby in the United Kingdom and McCollum in the United States (discovery of dietary vitamin D), whereas Huldshinsky, Chick, and Hume, Hess, and Weinstock discovered the curative effects of UV light (3,4,5,6,7,8). Steenbock discovered, around the same time, that UV irradiation of vegetarian oil produced the antirachitic substance vitamin D2 and patented this discovery.
Rickets, as a disease of children of either rich families (deliberately avoiding exposure to sunshine in countries such as the United Kingdom and India) or very poor families living in the slums of big European industrialized cities, gradually disappeared thereafter due either to better exposure to sunshine or to the oral use of vitamin D-rich cod liver oil. The chemical identification and chemical synthesis of vitamin D earned A. Windaus the Nobel Prize in 1938 (9).
The other major historic steps in the vitamin D saga started with the discovery of the complex metabolism of vitamin D into more than 41 metabolites, especially 25-hydroxyvitamin D (25-OHD) and 1,25-dihydroxyvitamin D [1,25-(OH)2D], and the complex regulation of the renal production of the active end product 1,25-(OH)2D as a true steroid hormone. Subsequently, the transport of vitamin D metabolites outside the cell [by lipoproteins, albumin, and vitamin D binding protein (DBP)] and inside the cell [vitamin D receptor (VDR)], and finally the identification of VDR as a nuclear transcription factor regulating a very large number of genes, further confirmed 1,25-(OH)2D as a classical calciotropic hormone (reviewed in Refs. 10,11,12,13,14,15,16,17,18,19,20,21,22).
The nearly ubiquitous presence of VDR, the extrarenal production of vitamin D metabolites, the regulation of multiple genes not involved in calcium metabolism, and finally the careful analysis of the phenotype of VDR-deficient mice and men broadened the scope or spectrum of activities of the vitamin D-VDR endocrine system. The present review will, therefore, primarily focus on the biological action profile of the vitamin D-VDR system, as revealed by studying animals with genetically modified expression of the VDR or other genes involved in vitamin D metabolism or actions. Of course, whenever possible, comparisons with human data will be included, especially those relevant to the (possible) consequences of vitamin D deficiency or resistance for human physiology or disease.
B. Vitamin D metabolism
Vitamin D derives from nutritional origin or is synthesized in the skin under influence of UV-B light. This is a purely photochemical reaction, and no enzymes are involved. However, the reaction requires a sufficiently large concentration of 7-dehydrocholesterol (7DHC) and UV-B 290–315 nm light. This 7DHC is the normal last step in the de novo synthesis of cholesterol and is usually present in low concentrations, unless the activity of 7DHC-Δ7-reductase is high. Information on the regulation of this enzyme is limited, except that total absence causes cholesterol deficiency and Smith-Lemli-Opitz syndrome. Increased activity of this enzyme (e.g., in feline species) eliminates the photoproduction of vitamin D, which then becomes a true vitamin. Vitamin D needs both 25- and 1α-hydroxylation to become the active hormone 1,25-(OH)2D. At least four enzymes, all microsomal cytochrome P450 (CYP) isoforms (CYP2DII, CYP2D25, CYP3A4, and CYP2R1), can accomplish the 25-hydroxylation of vitamin D in human liver cells, but the low-capacity, high-affinity microsomal CYP2R1 is most likely the key enzyme because a homozygous mutation was found in a patient with classical rickets and low circulating 25-OHD levels (23). The first putative liver 25-hydroxylase was purified by Russell’s group (24) and later cloned and renamed as CYP27A1. This mitochondrial enzyme is, however, mainly involved in cholesterol and bile acid metabolism and has only a low-affinity high-capacity 25-hydroxylase activity. CYP27A1 null mice and men therefore, exhibit (near) normal 25-OHD concentrations.
Little is known about the regulation of these (putative) 25-hydroxylases, but serum 25-OHD generally reflects the vitamin D nutritional status, and thus little feedback regulation is assumed. By contrast, there seems to be only one 25-OHD-1α-hydroxylase (CYP27B1), which is expressed at the highest concentration in the kidney, where its activity is regulated by calcium and phosphate as well as by their regulating hormones [calcium, PTH, calcitonin, GH, and IGF-I being positive regulators; phosphate, fibroblast growth factor 23 (FGF23), and 1,25-(OH)2D itself being negative regulators] (reviewed in Ref. 13).
Although the kidney seems to be the unique production site of serum 1,25-(OH)2D as originally described (25), exactly the same enzyme is expressed in many other tissues such as skin, monocytes, placenta, and bone cells, probably to produce 1,25-(OH)2D for autocrine and paracrine action (reviewed in Refs. 26 and 27). However, the local regulation of the CYP27B1 is quite different because immune stimuli, and not calcium regulating hormones, control monocyte CYP27B1 activity and expression (28). Inactivation of CYP27B1 gene causes a disease previously described in children as vitamin D-resistant rickets type 1 (29). These mice and children have severe rickets unresponsive to normal vitamin D replacement but curable by very high vitamin D or 25-OHD intake or normal 1α-hydroxylated vitamin D replacement doses (30,31). These diseases clearly demonstrate the essential role of this unique gene product (1α-hydroxylase) in calcium homeostasis. A large number of human mutations in the CYP27B1 gene have been described and can be used as a valuable model to identify the possible active sites (32).
Although a very large number of vitamin D metabolites have been identified in vitro and in vivo (12), only a single multifunctional CYP24A1 gene is responsible for the catabolism of 25-OHD and 1,25-(OH)2D, resulting in either calcitroic acid after initial 24-hydroxylation or a side chain lactone after initial 23-hydroxylation (32). Deletion of CYP24A1 in mice causes endogenous 1,25-(OH)2D excess and perinatal lethality, likely due to hypercalcemia in half of the newborns (33,34). Complete lack of CYP24A1 activity during development in CYP24A1 null pups, born from CYP24A1 null mothers, caused severe mineralization defects in intramembranous bones. This bone defect was normalized by genetic elimination of VDR signaling (34). No human cases of CYP24A1 deficiency have yet been described, but altered CYP24A1 gene expression has been associated with human diseases such as breast cancer (35) and asthma (36). Moreover, alternative gene splicing can produce an inactive enzyme and, therefore, expose cells locally to high 1,25-(OH)2D concentrations (37). The only noncytochrome enzyme known to metabolize vitamin D is responsible for the 3-epimerisation of vitamin D3 or its metabolites. The 3-epimers seem to have lower calcemic effects. Young children have considerably higher 3-epi-25-OHD serum levels than adults; however, the biological consequence of this is yet unknown (38).
Vitamin D and its metabolites are transported in the circulation bound to a plasma protein, DBP, which shares many structural and evolutionary similarities with albumin. DBP has at least a dual function, involving the depolymerization and binding of actin (39), in addition to the tight binding of 25-OHD (40). DBP is highly polymorphic in humans, and not a single case of total absence of DBP has been described in men. In DBP null mice, plasma concentrations of 25-OHD and 1,25-(OH)2D are extremely low, and their metabolic clearance is markedly increased. Consequently, mice lacking DBP are prone to rapidly develop vitamin D deficiency when fed a vitamin D-deficient diet (41). DBP is filtered in the glomerulus of the nephron, but it is reabsorbed together with 25-OHD in the renal tubuli by the bulk carrier transporter megalin. Megalin-deficient mice, therefore, cannot reabsorb DBP or 25-OHD in the nephron and, as a result, develop vitamin D-deficiency rickets (42).
C. Vitamin D receptor
The discovery of the binding of the active vitamin D hormone to a protein (43) coincided with the discovery of the renal origin and chemical identification of the hormone itself (25,44,45,46). The molecular cloning of VDR in chicken (47) and later in several other species confirmed that 1,25-(OH)2D functions like many other steroid hormones, by binding to and activating a nuclear transcription factor. VDR expression has been identified in virtually every human tissue. This universal presence of VDR was already a first, and important, hint for a wide spectrum of activities of the vitamin D-VDR endocrine system. Indeed, nearly all nucleated cells express the VDR, albeit at variable concentrations. The tissue and cell type localization of VDR has been confirmed by binding studies, mRNA in situ hybridization, autoradiography, and protein immunocytochemistry (48,49,50). The few cells or tissues that have low or absent VDR expression include red blood cells, mature striated muscle, and some highly differentiated brain cells, such as the Purkinje cells of the cerebellum (51). The molecular cloning identification of its structure and functional analysis of its structural domains (Fig. 1) clearly confirmed that the VDR belongs to a class of nuclear transcription factors. There are 48 nuclear receptors (NRs) in the human genome, and many of these have a distant evolutionary origin. These receptors probably started as unliganded DNA binding transcription factors. During evolution, most receptors adopted a specific ligand and segregated into transcription factors that remained homodimers or required heterodimerization [with retinoid X receptor (RXR)] (52,53,54).
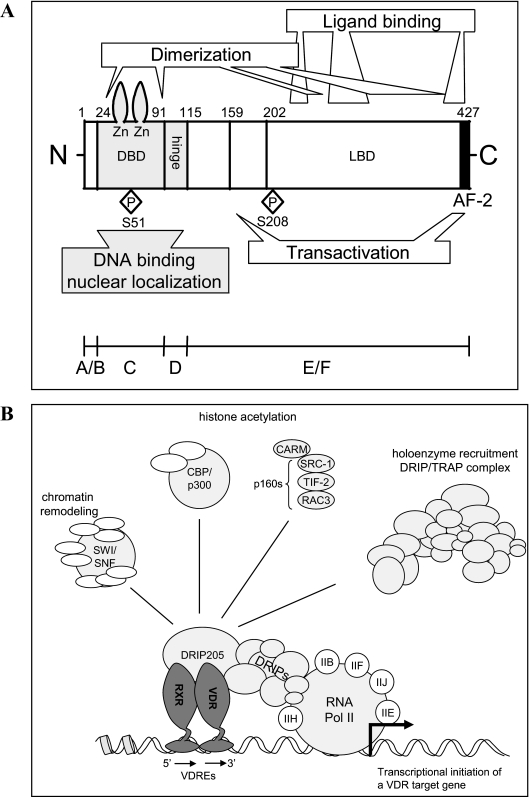
Figure 1.
Structure and structural domains of VDR. A, Schematic representation of the functional domains in the human VDR. A/B, Aminoterminal region. B, Schematic representation of the regulation of gene transcription by ligand-activated VDR-RXR heterodimers. Transcriptional activation requires the action of many multisubunit coactivator complexes that are recruited in a parallel and/or sequential manner (98).
The VDR has been found in mammals, birds, amphibians, and fish with a calcified skeleton (e.g., zebrafish), with a high degree of homology in structure, ligand binding, and functionality. The VDR is undetectable in nonchordate species, and this led to the initial hypothesis that a functional vitamin D endocrine system originated during evolution to allow the accumulation of calcium to build a calcified skeleton. However, the VDR was also detected in jawless primitive fish (lamprey or Petromyzon marinus) (55), indicating that the vitamin D system emerged before the development of calcified structures. In the NR family, the closest structural similarity to the VDR is found in the NR group with metabolic functions, such as the PXR, CAR, and FXR (all bile acid or xenobiotic receptors involved in bile acid homeostasis and detoxification) and the oxysteroid receptors LXR (α and β) (reviewed in Ref. 53). The three closely related receptors (VDR, PXR, and CAR) all share the ability to bind bile acid metabolites and are able to activate CYP enzymes involved in xenobiotic detoxication. One might thus speculate that the VDR originated during early evolution of vertebrates as a transcription factor involved in detoxication and only later became a major player in calcium homeostasis. However, the exact functional role of the VDR in primitive vertebrates or fish has not yet been elucidated.
Analogous to most members of group I NRs (56), the VDR functions through heterodimerization with any of the three RXR isoforms. Both apo RXR (without its specific ligand 9-cis-retinoic acid) and VDR acquire the active confirmation of the ligand binding domain (LBD) upon binding of 1,25-(OH)2D to VDR (see Section I.C).
The structure-function analysis of the VDR revealed the expected homology with other NRs containing five functional domains (Fig. 1). For the human VDR, a multitude of different transcripts was found; the transcripts vary in their 5′ untranslated region but mostly use the same translation initiation codon and thereby encode an identical 427 amino acid-long VDR protein (Table 1). A certain proportion of the VDR molecules is alternatively spliced. Of note is the 50 amino acid-extended VDRB1, which originates from alternative posttranscriptional splicing and the use of a more upstream in-frame start codon and is found in most humans cells (57). This isoform coexists with the previously identified “normal” VDR protein in humans. In kidney cells and in several cell lines, this extended variant constitutes up to 30% of the VDR. Functional differences between these two VDR isoforms have been described, including a difference in transactivation capacity of the promoter of the 24-hydroxylase gene (58) (Table 1). The AB domain of VDR is short, and its function has not been thoroughly defined. A polymorphic variant lacking the first three amino acids at the N terminus has increased transactivation potency due to better interaction with the transcription factor TFIIB (59). The two zinc fingers of the DNA binding domain (DBD) are absolutely essential because many mutations in this region cause VDR resistance in children. The elucidation of the functional domains of VDR by mutational analysis was of course preceded by many biochemical and cellular observations that predicted a specific function for the Zn fingers, activation domains, and interactions between VDR and regulatory DNA elements (reviewed in Refs. 21, 49, 60, and 61). Furthermore, the four VDR null mouse strains all lack either the first or second zinc finger, are fully VDR resistant, and, just like human patients, develop the characteristic phenotype, including alopecia. This region is also important for the nuclear translocation of VDR (62). The hinge region of VDR is 20–50 amino acids longer than in most other NRs and is important for the flexibility of VDR to allow DNA interaction via the DBD, as well as interaction of the LBD with the coactivator proteins. The LBD consists of 12 α-helices and three β-sheets and has a highly flexible unique extension between helix 1 and helix 3. This floppy part of the LBD impairs crystallization, and therefore a LBD without this extra loop was used for crystallization with 1,25-(OH)2D (63). However, this unique loop seems functionally silent because the loss of this loop in engineered human VDR-LBD or in natural zebrafish VDR has no functional consequences. Nevertheless, a C190W (cystine to tryptophan) mutation in this region caused vitamin D resistance, but this observation was only published in abstract form (cited in Ref. 19). The LBD of VDR is characterized by a large pocket (+/− 700 A3) in comparison with most other NR LBDs. This is probably related to the long and flexible side chain of the ligand (not present in other steroid hormones) and possibly explains the accommodation of many 1,25-(OH)2D analogs with a bulky or even a double side chain. Many of the human VDR mutations causing vitamin D resistance rickets are due to mutations in the LBD, thereby impairing or abolishing binding to 1,25-(OH)2D. When the mutation in the LBD only partially impairs ligand binding, rickets is less severe, and occasionally, hair growth is normal. The binding of ligand to the LBD changes the surface of the LBD necessary for improved heterodimerization with RXR. Mutations or absence of the activation factor-2 (AF-2) domain (Fig. 1) destroy VDR function, and this can best be explained by the function of helix 12 in the “mouse trap” model, whereby the ligand binding induces reorientation of helix 12/AF-2/F domain, which is necessary for the creation of a coactivator platform. As VDR is actively shuttling between the cytosol and the nucleus, genetic mutations impairing this translocation may also impair VDR action.
Table 1.
Properties of VDR
| Molecular mass | 50 kDa (human VDR) |
| Kd | |
| 1,25-(OH)2D | 10−10m |
| 25-OHD | 10−8m |
| Isoelectric point | 6.2 |
| Tissue concentration | 0.01–1 pmol/mg protein |
| 200–25,000 copies/cell | |
| Structural variants | |
| VDR-A (f-allele) | 427 Amino acids |
| VDR-A (F-allele) | 424 Amino acids, by alternative translation start site |
| VDR-B1 | 477 Amino acids, by N-terminal extension |
| Polymorphisms | |
| Large number in 5′ promoter | cdx-2 |
| N terminal | Fok I |
| C terminal | BsmI, ApaI, TaqI, TruI, polyA microsatellite |
Mutations causing gene deletion, frameshift, abnormal splice sites, or premature stop codons destroy either the expression or binding activity and, thus, abolish VDR action (19,64,65). Vitamin D/VDR resistance with a normal VDR has been described in New World monkeys and in a single human case due to abnormally high expression of an intracellular vitamin D responsive element (VDRE) binding protein (66).
The molecular activity of the VDR as transcription factor is largely based on the model of other NRs. The crystal structure of some holo- and apo-NRs provided the key information that ligand binding reorients the flexible position of helix 12 away from the core of the LBD into a closer compact structure of the LDB, thereby, together with small repositioning of H3 and H11, creating a hydrophobic cleft composed of helices 3, 4, and 12 and promoting interactions with the conserved LXXLL motif of the coactivators (67). Binding of a full receptor antagonist is thought to induce an alternative conformation in the receptor at H12 such that coactivator binding is compromised. The agonist position of VDR, in complex with 1,25-(OH)2D or ligands, has been confirmed by direct analysis of the appropriate VDR crystals, whereas the antagonist conformation is based on homology with other NRs and in silico modeling techniques.
In humans, multiple polymorphisms of the VDR gene have been identified and thoroughly studied (68,69). These polymorphisms are distributed throughout the complete VDR gene region and have been associated with disorders like cancer and autoimmune diseases. Among the VDR polymorphisms, the FokI single nucleotide polymorphism of the translation start site results in a VDR protein shortened by three amino acids (70) and is not linked to any of the other VDR polymorphisms (69). However, the clinical impact of this VDR FokI polymorphism remains unclear. Analysis of the multiple studies assessing the correlation between the VDR FokI polymorphism and genetic predisposition to bone-related disease, risk of cancer, and immune-mediated diseases [such as diabetes (71,72,73,74,75,76,77,78,79,80,81)] fails to reveal solid associations or to provide a clear functional explanation for the observed differences in disease prevalence (reviewed in Ref. 82).
D. VDRE
Upon 1,25-(OH)2D binding, the VDR is phosphorylated (Fig. 1), and its surface conformation is reconfigured, which is thought to result in the release of corepressors (see Section I.E). Moreover, in response to ligand, the VDR recruits its preferred dimerization partner, RXR, and binds to VDREs composed of two hexameric binding sites arranged either as direct repeats interspaced by a varying number of nucleotides (but generally three) or as inverted palindromes interspaced by nine nucleotides (49,83). (A/G)G(G/T)TCA is considered to be the consensus sequence for a VDRE half site, although considerable sequence diversity exists. The two receptors interact symmetrically with their LBD (both in helix 12 activated configuration), but their DBD is asymmetrical, with RXR as a more compact and VDR in an extended configuration between the LBD and DBD. This is probably due to the flexibility of the hinge region and allows the correct positioning of both receptors to the helix structure of DNA. Many genes regulated by vitamin D have multiple VDREs in their promoter, sometimes even far away from the coding region, and chromatin immunoprecipitation analysis has revealed that the receptor complex binds to different VDREs in cyclic waves (84,85).
E. Corepressors and coactivators
Modulation of gene expression is, however, not mediated directly by binding of VDR/RXR heterodimers to DNA, but rather is dependent upon the ability of this dimer to recruit coregulatory protein complexes, instead of being silenced by corepressors (86,87). Analogous to other NRs, unliganded VDR is probably kept transcriptionally silent, even when present in the nucleus and bound to chromatin by one or more corepressors [silent mediator for retinoid and thyroid hormone receptors (SMRT), NR corepressors (NCoR), Alien (88) or sin 3] that deacetylate histones (directly or indirectly) and, thus, keep the chromatin in a densely packed configuration that is inaccessible to the transcription protein complex (89).
1. Positive gene regulation.
Upon ligand binding-induced reconfiguration of the VDR, surface coactivators are able to bind VDR. Chromatin immunoprecipitation studies indicate that a coactivator exchange occurs in the transcriptional complex on NR-responsive promoters (85). First, coactivators of the CBP/p300 family and of the p160 protein family, including the steroid receptor coactivators (SRCs) (such as SRC-1), are recruited (90,91,92). These proteins possess intrinsic histone acetyltransferase (HAT) activity and, by acetylating histone tails, open up the chromatin structure, creating a chromatin environment permissive for gene transcription (93). In a second wave, the vitamin D receptor interacting protein (DRIP)/T3 receptor auxiliary protein (TRAP) multimeric complex is recruited, whereby DRIP205/TRAP220 binds directly to VDR/RXR heterodimers through one of two LXXLL motifs (94,95,96). Subsequently, basal transcription factors, as well as RNA polymerase II, are recruited to the transcription start sites, and as a result, target gene transcription is induced (97,98). Gene expression can also be affected by rearrangements in the nucleosome array, mediated by ATP-dependent chromatin remodeling complexes among which SWI/SNF-type and ISWI-type complexes and the multiprotein complex WINAC, the latter of which interacts directly with the VDR, via the Williams syndrome transcription factor (99,100,101). In addition, several other unrelated proteins with coactivator activity have been identified, among which NCoA-62/ski-interacting protein and peroxisome proliferator-activated receptor γ coactivator-1α (102,103).
2. Negative gene regulation.
The VDR not only directly activates gene transcription but also directly down-regulates the transcription of the genes encoding PTH, PTHrP, and CYP27B1 (104,105,106,107,108). Negative VDREs (nVDREs), which closely resemble the consensus VDRE sequence, have been mapped both in the human and rat PTH promoter, and in the human PTHrP gene promoter. These nVDREs are bound by either VDR homodimers or by VDR/RXR heterodimers where the VDR occupies the 5′-located half-site (109,110). A second group of nVDREs, composed of E-box type motifs (5′-CATCTG-3′) was identified in the human CYP27B1, as well as in the human PTH and PTHrP promoters (105,106). These nVDREs are transcriptionally active, even in the absence of 1,25-(OH)2D, and are bound by VDR-interacting repressor, a basic helix-loop-helix transcription factor that recruits CBP/p300 HAT activity upon phosphorylation by protein kinase A (107). Although the exact mechanisms responsible for transcriptional repression remain to be elucidated, different signal transduction mechanisms have been proposed. In the presence of 1,25-(OH)2D, ligand-induced coregulator switching occurs and is responsible for ligand-induced transcriptional repression (111). Liganded VDR/RXR heterodimers associate with the VDRE-bound VDR-interacting repressor, which results in the replacement of the CBP/p300 HAT coactivator by histone deacetylases and NCoR/SMRT corepressors. The WINAC multimeric complex facilitates chromatin remodeling during this replacement process (112).
Activated NRs can also bind to the binding sites of other transcription factors and, as such, negatively regulate gene transcription in an indirect manner, as was described for the negative regulation of IL-2 and IL-12 gene transcription (113,114).
F. Genomic and nongenomic actions of the vitamin D hormone
A broad set of microarray studies have been performed to unravel the molecular pathways involved in the biological action of 1,25-(OH)2D. These studies have used a variety of technology platforms (spotted cDNA arrays, Affymetrix Genechips, Agilent), although it is only in the last couple of years that arrays were used to profile the entire transcriptome. Gene expression profiles after treatment with 1,25-(OH)2D have been generated in classical [bone (115,116,117,118,119,120), kidney (121), and intestine (122,123)] and nonclassical [malignant, immune, and smooth muscle cells (124,125,126)] target tissues and cells. To gain more insight into the antiproliferative and prodifferentiating effect of 1,25-(OH)2D, genomic profiling has been performed in malignant cells such as prostate (127,128,129,130,131,132,133), breast (134,135,136), leukemia (137,138), colon (139), and ovarian cancer (140) cells, and squamous cell carcinoma cells (141). Although it is difficult to compare such studies due to variations in the experimental design (e.g., cell culture conditions, concentration of 1,25-(OH)2D, and time points after stimulation with 1,25-(OH)2D), the gene expression profiles all agree that 1,25-(OH)2D regulates, directly and/or indirectly, a very large number of genes (0.8–5% of the total genome) and appears to be involved in a variety of cellular functions including growth regulation, DNA repair, differentiation, apoptosis, membrane transport, metabolism, cell adhesion, and oxidative stress.
The mechanisms underlying the immunomodulatory effects of 1,25-(OH)2D have also been investigated using microarray technology on dendritic cells (DCs) (142) and T cells (143,144), as well as in tissues isolated from mice with autoimmune diseases such as inflammatory bowel disease [colon (145)] and experimental autoimmune encephalomyelitis [spinal cord (146)] after 1,25-(OH)2D treatment. Gene expression profiles were also examined in heart (147) and kidney (121) tissues isolated from VDR null mice and compared with VDR wild-type (WT) mice.
Until now, investigations on 1,25-(OH)2D action using proteomic analysis have been limited. Recently, 10 proteins (out of 270 proteins analyzed) were identified as being differentially expressed between MCF-7 breast cancer cells treated with 1,25-(OH)2D or vehicle using an antibody-based proteomics approach. The function of most of these differentially expressed proteins was consistent with the antiproliferative and proapoptotic effects of 1,25-(OH)2D in breast cancer cells (148).
Steroid hormones and many other ligands of NRs not only exert their function by directly regulating gene transcription but also display a wide variety of rapid nongenomic or nongenotropic actions. This activity includes a number of rapid (seconds to minutes) and usually transient changes in transmembrane transport of ions (such as calcium and chloride) or intracellular signaling pathways (such as changes in cAMP, protein kinase A, protein kinase C, phospholipase C, phosphatidylinositol-3 kinase, and MAPK activities) (reviewed in Refs. 149,150,151,152).
The structural requirements of the ligands, which bind to their membrane receptor and induce these rapid actions, differ from those involved in genomic actions (153). The molecular cloning of the putative membrane receptor for 1,25-(OH)2D is long overdue. The picture is further complicated by the involvement of the classical NR, VDR, in these rapid actions because the presence of VDR is necessary in this nongenomic pathway (154). The nongenomic and genomic activities of NR ligands, including 1,25-(OH)2D, may, however, complement each other because (rapid) activation of second messengers (ions or calmodulin-dependent kinase) may activate (e.g., phosphorylate) VDR and amplify its genomic activity (149,151).
An important tool to analyze the in vivo function of 1,25-(OH)2D was the generation of VDR and CYP27B1 (1α-hydroxylase) null mice. VDR null mice were generated by four independent groups in Tokyo (155), Boston (156), Leuven (157), and Munchen (158) by disruption of the DNA binding site using different targeting constructs and mice strains. One strain of VDR null mice was generated by using a cre-lox system by crossing VDRlox mice with phosphoglycerate kinase-cre mice (157). Two different groups have engineered 1α-hydroxylase null mice by targeting the protein’s heme binding and (part of) the hormone binding domains (159,160). The study of these VDR or 1α-hydroxylase null mice, or mice with these deletions in combination with other genetic mutations (Table 2), generated a wealth of information regarding the spectrum of activities of the VDR-vitamin D endocrine system (reviewed in Sections II to XI). These studies also revealed that the VDR may have ligand-independent functions, especially in the skin and the immune system.
Table 2.
Effect of genetic intervention in VDR or CYP27B1 null mice
| VDR: calcium and bone
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetic intervention | Calcium (re)absorption
|
Serum
|
Bone
|
Skin Alopecia | Remarks | Ref. | |||||
| Intestine | kidney | Ca | P | PTH | 1,25-OH)2D | Rickets | Osteomalacia | ||||
| VDR | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | Yes | Yes | Yes | 150,151,152,153 | |
| VDR/CYP24 | ? | ? | ? | ? | ? | ↑ | ? | ↓ | Yes | Rescue of decreased bone mineralisation | 34 |
| VDR/RXRγ | ? | ? | ↓ | ↓ | ? | ↑ | ↑↑ | Yes | Persistent skeletal abnormalities during rescue diet | 193 | |
| VDR/CaBP-28k | ? | ↓↓↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | Yes | Premature death, persistent skeletal abnormalities during rescue diet | 181 |
| VDR/FGF23 | ? | = | = | = | ↑ | ↑ | = | = | Yes | Rescue of mineral abnormalities and ectopic calcifications (rescue diet) | 222 |
| CYP27B1 | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↓↓↓ | Yes | Yes | ∼ VDR null mice | 154,155 |
| CYP27B1/VDR | ? | ? | ↓ | ↓ | ↑ | ↓↓↓ | + | Yes | Yes | Persistent skeletal abnormalities during rescue diet | 192 |
| CYP27B1/PTH | ? | ↓↓↓ | ↓↓ | ↑ | ↓↓↓ | ↓↓↓ | ↑↑ | / | Premature death due to severe hypocalcemia | 201 | |
| CYP27B1/FGF23 | ? | ? | ↓ | ↓ | ↑ | ↓↓↓ | Yes | Yes | / | Rescue of mineral abnormalities and ectopic calcifications | 220,221 |
| CYP27B1/Na/Pi-2a | ? | ↓ | = | ↓ | = | ↓↓↓ | ? | ? | / | Rescue of renal calcifications (data of 3-wk-old mice) | 227 |
| VDR: cancer, immune system and diabetes pathology | |||||||||||
| VDR × MMTV-Neu | Increased susceptibility to dysplasia of the mammary ductal epithelia and atrophy of the mammary ductal epithelia, associated with reduced survival | 302 | |||||||||
| VDR/IL-10 | Increased severity of inflammatory bowel disease | 415,416 | |||||||||
| VDR × NOD | No enhanced susceptibility to diabetes but aggravation of known immune abnormalities | 419 | |||||||||
↑, Increase; ↓, decrease; ?, not investigated; /, not present; and =, comparable values vs. WT mice; MMTV, mouse mammary tumor virus.
II. VDR-Vitamin D Endocrine System and Bone and Growth Plate
A. VDR or 1α-hydroxylase inactivation impairs calcium and bone homeostasis
Although the VDR is widely expressed during embryonic development in tissues involved in calcium homeostasis and bone development, VDR null mice were phenotypically normal at birth. Around weaning, they developed hypocalcemia, secondary hyperparathyroidism, and hypophosphatemia. At this age, VDR null mice also became growth retarded and developed severe rickets and osteomalacia (155,156,157,158). Mice deficient in 1α-hydroxylase displayed an analogous phenotype, with respect to calcium and bone homeostasis (159,160). However, they differed in serum levels of vitamin D metabolites. When the VDR was inactivated, serum 1,25-(OH)2D levels were increased due to secondary hyperparathyroidism, increased renal 1α-hydroxylase activity, and decreased 24-hydroxylase activity. Serum 24,25-(OH)2D levels are decreased after weaning. These changes are consistent with the 1,25-(OH)2D/VDR-mediated gene regulation observed in vitro: negative regulation of 1α-hydroxylase, whereas 24-hydroxylase gene expression is positively regulated by 1,25-(OH)2D-induced VDR action (see Section I.B). On the other hand, 1,25-(OH)2D levels were undetectable in 1α-hydroxylase null mice, whereas 25-OHD levels were elevated. Serum 24,25-(OH)2D levels were decreased, comparable to VDR null mice (155,161). These phenotypic characteristics could be largely corrected by supplementation of dietary calcium (in combination with high-lactose:rescue diet) in both knockout models or 1,25-(OH)2D treatment of 1α-hydroxylase null mice (157,161,162,163,164,165,166,167). These findings confirm previous observations in humans (168,169,170).
B. 1,25-(OH)2D-VDR action regulates calcium transport in intestine and kidney
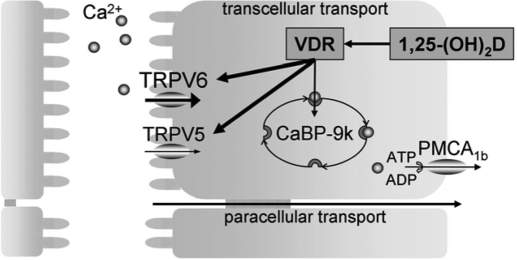
An important role of 1,25-(OH)2D is to regulate calcium absorption in the intestine and reabsorption in the kidney. Intestinal and renal calcium transfer consists of a passive concentration gradient-dependent paracellular transport and an active ATP-dependent transcellular transport (reviewed in Ref. 171). The latter is largely regulated by 1,25-(OH)2D and is critical when calcium supply is low. This transcellular calcium transport involves three sequential steps: 1) influx of calcium into the epithelial cells, mediated by apical calcium channels of the transient receptor potential vanilloid (TRPV) family and promoted by a steep electrochemical gradient across the apical membrane; 2) cytosolic transport of calcium bound to calcium binding proteins, calbindins (CaBPs); and 3) extrusion of calcium across the basolateral membrane into extracellular fluid by an energy-requiring process.
The intestine and kidney differ, however, in the site of active calcium transport relative to the location of passive transport. In the kidney, the fine tuning of calcium reabsorption is regulated by 1,25-(OH)2D and takes place in the distal nephron, subsequent to the ample combined calcium and sodium reabsorption in the early part of the nephron. In the intestine, the active 1,25-(OH)2D-regulated calcium absorption is mainly located in the duodenum, whereas the passive calcium absorption occurs in the jejunum. The molecular players regulating this transport also differ between intestine and kidney: TRPV6 is abundantly expressed in duodenum, whereas TRPV5 is the main apical calcium channel in the kidney; CaBP-9k (molecular mass, 9 kDa) is the only cytosolic calcium transporter in the intestine, whereas both CaBP-9k and CaBP-28k (molecular mass, 28 kDa) are present in the kidney; the plasma membrane calcium ATPase (PMCA1b) regulates calcium extrusion in intestine, whereas both Na+/Ca2+ exchanger (NCX1) and PMCA1b are involved in the kidney (171,172).
1. Intestinal calcium absorption.
As anticipated, intestinal calcium absorption was manifestly (60%) decreased in adult VDR null mice, as measured by accumulation of serum radioactive calcium after oral gavage (157) and by the in situ ligated loop technique (167). As already mentioned, hypocalcemia in VDR null mice develops at the time of weaning, which correlates with the observation that before weaning intestinal calcium absorption in rats occurs by a nonsaturable 1,25-(OH)2D-independent mechanism (173). This pathway becomes gradually replaced by a 1,25-(OH)2D-dependent saturable component. The lack of response of the intestine to 1,25-(OH)2D may be explained by the relative absence of the VDR during early neonatal life because intestinal VDR remains low during suckling and increases to adult levels 1 wk after weaning (174). However, the onset of growth retardation and bone disease correlates with weaning, even when weaning was accelerated (2 wk) or delayed (4 wk) (155).
The decrease in intestinal calcium absorption was associated with altered expression of several calcium transport proteins in the duodenum of VDR or 1α-hydroxylase null mice. TRPV6 mRNA levels were extremely low in VDR null mice and were not induced by a low-calcium diet or 1,25-(OH)2D injection, as observed in WT mice (157). Also CaBP-9k mRNA and protein levels were reduced in intestine, albeit at variable degrees in different VDR null strains (155,175,176,177). This decrease was already observed in normocalcemic 2-wk-old VDR null mice, which suggests that it was a consequence of VDR deficiency rather than hypocalcemia. Because CaBP-9k mRNA and protein levels decline with age in WT mice, but not in VDR null mice, the difference in CaBP-9k expression between genotypes becomes smaller in aged mice (178). Of note, 1α-hydroxylase null mice also showed a decrease in CaBP-9k expression (159,160). On the other hand, PMCA1b gene expression is unaltered in the duodenum of VDR null mice (157). When given a rescue diet (consisting of high lactose and high calcium), TRPV6 and CaBP-9k gene expression in duodenum decreased both in WT and VDR null mice, resulting in comparable levels between the two genotypes (157,175,176). These data suggest that, when paracellular calcium transport becomes sufficient to meet dietary calcium needs, the genes involved in active calcium transport are down-regulated by a still uncharacterized mechanism.
The importance of 1,25-(OH)2D-regulated genes for intestinal calcium absorption was shown for some of the molecular players. During normal calcium intake, both decreased and unaltered intestinal calcium absorption has been reported for TRPV6 null mice, although normal serum calcium levels could be maintained. When switched to a low-calcium diet, TRPV6 null mice were still able to increase intestinal calcium absorption, but not to the same extent as control mice, resulting in hypocalcemia (179,180). On the other hand, CaBP-9k ablation had no effect on intestinal calcium absorption or serum calcium levels. A likely explanation is that its role is compensated by other known and/or unknown calcium transport proteins (181,182). Ablation of both TRPV6 and CaBP-9K did not aggravate intestinal calcium absorption when calcium intake was sufficient, but it did aggravate the effect of TRPV6 deletion when switched to a low-calcium diet (179). Taken together, the VDR promotes adequate intestinal calcium absorption by regulating the expression of several known and/or unknown calcium transport proteins in the duodenum (Fig. 2). Recently, the expression of claudin 2 and claudin 12 was shown to be induced by 1,25-(OH)2D and decreased in the intestine of VDR null mice. These proteins are suggested to form paracellular calcium channels and highlight a new mechanism behind 1,25-(OH)2D-dependent calcium homeostasis (183).
Figure 2.
Model for transcellular intestinal calcium absorption. Uptake of calcium in the enterocyte is mediated by TRPV6 and TRPV5, followed by intracellular binding to CaBP-9k and energy-dependent, basolateral extrusion by PMCA1b. This process is stimulated by 1,25-(OH)2D/VDR signaling resulting in increased gene expression of TRPV6, TRPV5, and CaBP-9k.
2. Renal calcium reabsorption.
The kidney contributes to the regulation of the extracellular calcium homeostasis by reabsorbing a large fraction of filtered calcium. As mentioned, 1,25-(OH)2D/VDR action is involved in the active reabsorption of calcium in the distal tubule. In line herewith, VDR null mice showed inappropriately high urinary calcium excretion given the observed hypocalcemia, suggesting renal calcium wasting due to disturbed calcium reabsorption. This was especially evident in normocalcemic VDR null mice on a rescue diet, which exhibited a significant increase in calcium/creatinine ratio compared with WT mice (158,178).
Expression of CaBP-9k was consistently decreased in the kidney of both VDR and 1α-hydroxylase null mice fed a normal diet. However, CaBP-28k mRNA levels were only moderately decreased in the kidney of VDR null mice, whereas they were significantly decreased in 1α-hydroxylase null mice. A similar pattern was observed for TRPV5 and NCX1: normal in VDR null mice but reduced in 1α-hydroxylase null mice. The expression of PMCA1b was unaltered, irrespective of the genotype (155,157,158,160,164,176). Feeding these mice a rescue diet lowered CaBP-9k expression in WT and VDR or 1α-hydroxylase null mice, leading to comparable levels between all genotypes (157,164,167). On the other hand, normalization of 1,25-(OH)2D serum levels in 1α-hydroxylase null mice resulted in increased expression of renal calcium transport proteins and normalization of serum calcium levels (164). These data indicate that renal calcium reabsorption is impaired when genomic actions of 1,25-(OH)2D are lacking and that CaBP-9k may be an important component of this process.
The crucial role of 1,25-(OH)2D-regulated proteins in mediating renal calcium reabsorption was demonstrated in TRPV5 null mice (184). These mice displayed persistent hypercalciuria associated with increased 1,25-(OH)2D levels and intestinal (hyper)absorption of calcium. This effect on intestinal calcium absorption was thought to be dependent on the increased 1,25-(OH)2D levels because compound TRPV5/1α-hydroxylase null mice did not show increased intestinal expression of calcium transporters and were severely hypocalcemic (185). However, TRPV5 null mice remained normocalcemic and did not develop rickets, but showed a reduction in bone mass, particularly in cortical thickness.
On the other hand, genetic ablation of the CaBP-28k gene in mice did not affect calcium homeostasis because serum calcium and phosphate levels and urinary calcium excretion were normal. Only subtle effects on bone were observed (both decreased bone mineral density and increased bone volume were reported) (186,187,188,189). Furthermore, ablation of CaBP-28k in TRPV5 null mice did not aggravate the TRPV5 null phenotype, indicating that the role of CaBP-28k can be compensated by CaBP-9k (190). However, studies using VDR/CaBP-28k double null mice indicate that CaBP-28k is involved in maintaining calcium homeostasis and skeletal mineralization, but the studies also suggest that CaBP-9k can largely compensate for this role (189). Notably, the double null mice on a regular diet were more growth retarded than VDR null mice and died prematurely at 2.5–3 months of age. Compared with VDR null mice, they were as hypocalcemic but showed more severe hypercalciuria, hyperparathyroidism, and pronounced skeletal abnormalities. On the rescue diet, serum calcium levels were normalized in both VDR null and double null mice; however, in contrast to VDR null mice, the skeletal abnormalities were not completely corrected in the double null mice. Ablation of NCX1 or PMCA1b resulted in embryonic lethality due to their essential function in cell viability by maintaining normal intracellular calcium levels, thereby precluding studies of their contribution to calcium homeostasis (191,192,193,194).
Taken together, VDR action is required for adequate calcium reabsorption in the kidney, by regulating the expression of several calcium transport proteins among which TRPV5 plays a crucial role.
C. Direct vs. indirect role of 1,25-(OH)2D-VDR action in chondrocytes
The VDR is present in the fetal rat at the time of mesenchymal condensation of skeletal tissues (195). However, embryonic bone development is normal in the absence of 1,25-(OH)2D-VDR action. VDR null pups of heterozygote VDR mothers exhibited normal length, and the morphology and mineral content of long bones was not altered. Growth plate characteristics, including gene expression pattern, were comparable to WT littermates. Of note, VDR null fetuses of heterozygote VDR mothers displayed normal serum levels of calcium, phosphorus, and PTH, and placental 45Ca transfer was not disturbed. VDR null fetuses did have increased 1,25-(OH)2D levels, accompanied by increased CYP27B1 mRNA expression in kidney, but not in the placenta (196).
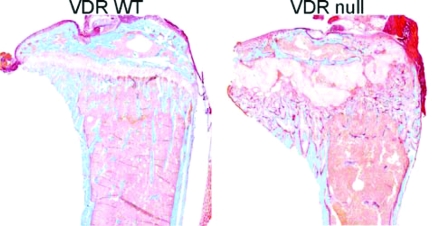
However, after weaning the longitudinal growth of long bones of VDR null and 1α-hydroxylase null mice was impaired and x-ray analysis revealed features of advanced rickets, including widening of the epiphyseal growth plate. Histology showed an increase in width and marked disorganization of the growth plate, including impaired mineralization of hypertrophic chondrocytes (Fig. 3) (155,156,158,159,160). Detailed analysis of the growth plate of VDR null mice demonstrated normal resting and proliferating chondrocyte layers. Accordingly, chondrocyte proliferation and expression of markers of chondrocyte differentiation, including collagen X and osteopontin, were not altered in VDR null mice. However, apoptosis of hypertrophic chondrocytes was markedly impaired in VDR null mice, resulting in an expansion of the growth plate that was already noticed shortly after weaning (197).
Figure 3.
Abnormal growth plate morphology and increased osteoid surface in VDR ablated mice. Goldner staining of tibiae showing abnormal growth plate and increased osteoid volume (dark pink) in VDR null mice compared with VDR WT mice.
By comparing several genetic models with dysregulated phosphate homeostasis, it was recently elucidated that circulating phosphate levels are crucial for hypertrophic chondrocyte apoptosis in vivo. Apoptosis of hypertrophic chondrocytes via activation of the caspase-9-mediated mitochondrial pathway was directly regulated by phosphate levels, as suggested by in vitro studies (198). In line with these findings, prevention of dysregulated mineral homeostasis by dietary intervention or 1,25-(OH)2D administration rescued the rachitic phenotype of VDR or 1α-hydroxylase null mice (161,162,163,165,166). Notably, the morphology and width of the growth plate were indistinguishable from those in WT controls, suggesting that the receptor-dependent actions of 1,25-(OH)2D are not required for normal growth plate development or maturation but that impaired mineral ion, and especially phosphate, homeostasis is the primary cause of the rachitic changes.
In accordance with these findings, chondrocyte-specific inactivation of the VDR did not alter chondrocyte development in the growth plate (199). VDR action in chondrocytes regulated bone development and phosphate homeostasis by inducing expression of paracrine factors. Particularly, vascular endothelial growth factor and receptor activator of nuclear factor κB (NFκB) ligand expression was decreased, leading to impaired vascular invasion and decreased osteoclast number in the metaphysis of long bones of juvenile mice. In addition, FGF23 expression in osteoblasts was reduced, resulting in increased serum levels of phosphate and 1,25-(OH)2D (see Section II.E.2). Nevertheless, persistent abnormality of the growth plate has been observed in normocalcemic 1α-hydroxylase null mice or double 1α-hydroxylase/VDR null mice on a rescue diet and has been reported in VDR/RXRγ double null mice (200,201). A possible explanation is that the correction of the calcium/phosphate homeostasis was quantitatively incomplete, precluding normalization of growth plate morphology. Alternatively, 1,25-(OH)2D may interact with a novel (unknown) NR in chondrocytes that heterodimerizes with RXRγ.
Taken together, 1,25-(OH)2D-VDR action influences growth plate morphology, mainly indirectly, by preventing hypophosphatemia that decreases chondrocyte apoptosis. On the other hand, VDR expression in chondrocytes affects trabecular bone mass and serum FGF23 levels during skeletal growth.
D. Changes in bone metabolism by interference with 1,25-(OH)2D-VDR action
After weaning, the characteristic features of osteomalacia became apparent in VDR null and 1α-hydroxylase null mice: the trabecular bone volume was increased due to an increase in the amount of unmineralized bone. The number of osteoblasts lining bone surfaces was increased and was associated with elevated serum levels of alkaline phosphatase. Mineral apposition was, however, markedly impaired. In most VDR null and 1α-hydroxylase null strains, the number of osteoclasts was not significantly altered, although a decrease was reported in one strain. Given the hyperparathyroidism, an increased level of osteoclasts would have been expected (161,162). Cocultures of osteoblasts and osteoclast progenitors, derived from WT and VDR null mice in different genetic combinations, demonstrated that 1,25-(OH)2D induces osteoclast formation by VDR-mediated actions in the osteoblasts, but that functionally intact osteoclasts can be formed without 1,25-(OH)2D action when other inducing agents like PTH are present (202). Biomechanical parameters demonstrated increased bone fragility in the hypocalcemic VDR null mice (162).
In VDR null mice fed a rescue diet, none of these parameters was significantly different from those in WT littermates raised under identical conditions; trabecular parameters and biomechanical competence of cortical bone were normal (162). Detailed dietary studies revealed that the Ca/P ratio in the rescue diet was important for bone mineralization because it affected intestinal calcium and phosphorus transport in VDR null mice (203). A comparable effect was seen in 1α-hydroxylase null mice after feeding a rescue diet or treating with 1,25-(OH)2D (161,163). Genetic evidence confirmed the importance of intestinal VDR action for bone homeostasis; introducing VDR specifically in the intestine of VDR null mice reversed the decrease in mineralized bone mass as well as the increase in osteoid volume (R. Masuyama, L. Lieben, R. Bouillon, and G. Carmeliet, unpublished observations). These data suggest that the skeletal consequences of VDR inactivation mainly result from impaired intestinal calcium absorption and/or the associated secondary hyperparathyroidism and hypophosphatemia.
In line with these findings, in vitro osteoblast differentiation of bone marrow stromal cells isolated from VDR null mice was comparable to WT controls. No difference was observed in alkaline phosphatase activity, gene expression of osteopontin, bone sialoprotein, and osteocalcin and calcium content of mineralized cell cultures (204). However, the number of bone marrow osteogenic progenitors seemed to decline in normocalcemic aging VDR or 1α-hydroxylase null mice compared with WT mice. This decrease was accompanied by reduced trabecular bone volume, suggesting that (direct) 1,25-(OH)2D-VDR action in bone may become critical with older age (200). Consistent herewith, mice overexpressing VDR in mature osteoblasts demonstrated increased bone volume due to enhanced cortical bone formation. Trabecular bone volume was also increased in these transgenic mice but was associated with decreased trabecular resorption, suggesting that VDR action in mature osteoblastic cells may inhibit osteoclastic bone resorption, in contrast to its effect in immature osteoblasts (205). However, ablation of VDR enhanced calvarial osteoblast differentiation, suggesting that other endocrine and paracrine factors modulate the effect of VDR on osteoblast differentiation in vivo (206).
Whereas the overall beneficial effects of vitamin D on bone have been known for about one century because it can prevent or cure rickets and osteomalacia, the direct effects of the 1,25-(OH)2D-VDR endocrine system are less evident and vary between: 1) beneficial, as demonstrated by increased bone mass and strength in osteocalcin promoter-driven VDR overexpression in osteoblasts (205); 2) neutral, because a simple rescue diet can normalize bone histology, bone mass, and strength (discussed in Section II.D); and 3) detrimental for bone, because VDR null bone transplanted into WT mice shows increased bone mass (and vice versa, WT bone transplanted into VDR null mice is more osteopenic) (207). Selective VDR deficiency in chondrocytes also increases bone mass as long as the growth plate remains functional (199). Only detailed phenotypic analysis of mice with cell-specific VDR deletion in osteoblasts, osteoclasts (precursors), or osteocytes will allow this question to be addressed appropriately. Preliminary data on osteoblast-specific VDR deletion suggests that cortical bone of these mice is increased (208).
E. Interaction of 1,25-(OH)2D-VDR pathway with phosphate-regulating hormones
1. Effects on parathyroid gland.
The hyperparathyroidism observed in VDR null and 1α-hydroxylase null mice was characterized by parathyroid hyperplasia, increased PTH mRNA levels in the parathyroids, and elevated serum PTH levels. Feeding these mice a rescue diet normalized circulating PTH levels, suggesting that ambient serum calcium, rather than 1,25-(OH)2D-VDR action itself, plays a key role in the pathogenesis of hyperparathyroidism (157,158,161,165). Of note, PTH production is negatively regulated by serum ionized calcium levels acting via the calcium-sensing receptor and by 1,25-(OH)2D serum levels. However, parathyroid gland hyperplasia persisted in the 1α-hydroxylase null mice fed a rescue diet but was normalized when mice were treated with 1,25-(OH)2D (200).
To investigate the contribution of PTH in the 1α-hydroxylase null phenotype, double null mice were generated (209). Single PTH null mice developed hypocalcemia, hyperphosphatemia, and low serum 1,25-(OH)2D levels (210). The double null mice died at 3 wk with tetany due to severe hypocalcemia, suggesting that secondary hyperparathyroidism contributes to control calcium homeostasis by its effect on bone and kidney (209). Treating these mice with PTHrP or PTH reduced hypocalcemia, apparently by increasing expression of renal calcium transporters, and additionally enhanced bone formation (211). Administration of 1,25-(OH)2D also increased serum calcium levels, presumably by its action on the intestine and kidney, and also improved bone formation independently of PTH (212).
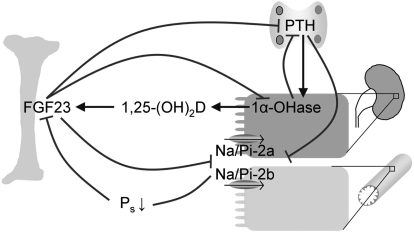
The hyperparathyroidism, in VDR null or 1α-hydroxylase null mice, may have contributed to the hypophosphatemia, because PTH suppresses apical Na/Pi-2a and Na/Pi-2b cotransporters, which mediate phosphate uptake in the proximal renal tubules and small intestine, respectively (213,214) (Fig. 4). In addition, 1,25-(OH)2D-responsive phosphate transport in the intestine and kidney has been described, presumably by regulating Na/Pi-2b and Na/Pi-2a protein expression, respectively (215,216). Accordingly, intestinal phosphate uptake was decreased in VDR null mice. However, feeding VDR null mice or 1α-hydroxylase null mice a low-phosphate diet increased both intestinal Na/Pi-2b and renal Na/Pi-2a protein levels, indicating that expression can also be regulated independently of VDR action (217,218).
Figure 4.
Vitamin D endocrine system, phosphate homeostasis, and FGF23. Increased serum phosphate or decreased serum calcium levels (not shown) induce PTH secretion by the parathyroid gland, which stimulates renal 1,25-(OH)2D synthesis. Increased 1,25-(OH)2D levels induce FGF23 production by osteoblasts and osteocytes. Both increased FGF23 and PTH levels reduce Na/Pi-2a and Na/Pi-2b expression in kidney and intestine, respectively, resulting in decreased phosphate (re)absorption and lower serum phosphate levels. FGF23 also down-regulates renal 1α-hydroxylase and decreases PTH secretion, creating a multiloop feedback system.
2. Effects on FGF23.
Another factor involved in phosphate homeostasis is the circulating phosphaturic factor, FGF23, which is produced by osteoblastic cells and leads to renal phosphate wasting. Like PTH, FGF23 can suppress expression of Na/Pi-2a and Na/Pi-2c cotransporters (219,220). In addition, FGF23 suppresses the expression of PTH and 1α-hydroxylase, thereby decreasing 1,25-(OH)2D levels (220,221). The expression of FGF23 is regulated by both 1,25-(OH)2D-dependent and VDR-independent signaling. Stimulation of the 1,25-(OH)2D-VDR pathway induces the expression of FGF23, as evidenced by increased FGF23 levels after 1,25-(OH)2D administration (222). In line with these findings, VDR null mice showed undetectable FGF23 levels (223,224). In addition, normalization of serum calcium and phosphate levels by dietary means increased FGF23 levels in VDR null mice, indicating that FGF23 expression is also regulated by a VDR-independent pathway, with phosphate being a potent stimulator (223,224). Moreover, FGF23 expression was inhibited by low serum phosphate levels, which resulted in increased Na/Pi-2a expression and renal phosphate reabsorption (217,224). To exert its biological function, FGF23 is not dependent on a functional 1,25-(OH)2D-VDR system. Notably, treatment of VDR null mice with FGF23 further decreased hypophosphatemia due to reduced renal and intestinal phosphate absorption, accompanied by decreased Na/Pi (natrim-dependent phosphate cotransporter)-2a, Na/Pi-2b, and 1α-hydroxylase expression (223,225,226).
As expected, FGF23 null mice showed hyperphosphatemia, moderate hypercalcemia, low PTH levels, and elevated levels of 1,25-(OH)2D. The adult bone phenotype was characterized by a disorganized growth plate lacking hypertrophic chondrocytes and decreased mineralized bone mass with increased osteoid, and it was associated with skeletal nodule formation and soft tissue calcifications (227,228). Loss of 1,25-(OH)2D activities from FGF23 null mice, by generating compound FGF23/1α-hydroxylase double null mice, resulted in the disappearance of abnormal skeletal nodule formation and soft tissue calcifications, suggesting that at least some of the anomalies found in FGF23 null mice are mediated through increased 1,25-(OH)2D activities. In addition, the severe hyperphosphatemia is reversed into hypophosphatemia, possibly attributable to hyperphosphaturia in compound null mice. The decreased activity of Na/Pi-2a in double mutants may be partly due to the elevated serum PTH levels (228,229). Comparable effects were observed when FGF23 null mice were crossed with VDR mutant mice and given a rescue diet (230). These data indicate that alterations in mineral metabolism in FGF23 null mice require an intact 1,25-(OH)2D-VDR signaling pathway.
The mineral and bone phenotype of FGF23 null mice is very comparable to the phenotype of Klotho null mice (231). Klotho is a transmembrane protein that enhances FGF23 binding to its receptor complex, implicating Klotho as a cofactor in FGF23-FGF receptor interaction (232). FGF23 serum levels are severely increased in Klotho null mice, but these mice show hyperphosphatemia and hypervitaminosis D, associated with increased expression of Na/Pi-2a and 1α-hydroxylase (229,233,234). A significant rescue of this phenotype was obtained when Klotho null mice were fed a vitamin D-deficient diet (234). The altered mineral ion homeostasis, and especially hyperphosphatemia, seemed to be the most important factor causing soft tissue calcifications. Accordingly, Na/Pi-2a null mice displayed hypophosphatemia, associated with secondary increased serum 1,25-(OH)2D and calcium levels leading to renal calcification, which could be rescued by blocking 1,25-(OH)2D activity by genetic means (235).
F. VDR, vitamin D, and tooth development
Teeth contain several types of calcium depositions: the very hard enamel formed by specialized cells that disappear after tooth eruption; dentin, connected with dentinoblasts that survive as long as the living teeth; and finally cementum, generated by cementoblasts, connected with periodontium to the bones of the upper and lower mandible. Thus, each cell type generates a specialized calcified tissue. The cell types responsible for generating these calcified matrixes are under the control of vitamin D, as revealed by selective up- or down-regulation of gene expression (e.g., VDR, MSX, osteocalcin, osteopontin, calbindin D proteins, and matrix proteins such as amelogenin) (236,237). Vitamin D or 1α-hydroxylase deficiency or VDR resistance during tooth development creates abnormal amelogenesis, dentinogenesis, and cementogenesis (238). The maturation and mineralization of enamel are especially decreased, generating a classic lifelong, irreversible enamel dysplasia. The dentin of VDR null mice (age, 70 d) was also clearly undermineralized and thinner, whereas the pulp chamber was enlarged, as revealed by micro-computed tomography scanning. The predentin area was enlarged in VDR null mice and the mineralization front was irregular. These data clearly indicate compromised dentin maturation in VDR null mice (239). Similar tooth abnormalities have been described in vitamin D- or 1α-hydroxylase-deficient or VDR-resistant children. These rachitic teeth are also more prone to periodontal abscesses. The effects of vitamin D status on periodontitis and tooth loss in adults and the elderly are less well evaluated. In a small-scale prospective trial with or without vitamin D and calcium supplementation for the prevention of osteoporosis, tooth loss was substantially lower (odds ratio, 0.4) in the calcium/vitamin D supplemented group (240).
G. Conclusion
VDR and 1α-hydroxylase null mice display severe hypocalcemia, rickets, and osteomalacia. These effects on calcium and bone homeostasis are largely mediated by 1,25-(OH)2D action on intestinal and renal calcium absorption because these processes are severely impaired in VDR-ablated mice. Normalization of serum calcium by genetic or dietary interventions corrects most of these bone abnormalities. Several intestinal and renal 1,25-(OH)2D-responsive genes have been identified, and studies using transgenic mice confirmed a crucial role for TRPV5 in renal calcium reabsorption. Intestinal calcium absorption involves several transporters (TRPV6, CaBP-9k, and PMCA1b), but inactivation of these genes in mice did not impair intestinal calcium absorption, suggesting redundancy or involvement of other molecules. Besides these indirect effects of 1,25-(OH)2D/VDR on bone, direct, although more subtle, positive and negative effects are also observed but still require detailed analysis. In addition, the 1,25-(OH)2D-VDR endocrine system also regulates phosphate balance, mainly by regulation of PTH and FGF23 levels, as shown by hypophosphatemia, secondary hyperparathyroidism, and undetectable FGF23 levels in VDR null mice.
III. VDR-Vitamin D Endocrine System and Skin
The skin is a unique organ in the vitamin D system because it can synthesize vitamin D in response to UV radiation, is capable of metabolic activation of vitamin D into 25-OHD and 1,25-(OH)2D, can inactive these metabolites by activation of CYP24A1, and finally can express the VDR and responds to VDR activation by induction or repression of a multitude of genes. Therefore, the skin is the sole tissue capable of vitamin D synthesis and activation, as well as autocrine/paracrine activation by the vitamin D hormone. Like calcium, 1,25-(OH)2D promotes differentiation of epidermal keratinocytes (241). Both in vitro and in vivo analyses demonstrate that these two pathways act synergistically. Studies in cultured keratinocytes demonstrate that both increases in calcium and treatment with 1,25-(OH)2D decrease keratinocyte proliferation and promote keratinocyte differentiation (241,242). In vivo analyses in VDR null mice demonstrate a significant abnormality in differentiation of epidermal keratinocytes after the second week of life (243). However, prevention of hypocalcemia in the VDR null mice prevents this phenotype (241), suggesting that normalization of calcium can compensate for the absence of the VDR in this regard. This observation is consistent with studies demonstrating that cultured keratinocytes isolated from VDR null mice fail to differentiate in response to 1,25-(OH)2D but differentiate normally in response to calcium (244). In addition to being expressed in epidermal keratinocytes, the VDR is also present in the outer root sheath and hair follicle bulb, as well as in the sebaceous glands (245,246).
A. VDR is essential for hair cycling
Humans with VDR mutations have not been reported to have abnormalities in epidermal keratinocyte differentiation, but, like VDR null mice, most kindreds develop alopecia (19). VDR null mice develop hypocalcemia, hypophosphatemia, hyperparathyroidism, and growth retardation by 21 d of age (Section II), followed shortly thereafter by perioral and periorbital hair loss and ultimately alopecia totalis, associated with large dermal cysts (155,156,157,158). Whereas the development of hyperparathyroidism and skeletal abnormalities in the VDR null mice is prevented by maintenance of normal mineral ions, alopecia is not (Fig. 5) (165). Furthermore, when WT and VDR null mice are raised under dietary and UV-free conditions that lead to undetectable circulating 25-OHD and 1,25-(OH)2D levels, VDR null mice develop alopecia whereas WT controls do not (247). Thus, the markedly elevated levels of 1,25-(OH)2D cannot be implicated in the pathogenesis of alopecia in the VDR null mice. Furthermore, the absence of alopecia in WT mice with undetectable circulating levels of vitamin D metabolites suggests that the absence of receptor and the absence of ligand have different effects on the hair follicle. In this experimental model, it is unlikely that local vitamin D is produced in the skin and subsequently converted to 1,25-(OH)2D because this process is dependent upon UV radiation. Furthermore, investigations in mice with targeted ablation of CYP27B1 do not exhibit a hair follicle phenotype (159,160), confirming that the effects of the VDR on the hair follicle do not require 1,25-(OH)2D. However, these studies do not preclude the possibility that a novel endogenous VDR ligand, synthesized in the skin, could maintain normal hair growth in the absence of vitamin D and 1,25-(OH)2D.
Figure 5.
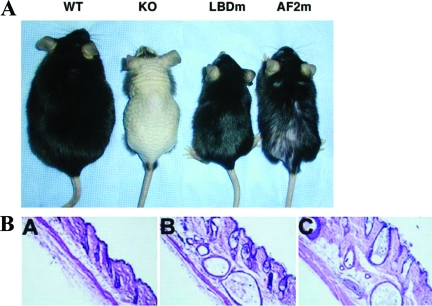
Skin phenotype of VDR null mice. A, Phenotype of 8-month-old VDR null mice with keratinocyte-specific VDR transgene expression. A WT control littermate is on the left, followed by a transgene negative VDR null mouse (KO). The VDR null mouse with keratinocyte-specific expression of a VDR with the LBD mutation (LBDm) does not have a cutaneous phenotype, whereas that expressing the VDR with the AF-2 domain mutation (AF-2m) exhibits significant hair loss. The smaller size of the three KO mice is due to growth retardation associated with abnormal mineral ion homeostasis. [Adapted from K. Skorija et al.: Mol Endocrinol 19:855–862, 2005 (251) Copyright The Endocrine Society.] B, Hematoxylin and eosin staining of the skin from a 70 day old WT mouse (A) and VDR null mice with abnormal (B) and normal (C) mineral ion homeostasis. [Adapted from Y.C. Li et al.: Endocrinology 139:4391–4396, 1998 (165) Copyright The Endocrine Society.]
Formation of hair follicles requires signaling between the mesodermal dermal papilla cells and the epithelial keratinocytes. The dermal papilla cells are thought to send signals to keratinocyte stem cells in the bulge region of the follicle, resulting in proliferation and subsequent differentiation into hair follicle keratinocytes (248). There are three defined stages in the hair cycle: anagen, catagen, and telogen. During anagen, cells proliferate rapidly, generating a mature hair follicle that forms a hair shaft. This is followed by regression (catagen) of the keratinocytes to the level of the hair follicle bulge, and finally the quiescent phase, telogen. It is thought that the approximation of the dermal papilla cells to the keratinocyte stem cells in the bulge, during telogen, enables signaling between these cells, resulting in anagen initiation. Although proliferation of neonatal keratinocytes was unaffected by the absence of the VDR, the VDR null mice were unable to respond to anagen-initiating stimuli after hair follicle morphogenesis is complete (the second week of life), suggesting that the VDR is critical for regulating postnatal hair cycles (244).
Hair reconstitution assays identified the cellular source of the hair follicle defect. In this assay, keratinocytes and dermal papilla cells isolated from neonatal WT and VDR null mice were coimplanted sc into nude mice. Generation of normal follicles was initially observed regardless of the VDR status of the cells used, confirming that the VDR is not essential for hair follicle morphogenesis. However, keratinocytes lacking the VDR were unable to undergo postmorphogenic hair cycles, regardless of the genotype of dermal papilla cells (247). In addition, keratinocyte-restricted VDR expression rescued alopecia, but not abnormalities in mineral ion or skeletal homeostasis, in the VDR null mice (249,250). Thus, the alopecia in the VDR null mice is due to impaired VDR action in the keratinocyte component of the hair follicle.
Studies using mutant VDR transgenes demonstrated that ligand binding is not required for the VDR to prevent alopecia. The mutation introduced into the LBD of the VDR prevents binding of lithocholic acid, an alternative VDR ligand, as well as that of 1,25-(OH)2D, suggesting that the effects of the VDR on the hair follicle are not due to interactions with a novel ligand. However, keratinocyte-specific expression of a VDR with a mutation in the NR coactivator binding domain (AF-2 domain) attenuated but did not prevent the development of alopecia. The VDR null mice with keratinocyte-specific expression of the AF-2 mutant VDR exhibited hair loss (Fig. 5), accompanied by dermal cysts and dilated piliary canals by 8 months of age (251). Thus, the actions of the VDR in the epidermis are independent of ligand binding but require interactions with nuclear factors.
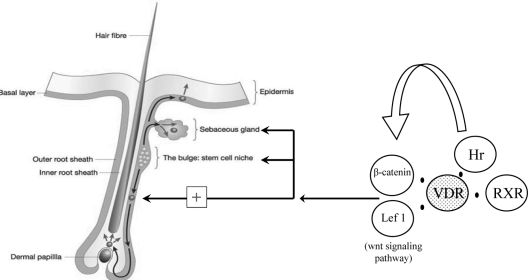
Mutations in the NR corepressor, Hairless, resulted in alopecia strikingly similar to that observed in the VDR null mice (252). Hairless binds to the VDR and represses VDR-mediated transactivation (253,254); however, neither of the VDR mutants studied in vivo interfered with VDR-Hairless interactions (251). Canonical Wnt signaling has been shown to play an important role in hair follicle development and to participate in postmorphogenic hair cycling (255,256,257). Interestingly, the dermal cysts and increase in sebaceous glands observed in the VDR null mice and Hairless mice were similar to that observed in mice expressing a keratinocyte-specific Lef1 transgene with a mutation that prevents its interactions with β-catenin (258). Confirming a functional interaction of the VDR with the canonical Wnt signaling pathway, transient gene expression assays in primary neonatal keratinocytes demonstrated that absence of the VDR prevented synergistic activation of a Wnt response element by β-catenin and Lef1 (259). Furthermore, immunoprecipitation studies demonstrated that VDR is present in a complex with β-catenin and Lef1. Hairless has also been shown to promote Wnt signaling (260), suggesting that abnormal canonical Wnt signaling may be the final common pathway by which VDR and Hairless mutations lead to cutaneous abnormalities (Fig. 6).
Figure 6.
Skin phenotype, VDR, VDR protein partners, and signaling pathways. The unliganded VDR is part of a multiprotein complex with its heterodimerization partner RXR and the corepressor Hairless (Hr). Each and all together are needed for long-term functional survival of skin stem cells. Moreover they are all three needed for orientation of the stem cells toward a functional hair follicle. VDR physically interacts with wnt target genes, β-catenin, and Lef-1. Both VDR and Hr promote wnt signaling to maintain normal hair cycling.
Keratinocytes in the bulge region of the hair follicle are thought to contain stem cells that can differentiate into epidermal keratinocytes, hair follicle keratinocytes, and sebaceous cells (261). Colony formation assays were performed to evaluate whether there was a defect in these stem cells in the absence of the VDR. Studies performed in neonatal mice, whose hair follicles are still undergoing morphogenesis, did not exhibit a defect in colony formation, whereas, by 4 wk of age, the keratinocytes of the VDR null mice were unable to form colonies characteristic of those formed by keratinocyte stem cells. Flow cytometric analyses for bulge keratinocytes demonstrated a normal number of keratinocyte stem cells at this age, suggesting a functional abnormality in these cells, based on their inability to form colonies in vitro (259) and their inability to regenerate hair follicles in vivo (244). However, a gradual decrease in the number of bulge keratinocytes was observed with age in the VDR null mice, suggesting a defect in keratinocyte stem cell self-renewal as well. The keratinocyte-specific VDR transgene prevented all these abnormalities in keratinocyte stem cells (259).
Thus, ligand-independent effects of the VDR are required for normal keratinocyte stem cell function. Although these data revealed novel functions for the VDR, they also raised a series of important questions that remain unanswered. The intriguing similarity of the Hairless and VDR mutant phenotypes suggests that these genes act in the same pathway or have similar, yet unidentified, targets in the keratinocyte stem cell. In an analogous fashion, the effects of the VDR and of the canonical Wnt signaling pathway on the hair follicle also suggest that synergistic interactions are essential for hair follicle homeostasis. Identification of the molecular partners of the VDR in keratinocyte stem cells, and of its targets, will undoubtedly reveal novel actions of this NR.
B. CYP27B1 is required for optimal epidermal differentiation
Compared with other tissues, renal tubular epithelial cells and keratinocytes express high levels of CYP27B1, the enzyme responsible for the conversion of 25-OHD to the active metabolite 1,25-(OH)2D. Therefore, several studies used the keratinocyte as a model system to investigate the role of 1α-hydroxylase activity and locally produced 1,25-(OH)2D in cell proliferation and differentiation. Although there was no gross skin phenotype in 1α-hydroxylase null mice, expression levels of the differentiation markers involucrin, profilaggrin, and loricrin were reduced in 1α-hydroxylase null mice (262). Furthermore, a pronounced retardation in the ability to recover normal barrier function after acute perturbation was found in 1α-hydroxylase null mice. These findings suggest that 1α-hydroxylase activity, most likely through the synthesis of active 1,25-(OH)2D, is essential for normal epidermal differentiation. Similar results were obtained by inactivation of both 1α-hydroxylase alleles in a ras-transformed keratinocyte cell line that typically produces squamous carcinoma in nude mice (263). In contrast to tumors from 1α-hydroxylase WT keratinocytes, tumors derived from 1α-hydroxylase null cells were not growth inhibited by locally administered 25-OHD, whereas they remained responsive to the antiproliferative and prodifferentiating effects of 1,25-(OH)2D, demonstrating the importance of 1α-hydroxylase activity in the autocrine regulation of growth and differentiation in keratinocytes. It should be noted, however, that in the absence of locally administered 25-OHD, tumors derived from 1α-hydroxylase null keratinocytes were not larger or heavier than those which originated from WT keratinocytes.
IV. VDR-Vitamin D Endocrine System and Cell Proliferation and Cancer
A. Mechanisms responsible for the antineoplastic effects of 1,25-(OH)2D
In 1981, Colston et al. demonstrated that the doubling time of malignant melanoma cells increased after incubation with 1,25-(OH)2D (264). In that same year, Abe et al. (265) demonstrated the differentiation of HL60 leukemia cells toward the macrophage lineage, after incubation with 1,25-(OH)2D. Since then, the antineoplastic activity of 1,25-(OH)2D was shown both in vitro and in vivo in a wide variety of malignancies, such as leukemia (266,267) and colon (268,269), breast (270,271,272), and prostate cancer (273,274). In a number of cell types, induction of programmed cell death contributes to the antineoplastic activity of 1,25-(OH)2D, but inhibition of angiogenesis and invasiveness may also contribute to its antitumor activity.
1. Inhibition of proliferation and induction of differentiation.
Growth inhibition of keratinocytes and myeloid leukemia cells, after incubation with 1,25-(OH)2D, is accompanied by the induction of a more differentiated phenotype (265). However, regulation of proliferation and differentiation is not always coupled, and the degree of cellular proliferation and/or differentiation is cell type-specific (267,275). In most cell types that express a functional VDR, incubation with 1,25-(OH)2D results in an accumulation of cells in the G0/G1 phase of the cell cycle (276). The exact sequence of events between VDR-mediated transactivation, or repression of target genes, and the actual G0/G1 arrest is probably cell type-specific and is the subject of intensive research. However, most of the proposed signaling pathways converge at the level of complex formation between the retinoblastoma family of pocket proteins, the E2F family of transcription factors, and the subsequent down-regulation of a large number of E2F-target genes that are required for normal cell cycle progression (276,277). The pocket proteins, p107 and p130, are crucial to the antiproliferative activity of 1,25-(OH)2D (277). Activation of the cyclin-dependent kinase inhibitors such as p18, p19, p21, or p27, repression of cyclin D1 expression, as well as down-regulation of the activity of complexes between cyclins and cyclin-dependent kinases, have been suggested to be early events, whether or not directly mediated by 1,25-(OH)2D, that occur upstream of the E2F-pathway and may be responsible for the growth-inhibitory effect of 1,25-(OH)2D (276,278,279,280,281). However, 1,25-(OH)2D may also inhibit cell growth by interfering with signaling pathways initiated by TGFβ, epidermal growth factor, IGF, prostaglandins (131), Wnt-ligands (282), as well as by intervening in other mitogenic signaling pathways (e.g., ERK/MAPK pathway and c-myc) (269,270,281,283,284,285,286,287) (Fig. 7).
Figure 7.
Cell cycle: 1,25-(OH)2D-induced signaling pathways involved in the regulation of cell proliferation and apoptosis. 1,25-(OH)2D blocks the progression of cells from the G1 to the S phase of the cell cycle either directly or through the induction of other growth factors (e.g., TGFβ). 1,25-(OH)2D induces the expression of different cyclin-dependent kinase inhibitors (p18, p19, p21, and p27), which inhibit the activity of cyclin/cdk complexes. Reduced cyclin expression after treatment with 1,25-(OH)2D also contributes to a reduced cyclin/cdk activity. As a result, the pocket proteins retinoblastoma (pRb), p107, and p130 remain in an underphosphorylated state and form complexes with the E2F family of transcription factors. Complexes between the repressor E2F family members E2F4 and E2F5 on the one hand and the pocket proteins p107 and p130 on the other hand are especially thought to associate with promoter regions of E2F target genes in cells that are treated with 1,25-(OH)2D. In some cell types, 1,25-(OH)2D is shown to affect cell proliferation through inhibition of EGF-induced Ras-signaling. 1,25-(OH)2D not only retards cell cycle progression but also induces apoptotic cell death either directly by inhibition of the antiapoptotic protein bcl-2 and by induction of the proapoptotic protein bax or by interfering with other signaling pathways (e.g., EGF, β-catenin, prostaglandins). Inhibition of prostaglandin (PGE2)-signaling, either by a reduction of prostaglandin synthesis or by a decrease in expression of prostaglandin receptors (EP1–4) after treatment with 1,25-(OH)2D, also contributes to the growth-inhibitory and proapoptotic effects of 1,25-(OH)2D. 1,25-(OH)2D also interacts with β-catenin-signaling. β-catenin is required for cell-cell adhesion and for the regulation of gene expression in response to Wnt-signaling. 1,25-(OH)2D is able to transrepress β-catenin/T cell factor (TCF) signaling through the rapid induction of VDR/β-catenin interaction and the subsequent expression of E-cadherin, which promotes the redistribution of β-catenin to the plasma membrane. On the other hand, high levels of β-catenin are shown to potentiate the ligand-dependent activation of VDR-regulated promoters.
2. Induction of apoptosis.
1,25-(OH)2D induces apoptosis in a number of tumor models, including carcinomas of the breast, colon, and prostate, but the underlying mechanisms of action are only partially elucidated (271,272,288). Alterations in the relative expression and/or cellular distribution of the Bcl-2 family of pro- and antiapoptotic proteins and the subsequent release of cytochrome c from the mitochondria have been suggested to contribute to the induction of apoptosis by 1,25-(OH)2D (272,289). However, depending on the cell type, 1,25-(OH)2D has also been shown to interact with other signaling pathways (e.g., IGF, TNFα) that may lead to the induction of programmed cell death (290,291).
Interestingly, after cellular stress or UV damage, and depending on the cell type, 1,25-(OH)2D was also able to exert antiapoptotic effects (292,293,294).
3. Inhibition of angiogenesis and invasiveness.
Because angiogenesis is an essential requirement for the growth of solid tumors, the antiangiogenic activity of 1,25-(OH)2D may represent an important mechanism responsible for its tumor suppressive activity. 1,25-(OH)2D was demonstrated to inhibit angiogenesis in both in vitro and in vivo experimental models (295,296). This antiangiogenic activity was suggested to be mediated by an inhibition of proliferation of tumor-derived endothelial cells, and/or by repression of the release of angiogenic factors, such as vascular endothelial growth factor (e.g., by repression of hypoxia-inducible factor-1 mediated transactivation), TGF-α, and epidermal growth factor (297,298).
Invasion of tumor cells to secondary sites requires degradation of the extracellular matrix, a process shown to be inhibited by 1,25-(OH)2D. Inhibition of serine proteinases (such as components of the plasminogen activator system), decreased activity of metalloproteinases, increased expression of E-cadherin, and inhibition of tenascin-C might all contribute to the antiinvasive effects of 1,25-(OH)2D (268,299,300,301).
B. Lessons from animal models: VDR null mice and cancer
1. Hyperproliferation of colonic cells in VDR null mice.
VDR and CYP27B1 are expressed in nonmalignant as well as malignant colon tissues from human origin. Interestingly, expression levels of both VDR and CYP27B1 are increased during early colorectal tumor progression, which could suggest a role for 1,25-(OH)2D in the paracrine/autocrine growth inhibition during early stages of colon tumor progression (302,303). This hypothesis is supported by in vivo findings in Tokyo VDR null mice, which were fed a lactose/calcium-enriched rescue diet to normalize serum mineral homeostasis, bone growth, and body weight. In the distal colon of these mice, complete loss of 1,25-(OH)2D-mediated growth control was accompanied by colonic hyperproliferation, as measured by expression of proliferating cell nuclear antigen, and by cyclin D1 (304,305). Moreover, direct DNA damage, evaluated by enhanced levels of 8-hydroxy-2′-deoxyguanosine, was enhanced in VDR null mice (304,305).
2. Skin of VDR null mice is hypersensitive to carcinogenesis.
In addition to a role in colon as a physiological growth regulator, 1,25-(OH)2D is thought to exert protective effects in skin transformation. Arguments in favor of this hypothesis are the finding that VDR and CYP27B1 are expressed in normal and malignant keratinocytes and the observation that 1,25-(OH)2D exerts antiproliferative, as well as prodifferentiating, effects on keratinocytes both in vitro and in vivo (243,306). Skin of Boston VDR null mice maintained on a rescue diet was found to be hypersensitive to tumorigenesis in response to oral administration of the carcinogen 7,12-dimethylbenzanthracene (DMBA) (307). Within 60 d of carcinogen exposure, 88% of VDR null mice developed persistent skin tumors, the majority of which were sebaceous, squamous, or follicular papillomas. With aging, skin of VDR null mice not exposed to DMBA became thick and wrinkled and displayed dermoid cysts.
Dysregulated cellular proliferation, as evidenced by basal cell proliferation and epidermal hyperplasia as well as aberrant differentiation in VDR null mice, demonstrated by decreased levels of epidermal differentiation markers such as involucrin, profilaggrin, and loricrin, might be responsible for the enhanced sensitivity to DMBA-induced skin tumorigenesis (243,307). However, despite the epidermal hyperproliferation, spontaneous cutaneous tumors were rarely detected in VDR null mice (307). The molecular basis for predisposing the skin to the development of tumors or basal cell carcinoma may be related to the overexpression of hedgehog signaling in normal and chemocarcinogen stimulated VDR null keratinocytes (308).
3. VDR ablation alters mammary gland morphology and tumorigenesis.
VDR is present in all major cell types of the mammary gland including basal and luminal epithelial cells, cap cells, and stromal cells. Within the mammary gland, higher VDR levels are found in differentiated cell populations compared with proliferating populations (309). VDR expression is dynamically regulated throughout the reproductive cycle, with peak levels observed during gestation and lactation (310). These findings suggest that VDR acts as physiological growth regulator of normal mammary epithelium. This hypothesis is strengthened by observations in Boston VDR null mice kept on a rescue diet. During puberty, glands from VDR null mice displayed increased branching, greater ductal extension, and higher numbers of undifferentiated end bud structures, compared with glands from WT mice. Furthermore, glands from pubertal VDR null mice exhibited enhanced growth in response to exogenous estrogen and progesterone, both in vivo and in organ culture, compared with glands from WT mice (309). During gestation, VDR ablation was accompanied by accelerated glandular development, whereas postlactational involution was delayed in VDR null mice (310). In line with these observations, which suggest a role for 1,25-(OH)2D in the control of mammary gland proliferation and differentiation, enhanced expression of VDR and CYP27B1 was found in breast tumors. However, growth control by 1,25-(OH)2D might be abrogated in breast tumors because the chromosomal region 20q13.2, which contains the CYP24A1 gene, was found to be amplified in breast tumors (35). In animal models, abrogation of 1,25-(OH)2D signaling by knocking out the VDR was found to lead to an enhanced susceptibility to tumorigenesis. In a first model, Boston VDR null mice were crossed with mouse mammary tumor virus-neu mice that specifically express the c-neu protooncogene in the mammary gland. Complete loss of VDR signaling in these mice was accompanied by pathological changes such as dysplasia of the mammary ductal epithelia, atrophy of the associated fat pad, and reduced survival. Although decreased survival of neu/VDR null mice hampered detailed evaluation of tumor development, dysregulated growth of alveolar and ductal cells was consistently observed in these mice. Furthermore, compared with neu-mice that express two intact VDR copies, haploinsufficiency of the VDR was found to accelerate c-neu-driven mammary tumorigenesis (311,312). In a second model, Boston VDR null mice and their WT littermates were exposed twice to the chemical carcinogen DMBA (313). Compared with WT mice, VDR null mice exhibited an increased incidence of glandular hyperplasia and altered tumor characteristics (hormone-independent tumors with squamous differentiation), but mammary tumor incidence, latency, and multiplicity were not affected by VDR ablation.
4. VDR ablation and other tumors.
VDR deficiency not only predisposes to epithelial tumors (colon, skin) but also results in greater susceptibility to chemocarcinogen-induced leukemia (311,313) (Table 3). Tumor development in ovary, uterus, lung, or liver was not different between VDR WT and null mice. However, lung metastatic cancer growth was extremely reduced in VDR null mice (300). Together, these findings suggest that optimal VDR signaling may contribute to suppression of tumorigenesis.
Table 3.
DMBA- and oncogene-induced carcinogenesis in Boston VDR null mice
| Tumor incidence (%)
|
||
|---|---|---|
| VDR WT mice | VDR null mice | |
| DMBA-induced carcinogenesis | ||
| Mammary hyperplasia and neoplasia | ||
| In situalveolar hyperplasia | 69 | 87 |
| In situductal hyperplasia | 83 | 98 |
| Palpable tumors | 75 | 74 |
| Skin tumors | <2 | 88 |
| Thymic and lymphoblastic lymphoma | 7 | 20 |
|
neu/VDR WT mice
|
neu/VDR +/− mice
|
|
| neu-induced carcinogenesis | ||
| Mammary tumors | 53 | 74 |
Chemocarcinogens (DMBA) were given twice (at 5 and 7 wk), and tumor occurrence was monitored for 6 months; oncogen-induced carcinogenesis was studied in VDR homo- and heterozygous mice crossed with mice overexpressing the neu protooncogen. [Adapted from G.M. Zinser and J. Welsh: Carcinogenesis 25:2361–2372, 2004 (312) with permission from Oxford University Press and G.M Zinser et al.: J Steroid Biochem Mol Biol97:153–164, 2005 (313) with permission from Elsevier.]
C. Lessons from epidemiological and intervention studies in humans
1. Vitamin D intake and exposure to sunshine in relation to cancer incidence.
The findings that 1,25-(OH)2D inhibits the proliferation of a wide range of cell types and that some cell types in the VDR null mice are characterized by dysregulated cell proliferation and differentiation are in support of the hypothesis that vitamin D levels, obtained either by exposure to sunshine or by vitamin D intake, can be associated with cancer risk in humans. The beneficial effect of sun exposure on cancer incidence was first suggested by Garland and Garland (314), who demonstrated that colon cancer mortality rates in the United States were higher in regions with less UV radiation. Additionally, a large number of studies have pointed to a decreased colon (314,315), breast (315,316,317,318,319), and prostate (315,320,321) cancer risk in regions with high UV radiation. However, the retrospective and subjective assessment of solar exposure may bias the outcome of these studies. Moreover, in both the United States and Europe, UV-B irradiation is shown to be a poor predictor of vitamin D status (322), which suggests that UV light exposure may not represent a surrogate marker of vitamin D status. The relationship between vitamin D intake and cancer risk has been studied extensively. Epidemiological data for breast cancer are sparse but seem to support the conclusion that higher vitamin D intake is associated with reduced risk (318,319,323,324). However, the results for colon cancer are too diverse to support any conclusion (325,326,327,328,329,330), whereas for prostate cancer, epidemiological data do not support the inverse relationship (331,332,333,334). Cross-sectional studies on the relation between nutritional vitamin D intake and cancer thus provide variable results, but this is not surprising because: 1) nutritional intake only partially explains the vitamin D status; 2) nutritional content and intake of vitamin D are notoriously difficult to evaluate, due to variable vitamin D content in a limited number of food items; and 3) there is a confounding factor of variable calcium intake, which may have an independent effect on cancer risk. Therefore, a more accurate and quantitative approach is to investigate whether the concentration of circulating vitamin D metabolites, which accounts for all sources of vitamin D, is associated with cancer risk.
2. Plasma 25-OHD concentrations in relation to cancer risk.
Numerous studies have addressed the question as to whether high levels of circulating vitamin D metabolites are associated with reduced cancer risk. Most reports agree that serum levels of 1,25-(OH)2D are poorly associated with cancer incidence (335,336,337). It is, however, generally accepted that, due to its relatively long half-life of approximately 2 to 3 wk, circulating 25-OHD level constitutes a better indicator of vitamin D status (12). Given the almost universal expression of CYP27B1, it is assumed that most cells are able to produce their own active 1,25-(OH)2D from 25-OHD, whereby the amount of locally produced active 1,25-(OH)2D is dependent on the serum concentration of its precursor (338). The relationship between serum 25-OHD and cancer incidence has been studied extensively for colorectal (314,335,337,339,340,341,342,343,344,345), prostate (346,347,348,349,350,351,352), and breast (336,353,354,355) cancer, with conflicting results. Different study outcomes may be related to divergent median 25-OHD in the investigated populations. Because it is suggested that the inverse association between serum 25-OHD levels and cancer incidence is more pronounced in the deficiency region (356), it is possible that, in populations with elevated circulating 25-OHD levels, no beneficial effects on cancer incidence are observed. Recent meta-analysis of studies addressing the association between 25-OHD levels and colorectal cancers revealed that individuals with serum 25-OHD levels equal to or greater than 82 nmol/liter had a 50% lower incidence of colorectal cancer than those with relatively low levels (≤30 nmol/liter) (357,358,359). The inverse association between colorectal cancer mortality and serum 25-OHD levels was confirmed by the results of the Third National Health and Nutrition Examination Survey (360). The association between plasma 25-OHD and prostate or breast cancer is more mixed because most studies show a negative relation, but a substantial number of studies show no relation, and a few even indicate a positive relation between (higher) 25-OHD levels and prostate cancer (357,358).
The final question is, of course, whether serum 25-OHD is a predictor or has a causative relation with the overall cancer risk. An association between total cancer mortality and baseline serum 25-OHD levels was not supported by a large survey (360). A few reports suggested that higher 25-OHD concentrations were associated with an increased cancer incidence (352,361,362). Some of the study results may be biased, due to the fact that only single measurements of 25-OHD are used in the studies, and these may not reflect long-term vitamin D status. Therefore, intervention studies in which vitamin D supplements are administered and cancer incidence is monitored may be more informative.
3. Intervention studies
a. Effect of vitamin D supplementation on cancer risk.
Recently, two randomized, double-blind, placebo-controlled trials have been completed in which the effect of vitamin D plus calcium supplementation on cancer risk was prospectively studied. In the Women’s Health Initiative (WHI) study, postmenopausal women received calcium (1 g) plus vitamin D (400 IU) or a matching placebo on a daily basis for 7 yr (363). Although a significant inverse relationship was found between baseline levels of serum 25-OHD and colorectal cancer risk, the incidence of invasive colorectal cancer did not differ significantly between women assigned to calcium plus vitamin D supplementation and those assigned to placebo. In a second, much smaller, 4-yr study in postmenopausal women, higher doses of calcium (1.4–1.5 g) and vitamin D (1100 IU) were administered, which raised serum 25-OHD to levels above 80 nmol/liter (364). Comparable to the WHI study, higher circulating 25-OHD levels were associated with a decreased cancer risk. However, in this study, improvement of the calcium and vitamin D nutritional status did significantly reduce overall cancer risk in postmenopausal women. In view of the small number of cancer deaths and a major confounding factor of calcium intake, which by itself seems to have contributed more than vitamin D intake, a much larger prospective study with substantial vitamin D supplementation is essential.
b. Application of vitamin D metabolites in the treatment of prostate cancer.
The effect of oral vitamin D3 (2000 IU daily) on prostate-specific antigen (PSA) levels was investigated in a small number of prostate cancer patients (n = 15) in whom local treatments had failed (365). In 9 of 15 patients, PSA levels decreased or remained stable after the initiation of vitamin D3 supplementation. Moreover, in 14 of 15 patients, a prolongation of PSA doubling time was observed.
In a small pilot trial in which 1,25-(OH)2D was administered on a daily basis to patients with rising PSA after radiation or prostatectomy, PSA doubling times tended to decrease (366). However, increased dosing of 1,25-(OH)2D was not possible due to hypercalcemia. Therefore, alternative dosing schedules have been evaluated. Weekly instead of daily administration of 1,25-(OH)2D allowed significant dose escalation, with minimal calcemic effects (367). Moreover, combination therapy with dexamethasone, estramustine, or taxotere plus dexamethasone was shown to be safe and well tolerated (368,369,370,371). In a setting of metastatic androgen-independent prostate cancer, addition of weekly high dose 1,25-(OH)2D to a regimen of docetaxel and dexamethasone had favorable effects on PSA response in a small patient cohort (30 of 37 patients achieved a PSA response) (368). Recently, the safety and activity of high dose 1,25-(OH)2D, in combination with docetaxel plus dexamethasone, was compared with placebo and docetaxel plus dexamethasone in a double-blinded randomized study in a large cohort of patients with androgen-independent prostate cancer (369). Addition of high dose 1,25-(OH)2D to docetaxel-based therapy proved to be safe and was associated with improved survival. However, no significant differences in PSA responses were observed.
These intervention studies illustrate the possible therapeutic application of 1,25-(OH)2D in cancer treatment. However, analogs of 1,25-(OH)2D with good systemic bioavailability, an enhanced potency to inhibit cell proliferation, and reduced calcemic effects may have a greater impact on the suppression of malignant cell growth. Vitamin D analogs have already been shown to be effective in the treatment of the hyperproliferative disorder psoriasis (372). Moreover, the potential clinical usefulness of vitamin D analogs in cancer has been demonstrated in different animal models of breast, prostate, and colon cancer (reviewed in Refs. 373 and 374). The vitamin D analog seocalcitol (EB1089), which showed therapeutic anticancer potential in various animal models, was evaluated in phase I (375) and phase II (376,377) clinical trials. However, at the maximal tolerable dosage, no objective antitumor activity of seocalcitol was observed in patients with advanced disease. To enhance the applicability of vitamin D analogs, several strategies have been suggested. To limit the calcemic activity, analogs with a greater dissociation between antiproliferative and calcemic activity might be used, but combination with compounds that limit bone resorption may also offer a valuable option. Alternatively, coadministration of chemotherapeutic cytotoxic agents and vitamin D analogs may have synergistic effects on cell growth, tumor angiogenesis, and metastasis and augment the efficacy of vitamin D analogs in the treatment of cancer.
D. Conclusion
Nearly all cell types express a functional VDR, and their growth is inhibited upon exposure to pharmacological doses of 1,25-(OH)2D, the active metabolite of vitamin D. However, also under physiological conditions, 1,25-(OH)2D is suggested to regulate normal cell growth because VDR null mice are predisposed to develop cancer when challenged with carcinogenic agents (Table 4). In parallel, epidemiological studies suggest that a poor vitamin D status is associated with a higher incidence of human cancer, further supporting the notion that 1,25-(OH)2D is an important regulator of normal cell proliferation. However, in a large study cohort, vitamin D supplementation (400 IU/d) was not associated with a decreased colorectal cancer risk. Therefore, long-term prospective studies with vitamin D (>1000 IU/d) are essential to evaluate the potential preventive effect of a better vitamin D status. In addition, selective VDR modulators, alone or in combination therapy, may constitute a better alternative for the treatment of existing cancers.
Table 4.
VDR-vitamin D endocrine system and cancer: summary
| 1. 1,25-(OH)2D is a potent inhibitor of cell cycle progression |
| Mainly as inhibitor of progression beyond G0/G1 stage |
| Coordinated action on many cell cycle pathways |
| Occurring in most normal and malignant cells |
| Variety of escape mechanisms in more malignant cell types |
| 2. VDR null mice are predisposed to carcinogen/oncogen induced cancers |
| 3. Large number of cross sectional and prospective epidemiological data link vitamin D deficiency with higher cancer incidence |
| 4. Some selective VDR modulators inhibitor tumor growth in rodent tumor models |
| 5. Proof of concept of therapeutic value of |
| Vitamin D supplementation: inconsistent results |
| Active vitamin D metabolites/analogs: ongoing studies |
A clear example of the potential clinical applications of the antiproliferative activity of 1,25-(OH)2D or its analogs is the now widespread use of calcipotriol or related analogs in the topical treatment of hyperproliferative skin diseases, such as psoriasis (378,379,380), although part of the clinical activity may also be due to their immune prospectives (381).
V. VDR-Vitamin D Endocrine System and Immunology
A. Vitamin D physiology and the immune system
The VDR is expressed in most cells of the immune system, including activated CD4+ and CD8+ T lymphocytes, as well as in antigen-presenting cells (APCs) such as macrophages and DCs (382). VDR expression in B lymphocytes is more controversial, with positive and negative reports (382,383). 1α-Hydroxylase or CYP27B1 is also expressed in macrophages, DCs, and even T and B lymphocytes (144,338,383,384,385). The 1α-hydroxylase present in immune cells is identical to the renal enzyme, but regulation of its expression and activity is different (Fig. 8). Whereas the renal enzyme is under control of calcemic and bone signals [such as PTH and 1,25-(OH)2D itself], the macrophage enzyme is primarily regulated by immune signals, with IFNγ and Toll-like receptor (TLR) agonists being powerful stimulators (28,385,386,387,388,389,390). Importantly, and in contrast with 1α-hydroxylase regulation in kidney, 1α-hydroxylase production in immune cells is not subjected to negative feedback signals from 1,25-(OH)2D itself (384,385). This explains the massive local production of 1,25-(OH)2D by disease-associated macrophages that is seen in patients with granulomatous diseases (sarcoidosis and tuberculosis), and the consequent possible spillover in the general circulation, eventually leading to systemic hypercalcemia. Up-regulation of 1α-hydroxylase, and therefore 1,25-(OH)2D synthesis, by immune cells and especially DCs typically occurs at later stages of immune activation, thus providing a late negative feedback loop, down-regulating immune responses. In parallel, VDR is down-regulated in macrophages and DCs at later stages of immune activation (391,392). It is of interest that a defect in up-regulation of 1α-hydroxylase in response to immune stimuli had been reported in nonobese diabetic (NOD) mice, an animal model of type 1 diabetes that spontaneously develops this autoimmune disease (385).
Figure 8.
Overview of vitamin D homeostasis in the immune system. Both 25-hydroxylase and 1α-hydroxylase are present in APCs such as DCs and macrophages resulting in the local production of active 1,25-(OH)2D. In the steady state and the early phase of inflammation, 1α-hydroxylase is absent or low. In IFNγ- and TLR ligand (TLR-L)-activated macrophages, 1α-hydroxylase is induced and 1,25-(OH)2D is generated. Under steady-state conditions, 1,25-(OH)2D induces 24-hydroxylase expression through a VDR-dependent mechanism creating a self-regulatory feedback loop and enabling 1,25-(OH)2D to fulfill its role in maintaining immune balance. In conditions of infection or inflammation, up-regulation of 24-hydroxylase is, however, hindered by IFNγ-induced STAT1α (signal transducers and activators of transcription-1α) activity resulting in continued high 1,25-(OH)2D levels and thus sustained and protective antimicrobial activity. In parallel, VDR is up-regulated in the early stage of inflammation after TLR activation and down-regulated in APCs at later stages of activation. Solid line, Stimulatory pathway; dotted line, inhibitory pathway.
The 25-hydroxylase and 24-hydroxylase enzymes are also expressed in immune cells (144,383,384,393). Whereas the expression of 25-hydroxylase is poorly regulated, the presence of 24-hydroxylase in immune cells is highly regulated and dependent on the differentiation status of the cell. Undifferentiated monocytes are highly susceptible to 1,25-(OH)2D-mediated 24-hydroxylase induction, whereas differentiated/activated macrophages are resistant due to an interplay between IFNγ- and 1,25-(OH)2D-mediated effects. STAT1α, involved in IFNγ-signaling, interacts with the DBD of the VDR, thereby prohibiting binding of the 1,25-(OH)2D/VDR/RXR-complex to the 24-hydroxylase promoter and induction of transcription (393). This impaired ability to initiate its own breakdown also contributes to the persistent overproduction of 1,25-(OH)2D in macrophages of patients with granulomatous diseases (386).
The regulated expression of all these components of the vitamin D endocrine system (VDR, and 25-, 1α-, and 24-hydroxylase) within immune cells strongly suggests a physiological role for 1,25-(OH)2D in the immune system.
B. VDR and the immune system
1. Signaling through the VDR and the immune system.
The discovery that the VDR is widely expressed in the immune system led to the recognition of a central immunomodulatory role for 1,25-(OH)2D (394,395). Here, as in other 1,25-(OH)2D-responsive tissues, the 1,25-(OH)2D/VDR complex can directly interact with VDREs in the promoter region of 1,25-(OH)2D target genes. Studies reporting a 1,25-(OH)2D-mediated increase in TNFα production by bone marrow cells demonstrated that this was partially mediated by direct binding of the liganded VDR-RXR complex to a VDRE in the promoter region of the TNFα gene (396). More recently, a VDRE was discovered in the promoter of the human cathelicidin antimicrobial peptide (CAMP) gene (397). An example of a negative VDRE was found in the promoter region of the IFNγ gene. Presumably IFNγ production is inhibited by binding of the liganded VDR-RXR complex to this negative VDRE, as well as by interacting with a crucial upstream enhancer element (398). In a unique manner, suppression of the granulocyte/macrophage recruiter, granulocyte macrophage colony stimulating factor, by 1,25-(OH)2D is achieved by binding of ligand-bound VDR monomers to functional repressive complexes in the promoter region of the cytokine gene (399,400).
Additionally, liganded but also unliganded VDR can interfere with signaling by other transcription factors, a mechanism by which 1,25-(OH)2D exerts most of its immune effects. The VDR/1,25-(OH)2D complex dose-dependently interferes with the signaling of transcription factors [such as nuclear factor of activated T cells (NFAT), NFκB, and activating protein-1 (AP-1)] that play a crucial role in regulating immunomodulatory genes (400,401,402) and whose dysregulation is suggested to be involved in the pathogenesis of different autoimmune diseases (403). As such, 1,25-(OH)2D impedes the activation of NFκB as well as its binding to consensus sequences, thus resulting in 1,25-(OH)2D-induced down-regulation of IL-8 and IL-12 (114,404). In DCs and many other cell types, this NFκB pathway has been proven to be very important in 1,25-(OH)2D-mediated effects (402,405,406,407). Notably, inhibition of phosphorylation (and subsequent ubiquitination and degradation) of the cytosolic inhibitor of NFκB, inhibition of NFκB nuclear translocation, binding (and therefore retention) of NFκB to VDR in the nucleus, and interference with DNA binding of the NFκB-complex are targets of 1,25-(OH)2D actions. Moreover, a VDRE has been found in the promoter of RelB, one of the NFκB family members, contributing to the 1,25-(OH)2D-mediated inhibition of NFκB-mediated maturation of DCs (408). Constitutive association of (unliganded) VDR with the RelB promoter is enhanced by ligand-binding but decreased by lipopolysaccharide (LPS). This process is controlled by chromatin remodeling by means of histone deacetylase 3 activity. Studies in mouse embryonic fibroblasts isolated from VDR null mice confirmed the direct involvement of VDR in the regulation of NFκB activity (409). VDR null cells exhibited reduced inhibitor of NFκB levels, leading to increased nuclear translocation of p65. Moreover, because of the lack of VDR, the physical interaction between VDR and p65 in the nucleus is absent, which may free p65 and again increase its activity. Together, these combined changes lead to the marked increase of NFκB activity in VDR null fibroblasts.
Similarly, the NFAT and AP-1 transcription factor pathways are involved in the 1,25-(OH)2D-mediated inhibition of IL-2 and IL-4 (113,410,411).
2. VDR and CYP27B1 polymorphisms and the immune system.
A vast amount of information has been collected over the years regarding the association of VDR polymorphisms with susceptibility to different autoimmune diseases and infection (412). The results obtained so far are conflicting, and the role of VDR polymorphisms remains unresolved. However, many immune and regulatory genes are located in close vicinity of the VDR gene on human chromosome 12q13.11. In type 1 diabetes, recent genome searches indicate the possible existence of additional disease susceptibility genes in this region. Although the VDR is a potentially good candidate diabetes susceptibility gene and an association between some VDR gene polymorphisms and type 1 diabetes susceptibility has been shown in different populations, a recent meta-analysis could not find any evidence for an association between VDR polymorphisms and type 1 diabetes risk in either case-control or family-transmission studies (413). In contrast, susceptibility to type 1 diabetes is associated with polymorphisms in the CYP27B1 gene (414), as well as the 25-hydroxylase (CYP2R1) gene (415). These findings of an association between vitamin D metabolism and diabetes risk are concordant with the association of vitamin D (deficiency) levels and type I diabetes.
Also, for many other autoimmune diseases (multiple sclerosis, rheumatoid arthritis, Graves’ disease, systemic lupus erythematosis, hepatitis, inflammatory bowel diseases, and thyroiditis), as well as for tuberculosis infection, the association with VDR polymorphisms has been investigated with conflicting results. A recent meta-analysis for tuberculosis risk demonstrated that the data gathered to date are inconclusive and studies are underpowered (416). One recent study reports an association of improved renal allograft survival with the FokI T allele (417), which gives rise to the long form of VDR, which has been shown to be less immunologically active (418). Indeed, we described that the shortened VDR FokI C allele exhibits higher transcriptional activity of immune-specific transcription factors, leading to changes in proliferation and cytokine synthesis of different immune cell types, correlating with a more activated immune system and thus, possibly predisposing to autoimmune diseases or graft rejection.
3. Absence of the VDR and the immune system.
Because the immune effects of 1,25-(OH)2D are mediated through the VDR, the immune system of VDR null mice became a focus of thorough investigation. Myelopoiesis, as well as cellular number and composition in all immune organs of VDR null mice, was shown to be normal (419,420). In humans with a mutation in the VDR gene resulting in vitamin D-resistant rickets type II, normal myelopoiesis was observed (421). Experiments performed with VDR null mice fed a normal diet revealed some intrinsic defects in T cell proliferation (to anti-CD3, a calcium dependent signal) and macrophage function (chemotaxis), which could all be remediated by normalizing calcium levels by a high-calcium, high-lactose rescue diet (419). These calcium-related effects include in vivo resistance to multiple low-dose streptozotocin-induced diabetes [MLDS-DM, a model of immune-mediated toxin-induced diabetes (422)] in hypocalcemic but not in normocalcemic VDR null mice. Other studies did, however, report some immune abnormalities in normocalcemic VDR null mice, each focusing on different cell types. When analyzing the cytokine production by splenic T cells, a defect in T helper 1 cell (Th1)-related IFNγ production was observed, presumably related to defective IL-18 production by macrophages and decreased STAT4 expression in T cells themselves (420). A study focusing on the phenotype of DCs in normocalcemic VDR null mice found some abnormalities in DCs from skin-draining lymph nodes (423). These lymph nodes were enlarged, with a higher proportion of more mature DCs (observed as higher major histocompatibility complex (MHC) II and CD40, CD80/86 expression). Yet another study demonstrated an accelerated maturation and increased responsiveness to IgE-mediated stimulation of mast cells from VDR null mice, the main Th2-type effector cells releasing histamine, upon stimulation mainly by IgE (424).
When comparing the studies investigating the prevalence of different autoimmune disease models in VDR null mice, some apparent contradictions surfaced. In different models of inflammatory bowel disease (whether chemically induced, induced by the transfer of CD45RBhigh+ pathogenic T cells, or occurring spontaneously in IL-10 null mice), exacerbated disease severity was always observed in VDR null mice (425,426,427). This was accompanied by increased expression of inflammatory and Th1-related cytokines and chemokines in the intestinal tract (e.g., IL-1, TNFα, IFNγ, IL-12, and MIP1α). In contrast, VDR null mice on a high calcium diet were found to be less susceptible to experimentally induced autoimmune encephalomyelitis, a multiple sclerosis model (428). More recently, we described the effects of lack of functional VDR in the animal model for type 1 diabetes, the NOD mouse (429). Analysis of the immune system revealed severe immune defects considered to be crucial in the development of NOD diabetes, but the VDR null NOD mice, fed a normal diet that did not rescue the hypocalcemia, did not display an enhanced susceptibility to diabetes. A clear decline in the number of immature TCR-α/β+CD4−CD8− NKT cells and regulatory CD4+CD25+ T cells was observed in immune organs of VDR null NOD mice. A relative deficiency of TCR-α/β+CD4−CD8− NKT cells and regulatory CD4+CD25+ T cells is postulated to contribute to an inability to maintain peripheral tolerance and lead to the development of diabetes in regular NOD mice (430,431). Moreover, inactivation of VDR in NOD mice resulted in disturbed cytokine and chemokine profile with extremely low IL-1, IL-6, and CCL2 transcripts reflecting a maturational defect of macrophages in NOD mice (432), in agreement with similar observations in vitamin D-deficient NOD mice (433,434). The thymus and lymph nodes of VDR null NOD mice contained even fewer mature CD11c+ DCs than WTs, and this could contribute to a defective elimination of diabetogenic T cells (435). The association of VDRR II and early onset type 1 diabetes has been reported once (436).
VDR null mice are resistant to experimentally induced airway inflammation and asthma (437,438). This was proposed to be due to a failure of the lung environment to respond to inflammation and attract pathogenic immune cells (Th2 cells and eosinophils), and not to defects in the priming and lung homing of the immune cells themselves. A robust Th2 response, characterized by IL-5 and IL-13 production, along with elevated IgE levels, could be induced in VDR null mice. The failure of trafficking of immune cells to the lung is in concordance with the recent rediscovery that 1,25-(OH)2D plays an important role in migration of immune cells. In addition to the long-known defects in macrophage chemotaxis caused by vitamin D deficiency, it was discovered more recently that 1,25-(OH)2D plays a role in microenvironmental positioning of T cells by altering their expression pattern of chemokine- and homing receptors (144). Increased expression of the skin-homing chemokine receptor, CCR10, and decreased expression of gut-homing CCR9 and α4β7 directed T cells toward the skin after 1,25-(OH)2D exposure. This tissue tropism of lymphocytes is proposed to be based on a model in which DCs process and “interpret” locally produced metabolites to program T cell homing and microenvironmental positioning with gut-tropism linked to vitamin A processing and skin-tropism linked to vitamin D processing (439). This altered T cell homing behavior could play a role not only in the resistance of VDR null mice to allergy but also in their increased susceptibility to inflammatory bowel disease.
VDR null mice are more resistant to parasitic Leishmania major infection (440). This infection model depends on the microbicidal function of IFNγ-activated macrophages, a host defense mechanism inhibited by 1,25-(OH)2D, as part of a negative feedback loop controlling inflammatory responses (441). In VDR null mice, this 1,25-(OH)2D-mediated inhibition is absent, resulting in continuous activation of macrophages and, consequently, enhanced microbicidal activity. This is in sharp contrast with the association between vitamin D deficiency and increased susceptibility for most nonparasitic infections. This discrepancy might be related to parasitic infections being more closely linked with Th2 defense mechanisms, whereas the innate immune system and Th1 arm of the immune system deals with other infections.
C. Vitamin D ligand deficiency and the immune system
In previous centuries, the medical world had already linked the presence of immune abnormalities to a deficiency in vitamin D, the precursor of the active VDR ligand. The oldest known effects of vitamin D deficiency on immune function are at the level of macrophage function (442) and resistance to infections, especially tuberculosis. A correlation between susceptibility to and disease progression of tuberculosis and serum vitamin D levels has been repeatedly observed (443,444,445,446,447). Moreover, oral administration of vitamin D has been shown to markedly improve tuberculosis outcome (448,449). A recent study on healthy tuberculosis contacts demonstrated that vitamin D treatment increases their monocytes’ capacity to mount a protective response to the bacillus Calmette-Guérin vaccine (450). It has been suggested that under conditions of low systemic 25-OHD levels, as is the case in vitamin D deficiency, only limited 1,25-(OH)2D can be produced locally by macrophages from its precursor 25-OHD, impeding the normal antimicrobial and antiinflammatory function attributed to 1,25-(OH)2D. This hypothesis is confirmed by the lower induction of the CAMP by monocytes incubated with 25-OHD-deficient serum from sunlight-deprived African-Americans (451). Defects in immune functions indispensable for antimicrobial activity have been observed in vitamin D-deficient mice (433,452). In addition, a disturbed delayed type hypersensitivity response has been reported in mice lacking vitamin D (453).
Furthermore, it has been suggested that in vitamin D deficiency the 1,25-(OH)2D-mediated attenuation of pathological Th1 immune responses is impaired, thus explaining the increased risk for Th1-mediated autoimmune diseases (454) such as inflammatory bowel disease (455,456), rheumatoid arthritis (457,458), systemic lupus erythematosus (459,460), multiple sclerosis (461,462,463), and type 1 diabetes (464). Many epidemiological data link vitamin D deficiency and increased prevalence of these autoimmune diseases with, for example, a 3-fold increase in type 1 diabetes when vitamin D deficiency was present in early life (464). Another strong argument linking vitamin D levels to immune function is the correlation between areas with low vitamin D supply (due to insufficient sunlight exposure time or nutritional vitamin D uptake) and increased incidences of different autoimmune diseases (465,466,467,468). The seasonal variation in onset and exacerbations of autoimmune diseases, with a peak in late winter/early spring when serum vitamin D levels are lowest, strengthen this correlation (469,470). In a very large prospective, nested case-control study among more than 7 million U.S. military personnel, a clear association between serum 25-OHD levels and risk of multiple sclerosis was observed (463). The risk of multiple sclerosis dose-dependently decreased with increasing serum 25-OHD levels, with the highest risk for serum 25-OHD levels below 75 nmol/ml, a marginally reduced risk for serum 25-OHD levels of 75–100 nmol/ml, and a significant 51% reduction of multiple sclerosis risk for serum 25-OHD levels above 100 nmol/ml.
In animal models of autoimmune diseases, aggravated disease presentation has also been demonstrated during vitamin D deficiency. In NOD mice, vitamin D deficiency during early life results in a more aggressive manifestation of the disease, with an earlier onset and a higher incidence (433,434). The thymus of vitamin D-deficient NOD mice had decreased CD8+ T cell and increased immature CD4+CD8+ T cell numbers, possibly pointing toward a T cell maturation problem. Moreover, numbers of regulatory CD4+CD62L+ T cells, already low in NOD mice, were further decreased in both thymus and peripheral lymph nodes. Besides these T cell abnormalities, vitamin D deficiency in NOD mice caused severe defects in macrophage function with lower respiratory burst capacity and a disturbed cytokine profile (increased IL-15 and extremely low IL-1 and IL-6). This aberrant inflammatory behavior of resident macrophages might, besides playing a role in antimicrobial activity, impair their migratory capacity, thus trapping these macrophages in the pancreas and leading to nonspecific damage to pancreatic β-cells, eventually triggering a β-cell destructive cascade. Accordingly, a highly inflamed state has been previously demonstrated in islets of NOD mice (471). Moreover, excessive expression of proinflammatory cytokines has been found in the islets of vitamin D-deficient NOD mice, indicative of a higher activation status of the infiltrating immune cells and thus a more aggressive presentation of the disease (433). Experimental autoimmune encephalomyelitis and inflammatory bowel disease are also accelerated and aggravated in vitamin D-deficient animals (465,472,473). Whereas vitamin D deficiency is strongly correlated with human immune abnormalities (454), the effects of supplements of vitamin D or 25-OHD in autoimmune diseases are less clear. Much depends on the initial vitamin D levels before supplementation—whether supplementation reverses existing vitamin D deficiency or adds extra vitamin D to already vitamin D-sufficient levels. For type 1 diabetes, multiple studies have evaluated the impact of vitamin D intake during pregnancy and/or early life and the prevalence of diabetes or diabetes-related autoimmunity (474,475). 25-OHD levels at onset of disease were also investigated (476,477,478). Supplementing already vitamin D-sufficient NOD mice or BB rats with 1000 IU of regular vitamin D at an early age does not protect from autoimmune diabetes (479,480). However, the insulin content in the islets of normal as well as diabetic NOD mice was increased. In the experimental autoimmune encephalomyelitis (EAE) model, vitamin D supplements fed to vitamin D-deficient mice conferred protection from disease (473).
The relation between vitamin D deficiency and asthma and allergy is more controversial. One study, however, demonstrated an inverse correlation between vitamin D levels and allergy or asthma (481). A link could be made not only to immune function but also to the development of the lung in utero and later on to its function. Some epidemiological data show that (higher) maternal vitamin D intake during pregnancy or early in life is inversely correlated with asthma and allergy later on (482,483). However, other studies report a detrimental effect of vitamin D supplementation on the development of allergy (484,485,486).
D. Discrepancy between loss of VDR and vitamin D deficiency in the immune system
The discrepancy between the consequences of absence of the NR vs. absence of ligand on autoimmune disease presentation is largely unexplained. Vitamin D-deficient animals and humans have an increased sensitivity to mycobacteria, probably related to deficient macrophage function (451), and consequent inability to induce CAMP expression (487). In contrast, macrophage chemotaxis, phagocytosis, and respiratory burst capacity were normal in VDR null NOD mice. Moreover, the DC phenotype was abnormal in VDR null mice on different backgrounds (419,429), whereas DCs from vitamin D-deficient mice did not present abnormalities (E. van Etten, G.B. Ferreira, A. Giuletti, and C. Mathieu, unpublished results). Changes in T lymphocyte behavior were observed in VDR null NOD mice, but not in vitamin D-deficient mice.
Not only are the effects of lacking ligand or lacking the NR dependent on the tissue, but they also affect the presentation of different diseases in different ways (Table 5). As stated before, in NOD mice only vitamin D deficiency (433) and not lack of VDR (429) aggravated diabetes presentation (higher incidence and earlier onset). In the multiple sclerosis model, experimental autoimmune encephalomyelitis, VDR null mice were protected from disease (428), whereas vitamin D deficiency accelerated disease development (472). In contrast, in models of acute experimental inflammatory bowel disease, more severe colitis was present in the absence of either vitamin D or its receptor (427). A clear explanation for these diverse disease phenotypes is lacking, but involvement of different immune cells in respective target organs that are affected in a different way by ligand or receptor loss may be responsible.
Table 5.
Comparison of the absence of VDR and vitamin D in the immune system: effects of the absence of the VDR, or vitamin D deficiency and the administration of pharmacological doses of the active 1,25-(OH)2D or analogs in different immune settings
| VDR KO | Vitamin D deficiency
|
1,25-(OH)2D or analogs | ||
|---|---|---|---|---|
| Animal studies | Human data | |||
| Autoimmune diseases | ||||
| Type 1 diabetes | = | + | + | − |
| MS/EAE | − | + | + | − |
| Crohn’s disease/IBD | + | + | + | − |
| RA/CIA | nd | nd | + | − |
| SLE | nd | nd | + | − |
| Allergy/asthma | − | (− / = ) | + | − / = /+ |
| Infections | ||||
| Leishmania | − | nd | nd | nd |
| Tuberculosis | nd | + | + | − |
=, No change in disease presentation; +, aggravation; −, protection; nd, no data available; MS, multiple sclerosis; EAE, experimental autoimmune encephalomyelitis; IBD, inflammatory bowel disease; RA, rheumatoid arthritis; CIA, collagen-induced arthritis; SLE, systemic lupus erythematosus.
This discrepancy of effects between lack of receptor and lack of ligand has also been observed for other NRs such as the thyroid receptor/thyroid hormone and the estrogen receptor/estradiol system (488). Repression by unliganded thyroid receptor has more profound effects on gene expression than absence of the thyroid receptor. Similarly, in the immune system, vitamin D deficiency often has more impact on disease outcome than absence of the VDR (Table 5). On the contrary, in the skin, absence of the VDR generates a more profound phenotype (alopecia) than absence of the ligand.
E. Pharmacological effects of 1,25-(OH)2D and analogs in the immune system
1. In vitro.
1,25-(OH)2D directly inhibits proliferation of human and murine T cells and inhibits the expression of several T cell cytokines (IL-2, IFNγ, and TNFα) (394,395,466,489,490,491). The greatest immunomodulating effects of 1,25-(OH)2D have been found in APCs, especially DC. Multiple in vitro studies using either human peripheral blood monocytes or murine bone marrow cells as precursors revealed that 1,25-(OH)2D can potently inhibit DC differentiation (492,493,494). Also, the in vitro and in vivo maturation process of DCs is seriously impaired by 1,25-(OH)2D, with decreased surface expression of MHC class II, costimulatory molecules (CD40, CD80, CD86), and other maturation-induced surface markers. Even 1,25-(OH)2D treatment can redirect already differentiated DCs toward a CD14+ cell type. In addition, 1,25-(OH)2D treatment of differentiating DCs disturbs their migratory capacity in response to inflammatory and lymph node homing chemokines, although the expression of the cognate chemokine receptors was unaffected (492). Furthermore, the cytokine expression pattern of DCs is altered by 1,25-(OH)2D, with strong inhibition of IL-12 and up-regulation of IL-10 expression (493,494).
Because DCs are, as professional APCs, the predominant cells linking innate to adaptive immune responses, the effect of 1,25-(OH)2D on DCs inevitably has major impact on T cell behavior (Fig. 9). Although 1,25-(OH)2D has direct effects on T cells, it is mainly by this indirect way that 1,25-(OH)2D influences T cell responses. By altering DC cytokine expression (suppression of Th1-directing IL-12 and up-regulation of counteracting IL-10), 1,25-(OH)2D skews the T cell differentiation toward a Th2 phenotype. More importantly, by modulating DC maturation and activation, 1,25-(OH)2D gives rise to tolerogenic DCs, thus contributing to the induction of regulatory T cells or even T cell anergy and apoptosis (495). This 1,25-(OH)2D-mediated induction of regulatory T cells has been shown in vitro and in vivo. When cultured together with 1,25-(OH)2D-modulated DCs, naive as well as committed autoreactive T cells showed complete hyporesponsiveness, as determined by decreased proliferation and IFNγ secretion, and even underwent antigen-dependent apoptosis (493,494,496).
Figure 9.
Overview of the pharmacological effects of 1,25-(OH)2D in the immune system. 1,25-(OH)2D inhibits the surface expression of MHC II-complexed antigen and of costimulatory molecules, as well as the production of the cytokine IL-12 in APCs (such as DC), thereby shifting the polarization of T cells from an (auto-)aggressive effector (Te) toward a protective or regulatory (Tr) phenotype. 1,25-(OH)2D exerts its immunomodulatory effects also directly on the level of T cells. Together, these immunomodulatory effects of 1,25-(OH)2D onto players of the adaptive immune system can lead to the protection of target tissues in autoimmune diseases and transplantation. In the innate immune system on the other hand, 1,25-(OH)2D strengthens the antimicrobial function of monocytes and macrophages, for example through enhanced expression of the CAMP, eventually leading to better clearance of pathogenic microorganisms.
2. In vivo.
High supraphysiological doses of 1,25-(OH)2D are needed to obtain immunomodulatory effects in vivo. Concomitant calcemic side effects are, therefore, observed, such as hypercalcemia, hypercalciuria, renal calcification, and increased bone resorption, prohibiting the clinical use of 1,25-(OH)2D as immunomodulator. To overcome this limitation, structural analogs of 1,25-(OH)2D have been developed showing higher immunomodulatory potency but lower calcemic activity (12,497). The therapeutic potential of treatments including 1,25-(OH)2D or its analogs has been explored in vivo in several animal models of autoimmune diseases, organ transplantation, and infection (reviewed in Refs. 394, 395, 491, and 498,499,500).
3. Autoimmune diseases.
1,25-(OH)2D and its analogs prevent diabetes and insulitis (the histological lesion in pancreatic islets caused by infiltrating immune cells and preceding the clinical presentation of diabetes) in NOD mice when treatment is started at an early age, before the onset of insulitis (501,502,503). The beneficial effects of 1,25-(OH)2D and analogs, in this model, are a consequence of 1) the restoration of defective suppressor cell activity; 2) the enhanced clearance of autoreactive T cells, by restoring apoptosis sensitivity in the central and peripheral immune system; and 3) a shift from a Th1 to a Th2 cytokine expression profile locally in the pancreas and in the pancreas-draining lymph nodes (504,505). 1,25-(OH)2D induces this immune shift in response to autoantigens but not to disease-irrelevant self or foreign antigens (505). When administered in mice of older age when autoimmune β-cell destruction is already taking place, analogs of 1,25-(OH)2D could still block diabetes progression, either alone or in combination with a short induction course of cyclosporin A (506,507). After syngeneic islet transplantation, although analogs of 1,25-(OH)2D in monotherapy can delay autoimmune diabetes recurrence in NOD mice, better protection from disease recurrence and less toxicity were obtained with combinations of 1,25-(OH)2D analogs and standard immunosuppressants (cyclosporin A, IFNβ) (508,509). For combinations with cyclosporin A, graft survival persisted, even after withdrawal of therapy, suggesting a reinduction of self-tolerance (508).
Many other examples of animal autoimmune disease models exist in which treatment, including 1,25-(OH)2D or its analogs, prevents or attenuates disease, such as systemic lupus erythematosus (510), experimental autoimmune encephalomyelitis (511,512,513,514,515,516), collagen-induced arthritis (517,518), inflammatory bowel disease (465), prostatitis (519), thyroiditis (520), and different forms of nephritis (521,522). A few clinical trials even demonstrated disease-ameliorating effects of 1,25-(OH)2D analogs in patients suffering from multiple sclerosis or rheumatoid arthritis (523,524). For the prostate, which is one of the known extrarenal sites of 1,25-(OH)2D synthesis and VDR expression, prostatitis (519) and prostate cancer (see Section IV) are not the only possible applications for 1,25-(OH)2D3. Recently, an analog of 1,25-(OH)2D3 proved to be protective in animal models of benign prostate hyperplasia, a complex syndrome comprising prostate overgrowth, urinary irritative syndrome, and an inflammatory component, by targeting prostatic growth, bladder function, and inflammation (525,526). A multicenter, double-blind, randomized, placebo-controlled phase IIa clinical study confirmed the inhibitory effects on prostate growth (527).
The mechanisms behind the 1,25-(OH)2D-induced protection from these different autoimmune diseases has not yet been fully elucidated. In EAE, the induction of Th2 responses, inhibition of Th1 action and effects on the blood-brain barrier could be involved (528,529,530,531,532). In the model of inflammatory bowel disease the protective effects of 1,25-(OH)2D and its analogs are partly mediated through inhibition of Th17 cells, together with an increase in regulatory T cells (533). Th17 cells are a recently defined helper T cell population, along with Th1, Th2 and regulatory T cells, which play a proinflammatory role in autoimmunity and tissue inflammation (534).
4. Transplant survival.
1,25-(OH)2D and its analogs can also prolong the survival of heart, aorta, kidney, liver, small bowel, pancreatic islet, and skin allografts (535,536,537,538,539,540). These effects of 1,25-(OH)2D and analogs were generally modest when used in monotherapy but are more robust when used in combination with subtherapeutic doses of classical immunosuppressants (cyclosporin, tacrolimus, sirolimus, mycophenolate, and glucocorticoids). Adding 1,25-(OH)2D or analogs to low doses of classical immunosuppressants resulted in a clear synergistic immunomodulation, as measured in vitro and in vivo (514). When 1,25-(OH)2D or analogs were administered in transplant recipients, an increased resistance to opportunistic infections was observed (541). Moreover, whereas none of the standard immunosuppressants is able to prevent chronic rejection, treatment with analogs of 1,25-(OH)2D does have an impact. Chronic rejection is caused by immunological and nonimmunological factors and is characterized by perivascular inflammation, fibrosis, and vascular narrowing, due to smooth muscle cell proliferation. In an aortic allograft model, which mimics the vascular lesions seen in human chronic allograft rejection, treatment with a 1,25-(OH)2D analog prevented chronic aortic allograft rejection and attenuated vascular damage (542,543).
5. Infections.
Exposing monocytes and macrophages to 1,25-(OH)2D improves their chemotactic and phagocytic capacity, features that are indispensable for their tumor cell cytotoxicity and microbacterial activity (544). Monocytes and macrophages are, in addition to their role as APCs in adaptive immune responses, key players in mounting innate immune responses against various infectious agents, detecting dangerous microbial invaders by means of their pattern-recognition receptors (e.g., TLRs), and subsequently producing antimicrobial peptides such as defensins and cathelicidins (545,546). 1,25-(OH)2D is able to induce the expression of human CAMP in human primary cells (monocytes, keratinocytes, neutrophils, and macrophages) and cell lines through direct interaction with VDRE sequences present in the promoter of the CAMP gene (397,487,547,548). In human monocytes, triggering of TLR2/1 results in the conversion of 25-OHD into active 1,25-(OH)2D and the consequent induction of CAMP (451), whereas at lower 25-OHD levels (for example, in serum of African-Americans), the induction of CAMP and subsequent antimicrobial capacity is reduced. In human keratinocytes, at sites of injury, the induction of CAMP by 1,25-(OH)2D requires SRC3 and is influenced by histone acetylation (549). In contrast with the human CAMP gene, no VDRE were detected in the promoter region of the mouse cathelicidin gene (548). Consistent with these findings, no differences in CAMP expression were observed between WT and VDR null mice. At later stages of infection (after 72 h), 1,25-(OH)2D suppresses the expression of TLR2 and TLR4 in human monocytes by a VDR-dependent mechanism (550), possibly representing a negative feedback mechanism, so as to prevent excessive TLR-activation and eventually sepsis.
F. Conclusion
The regulated presence in many immune cells of the key enzymes involved in the metabolism of 1,25-(OH)2D and the presence of VDR in almost all immune cells suggests a physiological role for the VDR-vitamin D endocrine system in immune homeostasis. Besides solid epidemiological evidence linking vitamin D deficiency to autoimmune diseases and increased susceptibility to infections, genetic studies also point toward associations between immune behavior and the different components of the VDR-vitamin D endocrine system. Intervention studies with pharmacological doses of 1,25-(OH)2D and some of its analogs have until now only been performed in animal models. These clearly demonstrate prevention of several autoimmune diseases, as well as prolongation of graft survival. All these data support the concept that the VDR-vitamin D endocrine system plays a role in the etiology of autoimmune diseases, such as type 1 diabetes, being involved in the genetic (genetic variations in CYP27B1), environmental (vitamin D deficiency), and immunological aspects [potent immunomodulatory effects of 1,25-(OH)2D] of their etiology. Moreover 1,25-(OH)2D can stimulate the native immune system and thus may offer a novel strategy to cope with (drug-resistant) infections.
The main practical conclusion from the studies on the VDR-vitamin D endocrine system and immunology to date is that avoiding vitamin D deficiency is important, not only for calcium and bone issues but also for a healthy immune system.
VI. VDR-Vitamin D Endocrine System and Glucose Homeostasis
Calcium fluxes and regulation of intracellular calcium stores are essential in the regulation of insulin secretion by the β-cells of the islets of Langerhans in the pancreas. This makes 1,25-(OH)2D, the central hormone in calcium regulation, a potential candidate for influencing normal β-cell function. Since the early observations in 1980 by Norman et al. (551) that pancreatic insulin secretion is inhibited by vitamin D deficiency, several reports have demonstrated an active role for vitamin D in regulating the function of the endocrine pancreas, especially the insulin-producing β-cells. The VDR is present in β-cells (552), and intriguing observations suggest the presence of a receptor localized in the membrane (553,554,555).
A. Vitamin D deficiency and VDR null mice
The earliest studies focused mainly on the effects of vitamin D deficiency in animal models and humans on insulin secretion and glucose tolerance (551,556,557,558,559,560,561,562,563,564,565). Vitamin D deficiency clearly impairs secretion of insulin (but not the other pancreatic hormones) and induces glucose intolerance, whereas vitamin D reverses the abnormalities (551,556,559,560,562,563,564). Data from VDR null mice are conflicting, with some groups reporting impaired glucose tolerance (565) while others report normal glucose metabolism (419,429). As in other systems, the background of the mouse in which the VDR deletion is introduced seems to be of critical importance. Recent in vitro data on β-cell function in islets from VDR null mice did not show any abnormalities (419,429). The pancreatic β-cell may, therefore, represent another target tissue with discrepancy between effects of the vitamin D ligand and the VDR itself. In NOD mice, calcium levels are essential in maintaining normal β-cell function because islets isolated from vitamin D-deficient, normocalcemic, NOD mice had normal insulin synthesis and secretion in response to glucose (433). Whether the effects of vitamin D deficiency and repletion or vitamin D resistance on glucose homeostasis in vivo are direct or indirect effects of vitamin D cannot be resolved by such studies because vitamin D-deficient animals do not have a normal food intake, thus losing weight and being unable to maintain normal plasma calcium levels. Moreover, these metabolic changes, per se, directly impair calcium handling in the β-cell and provoke β-cell dysfunction and glucose intolerance (556,564).
B. Effects of vitamin D metabolites in vitro
Convincing data come from in vitro studies where islets isolated from vitamin D-deficient animals show impaired insulin release when cultured in vitro and challenged with glucose (566,567), whereas these abnormalities can be prevented by culturing the islets in the presence of high concentrations of 1,25-(OH)2D (557,566,567,568). Moreover, high concentrations of 1,25-(OH)2D increase insulin synthesis and release upon glucose challenge in isolated pancreatic islets from normal animals (557,559,568,569,570,571,572,573,574,575,576,577). The mechanism by which 1,25-(OH)2D affects insulin secretion probably involves a significant rise in cytosolic calcium levels after incubation with 1,25-(OH)2D, resulting in insulin secretion. Controversy still exists as to whether this higher intracellular calcium comes from an influx of calcium via voltage-dependent calcium channels, or whether mobilization of calcium from intracellular organelles and activation of release-potentiating systems via protein kinase C/protein kinase A pathways are implicated (553,566,567).
In the pathogenesis of type 1 diabetes, β-cell damage by cytokines and other inflammatory agents might play an important role. Several studies demonstrate a protective effect of 1,25-(OH)2D (incubation periods ranging between 48 and 72 h) or its analogs on cytokine-induced β-cell dysfunction and death (572,575,577). A shorter exposure to 1,25-(OH)2D (24 h) did not confirm these data (574). We could not find protective effects of 1,25-(OH)2D on β-cell death but clearly demonstrated altered expression of chemokines in the β-cells, thus potentially altering their fate in type 1 diabetes (578) (see Section V).
C. Effects of vitamin D metabolites in vivo: clinical implications for type 2 diabetes
Vitamin D-deficient humans have reduced insulin secretion, whereas, as expected, vitamin D treatment (and calcium supplementation) restores glucose tolerance (559,570,573,579). Administration of vitamin D supplements or high doses of 1,25-(OH)2D, to vitamin D-replete patients with impaired glucose tolerance or frank type 2 diabetes yields conflicting results, with some reporting improvement (573) and others no effect (580), and one study even showed worsening of type 2 diabetes. Notably, Taylor and Wise (581) reported that vitamin D supplementation in three cases of British Asians with vitamin D deficiency and type 2 diabetes led to increased insulin resistance and deterioration of glycemic control.
Uremic patients are 1,25-(OH)2D-deficient and also show insulin resistance. Allegra et al. (569) clearly demonstrated that insulin resistance in uremic patients decreased after 1,25-(OH)2D therapy but that glucose intolerance was not completely reversed.
The relationship between vitamin D status and the metabolic syndrome has been evaluated in some small-scale studies, as well as in the large-scale Third National Health and Nutrition Examination Survey. 25-OHD levels above 19 ng/ml, and especially levels above 39 ng/ml, were associated with a significant lower odds ratio for the presence of metabolic syndrome and especially of abdominal obesity (582). Vitamin D deficiency is also associated with higher cardiovascular risk factors (see Section VII). A small-scale post hoc analysis of a bone study revealed that daily calcium (500 mg) and vitamin D (700 IU) supplementation for 3 yr prevented a further rise in fasting blood glucose in a subgroup with impaired fasting blood glucose (100–125 mg/dl) at baseline (583).
VII. VDR-Vitamin D Endocrine System and Cardiovascular System
Many cells that play a crucial role in the cardiovascular system express the VDR and respond to 1,25-(OH)2D with cell-specific gene regulation and functional consequences. These cells include vascular endothelial cells, cardiomyocytes, vascular smooth muscle cells, and monocytes/phagocytes (584) (Fig. 10). The juxtaglomerular cells of the nephron, which produce renin, are also 1,25-(OH)2D-sensitive (Fig. 11). Renin is a protease that cleaves angiotensinogen to angiotensin I, which is subsequently converted to angiotensin II. Angiotensin II activates specific receptors and regulates electrolyte, volume, and blood pressure homeostasis. In VDR null mice, increased renal renin mRNA levels have been observed, even after normalization of calcium homeostasis by a rescue diet (585). This leads to high plasma angiotensin II levels, systemic hypertension, and ultimately results in cardiac hypertrophy (586). The hypertensive effects of VDR deletion are blocked by angiotensin-converting enzyme inhibitors. A VDRE has been identified in the promoter of the renin gene, and direct inhibition of renin expression by 1,25-(OH)2D has been confirmed in vitro (586) and in vivo (121,587). In 1α-hydroxylase-deficient mice, renal renin expression was also increased. Renin, therefore, is likely to be negatively and directly regulated by 1,25-(OH)2D in mice, with cardiac repercussions (hypertrophy and decreased contractibility). Another group (588), however, found a nonsignificant increase in plasma renin activity in the same Boston VDR null strain, and no systemic hypertension. Even in the absence of hypertension, VDR null mice developed cardiac hypertrophy and fibrosis, suggesting that 1,25-(OH)2D also has direct effects on prevention of cardiocyte hypertrophy (588). Cardiac myocytes from VDR null mice showed accelerated rates of contraction and relaxation, compared with WT mice, and 1,25-(OH)2D directly affected contractility in WT but not in VDR null cardiac myocytes (589). An alternative mechanism contributing to cardiac hypertrophy in these VDR null mice could be increased extracellular matrix production and subsequent fibrosis, due to deficient expression of tissue inhibitors of metalloproteases-1 and -3 (147).
Figure 10.
1,25-(OH)2D-VDR effects on cardiovascular cells. The vitamin D endocrine system generates mostly positive direct (on cells of the vascular wall and cardiomyocytes) or indirect effects regulating the production of pro/antithrombotic/fibrinolytic factors. PAI-1, Plasminogen activator inhibitor 1; BNP,brain natriuretic peptide.
Figure 11.
VDR-vitamin D endocrine system and the renin-angiotensin system. 1,25-(OH)2D directly down-regulates the renal production of renin and thereby regulates the renin-angiotensin system, systemic blood pressure, and cardiac function.
The VDR-vitamin D effects on the renin-angiotensin system may also be involved in the renoprotective effects of 1,25-(OH)2D. VDR null mice exhibit an earlier onset and more severe nephropathy after streptozotocin-induced diabetes (590). This more severe diabetic nephropathy is characterized by increased proteinuria; higher renin, angiotensin, and angiotensin receptor expression; more mesangial sclerosis; lower nephrin expression; and decreased podocyte number. These renoprotective effects of the vitamin D endocrine system were also observed in patients with chronic renal failure because vitamin D analogs were able to reduce proteinuria (591).
In normotensive (592,593) and hypertensive patients (594,595), blood pressure is inversely correlated with plasma 1,25-(OH)2D and renin concentration. Vitamin D and calcium supplementation decreased blood pressure in short-term studies (596), and 1α-hydroxylated vitamin D therapy also reduced blood pressure in hypertensive patients (597).
The VDR-vitamin D endocrine system has several other beneficial effects on cells of the cardiovascular system. In cultured monocytic cells, 1,25-(OH)2D has anticoagulant effects by up-regulation of the anticoagulant glycoprotein, thrombomodulin, and down-regulation of a key regulator of a procoagulation factor, tissue factor (598,599). In VDR null mice (placed on a high-calcium rescue diet to avoid the effects of hypocalcemia), platelet aggregation was increased and LPS-induced, multiorgan thrombosis was also enhanced. This was consistent with increased tissue factor expression in liver and kidney and decreased thrombomodulin expression in several tissues (600).
In a LPS-induced disseminated intravascular coagulation rat model, administration of 1,25-(OH)2D reduced thrombosis (601). In a phase II human trial of intermittent high-dose 1,25-(OH)2D therapy to delay metastatic prostate cancer growth, a significant reduction of thromboembolic events was reported (602). Vascular smooth muscle cells, as well as endothelial cells, are responsive to 1,25-(OH)2D. Plasminogen activator inhibitor 1 and thrombospondulin 1 gene expression is indirectly suppressed by 1,25-(OH)2D (345,603). In addition 1,25-(OH)2D enhances smooth muscle cell relaxation and cardiac myocyte contractility (345,604). Based on in vitro effects of 1,25-(OH)2D and phenotypic analysis of VDR null mice, the combined effects of 1,25-(OH)2D on cells of the vascular wall and on cardiomyocytes provide synergistic beneficial effects on cardiovascular function.
Nevertheless, it is well known that vitamin D toxicity is associated with ectopic calcification, especially of the vascular wall. This has been observed in rodents and patients exposed to high doses of exogenous vitamin D, as well as in mice with endogenous overproduction of 1,25-(OH)2D due to targeted gene silencing of CYP24A1 (33,34), FGF23 (227), and Klotho (231) (Table 6). These ectopic calcifications are also observed in the kidney and other soft tissues, leading to organ failure and early lethality. The ectopic calcifications are partially a passive phenomenon due to oversaturation of Ca × P concentration, but in some tissues (e.g., vascular wall) a true cellular transformation of mesenchymal cells into osteoblast-like cells is induced by excess 1,25-(OH)2D or excess serum phosphate and membrane phosphate transporter Pit 1 (605). Of course, atherosclerotic intimal and medial artery as well as valvular calcifications can also be induced by a wide variety of factors, including exposure to oxidized LDL, inflammatory cytokines, and other proteins that enhance net calcium deposition. Treatment of secondary hyperparathyroidism with active vitamin D metabolites is frequently associated with increased risk for ectopic and, especially, vascular calcifications, because of potential direct effects of 1,25-(OH)2D on vascular calcifications and indirect effects on increasing the Ca × P product. Although this risk is real and the Ca × P product should be carefully monitored, treatment of severe secondary hyperparathyroidism with large doses of vitamin D may actually decrease vascular calcifications (606).
Table 6.
Vitamin D toxicity
| Major symptoms |
| Hypercalciuria, hypercalcemia, and hyperphosphatemia |
| Nephrocalcinosis → kidney failure |
| Ectopic (including vascular and valvular) calcifications |
| Premature death |
| Origin |
| 1. Exogenous vitamin D excess (25-OHD ≫100 ng/ml) |
| 2. Endogenous 1,25-(OH)2D overload by |
| a CYP24A1 gene deletion |
| a FGF23 gene deletion |
| → Renal phosphate reabsorption ↓ → serum P ↑ |
| → CYP27B1 ↑ → 1,25-(OH)2D ↑ → serum Ca ↑ |
| → Vascular calcifications |
| Nephrocalcinosis |
| Accelerated ageing |
| Decreased survival |
| a Klotho gene deletion |
| → FGF23 resistance |
| → Phenotype similar to FGF23 null mice |
| → Decreased survival |
Many abnormalities abolished by crossing with VDR or 1α-hydroxylase null mice. →, leading to; ↓, decrease; and ↑, increase.
Several clinical studies link mild vitamin D deficiency with increased cardiovascular risk (factors) such as hypertension, obesity, type 2 diabetes, and metabolic syndrome and even vascular calcifications (607,608,609,610,611). The largest prospective intervention trial (WHI) with calcium (1 g/d) and vitamin D (400 IU/d), however, revealed no change in coronary or cerebrovascular risk after 7 yr of follow-up (612). Patients with chronic renal failure on maintenance hemodialysis are highly vulnerable to diffuse vascular calcifications and are frequently deficient in both 25-OHD and 1,25-(OH)2D. Very large retrospective studies in Japanese and U.S. chronic renal failure patients, subsequently confirmed in smaller studies in other populations, revealed a survival benefit, especially cardiovascular, from treatment with a VDR agonist (613,614). Other observations in vitamin D-deficient patients with chronic renal failure confirm that 25-OHD deficiency is associated with atherosclerotic lesions, characterized by increased arterial stiffening and reduced flow-mediated dilatation (615).
In summary, based on in vitro cellular studies and observations in VDR and 1α-hydroxylase null mice, VDR or vitamin D deficiency seems to predispose to cardiovascular and metabolic risks. Cross-sectional studies and some prospective studies in humans also suggest such a link, but no prospective controlled trials have yet confirmed such causal beneficial effects of the vitamin D endocrine system. Moreover, endogenous or exogenous vitamin D excess can cause ectopic soft tissue calcifications resulting in severely impaired survival. Thus, both deficiency and excess are to be avoided, and the true therapeutic window of vitamin D metabolites, with regard to cardiovascular risk factors, has yet to be defined.
VIII. VDR-Vitamin D Endocrine System and Muscle
The VDR is expressed in muscle, especially during the early myoblast and myotube stages of muscle development, whereas its expression is much lower in fully mature muscle cells. Severe vitamin D deficiency, such as in rickets or osteomalacia and in chronic renal failure, is associated with muscle weakness or myopathy. It is unclear whether this is a direct or indirect consequence of lack of vitamin D action (616). This question has been addressed in VDR null mice on both normal and rescue diets (617). Muscle fibers (type I and type II) of all striated muscle are significantly smaller in VDR null mice (−20%), suggesting effects during a late stage of muscle development. This was confirmed by gene and protein expression analysis. Markers of early muscle development (myogenic transcription factors such as myf5, E2A, and myogenin) are still present in striated muscle of 3-wk-old VDR null mice, but not in WT muscle. Similarly, neonatal and embryonic myosin heavy chain isoforms remain much longer expressed in the cytoplasm of small muscle fibers of VDR null mice. These observations were present in normocalcemic VDR null mice, indicating a direct vitamin D–VDR effect. These genes (myf5, myogenin, and neonatal myosin heavy chain isoform) were all down-regulated by 1,25-(OH)2D in a myocyte cell line (617).
Myf-5/myo D double null mice show complete absence of skeletal muscle, and myogenin/MRF4 double null mice have a major deficit in the formation of mature multinucleated myotubes. It is unclear why VDR null muscle cells express more Myf- 5 and myogenin and yet have decreased muscle mass and size, but this nevertheless indicates that the vitamin D-VDR endocrine system is important for striated muscle. Apart from the genomic VDR-mediated effects, several rapid nongenomic effects of 1,25-(OH)2D on muscle cells have been reported, with rapid transient changes in intracellular calcium concentrations and other intracellular signaling pathways (151,618).
These findings may be relevant for the myopathy associated with severe vitamin D deficiency of rickets or renal osteodystrophy patients. Sarcopenia, or progressive loss of muscle mass and strength, is frequently seen in the elderly in association with vitamin D deficiency (619,620,621). Vitamin D supplementation of vitamin D-deficient elderly subjects has a modest effect on muscle function, body sway, and frequency of falls (622,623,624,625,626). The overall effect of vitamin D on falls is, however, modest and inconsistent (627) and cannot be fully dissociated from the frequent concomitant administration of calcium supplements. The combined supplementation with vitamin D and calcium clearly reduces the risk of fractures, according to several meta-analyses (628,629).
In conclusion, severe vitamin D deficiency in patients with rickets, osteomalacia, or renal osteodystrophy is frequently associated with myopathy. Milder vitamin D deficiency in the elderly is associated with decreased muscle mass and strength, and predisposes to falls. In vitro studies, especially muscle phenotyping of VDR null mice, suggest a direct effect of the VDR-vitamin D endocrine system on the later stages of muscle cell development and thus could potentially explain the observations in vitamin D-deficient patients. However, the molecular and cellular details of the mode of action of 1,25-(OH)2D on striated muscle are scarce.
IX. VDR-Vitamin D Endocrine System and Brain
VDR and key enzymes of vitamin D metabolism are expressed in nearly all regions of the rodent brain (630). The functionality of the vitamin D endocrine system has been demonstrated in vitro using neuronal or glial cell culture because 1,25-(OH)2D is a potent inducer of nerve growth factor, glial cell line-derived neurotropic factor, and neurotropin 3 (631,632,633). VDR null mice show abnormal behavior, especially muscle and motor behavior, but normal cognition, as evaluated by a wide variety of more than 40 behavioral screening tests (634). Because these animals were raised on a normal calcium intake and therefore developed hypocalcemia and growth delay, an indirect effect of hypocalcemia and muscle weakness on motor behavior cannot be excluded. Another extensive set of experiments, performed in Tuohimaa’s laboratory, revealed that VDR null mice on a normal calcium diet have an abnormal grooming pattern (higher frequency of grooming, but with incorrect transition and increased interruption of grooming behavior) (635,636). They confirmed impaired motor performance (637) and suggested that VDR null mice display an increased anxiety behavior (638). Most other behavioral tests including mating behavior were normal.
Simple vitamin D deficiency during early life also has neurobehavioral effects because brains of newborn rats born to vitamin D-deficient mothers are larger, with larger lateral ventricles, increased brain cell proliferation, yet decreased cortical brain thickness (639). At a molecular level, the expression of nerve growth factor and glial cell line-derived neurotrophic factor was decreased in vitamin D-deficient newborn rats. These effects were not transient because at 10 wk of age, the lateral ventricles were still enlarged and the expression of genes involved in neuronal function or structure (e.g., nerve growth factor, neurofilament, and γ-aminobutyric acid-A) were lower in brains of rats that were transiently vitamin D deficient during early development (640,641). A detailed proteomics analysis of prefrontal and hippocampus areas of brains from rats exposed to transient pre- and perinatal vitamin D deficiency identified dysregulation of more than 30 proteins involved in a variety of cellular functions. Half of these proteins are known to be misexpressed in schizophrenia or multiple sclerosis (642). A more detailed functional analysis of late consequences of developmental vitamin D deficiency in rats suggests persistent mild learning and memory dysfunction (643) and drug-induced hyperlocomotion (an animal model of schizophrenia) (644). Other mild behavioral abnormalities were found in two different strains of mice born to vitamin D-deficient mothers raised on a vitamin D-replete diet after weaning (645). The neuroprotective effects of 1,25-(OH)2D have also been demonstrated in rodent models of stroke and 6-hydroxy-dopamine toxicity (646,647). The rodent data thus indicate that pre/perinatal VDR-vitamin D deficiency can result in long-lasting changes in gene and protein expression in the brain and may be associated with variable, subtle, structural and behavioral abnormalities.
The human equivalent of vitamin D effects on early brain development has not been fully explored. Low levels of 25-OHD in pregnant mothers has been associated with increased risk of schizophrenia of their children (648). Retrospective analysis of the effects of vitamin D supplements during the first year of life in northern Finland revealed that regular or intermittent vitamin D supplements (especially ≥2000 IU/d) were associated with a reduced risk (RR, 0.23) of schizophrenia in males but not in females (at the age of 31 yr) (648). The absolute number of cases was too small to allow firm conclusions about the association with vitamin D deficiency, and thus additional studies are required. Low vitamin D status is also frequently observed in patients with Alzheimer’s disease and schizophrenia and in elderly subjects with cognitive dysfunction (649).
Thus, the rodent and human data on the effect of the VDR-vitamin D endocrine system on brain and behavioral function are far from conclusive in terms of a causal relationship, but nevertheless are sufficiently hypothesis generating to warrant further cellular, animal, and human studies.
X. VDR-Vitamin D Endocrine System and Reproduction
Not only did the first strain of VDR null mice develop the expected bone and hair phenotype, but female mice also showed uterine hypoplasia and impaired ovarian folliculogenesis (155). The fertility of vitamin D-deficient female rats was decreased by 75% (650). This reduced fertility persisted in vitamin D-deficient, yet calcium-repleted rats, suggesting a direct effect of vitamin D rather than an indirect effect mediated by hypocalcemia (651).
A possible mechanism may be the direct stimulatory effect of 1,25-(OH)2D on the aromatase gene expression in reproductive tissues of female and male mice (652,653). It is notable that male fertility was also reduced, as demonstrated by reduced sperm number and mobility and histological changes in testicular morphology (652). LH and FSH serum levels were increased in these mice, indicating primary hypergonadotropic hypogonadism. Supplementation with estradiol corrected the reproductive phenotype of VDR null mice, whereas partial correction of calcium homeostasis by a high-calcium diet was only partially effective (652). These data all indicate a direct effect of the VDR-vitamin D endocrine system on female and male reproduction. These observations are consistent with the presence of the VDR in reproductive tissues, including human sperm (654). Subsequent studies using the same Tokyo VDR null mice confirmed a lower reproductive efficacy in mice fed a normal or low-calcium diet, whereas on a high-calcium/lactose diet reproduction normalized (655), implying that hypocalcemia is the major driving factor for reduced fertility. The reduced conception rate (−90%) in VDR null mice was also confirmed in the Boston strain, but again, a rescue diet normalized the conception rate (196).
The effects of maternal VDR deficiency on fetal and postnatal development have been evaluated in vitamin D- and 1α-hydroxylase-deficient animals and VDR null mice. On a normal calcium diet, fetuses from these animals are growth retarded and usually, but not always, have abnormal serum calcium levels and skeletal mineralization defects (196,656,657,658). When abnormalities were observed in fetuses born from vitamin D-deficient or VDR null mothers, they were rescued by normalization of serum 1,25-(OH)2D and/or calcium levels (657,658). A more detailed analysis revealed that VDR null pups from VDR null mothers showed a more normal development than heterozygote VDR pups. These heterozygous pups responded to excess of 1,25-(OH)2D with decreased bone mineralization and early postnatal mortality (659).
The human equivalent of VDR or 1α-hydroxylase deficiency on reproduction is poorly studied. The effects of severe or even mild vitamin D deficiency on human reproduction are also poorly studied. However, vitamin D deficiency is quite frequent in pregnancy, frequently persists during lactation, and thus is not an absolute obstacle to successful reproduction.
Vitamin D deficiency during pregnancy has also been associated with an increased risk of preeclampsia (660,661), based on a large nested case-control study. This is reminiscent of the effects of VDR or vitamin D deficiency on systemic hypertension (see Section VII). Therefore, further studies are desirable in pregnant VDR or 1α-hydroxylase null mice, as well as intervention studies with vitamin D supplementation in pregnant women, especially in view of the high prevalence of vitamin D deficiency during pregnancy (662,663,664). Moreover, the long-term consequences of the prenatal and early postnatal effects of vitamin D deficiency might be very serious because developmental vitamin D deficiency is associated with decreased bone mass (665), enhanced risk for autoimmune disease (such as type 1 diabetes; see Section V), and brain dysfunction (see Section IX).
XI. VDR-Vitamin D Endocrine System and Adipocytes
Fat cells are very active endocrine cells that express the VDR and could, therefore, be potential targets for vitamin D action. VDR null mesenchymal cells from bone marrow express increased mRNA levels of peroxisome proliferator-activated receptor γ and other markers of adipogenic differentiation when cultured under adipogenic conditions (204). This is not totally unexpected because mesenchymal stem cells are preferentially oriented toward the osteoblastic lineage when exposed to 1,25-(OH)2D, whereas adipogenesis is impaired (666,667). This 1,25-(OH)2D –VDR effect on adipogenesis is mediated by down-regulation of C/EBPβ, DKK1, SFRP2 and, thus, enhancement of the canonical Wnt signaling pathway.
The in vivo effects of VDR or CYP27B1 deficiency on total body composition (muscle/fat/bone) independent from serum calcium homeostasis has not yet been fully explored.
XII. General Conclusions and Perspectives
The VDR-vitamin D endocrine system originated during the evolution of vertebrates, shortly before their skeletons became calcified. Its best known action is the regulation of extracellular calcium and phosphate homeostasis, especially by increasing the absorption or reabsorption of calcium at nearly all epithelia involved in calcium transport. The consequences of vitamin D deficiency or loss of vitamin D action (by a deficit in its supply, metabolic activation, or inactivation of its NR) generates a bone, growth plate, and tooth phenotype known as rickets (before end of puberty) or osteomalacia. The bone/growth plate/tooth phenotype of mice with genetically engineered lack of vitamin D activation (1α-hydroxylase or CYP27B1 null mice) or activity (VDR null mice) is, in all aspects, identical to the phenotype of humans with similar disorders or severe vitamin D deficiency. The growth plate of VDR or 1α-hydroxylase-deficient animals or men is structurally abnormal by widening of the hypertrophic chondrocytes and deficient mineralization of metaphyseal bone. Low levels of extracellular calcium and phosphate impair bone mineralization, whereas osteoclast and especially osteoblast activity is altered, resulting in undermineralization of bone and teeth. This explains, to a large extent, the clinical picture of rickets, characterized by stunted growth, enlargement of wrists and ribs, and decreased bone strength with bowing of weight bearing bones. The molecular and cellular mechanisms involved in the etiology of this bone/growth plate/tooth development are complex. However, normalization of this phenotype in mice or men with 1α-hydroxylase or VDR deficiency by a high calcium and lactose intake or by selective rescue of the VDR in the intestine of VDR null mice provides very strong arguments for the intestinal epithelium as the primary target for vitamin D action. Several gene products involved in transepithelial calcium transport are strongly vitamin D-dependent, but it seems that the three major candidates (TRPV5/6; CaBP-9/28k and PMCA pumps) cannot fully explain the molecular mechanism of 1,25-(OH)2D-VDR dependent calcium transport. The hunt for the key vitamin D-dependent genes for the most important vitamin D action is therefore still ongoing.
The VDR-vitamin D endocrine system is also functional in other calcium transporting or calcium-sensing tissues such as the kidney, all bone cells including growth plate chondrocytes, and parathyroid glands, with less convincing or absent effects on calcium transport in the placenta or mammary gland (Table 7). Due to the dominant effect of VDR/1α-hydroxylase deficiency on intestinal calcium and phosphate transport, it will require the generation and phenotypic analysis of tissue-specific transgenic animals to define the possible direct effects of the vitamin D endocrine system on other tissues involved in global calcium homeostasis (e.g., selective deletion or rescue of VDR in bone, kidney, or parathyroid cells). Selective VDR deletion in growth plate chondrocytes already demonstrated its redundancy for the growth plate itself but revealed a potential endocrine function of the growth plate VDR on bone and kidney.
Table 7.
Overview of the VDR null mouse phenotype
| Phenotype
|
|||
|---|---|---|---|
| Total VDR deletion | Total VDR deletion on rescue diet | Tissue-specific deletion/transgene | |
| Intestine | Impaired (active) calcium absorption | Not available | VDR-calbindin rescue, tissue-specific KO: rescue of bone phenotype in progress |
| Growth plate | Ricketic hypertrophy | Normal | Chondrocyte-specific KO: no growth plate phenotype increased bone mass |
| Bone | Impaired mineralization | Normal | Osteoblast specific KO: phenotype analysis in progress |
| Kidney | Relative hypercalciuria | Hypercalciuria | Not available |
| Parathyroid gland | Hyperparathyroidism | Normal | Tissue-specific KO: phenotype analysis in progress |
| Skin | |||
| Epithelium | Impaired differentiation | Normal | Not available |
| Impaired barrier function (CYP27B1 KO) | |||
| Hair follicle | Total alopecia | Total alopecia | Keratinocyte-specific VDR rescue: normalization of hair growth |
| Immune system | |||
| Innate (monocytes) | Impaired macrophage function | Resistance to Leishmania infection | Not available |
| Acquired | Resistance to LDS-induced diabetes | Prone to LDS-induced diabetes | Not available |
| Normal susceptibility to type 1 diabetes | More susceptible to IBD | ||
| Increased resistance to EAE | |||
| Mast cells | Increased activity | Not available | Not available |
| Allergy/asthma | Not available | Resistance to airway inflammation | Not available |
| Cell proliferation/cancer | Not available | Increased risk of chemocarcinogen or oncogen mediated cancer | Not available |
| Mammary gland | Not available | During puberty accelerated growth and branching morphogenesis, abnormal ductal morphological features, accelerated mammary gland development during pregnancy and delayed postlactational involution | Not available |
| Cardiovascular system | High renin hypertension | Idem | Not available |
| Cardiac hypertrophy | Idem | ||
| Increased thrombogenicity | Idem | ||
| Muscle | Impaired maturation of striated muscle | Idem | Not available |
| Brain | Subtle behavioral modification (grooming, maternal behavior, etc.) | Not available | Not available |
| Reproduction | |||
| Males | Reduced fertility | (Near) normal | Not available |
| Females | Reduced fertility | Impaired fertility in Tokyo KO | Not available |
LDS, Low-dose streptozotocin; IBD, inflammatory bowel disease; idem, phenotype identical to phenotype of total VDR deletion without a reserve diet.
The widespread presence of VDR in nearly all cells and tissues, the nearly equally widespread presence of vitamin D-metabolizing enzymes, and the very large number of genes (estimated at about 3% of the mouse or human genome) that are directly and/or indirectly responsive to VDR-1,25-(OH)2D all point toward a role for this endocrine system well beyond extracellular calcium and phosphate homeostasis (Table 7). This is, in fact, not the exception but the rule because nearly all ligands for NRs have a wide spectrum of activities beyond their primary targets (e.g., estrogens, androgens, glucocorticoids, thyroid hormones, retinoids, and peroxisome proliferator-activated receptor γ ligands).
The skin is the most obvious “noncalcemic” target for the VDR-vitamin D endocrine system because it was known from human vitamin D-resistant rickets (type II due to VDR mutations) that acquired generalized alopecia is part of the phenotype. This was confirmed in all VDR null mouse strains. Their hair cycle phenotype is purely dependent on VDR and not on the vitamin D ligand because it cannot be reproduced by vitamin D or 1α-hydroxylase deficiency. This is a first clear example of discordance between VDR and 1,25-(OH)2D deficiency and reminiscent of other examples in the NR field (e.g., thyroid hormone receptor). The molecular mechanisms that lead to alopecia are only partially understood and involve protein-protein interactions between VDR, RXR, hairless, and components of the wnt signaling. Although normocalcemic VDR and 1α-hydroxylase null mice do not develop a skin or epidermis phenotype beyond alopecia, they show, nevertheless, mild defects in the repair of the essential barrier function of their skin after acute skin injury.
The vitamin D hormone, 1,25-(OH)2D, is a potent inhibitor of cell cycle progression, with predominant effects on G0/G1 cell cycle arrest. This antiproliferative effect is coordinated by direct and indirect control of a very large number of genes involved in DNA replication and cell cycle progression. VDR null mice should, therefore, be more prone to develop cancer and, indeed, they develop more tumors or premalignant lesions when exposed to oncogenes or chemocarcinogens. Moreover, there are strong epidemiological links between vitamin D deficiency and a wide variety of common cancers in humans, especially of colon, breast, and prostate. All the cells (APCs and monocyte/macrophages, T and B cells, and mast cells) of the immune system express VDR either in the basal state or after appropriate immune stimuli. Some immune cells (especially monocytic cells) express CYP27B1 after exposure to combined immune stimuli. In the basal state, VDR or 1α-hydroxylase null mice do not show a major immune phenotype at least after normalization of serum calcium homeostasis, although there are many subtle cellular and molecular defects in their immune system. VDR- and/or vitamin D-deficient mice are, however, more prone to develop autoimmune diseases such as inflammatory bowel disease or type 1 diabetes. In contrast to the hyperreactive acquired immune system, the native immune defense system seems to be hyporeactive in vitamin D-resistant or -deficient animals and humans, and this may predispose to (bacterial) infections. Little is known about the immune abnormalities in humans with genetic defects in vitamin D action, but there are many suggestions for an increased prevalence of major autoimmune diseases (especially type 1 diabetes and multiple sclerosis) in vitamin D-deficient subjects, especially when deficiency is present during development (diabetes) or before adulthood (multiple sclerosis).
A wide spectrum of observations links vitamin D deficiency or resistance with increased cardiovascular risks. VDR and 1α-hydroxylase-deficient mice overexpress renin, and this leads to systemic hypertension and cardiac hypertrophy. VDR null mice are also more prone to diabetes-induced renal damage. VDR null mice also develop more inflammation-induced multiorgan thrombosis, probably related to an increased expression of prothrombotic and reduced expression of antithrombotic or fibrinolytic factors. 1,25-(OH)2D exerts beneficial effects on several cells of the vascular wall such as endothelial and smooth muscle cells. In line with these animal data, vitamin D deficiency in humans is associated with increased incidence of cardiovascular risk factors and metabolic syndrome, but causality has yet to be demonstrated by appropriate intervention studies. Nevertheless, vitamin D toxicity either due to exogenous vitamin D (metabolite) overdosing or by endogenous overproduction (e.g., by deletion of CYP24A1, FGF23, or Klotho gene) is mainly due to ectopic and, especially, vascular and valvular calcifications and subsequent organ failure. Therefore, vitamin D deficiency, as well as vitamin D excess, has negative cardiovascular effects, demonstrating the need for carefully optimizing the exposure to vitamin D.
VDR null mice, even when normocalcemic, show signs of impaired development of striated muscle. This may be reminiscent of the effects of severe vitamin D deficiency on muscle weakness, as seen in patients with rickets, osteomalacia, or chronic renal failure.
VDR is expressed in most brain areas, but VDR null mice do not have an obvious neurological phenotype. However, VDR null mice display a variety of subtle behavioral abnormalities such as abnormal grooming pattern and maternal behavior. Finally, VDR null mice have impaired male and female reproduction, which is correctable by a high-calcium diet in some, but not all, knockout (KO) strains.
The comparison of the actions of VDR and its natural ligands [1,25-(OH)2D and maybe lithocholic acid] revealed, as for other NRs, that VDR has some ligand-independent actions (e.g., alopecia in VDR but not in 1α-hydroxylase null mice; differences in immune effects between VDR and vitamin D deficiency). Moreover, some VDR-vitamin D endocrine effects may be limited to specific time periods, such as the action on thymic T cell or brain cell programming during late embryonic development, whereas for other tissues VDR becomes only activated at the end of weaning (e.g., intestinal calcium absorption). The generation of tissue- or cell-specific but also developmental stage-specific VDR transgenic mice may help to elucidate such phenomena.
The bone/growth plate/tooth phenotypes of human and mouse VDR or 1α-hydroxylase deficiency are perfectly overlapping. This raises the question whether this also applies to the spectrum of noncalcemic effects of the VDR-vitamin D endocrine system. This is certainly the case for the acquired total alopecia observed in case of VDR resistance. There are many similarities between the immune, cardiovascular, and muscle phenotype of VDR-resistant mice and observational data in humans with vitamin D deficiency. This also applies to the antiproliferative effects of 1,25-(OH)2D on most benign and malignant cells and the possible link between chronic vitamin D deficiency and subsequent cancer risk. Only large-scale and long-term prospective studies can reveal the clinical implications of (mild) vitamin D deficiency for major human diseases such as cancer, autoimmune disorders, and muscle or brain function. The VDR null phenotype and human epidemiological data are certainly sufficiently hypothesis generating to warrant such studies.
Acknowledgments
The authors thank all co-authors of their previous publications who contributed to the original data recorded here, with special thanks to Femke Baeke, Guy Eelen, Conny Gysemans, Ritsuko Masuyama, Lut Overbergh, and Sophie Van Cromphaut (Laboratory for Experimental Medicine and Endocrinology, Leuven, Belgium), and special thanks also to Yanchun Li, Cindy Chen, Kristi Skorija, Yoshiyuki Sakai, Megan Cox, and Luisella Cianferotti (Boston Laboratory, Boston, MA).
Footnotes
The work in the authors’ laboratories was supported by the University of Leuven (GOA/04/10, EF/05/007), Flanders Research Foundation [Fonds voor Wetenschappelijk onderzoek Vlaanderen (FWO), Grants G.0084.02, G.0233.04, G.0508.05, G.0552.06, G.0553.06, and G.0649.08], the Juvenile Diabetes Research Foundation (JDRF; Grant 4-2005-1237), and the Belgium Program on Interuniversity Poles of Attraction initiated by the Belgian State (Grants IUAP P5/17 and P6/34). C.M. is holder of a Clinical Research Fellowship (FWO, Belgium), E.v.E. holds a Postdoctoral Fellowship (JDRF, 3-2006-33), and L.L. is a fellow of the IWT-Flanders. The work in Boston was supported by National Institutes of Health Grant DK46974.
Disclosure Statement: G.C., L.V., E.v.E., A.V., H.F.L., L.L., C.M., and M.D. have nothing to disclose. R.B. received consulting fees (Lilly, Tibotec, BioXell) and lecture fees (Merck & Co, Amgen) and has intellectual property rights on a university patent on vitamin D analogs licensed to Hybrigenix, France.
First Published Online August 11, 2008
Abbreviations: AF-2, Activation function-2; APC, antigen-presenting cell; CaBP, calcium binding protein; CAMP, human cathelicidin antimicrobial peptide; CYP, cytochrome P450; DBD, DNA binding domain; DBP, vitamin D binding protein; DC, dendritic cell; 7-DHC, 7-dehydrocholesterol; DMBA, 7,12-dimethylbenzanthracene; DRIP, vitamin D receptor interacting protein; EAE, experimental autoimmune encephalomyelitis; EGF, epidermal growth factor; FGF23, fibroblast growth factor 23; HAT, histone acetyltransferase; KO, knockout; LPS, lipopolysaccharide; MHC, major histocompatibility complex; Na/Pi, natrium-dependent phosphate cotransporter; NCX1, Na+/Ca2+ exchanger; NFAT, nuclear factor of activated T cells; NFκB, nuclear factor κB; NOD, nonobese diabetic; NR, nuclear receptor; nVDRE, negative VDRE; 1,25-(OH)2D, 1,25-dihydroxyvitamin D3 or 1,25-dihydroxyvitamin D2; 25-OHD, 25-hydroxyvitamin D3 or 25-hydroxyvitamin D2; PMCA1b, plasma membrane calcium ATPase; PSA, prostate-specific antigen; RXR, retinoid X receptor; SRC, steroid receptor coactivator; Th, T helper cell; TLR, Toll-like receptor; TRAP, T3 receptor auxiliary protein; TRPV, transient receptor potential vanilloid; VDR, vitamin D receptor; VDRE, vitamin D responsive element; WT, wild-type.
References
- Tausk M 1945 Driehonderd jaar rachitis onderzoek. De Engelsche ziekte in de geneeskunde voorheen en thans. 1645–1945. Uitgave der N.V. Organon-OSS [Google Scholar]
- Ebstein W 1908 Über das Vorkommen rachitischer Skelettveränderungen im Altertum und im Mittelalter. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin (Berlin) 193:519 [Google Scholar]
- Chick H, Palzell EJ, Hume EM 1923 Studies of rickets in Vienna 1919–1922. Special Report 77. Medical Research Council [Google Scholar]
- Hess A 1922 Influence of light on the prevention of rickets. Lancet 2:1222 [Google Scholar]
- Huldshinsky K 1919 Heilung von Rachitis durch künstliche Höhensonne. Dtsch Med Wochenschr 45:712–713 [Google Scholar]
- McCollum EV, Simmonds N, Pitz W 1916 The relation of unidentified dietary factors, the fat-soluble A and water-soluble B of the diet to the growth promoting properties of milk. J Biol Chem 27:33–38 [Google Scholar]
- Mellanby E, Cantag MD 1919 Experimental investigation on rickets. Lancet 196:407–412 [Google Scholar]
- Park EA 1923 The etiology of rickets. Physiol Rev 3:106–163 [Google Scholar]
- Windaus A, Linsert O 1928 Vitamin D1. Ann Chem 465:148 [Google Scholar]
- Bikle DD 1992 Clinical counterpoint: vitamin D—new actions, new analogs, new therapeutic potential. Endocr Rev 13:765–784 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Pillai S 1993 Vitamin D, calcium, and epidermal differentiation. Endocr Rev 14:3–19 [DOI] [PubMed] [Google Scholar]
- Bouillon R, Okamura WH, Norman AW 1995 Structure-function relationships in the vitamin D endocrine system. Endocr Rev 16:200–257 [DOI] [PubMed] [Google Scholar]
- Bouillon R 2005 Vitamin D: from photosynthesis, metabolism, and action to clinical applications. In: DeGroot LJ, Jameson JL, eds. Endocrinology. Vol 2. Philadelphia: Elsevier Saunders; 1435–1463 [Google Scholar]
- Brommage R, DeLuca HF 1985 Evidence that 1,25-dihydroxyvitamin D3 is the physiologically active metabolite of vitamin D3. Endocr Rev 6:491–511 [DOI] [PubMed] [Google Scholar]
- DeLuca HF 2004 Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80:1689S–1696S [DOI] [PubMed] [Google Scholar]
- Fraser DR 1980 Regulation of the metabolism of vitamin D. Physiol Rev 60:551–613 [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR 2001 Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord 2:203–216 [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Pike JW, Feldman D 1999 The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev 20:156–188 [DOI] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R 2005 Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26:662–687 [DOI] [PubMed] [Google Scholar]
- Norman AW, Roth J, Orci L 1982 The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev 3:331–366 [DOI] [PubMed] [Google Scholar]
- Norman AW 2006 Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147:5542–5548 [DOI] [PubMed] [Google Scholar]
- Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW 2004 Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 101:7711–7715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Davis DL, Dahlback H, Jornvall H, Russell DW 1989 Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem 264:8222–8229 [PubMed] [Google Scholar]
- Fraser DR, Kodicek E 1970 Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature 228:764–766 [DOI] [PubMed] [Google Scholar]
- Bikle DD 2007 What is new in vitamin D: 2006–2007. Curr Opin Rheumatol 19:383–388 [DOI] [PubMed] [Google Scholar]
- Jones G 2007 Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1α-hydroxylase in the classical and nonclassical actions of 1α,25-dihydroxyvitamin D(3). Semin Dial 20:316–324 [DOI] [PubMed] [Google Scholar]
- Overbergh L, Stoffels K, Waer M, Verstuyf A, Bouillon R, Mathieu C 2006 Immune regulation of 25-hydroxyvitamin D-1α-hydroxylase in human monocytic THP1 cells: mechanisms of interferon-γ-mediated induction. J Clin Endocrinol Metab 91:3566–3574 [DOI] [PubMed] [Google Scholar]
- Prader A, Illig R, Heierle E 1961 [An unusual form of primary vitamin D-resistant rickets with hypocalcemia and autosomal-dominant hereditary transmission: hereditary pseudo-deficiency rickets.]. Helv Paediatr Acta 16:452–468 [PubMed] [Google Scholar]
- St Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH 1997 The 25-hydroxyvitamin D 1-α-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J Bone Miner Res 12:1552–1559 [DOI] [PubMed] [Google Scholar]
- Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S 1997 25-Hydroxyvitamin D3 1α-hydroxylase and vitamin D synthesis. Science 277:1827–1830 [DOI] [PubMed] [Google Scholar]
- Prosser DE, Jones G 2004 Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29:664–673 [DOI] [PubMed] [Google Scholar]
- St Arnaud R 1999 Targeted inactivation of vitamin D hydroxylases in mice. Bone 25:127–129 [DOI] [PubMed] [Google Scholar]
- St Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH 2000 Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology 141:2658–2666 [DOI] [PubMed] [Google Scholar]
- Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW, Pinkel D 2000 Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet 25:144–146 [DOI] [PubMed] [Google Scholar]
- Wjst M, Altmuller J, Faus-Kessler T, Braig C, Bahnweg M, Andre E 2006 Asthma families show transmission disequilibrium of gene variants in the vitamin D metabolism and signalling pathway. Respir Res 7:60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M 2005 Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem 280:20604–20611 [DOI] [PubMed] [Google Scholar]
- Singh RJ, Taylor RL, Reddy GS, Grebe SK 2006 C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 91:3055–3061 [DOI] [PubMed] [Google Scholar]
- Van Baelen H, Bouillon R, De Moor P 1980 Vitamin D-binding protein (Gc-globulin) binds actin. J Biol Chem 255:2270–2272 [PubMed] [Google Scholar]
- Verboven C, Rabijns A, De Maeyer M, Van Baelen H, Bouillon R, De Ranter C 2002 A structural basis for the unique binding features of the human vitamin D-binding protein. Nat Struct Biol 9:131–136 [DOI] [PubMed] [Google Scholar]
- Cooke NE, Haddad JG 1989 Vitamin D binding protein (Gc-globulin). Endocr Rev 10:294–307 [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE 1999 An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96:507–515 [DOI] [PubMed] [Google Scholar]
- Brumbaugh PF, Haussler MR 1975 Specific binding of 1α,25-dihydroxycholecalciferol to nuclear components of chick intestine. J Biol Chem 250:1588–1594 [PubMed] [Google Scholar]
- Holick MF, Schnoes HK, DeLuca HF, Suda T, Cousins RJ 1971 Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry 10:2799–2804 [DOI] [PubMed] [Google Scholar]
- Lawson DE, Fraser DR, Kodicek E, Morris HR, Williams DH 1971 Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature 230:228–230 [DOI] [PubMed] [Google Scholar]
- Norman AW, Myrtle JF, Midgett RJ, Nowicki HG, Williams V, Popjak G 1971 1,25-Dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine. Science 173:51–54 [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O'Malley BW 1987 Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science 235:1214–1217 [DOI] [PubMed] [Google Scholar]
- Clemens TL, Garrett KP, Zhou XY, Pike JW, Haussler MR, Dempster DW 1988 Immunocytochemical localization of the 1,25-dihydroxyvitamin-D3 receptor in target cells. Endocrinology 122:1224–1230 [DOI] [PubMed] [Google Scholar]
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW 1998 The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J Bone Miner Res 13:325–349 [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, Clark SA, DeLuca HF 1982 Brain target sites for 1,25-dihydroxyvitamin D3. Science 215:1403–1405 [DOI] [PubMed] [Google Scholar]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ 2005 Distribution of the vitamin D receptor and 1 α-hydroxylase in human brain. J Chem Neuroanat 29:21–30 [DOI] [PubMed] [Google Scholar]
- Gronemeyer H, Laudet V 1995 Transcription factors 3: nuclear receptors. Protein Profile 2:1173–1308 [PubMed] [Google Scholar]
- Moore DD, Kato S, Xie W, Mangelsdorf DJ, Schmidt DR, Xiao R, Kliewer SA 2006 International Union of Pharmacology. LXII. The NR1H and NR1I receptors: constitutive androstane receptor, pregnene X receptor, farnesoid X receptor α, farnesoid X receptor β, liver X receptor α, liver X receptor β, and vitamin D receptor. Pharmacol Rev 58:742–759 [DOI] [PubMed] [Google Scholar]
- Rochel N, Moras D 2006 Ligand binding domain of vitamin D receptors. Curr Top Med Chem 6:1229–1241 [DOI] [PubMed] [Google Scholar]
- Whitfield GK, Dang HT, Schluter SF, Bernstein RM, Bunag T, Manzon LA, Hsieh G, Dominguez CE, Youson JH, Haussler MR, Marchalonis JJ 2003 Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology 144:2704–2716 [DOI] [PubMed] [Google Scholar]
- Robinson-Rechavi M, Carpentier AS, Duffraisse M, Laudet V 2001 How many nuclear hormone receptors are there in the human genome? Trends Genet 17:554–556 [DOI] [PubMed] [Google Scholar]
- Sunn KL, Cock TA, Crofts LA, Eisman JA, Gardiner EM 2001 Novel N-terminal variant of human VDR. Mol Endocrinol 15:1599–1609 [DOI] [PubMed] [Google Scholar]
- Esteban LM, Fong C, Amr D, Cock TA, Allison SJ, Flanagan JL, Liddle C, Eisman JA, Gardiner EM 2005 Promoter-, cell-, and ligand-specific transactivation responses of the VDRB1 isoform. Biochem Biophys Res Commun 334:9–15 [DOI] [PubMed] [Google Scholar]
- Jurutka PW, Remus LS, Whitfield GK, Thompson PD, Hsieh JC, Zitzer H, Tavakkoli P, Galligan MA, Dang HT, Haussler CA, Haussler MR 2000 The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol 14:401–420 [DOI] [PubMed] [Google Scholar]
- Haussler MR 1986 Vitamin D receptors: nature and function. Annu Rev Nutr 6:527–562 [DOI] [PubMed] [Google Scholar]
- Haussler MR, McCain TA 1977 Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med 297:974–983 [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Shimizu Y, Minoshima S, Shimizu N, Haussler CA, Jurutka PW, Haussler MR 1998 Novel nuclear localization signal between the two DNA-binding zinc fingers in the human vitamin D receptor. J Cell Biochem 70:94–109 [PubMed] [Google Scholar]
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D 2000 The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell 5:173–179 [DOI] [PubMed] [Google Scholar]
- Hochberg Z 2003 Introduction. Rickets—past and present. Endocr Dev 6:1–13 [DOI] [PubMed] [Google Scholar]
- Malloy PJ, Feldman D 2003 Hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Dev 6:175–199 [DOI] [PubMed] [Google Scholar]
- Chen H, Hewison M, Hu B, Adams JS 2003 Heterogeneous nuclear ribonucleoprotein (hnRNP) binding to hormone response elements: a cause of vitamin D resistance. Proc Natl Acad Sci USA 100:6109–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Ribeiro RC, Wagner RL, Nguyen H, Apriletti JW, Fletterick RJ, Baxter JD, Kushner PJ, West BL 1998 Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280:1747–1749 [DOI] [PubMed] [Google Scholar]
- Fang Y, van Meurs JB, d'Alesio A, Jhamai M, Zhao H, Rivadeneira F, Hofman A, van Leeuwen JP, Jehan F, Pols HA, Uitterlinden AG 2005 Promoter and 3′-untranslated-region haplotypes in the vitamin D receptor gene predispose to osteoporotic fracture: the Rotterdam study. Am J Hum Genet 77:807–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejentsev S, Godfrey L, Snook H, Rance H, Nutland S, Walker NM, Lam AC, Guja C, Ionescu-Tirgoviste C, Undlien DE, Ronningen KS, Tuomilehto-Wolf E, Tuomilehto J, Newport MJ, Clayton DG, Todd JA 2004 Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet 13:1633–1639 [DOI] [PubMed] [Google Scholar]
- Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, Tonai T, Nishisho T, Mori S, Takeda E 1997 A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res 12:915–921 [DOI] [PubMed] [Google Scholar]
- Audi L, Marti G, Esteban C, Oyarzabal M, Chueca M, Gussinye M, Yeste D, Fernandez-Cancio M, Andaluz P, Carrascosa A 2004 VDR gene polymorphism at exon 2 start codon (FokI) may have influenced type 1 diabetes mellitus susceptibility in two Spanish populations. Diabet Med 21:393–394 [DOI] [PubMed] [Google Scholar]
- Capoluongo E, Pitocco D, Concolino P, Santonocito C, Di Stasio E, d'Onofrio G, Manto A, Giardina B, Ghirlanda G, Ameglio F, Zuppi C 2006 Slight association between type 1 diabetes and “ff” VDR FokI genotype in patients from the Italian Lazio region. Lack of association with diabetes complications. Clin Biochem 39:888–892 [DOI] [PubMed] [Google Scholar]
- Eerligh P, Koeleman BP, Dudbridge F, Jan BG, Roep BO, Giphart MJ 2004 Functional genetic polymorphisms in cytokines and metabolic genes as additional genetic markers for susceptibility to develop type 1 diabetes. Genes Immun 5:36–40 [DOI] [PubMed] [Google Scholar]
- Fassbender WJ, Goertz B, Weismuller K, Steinhauer B, Stracke H, Auch D, Linn T, Bretzel RG 2002 VDR gene polymorphisms are overrepresented in German patients with type 1 diabetes compared to healthy controls without effect on biochemical parameters of bone metabolism. Horm Metab Res 34:330–337 [DOI] [PubMed] [Google Scholar]
- Guja C, Marshall S, Welsh K, Merriman M, Smith A, Todd JA, Ionescu-Tirgoviste C 2002 The study of CTLA-4 and vitamin D receptor polymorphisms in the Romanian type 1 diabetes population. J Cell Mol Med 6:75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorffy B, Vasarhelyi B, Krikovszky D, Madacsy L, Tordai A, Tulassay T, Szabo A 2002 Gender-specific association of vitamin D receptor polymorphism combinations with type 1 diabetes mellitus. Eur J Endocrinol 147:803–808 [DOI] [PubMed] [Google Scholar]
- Koeleman BP, Valdigem G, Eerligh P, Giphart MJ, Roep BO 2002 Seasonality of birth in patients with type 1 diabetes. Lancet 359:1246–1247 [DOI] [PubMed] [Google Scholar]
- Nejentsev S, Cooper JD, Godfrey L, Howson JM, Rance H, Nutland S, Walker NM, Guja C, Ionescu-Tirgoviste C, Savage DA, Undlien DE, Ronningen KS, Tuomilehto-Wolf E, Tuomilehto J, Gillespie KM, Ring SM, Strachan DP, Widmer B, Dunger D, Todd JA 2004 Analysis of the vitamin D receptor gene sequence variants in type 1 diabetes. Diabetes 53:2709–2712 [DOI] [PubMed] [Google Scholar]
- Pani MA, Knapp M, Donner H, Braun J, Baur MP, Usadel KH, Badenhoop K 2000 Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes 49:504–507 [DOI] [PubMed] [Google Scholar]
- Turpeinen H, Hermann R, Vaara S, Laine AP, Simell O, Knip M, Veijola R, Ilonen J 2003 Vitamin D receptor polymorphisms: no association with type 1 diabetes in the Finnish population. Eur J Endocrinol 149:591–596 [DOI] [PubMed] [Google Scholar]
- Zemunik T, Skrabic V, Boraska V, Diklic D, Terzic IM, Capkun V, Peruzovic M, Terzic J 2005 FokI polymorphism, vitamin D receptor, and interleukin-1 receptor haplotypes are associated with type 1 diabetes in the Dalmatian population. J Mol Diagn 7:600–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitterlinden AG, Fang Y, van Meurs JB, Pols HA, van Leeuwen JP 2004 Genetics and biology of vitamin D receptor polymorphisms. Gene 338:143–156 [DOI] [PubMed] [Google Scholar]
- Schrader M, Kahlen JP, Carlberg C 1997 Functional characterization of a novel type of 1α,25-dihydroxyvitamin D3 response element identified in the mouse c-fos promoter. Biochem Biophys Res Commun 230:646–651 [DOI] [PubMed] [Google Scholar]
- Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW 2006 The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20:1447–1461 [DOI] [PubMed] [Google Scholar]
- Kim S, Shevde NK, Pike JW 2005 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res 20:305–317 [DOI] [PubMed] [Google Scholar]
- Glass CK, Rosenfeld MG 2000 The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 14:121–141 [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW 2002 Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474 [DOI] [PubMed] [Google Scholar]
- Polly P, Herdick M, Moehren U, Baniahmad A, Heinzel T, Carlberg C 2000 VDR-Alien: a novel, DNA-selective vitamin D(3) receptor-corepressor partnership. FASEB J 14:1455–1463 [DOI] [PubMed] [Google Scholar]
- Tagami T, Lutz WH, Kumar R, Jameson JL 1998 The interaction of the vitamin D receptor with nuclear receptor corepressors and coactivators. Biochem Biophys Res Commun 253:358–363 [DOI] [PubMed] [Google Scholar]
- Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM 1996 Role of CBP/P300 in nuclear receptor signalling. Nature 383:99–103 [DOI] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG 1996 A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403–414 [DOI] [PubMed] [Google Scholar]
- Rachez C, Freedman LP 2000 Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene 246:9–21 [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O'Malley BW 1997 Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194–198 [DOI] [PubMed] [Google Scholar]
- Burakov D, Wong CW, Rachez C, Cheskis BJ, Freedman LP 2000 Functional interactions between the estrogen receptor and DRIP205, a subunit of the heteromeric DRIP coactivator complex. J Biol Chem 275:20928–20934 [DOI] [PubMed] [Google Scholar]
- Sharma D, Fondell JD 2002 Ordered recruitment of histone acetyltransferases and the TRAP/mediator complex to thyroid hormone-responsive promoters in vivo. Proc Natl Acad Sci USA 99:7934–7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhooke JL, Benning MM, Bauer CB, Pike JW, DeLuca HF 2004 Molecular structure of the rat vitamin D receptor ligand binding domain complexed with 2-carbon-substituted vitamin D3 hormone analogues and a LXXLL-containing coactivator peptide. Biochemistry 43:4101–4110 [DOI] [PubMed] [Google Scholar]
- Rachez C, Gamble M, Chang CP, Atkins GB, Lazar MA, Freedman LP 2000 The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol Cell Biol 20:2718–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK 2006 Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 20:1405–1428 [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Fujiki R, Yoshimura K, Mezaki Y, Uematsu Y, Matsui D, Ogawa S, Unno K, Okubo M, Tokita A, Nakagawa T, Ito T, Ishimi Y, Nagasawa H, Matsumoto T, Yanagisawa J, Kato S 2003 The chromatin-remodeling complex WINAC targets a nuclear receptor to promoters and is impaired in Williams syndrome. Cell 113:905–917 [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL 2007 The role of chromatin during transcription. Cell 128:707–719 [DOI] [PubMed] [Google Scholar]
- Villagra A, Cruzat F, Carvallo L, Paredes R, Olate J, van Wijnen AJ, Stein GS, Lian JB, Stein JL, Imbalzano AN, Montecino M 2006 Chromatin remodeling and transcriptional activity of the bone-specific osteocalcin gene require CCAAT/enhancer-binding protein β-dependent recruitment of SWI/SNF activity. J Biol Chem 281:22695–22706 [DOI] [PubMed] [Google Scholar]
- Baudino TA, Kraichely DM, Jefcoat Jr SC, Winchester SK, Partridge NC, MacDonald PN 1998 Isolation and characterization of a novel coactivator protein, NCoA-62, involved in vitamin D-mediated transcription. J Biol Chem 273:16434–16441 [DOI] [PubMed] [Google Scholar]
- Savkur RS, Bramlett KS, Stayrook KR, Nagpal S, Burris TP 2005 Coactivation of the human vitamin D receptor by the peroxisome proliferator-activated receptor γ coactivator-1α. Mol Pharmacol 68:511–517 [DOI] [PubMed] [Google Scholar]
- Dusso AS 2003 Vitamin D receptor: mechanisms for vitamin D resistance in renal failure. Kidney Int Suppl 85:S6–S9 [DOI] [PubMed] [Google Scholar]
- Kim MS, Fujiki R, Murayama A, Kitagawa H, Yamaoka K, Yamamoto Y, Mihara M, Takeyama K, Kato S 2007 1α,25(OH)2D3-induced transrepression by vitamin D receptor through E-box-type elements in the human parathyroid hormone gene promoter. Mol Endocrinol 21:334–342 [DOI] [PubMed] [Google Scholar]
- Murayama A, Takeyama K, Kitanaka S, Kodera Y, Hosoya T, Kato S 1998 The promoter of the human 25-hydroxyvitamin D3 1 α-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1 α,25(OH)2D3. Biochem Biophys Res Commun 249:11–16 [DOI] [PubMed] [Google Scholar]
- Murayama A, Kim MS, Yanagisawa J, Takeyama K, Kato S 2004 Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching. EMBO J 23:1598–1608 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M 2001 Extrarenal expression of 25-hydroxyvitamin d(3)-1 α-hydroxylase. J Clin Endocrinol Metab 86:888–894 [DOI] [PubMed] [Google Scholar]
- Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM 1992 Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 89:8097–8101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J, Ashok S, Koszewski NJ 1999 Vitamin D receptor interactions with the rat parathyroid hormone gene: synergistic effects between two negative vitamin D response elements. J Bone Miner Res 14:1828–1837 [DOI] [PubMed] [Google Scholar]
- Fujiki R, Kim MS, Sasaki Y, Yoshimura K, Kitagawa H, Kato S 2005 Ligand-induced transrepression by VDR through association of WSTF with acetylated histones. EMBO J 24:3881–3894 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Turunen MM, Dunlop TW, Carlberg C, Vaisanen S 2007 Selective use of multiple vitamin D response elements underlies the 1α,25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res 35:2734–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroy I, Towers TL, Freedman LP 1995 Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol 15:5789–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P 1998 Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-κB down-regulation in transcriptional repression of the p40 gene. J Clin Invest 101:252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque G, Macoritto M, Kremer R 2004 Vitamin D treatment of senescence accelerated mice (SAM-P/6) induces several regulators of stromal cell plasticity. Biogerontology 5:421–429 [DOI] [PubMed] [Google Scholar]
- Eelen G, Verlinden L, Van Camp M, Van Hummelen P, Marchal K, De Moor B, Mathieu C, Carmeliet G, Bouillon R, Verstuyf A 2004 The effects of 1α,25-dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res 19:133–146 [DOI] [PubMed] [Google Scholar]
- Farach-Carson MC, Xu Y 2002 Microarray detection of gene expression changes induced by 1,25(OH)(2)D(3) and a Ca(2+) influx-activating analog in osteoblastic ROS 17/2.8 cells. Steroids 67:467–470 [DOI] [PubMed] [Google Scholar]
- Pochampally RR, Ylostalo J, Penfornis P, Matz RR, Smith JR, Prockop DJ 2007 Histamine receptor H1 and dermatopontin: new downstream targets of the vitamin D receptor. J Bone Miner Res 22:1338–1349 [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Graham EJ, Wang L, Lossdorfer S, Gay I, Johnson-Pais TL, Carnes DL, Sylvia VL, Boyan BD 2005 Phospholipase A2 activating protein (PLAA) is required for 1α,25(OH)2D3 signaling in growth plate chondrocytes. J Cell Physiol 203:54–70 [DOI] [PubMed] [Google Scholar]
- Verlinden L, Eelen G, Van Hellemont R, Engelen K, Beullens I, Van Camp M, Marchal K, Mathieu C, Bouillon R, Verstuyf A 2007 1α,25-Dihydroxyvitamin D3-induced down-regulation of the checkpoint proteins, Chk1 and Claspin, is mediated by the pocket proteins p107 and p130. J Steroid Biochem Mol Biol 103:411–415 [DOI] [PubMed] [Google Scholar]
- Li X, Zheng W, Li YC 2003 Altered gene expression profile in the kidney of vitamin D receptor knockout mice. J Cell Biochem 89:709–719 [DOI] [PubMed] [Google Scholar]
- Kutuzova GD, DeLuca HF 2004 Gene expression profiles in rat intestine identify pathways for 1,25-dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch Biochem Biophys 432:152–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzova GD, DeLuca HF 2007 1,25-Dihydroxyvitamin D3 regulates genes responsible for detoxification in intestine. Toxicol Appl Pharmacol 218:37–44 [DOI] [PubMed] [Google Scholar]
- Bosse Y, Maghni K, Hudson TJ 2007 1α,25-Dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics 29:161–168 [DOI] [PubMed] [Google Scholar]
- Shalhoub V, Shatzen E, Henley C, Boedigheimer M, McNinch J, Manoukian R, Damore M, Fitzpatrick D, Haas K, Twomey B, Kiaei P, Ward S, Lacey DL, Martin D 2006 Calcification inhibitors and Wnt signaling proteins are implicated in bovine artery smooth muscle cell calcification in the presence of phosphate and vitamin D sterols. Calcif Tissue Int 79:431–442 [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE 2007 VDR-mediated gene expression patterns in resting human coronary artery smooth muscle cells. J Cell Biochem 100:1395–1405 [DOI] [PubMed] [Google Scholar]
- Guzey M, Luo J, Getzenberg RH 2004 Vitamin D3 modulated gene expression patterns in human primary normal and cancer prostate cells. J Cell Biochem 93:271–285 [DOI] [PubMed] [Google Scholar]
- Ikezoe T, Gery S, Yin D, O'Kelly J, Binderup L, Lemp N, Taguchi H, Koeffler HP 2005 CCAAT/enhancer-binding protein delta: a molecular target of 1,25-dihydroxyvitamin D3 in androgen-responsive prostate cancer LNCaP cells. Cancer Res 65:4762–4768 [DOI] [PubMed] [Google Scholar]
- Khanim FL, Gommersall LM, Wood VH, Smith KL, Montalvo L, O'Neill LP, Xu Y, Peehl DM, Stewart PM, Turner BM, Campbell MJ 2004 Altered SMRT levels disrupt vitamin D3 receptor signalling in prostate cancer cells. Oncogene 23:6712–6725 [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Shinghal R, Raghavachari N, Brooks JD, Peehl DM, Feldman D 2004 Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate 59:243–251 [DOI] [PubMed] [Google Scholar]
- Moreno J, Krishnan AV, Peehl DM, Feldman D 2006 Mechanisms of vitamin D-mediated growth inhibition in prostate cancer cells: inhibition of the prostaglandin pathway. Anticancer Res 26:2525–2530 [PubMed] [Google Scholar]
- Peehl DM, Shinghal R, Nonn L, Seto E, Krishnan AV, Brooks JD, Feldman D 2004 Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol 92:131–141 [DOI] [PubMed] [Google Scholar]
- Qiao S, Tuohimaa P 2004 The role of long-chain fatty-acid-CoA ligase 3 in vitamin D3 and androgen control of prostate cancer LNCaP cell growth. Biochem Biophys Res Commun 319:358–368 [DOI] [PubMed] [Google Scholar]
- Kawata H, Kamiakito T, Takayashiki N, Tanaka A 2006 Vitamin D3 suppresses the androgen-stimulated growth of mouse mammary carcinoma SC-3 cells by transcriptional repression of fibroblast growth factor 8. J Cell Physiol 207:793–799 [DOI] [PubMed] [Google Scholar]
- Lyakhovich A, Aksenov N, Pennanen P, Miettinen S, Ahonen MH, Syvala H, Ylikomi T, Tuohimaa P 2000 Vitamin D induced up-regulation of keratinocyte growth factor (FGF-7/KGF) in MCF-7 human breast cancer cells. Biochem Biophys Res Commun 273:675–680 [DOI] [PubMed] [Google Scholar]
- Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D 2003 Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat 80:49–62 [DOI] [PubMed] [Google Scholar]
- Song JH, Kim JM, Kim SH, Kim HJ, Lee JJ, Sung MH, Hwang SY, Kim TS 2003 Comparison of the gene expression profiles of monocytic versus granulocytic lineages of HL-60 leukemia cell differentiation by DNA microarray analysis. Life Sci 73:1705–1719 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tazoe H, Taguchi K, Koyama Y, Ichikawa H, Hayakawa S, Munakata H, Isemura M 2006 DNA microarray analysis of changes in gene expression induced by 1,25-dihydroxyvitamin D3 in human promyelocytic leukemia HL-60 cells. Biomed Res 27:99–109 [DOI] [PubMed] [Google Scholar]
- Wood RJ, Tchack L, Angelo G, Pratt RE, Sonna LA 2004 DNA microarray analysis of vitamin D-induced gene expression in a human colon carcinoma cell line. Physiol Genomics 17:122–129 [DOI] [PubMed] [Google Scholar]
- Zhang X, Li P, Bao J, Nicosia SV, Wang H, Enkemann SA, Bai W 2005 Suppression of death receptor-mediated apoptosis by 1,25-dihydroxyvitamin D3 revealed by microarray analysis. J Biol Chem 280:35458–35468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH 2005 Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol 19:2685–2695 [DOI] [PubMed] [Google Scholar]
- Griffin MD, Xing N, Kumar R 2004 Gene expression profiles in dendritic cells conditioned by 1α,25-dihydroxyvitamin D3 analog. J Steroid Biochem Mol Biol 89–90:443–448 [DOI] [PubMed] [Google Scholar]
- Mahon BD, Wittke A, Weaver V, Cantorna MT 2003 The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem 89:922–932 [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC 2007 DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8:285–293 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Mahon BD 2005 D-hormone and the immune system. J Rheumatol Suppl 76:11–20 [PubMed] [Google Scholar]
- Spach KM, Pedersen LB, Nashold FE, Kayo T, Yandell BS, Prolla TA, Hayes CE 2004 Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics 18:141–151 [DOI] [PubMed] [Google Scholar]
- Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU 2007 Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol 103:416–419 [DOI] [PubMed] [Google Scholar]
- Byrne B, Welsh J 2007 Identification of novel mediators of vitamin D signaling and 1,25(OH)2D3 resistance in mammary cells. J Steroid Biochem Mol Biol 103:703–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison TI, Dowd DR, MacDonald PN 2005 Calmodulin-dependent kinase IV stimulates vitamin D receptor-mediated transcription. Mol Endocrinol 19:2309–2319 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC 2001 Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP 2004 Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov 3:27–41 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW, Okamura WH, Hammond MW, Bishop JE, Dormanen MC, Bouillon R, Van Baelen H, Ridall AL, Daane E, Khoury R, Farach-Carson MC 1997 Comparison of 6-s-cis- and 6-s-trans-locked analogs of 1α,25-dihydroxyvitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-s-cis- nor 6-s-trans-locked analogs are preferred for genomic biological responses. Mol Endocrinol 11:1518–1531 [DOI] [PubMed] [Google Scholar]
- Zanello LP, Norman AW 2004 Rapid modulation of osteoblast ion channel responses by 1α,25(OH)2-vitamin D3 requires the presence of a functional vitamin D nuclear receptor. Proc Natl Acad Sci USA 101:1589–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S 1997 Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16:391–396 [DOI] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB 1997 Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94:9831–9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G 2001 Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98:13324–13329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R, Moller G, Adamski J, Balling R 2002 Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol Endocrinol 16:1524–1537 [DOI] [PubMed] [Google Scholar]
- Dardenne O, Prud'homme J, Arabian A, Glorieux FH, St Arnaud R 2001 Targeted inactivation of the 25-hydroxyvitamin D(3)-1(α)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology 142:3135–3141 [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D 2001 Targeted ablation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA 98:7498–7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne O, Prud'homme J, Hacking SA, Glorieux FH, St Arnaud R 2003 Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). Bone 32:332–340 [DOI] [PubMed] [Google Scholar]
- Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB 1999 Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140:4982–4987 [DOI] [PubMed] [Google Scholar]
- Dardenne O, Prudhomme J, Hacking SA, Glorieux FH, St Arnaud R 2003 Rescue of the pseudo-vitamin D deficiency rickets phenotype of CYP27B1-deficient mice by treatment with 1,25-dihydroxyvitamin D3: biochemical, histomorphometric, and biomechanical analyses. J Bone Miner Res 18:637–643 [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Dardenne O, van Abel M, van der Kemp AW, van Os CH, Arnaud R, Bindels RJ 2002 Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3–1α-hydroxylase knockout mice. FASEB J 16:1398–1406 [DOI] [PubMed] [Google Scholar]
- Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB 1998 Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology 139:4391–4396 [DOI] [PubMed] [Google Scholar]
- Rowling MJ, Gliniak C, Welsh J, Fleet JC 2007 High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr 137:2608–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Kato S, Fleet JC 2003 Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr 133:374–380 [DOI] [PubMed] [Google Scholar]
- Balsan S, Garabedian M, Larchet M, Gorski AM, Cournot G, Tau C, Bourdeau A, Silve C, Ricour C 1986 Long-term nocturnal calcium infusions can cure rickets and promote normal mineralization in hereditary resistance to 1,25-dihydroxyvitamin D. J Clin Invest 77:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Z, Tiosano D, Even L 1992 Calcium therapy for calcitriol-resistant rickets. J Pediatr 121:803–808 [DOI] [PubMed] [Google Scholar]
- Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S 1998 Inactivating mutations in the 25-hydroxyvitamin D3 1α-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med 338:653–661 [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ 2005 Calcium absorption across epithelia. Physiol Rev 85:373–422 [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Hartog A, Stuiver M, Doucet A, Willems PH, Bindels RJ 2000 Localization of the epithelial Ca(2+) channel in rabbit kidney and intestine. J Am Soc Nephrol 11:1171–1178 [DOI] [PubMed] [Google Scholar]
- Dostal LA, Toverud SU 1984 Effect of vitamin D3 on duodenal calcium absorption in vivo during early development. Am J Physiol 246:G528–G534 [DOI] [PubMed] [Google Scholar]
- Halloran BP, DeLuca HF 1981 Appearance of the intestinal cytosolic receptor for 1,25-dihydroxyvitamin D3 during neonatal development in the rat. J Biol Chem 256:7338–7342 [PubMed] [Google Scholar]
- Bolt MJ, Cao LP, Kong J, Sitrin MD, Li YC 2005 Vitamin D receptor is required for dietary calcium-induced repression of calbindin-D9k expression in mice. J Nutr Biochem 16:286–290 [DOI] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Demay MB 1998 Analysis of vitamin D-dependent calcium-binding protein messenger ribonucleic acid expression in mice lacking the vitamin D receptor. Endocrinology 139:847–851 [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Rummens K, Stockmans I, Van Herck E, Dijcks FA, Ederveen AG, Carmeliet P, Verhaeghe J, Bouillon R, Carmeliet G 2003 Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res 18:1725–1736 [DOI] [PubMed] [Google Scholar]
- Li YC, Bolt MJ, Cao LP, Sitrin MD 2001 Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol Endocrinol Metab 281:E558–E564 [DOI] [PubMed] [Google Scholar]
- Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S 2008 Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-d9k. Endocrinology 149:3196–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, Zhuang L, Freeman MR, Gouveia CH, Wu J, Luo H, Mauro T, Brown EM, Hediger MA 2007 Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res 22:274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuzova GD, Akhter S, Christakos S, Vanhooke J, Kimmel-Jehan C, DeLuca HF 2006 Calbindin D(9k) knockout mice are indistinguishable from wild-type mice in phenotype and serum calcium level. Proc Natl Acad Sci USA 103:12377–12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GS, Lee KY, Choi KC, Ryu YH, Paik SG, Oh GT, Jeung EB 2007 Phenotype of a calbindin-d9k gene knockout is compensated for by the induction of other calcium transporter genes in a mouse model. J Bone Miner Res 22:1968–1978 [DOI] [PubMed] [Google Scholar]
- Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H, Yamashita T, Kato S, Sawada N, Chiba H 2008 Tight junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell 19:1912–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ 2003 Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112:1906–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkema KY, Nijenhuis T, van der Eerden BC, van der Kemp AW, Weinans H, van Leeuwen JP, Bindels RJ, Hoenderop JG 2005 Hypervitaminosis D mediates compensatory Ca2+ hyperabsorption in TRPV5 knockout mice. J Am Soc Nephrol 16:3188–3195 [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M 1997 Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28k gene. Proc Natl Acad Sci USA 94:1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis DS, Kim D, Szivek JA, Lai LW, Lien YH 2006 Functionally improved bone in calbindin-D28k knockout mice. Bone 39:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooy K, Kohut J, Christakos S 2000 The role of calbindin and 1,25dihydroxyvitamin D3 in the kidney. Curr Opin Nephrol Hypertens 9:341–347 [DOI] [PubMed] [Google Scholar]
- Zheng W, Xie Y, Li G, Kong J, Feng JQ, Li YC 2004 Critical role of calbindin-D28k in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28k. J Biol Chem 279:52406–52413 [DOI] [PubMed] [Google Scholar]
- Gkika D, Hsu YJ, van der Kemp AW, Christakos S, Bindels RJ, Hoenderop JG 2006 Critical role of the epithelial Ca2+ channel TRPV5 in active Ca2+ reabsorption as revealed by TRPV5/calbindin-D28K knockout mice. J Am Soc Nephrol 17:3020–3027 [DOI] [PubMed] [Google Scholar]
- Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, Conway SJ 2001 Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 15:1209–1211 [DOI] [PubMed] [Google Scholar]
- Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O'Connor KT, Neumann JC, Andringa A, Miller DA, Prasad V, Doetschman T, Paul RJ, Shull GE 2004 Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem 279:33742–33750 [DOI] [PubMed] [Google Scholar]
- Reuter H, Henderson SA, Han T, Ross RS, Goldhaber JI, Philipson KD 2002 The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ Res 90:305–308 [DOI] [PubMed] [Google Scholar]
- Wakimoto K, Kobayashi K, Kuro O, Yao A, Iwamoto T, Yanaka N, Kita S, Nishida A, Azuma S, Toyoda Y, Omori K, Imahie H, Oka T, Kudoh S, Kohmoto O, Yazaki Y, Shigekawa M, Imai Y, Nabeshima Y, Komuro I 2000 Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem 275:36991–36998 [DOI] [PubMed] [Google Scholar]
- Johnson JA, Grande JP, Roche PC, Kumar R 1996 Ontogeny of the 1,25-dihydroxyvitamin D3 receptor in fetal rat bone. J Bone Miner Res 11:56–61 [DOI] [PubMed] [Google Scholar]
- Kovacs CS, Woodland ML, Fudge NJ, Friel JK 2005 The vitamin D receptor is not required for fetal mineral homeostasis or for the regulation of placental calcium transfer in mice. Am J Physiol Endocrinol Metab 289:E133–E144 [DOI] [PubMed] [Google Scholar]
- Donohue MM, Demay MB 2002 Rickets in VDR null mice is secondary to decreased apoptosis of hypertrophic chondrocytes. Endocrinology 143:3691–3694 [DOI] [PubMed] [Google Scholar]
- Sabbagh Y, Carpenter TO, Demay MB 2005 Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA 102:9637–9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G 2006 Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest 116:3150–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, Goltzman D 2004 Inactivation of the 25-hydroxyvitamin D 1α-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem 279:16754–16766 [DOI] [PubMed] [Google Scholar]
- Yagishita N, Yamamoto Y, Yoshizawa T, Sekine K, Uematsu Y, Murayama H, Nagai Y, Krezel W, Chambon P, Matsumoto T, Kato S 2001 Aberrant growth plate development in VDR/RXR γ double null mutant mice. Endocrinology 142:5332–5341 [DOI] [PubMed] [Google Scholar]
- Takeda S, Yoshizawa T, Nagai Y, Yamato H, Fukumoto S, Sekine K, Kato S, Matsumoto T, Fujita T 1999 Stimulation of osteoclast formation by 1,25-dihydroxyvitamin D requires its binding to vitamin D receptor (VDR) in osteoblastic cells: studies using VDR knockout mice. Endocrinology 140:1005–1008 [DOI] [PubMed] [Google Scholar]
- Masuyama R, Nakaya Y, Tanaka S, Tsurukami H, Nakamura T, Watanabe S, Yoshizawa T, Kato S, Suzuki K 2001 Dietary phosphorus restriction reverses the impaired bone mineralization in vitamin D receptor knockout mice. Endocrinology 142:494–497 [DOI] [PubMed] [Google Scholar]
- Cianferotti L, Demay MB 2007 VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J Cell Biochem 101:80–88 [DOI] [PubMed] [Google Scholar]
- Gardiner EM, Baldock PA, Thomas GP, Sims NA, Henderson NK, Hollis B, White CP, Sunn KL, Morrison NA, Walsh WR, Eisman JA 2000 Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J 14:1908–1916 [DOI] [PubMed] [Google Scholar]
- Sooy K, Sabbagh Y, Demay MB 2005 Osteoblasts lacking the vitamin D receptor display enhanced osteogenic potential in vitro. J Cell Biochem 94:81–87 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seino Y 2004 Direct action of 1,25-dihydroxyvitamin D on bone: VDRKO bone shows excessive bone formation in normal mineral condition. J Steroid Biochem Mol Biol 89–90:343–345 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yoshizawa T, Fukuda T, Kawano H, Nakamura T, Yamada T, Karsenty G, Kato A A genetic evidence of direct VDR function in osteoblasts. Generation and analysis of osteoblast-specific VDR KO mice. p S26 (Abstract) [Google Scholar]
- Xue Y, Karaplis AC, Hendy GN, Goltzman D, Miao D 2005 Genetic models show that parathyroid hormone and 1,25-dihydroxyvitamin D3 play distinct and synergistic roles in postnatal mineral ion homeostasis and skeletal development. Hum Mol Genet 14:1515–1528 [DOI] [PubMed] [Google Scholar]
- Miao D, He B, Karaplis AC, Goltzman D 2002 Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest 109:1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Zhang Z, Karaplis AC, Hendy GN, Goltzman D, Miao D 2005 Exogenous PTH-related protein and PTH improve mineral and skeletal status in 25-hydroxyvitamin D-1α-hydroxylase and PTH double knockout mice. J Bone Miner Res 20:1766–1777 [DOI] [PubMed] [Google Scholar]
- Xue Y, Karaplis AC, Hendy GN, Goltzman D, Miao D 2006 Exogenous 1,25-dihydroxyvitamin D3 exerts a skeletal anabolic effect and improves mineral ion homeostasis in mice that are homozygous for both the 1α-hydroxylase and parathyroid hormone null alleles. Endocrinology 147:4801–4810 [DOI] [PubMed] [Google Scholar]
- Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA 2006 The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int 69:495–503 [DOI] [PubMed] [Google Scholar]
- Tenenhouse HS 2005 Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr 25:197–214 [DOI] [PubMed] [Google Scholar]
- Hattenhauer O, Traebert M, Murer H, Biber J 1999 Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am J Physiol 277:G756–G762 [DOI] [PubMed] [Google Scholar]
- Xu H, Bai L, Collins JF, Ghishan FK 2002 Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)(2) vitamin D(3). Am J Physiol Cell Physiol 282:C487–C493 [DOI] [PubMed] [Google Scholar]
- Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, St Arnoud R, Murer H, Biber J 2005 Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1αOHase-deficient mice. Am J Physiol Cell Physiol 288:C429–C434 [DOI] [PubMed] [Google Scholar]
- Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K 2004 Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol 287:F39–F47 [DOI] [PubMed] [Google Scholar]
- Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB 2004 Transgenic mice expressing fibroblast growth factor 23 under the control of the α1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145:3087–3094 [DOI] [PubMed] [Google Scholar]
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T 2004 FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435 [DOI] [PubMed] [Google Scholar]
- Ben Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro O, Mohammadi M, Sirkis R, Naveh-Many T, Silver J 2007 The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK 2005 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289:G1036–G1042 [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K, Fujita T, Fukumoto S, Yamashita T 2005 Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289:F1088–F1095 [DOI] [PubMed] [Google Scholar]
- Yu X, Sabbagh Y, Davis SI, Demay MB, White KE 2005 Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone 36:971–977 [DOI] [PubMed] [Google Scholar]
- Inoue Y, Segawa H, Kaneko I, Yamanaka S, Kusano K, Kawakami E, Furutani J, Ito M, Kuwahata M, Saito H, Fukushima N, Kato S, Kanayama HO, Miyamoto K 2005 Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem J 390:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Ito M, Kuwahata M, Kato S, Segawa H 2005 Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial 9:331–335 [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T 2004 Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitara D, Razzaque MS, St Arnaud R, Huang W, Taguchi T, Erben RG, Lanske B 2006 Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol 169:2161–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque MS, Sitara D, Taguchi T, St Arnaud R, Lanske B 2006 Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J 20:720–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M, Frohlich LF, Zeitz U, Lanske B, Erben RG 2007 Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol 26:75–84 [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI 1997 Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390:45–51 [DOI] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T 2006 Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774 [Google Scholar]
- Razzaque MS, Lanske B 2006 Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med 12:298–305 [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y 2003 Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17:2393–2403 [DOI] [PubMed] [Google Scholar]
- Tenenhouse HS, Gauthier C, Chau H, St Arnaud R 2004 1α-Hydroxylase gene ablation and Pi supplementation inhibit renal calcification in mice homozygous for the disrupted Npt2a gene. Am J Physiol Renal Physiol 286:F675–F681 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Price PA, Schrijvers A, Bervoets TJ, Karsenty G 1998 Studies of osteocalcin function in dentin formation in rodent teeth. Eur J Oral Sci 106:795–807 [DOI] [PubMed] [Google Scholar]
- Lezot F, Descroix V, Mesbah M, Hotton D, Blin C, Papagerakis P, Mauro N, Kato S, MacDougall M, Sharpe P, Berdal A 2002 Cross-talk between Msx/Dlx homeobox genes and vitamin D during tooth mineralization. Connect Tissue Res 43:509–514 [DOI] [PubMed] [Google Scholar]
- Berdal A, Bailleul-Forestier I, Davideau J-L, Lezot F 2005 Dento-alveolar bone complex and vitamin D. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. San Diego: Elsevier Academic Press; 599–607 [Google Scholar]
- Zhang X, Rahemtulla FG, MacDougall MJ, Thomas HF 2007 Vitamin D receptor deficiency affects dentin maturation in mice. Arch Oral Biol 52:1172–1179 [DOI] [PubMed] [Google Scholar]
- Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B 2001 Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med 111:452–456 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Oda Y, Xie Z 2004 Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J Steroid Biochem Mol Biol 89–90:355–360 [DOI] [PubMed] [Google Scholar]
- Bikle DD 2004 Vitamin D regulated keratinocyte differentiation. J Cell Biochem 92:436–444 [DOI] [PubMed] [Google Scholar]
- Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD 2002 Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol 118:11–16 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Demay MB 2000 Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology 141:2043–2049 [DOI] [PubMed] [Google Scholar]
- Reichrath J, Schilli M, Kerber A, Bahmer FA, Czarnetzki BM, Paus R 1994 Hair follicle expression of 1,25-dihydroxyvitamin D3 receptors during the murine hair cycle. Br J Dermatol 131:477–482 [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF 1979 Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science 206:1188–1190 [DOI] [PubMed] [Google Scholar]
- Sakai Y, Kishimoto J, Demay MB 2001 Metabolic and cellular analysis of alopecia in vitamin D receptor knockout mice. J Clin Invest 107:961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugosz A 1999 The Hedgehog and the hair follicle: a growing relationship. J Clin Invest 104:851–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Sakai Y, Demay MB 2001 Targeting expression of the human vitamin D receptor to the keratinocytes of vitamin D receptor null mice prevents alopecia. Endocrinology 142:5386–5389 [DOI] [PubMed] [Google Scholar]
- Kong J, Li XJ, Gavin D, Jiang Y, Li YC 2002 Targeted expression of human vitamin D receptor in the skin promotes the initiation of the postnatal hair follicle cycle and rescues the alopecia in vitamin D receptor null mice. J Invest Dermatol 118:631–638 [DOI] [PubMed] [Google Scholar]
- Skorija K, Cox M, Sisk JM, Dowd DR, MacDonald PN, Thompson CC, Demay MB 2005 Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol Endocrinol 19:855–862 [DOI] [PubMed] [Google Scholar]
- Miller J, Djabali K, Chen T, Liu Y, Ioffreda M, Lyle S, Christiano AM, Holick M, Cotsarelis G 2001 Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol 117:612–617 [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR, Thompson CC 2003 Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem 278:38665–38674 [DOI] [PubMed] [Google Scholar]
- Xie Z, Chang S, Oda Y, Bikle DD 2006 Hairless suppresses vitamin D receptor transactivation in human keratinocytes. Endocrinology 147:314–323 [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E 1998 De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95:605–614 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W 2001 β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545 [DOI] [PubMed] [Google Scholar]
- Lo CC, Prowse DM, Watt FM 2004 Transient activation of β-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131:1787–1799 [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E 2001 Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev 15:1688–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianferotti L, Cox M, Skorija K, Demay MB 2007 Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci USA 104:9428–9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin III GM, Sisk JM, Coulombe PA, Thompson CC 2005 Hairless triggers reactivation of hair growth by promoting Wnt signaling. Proc Natl Acad Sci USA 102:14653–14658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM 2000 Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102:451–461 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM 2004 25-Hydroxyvitamin D 1α-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol 122:984–992 [DOI] [PubMed] [Google Scholar]
- Huang DC, Papavasiliou V, Rhim JS, Horst RL, Kremer R 2002 Targeted disruption of the 25-hydroxyvitamin D3 1α-hydroxylase gene in ras-transformed keratinocytes demonstrates that locally produced 1α,25-dihydroxyvitamin D3 suppresses growth and induces differentiation in an autocrine fashion. Mol Cancer Res 1:56–67 [PubMed] [Google Scholar]
- Colston K, Colston MJ, Feldman D 1981 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology 108:1083–1086 [DOI] [PubMed] [Google Scholar]
- Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T 1981 Differentiation of mouse myeloid leukemia cells induced by 1 α,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 78:4990–4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WH, Seol JG, Kim ES, Jung CW, Lee CC, Binderup L, Koeffler HP, Kim BK, Lee YY 2000 Cell cycle arrest induced by the vitamin D(3) analog EB1089 in NCI-H929 myeloma cells is associated with induction of the cyclin-dependent kinase inhibitor p27. Exp Cell Res 254:279–286 [DOI] [PubMed] [Google Scholar]
- Studzinski GP, Rathod B, Wang QM, Rao J, Zhang F 1997 Uncoupling of cell cycle arrest from the expression of monocytic differentiation markers in HL60 cell variants. Exp Cell Res 232:376–387 [DOI] [PubMed] [Google Scholar]
- Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A 2001 Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol 154:369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong WM, Kallay E, Hofer H, Hulla W, Manhardt T, Peterlik M, Cross HS 1998 Growth regulation of human colon cancer cells by epidermal growth factor and 1,25-dihydroxyvitamin D3 is mediated by mutual modulation of receptor expression. Eur J Cancer 34:2119–2125 [DOI] [PubMed] [Google Scholar]
- Vink-van Wijngaarden T, Pols HA, Buurman CJ, Birkenhager JC, van Leeuwen JP 1996 Inhibition of insulin- and insulin-like growth factor-I-stimulated growth of human breast cancer cells by 1,25-dihydroxyvitamin D3 and the vitamin D3 analogue EB1089. Eur J Cancer 32A:842–848 [DOI] [PubMed] [Google Scholar]
- Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J 1996 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol 58:367–376 [DOI] [PubMed] [Google Scholar]
- James SY, Mackay AG, Colston KW 1996 Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J Steroid Biochem Mol Biol 58:395–401 [DOI] [PubMed] [Google Scholar]
- Getzenberg RH, Light BW, Lapco PE, Konety BR, Nangia AK, Acierno JS, Dhir R, Shurin Z, Day RS, Trump DL, Johnson CS 1997 Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology 50:999–1006 [DOI] [PubMed] [Google Scholar]
- Skowronski RJ, Peehl DM, Feldman D 1993 Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 132:1952–1960 [DOI] [PubMed] [Google Scholar]
- Bergh G, Telleus A, Fritzon A, Kornfalt S, Johnson E, Olsson I, Gullberg U 2001 Forced expression of the cyclin-dependent kinase inhibitor p16(INK4A) in leukemic U-937 cells reveals dissociation between cell cycle and differentiation. Exp Hematol 29:1382–1391 [DOI] [PubMed] [Google Scholar]
- Jensen SS, Madsen MW, Lukas J, Binderup L, Bartek J 2001 Inhibitory effects of 1α,25-dihydroxyvitamin D(3) on the G(1)-S phase-controlling machinery. Mol Endocrinol 15:1370–1380 [DOI] [PubMed] [Google Scholar]
- Verlinden L, Eelen G, Beullens I, Van Camp M, Van Hummelen P, Engelen K, Van Hellemont R, Marchal K, De Moor B, Foijer F, Te RH, Beullens M, Bollen M, Mathieu C, Bouillon R, Verstuyf A 2005 Characterization of the condensin component Cnap1 and protein kinase Melk as novel E2F target genes down-regulated by 1,25-dihydroxyvitamin D3. J Biol Chem 280:37319–37330 [DOI] [PubMed] [Google Scholar]
- Gedlicka C, Hager G, Weissenbock M, Gedlicka W, Knerer B, Kornfehl J, Formanek M 2006 1,25(OH)2Vitamin D3 induces elevated expression of the cell cycle inhibitor p18 in a squamous cell carcinoma cell line of the head and neck. J Oral Pathol Med 35:472–478 [DOI] [PubMed] [Google Scholar]
- Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP 1996 Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev 10:142–153 [DOI] [PubMed] [Google Scholar]
- Wang QM, Jones JB, Studzinski GP 1996 Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res 56:264–267 [PubMed] [Google Scholar]
- Verlinden L, Verstuyf A, Convents R, Marcelis S, Van Camp M, Bouillon R 1998 Action of 1,25(OH)2D3 on the cell cycle genes, cyclin D1, p21 and p27 in MCF-7 cells. Mol Cell Endocrinol 142:57–65 [DOI] [PubMed] [Google Scholar]
- Aguilera O, Pena C, Garcia JM, Larriba MJ, Ordonez-Moran P, Navarro D, Barbachano A, Lopez dS, I, Ballestar E, Fraga MF, Esteller M, Gamallo C, Bonilla F, Gonzalez-Sancho JM, Munoz A 2007 The Wnt antagonist DICKKOPF-1 gene is induced by 1α,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 28:1877–1884 [DOI] [PubMed] [Google Scholar]
- Reitsma PH, Rothberg PG, Astrin SM, Trial J, Bar-Shavit Z, Hall A, Teitelbaum SL, Kahn AJ 1983 Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature 306:492–494 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Hashimoto K, Nishida Y, Hashiro M, Yoshikawa K 1990 Growth-inhibitory effects of 1,25-dihydroxyvitamin D3 on normal human keratinocytes cultured in serum-free medium. Biochem Biophys Res Commun 166:916–923 [DOI] [PubMed] [Google Scholar]
- Mercier T, Chaumontet C, Gaillard-Sanchez I, Martel P, Heberden C 1996 Calcitriol and lexicalcitol (KH1060) inhibit the growth of human breast adenocarcinoma cells by enhancing transforming growth factor-β production. Biochem Pharmacol 52:505–510 [DOI] [PubMed] [Google Scholar]
- Wu Y, Haugen JD, Zinsmeister AR, Kumar R 1997 1α,25-Dihydroxyvitamin D3 increases transforming growth factor and transforming growth factor receptor type I and II synthesis in human bone cells. Biochem Biophys Res Commun 239:734–739 [DOI] [PubMed] [Google Scholar]
- Rozen F, Pollak M 1999 Inhibition of insulin-like growth factor I receptor signaling by the vitamin D analogue EB1089 in MCF-7 breast cancer cells: a role for insulin-like growth factor binding proteins. Int J Oncol 15:589–594 [DOI] [PubMed] [Google Scholar]
- Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Binderup L, Koeffler HP, Kim BK, Lee YY 2000 Induction of apoptosis by vitamin D3 analogue EB1089 in NCI-H929 myeloma cells via activation of caspase 3 and p38 MAP kinase. Br J Haematol 109:576–583 [DOI] [PubMed] [Google Scholar]
- Wagner N, Wagner KD, Schley G, Badiali L, Theres H, Scholz H 2003 1,25-Dihydroxyvitamin D3-induced apoptosis of retinoblastoma cells is associated with reciprocal changes of Bcl-2 and bax. Exp Eye Res 77:1–9 [DOI] [PubMed] [Google Scholar]
- Xie SP, James SY, Colston KW 1997 Vitamin D derivatives inhibit the mitogenic effects of IGF-I on MCF-7 human breast cancer cells. J Endocrinol 154:495–504 [DOI] [PubMed] [Google Scholar]
- McGuire TF, Trump DL, Johnson CS 2001 Vitamin D(3)-induced apoptosis of murine squamous cell carcinoma cells. Selective induction of caspase-dependent MEK cleavage and up-regulation of MEKK-1. J Biol Chem 276:26365–26373 [DOI] [PubMed] [Google Scholar]
- De Haes P, Garmyn M, Degreef H, Vantieghem K, Bouillon R, Segaert S 2003 1,25-Dihydroxyvitamin D3 inhibits ultraviolet B-induced apoptosis, Jun kinase activation, and interleukin-6 production in primary human keratinocytes. J Cell Biochem 89:663–673 [DOI] [PubMed] [Google Scholar]
- Rocker D, Ravid A, Liberman UA, Garach-Jehoshua O, Koren R 1994 1,25-Dihydroxyvitamin D3 potentiates the cytotoxic effect of TNF on human breast cancer cells. Mol Cell Endocrinol 106:157–162 [DOI] [PubMed] [Google Scholar]
- Wong G, Gupta R, Dixon KM, Deo SS, Choong SM, Halliday GM, Bishop JE, Ishizuka S, Norman AW, Posner GH, Mason RS 2004 1,25-Dihydroxyvitamin D and three low-calcemic analogs decrease UV-induced DNA damage via the rapid response pathway. J Steroid Biochem Mol Biol 89–90:567–570 [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T 2005 1α,25-Dihydroxyvitamin D(3) is a preventive factor in the metastasis of lung cancer. Carcinogenesis 26:429–440 [DOI] [PubMed] [Google Scholar]
- Majewski S, Skopinska M, Marczak M, Szmurlo A, Bollag W, Jablonska S 1996 Vitamin D3 is a potent inhibitor of tumor cell-induced angiogenesis. J Investig Dermatol Symp Proc 1:97–101 [PubMed] [Google Scholar]
- Flynn G, Chung I, Yu WD, Romano M, Modzelewski RA, Johnson CS, Trump DL 2006 Calcitriol (1,25-dihydroxycholecalciferol) selectively inhibits proliferation of freshly isolated tumor-derived endothelial cells and induces apoptosis. Oncology 70:447–457 [DOI] [PubMed] [Google Scholar]
- Ben Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ 2007 1α,25-Dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther 6:1433–1439 [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Sasaki Y, Kato S, Kubodera N, Okano T 2005 22-Oxa-1α,25-dihydroxyvitamin D3 inhibits metastasis and angiogenesis in lung cancer. Carcinogenesis 26:1044–1054 [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Kawaura A, Kato S, Takeda E, Okano T 2004 Metastatic growth of lung cancer cells is extremely reduced in vitamin D receptor knockout mice. J Steroid Biochem Mol Biol 89–90:545–547 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sancho JM, Alvarez-Dolado M, Munoz A 1998 1,25-Dihydroxyvitamin D3 inhibits tenascin-C expression in mammary epithelial cells. FEBS Lett 426:225–228 [DOI] [PubMed] [Google Scholar]
- Cross HS, Bareis P, Hofer H, Bischof MG, Bajna E, Kriwanek S, Bonner E, Peterlik M 2001 25-Hydroxyvitamin D(3)-1α-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids 66:287–292 [DOI] [PubMed] [Google Scholar]
- Cross HS, Bajna E, Bises G, Genser D, Kallay E, Potzi R, Wenzl E, Wrba F, Roka R, Peterlik M 1996 Vitamin D receptor and cytokeratin expression may be progression indicators in human colon cancer. Anticancer Res 16:2333–2337 [PubMed] [Google Scholar]
- Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, Bieglmayer C, Kato S, Cross HS 2001 Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis 22:1429–1435 [DOI] [PubMed] [Google Scholar]
- Kallay E, Bareis P, Bajna E, Kriwanek S, Bonner E, Toyokuni S, Cross HS 2002 Vitamin D receptor activity and prevention of colonic hyperproliferation and oxidative stress. Food Chem Toxicol 40:1191–1196 [DOI] [PubMed] [Google Scholar]
- Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T 1983 Regulation of terminal differentiation of cultured mouse epidermal cells by 1 α,25-dihydroxyvitamin D3. Endocrinology 113:1950–1957 [DOI] [PubMed] [Google Scholar]
- Zinser GM, Sundberg JP, Welsh J 2002 Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis 23:2103–2109 [DOI] [PubMed] [Google Scholar]
- Teichert A, Welsh J, Bikle DD The vitamin D receptor, Hedgehog signaling and epidermal carcinogenesis. p WG1 (Abstract) [Google Scholar]
- Zinser G, Packman K, Welsh J 2002 Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Development 129:3067–3076 [DOI] [PubMed] [Google Scholar]
- Zinser GM, Welsh J 2004 Accelerated mammary gland development during pregnancy and delayed postlactational involution in vitamin D3 receptor null mice. Mol Endocrinol 18:2208–2223 [DOI] [PubMed] [Google Scholar]
- Welsh J 2004 Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr 80:1721S–1724S [DOI] [PubMed] [Google Scholar]
- Zinser GM, Welsh J 2004 Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis 25:2361–2372 [DOI] [PubMed] [Google Scholar]
- Zinser GM, Suckow M, Welsh J 2005 Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol 97:153–164 [DOI] [PubMed] [Google Scholar]
- Garland CF, Garland FC 1980 Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol 9:227–231 [DOI] [PubMed] [Google Scholar]
- Robsahm TE, Tretli S, Dahlback A, Moan J 2004 Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control 15:149–158 [DOI] [PubMed] [Google Scholar]
- Garland FC, Garland CF, Gorham ED, Young JF 1990 Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med 19:614–622 [DOI] [PubMed] [Google Scholar]
- Gorham ED, Garland FC, Garland CF 1990 Sunlight and breast cancer incidence in the USSR. Int J Epidemiol 19:820–824 [DOI] [PubMed] [Google Scholar]
- John EM, Schwartz GG, Dreon DM, Koo J 1999 Vitamin D and breast cancer risk: the NHANES I epidemiologic follow-up study, 1971–1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev 8:399–406 [PubMed] [Google Scholar]
- Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R 2007 Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 16:422–429 [DOI] [PubMed] [Google Scholar]
- John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA 2005 Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res 65:5470–5479 [DOI] [PubMed] [Google Scholar]
- John EM, Koo J, Schwartz GG 2007 Sun exposure and prostate cancer risk: evidence for a protective effect of early-life exposure. Cancer Epidemiol Biomarkers Prev 16:1283–1286 [DOI] [PubMed] [Google Scholar]
- van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA 1995 Serum vitamin D concentrations among elderly people in Europe. Lancet 346:207–210 [DOI] [PubMed] [Google Scholar]
- Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM 2007 Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med 167:1050–1059 [DOI] [PubMed] [Google Scholar]
- Robien K, Cutler GJ, Lazovich D 2007 Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women’s Health Study. Cancer Causes Control 18:775–782 [DOI] [PubMed] [Google Scholar]
- Ferraroni M, La Vecchia C, D'Avanzo B, Negri E, Franceschi S, Decarli A 1994 Selected micronutrient intake and the risk of colorectal cancer. Br J Cancer 70:1150–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O 1985 Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet 1:307–309 [DOI] [PubMed] [Google Scholar]
- Kesse E, Boutron-Ruault MC, Norat T, Riboli E, Clavel-Chapelon F 2005 Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int J Cancer 117:137–144 [DOI] [PubMed] [Google Scholar]
- Levi F, Pasche C, Lucchini F, La Vecchia C 2000 Selected micronutrients and colorectal cancer. A case-control study from the canton of Vaud, Switzerland. Eur J Cancer 36:2115–2119 [DOI] [PubMed] [Google Scholar]
- Oh K, Willett WC, Wu K, Fuchs CS, Giovannucci EL 2007 Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am J Epidemiol 165:1178–1186 [DOI] [PubMed] [Google Scholar]
- Pritchard RS, Baron JA, Gerhardsson de Verdier M 1996 Dietary calcium, vitamin D, and the risk of colorectal cancer in Stockholm, Sweden. Cancer Epidemiol Biomarkers Prev 5:897–900 [PubMed] [Google Scholar]
- Chan JM, Giovannucci E, Andersson SO, Yuen J, Adami HO, Wolk A 1998 Dairy products, calcium, phosphorous, vitamin D, and risk of prostate cancer (Sweden). Cancer Causes Control 9:559–566 [DOI] [PubMed] [Google Scholar]
- Kristal AR, Cohen JH, Qu P, Stanford JL 2002 Associations of energy, fat, calcium, and vitamin D with prostate cancer risk. Cancer Epidemiol Biomarkers Prev 11:719–725 [PubMed] [Google Scholar]
- Tavani A, Bertuccio P, Bosetti C, Talamini R, Negri E, Franceschi S, Montella M, La Vecchia C 2005 Dietary intake of calcium, vitamin D, phosphorus and the risk of prostate cancer. Eur Urol 48:27–33 [DOI] [PubMed] [Google Scholar]
- Tseng M, Breslow RA, Graubard BI, Ziegler RG 2005 Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr 81:1147–1154 [DOI] [PubMed] [Google Scholar]
- Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, Giovannucci EL 2004 Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev 13:1502–1508 [PubMed] [Google Scholar]
- Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, Colston KW 2005 Plasma 25-hydroxy vitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer 41:1164–1169 [DOI] [PubMed] [Google Scholar]
- Tangrea J, Helzlsouer K, Pietinen P, Taylor P, Hollis B, Virtamo J, Albanes D 1997 Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control 8:615–625 [DOI] [PubMed] [Google Scholar]
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS 2007 Extra-renal 25-hydroxyvitamin D3–1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 103:316–321 [DOI] [PubMed] [Google Scholar]
- Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW 1995 Colon cancer and serum vitamin D metabolite levels 10–17 years prior to diagnosis. Am J Epidemiol 142:608–611 [DOI] [PubMed] [Google Scholar]
- Peters U, McGlynn KA, Chatterjee N, Gunter E, Garcia-Closas M, Rothman N, Sinha R 2001 Vitamin D, calcium, and vitamin D receptor polymorphism in colorectal adenomas. Cancer Epidemiol Biomarkers Prev 10:1267–1274 [PubMed] [Google Scholar]
- Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, Heber D 2003 Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst 95:1765–1771 [DOI] [PubMed] [Google Scholar]
- Jacobs ET, Alberts DS, Benuzillo J, Hollis BW, Thompson PA, Martinez ME 2007 Serum 25(OH)D levels, dietary intake of vitamin D, and colorectal adenoma recurrence. J Steroid Biochem Mol Biol 103:752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S 2007 Plasma vitamin D and risk of colorectal cancer: the Japan Public Health Center-Based Prospective Study. Br J Cancer 97:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz EA, Hankinson SE, Hollis BW, Colditz GA, Hunter DJ, Speizer FE, Giovannucci E 2000 Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and adenomatous polyps of the distal colorectum. Cancer Epidemiol Biomarkers Prev 9:1059–1065 [PubMed] [Google Scholar]
- Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci EL 2007 A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst 99:1120–1129 [DOI] [PubMed] [Google Scholar]
- Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P 2000 Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 11:847–852 [DOI] [PubMed] [Google Scholar]
- Corder EH, Guess HA, Hulka BS, Friedman GD, Sadler M, Vollmer RT, Lobaugh B, Drezner MK, Vogelman JH, Orentreich N 1993 Vitamin D and prostate cancer: a prediagnostic study with stored sera. Cancer Epidemiol Biomarkers Prev 2:467–472 [PubMed] [Google Scholar]
- Gann PH, Ma J, Hennekens CH, Hollis BW, Haddad JG, Stampfer MJ 1996 Circulating vitamin D metabolites in relation to subsequent development of prostate cancer. Cancer Epidemiol Biomarkers Prev 5:121–126 [PubMed] [Google Scholar]
- Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J 2007 A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med 4:e103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura AM, Stemmermann GN, Lee J, Kolonel LN, Chen TC, Turner A, Holick MF 1998 Serum vitamin D metabolite levels and the subsequent development of prostate cancer (Hawaii, United States). Cancer Causes Control 9:425–432 [DOI] [PubMed] [Google Scholar]
- Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E 2004 Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control 15:255–265 [DOI] [PubMed] [Google Scholar]
- Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, Stattin P, Harvei S, Hakulinen T, Luostarinen T, Dillner J, Lehtinen M, Hakama M 2004 Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer 108:104–108 [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE 2005 Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 14:1991–1997 [DOI] [PubMed] [Google Scholar]
- Colston KW, Lowe LC, Mansi JL, Campbell MJ 2006 Vitamin D status and breast cancer risk. Anticancer Res 26:2573–2580 [PubMed] [Google Scholar]
- Hiatt RA, Krieger N, Lobaugh B, Drezner MK, Vogelman JH, Orentreich N 1998 Prediagnostic serum vitamin D and breast cancer. J Natl Cancer Inst 90:461–463 [DOI] [PubMed] [Google Scholar]
- Vieth R 2002 Dairy products, calcium, and prostate cancer risk in the Physicians’ Health Study. Am J Clin Nutr 76:490–491 [DOI] [PubMed] [Google Scholar]
- Davis CD, Hartmuller V, Freedman DM, Hartge P, Picciano MF, Swanson CA, Milner JA 2007 Vitamin D and cancer: current dilemmas and future needs. Nutr Rev 65:S71–S74 [DOI] [PubMed] [Google Scholar]
- Giovannucci E 2007 Strengths and limitations of current epidemiologic studies: vitamin D as a modifier of colon and prostate cancer risk. Nutr Rev 65:S77–S79 [DOI] [PubMed] [Google Scholar]
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF 2007 Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med 32:210–216 [DOI] [PubMed] [Google Scholar]
- Freedman DM, Looker AC, Chang SC, Graubard BI 2007 Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst 99:1594–1602 [DOI] [PubMed] [Google Scholar]
- Abnet CC, Chen W, Dawsey SM, Wei WQ, Roth MJ, Liu B, Lu N, Taylor PR, Qiao YL 2007 Serum 25(OH)-vitamin D concentration and risk of esophageal squamous dysplasia. Cancer Epidemiol Biomarkers Prev 16:1889–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg-Solomon RZ, Vieth R, Azad A, Pietinen P, Taylor PR, Virtamo J, Albanes D 2006 A prospective nested case-control study of vitamin D status and pancreatic cancer risk in male smokers. Cancer Res 66:10213–10219 [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE 2006 Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 354:684–696 [DOI] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP 2007 Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85:1586–1591 [DOI] [PubMed] [Google Scholar]
- Woo TC, Choo R, Jamieson M, Chander S, Vieth R 2005 Pilot study: potential role of vitamin D (cholecalciferol) in patients with PSA relapse after definitive therapy. Nutr Cancer 51:32–36 [DOI] [PubMed] [Google Scholar]
- Gross C, Stamey T, Hancock S, Feldman D 1998 Treatment of early recurrent prostate cancer with 1,25-dihydroxyvitamin D3 (calcitriol). J Urol 159:2035–2039 [DOI] [PubMed] [Google Scholar]
- Beer TM, Javle M, Lam GN, Henner WD, Wong A, Trump DL 2005 Pharmacokinetics and tolerability of a single dose of DN-101, a new formulation of calcitriol, in patients with cancer. Clin Cancer Res 11:7794–7799 [DOI] [PubMed] [Google Scholar]
- Beer TM, Eilers KM, Garzotto M, Egorin MJ, Lowe BA, Henner WD 2003 Weekly high-dose calcitriol and docetaxel in metastatic androgen-independent prostate cancer. J Clin Oncol 21:123–128 [DOI] [PubMed] [Google Scholar]
- Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, Redfern CH, Fehrenbacher L, Saleh MN, Waterhouse DM, Carducci MA, Vicario D, Dreicer R, Higano CS, Ahmann FR, Chi KN, Henner WD, Arroyo A, Clow FW 2007 Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol 25:669–674 [DOI] [PubMed] [Google Scholar]
- Tiffany NM, Ryan CW, Garzotto M, Wersinger EM, Beer TM 2005 High dose pulse calcitriol, docetaxel and estramustine for androgen independent prostate cancer: a phase I/II study. J Urol 174:888–892 [DOI] [PubMed] [Google Scholar]
- Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS 2006 Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 106:2136–2142 [DOI] [PubMed] [Google Scholar]
- Masuda S, Jones G 2003 Vitamin D analogs–drug design based on proteins involved in vitamin D signal transduction. Curr Drug Targets Immune Endocr Metabol Disord 3:43–66 [PubMed] [Google Scholar]
- Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A 2006 Vitamin D and cancer. J Steroid Biochem Mol Biol 102:156–162 [DOI] [PubMed] [Google Scholar]
- Masuda S, Jones G 2006 Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther 5: 797–808 [DOI] [PubMed] [Google Scholar]
- Gulliford T, English J, Colston KW, Menday P, Moller S, Coombes RC 1998 A phase I study of the vitamin D analogue EB 1089 in patients with advanced breast and colorectal cancer. Br J Cancer 78:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhoff K, Dancey J, Astrup L, Skovsgaard T, Hamberg KJ, Lofts FJ, Rosmorduc O, Erlinger S, Bach HJ, Steward WP, Skov T, Burcharth F, Evans TR 2003 A phase II study of the vitamin D analogue seocalcitol in patients with inoperable hepatocellular carcinoma. Br J Cancer 89:252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TR, Colston KW, Lofts FJ, Cunningham D, Anthoney DA, Gogas H, de Bono JS, Hamberg KJ, Skov T, Mansi JL 2002 A phase II trial of the vitamin D analogue seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br J Cancer 86:680–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaert S, Duvold LB 2006 Calcipotriol cream: a review of its use in the management of psoriasis. J Dermatolog Treat 17:327–337 [DOI] [PubMed] [Google Scholar]
- Menter A, Griffiths CE 2007 Current and future management of psoriasis. Lancet 370:272–284 [DOI] [PubMed] [Google Scholar]
- Fogh K, Kragballe K 2004 New vitamin D analogs in psoriasis. Curr Drug Targets Inflamm Allergy 3:199–204 [DOI] [PubMed] [Google Scholar]
- Reichrath J 2007 Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol 16:618–625 [DOI] [PubMed] [Google Scholar]
- Veldman CM, Cantorna MT, DeLuca HF 2000 Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys 374:334–338 [DOI] [PubMed] [Google Scholar]
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE 2007 Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 179:1634–1647 [DOI] [PubMed] [Google Scholar]
- Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R 2003 Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol 170:5382–5390 [DOI] [PubMed] [Google Scholar]
- Overbergh L, Decallonne B, Valckx D, Verstuyf A, Depovere J, Laureys J, Rutgeerts O, Saint-Arnaud R, Bouillon R, Mathieu C 2000 Identification and immune regulation of 25-hydroxyvitamin D-1-α-hydroxylase in murine macrophages. Clin Exp Immunol 120:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusso AS, Kamimura S, Gallieni M, Zhong M, Negrea L, Shapiro S, Slatopolsky E 1997 γ-Interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab 82:2222–2232 [DOI] [PubMed] [Google Scholar]
- Esteban L, Vidal M, Dusso A 2004 1α-Hydroxylase transactivation by γ-interferon in murine macrophages requires enhanced C/EBPβ expression and activation. J Steroid Biochem Mol Biol 89–90:131–137 [DOI] [PubMed] [Google Scholar]
- Monkawa T, Yoshida T, Hayashi M, Saruta T 2000 Identification of 25-hydroxyvitamin D3 1α-hydroxylase gene expression in macrophages. Kidney Int 58:559–568 [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Pavlovitch J, Papiernik M, Guillozo H, Walrant-Debray O, Pontoux C, Garabedian M 1997 Changes in 1,25-(OH)2D3 synthesis and its receptor expression in spleen cell subpopulations of mice infected with LPBM5 retrovirus. Endocrinology 138:5505–5510 [DOI] [PubMed] [Google Scholar]
- Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C 2006 Immune regulation of 25-hydroxyvitamin-D3-1α-hydroxylase in human monocytes. J Bone Miner Res 21:37–47 [DOI] [PubMed] [Google Scholar]
- Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M 2003 Regulation of 25-hydroxyvitamin D3-1 α-hydroxylase and production of 1 α,25-dihydroxyvitamin D3 by human dendritic cells. Blood 102:3314–3316 [DOI] [PubMed] [Google Scholar]
- Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H 1993 1,25-Dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood 82:1300–1307 [PubMed] [Google Scholar]
- Vidal M, Ramana CV, Dusso AS 2002 Stat1-vitamin D receptor interactions antagonize 1,25-dihydroxyvitamin D transcriptional activity and enhance stat1-mediated transcription. Mol Cell Biol 22:2777–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Adorini L 2002 The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med 8:174–179 [DOI] [PubMed] [Google Scholar]
- van Etten E, Mathieu C 2005 Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 97:93–101 [DOI] [PubMed] [Google Scholar]
- Hakim I, Bar-Shavit Z 2003 Modulation of TNF-α expression in bone marrow macrophages: involvement of vitamin D response element. J Cell Biochem 88:986–998 [DOI] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH 2004 Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912 [DOI] [PubMed] [Google Scholar]
- Cippitelli M, Santoni A 1998 Vitamin D3: a transcriptional modulator of the interferon-γ gene. Eur J Immunol 28:3017–3030 [DOI] [PubMed] [Google Scholar]
- Towers TL, Freedman LP 1998 Granulocyte-macrophage colony-stimulating factor gene transcription is directly repressed by the vitamin D3 receptor. Implications for allosteric influences on nuclear receptor structure and function by a DNA element. J Biol Chem 273:10338–10348 [DOI] [PubMed] [Google Scholar]
- Towers TL, Staeva TP, Freedman LP 1999 A two-hit mechanism for vitamin D3-mediated transcriptional repression of the granulocyte-macrophage colony-stimulating factor gene: vitamin D receptor competes for DNA binding with NFAT1 and stabilizes c-Jun. Mol Cell Biol 19:4191–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine M, Watabe Y, Shimaoka S, Sato F, Kake K, Nishina H, Ohtsuki M, Nakagawa H, Tamaki K 1999 The action of a novel vitamin D3 analogue, OCT, on immunomodulatory function of keratinocytes and lymphocytes. Arch Dermatol Res 291:500–506 [DOI] [PubMed] [Google Scholar]
- Yu XP, Bellido T, Manolagas SC 1995 Down-regulation of NF-κB protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 92:10990–10994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert M, Kluter A, Zettl UK, Neeck G 2004 Transcription factors in autoimmune diseases. Curr Pharm Des 10:2787–2796 [DOI] [PubMed] [Google Scholar]
- Harant H, Wolff B, Lindley IJ 1998 1α,25-Dihydroxyvitamin D3 decreases DNA binding of nuclear factor-κB in human fibroblasts. FEBS Lett 436:329–334 [DOI] [PubMed] [Google Scholar]
- Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L 2004 A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol 173:2280–2287 [DOI] [PubMed] [Google Scholar]
- Heine G, Anton K, Henz BM, Worm M 2002 1α,25-Dihydroxyvitamin D3 inhibits anti-CD40 plus IL-4-mediated IgE production in vitro. Eur J Immunol 32:3395–3404 [DOI] [PubMed] [Google Scholar]
- Riis JL, Johansen C, Gesser B, Moller K, Larsen CG, Kragballe K, Iversen L 2004 1α,25(OH)(2)D(3) regulates NF-κB DNA binding activity in cultured normal human keratinocytes through an increase in IκBα expression. Arch Dermatol Res 296:195–202 [DOI] [PubMed] [Google Scholar]
- Dong X, Lutz W, Schroeder TM, Bachman LA, Westendorf JJ, Kumar R, Griffin MD 2005 Regulation of relB in dendritic cells by means of modulated association of vitamin D receptor and histone deacetylase 3 with the promoter. Proc Natl Acad Sci USA 102:16007–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto FL, Sun J, Kong J, Duan Y, Liao A, Madara JL, Li YC 2007 Involvement of the vitamin D receptor in the regulation of NF-κB activity in fibroblasts. J Steroid Biochem Mol Biol 103:563–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeva-Vieira TP, Freedman LP 2002 1,25-Dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol 168:1181–1189 [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Reddy GS, Kobayashi T, Okano T, Park J, Sharma S 1998 Nuclear factor of activated T cells (NFAT) as a molecular target for 1α,25-dihydroxyvitamin D3-mediated effects. J Immunol 160:209–218 [PubMed] [Google Scholar]
- Valdivielso JM, Fernandez E 2006 Vitamin D receptor polymorphisms and diseases. Clin Chim Acta 371:1–12 [DOI] [PubMed] [Google Scholar]
- Guo SW, Magnuson VL, Schiller JJ, Wang X, Wu Y, Ghosh S 2006 Meta-analysis of vitamin D receptor polymorphisms and type 1 diabetes: a HuGE review of genetic association studies. Am J Epidemiol 164:711–724 [DOI] [PubMed] [Google Scholar]
- Bailey R, Cooper JD, Zeitels L, Smyth DJ, Yang JH, Walker NM, Hypponen E, Dunger DB, Ramos-Lopez E, Badenhoop K, Nejentsev S, Todd JA 2007 Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 56:2616–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lopez E, Bruck P, Jansen T, Herwig J, Badenhoop K 2007 CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabetes Metab Res Rev 23:631–636 [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Baker I, Davey SG 2005 Meta-analysis of vitamin D receptor polymorphisms and pulmonary tuberculosis risk. Int J Tuberc Lung Dis 9:1174–1177 [PubMed] [Google Scholar]
- Lavin PJ, Laing ME, O'Kelly P, Moloney FJ, Gopinathan D, Aradi AA, Shields DC, Murphy GM, Conlon PJ 2007 Improved renal allograft survival with vitamin D receptor polymorphism. Ren Fail 29:785–789 [DOI] [PubMed] [Google Scholar]
- van Etten E, Verlinden L, Giulietti A, Ramos-Lopez E, Branisteanu DD, Ferreira GB, Overbergh L, Verstuyf A, Bouillon R, Roep BO, Badenhoop K, Mathieu C 2007 The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol 37:395–405 [DOI] [PubMed] [Google Scholar]
- Mathieu C, van Etten E, Gysemans C, Decallonne B, Kato S, Laureys J, Depovere J, Valckx D, Verstuyf A, Bouillon R 2001 In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J Bone Miner Res 16:2057–2065 [DOI] [PubMed] [Google Scholar]
- O'Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP 2002 Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest 109:1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler A, Merchav S, Fabian I, Tatarsky I, Weisman Y, Hochberg Z 1987 Myeloid progenitors from the bone marrow of patients with vitamin D resistant rickets (type II) fail to respond to 1,25(OH)2D3. Br J Haematol 67:267–271 [DOI] [PubMed] [Google Scholar]
- Lukic ML, Stosic-Grujicic S, Shahin A 1998 Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev Immunol 6:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R 2001 Dendritic cell modulation by 1α,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA 98:6800–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni E, Biffi M, Benigni F, Monno A, Carlucci D, Carmeliet G, Bouillon R, D'Ambrosio D 2007 VDR-dependent regulation of mast cell maturation mediated by 1,25-dihydroxyvitamin D3. J Leukoc Biol 81:250–262 [DOI] [PubMed] [Google Scholar]
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT 2003 A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 17:2386–2392 [DOI] [PubMed] [Google Scholar]
- Froicu M, Zhu Y, Cantorna MT 2006 Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology 117:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M, Cantorna MT 2007 Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol 8:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan TF, DeLuca HF 2002 The vitamin D receptor is necessary for 1α,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Arch Biochem Biophys 408:200–204 [DOI] [PubMed] [Google Scholar]
- Gysemans C, van Etten E, Overbergh L, Giulietti A, Eelen G, Waer M, Verstuyf A, Bouillon R, Mathieu C 2008 Unaltered diabetes presentation in NOD mice lacking the vitamin D receptor. Diabetes 57:269–275 [DOI] [PubMed] [Google Scholar]
- Alard P, Manirarora JN, Parnell SA, Hudkins JL, Clark SL, Kosiewicz MM 2006 Deficiency in NOD antigen-presenting cell function may be responsible for suboptimal CD4+CD25+ T-cell-mediated regulation and type 1 diabetes development in NOD mice. Diabetes 55:2098–2105 [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kinder SJ, Silvera P, Baxter AG 1997 Flow cytometric study of T cell development in NOD mice reveals a deficiency in αβTCR+CDR−CD8− thymocytes. J Autoimmun 10:279–285 [DOI] [PubMed] [Google Scholar]
- Serreze DV, Gaedeke JW, Leiter EH 1993 Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: defective regulation of cytokine receptors and protein kinase C. Proc Natl Acad Sci USA 90:9625–9629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulietti A, Gysemans C, Stoffels K, van Etten E, Decallonne B, Overbergh L, Bouillon R, Mathieu C 2004 Vitamin D deficiency in early life accelerates type 1 diabetes in non-obese diabetic mice. Diabetologia 47:451–462 [DOI] [PubMed] [Google Scholar]
- Zella JB, DeLuca HF 2003 Vitamin D and autoimmune diabetes. J Cell Biochem 88:216–222 [DOI] [PubMed] [Google Scholar]
- Dahlen E, Hedlund G, Dawe K 2000 Low CD86 expression in the nonobese diabetic mouse results in the impairment of both T cell activation and CTLA-4 up-regulation. J Immunol 164:2444–2456 [DOI] [PubMed] [Google Scholar]
- Nguyen M, d'Alesio A, Pascussi JM, Kumar R, Griffin MD, Dong X, Guillozo H, Rizk-Rabin M, Sinding C, Bougneres P, Jehan F, Garabedian M 2006 Vitamin D-resistant rickets and type 1 diabetes in a child with compound heterozygous mutations of the vitamin D receptor (L263R and R391S): dissociated responses of the CYP-24 and rel-B promoters to 1,25-dihydroxyvitamin D3. J Bone Miner Res 21:886–894 [DOI] [PubMed] [Google Scholar]
- Wittke A, Weaver V, Mahon BD, August A, Cantorna MT 2004 Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J Immunol 173:3432–3436 [DOI] [PubMed] [Google Scholar]
- Wittke A, Chang A, Froicu M, Harandi OF, Weaver V, August A, Paulson RF, Cantorna MT 2007 Vitamin D receptor expression by the lung micro-environment is required for maximal induction of lung inflammation. Arch Biochem Biophys 460:306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius RE 2007 Vitamins in control of lymphocyte migration. Nat Immunol 8:229–230 [DOI] [PubMed] [Google Scholar]
- Ehrchen J, Helming L, Varga G, Pasche B, Loser K, Gunzer M, Sunderkotter C, Sorg C, Roth J, Lengeling A 2007 Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J 21:3208–3218 [DOI] [PubMed] [Google Scholar]
- Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, Probst-Kepper M, Balling R, Lengeling A 2005 1α,25-Dihydroxyvitamin D3 is a potent suppressor of interferon γ-mediated macrophage activation. Blood 106:4351–4358 [DOI] [PubMed] [Google Scholar]
- Gavison R, Bar-Shavit Z 1989 Impaired macrophage activation in vitamin D3 deficiency: differential in vitro effects of 1,25-dihydroxyvitamin D3 on mouse peritoneal macrophage functions. J Immunol 143:3686–3690 [PubMed] [Google Scholar]
- Chan TY 2000 Vitamin D deficiency and susceptibility to tuberculosis. Calcif Tissue Int 66:476–478 [DOI] [PubMed] [Google Scholar]
- Davies PD, Brown RC, Woodhead JS 1985 Serum concentrations of vitamin D metabolites in untreated tuberculosis. Thorax 40:187–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange JM, Davies PD, Brown RC, Woodhead JS, Kardjito T 1985 A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle 66:187–191 [DOI] [PubMed] [Google Scholar]
- Waters WR, Palmer MV, Nonnecke BJ, Whipple DL, Horst RL 2004 Mycobacterium bovis infection of vitamin D-deficient NOS2−/− mice. Microb Pathog 36:11–17 [DOI] [PubMed] [Google Scholar]
- Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN 2000 Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 355:618–621 [DOI] [PubMed] [Google Scholar]
- Morcos MM, Gabr AA, Samuel S, Kamel M, el Baz M, el Beshry M, Michail RR 1998 Vitamin D administration to tuberculous children and its value. Boll Chim Farm 137:157–164 [PubMed] [Google Scholar]
- Nursyam EW, Amin Z, Rumende CM 2006 The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones 38:3–5 [PubMed] [Google Scholar]
- Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ 2007 A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med 176:208–213 [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL 2006 Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- Kankova M, Luini W, Pedrazzoni M, Riganti F, Sironi M, Bottazzi B, Mantovani A, Vecchi A 1991 Impairment of cytokine production in mice fed a vitamin D3-deficient diet. Immunology 73:466–471 [PMC free article] [PubMed] [Google Scholar]
- Yang S, Smith C, Prahl JM, Luo X, DeLuca HF 1993 Vitamin D deficiency suppresses cell-mediated immunity in vivo. Arch Biochem Biophys 303:98–106 [DOI] [PubMed] [Google Scholar]
- Peterlik M, Cross HS 2005 Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest 35:290–304 [DOI] [PubMed] [Google Scholar]
- Lim WC, Hanauer SB, Li YC 2005 Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol 2:308–315 [DOI] [PubMed] [Google Scholar]
- Pappa HM, Grand RJ, Gordon CM 2006 Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis 12:1162–1174 [DOI] [PubMed] [Google Scholar]
- Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B 2007 Vitamin D in rheumatoid arthritis. Autoimmun Rev 7:59–64 [DOI] [PubMed] [Google Scholar]
- Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG 2004 Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum 50:72–77 [DOI] [PubMed] [Google Scholar]
- Grant WB 2004 Solar UV-B radiation is linked to the geographic variation of mortality from systemic lupus erythematosus in the USA. Lupus 13:281–282 [DOI] [PubMed] [Google Scholar]
- Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS 2006 Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev 5:114–117 [DOI] [PubMed] [Google Scholar]
- Embry AF 2004 Vitamin D supplementation in the fight against multiple sclerosis. J Orthomolec Med 19:27–38 [Google Scholar]
- Munger KL, Zhang SM, O'Reilly E, Hernan MA, Olek MJ, Willett WC, Ascherio A 2004 Vitamin D intake and incidence of multiple sclerosis. Neurology 62:60–65 [DOI] [PubMed] [Google Scholar]
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A 2006 Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296:2832–2838 [DOI] [PubMed] [Google Scholar]
- Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM 2001 Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358:1500–1503 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Munsick C, Bemiss C, Mahon BD 2000 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130:2648–2652 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Mahon BD 2004 Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 229:1136–1142 [DOI] [PubMed] [Google Scholar]
- Hayes CE, Cantorna MT, DeLuca HF 1997 Vitamin D and multiple sclerosis. Proc Soc Exp Biol Med 216:21–27 [DOI] [PubMed] [Google Scholar]
- Ponsonby AL, McMichael A, van der Mei I 2002 Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology 181–182:71–78 [DOI] [PubMed] [Google Scholar]
- Auer DP, Schumann EM, Kumpfel T, Gossl C, Trenkwalder C 2000 Seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 47:276–277 [PubMed] [Google Scholar]
- Iikuni N, Nakajima A, Inoue E, Tanaka E, Okamoto H, Hara M, Tomatsu T, Kamatani N, Yamanaka H 2007 What’s in season for rheumatoid arthritis patients? Seasonal fluctuations in disease activity. Rheumatology (Oxford) 46:846–848 [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL 2003 IL-1β and IFN-γ induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia 46:255–266 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF 1996 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA 93:7861–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach KM, Hayes CE 2005 Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol 175:4119–4126 [DOI] [PubMed] [Google Scholar]
- Brekke HK, Ludvigsson J 2007 Vitamin D supplementation and diabetes-related autoimmunity in the ABIS study. Pediatr Diabetes 8:11–14 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Gysemans C, Giulietti A, Bouillon R 2005 Vitamin D and diabetes. Diabetologia 48:1247–1257 [DOI] [PubMed] [Google Scholar]
- Littorin B, Blom P, Scholin A, Arnqvist HJ, Blohme G, Bolinder J, Ekbom-Schnell A, Eriksson JW, Gudbjornsdottir S, Nystrom L, Ostman J, Sundkvist G 2006 Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 49:2847–2852 [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Manfrini S, Crino A, Picardi A, Leomanni C, Cherubini V, Valente L, Khazrai M, Visalli N 2005 Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res 37:680–683 [DOI] [PubMed] [Google Scholar]
- Soilu-Hanninen M, Airas L, Mononen I, Heikkila A, Viljanen M, Hanninen A 2005 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler 11:266–271 [DOI] [PubMed] [Google Scholar]
- Mathieu C, van Etten E, Gysemans C, Decallonne B, Bouillon R 2002 Seasonality of birth in patients with type 1 diabetes. Lancet 359:1248 [DOI] [PubMed] [Google Scholar]
- Mathieu C, van Etten E, Decallonne B, Guilietti A, Gysemans C, Bouillon R, Overbergh L 2004 Vitamin D and 1,25-dihydroxyvitamin D3 as modulators in the immune system. J Steroid Biochem Mol Biol 89–90:449–452 [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Weiss ST 2007 Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 120:1031–1035 [DOI] [PubMed] [Google Scholar]
- Camargo Jr CA, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW 2007 Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 85:788–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux G 2007 Early life events in asthma—diet. Pediatr Pulmonol 42:663–673 [DOI] [PubMed] [Google Scholar]
- Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C 2008 Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 62:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR 2004 Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann NY Acad Sci 1037:84–95 [DOI] [PubMed] [Google Scholar]
- Wjst M, Hypponen E 2007 Vitamin D serum levels and allergic rhinitis. Allergy 62:1085–1086 [DOI] [PubMed] [Google Scholar]
- Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ 2007 IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol 178:7190–7198 [DOI] [PubMed] [Google Scholar]
- Brent GA 2000 Tissue-specific actions of thyroid hormone: insights from animal models. Rev Endocr Metab Disord 1:27–33 [DOI] [PubMed] [Google Scholar]
- Adorini L 2005 Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol 233:115–124 [DOI] [PubMed] [Google Scholar]
- Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A 2001 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol 167:4974–4980 [DOI] [PubMed] [Google Scholar]
- Griffin MD, Xing N, Kumar R 2003 Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr 23:117–145 [DOI] [PubMed] [Google Scholar]
- Gauzzi MC, Purificato C, Donato K, Jin Y, Wang L, Daniel KC, Maghazachi AA, Belardelli F, Adorini L, Gessani S 2005 Suppressive effect of 1α,25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J Immunol 174:270–276 [DOI] [PubMed] [Google Scholar]
- Penna G, Adorini L 2000 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164:2405–2411 [DOI] [PubMed] [Google Scholar]
- van Halteren AG, van Etten E, de Jong EC, Bouillon R, Roep BO, Mathieu C 2002 Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3). Diabetes 51:2119–2125 [DOI] [PubMed] [Google Scholar]
- D'Ambrosio D 2006 Regulatory T cells: how do they find their space in the immunological arena? Semin Cancer Biol 16:91–97 [DOI] [PubMed] [Google Scholar]
- van Halteren AG, Tysma OM, van Etten E, Mathieu C, Roep BO 2004 1α,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J Autoimmun 23:233–239 [DOI] [PubMed] [Google Scholar]
- Bouillon R, Verlinden L, Eelen G, De Clercq P, Vandewalle M, Mathieu C, Verstuyf A 2005 Mechanisms for the selective action of vitamin D analogs. J Steroid Biochem Mol Biol 97:21–30 [DOI] [PubMed] [Google Scholar]
- Adorini L 2002 1,25-Dihydroxyvitamin D3 analogs as potential therapies in transplantation. Curr Opin Investig Drugs 3:1458–1463 [PubMed] [Google Scholar]
- Baeke F, van Etten E, Overbergh L, Mathieu C 2007 Vitamin D3 and the immune system: maintaining the balance in health and disease. Nutr Res Rev 20:106–118 [DOI] [PubMed] [Google Scholar]
- DeLuca HF, Cantorna MT 2001 Vitamin D: its role and uses in immunology. FASEB J 15:2579–2585 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Laureys J, Sobis H, Vandeputte M, Waer M, Bouillon R 1992 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 41:1491–1495 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R 1994 Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 37:552–558 [DOI] [PubMed] [Google Scholar]
- Mathieu C, Waer M, Casteels K, Laureys J, Bouillon R 1995 Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin D3, KH1060. Endocrinology 136:866–872 [DOI] [PubMed] [Google Scholar]
- Casteels K, Waer M, Bouillon R, Depovere J, Valckx D, Laureys J, Mathieu C 1998 1,25-Dihydroxyvitamin D3 restores sensitivity to cyclophosphamide-induced apoptosis in non-obese diabetic (NOD) mice and protects against diabetes. Clin Exp Immunol 112:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbergh L, Decallonne B, Waer M, Rutgeerts O, Valckx D, Casteels KM, Laureys J, Bouillon R, Mathieu C 2000 1α,25-Dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543). Diabetes 49:1301–1307 [DOI] [PubMed] [Google Scholar]
- Casteels KM, Mathieu C, Waer M, Valckx D, Overbergh L, Laureys JM, Bouillon R 1998 Prevention of type I diabetes in nonobese diabetic mice by late intervention with nonhypercalcemic analogs of 1,25-dihydroxyvitamin D3 in combination with a short induction course of cyclosporin A. Endocrinology 139:95–102 [DOI] [PubMed] [Google Scholar]
- Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L 2002 A 1α,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51:1367–1374 [DOI] [PubMed] [Google Scholar]
- Casteels K, Waer M, Laureys J, Valckx D, Depovere J, Bouillon R, Mathieu C 1998 Prevention of autoimmune destruction of syngeneic islet grafts in spontaneously diabetic nonobese diabetic mice by a combination of a vitamin D3 analog and cyclosporine. Transplantation 65:1225–1232 [DOI] [PubMed] [Google Scholar]
- Gysemans C, van Etten E, Overbergh L, Verstuyf A, Waer M, Bouillon R, Mathieu C 2002 Treatment of autoimmune diabetes recurrence in non-obese diabetic mice by mouse interferon-β in combination with an analogue of 1α,25-dihydroxyvitamin-D3. Clin Exp Immunol 128:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi T, Nakao Y, Matsui T, Nakagawa T, Matsuda S, Komoriya K, Kanai Y, Fujita T 1985 Effects of corticosteroid and 1,24R-dihydroxy-vitamin D3 administration on lymphoproliferation and autoimmune disease in MRL/MP-lpr/lpr mice. Int Arch Allergy Appl Immunol 77:396–404 [DOI] [PubMed] [Google Scholar]
- Branisteanu DD, Waer M, Sobis H, Marcelis S, Vandeputte M, Bouillon R 1995 Prevention of murine experimental allergic encephalomyelitis: cooperative effects of cyclosporine and 1α,25-(OH)2D3. J Neuroimmunol 61:151–160 [DOI] [PubMed] [Google Scholar]
- Branisteanu DD, Mathieu C, Bouillon R 1997 Synergism between sirolimus and 1,25-dihydroxyvitamin D3 in vitro and in vivo. J Neuroimmunol 79:138–147 [DOI] [PubMed] [Google Scholar]
- Lemire JM, Archer DC 1991 1,25-Dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest 87:1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Etten E, Branisteanu DD, Verstuyf A, Waer M, Bouillon R, Mathieu C 2000 Analogs of 1,25-dihydroxyvitamin D3 as dose-reducing agents for classical immunosuppressants. Transplantation 69:1932–1942 [DOI] [PubMed] [Google Scholar]
- van Etten E, Branisteanu DD, Overbergh L, Bouillon R, Verstuyf A, Mathieu C 2003 Combination of a 1,25-dihydroxyvitamin D3 analog and a bisphosphonate prevents experimental autoimmune encephalomyelitis and preserves bone. Bone 32:397–404 [DOI] [PubMed] [Google Scholar]
- van Etten E, Gysemans C, Branisteanu DD, Verstuyf A, Bouillon R, Overbergh L, Mathieu C 2007 Novel insights in the immune function of the vitamin D system: synergism with interferon-β. J Steroid Biochem Mol Biol 103:546–551 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF 1998 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr 128:68–72 [DOI] [PubMed] [Google Scholar]
- Larsson P, Mattsson L, Klareskog L, Johnsson C 1998 A vitamin D analogue (MC 1288) has immunomodulatory properties and suppresses collagen-induced arthritis (CIA) without causing hypercalcaemia. Clin Exp Immunol 114:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna G, Amuchastegui S, Cossetti C, Aquilano F, Mariani R, Sanvito F, Doglioni C, Adorini L 2006 Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol 177:8504–8511 [DOI] [PubMed] [Google Scholar]
- Fournier C, Gepner P, Sadouk M, Charreire J 1990 In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis. Clin Immunol Immunopathol 54:53–63 [DOI] [PubMed] [Google Scholar]
- Branisteanu DD, Leenaerts P, Van Damme B, Bouillon R 1993 Partial prevention of active Heymann nephritis by 1 α, 25 dihydroxyvitamin D3. Clin Exp Immunol 94:412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire JM, Ince A, Takashima M 1992 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 12:143–148 [DOI] [PubMed] [Google Scholar]
- Andjelkovic Z, Vojinovic J, Pejnovic N, Popovic M, Dujic A, Mitrovic D, Pavlica L, Stefanovic D 1999 Disease modifying and immunomodulatory effects of high dose 1 α (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheumatol 17:453–456 [PubMed] [Google Scholar]
- Wingerchuk DM, Lesaux J, Rice GP, Kremenchutzky M, Ebers GC 2005 A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 76:1294–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescioli C, Ferruzzi P, Caporali A, Scaltriti M, Bettuzzi S, Mancina R, Gelmini S, Serio M, Villari D, Vannelli GB, Colli E, Adorini L, Maggi M 2004 Inhibition of prostate cell growth by BXL-628, a calcitriol analogue selected for a phase II clinical trial in patients with benign prostate hyperplasia. Eur J Endocrinol 150:591–603 [DOI] [PubMed] [Google Scholar]
- Crescioli C, Morelli A, Adorini L, Ferruzzi P, Luconi M, Vannelli GB, Marini M, Gelmini S, Fibbi B, Donati S, Villari D, Forti G, Colli E, Andersson KE, Maggi M 2005 Human bladder as a novel target for vitamin D receptor ligands. J Clin Endocrinol Metab 90:962–972 [DOI] [PubMed] [Google Scholar]
- Colli E, Rigatti P, Montorsi F, Artibani W, Petta S, Mondaini N, Scarpa R, Usai P, Olivieri L, Maggi M 2006 BXL628, a novel vitamin D3 analog arrests prostate growth in patients with benign prostatic hyperplasia: a randomized clinical trial. Eur Urol 49:82–86 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Woodward WD, Hayes CE, DeLuca HF 1998 1,25-Dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-β 1 and IL-4. J Immunol 160:5314–5319 [PubMed] [Google Scholar]
- Cantorna MT, Humpal-Winter J, DeLuca HF 2000 In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D(3). Arch Biochem Biophys 377:135–138 [DOI] [PubMed] [Google Scholar]
- Carswell S 1997 Vitamin D in the nervous system: actions and therapeutic potential. In: Feldman D, Glorieux FH, Pike JW, eds. Vitamin D. San Diego: Academic Press; 1197–1211 [Google Scholar]
- Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, Martino G, Panina-Bordignon P, Adorini L 2000 Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur J Immunol 30:498–508 [DOI] [PubMed] [Google Scholar]
- Nashold FE, Hoag KA, Goverman J, Hayes CE 2001 Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D(3) prevention of experimental autoimmune encephalomyelitis. J Neuroimmunol 119:16–29 [DOI] [PubMed] [Google Scholar]
- Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM 2008 Immune modulatory treatment of TNBS colitis with calcitriol is associated with a change of a Th1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther 324:23–33 [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK 2007 T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 8:345–350 [DOI] [PubMed] [Google Scholar]
- Bertolini DL, Araujo PR, Silva RN, Duarte AJ, Tzanno-Martins CB 1999 Immunomodulatory effects of vitamin D analog KH1060 on an experimental skin transplantation model. Transplant Proc 31:2998–2999 [DOI] [PubMed] [Google Scholar]
- Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L 2001 Regulatory T cells induced by 1 α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 167:1945–1953 [DOI] [PubMed] [Google Scholar]
- Hullett DA, Cantorna MT, Redaelli C, Humpal-Winter J, Hayes CE, Sollinger HW, DeLuca HF 1998 Prolongation of allograft survival by 1,25-dihydroxyvitamin D3. Transplantation 66:824–828 [DOI] [PubMed] [Google Scholar]
- Johnsson C, Tufveson G 1994 MC 1288—a vitamin D analogue with immunosuppressive effects on heart and small bowel grafts. Transpl Int 7:392–397 [DOI] [PubMed] [Google Scholar]
- Redaelli CA, Wagner M, Tien YH, Mazzucchelli L, Stahel PF, Schilling MK, Dufour JF 2001 1 α,25-Dihydroxycholecalciferol reduces rejection and improves survival in rat liver allografts. Hepatology 34:926–934 [DOI] [PubMed] [Google Scholar]
- Redaelli CA, Wagner M, Gunter-Duwe D, Tian YH, Stahel PF, Mazzucchelli L, Schmid RA, Schilling MK 2002 1α,25-Dihydroxyvitamin D3 shows strong and additive immunomodulatory effects with cyclosporine A in rat renal allotransplants. Kidney Int 61:288–296 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hullett DA, Redaelli C, Brandt CR, Humpal-Winter J, Sollinger HW, DeLuca HF 1998 1,25-Dihydroxyvitamin D3 prolongs graft survival without compromising host resistance to infection or bone mineral density. Transplantation 66:828–831 [DOI] [PubMed] [Google Scholar]
- Amuchastegui S, Daniel KC, Adorini L 2005 Inhibition of acute and chronic allograft rejection in mouse models by BXL-628, a nonhypercalcemic vitamin D receptor agonist. Transplantation 80:81–87 [DOI] [PubMed] [Google Scholar]
- Raisanen-Sokolowski AK, Pakkala IS, Samila SP, Binderup L, Hayry PJ, Pakkala ST 1997 A vitamin D analog, MC1288, inhibits adventitial inflammation and suppresses intimal lesions in rat aortic allografts. Transplantation 63:936–941 [DOI] [PubMed] [Google Scholar]
- Xu H, Soruri A, Gieseler RK, Peters JH 1993 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol 38:535–540 [DOI] [PubMed] [Google Scholar]
- Ganz T 2003 Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720 [DOI] [PubMed] [Google Scholar]
- Zasloff M 2002 Antimicrobial peptides of multicellular organisms. Nature 415:389–395 [DOI] [PubMed] [Google Scholar]
- Adams JS, Liu PT, Chun R, Modlin RL, Hewison M 2007 Vitamin D in defense of the human immune response. Ann NY Acad Sci 1117:94–105 [DOI] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP 2005 Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077 [DOI] [PubMed] [Google Scholar]
- Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, Bikle DD, Gallo RL 2008 Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D(3). J Invest Dermatol 128:816–824 [DOI] [PubMed] [Google Scholar]
- Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zugel U, Steinmeyer A, Pollak A, Roth E, Boltz-Nitulescu G, Spittler A 2006 Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol 36:361–370 [DOI] [PubMed] [Google Scholar]
- Norman AW, Frankel JB, Heldt AM, Grodsky GM 1980 Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 209:823–825 [DOI] [PubMed] [Google Scholar]
- Lee S, Clark SA, Gill RK, Christakos S 1994 1,25-Dihydroxyvitamin D3 and pancreatic β-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology 134:1602–1610 [DOI] [PubMed] [Google Scholar]
- de Boland AR, Norman AW 1990 Influx of extracellular calcium mediates 1,25-dihydroxyvitamin D3-dependent transcaltachia (the rapid stimulation of duodenal Ca2+ transport). Endocrinology 127:2475–2480 [DOI] [PubMed] [Google Scholar]
- Norman AW, Okamura WH, Bishop JE, Henry HL 2002 Update on biological actions of 1α,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3. Mol Cell Endocrinol 197:1–13 [DOI] [PubMed] [Google Scholar]
- Sergeev IN, Rhoten WB 1995 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic β-cell line. Endocrinology 136:2852–2861 [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Kestekian R, Havrankova J, Gascon-Barre M 1993 Calcium is essential in normalizing intolerance to glucose that accompanies vitamin D depletion in vivo. Diabetes 42:35–43 [DOI] [PubMed] [Google Scholar]
- Cade C, Norman AW 1986 Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 119:84–90 [DOI] [PubMed] [Google Scholar]
- Chertow BS, Sivitz WI, Baranetsky NG, Clark SA, Waite A, DeLuca HF 1983 Cellular mechanisms of insulin release: the effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology 113:1511–1518 [DOI] [PubMed] [Google Scholar]
- Gedik O, Akalin S 1986 Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia 29:142–145 [DOI] [PubMed] [Google Scholar]
- Isaia G, Giorgino R, Adami S 2001 High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care 24:1496 [DOI] [PubMed] [Google Scholar]
- Kadowaki S, Norman AW 1984 Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest 73:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyomba BL, Bouillon R, De Moor P 1984 Influence of vitamin D status on insulin secretion and glucose tolerance in the rabbit. Endocrinology 115:191–197 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Seino Y, Ishida M, Yamaoka K, Yabuuchi H, Ishida H, Seino S, Seino Y, Imura H 1984 Effect of vitamin D3 on the pancreatic secretion of insulin and somatostatin. Acta Endocrinol (Copenh) 105:528–533 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Seino Y, Ishida M, Yamaoka K, Satomura K, Yabuuchi H, Seino Y, Imura H 1986 Effect of 1,25-dihydroxyvitamin D3 on insulin secretion: direct or mediated? Endocrinology 118:1971–1976 [DOI] [PubMed] [Google Scholar]
- Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG 2003 Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 17:509–511 [DOI] [PubMed] [Google Scholar]
- Billaudel BJ, Faure AG, Sutter BC 1990 Effect of 1,25 dihydroxyvitamin D3 on isolated islets from vitamin D3-deprived rats. Am J Physiol 258:E643–E648 [DOI] [PubMed] [Google Scholar]
- Billaudel BJ, Delbancut AP, Sutter BC, Faure AG 1993 Stimulatory effect of 1,25-dihydroxyvitamin D3 on calcium handling and insulin secretion by islets from vitamin D3-deficient rats. Steroids 58:335–341 [DOI] [PubMed] [Google Scholar]
- Kadowaki S, Norman AW 1985 Time course study of insulin secretion after 1,25-dihydroxyvitamin D3 administration. Endocrinology 117:1765–1771 [DOI] [PubMed] [Google Scholar]
- Allegra V, Luisetto G, Mengozzi G, Martimbianco L, Vasile A 1994 Glucose-induced insulin secretion in uremia: role of 1 α,25(HO)2-vitamin D3. Nephron 68:41–47 [DOI] [PubMed] [Google Scholar]
- Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ 1995 Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia 38:1239–1245 [DOI] [PubMed] [Google Scholar]
- d'Emden MC, Dunlop M, Larkins RG, Wark JD 1989 The in vitro effect of 1 α,25-dihydroxyvitamin D3 on insulin production by neonatal rat islets. Biochem Biophys Res Commun 164:413–418 [DOI] [PubMed] [Google Scholar]
- Hahn HJ, Kuttler B, Mathieu C, Bouillon R 1997 1,25-Dihydroxyvitamin D3 reduces MHC antigen expression on pancreatic β-cells in vitro. Transplant Proc 29:2156–2157 [DOI] [PubMed] [Google Scholar]
- Kumar S, Davies M, Zakaria Y, Mawer EB, Gordon C, Olukoga AO, Boulton AJ 1994 Improvement in glucose tolerance and β-cell function in a patient with vitamin D deficiency during treatment with vitamin D. Postgrad Med J 70:440–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio D, Andersen H, Karlsen A, Mandrup-Poulsen T, Nerup J Effect of steroidal hormones on interleukin-1β action on rat islets. p A80 (Abstract) [Google Scholar]
- Riachy R, Vandewalle B, Belaich S, Kerr-Conte J, Gmyr V, Zerimech F, d'Herbomez M, Lefebvre J, Pattou F 2001 Beneficial effect of 1,25 dihydroxyvitamin D3 on cytokine-treated human pancreatic islets. J Endocrinol 169:161–168 [DOI] [PubMed] [Google Scholar]
- Riachy R, Vandewalle B, Kerr CJ, Moerman E, Sacchetti P, Lukowiak B, Gmyr V, Bouckenooghe T, Dubois M, Pattou F 2002 1,25-Dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the antiapoptotic protein A20. Endocrinology 143:4809–4819 [DOI] [PubMed] [Google Scholar]
- Sandler S, Buschard K, Bendtzen K 1994 Effects of 1,25-dihydroxyvitamin D3 and the analogues MC903 and KH1060 on interleukin-1 β-induced inhibition of rat pancreatic islet β-cell function in vitro. Immunol Lett 41:73–77 [DOI] [PubMed] [Google Scholar]
- Gysemans CA, Cardozo AK, Callewaert H, Giulietti A, Hulshagen L, Bouillon R, Eizirik DL, Mathieu C 2005 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology 146:1956–1964 [DOI] [PubMed] [Google Scholar]
- Pittas AG, Dawson-Hughes B, Li T, van Dam RM, Willett WC, Manson JE, Hu FB 2006 Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 29:650–656 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Riddle M, Prince M 1994 Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. Am J Clin Nutr 59:1083–1087 [DOI] [PubMed] [Google Scholar]
- Taylor AV, Wise PH 1998 Vitamin D replacement in Asians with diabetes may increase insulin resistance. Postgrad Med J 74:365–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Ajani UA, McGuire LC, Liu S 2005 Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 28:1228–1230 [DOI] [PubMed] [Google Scholar]
- Pittas AG, Harris SS, Stark PC, Dawson-Hughes B 2007 The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 30:980–986 [DOI] [PubMed] [Google Scholar]
- Ruth Wu-Wong J, Nakane M, Ma J, Cook AL 2005 Vitamin D analogs down-regulate plasminogen activator inhibitor-1 in human coronary artery smooth muscle cells. J Thromb Haemost 3:1545–1546 [DOI] [PubMed] [Google Scholar]
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP 2002 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC 2005 Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 288:E125–E132 [DOI] [PubMed] [Google Scholar]
- Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J 2004 Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 89–90:387–392 [DOI] [PubMed] [Google Scholar]
- Simpson RU, Hershey SH, Nibbelink KA 2007 Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol 103:521–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU 2008 Functional vitamin D receptor in the T-tubules of cardiac myocytes. Endocrinology 149:558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC 2008 Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 73:163–171 [DOI] [PubMed] [Google Scholar]
- Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D 2005 Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68:2823–2828 [DOI] [PubMed] [Google Scholar]
- Kristal-Boneh E, Froom P, Harari G, Ribak J 1997 Association of calcitriol and blood pressure in normotensive men. Hypertension 30:1289–1294 [DOI] [PubMed] [Google Scholar]
- Lind L, Hanni A, Lithell H, Hvarfner A, Sorensen OH, Ljunghall S 1995 Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens 8:894–901 [DOI] [PubMed] [Google Scholar]
- Burgess ED, Hawkins RG, Watanabe M 1990 Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens 3:903–905 [DOI] [PubMed] [Google Scholar]
- Resnick LM, Muller FB, Laragh JH 1986 Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med 105:649–654 [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C 2001 Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab 86:1633–1637 [DOI] [PubMed] [Google Scholar]
- Lind L, Wengle B, Wide L, Ljunghall S 1989 Reduction of blood pressure during long-term treatment with active vitamin D (alphacalcidol) is dependent on plasma renin activity and calcium status. A double-blind, placebo-controlled study. Am J Hypertens 2:20–25 [DOI] [PubMed] [Google Scholar]
- Ohsawa M, Koyama T, Yamamoto K, Hirosawa S, Kamei S, Kamiyama R 2000 1α,25-Dihydroxyvitamin D(3) and its potent synthetic analogs down-regulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation 102:2867–2872 [DOI] [PubMed] [Google Scholar]
- Koyama T, Shibakura M, Ohsawa M, Kamiyama R, Hirosawa S 1998 Anticoagulant effects of 1α,25-dihydroxyvitamin D3 on human myelogenous leukemia cells and monocytes. Blood 92:160–167 [PubMed] [Google Scholar]
- Aihara K, Azuma H, Akaike M, Ikeda Y, Yamashita M, Sudo T, Hayashi H, Yamada Y, Endoh F, Fujimura M, Yoshida T, Yamaguchi H, Hashizume S, Kato M, Yoshimura K, Yamamoto Y, Kato S, Matsumoto T 2004 Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem 279:35798–35802 [DOI] [PubMed] [Google Scholar]
- Asakura H, Aoshima K, Suga Y, Yamazaki M, Morishita E, Saito M, Miyamoto K, Nakao S 2001 Beneficial effect of the active form of vitamin D3 against LPS-induced DIC but not against tissue-factor-induced DIC in rat models. Thromb Haemost 85:287–290 [PubMed] [Google Scholar]
- Beer TM, Venner PM, Ryan CW, Petrylak DP, Chatta G, Ruether JD, Chi KN, Curd JG, Deloughery TG 2006 High dose calcitriol may reduce thrombosis in cancer patients. Br J Haematol 135:392–394 [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Nakane M, Ma J 2007 Vitamin D analogs modulate the expression of plasminogen activator inhibitor-1, thrombospondin-1 and thrombomodulin in human aortic smooth muscle cells. J Vasc Res 44:11–18 [DOI] [PubMed] [Google Scholar]
- Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV 2006 Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 41:350–359 [DOI] [PubMed] [Google Scholar]
- Johnson RC, Leopold JA, Loscalzo J 2006 Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99:1044–1059 [DOI] [PubMed] [Google Scholar]
- Verberckmoes R, Bouillon R, Krempien B 1975 Disappearance of vascular calcifications during treatment of renal osteodystrophy. Two patients treated with high doses of vitamin D and aluminum hydroxide. Ann Intern Med 82:529–533 [DOI] [PubMed] [Google Scholar]
- Autier P, Gandini S 2007 Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–1737 [DOI] [PubMed] [Google Scholar]
- Doherty TM, Tang W, Dascalos S, Watson KE, Demer LL, Shavelle RM, Detrano RC 1997 Ethnic origin and serum levels of 1α,25-dihydroxyvitamin D3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation 96:1477–1481 [DOI] [PubMed] [Google Scholar]
- Pittas AG, Lau J, Hu FB, Dawson-Hughes B 2007 The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL 1997 Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation 96:1755–1760 [DOI] [PubMed] [Google Scholar]
- Zittermann A, Schleithoff SS, Koerfer R 2007 Vitamin D and vascular calcification. Curr Opin Lipidol 18:41–46 [DOI] [PubMed] [Google Scholar]
- Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M 2007 Calcium/vitamin D supplementation and cardiovascular events. Circulation 115:846–854 [DOI] [PubMed] [Google Scholar]
- Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y 2004 Lower risk for cardiovascular mortality in oral 1α-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant 19:179–184 [DOI] [PubMed] [Google Scholar]
- Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R 2003 Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349:446–456 [DOI] [PubMed] [Google Scholar]
- London GM, Guerin AP, Verbeke FH, Pannier B, Boutouyrie P, Marchais SJ, Metivier F 2007 Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol 18:613–620 [DOI] [PubMed] [Google Scholar]
- Boland R 1986 Role of vitamin D in skeletal muscle function. Endocr Rev 7:434–448 [DOI] [PubMed] [Google Scholar]
- Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T 2003 Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144:5138–5144 [DOI] [PubMed] [Google Scholar]
- Vazquez G, de Boland AR, Boland RL 1998 1α,25-Dihydroxy-vitamin-D3-induced store-operated Ca2+ influx in skeletal muscle cells. Modulation by phospholipase c, protein kinase c, and tyrosine kinases. J Biol Chem 273:33954–33960 [DOI] [PubMed] [Google Scholar]
- Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, Allain TJ 2002 Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res 17:891–897 [DOI] [PubMed] [Google Scholar]
- Visser M, Deeg DJ, Lips P 2003 Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 88:5766–5772 [DOI] [PubMed] [Google Scholar]
- Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P 2007 Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab 92:2058–2065 [DOI] [PubMed] [Google Scholar]
- Kenny AM, Biskup B, Robbins B, Marcella G, Burleson JA 2003 Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J Am Geriatr Soc 51:1762–1767 [DOI] [PubMed] [Google Scholar]
- Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, Thomas J, Lowndes C, Hopper JL, Wark JD 2005 Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc 53:1881–1888 [DOI] [PubMed] [Google Scholar]
- Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP 2007 A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc 55:234–239 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, Wong JB 2004 Effect of Vitamin D on falls: a meta-analysis. JAMA 291:1999–2006 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B 2006 Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med 166:424–430 [DOI] [PubMed] [Google Scholar]
- Law M, Withers H, Morris J, Anderson F 2006 Vitamin D supplementation and the prevention of fractures and falls: results of a randomised trial in elderly people in residential accommodation. Age Ageing 35:482–486 [DOI] [PubMed] [Google Scholar]
- Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P 2007 Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab 92:1415–1423 [DOI] [PubMed] [Google Scholar]
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A 2007 Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 370:657–666 [DOI] [PubMed] [Google Scholar]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D 2002 New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 13:100–105 [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Wion D, Brachet P 1996 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 7:2171–2175 [DOI] [PubMed] [Google Scholar]
- Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M 1994 1,25-Dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport 6:124–126 [DOI] [PubMed] [Google Scholar]
- Wion D, MacGrogan D, Neveu I, Jehan F, Houlgatte R, Brachet P 1991 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res 28:110–114 [DOI] [PubMed] [Google Scholar]
- Burne TH, McGrath JJ, Eyles DW, Mackay-Sim A 2005 Behavioural characterization of vitamin D receptor knockout mice. Behav Brain Res 157:299–308 [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Lou YR, Laaksi I, Tuohimaa P 2004 Increased grooming behavior in mice lacking vitamin D receptors. Physiol Behav 82:405–409 [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Lou YR, Laaksi I, Tuohimaa P 2005 Abnormal behavioral organization of grooming in mice lacking the vitamin D receptor gene. J Neurogenet 19:1–24 [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Lou YR, Laaksi I, Tuohimaa P 2004 Impaired motor performance in mice lacking neurosteroid vitamin D receptors. Brain Res Bull 64:25–29 [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Lou YR, Laaksi I, Tuohimaa P 2004 Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport 15:1271–1274 [DOI] [PubMed] [Google Scholar]
- Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F 2003 Vitamin D3 and brain development. Neuroscience 118:641–653 [DOI] [PubMed] [Google Scholar]
- Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, Feron F 2007 Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 103:538–545 [DOI] [PubMed] [Google Scholar]
- Feron F, Burne TH, Brown J, Smith E, McGrath JJ, Mackay-Sim A, Eyles DW 2005 Developmental vitamin D3 deficiency alters the adult rat brain. Brain Res Bull 65:141–148 [DOI] [PubMed] [Google Scholar]
- Almeras L, Eyles D, Benech P, Laffite D, Villard C, Patatian A, Boucraut J, Mackay-Sim A, McGrath J, Feron F 2007 Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics 7:769–780 [DOI] [PubMed] [Google Scholar]
- Becker A, Eyles DW, McGrath JJ, Grecksch G 2005 Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res 161:306–312 [DOI] [PubMed] [Google Scholar]
- Kesby JP, Burne TH, McGrath JJ, Eyles DW 2006 Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: an animal model of schizophrenia. Biol Psychiatry 60:591–596 [DOI] [PubMed] [Google Scholar]
- Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH 2008 Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res 187:343–350 [DOI] [PubMed] [Google Scholar]
- Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, Wang Y 2001 Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res 904:67–75 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, Lin SZ 2000 Vitamin D(3) attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology 39:873–880 [DOI] [PubMed] [Google Scholar]
- McGrath J, Eyles D, Mowry B, Yolken R, Buka S 2003 Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res 63:73–78 [DOI] [PubMed] [Google Scholar]
- Przybelski RJ, Binkley NC 2007 Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys 460:202–205 [DOI] [PubMed] [Google Scholar]
- Halloran BP, DeLuca HF 1980 Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr 110:1573–1580 [DOI] [PubMed] [Google Scholar]
- Kwiecinksi GG, Petrie GI, DeLuca HF 1989 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol 256:E483–E487 [DOI] [PubMed] [Google Scholar]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y 2000 Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 141:1317–1324 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Haji M, Takayanagi R, Tanaka S, Sugioka Y, Nawata H 1996 1,25-Dihydroxyvitamin D3 enhances the enzymatic activity and expression of the messenger ribonucleic acid for aromatase cytochrome P450 synergistically with dexamethasone depending on the vitamin D receptor level in cultured human osteoblasts. Endocrinology 137:1860–1869 [DOI] [PubMed] [Google Scholar]
- Corbett ST, Hill O, Nangia AK 2006 Vitamin D receptor found in human sperm. Urology 68:1345–1349 [DOI] [PubMed] [Google Scholar]
- Johnson LE, DeLuca HF 2001 Vitamin D receptor null mutant mice fed high levels of calcium are fertile. J Nutr 131:1787–1791 [DOI] [PubMed] [Google Scholar]
- Lachenmaier-Currle U, Harmeyer J 1989 Placental transport of calcium and phosphorus in pigs. J Perinat Med 17:127–136 [DOI] [PubMed] [Google Scholar]
- Rummens K, van Bree R, Van Herck E, Zaman Z, Bouillon R, Van Assche FA, Verhaeghe J 2002 Vitamin D deficiency in guinea pigs: exacerbation of bone phenotype during pregnancy and disturbed fetal mineralization, with recovery by 1,25(OH)2D3 infusion or dietary calcium-phosphate supplementation. Calcif Tissue Int 71:364–375 [DOI] [PubMed] [Google Scholar]
- Rummens K, Van Cromphaut SJ, Carmeliet G, Van Herck E, van Bree R, Stockmans I, Bouillon R, Verhaeghe J 2003 Pregnancy in mice lacking the vitamin D receptor: normal maternal skeletal response, but fetal hypomineralization rescued by maternal calcium supplementation. Pediatr Res 54:466–473 [DOI] [PubMed] [Google Scholar]
- Lieben L, Van Looveren R, Bouillon R, Carmeliet G Fetal hypervitaminosis D results in neonatal lethality. p S183 (Abstract) [Google Scholar]
- Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM 2007 Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92:3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely EW 2007 Calciotropic hormones in preeclampsia: a renewal of interest. J Clin Endocrinol Metab 92:3402–3403 [DOI] [PubMed] [Google Scholar]
- Dawodu A, Wagner CL 2007 Mother-child vitamin D deficiency: an international perspective. Arch Dis Child 92:737–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra SH, van Beek A, Janssen JW, de Vleeschouwer LH, Huysman WA, van den Akker EL 2007 High prevalence of vitamin D deficiency in newborn infants of high-risk mothers. Arch Dis Child 92:750–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer I, Karamali NS, Boeke AJ, Lips P, Middelkoop BJ, Verhoeven I, Wuister JD 2006 High prevalence of vitamin D deficiency in pregnant non-Western women in The Hague, Netherlands. Am J Clin Nutr 84:350–353 [DOI] [PubMed] [Google Scholar]
- Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, Arden NK, Godfrey KM, Cooper C 2006 Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367:36–43 [DOI] [PubMed] [Google Scholar]
- Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN 2006 Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3–L1 cells. J Biol Chem 281:11205–11213 [DOI] [PubMed] [Google Scholar]
- Sato M, Hiragun A 1988 Demonstration of 1 α,25-dihydroxyvitamin D3 receptor-like molecule in ST 13 and 3T3 L1 preadipocytes and its inhibitory effects on preadipocyte differentiation. J Cell Physiol 135:545–550 [DOI] [PubMed] [Google Scholar]