Abstract
Aim
The complex pathobiology of traumatic brain injury (TBI) offers numerous targets for potential neuroprotective agents. We evaluate the clinical benefit after creatine (Cr) administration in children and adolescents
Methods
A prospective, randomized, comparative, open- labelled pilot study of the possible neuroprotective effect of Cr was carried out on 39 children and adolescents, aged between 1 and 18 years of age, with TBI. The Cr was administered for 6 months, at a dose of 0.4 g/kg in an oral suspension form every day. For categorical variables, we used the Chi-square test to identify differences between controls and cases. Statistical significance was defined as a p-value < 0.05 and not statistically significant if p-value > 0.1.
Results
The administration of Cr to children and adolescents with TBI improved results in several parameters, including duration of post traumatic amnesia (PTA), duration of intubation, intensive care unit stay. Significant improvement was recorded in the categories of headache (p < 0.001), dizziness (p = 0.005) and fatigue (p < 0.001), aspects in all patients. No side effects were seen due to Cr administration.
Conclusion
More specific examinations including brain spectroscopy for in vivo evaluation of Cr can be done, in order to draw conclusions for the optimal duration and manner of Cr supply, as well as its possible role for the prevention of TBI complications, in double blind studies.
INTRODUCTION
The most common cause of death and disability after severe trauma in childhood is traumatic brain injury (TBI). Of all trauma deaths, 25% are caused by head injury (1). Much is yet to be learned regarding the effects of severe head trauma on brain development, but available studies suggest that neuropsychiatric recovery after significant head injury takes a long time following resolution of the immediate effects of the injury.
Functional deficits result from primary and secondary mechanisms after TBI. The primary damage occurs at the time of impact and results from the mechanical insult itself. The secondary injury, defined as cellular damage not immediately apparent after the trauma but developing within minutes, hours or even days, seems to be related to mitochondria dysfunction associated with the disruption in cellular calcium homeostasis that is known to occur after TBI. Maintenance of cellular calcium homeostasis is intimately related to adenosine triphosphate (ATP) use and synthesis, which are key to proper brain functioning. Enhanced neuron survival may be improved by providing an adequate supply of ATP immediately after trauma (2).
Creatine ([Cr] a-methyl-guanidinoacetic acid) is an amino acid endogenously produced from glycine, methionine and arginine in the liver, kidney and pancreas (1). Cr supplementation increases intramuscular and cerebral stores with both Cr, and its phosphorylated form, phosphocreatine [PCr] (3). The increase of these stores may offer therapeutic benefits by stimulating protein synthesis or reducing protein degradation, stabilizing biological membranes and preventing ATP depletion, which occurs in patients with TBI (4,5). Recent findings in animal models have demonstrated that Cr affords significant neuroprotection against experimental brain injury (6,7).
Based on these experimental facts, we studied administration of Cr to patients with TBI (8–13).
SUBJECTS AND METHODS
The institutional ethics committee of the University Hospital of Heraklion approved the study protocol. A prospective study was conducted in the Paediatric Surgical Department of Crete and Thessaloniki from February 2000 to March 2004. Thirty-nine children and adolescents were enrolled after obtaining informed consent from their parents. They were randomized to study and control groups, with the study group receiving Cr using Glasgow Coma Scale (GCS) scores as the randomization factor (3–5 vs. 6–9). Pearson's Chi-square test showed that there is no statistically significant difference between the two groups p = 0.835. We assessed power estimation for categorical variables (disability or death vs. good recovery). Initial considerations were: Type I error was set at 0.05 and 20 patients per arm were selected. The probability for a disability outcome was set at 85%. This percentage was estimated from previously published studies (14–16). The disability outcome for Cr group was set at 0.50. The power of this estimation was calculated at 66, 8%.
Entry and exclusion criteria
Patients eligible for the study had to satisfy the following inclusion criteria:
All patients should have age between 1 and 18 years,
GCS should be between 3 and 9 on admission,
a paediatric trauma score (PTS) should be between −4 and 12 on admission and
treatment could be initiated within 4 h from the time of injury.
Exclusion criteria were: 1. history of previous admission to hospital for head injury, 2. known psychiatric disorder or mental retardation, 3. received other medication within 30 days of enrolment. In general, patients were admitted directly to the hospital within 1–4 h after injury. The GCS and PTS were assessed as part of the neurological examination.
Therapy
According to random numbers, patients were allocated to receive either Cr at a dosage of 0.4 g/kg in an oral suspension form every day for 6 months or nothing. Cr was mixed with water or apple juice then flushed through nasogastric tube or given with a spoon.
Follow-up
After discharge from the hospital a follow-up was done at 6 months after injury by the same blinded surgeon in each hospital. During each visit a checklist of complaints was filled out, together with a structured interview and a neurological examination.
Statistical analysis
A parametric (Student's t-test) and a nonparametric test (Mann–Whitney U-test) were used to identify if there were differences between selected continuous variables in controls and cases.
For categorical variables, we used the Chi-square test to identify differences between controls and cases. Statistically significance was defined as a p-value < 0.05, trend towards significant if 0.05 < p-value < 0.1 and not statistically significant if p-value > 0.1.
RESULTS
Forty-eight children with TBI were admitted to our hospitals during the study. Three children were not eligible and the parents of four children refused to have their children participate in the study. A total of 39 eligible children were randomly selected.
There were 19 children (patients) in group I (controls) and 20 children in group II (Cr). Four children died during the time period of the study, (within 3 months), two from each group.
Twenty of the children in the study group received Cr at a dosage of 0.4 g/kg in an oral suspension form every day for 6 months, and 19 children received nothing. There were no significant differences between the two study groups. The patient characteristics are given in Table S1.
Using the Chi-square test, there were no statistically significant differences between the CT/MRI of brain, concomitant injuries, electroencephalogram, whether or not an operation was carried out, the GCS and the PTS between the two groups.
Outcome assessment (short term)
The mean duration of intubation, length of stay in intensive care (ICU), hospital stay and post traumatic amnesia (PTA) was compared between the two groups and the results are given in Table S2.
Outcome assessment (long term)
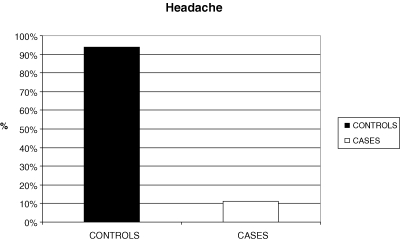
There was a statistically significant difference between the controls and cases and the groups of ‘headache’ (χ2= 23.139; df = 1; p < 0.001).
More specifically, the proportion of children having headache is significantly higher in controls than cases (93.8% vs. 11.1%), while the proportion of children having no headache is significantly higher in cases than controls (88.9% vs. 6.3%). (Fig. 1).
Figure 1.
Bar chart for Headache of controls and cases.
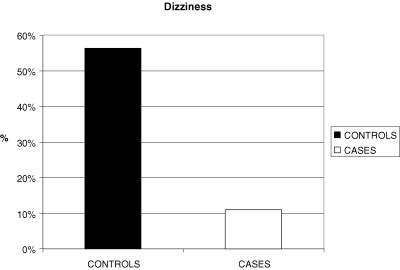
There was a statistically significant difference between the controls and cases and the groups of ‘dizziness’ (χ2= 7.886; df = 1; p = 0.005).
More specifically, the proportion of children having ‘dizziness’ is significantly higher in controls than cases (56.3% vs. 11.1%) while the proportion of children having no ‘dizziness’ is significantly higher in cases than controls (88.9% vs. 43.8%; Fig. 2).
Figure 2.
Bar chart for dizziness of controls and cases.
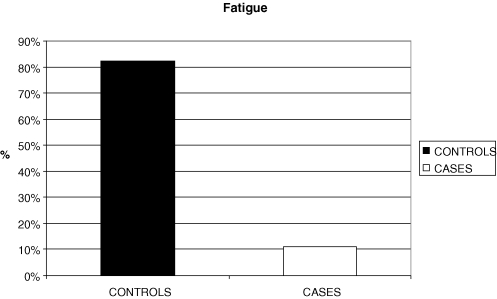
There was a statistically significant difference between the controls and cases and the groups of ‘fatigue’ (χ2= 17.881; df = 1; p < 0.001).
More specifically, the proportion of children having no fatigue is significantly higher in cases than controls (88.9% vs. 17.6%) while the proportions of children having fatigue is significantly higher in controls than cases (82.4% vs. 11.1%; Fig. 3).
Figure 3.
Bar chart for fatigue of controls and cases.
DISCUSSION
The Cr supplementation in children with TBI represents an attempt to provide neuroprotection for these children. In this study, the Cr administration gave encouraging results in several parameters, including posttraumatic headache, dizziness and fatigue, which are some of the most common symptoms after closed head injuries (2–4).
Posttraumatic headache can occur after mild, moderate or severe craniocerebral trauma. The headache may have features of usually two types: 1. tension-type headache that may be daily or episodic, 2. migraine headaches that are usually more severe lasting hours to days and 3. mixed headaches, both chronic daily headache and migraines (14). If the headaches develop within 2 weeks of the event, and persist for more than several months, we consider this to be the chronic phase of the posttraumatic headache syndrome (14,15). The fact that, at 6 months follow up, the Cr group had a statistically significant difference regarding the posttraumatic headache, provides evidence that the supply of Cr may be an adequate preventive treatment even for the rebound-headaches that may develop from the overuse of analgesic medications (16), as part of a multidimensional management approach including pharmacologic intervention, physical rehabilitation and cognitive behavioural therapy as used in the adult population (17). Positional vertigo, and particularly Bening Paroxysmal Positional Vertigo (BPPV), is the most common type of severe dizziness, and it is almost common after head injury. It is easily recognized by the pattern of dizziness that is brought only when head is placed in a certain position. Dizziness can be accompanied by noises in the ear, fullness or hearing changes as in posttraumatic Meniere's syndrome. Also, it can be accompanied by hearing loss, a nystagmus or peripheral facial weakness as in temporal bone fracture and labyrinthine concussion. A specialized examination is very useful in determining the character of the dizziness like measurement of balance, searching for nystagmus related to head and/or neck position or to vibration of the neck. Finally, in most instances an audiogram and an MRI scan or CT scan of the inner ear is included in the laboratory tests (18,19).
Generally, fatigue is defined as a sense of weakness, described by patients variously as exhaustion, tiredness, lack of energy and/or low vitality. It is also important to distinguish between cognitive/mental fatigue versus physical/somatic fatigue. Many times, persons with TBI will experience both of these phenomena. Some of the most important comorbidities of TBI, especially as they related to their role in generating fatigue include major depression and/or anxiety disorder and chronic pain that has clearly been shown to be of high prevalence after TBI (20).
Cr can play an important role as a neuroprotective agent for the prevention of these symptoms that are part of the posttraumatic syndrome, that affect the quality of life of both children and their families.
How Cr works remains to be determined. Work done in animal models suggested that the mechanical basis for the neuroprotective effects of Cr might involve alterations of the insult-induced depletions of cellular ATP. Chronic ingestion of Cr results in increased brain levels of PCr (21). Similar results regarding maintenance of cellular ATP levels have been demonstrated in animal models receiving Cr before TBI, particularly the effect of chronic administration of Cr ameliorated the extent of cortical damage by such as 36% in mice and 50% in rats (2). Lactate and free fatty acids, which are markers of secondary cellular injury following TBI, have been found to be lower in animals treated with Cr before TBI (3). Other mechanisms underlying this neuroprotection may involve the maintenance of mitochondrial integrity (22,23). The Cr action may prevent structural mitochondrial changes, as was shown in experimental work with adult rat cardiomyocytes cultured in Cr-deficient medium (24). The possible beneficial effect of Cr on mitochondrial function is also demonstrated in different clinical studies about Cr action on mitochondriopathies (22,23).
There is no doubt that the supply of Cr to patients with TBI needs further investigation, with more patients in double-blind studies and with longer follow-up. More specific examinations including brain spectroscopy for in vivo evaluation of Cr can be done, in order to draw conclusions for the optimal duration and manner of Cr supply, as well as its possible role for the prevention of TBI complications.
Acknowledgments
We thank the physician, nursing staff of the adult's intensive care unit of the University Hospital of Heraklion for the aid in subject enrolment and data collection.
Supplementary material
The following supplementary material is available for this article:
Patient characteristics
Short term outcome assessment
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1651-2227.2007.00529.x (This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Tegas JJ, III, DiScala C, Ramenofsky MC, Barlow B. Mortality and head injury: the paediatric perspective. J Pediatr Surg. 1990;25:92–6. doi: 10.1016/s0022-3468(05)80170-8. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan G, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–9. [PubMed] [Google Scholar]
- 3.Scheff SW, Dhillon HS. Creatine-enhanced diet alters levels of lactate and free fatty acids after experimental brain injury. Neurochem Res. 2004;29:469–79. doi: 10.1023/b:nere.0000013753.22615.59. [DOI] [PubMed] [Google Scholar]
- 4.Gasparovic C, Arfai N, Smid N, Feeney DM. Decrease and recovery of N-acetylaspartase/creatine in rat brain remote from focal injury. J Neurotrauma. 2001;18:241–6. doi: 10.1089/08977150151070856. [DOI] [PubMed] [Google Scholar]
- 5.Stockler S, Marescau B, De Deyn PP, Trijbels JM, Hanefeld F. Guanidino compounds in guanidinoacetate methyltransferase deficiency, a new inborn error of creatine synthesis. Metabolism. 1997;46:1189–93. doi: 10.1016/s0026-0495(97)90215-8. [DOI] [PubMed] [Google Scholar]
- 6.Schulze A, Bachert P, Schlemmer H, Harting I, Polster T, Salomons GS, et al. Lack of creatine in muscle and brain in an adult with GAMT deficiency. Ann Neurol. 2003;53:248–51. doi: 10.1002/ana.10455. [DOI] [PubMed] [Google Scholar]
- 7.Tarnopolsky MA, Roy BD, MacDonald JR. A randomized controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve. 1997;20:1502–9. doi: 10.1002/(sici)1097-4598(199712)20:12<1502::aid-mus4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Berger R, Middelanis J, Vaihinger HM, Mies G, Wilken B, Jensen A. Creatine protects the immature brain from hypoxic damage: dose-effect relationship. Brain Res. 1999;816:124–30. [Google Scholar]
- 9.Balestrino M, Rebaudo R, Lunardi G. Exogenous creatine delays anoxic depolarization and protects from hypoxic damage: dose-effect relationship. Brain Res. 1999;816:124–30. doi: 10.1016/s0006-8993(98)01131-7. [DOI] [PubMed] [Google Scholar]
- 10.Felber S, Skladal D, Wyss M, Kremser C, Koller A, Sperl W. Oral creatine supplementation in Duchenne muscular dystrophy: a clinical and 31P magnetic resonance spectroscopy study. Neurol Res. 2000;22:145–50. doi: 10.1080/01616412.2000.11741051. [DOI] [PubMed] [Google Scholar]
- 11.Gordon A, Hultmann E, Kaijser L, Kristjansson S, Rolf CJ, Nyquist O, Sylven C. Creatine supplementation in chronic heart failure increases skeletal muscles creatine phosphate and muscle performance. Cardiovascular Res. 1995;30:413–8. [PubMed] [Google Scholar]
- 12.Holtzman D, Togliatti A, Khait I, Jensen F. Creatine increases survival and suppresses seizures in the hypoxic immature rat. Paediatric Res. 1998;44:410–4. doi: 10.1203/00006450-199809000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Sakellaris G, Kotsiou M, Tamiolaki M, Kalostos G, Tsapaki E, Spanaki M, et al. Prevention of complications related to traumatic brain injury in children and adolescents with creatine administration. An open label randomised pilot study. J. Trauma. 2006;61:322–9. doi: 10.1097/01.ta.0000230269.46108.d5. [DOI] [PubMed] [Google Scholar]
- 14.Martelli MF, Grayson RL, Zasler ND. Posttraumatic headache: neuropsychological and psychological effects and treatment implications. J Head Trauma Rehabil. 1999;14:49–69. doi: 10.1097/00001199-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Evans RW. Post-traumatic headaches. Neurol Clin. 2004;22:237–49. doi: 10.1016/S0733-8619(03)00097-5. viii. [DOI] [PubMed] [Google Scholar]
- 16.Lane JC, Arciniegas DB. Post-traumatic headache. Curr Treat Options Neurol. 2002;4:89–104. doi: 10.1007/s11940-002-0007-3. [DOI] [PubMed] [Google Scholar]
- 17.Wilson MC, Krolczyk SJ. Pediatric post-traumatic headache. Curr Pain Headache Rep. 2006;10:387–90. doi: 10.1007/s11916-006-0065-4. [DOI] [PubMed] [Google Scholar]
- 18.Ylikoski J, Palva T, Sanna M. Dizziness after head trauma: clinical and morphologic findings. Am J Otol. 1982;3:343–52. [PubMed] [Google Scholar]
- 19.Eviatar L, Bergtraum M, Randel RM. Post-traumatic vertigo in children: a diagnostic approach. Paediatric Neurol. 1986;2:61–6. doi: 10.1016/0887-8994(86)90058-5. [DOI] [PubMed] [Google Scholar]
- 20.Stulemeijer M, van der Werf S, Bleijenberg G, Biert J, Brauer J, E Vos P. Recovery from mild traumatic brain injury: a focus on fatigue. J Neurol. 2006;253:1041–7. doi: 10.1007/s00415-006-0156-5. Epub 2006 May 17. [DOI] [PubMed] [Google Scholar]
- 21.Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5:347–50. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 22.Lifshitz J, Friberg H, Neumar RW, Raghupathi R, Welsh FA, Janmey P, et al. Structural and functional damage sustained by mitochondria after traumatic brain injury in the rat: evidence for differentially sensitive populations in the cortex and hippocampus. J Cereb Blood Flow Metab. 2003;23:219–31. doi: 10.1097/01.WCB.0000040581.43808.03. [DOI] [PubMed] [Google Scholar]
- 23.Borges N, Cerejo A, Santos A, Sarmento A, Azevedo I. Changes in rat cerebral mitochondrial succinate dehydrogenase activity after brain trauma. Int J Neurosci. 2004;114:217–27. doi: 10.1080/00207450490249419. [DOI] [PubMed] [Google Scholar]
- 24.Eppenberger-Eberhardt M, Riesinger I, Messerli M, Schwarb P, Muller M, Eppenberger HM, et al. Adult rat cardiomyocytes cultured in creatine-deficient medium display large mitochondria with paracrystalline inclusions, enriched for creatine kinase. J Cell Biol. 1991;113:289–302. doi: 10.1083/jcb.113.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient characteristics
Short term outcome assessment