Abstract
Emerging evidence indicates that chemokines can regulate both the physiology and biochemistry of CNS neurons and glia. In the current study, Western blot analysis showed that in rat hippocampal neuronal/glial cultures the signal transduction pathway activated by CCL2, a chemokine expressed in the normal brain and at elevated levels during neuroinflammation, involves a G-protein coupled receptor, p38 MAPK as well as its immediate upstream kinase MKK3/6, and the downstream transcription factor CREB. ERK 1/2 and the transcription factors STAT1 and STAT3 do not play a prominent role. CCL2 also altered Ca2+ influx and synaptic network activity in the hippocampal neurons. These results suggest an important role for p38 MAPK and CREB in hippocampal actions of CCL2.
Keywords: Chemokines, CREB, MAP kinase pathways, Hippocampal neurons, CCR2
1. Introduction
The chemokine CCL2, a 14 kDa protein previously known as monocyte chemoattractive protein-1, was originally described in the immune system as a potent chemoattractant for monocytes and macrophages (Matsushima et al., 1989). CCL2 and its primary receptor CCR2 are also widely expressed within the central nervous system (CNS), implicating roles for CCL2 in the CNS as well as in the immune system (Ambrosini and Aloisi, 2004; Bajetto et al., 2002). Sources of CCL2 within the CNS include microglia and astrocytes, although some neurons can also produce CCL2 (Babcock et al., 2003; Banisadr et al., 2005a; Farina et al, 2007; Josselyn and Nguyen, 2005; Kielian et al.., 2002; Weiss and Berman, 1998)
Emerging evidence supports both physiological and pathological roles for CCL2 in the CNS. CCL2 expression in the normal CNS starts at an early stage of development, suggesting a physiological role for CCL2 in CNS development (Geppert, 2003; Meng et al., 1999). However, a significant upregulation or dysregulation of CNS expression of CCL2 occurs in a variety of acute and chronic neuroinflammatory and neurodegenerative CNS disorders, implicating a role for CCL2 in pathological conditions. For example, CCL2 levels in the CNS are elevated in brain injury (Little et al., 2006), cerebral ischemia (Che et al., 2001; Minami et al., 2006), multiple sclerosis (Mahad and Ransohoff, 2003; McManus et al., 1998), human immunodeficiency virus (HIV)-associated dementia (Cinque et al., 1998) and Alzheimer’s disease (Galimberti et al., 2006). Both neurotoxic and neuroprotective roles for CCL2 have been proposed in these conditions.
CCL2 and other chemokines elicit their biological effects through interactions with G protein-coupled receptors. CCL2 preferentially binds to the chemokine receptor CCR2 (Bajetto et al., 2002). Chemokine receptors are linked to a number of downstream signal transduction pathways that mediate their biological effects (Bajetto et al., 2002). In immune cells, CCL2 signaling is reported to involve Gi/Go proteins and cellular calcium flux or activation of phospholipase C (Cambien et al., 2001; Dubois et al., 1996; Kuang et al., 1996). CCL2 has also been shown to trigger tyrosine phosphorylation and activation of Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) pathway in immune cells (Mellado et al., 1998) and to activate mitogen-activated protein kinases (MAPK) (Dubois et al., 1996; Jimenez-Sainz et al., 2003).
CCR2 is expressed in several regions of the normal CNS including in the cortex, hippocampus, caudate putamen, amygdala, hypothalamus, and cerebellum (Banisadr et al., 2002). Relatively little is known about the downstream signal transduction pathways linked to CCR2 in these brain regions or the biological consequences of receptor activation. This information is central to an understanding of the roles played by CCL2 in the CNS under physiological or pathological conditions. As a first step toward addressing these issues, we examined the signal transduction pathways activated by CCL2 in primary cultures of rat hippocampal cells. Because MAPK pathways are one of the major signaling pathways utilized by chemokines and are pathways that play a central role in hippocampal function, our studies focused on these pathways. Results show that CCL2 dramatically induces phosphorylation of p38 MAPK, which then acts to increase the level of phosphorylation of the transcription factor cAMP response element-binding protein (CREB). CCL2 also altered Ca2+ influx and synaptic network activity in the hippocampal neurons.
Experimental procedures
Animals
Timed-pregnant Sprague-Dawley rats for hippocampal culture were obtained from Charles River (Wilmington, MD, USA). The animal procedures were performed in accordance with the National Institutes of Health Guideline for the Care and Use of Laboratory Animals. Animal facilities and experimental protocols were in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
Primary cultures of rat hippocampal cells
Cultures were prepared from hippocampi of Sprague-Dawley rat embryos at 20 days of gestation and maintained as previously described (Nelson and Gruol, 2004). In brief, hippocampi were isolated, minced, and triturated through fire-polished Pasteur pipettes in saline containing (final concentrations in mM): 137 NaCl, 5.4 KCl, 0.17 Na2HPO4, 0.22 KH2PO4, 33.3 D-glucose, 43.8 sucrose, 10 HEPES-NaOH (pH 7.3). The cell suspension was plated at a density of 1 hippocampus per glass bottom culture dish (for immunohistochemistry; MatTek Corporation, Ashland, MA, USA) or 2.5 hippocampi per 35 mm culture dish (for Western blotting; Falcon Plastics, Franklin Lakes, NJ, USA) pre-coated with Matrigel (BD Biosciences, Bedford, MA, USA) in minimum essential medium (MEM) with Earle’s salts and L-glutamine (Gibco-Invitrogen, Carlsbad, CA, USA) supplemented with D-glucose (final concentration, 5.0 g/L) and 5% heat-inactivated fetal calf serum and 10% horse serum (Gibco-Invitrogen). The culture medium was exchanged twice weekly with a medium containing the same composition as above without fetal calf serum. Cultures were maintained at 37°C in a humidified atmosphere of 95% air/5% CO2. Proliferation of non-neuronal cells in cultures was arrested by the treatment with the anti-mitotic agent 5-fluorodeoxyuridine (20 μg/ml) at 3 days in vitro (DIV) for 3 days. For all studies, chemicals were of reagent grade or higher and were obtained from Sigma (St. Louis, MO, USA) unless noted otherwise.
CCL2 treatment
Acute treatment with various concentrations of recombinant rat CCL2/MCP-1 (PeproTech Inc. Rocky Hill, NJ, USA) was carried out at 13 DIV. The biological activity of the CCL2 used, determined by chemoattraction of human monocytes, was 10–100 ng/ml (0.7–7 nM; PeproTech Inc). We tested concentrations ranging from 1–25 nM (14–357 ng/ml) to provide dose-response information. Based on the reported biological activity of the CCL2 in the immune cells, this range is likely to reflect physiological to supraphysiological concentrations of CCL2. Concentration dependent effects of CCL2 with an EC50 of 5.5 nM have been reported for CCL2 depression of GABA-A currents in spinal neurons (Gosselin et al., 2005), consistent with physiological actions of CCL2 in the low nM range in neurons.
Immunohistochemistry
Immunohistochemical staining of the hippocampal cultures was performed at 15–23 DIV according to the methods described previously (Nelson and Gruol, 2004; van Gassen et al., 2005), using the affinity-purified goat polyclonal antibody raised against a peptide mapping at the carboxy terminus of the C-C chemokine receptor gene type 2B (CKR-2B) of mouse origin. In brief, cultures were rinsed with phosphate-buffered saline (PBS, 100 mM, pH 7.3) and fixed with 4% paraformaldehyde in PBS for 15 min, and permeablized with 0.05% Triton X-100 in PBS for 30 min. Endogenous biotin was blocked using the materials and methods provided in the endogenous blocking kit (Molecular Probes, Eugene, OR, USA). Cultures were incubated overnight at 4°C in PBS containing the primary antibody (1:100 dilution) and 0.05% BSA as a blocking agent. Immunoreactivity was detected by an immunoperoxidase reaction using Vectastain Elite ABC kit (Vector Laboratories (Burlingame, CA, USA) according to the manufacturer’s instruction. As a control for non-specific staining, sister cultures were co-incubated with the primary antibody and the antigenic peptide (1:50 dilution) used to produce the primary antibody.
Western blotting analyses
Hippocampal cultures were serum-starved for 20 h and treated with CCL2 at the concentrations indicated for the given periods of time as described in the figure legends. Then the cultures were rinsed 3 times with ice-cold PBS, lysed in the lysis buffer containing 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 4.5 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 1 mM NaF, 1 mM Na3VO4, 1% Triton X-100, 0.5% NP-40, and protease inhibitor cocktail (Boehringer, Indianapolis, IN, USA) for 30 min at 4°C, and then centrifuged at 14,000 rpm for 30 min. Protein concentrations in the supernatant were determined using a BioRad Dc protein assay kit (BioRad, Hercules, CA, USA). Equal amounts of protein (5–20 μg per lane) were separated by SDS-PAGE using 10% Novex NuPage Bis-Tris gels (Invitrogen Life Technologies, Carlsbad, CA, USA) and transferred overnight onto Immobilon-P membranes (Millipore, Billerica, MA, USA). Uniform transfer was confirmed by Ponceau S staining. The membranes were blocked for 1 h at room temperature in Tris-buffered saline (TBS), 0.05% Casein (Pierce Biotechnology, Rockford, IL, USA) and 0.1% Tween 20, and incubated overnight at 4°C with primary antibodies. After washing, the membranes were incubated for 1 h with anti-goat secondary IgG coupled to HRP, and immunoreactive bands were visualized on photographic film (Kodak, VWR Scientific Products, San Diego, CA, USA) using the ECL detection system (Pierce Biotechnology).
To determine the relative level of protein loaded, membranes were stripped with Pierce Restore Stripping buffer (Pierce Biotechnology, Rockford, Ill., USA) for 20 min, washed 5 times with 0.1% Tween 20 in PBS, and reprobed using anti-β-actin. The density of each band was quantified and normalized against the corresponding density of β-actin in the same lane. Normalized data from CCL2-treated cultures were then normalized to the normalized values for control cultures. Protein signals were quantified from photographic film using the public domain NIH image program (available on the Internet at http://rsb.info.nih.gov/nih-image/).
In some cases, the same membranes used for immunoblotting with the antibodies recognizing phosphorylated kinases were stripped and reprobed for total kinase (phosphorylated plus non-phosphorylated forms). Total kinase levels (normalized to β-actin levels in the same lane) were similar across culture dishes within a culture set. The immunoblots shown are the representatives of at least 3 separate experiments. The relevant regions of the blots are shown in the figures.
Antibodies
The antibodies against human phospho-p38 MAP kinase (#9215, Cell signaling Technology, Danvers, MA, USA, 1:1000 dilution) and human phospho-ERK 1/2 (#4377, Cell signaling Technology, 1:1000 dilution) are rabbit monoclonal and specifically recognize the corresponding kinases that are dually phosphorylated on tyrosine and threonine residues. The affinity purified rabbit polyclonal antibody against human phospho-mitogen-activated protein kinase kinase (MKK) 3/6 recognizes the kinase phosphorylated on serine residues at 189 and 207 (#9231, Cell Signaling Technology; 1:1000 dilution). The rabbit monoclonal antibody against human pCREB recognizes the amino acid sequence containing phospho-serine corresponding to residue 133 (#9198, Cell Signaling Technology; 1:750 dilution). The affinity purified rabbit polyclonal antibody against human phospho-STAT1 detects p91 STAT1 phosphorylated at tyrosine 701 and also the p84 splice varient (#9171, Cell Signaling Technology; 1:1000 dilution). The affinity purified rabbit polyclonal antibody against mouse phospho-STAT3 detects STAT3 phosphorylated at tyrosine 705 (#9131, Cell Signaling Technology; 1:1000 dilution). Other antibodies used are: an affinity purified rabbit polyclonal antibody raised against a synthetic peptide derived from the sequence of human p38 MAPK (#9212, Cell Signaling Technology; 1:1000 dilution); a mouse monoclonal antibody raised against a synthetic peptide derived from the sequence of rat p44/42 MAPK (#4696, Cell Signaling Technology; 1:1000 dilution); an affinity purified rabbit polyclonal antibody raised against a synthetic peptide derived from the sequence of human MKK3 (#9232, Cell Signaling Technology; 1:1000 dilution); an affinity purified rabbit polyclonal antibody raised against a synthetic peptide derived from the sequence of human CREB (#9192, Cell Signaling Technology; 1:1000 dilution); an affinity purified rabbit polyclonal antibody raised against a synthetic peptide corresponding to residues 712 to 750 of human STAT1α (KAP-TF001, StressGen Biotechnologies Corp, Victoria, Canada; 1–1000 dilution); an affinity purified rabbit polyclonal antibody raised against a synthetic peptide corresponding to residues 686 to 709 of human STAT3; a monoclonal antibody against β-actin (A5441; Sigma, St. Louis, MO, USA, 1:5000 dilution); an affinity purified goat antibody raised against a peptide mapping at the C-terminus of human CKR-2B (CCR2: #sc-6228, Santa Cruz Biotechnology, INC, Santa Cruz, CA, USA; 1:100 dilution); an affinity purified rabbit polyclonal antibody raised against a peptide mapping at the amino terminus of human BDNF of human origin (#sc-546, Santa Cruz Biotechnology, INC, 1:500 dilution); horseradish peroxidase (HRP)-conjugated secondary antibodies (Southern Biotech, Birmingham, AL, USA; 1:10,000 dilution).
Calcium imaging
Standard microscopic digital imaging techniques were used to measure intracellular Ca2+ levels as described previously (Przewlocki et al., 1999). The hippocampal cultures were loaded with the Ca2+ sensitive dye fura-2/AM (Molecular Probes) at 3 μM in physiological saline containing 0.02% pluronic F-127 (Molecular Probes) for 30 minutes at room temperature. The composition of the physiological saline was (in mM): 140 NaCl, 3.5 KCl, 0.4 KH2PO4, 1.25 Na2HPO4, 2.2 CaCl2, 2 MgSO4, 10 glucose and 10 HEPES. After removal of the fura-2, the cultures were incubated in dye-free saline for at least 45 minutes to allow for the de-esterification of the fura-2/AM.
Fields were selected for study and live fluorescence images acquired at excitation wavelengths of 340 and 380 nm using a SIT-66 video camera (DAGE-MTI, Dage, Michigan City, IN). Images were digitized for real-time display using the MCID imaging software. Data were collected at 0.8 to 1 s intervals (4 frames per wavelength were averaged for each time point). Measurements of fluorescence levels were made in individual neurons and intracellular Ca2+ levels were calculated by converting the fluorescence ratios (340/380 nm) to intracellular Ca2+ concentrations. The following formula was used for this conversion: [Ca2+]i = Kd(R-Rmin)/(Rmax - R)*Fo/Fs, where R is the ratio value, Rmin is the ratio for a Ca2+ free solution, Rmax is the ratio for a saturated Ca2+ solution, Kd is 225 (the dissociation constant for fura-2), Fo is the intensity of a Ca2+ free solution at 380 nm and Fs is the intensity of a saturated Ca2+ solution at 380 nm. The low level of background fluorescence and adjustment of the black level of the SIT camera eliminated the need for background subtraction methods. Calibration was done using fura-2 salt (100 μM) in solutions of known Ca2+ concentration (Molecular Probes kit #C-3009). Recordings were made at room temperature. Data were analyzed with AxoGraph software (Axon Instruments, Foster City, CA).
Ca2+ imaging experiments were performed in physiological saline or physiological saline with reduced Mg2+ (final concentration, 30 μM) plus 5 μM glycine. The reduced Mg2+ saline was used to facilitate activation of NMDA receptors. In some experiments, transmitter receptor antagonists were added to the recording saline including 5 μM NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide disodium; Tocris Cookson, Ltd., Langford, Bristol, UK), 50 μM dAPV (D(-)-2-amino-5-phosphonopentanoic acid; Tocris Cookson), 100 μM picrotoxin (Sigma), and 1 μM CGP55854A (CGP; Novartis, Basel, Switzerland) to block AMPA, NMDA, GABAA, and GABAB receptors, respectively. In some studies the bath also contained 0.2 μM tetrodotoxin (TTX, Sigma) to block Na+ based action potentials.
Statistical analyses
All experiments were performed at least 3 times. Quantitative data are expressed as the mean ± S.E.M. Statistical analysis was performed by ANOVA or the unpaired t-test. P values of less than 0.05 were considered statistically significant.
Results
CCL2 activates p38 MAP kinase in hippocampal cultures
To determine if CCL2 activates p38 MAPK or p44/42 MAPK (also known as ERK1/2) in hippocampal cells, we measured the level of phosphorylated p38 MAPK (p-p38MAPK) and phosphorylated ERK1/2 (p-ERK1/2) in rat hippocampal cultures under control conditions and after acute exposure to CCL2. Activation of p38 MAPK and ERK1/2 occurs by phosphorylation of specific residues and the relative level of phosphorylation can be assessed by Western blot using phospho-state specific antibodies.
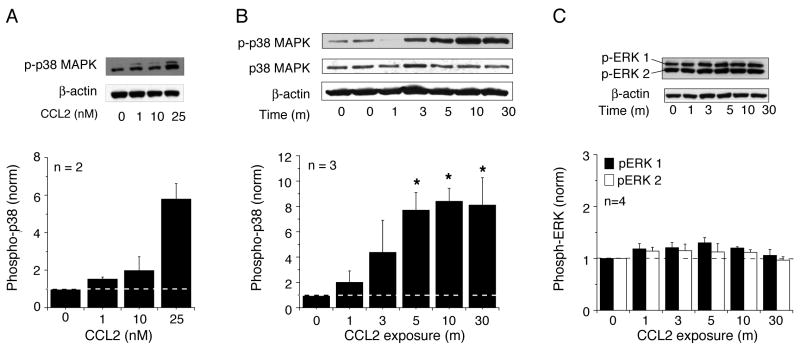
Acute exposure to CCL2 induced a concentration-dependent increase in the level of p-p38 MAPK in the hippocampal cultures (Fig. 1 A). The increase was prominent at 25 nM CCL2 and this concentration was utilized for additional studies. At 25 nM, CCL2 produced a rapid, time-dependent increase in the level of p-p38 MAPK starting as early as 1 min of exposure (Fig. 1B). Maximal levels of p-p38 MAPK were observed by 5–10 min of CCL2 exposure and the levels remained elevated at 30 min (Fig. 1 B). In contrast to p38 MAPK, CCL2 had only a small, non-significant effect on the level of p-ERK1/2 in the hippocampal cultures (Fig. 1C).
Figure 1.
Effect of CCL2 on the level of phosphorylated MAPK in hippocampal cultures. (A) Concentration-dependent effect of CCL2 (10 min exposure) on the level of phosphorylated p38 MAPK (p-p38 MAPK). (B) Time dependent effect of CCL2 (25 nM) on the level of p-p38 MAPK. (C) Time dependent effect of CCL2 (25 nM) on the level of phosphorylated ERK1/2 (p-ERK1/2). For all studies, representative immunoblots and a graph of mean values are shown. CCL2 significantly increased the level of p-p38 MAPK in the hippocampal cultures but not the level of p-ERK1/2. Methods were similar for all studies. Hippocampal cultures were serum-starved overnight and treated at 13 DIV with CCL2 (0–25 nM) for the times specified. Whole cell lysates were prepared and 20 μg of total protein per lane was separated on the SDS-PAGE and immunoblotted with the antibody specific for p-p38 MAPK or p-ERK1/2. The blot was stripped and reprobed with the antibody specific for β-actin. The density of each band was quantified and normalized against the corresponding density of β-actin in the same lane. All data were then normalized to the normalized value (i.e., p38 MAPK/b-actin) for control conditions (0 CCL2). Densitometry data are presented as the mean ± S.E.M. *, P < 0.05 vs control conditions. n = the number of independent samples tested. Samples were derived from at least 3 different culture sets.
CCL2 activates MKK 3/6 in hippocampal cultures
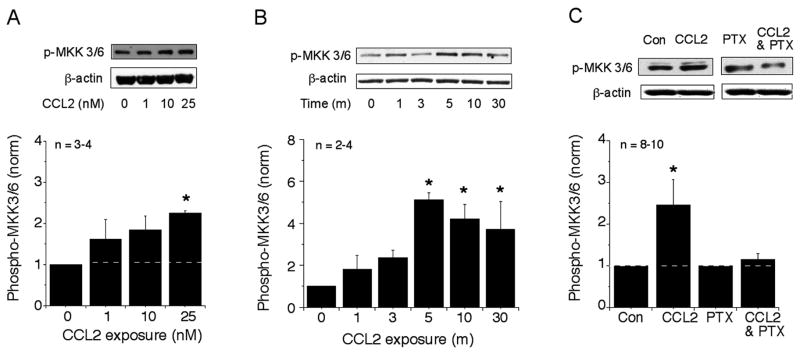
The immediate upstream kinases that activate p38 MAPK are mitogen-activated protein kinase kinase (MKK) 3 and 6. MKK3 and MKK6 (MKK3/6) activate p38 MAPK by phosphorylation and are highly selective for p38 MAPK (Kyriakis and Avruch, 2001). We examined the effect of CCL2 on the level of phosphorylated MKK3/6 (p-MKK3/6) to determine if CCR2 signal transduction in the hippocampal cells involved a role for this kinase. Acute exposure of the hippocampal cultures to CCL2 induced a rapid and prominent concentration- and time-dependent increase in the level of p-MKK3/6 (Fig. 2A,B). The increased level of p-MKK3/6 was observed as early as 1 min of exposure and the maximal activation was achieved at 5 min (Fig. 2B). Thus, p38 MAPK and MKK3/6 show a similar time course for the increase in the level of the phosphorylated form of the protein following CCL2 exposure, consistent with the known role of MKK3/6 in p38 MAPK activation.
Figure 2.
Effect of CCL2 on the level of p-MKK3/6 in hippocampal cultures. (A) Concentration-dependent effect of CCL2 (10 min exposure) on the level of p-MKK3/6. (B) Time-dependent effect of CCL2 (25 nM) on the level of p-MKK3/6. (C) Effect of PTX (100 ng/ml) on the CCL2-induced increases in the levels of p-MKK3/6. Representative immunoblots and a graph of mean values are shown for all studies. The methods were similar to the methods described in Figure 1 for p38 MAPK. Cultures were pretreated with PTX overnight. In C, data from CCL2-treated cultures were normalized to data from untreated control cultures; data from CCL2- and PTX-treated cultures was normalized to data from cultures treated with PTX but no CCL2.
CCR2, the receptor for CCL2, is a Gi/Go-coupled protein. To determine if a Gi/Go protein was involved in the CCL2-induced increase in the level of p-MKK3/6 in the hippocampal cultures, we tested the effect of pertussis toxin (PTX), a toxin that inactivates Gi/Go by ADP ribosylation. As shown in Fig. 2C, pretreatment with PTX blocked the CCL2-induced activation of MKK3/6, implying the involvement of Gi/Go protein in CCL2 signaling. PTX alone had no effect on the level of p-MKK3/6 (Fig. 2C).
CCL2 activates CREB in hippocampal cultures
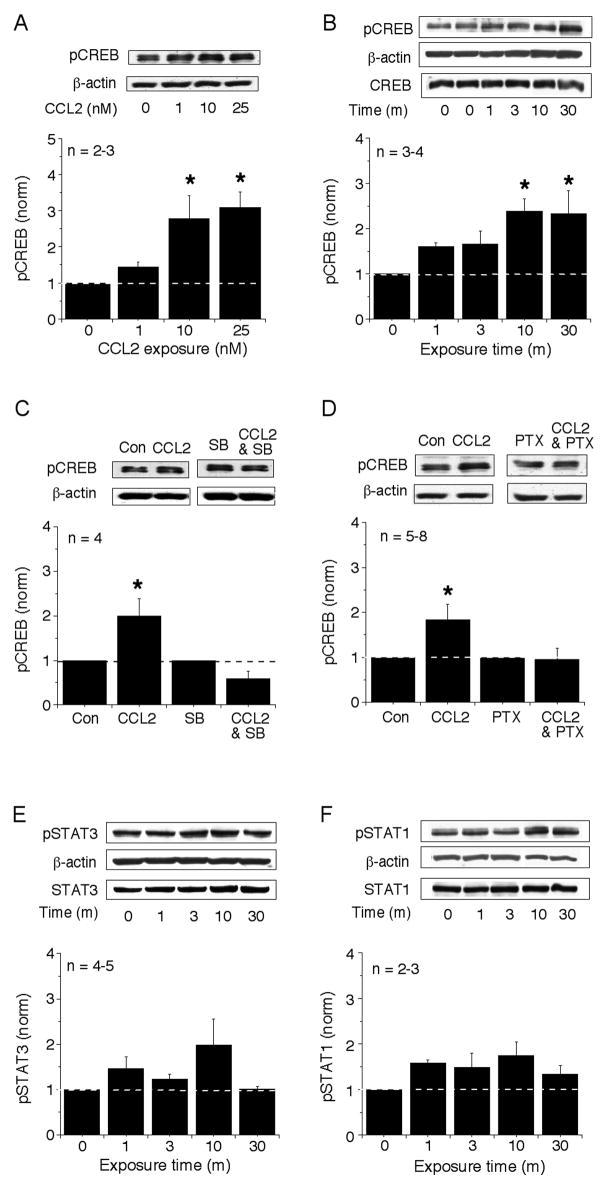
Downstream targets of p38 MAPK in immune and endothelial cells or cell lines include transcription factors such as CREB (Gustin et al., 2004; Nemeth et al., 2003) and STAT1 and STAT3 (Goh et al., 1999; Xu et al., 2003). To determine if the signal transduction pathway activated by CCL2 in the hippocampal cultures involves these transcription factors, we determined the relative level of phosphorylation and therefore activation of these proteins in control and CCL2-treated cultures using Western blots analysis and phospho-specific antibodies. Acute exposure to CCL2 induced a rapid, concentration- (Fig. 3A) and time-dependent (Fig. 3B) increase in the level of phosphorylated CREB (p-CREB) in the hippocampal cultures that reached a maximum at 10 min and remained elevated at 30 m of exposure (Fig. 3B). In contrast, no significant effect on the level of phosphorylated STAT1 (Fig. 3E) or phosphorylated STAT3 (Fig. 3F) was observed.
Figure 3.
Effect of CCL2 on the levels of phosphorylated CREB, STAT1 and STAT3 in hippocampal cultures. (A) Concentration-dependent effect of CCL2 (10 min exposure) on the level of p-CREB. (B, E, F) Time-dependent effect of CCL2 (25 nM) on the level of p-CREB (B), p-STAT3 (E) and p-STAT1 (F). CCL2 significantly increased the levels of p-CREB in a time-dependent manner. No significant effect on levels of p-STAT1 and p-STAT3 was observed. (C,D) Effects of PTX (100 ng/ml) and SB203580 (2 μM) on the CCL2-induced increase in the levels of p-CREB. Both inhibition of Gi/Go by PTX and inhibition of p38 MAPK by SB203580 blocked the CCL2-induced increase in the level of p-CREB, consistent with the involvement of a Gi/Go-coupled receptor and p38 MAPK in the actions of CCL2. Cultures were pretreated with either PTX overnight or SB203580 for 30 min, and the methods were similar to the methods described in the Figure 1. In C, data from CCL2-treated cultures were normalized to data from untreated control cultures; data from CCL2-and PTX-treated cultures was normalized to data from cultures treated with PTX but no CCL2. In D, data from CCL2-treated cultures were normalized to data from untreated control cultures; data from CCL2- and SB203580-treated cultures were normalized to data from cultures treated with SB203580 but no CCL2. For all studies, representative immunoblots and a graph of mean values are shown.
To determine if the pathway involves p38 MAPK and Gi/Go protein in the CCL2-induced increase in the level of p-CREB, we tested the effect of the Gi/Go protein inactivator PTX and the selective p38 MAPK inhibitor SB203580. The cultures were pretreated with PTX (100 ng/ml) overnight or with SB203580 (2 μM) for 30 min prior to CCL2 exposure. The CCL2-induced phosphorylation of CREB was blocked by the pretreatment with either SB203580 (Fig. 3C) or PTX (Fig. 3D).
CREB activation is often associated with increases in the level of the downstream protein brain derived neurotrophic factor (BDNF)(Tao et al., 1998). To determine if this association also occurred in the hippocampal cultures, we measured the relative level of the mature form of BDNF (21 kDa) in cultures treated with 25 nM CCL2 (10–30 min) using Western blot analysis. BDNF levels showed a 2 fold (2.3 ± 0.3, n=6; p< 0.05, unpaired t-test) increase in the CCL2-treated cultures compared with BDNF levels in control cultures (not shown).
CCR2 is expressed in hippocampal neurons
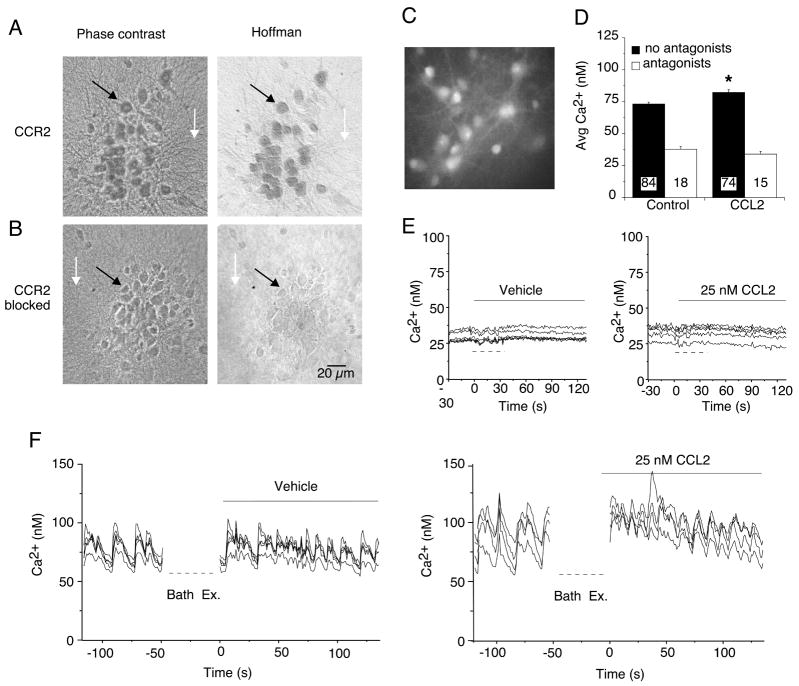
The hippocampal cultures contain both neurons and glial cells and both cell types have been reported to express CCR2 (Banisadr et al., 2005b). Therefore, both cell types could contribute to the increase in the level of p-p38 MAPK produced by CCL2 exposure. To determine if both neurons and glial cells expressed CCR2 in the hippocampal cultures, we immunostained the cultures with an antibody directed against the carboxy terminal of the C-C chemokine receptor gene type 2B (CCR2B). Immunoreactivity for CCR2 was prominent in the somatic region of the cultured neurons and was also evident in the dendrites (Fig. 4A). Under conditions where strong immunostaining was observed in the neurons, no immunoreactivity for CCR2 was evident in the glial cell layer (primarily astrocytes), which forms the substratum upon which the neurons grow (Fig. 4A). These results suggest that CCR2 expression is primarily in the neuronal population. Consistent with this result, immunostaining for CCR2 in spinal cord cultures was reported to be intense in neurons with no significant immunostaining of astrocytes (Gosselin et al., 2005). Neurons and glial cells in the hippocampal cultures were identified by morphological criteria developed in previous studies using cell specific antibodies carried out in parallel with electrophysiological or Ca2+ imaging recordings from the neurons (Nelson and Gruol, 2004; Vereyken et al., 2007). Blocking the antibody with the antigenic peptide used to produce the CCR2 antibody completely eliminated immunostaining for CCR2, indicating that the immunoreactivity was specific for the antigenic sequence (Fig. 4B).
Figure 4.
Hippocampal neurons express functional CCR2. (A) Immunocytochemical staining of rat hippocampal cultures using the antibody specific against CCR2B (1:100 dilution). Prominent immunostaining was observed in the neurons (e.g., black arrow) but not in the underlying glial cell layer (e.g., white arrow). Neurons were identified by morphological criteria established by immunostaining with cell type specific antibodies. The phase contrast images (left panel) show all cells in the microscopic field. The Hoffman optics images (right panel) show the immunostained cells in the field. (B) Specific immunostaining for CCR2 was blocked by preincubation of the antibody with the antigenic peptide used to raise the CCR2 antibody. (C–F) Effect of CCL2 on Ca2+ signals generated by spontaneous network synaptic activity in hippocampal neurons. (C) Gray scale digitized image showing a representative field of fura-2 loaded hippocampal neurons. (D) Mean (SEM) values for average Ca2+ levels under control conditions and after addition of CCL2 (25 nM) to the bath saline. Recordings were made in the presence (antagonists) and absence (no antagonists) of TTX plus glutamate and GABA receptor antagonists. Numbers in bars indicate the number of cells measured. (E, F) Representative recordings of intracellular Ca2+ levels before and after (solid bars) bath exchange. Dashed lines below the traces indicate when the bath was exchanged during the recording. In F, the recording was stopped during the bath exchange. In E, the bath contained TTX plus receptor antagonists to block synaptic network activity. In F, TTX and receptor antagonists were not used. The vehicle control bath exchange was used to identify potential changes in Ca2+ signals due to mechanical stimuli caused by the bath exchange.
To determine if activation of CCR2 in the hippocampal neurons could alter neuronal function, we tested the effect of acute bath application of CCL2 on spontaneous synaptic network activity exhibited by the cultured hippocampal neurons. The network activity involves both excitatory and inhibitory synaptic transmission and is associated with Ca2+ signals produced by Ca2+ influx through NMDA receptors and voltage-gated Ca2+ channels that contribute to the network activity (Przewlocki et al., 1999). The Ca2+ signals are often synchronized among neurons in a microscopic field, particularly when the Mg2+ in the bath saline is reduced to enable full activation of NMDA receptors, as was done in our experiments. The Ca2+ signals were measured in the individual neurons using fura-2 based Ca2+ imaging under baseline conditions and after addition of CCL2 to the bath and quantified by measurement of average Ca2+ levels measured during a standardized recording period. CCL2 was tested at 25 nM, a concentration shown to affect neuronal activity in our previous studies (van Gassen et al., 2005).
CCL2 significantly increased average Ca2+ levels in the cultured neurons (Fig. 4 C,D,G,H). However, when the synaptic network activity was blocked by addition of GABA (picrotoxin, CGP) and glutamate (NBQX, dAPV) receptor antagonists and TTX to the bath saline, there was no effect of CCL2 on intracellular Ca2+ levels. These results suggest that hippocampal neurons express functional CCR2 and that activation of these receptors can alter synaptic network activity.
Discussion
Recent studies show that CCL2 and its receptor CCR2 are expressed in the normal hippocampus and that the levels of expression increase during neuroinflammatory conditions associated with CNS injury and disease (Banisadr et al., 2005a; Banisadr et al., 2005b; Galasso et al., 2000; Kalehua et al., 2004; Little et al., 2002; Sakurai-Yamashita et al., 2006; Sheehan et al., 2007; Szaflarski et al., 1998). These results implicate a role for CCL2 in the hippocampus, particularly during neuroinflammatory conditions. However, the functional consequence of CCR2 activation by CCL2 in the hippocampus and the cellular pathways that mediate the downstream actions of CCL2 are relatively unexplored areas. As a first step toward addressing these issues, we investigated the signal transduction pathways associated with CCR2 activation in cultured rodent hippocampal cells.
Chemokine receptors such as CCR2 are members of the G protein-coupled receptor family and are associated with several different signal transduction pathways including MAPKs (Bajetto et al., 2002). MAPKs are recognized to be one of the major signaling pathways that link activation of G protein-coupled receptor to the nucleus (Bajetto et al., 2002; Marinissen and Gutkind, 2001). Our results show that in cultures of rat hippocampus CCL2 activation of CCR2 results in an increased level of phosphorylated p38 MAPK. In contrast, the level of phosphorylated ERK1/2 was not significantly altered by exposure of the cultures to CCL2. ERK1/2 has been reported to be activated by CCL2 in the immune cell system such as Murine T cell hybrid cell line and monocytes (Dubois et al., 1996; Jimenez-Sainz et al., 2003). In human endothelial cells, both ERK1/2 and p38 MAPK were shown to be activated by CCL2 (Werle et al., 2002). These reports and our findings in the present study showing the activation of p38 MAPK, not ERK1/2, in hippocampal cells imply that CCL2 signaling may be cell type-dependent.
Consistent with an involvement of p38 MAPK in the signal transduction pathway activated by CCL2 in hippocampal cells, the level of phosphorylated MKK3/6, the upstream kinase that specifically phosphorylates and thereby activates p38 MAPK, was also increased by CCL2 exposure. The Gi/Go protein inhibitor PTX blocked the CCL2-induced increase in the level of phosphorylated MKK3/6, indicating that a G protein-coupled receptor was involved in the actions of CCL2. These results show that the p38 MAPK pathway is an important component of the signal transduction linked to CCR2 in the hippocampus.
Activation of p38 MAPK can lead to gene expression through the phosphorylation and therefore activation of various transcription factors including CREB (Gustin et al., 2004; Kyriakis and Avruch, 2001; Nemeth et al., 2003). Of the three proteins studied, CREB, STAT1 and STAT3, only the level of phosphorylated CREB was significantly altered by exposure of the cultures to CCL2. The CCL2-induced increase in the level of phosphorylated CREB was blocked by inactivation of Gi/Go with PTX and by the selective p38 MAPK inhibitor SB203580, confirming the involvement of these proteins in the downstream effects CCR2 activation in hippocampal cells. Our studies also showed an increase in the level of the neurotrophic factor BDNF, a protein whose level of expression can be regulated by CREB (Tao et al., 1998). Thus, altered levels of BDNF could be one of the downstream effects of CCL2 exposure in the hippocampus.
It has previously been reported that CCR2 is expressed on several types of neurons such as human fetal neurons and NT2.N cells (Coughlan et al., 2000) and spinal cord neurons (Gosselin et al., 2005), and that it is constitutively expressed in several regions of the adult rat brain including hippocampus (Banisadr et al., 2005b). The expression of CCR2 has also been reported previously for astrocytes (Andjelkovic et al., 2002; Rezaie et al., 2002). In our hippocampal cultures, the primary cell type that showed immunostaining for CCR2 was the neurons. No prominent glial immunostaining was observed. These results suggest that neurons are the primary cell type responding to CCL2 in our cultures. A similar result was reported for spinal cord cultures (Gosselin et al., 2005)
The functional consequence of CCR2 expression in the hippocampus and CCL2 activation of the p38 MAPK pathway remain to be determined. Few studies on the effects of CCL2 on hippocampal neurons have appeared, although one report showed that CCL2 can induce Ca2+ transients in cultured neurons from several brain regions including the hippocampus (Banisadr et al., 2005b). CCL2 has also been reported to alter neuronal excitability and Ca2+ signaling in cerebellar neurons (van Gassen et al., 2005) and to reduce inhibitory responses mediated by GABA receptors in spinal cord neurons (Gosselin et al., 2005). In our studies, CCL2 increased Ca2+ signals associated with spontaneous synaptic network activity indicating that CCL2 can affect hippocampal neuronal function. The mechanisms mediating this effect were not investigated but could involve a reduction of inhibitory responses mediated by GABA receptors that contribute to the synaptic network activity. Future studies will test this possibility. It is unknown if the increase in p38 MAPK observed in our studies played a role in the CCL2-induced increase in synaptic network activity. Both p38 MAPK and CREB have been shown to play a key regulatory role in synaptic transmission, synaptic plasticity and memory mechanisms (Alonso et al., 2003; Brust et al., 2006; Butler et al., 2004; Josselyn and Nguyen, 2005; Rossato et al., 2006; Wang et al., 2007). Thus, these hippocampal synaptic functions may be an important target of CCL2 in the normal brain or during conditions associated with neuroinflammation and p38 MAPK may play a central role in the effects of CCL2 on these functions. Future studies will test these possibilities.
Acknowledgments
This research was supported by NIH grants MH63712 and P30 MH62261, a grant (E00015) from Korea Research Foundation, and a grant (PF06216-00) from the Plant Diversity Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology of Korean government.
Abbreviations
- CREB
cAMP response element-binding protein
- BDNF
brain derived neurotrophic factor
- CNS
central nervous system
- HIV
human immunodeficiency virus
- JAK2
Janus kinase 2
- STAT
signal transducer and activator of transcription
- MAPK
mitogen-activated protein kinases
- MKK
mitogen-activated protein kinase kinase
- PTX
pertussis toxin
- PBS
phosphate-buffered saline
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso M, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Memory formation requires p38MAPK activity in the rat hippocampus. Neuroreport. 2003;14:1989–1992. doi: 10.1097/00001756-200310270-00022. [DOI] [PubMed] [Google Scholar]
- Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Song L, Dzenko KA, Cong H, Pachter JS. Functional expression of CCR2 by human fetal astrocytes. J Neurosci Res. 2002;70:219–231. doi: 10.1002/jnr.10372. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: Evidence for its colocalization with neurotransmitters and neuropeptides. J Comp Neurol. 2005a;489:275–292. doi: 10.1002/cne.20598. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol. 2005b;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Queraud-Lesaux F, Boutterin MC, Pelaprat D, Zalc B, Rostene W, Haour F, Parsadaniantz SM. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem. 2002;81:257–269. doi: 10.1046/j.1471-4159.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Brust TB, Cayabyab FS, Zhou N, MacVicar BA. p38 mitogen-activated protein kinase contributes to adenosine A1 receptor-mediated synaptic depression in area CA1 of the rat hippocampus. J Neurosci. 2006;26:12427–12438. doi: 10.1523/JNEUROSCI.4052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–326. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Cambien B, Pomeranz M, Millet MA, Rossi B, Schmid-Alliana A. Signal transduction involved in MCP-1-mediated monocytic transendothelial migration. Blood. 2001;97:359–366. doi: 10.1182/blood.v97.2.359. [DOI] [PubMed] [Google Scholar]
- Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902:171–177. doi: 10.1016/s0006-8993(01)02328-9. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. Aids. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience. 2000;97:591–600. doi: 10.1016/s0306-4522(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Dubois PM, Palmer D, Webb ML, Ledbetter JA, Shapiro RA. Early signal transduction by the receptor to the chemokine monocyte chemotactic protein-1 in a murine T cell hybrid. J Immunol. 1996;156:1356–1361. [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Galasso JM, Miller MJ, Cowell RM, Harrison JK, Warren JS, Silverstein FS. Acute excitotoxic injury induces expression of monocyte chemoattractant protein-1 and its receptor, CCR2, in neonatal rat brain. Exp Neurol. 2000;165:295–305. doi: 10.1006/exnr.2000.7466. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, Guidi I, Blankenstein MA, Bresolin N, Scarpini E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- Geppert AM. Constitutive patterns of RANTES, MCP-1 and MIP-1 alpha expression at the mRNA and protein level during postnatal development of the rat brain. Folia Neuropathol. 2003;41:79–88. [PubMed] [Google Scholar]
- Goh KC, Haque SJ, Williams BR. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. Embo J. 1999;18:5601–5608. doi: 10.1093/emboj/18.20.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, Melik-Parsadaniantz S. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem. 2005;95:1023–1034. doi: 10.1111/j.1471-4159.2005.03431.x. [DOI] [PubMed] [Google Scholar]
- Gustin JA, Pincheira R, Mayo LD, Ozes ON, Kessler KM, Baerwald MR, Korgaonkar CK, Donner DB. Tumor necrosis factor activates CRE-binding protein through a p38 MAPK/MSK1 signaling pathway in endothelial cells. Am J Physiol Cell Physiol. 2004;286:C547–555. doi: 10.1152/ajpcell.00332.2002. [DOI] [PubMed] [Google Scholar]
- Jimenez-Sainz MC, Fast B, Mayor F, Jr, Aragay AM. Signaling pathways for monocyte chemoattractant protein 1-mediated extracellular signal-regulated kinase activation. Mol Pharmacol. 2003;64:773–782. doi: 10.1124/mol.64.3.773. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Nguyen PV. CREB, synapses and memory disorders: past progress and future challenges. Curr Drug Targets CNS Neurol Disord. 2005;4:481–497. doi: 10.2174/156800705774322058. [DOI] [PubMed] [Google Scholar]
- Kalehua AN, Nagel JE, Whelchel LM, Gides JJ, Pyle RS, Smith RJ, Kusiak JW, Taub DD. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 are involved in both excitotoxin-induced neurodegeneration and regeneration. Exp Cell Res. 2004;297:197–211. doi: 10.1016/j.yexcr.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Kielian T, van Rooijen N, Hickey WF. MCP-1 expression in CNS-1 astrocytoma cells: implications for macrophage infiltration into tumors in vivo. J Neurooncol. 2002;56:1–12. doi: 10.1023/a:1014495613455. [DOI] [PubMed] [Google Scholar]
- Kuang Y, Wu Y, Jiang H, Wu D. Selective G protein coupling by C-C chemokine receptors. J Biol Chem. 1996;271:3975–3978. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Little AR, Benkovic SA, Miller DB, O’Callaghan JP. Chemically induced neuronal damage and gliosis: enhanced expression of the proinflammatory chemokine, monocyte chemoattractant protein (MCP)-1, without a corresponding increase in proinflammatory cytokines(1) Neuroscience. 2002;115:307–320. doi: 10.1016/s0306-4522(02)00359-7. [DOI] [PubMed] [Google Scholar]
- Little AR, Sriram K, O’Callaghan JP. Corticosterone regulates expression of CCL2 in the intact and chemically injured hippocampus. Neurosci Lett. 2006;399:162–166. doi: 10.1016/j.neulet.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J Neuroimmunol. 1998;86:20–29. doi: 10.1016/s0165-5728(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Mellado M, Rodriguez-Frade JM, Aragay A, del Real G, Martin AM, Vila-Coro AJ, Serrano A, Mayor F, Jr, Martinez AC. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J Immunol. 1998;161:805–813. [PubMed] [Google Scholar]
- Meng SZ, Oka A, Takashima S. Developmental expression of monocyte chemoattractant protein-1 in the human cerebellum and brainstem. Brain Dev. 1999;21:30–35. doi: 10.1016/s0387-7604(98)00065-5. [DOI] [PubMed] [Google Scholar]
- Minami M, Katayama T, Satoh M. Brain cytokines and chemokines: roles in ischemic injury and pain. J Pharmacol Sci. 2006;100:461–470. doi: 10.1254/jphs.crj06005x. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Gruol DL. The chemokine CXCL10 modulates excitatory activity and intracellular calcium signaling in cultured hippocampal neurons. J Neuroimmunol. 2004;156:74–87. doi: 10.1016/j.jneuroim.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Nemeth ZH, Leibovich SJ, Deitch EA, Sperlagh B, Virag L, Vizi ES, Szabo C, Hasko G. Adenosine stimulates CREB activation in macrophages via a p38 MAPK-mediated mechanism. Biochem Biophys Res Commun. 2003;312:883–888. doi: 10.1016/j.bbrc.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Parsons KL, Sweeney DD, Trotter C, Netzeband JG, Siggins GR, Gruol DL. Opioid enhancement of calcium oscillations and burst events involving NMDA receptors and L-type calcium channels in cultured hippocampal neurons. J Neurosci. 1999;19:9705–9715. doi: 10.1523/JNEUROSCI.19-22-09705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie P, Trillo-Pazos G, Everall IP, Male DK. Expression of beta-chemokines and chemokine receptors in human fetal astrocyte and microglial co-cultures: potential role of chemokines in the developing CNS. Glia. 2002;37:64–75. doi: 10.1002/glia.1128. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Lima RH, Medina JH, Izquierdo I, Cammarota M. On the participation of hippocampal p38 mitogen-activated protein kinase in extinction and reacquisition of inhibitory avoidance memory. Neuroscience. 2006;143:15–23. doi: 10.1016/j.neuroscience.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Sakurai-Yamashita Y, Shigematsu K, Yamashita K, Niwa M. Expression of MCP-1 in the Hippocampus of SHRSP with Ischemia-Related Delayed Neuronal Death. Cell Mol Neurobiol. 2006;26:821–829. doi: 10.1007/s10571-006-9077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JJ, Zhou C, Gravanis I, Rogove AD, Wu YP, Bogenhagen DF, Tsirka SE. Proteolytic activation of monocyte chemoattractant protein-1 by plasmin underlies excitotoxic neurodegeneration in mice. J Neurosci. 2007;27:1738–1745. doi: 10.1523/JNEUROSCI.4987-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J, Ivacko J, Liu XH, Warren JS, Silverstein FS. Excitotoxic injury induces monocyte chemoattractant protein-1 expression in neonatal rat brain. Brain Res Mol Brain Res. 1998;55:306–314. doi: 10.1016/s0169-328x(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- van Gassen KL, Netzeband JG, de Graan PN, Gruol DL. The chemokine CCL2 modulates Ca2+ dynamics and electrophysiological properties of cultured cerebellar Purkinje neurons. Eur J Neurosci. 2005;21:2949–2957. doi: 10.1111/j.1460-9568.2005.04113.x. [DOI] [PubMed] [Google Scholar]
- Vereyken EJ, Bajova H, Chow S, de Graan PN, Gruol DL. Chronic interleukin-6 alters the level of synaptic proteins in hippocampus in culture and in vivo. Eur J Neurosci. 2007;25:3605–3616. doi: 10.1111/j.1460-9568.2007.05615.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chang L, Rowan MJ, Anwyl R. Developmental dependence, the role of the kinases p38 MAPK and PKC, and the involvement of tumor necrosis factor-R1 in the induction of mGlu-5 LTD in the dentate gyrus. Neuroscience. 2007;144:110–118. doi: 10.1016/j.neuroscience.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Berman JW. Astrocyte expression of monocyte chemoattractant protein-1 is differentially regulated by transforming growth factor beta. J Neuroimmunol. 1998;91:190–197. doi: 10.1016/s0165-5728(98)00183-0. [DOI] [PubMed] [Google Scholar]
- Werle M, Schmal U, Hanna K, Kreuzer J. MCP-1 induces activation of MAP-kinases ERK, JNK and p38 MAPK in human endothelial cells. Cardiovasc Res. 2002;56:284–292. doi: 10.1016/s0008-6363(02)00600-4. [DOI] [PubMed] [Google Scholar]
- Xu B, Bhattacharjee A, Roy B, Xu HM, Anthony D, Frank DA, Feldman GM, Cathcart MK. Interleukin-13 induction of 15-lipoxygenase gene expression requires p38 mitogen-activated protein kinase-mediated serine 727 phosphorylation of Stat1 and Stat3. Mol Cell Biol. 2003;23:3918–3928. doi: 10.1128/MCB.23.11.3918-3928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]