Abstract
A 10-week-old, intact female, Labrador retriever was presented for progressive extensor rigidity, facial swelling, and difficulty in walking. Generalized tetanus was diagnosed and treated successfully.
Résumé
Tétanos généralisé chez un Labrador retriever. Une Labrador retriever entière, âgée de 10 semaines, a été présentée pour rigidité progressive d’extension, de tuméfaction faciale et de difficulté à marcher. Un tétanos généralisé a été diagnostiqué et traité avec succès.
(Traduit par Docteur André Blouin)
A 10-week-old, intact female, yellow Labrador retriever was presented to the Morden Veterinary Clinic in Morden, Manitoba, for acute swelling of the entire face. No wound could be identified and a presumptive diagnosis was made of insect bite hypersensitivity. Diphenhydramine (PMS Diphenhydramine hydrochloride capsule USP; Pharmascience, Montreal, Quebec), 2 mg/kg bodyweight (BW), PO, was administered, and the puppy was sent home with instructions to the owner to administer ketoprofen (Anafen; Merial Canada, Baie-d’Urfé, Quebec), 0.5 mg/kg BW, PO, q24h for 4 d.
Thirty-six hours later, the puppy was presented again for a stiff gait. There was moderate extensor muscle rigidity that induced a splay-legged appearance. The facial swelling had persisted, and it was difficult to open the mouth. All other physical findings were normal. The puppy was hospitalized and treated once for a presumed immune-mediated polymyositis with dexamethasone (Dexamethasone 2; Vétoquinol, Lavaltrie, Quebec), 0.1 mg/kg BW, IM, and penicillin G procaine (Pen G Injection; Vétoquinol, Kambridge, Ontario), 45 000 units/kg BW, IM. Diazepam (Diazepam; Sandoz Canada, Quebec City, Quebec), 1 mg/kg BW, IV, was administered to decrease extensor rigidity, but the abnormalities continued to progress overnight.
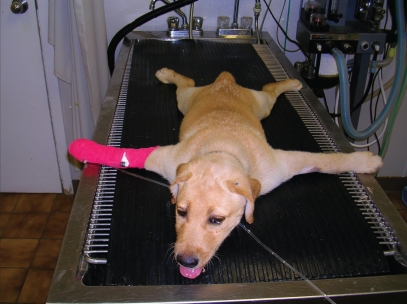
The next morning, severe extensor rigidity was present in all limbs and the puppy remained in sternal recumbency with all 4 limbs abducted (Figure 1). The limbs could not be flexed, both ears were held erect, and the facial musculature was contracted, drawing the lips back. Tachypnea was present (60 breaths/min), but the heart rate and temperature were normal. The puppy whimpered intermittently as muscle spasms occurred.
Figure 1.
A photograph of a 10-week-old, intact female, yellow Labrador retriever with generalized tetanus exhibiting extended limbs, a swollen face, and a wrinkled forehead.
Results from a complete blood (cell) count (CBC) revealed a mild anemia (PCV of 0.31 L/L; reference range: 0.37 to 0.55 L/L), and a moderate mature neutrophilia (17.2 × 109/L; reference range: 3.5 to 12 × 109/L). Results from a serum chemical panel were within normal reference ranges. The most appropriate clinical diagnosis was generalized tetanus, although a wound could not be documented.
Treatment was initiated with sodium ampicillin (Ampicillin sodium for injection USP; Novopharm Limited, Toronto, Ontario), 25 mg/kg BW, IV, q8h, and diazepam, 0.5 mg/kg BW, IV, as needed, for muscle relaxation. Antitoxin was not available, because the drug distribution center was closed for the weekend. The puppy was kept in a well-padded kennel, in a dark, quiet area. Intravenous fluids (Lactated Ringer’s Injection USP; Baxter, Toronto, Ontario) were administered at 50 mL/kg BW/d. Since the puppy was unable to open her mouth, soft dog food (Hills a/d; Hills Pet Nutrition, Topeka, Kansas, USA), was mixed with water and trickle-fed via a teat cannula attached to a syringe with the puppy being held upright to prevent regurgitation or aspiration. By Day 4, the puppy could lap food off a plate, but she was still recumbent with marked extensor rigidity. Meloxicam (Metacam; Boehringer Ingelheim Canada, Burlington, Ontario), 0.1 mg/kg BW, PO, q24h, was administered for analgesia. On Day 5, penicillin G procaine, 36 000 units/kg BW, IM, q12h, replaced the IV sodium ampicillin. Later that day, there was some muscular relaxation and the joints could be flexed manually.
Over the next 3 d, the puppy’s joints became more flexible and she was able to move her head, neck, and hindlimbs. Physical therapy was performed twice daily. Decubital ulcers had developed on the medial aspect of both stifles; they were cleansed with chlorhexidine gluconate solution (Stanhexidine; Omega Laboratories, Montreal, Quebec) and then coated with nystatin neomycin sulfate thiostreptin triamcinolone acetonide ointment (Panalog ointment for dogs; Novartis, Mississauga, Ontario), q8h.
The puppy was discharged from hospital on day 9. Administration of penicillin G procaine, 36 000 units/kg BW, IM, q12h, was continued for 21 d, and meloxicam, 0.1 mg/kg BW, PO, q24h, was continued for 14 d. The owners were encouraged to keep the puppy in a quiet, confined area with a well-padded bed and to perform a range of motion exercises with her limbs every day to improve mobility. Within 3 wk, the puppy could walk, and within 5 wk of initial presentation, the puppy had made a full recovery.
Tetanus is a disorder caused by the action of the neurotoxin tetanospasmin, produced during the vegetative growth phase of Clostridium tetani (1–5). Clostridium tetani is a gram-positive, spore-producing anaerobe (1–3). Spores of C. tetani can be found in the soil worldwide (1).
Tetanus develops after a wound becomes contaminated with C. tetani spores (1). It has been recognized in association with skin wounds, surgical sites, puncture wounds, tick bites, and teething (1–5,6). No wounds were found in this puppy, suggesting that the route of infection may have been mucosal breaks associated with teething (6). Under anaerobic conditions in the wound, the spores can germinate into the vegetative form and multiply (1–4,7). Exotoxins are produced locally at the site of the wound (1). The most potent and abundant toxin is tetanospasmin (1,2,7), which is responsible for the profound neurological and muscular effects (1,7). Other toxins, like tetanolepsin, are also produced, but they are clinically insignificant (1–3,7). Tetanospasmin ascends the axons of motor nerves located adjacent to the wound and after reaching cell bodies within the spinal cord, it ascends to the brain (1,2). Circulating tetanospasmin can also enter the brain directly through the blood-brain barrier (1,2). Tetanospasmin inhibits the release of the inhibitory neurotransmitters glycine and γ-aminobutyric acid (GABA) at interneurons in the spinal cord and brain (1–3). This leads to constant excitation of the nerves, causing the classical signs of tetanus (1).
Tetanus is rare in dogs and cats, compared with many other domestic species and humans, because of a relative inability of the toxin to penetrate and bind to nervous tissue in these species (1,4,7). Cats are approximately 10 times more resistant than dogs, and dogs are 600 times more resistant to tetanus than horses (1). The incubation period of tetanus is about 9 d, with a range of 3 to 18 d (1,6). Two forms of tetanus can be seen clinically, localized and generalized (1).
Patients with localized tetanus have 1 muscle or limb near the site of infection becoming stiff (1,2). The stiffness usually spreads to the opposite limb, and if left untreated, it will eventually involve the whole central nervous system and mimic generalized tetanus (1,2). Individuals with generalized tetanus are commonly presented with either swelling of the face or muscle rigidity, which quickly progresses to severe muscle rigidity, a stiff gait, and the inability to rise or stand (1,6). Other characteristic clinical signs include hyperextension of the limbs, extension of the tail, contracted facial musculature, erect ears, wrinkled forehead, trismus, risus sardonicus, miosis, enophthalmos, and prolapse of the 3rd eyelid (1,2,4–7). Opisthotonos and seizures also develop (1). Dogs commonly have reflex muscle spasms associated with auditory or tactile stimulation and may vocalize during spasms because of pain (1,2).
A presumptive diagnosis of tetanus can usually be made, based on the history, clinical signs, and progression of the disorder, as seen in this case (1–4,6,7). The presence of a wound helps to substantiate a diagnosis, but often a wound is not found due to the long incubation period (3). Specific diagnostic tests can be performed to confirm the diagnosis, but these are rarely done (2). Circulating antibody against tetanus toxin can be demonstrated in the serum and compared with levels in control animals (1,3). Clostridium tetani can sometimes be cultured from the wound, but this is very difficult, due to the need for strict anaerobic culture conditions and the low numbers of C. tetani organisms present (1,3). Recently, electromyography (EMG) has been described as a method to support the diagnosis of localized tetanus, but this equipment is not available in most practices and the findings are not pathognomonic for tetanus (1,3). Differential diagnoses considered in patients with tetanus could include immune-mediated polymyositis, strychnine toxicity, spinal trauma, hypocalcemia, or meningoencephalitis (1,2,5). In this case, immune-mediated polymyositis could be ruled out, based on the progression of the disease, as well as the clinical signs. Strychnine toxicity and spinal trauma were ruled out, based on the history. Blood calcium levels were normal on the serum biochemical panel. Meningoencephalitis was ruled out, based on the signalment, as well as a lack of pain and the lack of severe neurological abnormalities.
Treatment of tetanus is based on 3 principles: 1) neutralize the circulating tetanospasmin with antitoxin, 2) inhibit the growth of C. tetani with antimicrobial drugs, and 3) provide supportive care until the toxin’s effects have worn off (2,5,8). Antitoxin should be a routine part of early treatment, when available, because it neutralizes circulating unbound toxin and toxin yet to be produced, but it is ineffective in neutralizing already bound toxin (1,2,4,5). Antitoxin is best administered, IV, to rapidly achieve high levels in the circulation (1,2,5). One IV dose of 100 units/kg BW (up to a maximum of 20 000 units) will achieve therapeutic blood levels for 14 d (1,2,5). An IM injection of 1000 units of antitoxin adjacent to the wound can also be beneficial (1,2). A test dose of antitoxin (0.1 to 0.2 mL, intradermally) is often administered prior to the IV dose to help in predicting anaphylaxis (1,2). Penicillin is the antibiotic treatment of choice for tetanus infections and it should be administered both parenterally and at the site of the wound (1,2). Either IV aqueous penicillin (potassium or sodium salt) or IM procaine penicillin G may be used (1). Ampicillin is not as effective as penicillin against C. tetani (1,2), which probably explains the slow response to initial treatment in this case. Tetracyclines or clindamycin may also be effective (1,2). Recent studies have shown that metronidazole may be more effective than penicillin, because it reaches bactericidal concentrations in the anaerobic wound environment and has a strict anaerobic spectrum of activity, but it may be associated with a higher risk of toxicity (1,2,6–8).
Supportive care and monitoring are very important in the management of a tetanus patient (1,2,6). If a wound is identified, it should be debrided aggressively and then flushed with hydrogen peroxide (1,2). Sedatives are used to control convulsions and muscle spasms, to provide some muscle relaxation, and to limit the hyperthermia associated with increased muscle activity (1,2). A combination of a phenothiazine (chlorpromazine) with a barbituate (phenobarbital) or benzodiazepine (diazepam) can be used (1,2,7,8). Narcotics should be avoided, as they depress respiration and may cause CNS stimulation (1,6). To decrease sensory stimulation and resultant muscle spasms, patients should be kept in a quiet, dark room (1,2). In order to prevent pressure sores, a well-padded bed should be provided and the patient should be turned frequently (1,2). Eating and drinking should be encouraged, but the food may have to be blended and fed through the clenched mouth or administered through an esophageal or gastrostomy tube (1,2). Balanced, isotonic maintenance fluids should be administered, IV, to maintain hydration (1,2). Physiotherapy can improve blood and lymph flow to and from the muscles, promote muscle relaxation, decrease discomfort, and aid return of muscle function (3,4,7).
Complications are common and include pressure sores, hyperthermia, and dysuria (1,6). Less commonly, megaesophagus due to a hiatal hernia, aspiration pneumonia, fractures, and laryngeal spasm may occur (1,6,7).
Approximately 58% to 77% of tetanus patients recover (6). Localized tetanus carries a more favorable prognosis than generalized tetanus (1). Most patients will show some improvement 1 wk after the initiation of treatment and a full recovery is often seen within 4 wk, but there are reports of stiffness persisting for up to 4 mo (1).
No tetanus vaccine is available for dogs because of their relative resistance to the disease (1,2,6). Natural infection does not provide effective, long-lasting immunity (1,2). The best way to prevent tetanus is through rapid appropriate wound management and antimicrobial selection (1,2,6).
This case describes the clinical course of a rare disease in dogs and illustrates how these patients can be managed effectively in a practice setting. The key is in recognizing the disorder early and treating it appropriately (1,2,6).
Acknowledgments
The author thanks Drs. Hamilton, Pouteau, and VanAssen, as well as the staff at the Morden Veterinary Clinic, Morden, Manitoba, for their assistance. The author also thanks Dr. Taylor for her advice. CVJ
Footnotes
Dr. Sprott will receive 50 reprints of her article free of charge, courtesy of The Canadian Veterinary Journal.
References
- 1.Greene CE. Tetanus. In: Greene CE, editor. Infectious Diseases of the Dog and Cat. 3. St. Louis, Missouri: Saunders Elsevier; 2006. pp. 395–402. [Google Scholar]
- 2.Merrett DJ. Canine tetanus. Vet Annu. 1993;33:209–219. [Google Scholar]
- 3.DeRisio L, Zavattiero S, Venzi C, DelBue M, Poncelet L. Focal canine tetanus: Diagnostic value of electromyography. J Small Anim Pract. 2006;47:278–280. doi: 10.1111/j.1748-5827.2006.00046.x. [DOI] [PubMed] [Google Scholar]
- 4.Matthews BR, Forbes DC. Tetanus in a dog. Can Vet J. 1985;26:159–161. [PMC free article] [PubMed] [Google Scholar]
- 5.Strogdale L. Canine tetanus. J S Afr Vet Assoc. 1976;47:299–302. [PubMed] [Google Scholar]
- 6.Burkitt JM, Sturges BK, Jandrey KE, Kass PH. Risk factors associated with outcome in dogs with tetanus: 38 cases (1987–2005) J Am Vet Med Assoc. 2007;230:76–83. doi: 10.2460/javma.230.1.76. [DOI] [PubMed] [Google Scholar]
- 7.Acke E, Jones BR, Breathnach R, McAllister H, Mooney CT. Tetanus in the dog: Review and a case-report of concurrent tetanus with hiatal hernia. Irish Vet J. 2004;57:593–597. doi: 10.1186/2046-0481-57-10-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmadsyah I, Salim A. Treatment of tetanus: An open study to compare the efficacy of procaine penicillin and metronidazole. Br Med J. 1985;291:648–650. doi: 10.1136/bmj.291.6496.648. [DOI] [PMC free article] [PubMed] [Google Scholar]