Abstract
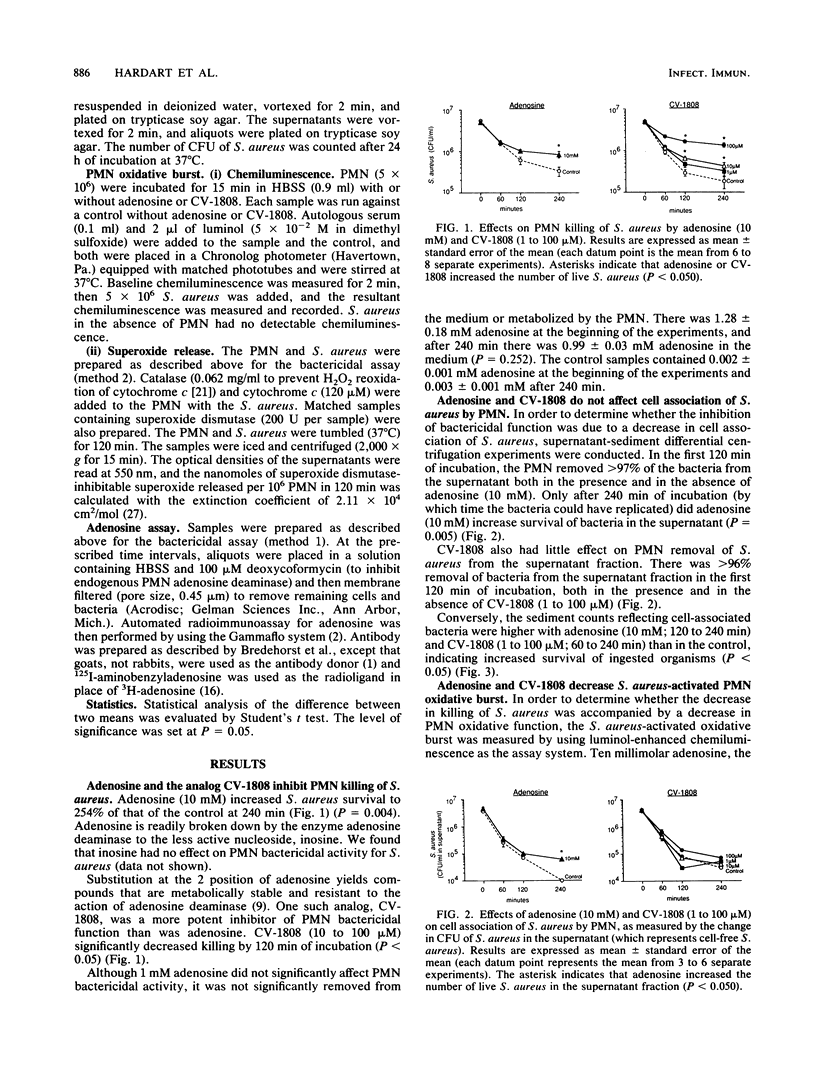
Adenosine is a natural autocoid and immunomodulator that serves an anti-inflammatory role. Stimulation of polymorphonuclear neutrophils (PMN) with soluble stimuli has been shown to inhibit the PMN oxidative burst. We examined the effects of adenosine and the adenosine analog 2-phenylaminoadenosine (CV-1808) on PMN bactericidal function. Adenosine (10 mM) and CV-1808 (10 to 100 microM) inhibited PMN killing of Staphylococcus aureus. There were more surviving bacteria after 240 min of incubation of PMN with S. aureus and adenosine (10 mM) or CV-1808 (100 microM) (254% +/- 45% and 739% +/- 88% of control, respectively) (P less than 0.05) than there were in the control. In contrast, inosine (10 mM), the major degradation product of adenosine, did not affect killing. Adenosine and CV-1808 did not alter cell association of S. aureus, but S. aureus-activated PMN superoxide release was decreased by adenosine (10 microM) and CV-1808 (10 microM) to 67% +/- 7% and 32% +/- 12% that of the control, respectively (P less than 0.05). Since adenosine inhibited PMN bactericidal function only at approximately 10,000 times peak physiological concentrations, endogenous adenosine levels would not be expected to adversely affect PMN bactericidal function. On the other hand, pharmacological concentrations of adenosine derivatives may decrease the oxidative burst and killing sufficiently to increase host susceptibility to infection.
Full text
PDF
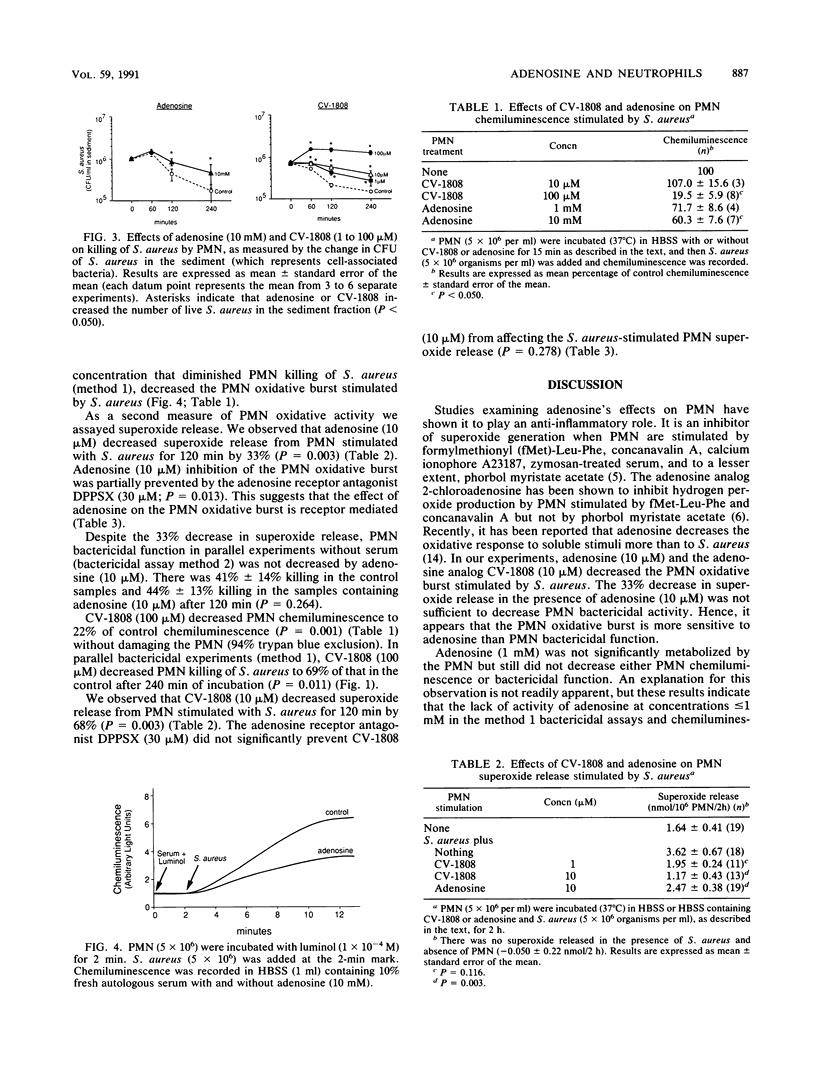


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredehorst R., Wielckens K., Kupper E. W., Schnabel W., Hilz H. Quantification without purification of blood and tissue adenosine by radioimmunoassay. Anal Biochem. 1983 Nov;135(1):156–164. doi: 10.1016/0003-2697(83)90745-5. [DOI] [PubMed] [Google Scholar]
- Brooker G., Terasaki W. L., Price M. G. Gammaflow: a completely automated radioimmunoassay system. Science. 1976 Oct 15;194(4262):270–276. doi: 10.1126/science.184530. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Rosenstein E. D., Korchak H. M., Weissmann G., Hirschhorn R. Occupancy of adenosine receptors raises cyclic AMP alone and in synergy with occupancy of chemoattractant receptors and inhibits membrane depolarization. Biochem J. 1988 Jun 15;252(3):709–715. doi: 10.1042/bj2520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kubersky S. M., Weissmann G., Hirschhorn R. Engagement of adenosine receptors inhibits hydrogen peroxide (H2O2-) release by activated human neutrophils. Clin Immunol Immunopathol. 1987 Jan;42(1):76–85. doi: 10.1016/0090-1229(87)90174-7. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Rosenstein E. D., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985 Aug;135(2):1366–1371. [PubMed] [Google Scholar]
- Daly J. W., Bruns R. F., Snyder S. H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981 May 11;28(19):2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Jacobson K. A., Ukena D. Adenosine receptors: development of selective agonists and antagonists. Prog Clin Biol Res. 1987;230:41–63. [PubMed] [Google Scholar]
- Dunwiddie T. V. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Fiederlein R. L., Glasser L., Galgiani J. N. Bordetella pertussis adenylate cyclase: effects of affinity-purified adenylate cyclase on human polymorphonuclear leukocyte functions. Infect Immun. 1987 Jan;55(1):135–140. doi: 10.1128/iai.55.1.135-140.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Cytoplasmic pH regulation in activated human neutrophils: effects of adenosine and pertussis toxin on Na+/H+ exchange and metabolic acidification. Biochim Biophys Acta. 1986 Dec 19;889(3):301–309. doi: 10.1016/0167-4889(86)90192-8. [DOI] [PubMed] [Google Scholar]
- Hand W. L., Hand D. L., King-Thompson N. L. Antibiotic inhibition of the respiratory burst response in human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1990 May;34(5):863–870. doi: 10.1128/aac.34.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M. Adenosine: emerging role as an immunomodifying agent. J Lab Clin Med. 1987 Sep;110(3):255–256. [PubMed] [Google Scholar]
- Linden J., Patel A., Earl C. Q., Craig R. H., Daluge S. M. 125I-labeled 8-phenylxanthine derivatives: antagonist radioligands for adenosine A1 receptors. J Med Chem. 1988 Apr;31(4):745–751. doi: 10.1021/jm00399a010. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone G., Petracca R., Vigorita S. Adenosine receptors on human inflammatory cells. Int Arch Allergy Appl Immunol. 1985;77(1-2):259–263. doi: 10.1159/000233805. [DOI] [PubMed] [Google Scholar]
- Nauseef W. M., Metcalf J. A., Root R. K. Role of myeloperoxidase in the respiratory burst of human neutrophils. Blood. 1983 Mar;61(3):483–492. [PubMed] [Google Scholar]
- Nishida Y., Honda Z., Miyamoto T. Suppression of human polymorphonuclear leukocyte phagocytosis by adenosine analogs. Inflammation. 1987 Sep;11(3):365–369. doi: 10.1007/BF00915840. [DOI] [PubMed] [Google Scholar]
- Roberts P. A., Newby A. C., Hallett M. B., Campbell A. K. Inhibition by adenosine of reactive oxygen metabolite production by human polymorphonuclear leucocytes. Biochem J. 1985 Apr 15;227(2):669–674. doi: 10.1042/bj2270669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier D. J., Imre K. M. The effects of adenosine agonists on human neutrophil function. J Immunol. 1986 Nov 15;137(10):3284–3289. [PubMed] [Google Scholar]
- Sollevi A. Cardiovascular effects of adenosine in man; possible clinical implications. Prog Neurobiol. 1986;27(4):319–349. doi: 10.1016/0301-0082(86)90005-5. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


