1. Introduction
Nucleosides are structural modules of nucleic acids and therefore of fundamental importance in all living systems [1,2]. They have been playing a major role in treating tumor and virus either as selective inhibitors of certain obligatory enzymes for cancer or viral replication [3], or as nucleic acid chain terminators which interrupt the replication of cancer cells or a virus [4]. The emergence of acquired immunodeficiency syndrome (AIDS) stimulated extensive antiviral research in the past several decades. The study of anti-HIV chemotherapy has promoted a rapid progress of medicinal chemistry, molecular virology as well as an understanding of the mechanism of action of antiviral agents. Currently, about 40 compounds have been approved by the FDA for treatment of AIDS, hepatitis B and C, and infections by herpes viruses. Among the antiviral agents, nucleoside analogs have played a major role.
The selective introduction of a fluoro group into in biologically active molecules has received much attention by medicinal chemists. Several efficient synthetic methodologies for the selective formation of the C-F bond have been developed [5]. The reason for the incorporation of fluorine(s) into biologically active molecules is based on the following characteristics of fluorinated compounds: 1) Fluorine is the second smallest atom and closely mimics hydrogen without much distortion of the geometry; 2) Fluorine is the most electronegative element which can serve as an isopolar and isosteric mimic of a hydroxyl group since the C-F bond length (1.35 Å) is close to the C-O bond length (1.43 Å), as well as fluorine is a hydrogen-bond acceptor; 3) The strength of the C-F bond exceeds that of the C-H bond which often results in increased biological and chemical stability of organofluorine compounds. Therefore, the selective introduction of fluorine atom(s) into a bioactive nucleoside as an isosteric replacement of hydrogen or as an isopolar mimic of hydroxyl group, frequently leads to a dramatic change in biological activities and becomes an important strategy in the design and discovery of novel drug candidates. Currently, there are eight fluorinated nucleoside analogs being used for the treatment of viral infections and cancer, and the additional fluoro-analogs are also undergoing in clinical trials.
In view of the progress in the medicinal chemistry of fluorinated nucleosides and the applications of newly developed methodologies in fluorination in this field, several excellent reviews on the synthetic aspects of sugar-fluorinated nucleosides have been recently published [6]. The present review deals with the synthetic methodology, structural and biological implication of carbohydrate modified fluoronuclesides.
2. Synthesis of carbohydrate fluorinated nucleosides
In principle, fluorinated nucleosides can be synthesized by either fluorination of a preformed nucleoside or by the condensation of a fluorine-substituted glycone with suitable heterocyclic bases. The first approach is a linear synthetic method, which provides the original configuration of starting nucleosides, and the second approach is to condense the fluorine-containing carbohydrates with various heterocyclic bases. The second methodology can provide a variety of fluoro-nucleosides, however, the main limitation of this approach is the poor stetreoselectivity in glycosylation unless the carbohydrates possess a group at the C2-position, which can promotes the steroselectivity for glycosylation [7]. Therefore, the glycosylation reaction of a 2′-deoxy or arabinosyl sugar is, in general, cumbersome in synthetic nucleoside chemistry [8].
There are two classes of fluorinating agents (Figure 1): i) nucleophilic reagents with a fluoride ion as a donor, e.g. DAST [(diethylamino)sulfur trifluoride Et2NSF3; ii) electrophilic reagents, equivalents of F2 with a general structure of (RSO2)2N-F or R3N+-F, among which selectfluor is the best representative.
Figure 1.
Common fluorinating agents
2.1.1 Nucleophilic fluorinating reagents
Fluoride ion is the smallest anion with the largest negative charge density, so it generally acts as a hydrogen-bond acceptor rather than as a nucleophilic agent. Depending on the reaction environment, the fluoride ion can act either as a poor nucleophile (in a protic solvent) or as a good nucleophile (in polar aprotic solvents, especially with large lipophilic cations). Activation of alcohols with good leaving groups, such as mesylate, tosylate or triflate, followed by a SN2 substitution by a fluoride ion has become a standard method to replace OH with F.
i) Olah’s reagents: Py.nHF and iPr2NH.3HF
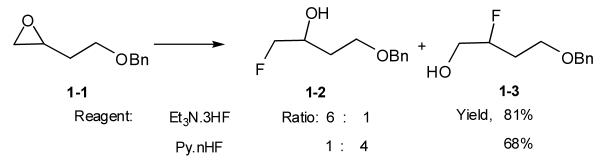
Secondary and tertiary alcohols can be converted to the corresponding fluorides by Olah’s reagent and a preferential fluorination of tertiary alcohols over secondary hydroxyl groups is possible. Py.nHF is more acidic than iPr2NH.3HF, and the difference leads to their opposite regioselectivity when opening epoxides 1-1 to fluorohydrins 1-2 & 1-3 (Scheme 1) and opening aziridines to β-fluoroamine [9]. The acidic HF and Py.nHF will open the epoxide ring by H+ and neutralization of the secondary carbocation by F-, while in case of iPr2NH.3HF, the epoxide is preferably attacked by F- at the least hindered carbon atom in an SN2 process.
Scheme 1.
ii) DAST ( Et2NSF3)
DAST is the most versatile reagent for fluorination in nucleoside chemistry for a one-step exchange of a hydroxyl group by fluorine. It can replace primary, secondary, tertiary and allylic hydroxyl groups by fluorine in excellent yields. For most substrates, SN2 displacement of hydroxyl by fluorine occurs with a complete inversion of configuration. DAST can also convert most aldehydes and ketones to the corresponding gem-difluorides [10].
2.1.2. Electrophilic fluorinating reagents
When a fluorine atom binds to an electronegative nitrogen (F-X) which is activated either by strongly electron-withdrawing groups such as sulfonyl or by positive charges in the same molecule, it can act as a ‘real’ electrophilic F+ for the addition to an electron-rich double bond or the replacement of a proton from an aromatic system (Scheme 2) [11].
Scheme 2.

As a dication, selecfluor has much higher reactivity than NFSI (Figure 1). It is a stable, easily handled, solid electrophilic fluorinating agent and soluble in polar solvents, such as MeCN, DMF, MeNO2 and H2O. Selecfluor is an equivalent of F2, but much more effective and selective. A number of fluorinations of resonance-stabilized carbanions, phosphonate, 1,3-dicarbonyls, enol acetates, enol silyl ethers, enamines, aromatics, double bonds and compounds containing C-S bonds have been fluorinated by selecfluor under mild conditions. Asymmetric fluorination is also achieved in the presence of various chiral catalysts [10].
In nucleoside chemistry, selecfluor is widely used to introduce a fluorine atom into heterocyclic bases via electrophilic substitution (Scheme 3).
Scheme 3.

Selecfluor can also selectively fluorinate certain sugar moieties, which possess electron-rich double bonds via an electrophilic addition (Scheme 4).
Scheme 4.
The conformation of a furanosyl moiety is believed to play a critical role with respect to the biological activity of nucleosides. The structural change caused by the replacement of oxygen or hydrogen by fluorine is significant and has been employed to promote biologically active conformations of nucleosides. In the following section, the synthetic methodology will be focused on several fluorinated nucleosides at different carbohydrate positions.
2.2 Nucleosides fluorinated at C2′
2.2.1 Synthesis of 2′-α-fluoro nucleosides
The unique role of the substituent (hydroxyl or hydrogen) on the C2′-position of nucleic acids as the distinguishing feature between deoxyribonucleic acids (DNA) and ribonucleic acids (RNA) promoted the investigation of the biological properties of nucleosides containing substituents other than hydrogen or hydroxyl groups at this position. Fox and coworkers [16] synthesized a series of 2′-deoxy-2′-fluoro analogs of uridine, 5-fluorouridine, thymidine and cytidine by treatment of 2,2′-anhydro nucleosides with hydrogen fluoride (Scheme 5).
Scheme 5.
This is the first example of a nucleoside with a fluorinated carbohydrate, and the SN2 reaction of a 2,2′-anhydro nucleoside by anhydrous HF became the standard method to introduce a 2′-α-F moiety into pyrimidine nucleosides [16]. Because HF itself is a hazardous reagent, the “tamed hydrofluoric acid”, Olah’s reagent, was used by Shi et al. [17] to fluorinate 2,2′-anhydro nucleosides (Scheme 6).
Scheme 6.
In comparison to hydrofluoric acid, Py.nHF is a milder reagent which can directly fluorinate 2,2′-anhydro-5-fluorouridine (6-1c). However, an arabinonucleoside (6-3), which can be readily prepared from (6-1c), can be fluorinated by DAST in excellent yield. It is noteworthy that 2′-fluoro-2′-deoxycytosine (FdC) (6-6a) was found to be a potent anti-HCV agent with EC90 value of 5 μM, which is more potent than ribavirin, an FDA approved anti-HCV drug [18]. FdC (6-6a) also exhibits potent antiviral activity against Borna disease virus (BDV) [19].
Although the fluorination of 2,2′-anhydronucleosides is a short and direct method to introduce 2′-α-F, the DAST fluorination of an arabinonucleoside is more versatile. 2′-α-Fluorination of purine nucleosides [20] and 2′-branch nucleosides [21] can be achieved only by the second approach (Scheme 7). 2′-Fluoro-2′-methyl cytosine (7-14) demonstrated potent and selective inhibition of HCV replication with an EC90 value of 5.4 μM without any toxicity up to 100μM [21, 22].
Scheme 7.
Taking advantage of electrophilic NFSI, Liotta and coworkers[23] recently reported a novel 2′-αfluorinating method (Scheme 8).
Scheme 8.
NFSI (Figure 1) is an air stable and easily handlable solid with a sufficient steric bulk to stereoselectively fluorinate the enolate of silyl-protected lactone (8-2) in 100% de. The 2′-αfluorolactone (8-3) was used as a common intermediate to synthesize a number of 2′,3′-dideoxy-2′-fluoro nucleosides [23].
2.2.2 Synthesis of 2′-β-fluoro nucleosides
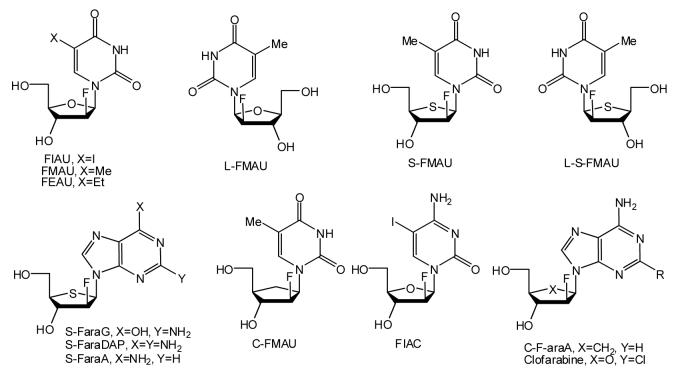
Introduction of a fluorine atom at the 2′-β-position of nucleoside analogs has produced a variety of interesting antiviral agents (Figure 2). FIAC, FEAU, FMAU and their congeners were first synthesized in Fox’s group (Scheme 9 and 10) [24] and showed potent activity against HSV and also demonstrated excellent activity against hepatitis-B virus (HBV), Varicella zoster virus (VZV), cytomegalovirus (CMV), and Epstein-Barr virus (EBV). In addition, FMAU showed significant activity against murine leukemias.
Figure 2.
Scheme 9.
Scheme 10.
F-ara-A (9-6) was first synthesized by Wright et al. (Scheme 9) [24a] as a fluoro derivative of β-D-arabinofuranosyladenine (ara-A), an agent approved by the FDA for HSV-1/2 treatment. Methyl 2,3-anhydro-5-O-benzyl-β-D-riboside (9-1) was treated with KHF2, which gave a mixture of the 3′- and 2′-fluoro-D-arabinofuranoside (9-2a) and (9-2b) with the 3′-β-F isomer as the major product. 2′-β-Fluorinated sugar (9-4) was used for coupling with 2,6-dichloropurine to give nucleosides (9-5) and subsequently afforded F-ara-A (9-6). Due to the low yield of the 2-fluoro sugar (9-2b), Watanabe et al. [24b,c] subsequently developed a practical synthetic approach to 2-deoxy-2-fluoro-D-arabinose (10-6) and (10-7) from a readily-available D-glucose derivative (10-1, Scheme 10).
The crucial steps were the fluorination of (10-1) by KF in acetamide as well as the conversion of 6-O-benzoyl-3-deoxy-3-fluoro-D-glucofuranose (10-5) into 5-O-benzoyl-2-deoxy-2-fluoroarabinofuranose (10-6) by periodate oxidation. A series of pyrimidine nucleosides, such as FMAU, FEAU and FIAU, were prepared in a large scale for biological evaluation (Figure 2). Unfortunately, the phase I trials of FMAU as an antileukemic agent were terminated due to neurological toxicity [25]. FIAU also exhibited delayed toxicities due to the interference of mitochondrial function resulting in lactic acidosis and hepatic failure [26].
Because of the broad spectrum of biological activities of 2′-F-arabinofuranosyl nucleosides, a number of structural modifications of these analogs have been carried out. Chu and co-workers have demonstrated that the enantiomer of FMAU, L-FMAU (Figure 2), is a promising agent against HBV [27]. It showed low toxicity in rats and woodchucks, potent in vivo antiviral activity against chronically infected woodchuck (WHV), respectable bioavailability and showed no significant virus rebound up to 36 weeks after cessation of the drug treatment. L-FMAU was approved as anti-HBV drug by the Korean FDA in Nov. 2006 and is under phase III clinical trials in the USA and Europe. Another interesting congener is clofarabine which was approved by FDA for the treatment of acute lymphoblastic leukemia [28].
4′-Thio substituted 2′-β-F nucleosides also retain their antiviral activity. Machida et al. have reported that 2′-fluoro-4′-thioarabinonucleosides are active against HSV-1, HSV-2, VZV, and HCMV [29]. S-FaraG (Figure 2) and S-FaraDAP are particularly potent against all types of herpes viruses and showed a 6-fold lower EC50 value in comparison to ganciclovir against HCMV. A carbocyclic derivative of FMAU and C-FMAU also showed moderate anti-HSV-1 activity [30], whereas C-F-araA (Figure 2) is 10-fold more active than cyclaradine itself against HSV-1 and HSV-2, and is more active than acyclovir against HSV-2 in mice [31].
The potent antiviral activities of FIAC and FMAU prompted Tann et al. [32] to develop an efficient method for the construction of the 2′-β-F arabino configuration (Scheme 11). The direct displacement of 2-imidazoylsulfonate (11-3) with KHF2 gave a 63% yield of 2′-β-F sugar (11-4), and subsequent bromination with 30% HBr in HOAc produced a quantitative conversion to the 1-α-bromo sugar (11-5). The exclusive formation of the 1-α-glycosyl bromide (11-5), attributed to the stabilizing interaction between the unshared electron pairs on fluorine and oxygen with the σ* orbital for the axial C-Br bond, the so-called “anomeric effect” [33], leads to preferential formation of β-nucleosides, and (11-5) is being used as a standard sugar for the preparation of 2′-deoxy-2′-fluoro-arabinonucleosides, in particular for pyrimidine nucleosides. It is noteworthy that the condensation of bromosugar (11-5) or (10-7) with 6-chloropurines is usually difficult [6,34]. In fact, the reactions of the sugar (10-7) with some purine bases, such as 6-chloropurine and 2,6-dichloropurine, gives a complicated mixture of four isomers (7-, or 9-substitued, α,βanomers) from which the desired isomer was separated in low yield [28a,35]. Starting from adenosine, after protecting the 3′,5′-dihydroxyls with a bulky trityl group to make the furanose ring adopt the C-2′-endo conformation, Watanabe and coworkers[36] synthesized 2′-deoxy-2′fluoro arabinoadenosine (12-3) by TASF fluorination of the 2′-OH in 30% yield (Scheme 12). Accordingly, Mikhailopulo et al. [37] chose a bulky pivaloyl group to protect adenosine, from which F-araA derivative (12-6) was prepared in 31% yield via DAST fluorination of the 2′-OH.
Scheme 11.
Scheme 12.
Although the direct coupling of the bromosugar (10-7) with 2,6-dichloropurine gave an unacceptable yield [28a], Montgomery et al. [28b] synthesized and evaluated various 2-halo-9-(2-deoxy-2-fluoro-β-D-arabinofuranosyl)adenines and found that clofarabine (Figure 2) possessed potent anti-tumor activity. Recently, Bauta et al. [28c] reported an efficient manufacturing process for clofarabine starting from commercially available bromosugar (11-5) (Scheme 13).
Scheme 13.
In view of the interesting discovery of L-nucleosides as biologically active compounds, the Chu’s group synthesized L-FMAU and its derivatives [38,39] (Scheme 14) and evaluated these compounds as potential antiviral agents. From this effort, they discovered L-FMAU as a potent antiviral agent against HBV as well as Epstein-Barr virus.
Scheme 14.
2′,3′-Dideoxy nucleosides (ddNs) exhibit potent anti-HIV activity and several in this class of nucleosides, such as ddI and ddC, are in clinical use. However, the instability of these compounds in an acidic media complicates oral administration. Since the fluorine at the 2′-position of a carbohydrate moiety can provide acidic and enzymatic stabilities to the glycosidic bond of nucleosides, a number of 2′-F ddNs have been prepared and evaluated. The 2′-α-F isomers of ara-ddI and ara-ddA were inactive, but the 2′-β-F isomers, F-ara-ddI and F-ara-ddA, were just as potent as parent drugs [6a].
As an efficient method to synthesize 2′-β-F ddNs, a standard sugar moiety (11-5) [40] and related 2′-deoxy-2′-fluoro arabinonucleosides (15-1) [41] were extensively used to synthesize Fara-ddNs (15-3) via Barton deoxygenation of 3′-OH (Scheme 15). 2,2′-Anhydro nucleoside (15-4) can also be used to synthesize a 2′-F-d4T analog (15-5) and 2′,3′-dideoxy- 2′,3′-difluoro arabinonucleoside (15-6) [41].
Scheme 15.
The requirement for tributyltin hydride in the deoxygenation step is especially troublesome as it results in tin contamination of the final product. Therefore, some convergent methodologies, which removed the C-3 hydroxyl group in an early stage of sugar preparation, were developed to synthesize 2′-β-F ddNs (F-ara-ddNs).
Taking advantage of the anomeric effect of 2′-β-F (Scheme 11), Chu and co-workers [42] developed two practical routes to key intermediates, 1′-α-Br-2′-β-F sugar moieties, (16-4) and (16-8), for the synthesis of D- and L-2′,3′-dideoxy-2′-fluoro arabinonucleosides, respectively (Scheme 16). Ocabe et al. at Roche [43] also described another efficient procedure to prepare a similar sugar moiety (Scheme 17).
Scheme 16.
Scheme 17.
In fact, for the synthesis of anti-HIV agents, F-ara-ddA, F-ara-ddI and their analogs, a variety of methods were developed, such as fluorination at the C2′-β-position of the purine 3′-deoxynucleosides [44] and hydrogenation of 2′-F d4Ns [45].
2.2.3 Synthesis of 2′,2′-difluro nucleosides
Gemcitabine (2′-deoxy-2′,2′-difluorocytidine) is a clinically effective anti-cancer agent for the treatment of pancreatic cancer. It has also shown anti-tumor activity against a wide spectrum of human solid tumors. Researchers at Lilly [46] described the synthesis of gemcitabine, which constructed the 2,2-difluoro-D-ribose moietey using a CF2-containing building block (Scheme 18). Thus, glyceraldehyde (18-1) was coupled with ethyl bromodifluoroacetate under Reformatski conditions to give a 1:3 mixture of (18-2) and (18-3). The major isomer was hydrolyzed to give lactone (18-4), which was further converted to 2,2-difluoro-D-ribose (18-6). Due to the strong electron-withdrawing effect of the 2-gem-difluoro group, the intermediate (18-6) was converted to the 1-O-mesylate (18-7), which was condensed with cytosine to afford gemcitabine.
Scheme 18.
Similarly, Chu and coworkers synthesized a series of L-2′,2′-difluoro ribonucleosides [47] and their 3′-deoxy derivatives. However, none of those showed any antiviral activity against HIV, HSV and HBV [48].
2.2.4 Synthesis of 2′-fluoro-2′,3′-didehydro-2′,3′-dideoxy nucleosides (2′F-d4Ns)
2′,3′-Unsaturated nucleosides, d4T and abacavir, are clinically effective anti-HIV agents and a number of 2′- or 3′-substituted d4N analogs also retain their anti-HIV activity. In particular, introduction of a fluorine atom at the 2′-position produces compounds with anti-HIV and anti-HBV activity. Chu and coworkers [49] demonstrated that a series of 2′F-d4Ns have interesting biological profiles and L-2′F-d4C and L-2′F-d4FC are among the most potent anti-HBV agents (Table 1),.
Table 1.
Anti-HIV and anti-HBV activity of 2′F-d4N analogs
 | |||||
|---|---|---|---|---|---|
| Compound | Activity (EC50, μM) | Toxicity (IC50, μM) | |||
| HIV | HBV | PMB | CEM | Vero | |
| D-2′F-d4C | 0.82 | - | >100 | >100 | >100 |
| D-2′F-d4A | 0.44 | - | >100 | >100 | >100 |
| L-2′F-d4C | 0.51 | 0.002 | >100 | >100 | >100 |
| L-2′F-d4FC | 0.17 | 0.004 | >100 | >100 | >100 |
| D-S-2′F-d4C | 0.12 | - | >100 | >100 | >100 |
| L-S-2′F-d4FC | 0.15 | - | >100 | >100 | >100 |
| L-C-2′F-d4A | 0.77 | - | >100 | >100 | >100 |
As shown in Scheme 19, L- and D-glyceraldehyde acetonide were subjected to Horner-Emmons reaction in the presence of fluorophosphonoacetate and NaN(TMS)2 to give 2-fluoro-α,β-unsaturated carboxylate (19-2) [49a] and (19-6) [49b], respectively. The esters (17-2) and (19-6) were cyclized under acidic conditions to 2-fluorobutenolides, and then transformed to the key intermediates, acetates (19-4) and (19-8), respectively. Condensations of the sugar moieties (19-4) and (19-8) with various silylated purines and pyrimidines was carried out to furnish L- and D-2′F-d4Ns, respectively.
Scheme 19.
The synthesis of D- and L-S-2′F-d4Ns [49c,d] started from (S)- and (R)-2-fluorobutenolide (19-7) and (19-3), respectively (Scheme 20). Lactone (19-7) was hydrogenated to give butyrolactone (20-1). The iodo ester (20-2) was generated from compound (20-1) which was transformed to thiolactone (20-3). The key intermediate (20-6), 4-thio-D-ribofuranoside, was obtained after several manipulations. The condensation of sugar (20-6) with bases gave exclusive β-nucleosides by virtue of the bulky α-phenylselenyl group. Oxidation with mCPBA followed by pyridine catalyzed elimination afforded D-S-2′F-d4Ns.
Scheme 20.
The synthesis of D- and L-C-2′F-d4Ns is accomplished through a lengthy sequence (Scheme 21) [49e]. The Ketone (21-1), prepared from D-ribose in 17-step, was fluorinated with DAST to give a difluorinated compound (21-2), which underwent elimination in the presence of potassium tertbutoxide to an allylic ether (21-3). After debenzylation using sodium metal in liquid ammonia, the key intermediate (21-4) was obtained which was coupled with purines and pyrimidines under Mitsunobu conditions.
Scheme 21.
2.3. Nucleosides fluorinated at C3′
2.3.1 Mono-fluorinated at C3′
The effect of a single fluorine atom at various positions of the carbohydrate moiety in dideoxynucleosides (ddNs) as reverse transcriptase inhibitors has been investigated extensively. The structure-activity relationships for these mono-fluoro ddNs shows that a sugar moiety, a fluorine atom at positions 3′-“down” or 2′-“up” correlates well with anti-HIV activity. On the other hand, fluorine atoms at the same positions, but with inverted stereochemistry, namely 3′-β-F or 2′-α-F configurations, generally produced inactive compounds [50].
A number of 3′α-fluorinated ddNs display potent antiviral activity (Figure 3). 3′α-Fluoro- 2′,3′-dideoxyuridine (FddU) demonstrated anti-HIV whereas ddU is inactive, and the 3′α-F substitution of ddG (FLG) has higher anti-HIV activity in comparison to the parent nucleoside in MT-4 and CH3 cells [51]. FddT (FLT) is one of the most potent inhibitors of HIV RT [52]. Unfortunately, FLT produces profound toxic effects. However FddClU demonstrated significant ant-HIV activity with low cytotoxicity in human leukemic cells as well as in bone marrow progenitor cells [53a]. FddClU is also active against HIV strains resistant to AZT, ddI, ddC and 3TC [53b]. FddG and L-FddC have also demonstrated their anti-HBV activity [54,55].
Figure 3.
Two approaches are used for the synthesis of 3′αF-ddNs: (i) transformation of natural 2′-deoxyribonucleosides [56] or ribonucleosides [57] to the desired dideoxyfluoro derivatives, and (ii) coupling of heterocyclic bases with suitable sugar moieties containing 3′ αF- [58] or a C3-xylo-hydroxyl group [59], which may be replaced by a fluorine atom. Natural ribonucleosides, although being the most synthetically accessible nucleosides, are less amenable for the synthesis of 3′αF-ddNs, probably due to lengthy transformation procedures. However, two recently published methods are of interest, in which Robins et al. [60] developed a facile sequence with deoxygenative [1,2]-H shift rearrangement as the key step (Scheme 22). The transformation from ribonucleoside (22-2) to 2′-deoxy xylonucleoside (22-3) consists of a [1,2]-hydride shift with the departure of a leaving group from the opposite face. Transient formation of a C=O group is followed by rapid transfer of a hydride-equivalent from the same face from which the leaving group departed, which results in a double inversion of the stereochemistry at the two vicinal carbon atoms. Thus, 3′αF-ddNs (22-6) were prepared from ribonucleoside (22-1) in 6 steps in good yield.
Scheme 22.
Takamatsu et al. [61] described another facile 6-step synthesis of 3′αF-ddNs using a similar deoxygenative rearrangement which involved a [1,2]-bromo shift during 3′α fluorination (Scheme 23).
Scheme 23.
2.3.2. 3′,3′-Difluoro and 3′-fluoro-2′,3′-didehydra-2′,3′-dideoxy nucleosides (3′F-d4Ns)
For the synthesis of 3′,3′-difluoronucleosides, two methods are used for a CF2 introduction: (i) difluorination of a carbonyl group (C=O); (ii) construction of appropriate sugar moieties with CF2-containing building blocks. The former is a direct and facile approach, but only applicable to cyclic ketone substrates with less steric hinderence (no substituent adjacent to carbonyl group). Chu and coworkers [62] developed a general synthetic method to prepare both D- and L-2′,3′-dideoxy-3′,3′-difluoronucleoside and their analogs (Scheme 24). Resorting to difluorination of an appropriate ketone, the key CF2-containing sugar intermediates (24-5) or (24-9) were prepared, which were subsequently condensed with bases to afford the corresponding 3′,3′-difluoronucleosides (24-10) and (24-11), respectively.
Scheme 24.
Using the same strategy, Chu and coworkers also synthesized thiosugar [63] as well as carbocyclic analogs [64] of 2′,3′-dideoxy-3′,3′-difluoro nucleosides (Scheme 25). None of the synthesized nucleosides showed any antiviral activity.
Scheme 25.
For the preparation of 3′,3′-difluoro nucleosides bearing vicinal 2′-OH, Qing et al. [65] provided a stereoselective procedure using a CF2-containing building block (Scheme 26). The method involves a sequence that includes a coupling reaction of a gem-difluorohomoallylic metallic reagent with D-glyceraldehyde acetonide, Sharpless asymmetric dihydroxylation of the resulting homoallylic alcohol, followed by a stereoselective ring forming reaction provided both D- and L-3′-deoxy-3′,3′-difluororibonucleosides (26-6) and (26-8).
Scheme 26.
Like 2′F-d4Ns (Table 1), 3′-fluoro-2′,3′-unsaturated nucleosides and their 4′-thiofuranosyl analogs as well as carbocyclic analogs were synthesized via an elimination reaction of 3′,3′-difluoronucleosides, which were synthesized as described in Scheme 24 and 25, in the presence of tBuOK (Scheme 27), and their antiviral activity were evaluated [62-64] (Table 2).
Scheme 27.
Table 2.
Anti-HIV and anti-HBV activity of 2′F-d4N analogs
 | |||||
|---|---|---|---|---|---|
| Compound | Activity (EC50, μM) | Toxicity (IC50, μM) | |||
| HIV-1 | M184V | PBM | CEM | Vero | |
| L-3′F-d4C | 0.089 | 2.4 | >100 | >100 | >100 |
| L-3′F-d4FC | 0.018 | - | >100 | >100 | >100 |
| L-S-3′F-d4C | 0.13 | - | >100 | >100 | >100 |
| L-S-3′F-d4FC | 0.031 | - | >100 | >100 | >100 |
| D-C-3′F-d4G | 0.41 | 3.8 | >100 | >100 | >100 |
The complete SAR study of the synthesized 3′F-d4Ns showed that several compounds possess potent anti-HIV activity (Table 2) [62-64]; D-3′F-d4C and D-3′F-d4A show modest anti-HIV activity in H9 cells, whereas L-3′F-d4C, L-3′F-d4FC and their 4′-thio analogs, L-S-3′F-d4C, LS-3′F-d4FC, exhibit potent anti-HIV agents without significant toxicity. Carbocyclic D-3′F-d4G also exhibited potent anti-HIV activity (EC50 0.4 μM). In addition, D-C-3′F-d4G and L-3′F-d4C showed significant activity against lamivudine-resistant virus (HIV-1M184V) in PBM cells.
The 2',3′-Double bond along with the fluoro group significantly enhanced the antiviral activity of the nucleosides (Table 1 and 2) [66]. Recent modeling studies (Figure 4a) [67] highlighted the additional hydrophobic interaction mediated by the 2′,3′-double bond with the active site residue (Tyr115) of HIV-1 reverse transcriptase to enhance the overall binding affinity. Further, the fluoro group at 2′-position orients the sugar moiety towards Met184 residue and reduces antiviral potency in M184V (Figure 4b) [67]. In another study, the fluoro group at the 3′-position reveals a different biological profile for D- and L-nucleoside. The L-nucleosides such as L-S-3′F-d4C showed potent anit-HIV activity while D-S-3′F-d4C was found to be inactive. Modeling studies (Figure 5) [63] suggest the favourable binding mode of L-S-3′F-d4C in the active site without any steric hindrance with Met184, while the D-S-3′F-d4C displays an unfavorable overall conformation due to presence of the intramolecular interaction between 3′-F and β-phosphate.
Figure 4a.

2′,3′-double bonds of (D4T, D4FC, 2′Fd4C, 2′F-4′Sd4C and Carbovir) are separated from the aromatic side chain of Tyr115 by 3.5 Å situated in parallel by the hydrophobic π—π interaction.
Figure 4b.

No steric hindrance is found between 4′-CH2 group (blue) of carbovir with Val184. However, 2′F-4′Sd4C with 4′-sulfur (red) experiences steric hindrance. L-Configured 3TC (yellow) also has steric hindrance with Val184.
Figure 5.

a) L-3′F-4′Sd4C binds in the active site without steric hindrance with Met184. 3′-F showing H-bond with NH of Asp185. b) L-3′F-4′Sd4C in M184V HIV-1-RT, showing steric hindrance with V184 c) 3′-F group of D-3′F-4′Sd4C showing intramolecular interaction with β-phosphate causing unfavourable binding conformation
4′-Subsituted nucleosides are believed to function as ‘delayed chain terminators’ [68] which arise from the rigid north-conformation of their sugar rings [69] and have a novel anti-HIV mechanism which differs from the conventional 2′,3′-dideoxy nucleosides. Ohrui et al. [70a] reported a number of 4′-ethynyl-2′-deoxy nucleosides, some of which were identified as among the most potent anti-HIV compounds which blocked the replication of a wide spectrum of laboratory and clinical HIV-1 strains. In view of this observation, Chu and coworkers [71] also synthesized D- and L-4′-ethynyl-3′-fluoro 2′,3′-unsaturated nucleosides (28-11) and (28-12)(Scheme 28).
Scheme 28.
Fluorine-containing lactone (20-1), which was prepared form D-glyceraldehyde acetonide (19-5), was converted to the α, β-unsaturated ketone (28-4), the key intermediate, which was subjected to the standard Grignard reaction with ethynylmagnesium bromide to give a separable mixture of (28-5) and (28-6) in a ratio of 3:2 with a total yield of 72%. Tertiary alcohol (28-5) and (26-6) underwent a series of reactions including the deprotection, oxidation, and acetylation to furnish the D- and L-3-fluoro-2,3-unsaturated-4-ethynylfuranose (28-9) and (28-10), respectively. D- and L-4′-Ethynyl-3′-fluoro-2′,3′-unsaturated nucleosides were thus synthesized from (28-9) and (28-10). Unfortunately, none of these nucleosides showed any potent anti-HIV activity.
2.4. Nucleosides fluorinated at C4′
Although natural nucleocidin (Scheme 29) was isolated in 1957, 4′-substituted nucleosides was not identified as a new class of anti-HIV nucleosides until Maag et al. [72] found that 4′-azido-2′-deoxythymidine (4′AZT) exerted potent activity against HIV-1 in 1992. Extensive structure-activity studies found that other substituents, such as methyl, fluoromethyl, ethynyl and cyano at 4′-position also exhibited high antiviral activity against HIV [73]. The unique anti-HIV activity of these nucleosides may be due to the rigid north-conformation of their sugar rings which related to 4′-substitution.
Scheme 29.
As shown in Scheme 29, Moffat et al. [74] developed a method for the introduction of fluorine atom at the C4′-position from 4′,5′-dehydronucleoside (29-2). Thus, the 4′,5′-olefin (29-2) was subjected to electrophilic addition of iodine fluoride which gave a mixture of 4′- and 5′-fluorinated nucleosides unselectively. The desired 4′-fuoro-5′-iodo nucleoside (29-3) is the key intermediate for nucleocidin. The 4′,5′-dehydronucleoside can also be converted to the 4′-β-OH intermediate (29-4), followed by selective introduction of the 4′-α-F by fluorination with DAST. Hong et al. [75] reported the synthesis of a chiral fluorine-containing building block (30-3), which was utilized in the convergent synthesis of 4′-fluorinated apionucleosides (30-6) [75] and carbocyclic 2′,3′-didexoy nucleosides (30-12) [76] (Scheme 30).
Scheme 30.
2.5. Nucleosides fluorinated at other positions
2.5.1. C6′-fluorinated carbocyclic nucleosides
Carbocyclic nucleosides are quite unique and as a result it is difficult to identify biologically active agents with respect to their furanose counterparts [77], probably due to their different conformational factors of the carbocyclic ring. The way to make the conformation of the carbocyclic ring similar to their furanose equivalents would be to put electronegative substituent(s) such as a fluorine atom into the ring. The CF2 group has been suggested by Blackburn [78] as an isopolar and isoteric subsituent for oxygen. Thus Qing and coworkers [79] synthesized 6′,6′-difluoro d4U (Scheme 31). The key intermediate, gem-difluorinated 2,3-unsaturated cyclopentyl alcohol (31-5), was prepared from (Z)-but-2-ene-1,4-diol via ring-closing metathesis, and the introduction of gem-difluoromethylene group was accomplished by a silicon-induced Reformatskii-Claisen reaction of chlorodifluoroacetic ester (31-1).
Scheme 31.
α-Fluorination of the cyclopentanone derivative with Selecfluor is another way to synthesize 6′-fluoro carbocyclic nucleosides. Samuelsson et al. [80] described an asymmetric synthesis of 6′-α- and 6′-β-monofluorinated carbanucleosides (Scheme 32). Thus, silylenol ether (32-2) which was prepared from cyclopentanone was treated with Selecfluor in DMF to give 1:1 inseparable mixture of the fluoroketones (32-3) and (32-4). Selective reduction of the ketone gave two diastereomeric alcohols, the key intermediate (32-5) and (32-6) for 6′-fluoronucleosides, in 41% and 49%, respectively.
Scheme 32.
2.5.2. Nucleosides bearing exocyclic fluorocarbon substituents
The influence of a trifluoromethyl group in biologically active molecules is often associated with its increased lipophilicity. In addition, its electronegativity (similar to fluorine) and the size (in between methyl and isoppropyl) are also contributing factors. The unique nature of the fluoromethyl group has prompted interest in the synthesis of nucleosides bearing exocyclic fluorocarbon substituents.
Several methods have been described in the literature for introducing a CF3 group into nucleoside analogs [81]. However, the utilization of Ruppert’s reagent, (trfluoromethyl)trimethylsilane (TMSCF3), as a nucleophilic trifluoromethylating reagent is rapidly becoming the method of choice. Such methodology requires the previous preparation of a suitable 2- or 3-keto sugar intermediate, which then accept the nucleophilic attack of CF3 carbanion. 3′-Trifluoromethyl nucleosides were reported [82] (Scheme 33) via this method. The Barton-type deoxygenation of the unreactive tertiary hydroxyl function using imidazole-1-thiocarbonyl or the phenoxythiocarbonyl derivative was unsuccessful. However, with a modification of the radical deoxygenation using the methyloxalyl ester derivative, the tertiary hydroxyl group (33-2) underwent stereospecific deoxygenation wherein the hydrogen atom abstraction from Bu3SnH occurred from the β-face to give 3-trifluoromethylated sugar (33-3) in good yield. Similarly, a CF3 group can be introduced at the C2 position of a sugar to prepare 2′-trifluoromethylated nucleosides [83].
Scheme 33.
The carbonyl group in the sugar unit can also be utilized to introduce a difluoromethyl group via a Wittig-type reaction or a nucleophilic addition with a CF2-containing reagent. Serafinowski et al. [84] treated 2′-oxo (or 3′-oxo) nucleosides with bromodifluoro[tris(dimethylamino)] phosphonium bromide and zinc to afford the corresponding 2′- (or 3′-) difluoromethylene nucleosides (Scheme 34). The difluoromethylene function of (34-5) can be transformed to a difluoromethyl group by hydrogenation or by TBAF mediated rearrangement.
Scheme 34.
Besides the above-mentioned Wittig-type olefination with the reagent generated in situ from CF2Br2/(Me2N)3P, McCarthy et al. [85] reported a modified Julia’s approach to introduce a difluoromethylene function at the 2′-keto position (Scheme 35). The α-face addition of the carbanion of difluoromethyl phenyl sulfone, PhSO2CF2H, into the protected 2-keto nucleoside (35-1) gave a sulfone (35-2) in 85% yield. The subsequent reductive elimination of tertiary mesylate (35-4), prepared from (35-2) in 70% yield using SmI2, was effective to build up the desired difluoromethylene group at C2′ position.
Scheme 35.
By choosing a different reductive reagent, sodium amalgam, Piccirilli et al. [86] transformed a CF2-containing sulfone into the difluoromethyl group instead of difluoromethylene (Scheme 36), and thus prepared 2′-difluoromethyl ribonucleosides (36-4). The key steps included nucleophilic addition of difluoromethyl phenyl sulfone to 2-keto-ribose (36-1) followed by mild and efficient reductive desulfonation.
Scheme 36.
The terminal monofluoro olefinic group (CHF=C-) is a useful functionality in the design of mechanism-based enzyme inhibitors. McCarthy et al. [87] developed a stereospecific method to construct a terminal E- and Z-fluoromethylene group from a carbonyl moiety and found that (E)-2′-fluoromethylene-2′-deoxycytidine, (E)-FMC (Scheme 37) shows potent anti-tumor activity against leukemias and solid tumors. Using the Hornor-Wittig reaction, 2′-ketonucleoside (37-2) was converted to a mixture of readily separable fluorovinyl sulfones E- and Z-(37-3), which can be transformed to fluorovinylstannanes with tributyltin hydride with retention of configuration. (E)-FMC was obtained directly from E-fluorovinylstannane in excellent yield.
Scheme 37.
3. Biological applications of fluorinated nucleosides
Fluorinated nucleosides (Figure 6) exhibit a wide variety of biological activity and have been used extensively as anti-tumor [88] and antiviral [89] agents. In many cases, the stability of the nucleoside analogue, particularly the stability of the glycosyl bond, is an important factor determining the biological activity as well as the therapeutic usefulness of nucleosides as drug candidate. Fluorine substitution at the 2′- or 3′-position of a sugar is known to increase the chemical stability of nucleoside analogues, particularly in an acidic environment [49b]. Fluorine substitution also has a favorable affect of increasing the metabolic stability [62]. These findings suggest the importance of a fluorine moiety in the nucleoside as therapeutic agents. Thymidylate synthase, ribonucleotide diphosphate reductase (RDPR) and viral polymerases are the major targets by fluoronucleosides and fluoroheterocyclic bases [90].
Figure 6.
Some important biologically useful fluorinated nucleosides & their precursors
3.1 Antiviral activity of fluoronucleosides
Viral polymerase is one of the most common and established targets of nucleoside reverse transcriptase inhibitors (NRTIs) in antiviral therapy [91], and the presence of a fluoro group in the nucleoside makes it unique in terms of their chemical, biological and structural properties [92]. For the past several years, Chu and coworkers have been involved in understanding the molecular mechanism of HIV and HBV polymerases and their interaction with nucleoside triphosphates using molecular modeling techniques [93]. Several fluoro nucleosides have been also investigated in terms of their structural and biological profile as polymerase inhibitors. A right hand structure (fingers, palm, and thumb subdomains) were assigned for the structural model of polymerase (Figure 7) with respect to HIV reverse transcriptase (HIV-RT) crystal structure [94].
Figure 7.
A viral polymerase model structure with DNA double strand (primer and template) and natural nucleoside,highlighting the position of catalytic polymerase site.
Polymerase catalytic residues are highly conserved [95] and are responsible for the interaction with the template, primer and incoming nucleoside triphosphate. The NRTIs include those compounds which can mimic endogenous pyrimidine or purine nucleosides, and all of them require an intracellular phosphorylation to their triphosphate forms for activity. The first phosphorylation step, often considered as rate limiting, is dependent on deoxynucleoside kinases, such as thymidine kinase, deoxycytidine kinase (dCK) and deoxyguanosine kinase (dGK). Deoxycytidine kinase does not discriminate between the enantiomeric (D & L) forms of substrates and is able to phosphorylate L-nucleosides in a number of cases [96].
Clevudine (L-FMAU a β-L-2′-fluoro-thymidine) is an L-nucleoside of thymidine with potent anti-HBV activity [97]. L-FMAU requires step-wise biotransformation to its triphosphate form to exert its pharmacological activities (Figure 8) [98]. The mono phosphorylation is catalyzed by thymidine kinase (TK) along with deoxycytidine kinase (dCK) [99]. The diphosphate formation may be associated with thymidylate kinase (TMPK) activity [100] and finally triphosphate formation is carried out by 3-phosphoglycerate kinase [101]. Experimental evidence suggests that there is no incorporation of L-FMAU into DNA (Scheme 38) [102], an observation that makes this molecule unique in contrast to other nucleoside anti-HBV agents.
Scheme 38.
A plausible metabolic path of L-FMAU as a HBV polymerase inhibitor
In addition, L-FMAU does not inhibit human cellular polymerases and does affect mitochondrial function or host DNA synthetic machinery [102]. The toxicological studies in mice (50 mg/Kg/day) and woodchucks (10 mg/Kg/day) suggest no apparent toxicity [97]. The short-term oral administration of L-FMAU to a duck model induced a significant decrease in the level of viremia, without liver toxicity [103]. Another study that included dose-dependent responses in woodchuck showed no drug-related toxicity and exceptional potency against chronic HBV infection [104].
L-FMAU (clevudine) is an oral antiviral agent for the treatment of chronic HBV that has been shown to be well tolerated and has demonstrated potent antiviral activity in clinical studies [105]. Clevudine has been approved for the treatment of chronic hepatitis B virus infection in South Korea in 2006 and is currently undergoing Phase III clinical trials in the US and Europe. Recent phase III clinical trials for 24 weeks [106] at 30 mg clearly demonstrated a potent and sustained antiviral effect associated with sustained normalization of alanine aminotransferase (ALT) levels. This sustained antiviral effect after discontinuation of the drug makes this molecule a unique antiviral agent in comparison to other approved anti-HBV agents. The plausible mechanism of sustained viral suppression may be attributed to the reduction of intrahepatic cccDNA [107]. In addition, immunological factors might be another reason for a sustained anti-HBV activity. These combined mechanisms along with the favorable pharmacokinetic properties may be the reason for the unique antiviral properties of clevudine. Emtricitabine (Figure 6) is another important 5-fluorinated nucleoside that acts as a NRTI, approved for the treatment of HIV infection. A potential disadvantage of this agent is that it may be ineffective against lamivudine-resistant HBV strains and may be associated with the development of similar HBV-resistant mutants. Elvucitabine (β-L-Fd4C) is another fluoro nucleoside in phase II as a NRTI.
3.2 Anticancer activity of fluoronucleosides
Among the important nucleoside analogs [108], fluornucleosides are well documented and explored as potential anticancer agent [109,110]. It has been suggested that the induction of apoptosis is the final pathway through which these drugs exert their anticancer activity [111]. These nucleoside analogs mimic natural nucleosides in terms of uptake and metabolism. Further, they can incorporate into newly synthesized DNA resulting in chain termination. Some of these drugs also inhibit key enzymes involved in the biosynthesis of purine and pyrimidine nucleotides and RNA synthesis as well as directly activate the apoptosis as an anti-cancer agent.
A recent study showed the importance of one of the fluoro nucleoside, clofarabine [2-chloro-(2-deoxy-fluoro-β-D-arabinofuranosyl)adenine], as a developed new-generation nucleoside analog for acute lymphoblastic leukemia (ALL) [112]. Like other nucleoside analogs, clofarabine exerts its anti-cancer effect by inducing “apoptosis” by blocking DNA synthesis as well as through inhibition of DNA repair [113].
Clofarabine is a newer generation nucleoside anticancer agent related to fludurabine. Fludarabine phosphate (Figure 6) [114] is a C2-fluorinated analog of a purine nucleoside antiviral agent vidarabine (ara-A). Unlike vidarabine, fludarabine phosphate is resistant to deamination by adenosine deaminase. Fludarabine phosphate is a water-soluble prodrug that is rapidly dephosphorylated to 2-fluoro-vidarabine (2F-ara-A). 2F-ara-A is actively transported into cells and is then rephosphorylated via deoxycytidine kinase to the active triphosphate derivative 2F-ara-ATP. 2F-ara-ATP competitively inhibits DNA synthesis via inhibition of DNA polymerase, ribonucleotide reductase, DNA primase, and DNA ligase. 2F-ara-ATP prevents elongation of DNA strands through direct incorporation into DNA as a fraudulent nucleotide. The degree of inhibition of these enzymes is stronger in the case of clofarabine. The mono phosphorylation of clofarabine is substantially more efficient than that of fludarabine [115]. In addition, intracellular retention of clofarabine triphosphate in acute myeloid leukemia (AML) is longer than that of fludarabine, which contributes to the higher potency of clofarabine as an anticancer agent. In addition to its potential effect it can also be given in combination with other anti-cancer agent such as cytarabine [116]. In recent years, a combination of radiotherapy and chemotherapy has emerged as a potential approach in cancer therapy. The current experimental evidence showed that a moderate dose of clofarabine along with radiotherapy led to a significant inhibition of tumor growth [117]. This result suggests the potential of clofarabine as a significant radiosensitizer.
Other well know fluoronucleoside, such as 5-fluorouracil (5-FU), have been used for the treatment of cancer for about 60 years [118]. 5-FU inhibits thymidylate synthase (TS), interrupting the action of an enzyme that converts 2′-deoxyuracilmonophoshate (dUMP) to 2′-deoxythyminemonophosphate (dTMP) via C5-methylation. Some of 5-FU’s principal use is in treatment of colorectal cancer as well as pancreatic cancer. 5-Fluorocytosine (flucytosine) is an inhibitor of sterol C-14 demethylase, an enzyme involved in the biosynthesis of ergosterol, an element of fungal cell wall. It is being marketed as an anti-fungal agent [119]. Trifluridine is an anti-herpesvirus drug acting on thymidylate synthase [120]. It is phosphorylated to the triphosphate by the virus-encoded thymidine kinase. The triphosphate inactivates the enzyme thymidine synthetase thus inhibiting DNA synthesis, both in the virus as well as in the host cell. It also competes with naturally occurring thymidine and gets integrated into DNA chain, thus producing fradulant DNA and thus inhibiting virus replication. Tezacitabine is currently in phase III trial for the treatment of solid tumors, such as lung, prostate, ovary, cervix and hematopoietic tissues. Its cytotoxicity has been proposed to originate from synergistic effects between its triphosphate, a DNA chain terminator, and its diphosphate, a stoichiometric inactivator of ribonucleotide reductase.
A another milestone in fluoronucleoside is the development of gemcitabine (2′,2′-difluorodeoxycytidine, dFdC) as an anticancer agent with a broad spectrum of activity that includes activity against solid tumors such as pancreatic cancer. It acts by inhibiting DNA synthesis via competitive incorporation into the growing DNA [121]. It has also a potential role in the treatment of central nervous system malignancies both as a cytotoxic agent as well as a potent radiosensitizer [122, 123].
Gemcitabine (Figure 6) [124] was launched as Gemzar® by Eli Lilly in 1996 and is currently approved as a first-line treatment for the pancreas adenocarcinoma. It is being used in combination with cisplatin for the treatment of non-small cell lung cancer and with paclitaxel for the treatment of breast cancer. It is proposed as an inhibitor of the ribonucleotide diphosphate reductase, a homodimeric enzyme catalyzing the conversion of nucleotides into deoxynucleotides [125]. It has also been investigated for use in esophageal cancer, and is also used experimentally in lymphomas and various other tumor types. A recent study suggests a differential higher uptake of gemcitabine in brain tumors [126] and it can facilitate higher cytotoxicity to brain tumor cells.
4. Conclusion
As highlighted in this review, a number of important pharmaceuticals have been discovered and developed based on fluorinated analogs of biologically active nucleosides. The introduction of fluorine atom(s) into a nucleoside structure at an appropriate position, as a mimic of hydrogen or hydroxyl group or as a fluorinated methyl group, has modulated and/or improved the pharmacological properties of a molecule with minor stereochemistry changes. With progress in synthetic methodologies for selective fluorine-introduction, fluorinated nucleosides will likely continue to be an excellent lead in drug discovery.
Acknowledgement
This review is partially supported by the grant (AI25899) from the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Voet D, Voet JG. Biochemistry. 2nd. John Wiley & Sons; New York: 1995. [DOI] [PubMed] [Google Scholar]
- 2.Gumina G, Chong Y, Choo H, Song G, Chu CK. Curr. Topics Med. Chem. 2002;2:1065–1086. doi: 10.2174/1568026023393138. [DOI] [PubMed] [Google Scholar]
- 3.Orr DC, Figueiredo HT, Mo CL, Penn CR, Cameron JM. J. Bio. Chem. 1992;267:4177–4182. [PubMed] [Google Scholar]
- 4 a).Turner MA, Yang X, Yin D, Kuczera K, Borchardt RT, Howell PL. Cell Biochem. Biophys. 2000;33:101–125. doi: 10.1385/CBB:33:2:101. [DOI] [PubMed] [Google Scholar]; b) Kitade Y, Kozaki C, Miwa T, Nakanishi M. Tetrahedron. 2002;58:1271–1277. [Google Scholar]; c) Anderson KS. Biochim. Biophys. Acta. 2002;1587:296–299. doi: 10.1016/s0925-4439(02)00092-3. [DOI] [PubMed] [Google Scholar]; Maga G, Spadari S. Curr. Drug Metab. 2002;3:73–96. doi: 10.2174/1389200023337982. [DOI] [PubMed] [Google Scholar]
- 5 a).Olah G, Chambers R, Prakash GKS, editors. Synthetic Fluorine Chemistry. John Wiley and Sons; New York: 1992. [Google Scholar]; Welch JT. Tetrahedron. 1987;43:3123–3197. [Google Scholar]; b) Wilkinson JA. Chem. Rev. 1992;92:505–519. [Google Scholar]; c) Purrington ST, Kagen BS. Chem. Rev. 1986;86:997–1018. [Google Scholar]; d) Schlosser M. Tetrahedron. 1978;34:3–17. [Google Scholar]
- 6 a).Pankiewicz KR. Carbohydr. Res. 2000;327:87–105. doi: 10.1016/s0008-6215(00)00089-6. [DOI] [PubMed] [Google Scholar]; b) Meng W-D, Qing F-L. Curr. Topics Med. Chem. 2006;6:1499–1528. doi: 10.2174/156802606777951082. [DOI] [PubMed] [Google Scholar]; c) Ying C, Clercq ED, Neyts J. Curr. Med. Chem. Anti-infective Agents. 2003;2:227–240. [Google Scholar]
- 7.Wilson LJ, Hager MW, El-Kattan YA, Liotta DC. Synthesis. 1995:146–150. [Google Scholar]
- 8.Vorbrüggen H, Ruh-Pohlenz C, Paquette LA, et al. Organic Reactions Ed. John Wiley and Sons; New York: 2000. [Google Scholar]
- 9.Muehlbacher M, Poulter CD. J. Org. Chem. 1988;53:1026–1030. [Google Scholar]
- 10 a).Hudlicky M. Org. Reactions. 1988;35:513–637. [Google Scholar]; b) Wang CJ. Org. Reactions. 1985;34:319–400. [Google Scholar]; c) Boswell GA, Ripka WC, Scribner RM, Tullock CW. Org. Reactions. 1974;21:1–124. [Google Scholar]
- 11 a).Lal SG, Pez GP, Syvret RG. Chem. Rev. 1996;96:1737–1756. doi: 10.1021/cr941145p. [DOI] [PubMed] [Google Scholar]; b) Taylor SD, Kotoris CC, Hum G. Tetrahedron. 1999:12431–12477. [Google Scholar]; c) Gilicinski AG, Pez GP, Syvret RG, Lal GS. J. Fluorine Chem. 1992;59:157–162. [Google Scholar]
- 12.Wang X, Seth PP, Ranken R, Swayze EE, Migawa MT. Nucleoside Nucleotides Nucleic Acids. 2004;23:161–170. doi: 10.1081/ncn-120027825. [DOI] [PubMed] [Google Scholar]
- 13.Visser GWM, Herder RE, Noordhuis P, Zwaagstra O, Jacobus DM, De Kanter FJJ. J. Chem. Soc. Perkin Trans. 1988;I:2547–2550. [Google Scholar]
- 14.Burkart MD, Zhang Z, Hung S-C, Wong C-H. J. Am. Chem. Soc. 1997;119:11743–11746. [Google Scholar]
- 15.Li Y, Mao S, Hager MW, Becnel KD, Schinazi RF, Liotta DC. Bioorg. Med. Chem. Lett. 2007;17:3398–3401. doi: 10.1016/j.bmcl.2007.03.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Codington JF, Doerr IL, Fox JJ. J. Org. Chem. 1964;29:558–564. [Google Scholar]
- 17.Shi J, Du J, Ma T, Pankiewicz KW, Patterson SE, Tharnish PM, McBrayer TR, Stuyver LJ, Otto MJ, Chu CK, Schinazi RF, Watanabe KA. Bioorg. Med. Chem. 2005;13:1641–1652. doi: 10.1016/j.bmc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Stuyver LJ, McBrayer TR, Whitaker T, Tharnish PM, Ramesh M, Lostia S, Cartee L, Shi J, Hobbs A, Schinazi RF, Watanabe KA, Otto MJ. Antimicrob. Agents Chemother. 2004;48:651–654. doi: 10.1128/AAC.48.2.651-654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajramovic JJ, Volmer R, Syan S, Pochet S, Gonzalez-Dunia1 D. Antimicrob. Agents Chemother. 2004;48:1422–1425. doi: 10.1128/AAC.48.4.1422-1425.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pankiewicz KW, Krzeminski J, Ciszewski LA, Ren W, Watanabe KA. J. Org. Chem. 1992;57:553–559. [Google Scholar]; b) Choi Y, Li L, Grill S, Gullen E, Lee CS, Gumina G, Tsujii E, Cheng Y-C, Chu CK. J. Med. Chem. 2000;43:2538–2546. doi: 10.1021/jm990543n. [DOI] [PubMed] [Google Scholar]
- 21 a).Clark JL, Mason JC, Hollecker L, Stuyver LJ, Tharnish PM, McBrayer TR, Otto MJ, Furman PA, Schinazi RF, Watanabe KA. Bioorg. Med. Chem. Lett. 2006;16:1712–1715. doi: 10.1016/j.bmcl.2005.12.002. [DOI] [PubMed] [Google Scholar]; b) Clark JL, Hollecker L, Mason JC, Stuyver LJ, Tharnish PM, Lostia S, McBrayer TR, Schinazi RF, Watanabe KA, Otto MJ, Furman PA, Stec WJ, Patterson SE, Pankiewicz KW. J. Med. Chem. 2005;48:5504–5508. doi: 10.1021/jm0502788. [DOI] [PubMed] [Google Scholar]
- 22.Murakami E, Bao H, Ramesh M, McBrayer TR, Whitaker T, Steuer HMM, Schinazi RF, Stuyver LJ, Obikhod A, Otto MJ, Furman PA. Antimicrob. Agents Chemother. 2007;51:503–509. doi: 10.1128/AAC.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAtee JJ, Schinazi RF, Liotta DC. J. Org. Chem. 1998;63:2161–2167. [Google Scholar]
- 24 a).Wright JA, Taylor NF, Fox JJ. J. Org. Chem. 1969;34:2632–2636. doi: 10.1021/jo01261a031. [DOI] [PubMed] [Google Scholar]; b) Reichman U, Watanabe YA, Fox JJ. Carbohydr. Res. 1975;42:233–240. doi: 10.1016/s0008-6215(00)84265-2. [DOI] [PubMed] [Google Scholar]; c) Watanabe KA, Su T-L, Klein RS, Chu CK, Matsuda A, Chun MW, Lopez C, Fox JJ. J. Med. Chem. 1983;26:152–156. doi: 10.1021/jm00356a007. [DOI] [PubMed] [Google Scholar]; d) Watanabe KA, Su T-L, Reichman U, Greenberg N, Lopez C, Fox JJ. J. Med. Chem. 1984;27:91–94. doi: 10.1021/jm00367a020. [DOI] [PubMed] [Google Scholar]; e) Perlman ME, Watanabe KA, Schinazi RF, Fox JJ. J. Med. Chem. 1985;28:741–748. doi: 10.1021/jm00383a009. [DOI] [PubMed] [Google Scholar]; f) Su T-L, Watanabe KA, Schinazi RF, Fox JJ. J. Med. Chem. 1986;29:151–154. doi: 10.1021/jm00151a025. [DOI] [PubMed] [Google Scholar]
- 25.Abbruzzese JL, Schmidt S, Raber MN, Levy JK, Castellanos AM, Legha SS, Krakoff IH. Invest. New Drugs. 1989;7:195–197. doi: 10.1007/BF00170857. [DOI] [PubMed] [Google Scholar]
- 26.McKenzie R, Fried MW, Sallie R, Varam H. ConJee, Di Bisceglie AM, Park Y, Savararese B, Kleiner D, Tsokos M, Luciano C, Pruett T, Stotka JL, Straus SE, Hoofnagle JH. N. Engl. J. Med. 1995;333:1099–1116. doi: 10.1056/NEJM199510263331702. [DOI] [PubMed] [Google Scholar]
- 27.Chu CK, Ma T, Shanmuganathan K, Wang C, Xiang Y, Pai SB, Tao G-Q, Sommadossi J-P, Cheng Y-C. Antimicrob. Agents Chemother. 1995;39:979–981. doi: 10.1128/aac.39.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28 a).Montgomery JA, Shortnacy AT, Carson DA, Secrist JA., III J. Med. Chem. 1986;29:2389–2392. doi: 10.1021/jm00161a041. [DOI] [PubMed] [Google Scholar]; b) Montgomery JA, Shortnacy AT, Clayton SD, Riordan JM, Secrist JA., III J. Med. Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]; c) Bauta WE, Schulmeier BE, Burke B, Puente JF, Cantrell WR, Lovett D, Goebel J, Anderson B, Ionescu D, Guo R. Org. Proc. Res. Dev. 2004;8:889–896. [Google Scholar]; d) Bonate PL, Arthaud L, Cantrell WR, Jr., Stephenson K, Secrist JA, Weitman S. Nature Reviews. 2006;5:855–857. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 29 a).Machida H, Ashida N, Miura S, Endo M, Yamada K, Kitano K, Yoshimura Y, Sakata S, Ijichi O, Eizuru Y. Antiviral Res. 1998;39:129–137. doi: 10.1016/s0166-3542(98)00042-4. [DOI] [PubMed] [Google Scholar]; b) Satoh H, Yashimura Y, Sakata S, Miura S, Machida H. Bioorg. Med. Chem. Lett. 1998;8:989–992. doi: 10.1016/s0960-894x(98)00161-9. [DOI] [PubMed] [Google Scholar]
- 30.Borthwick AD, Evans DN, Kirk BE, Biggadike K, Exall AM, Roberts AM, Youds PM, Knight J, Coates JA. J. Med. Chem. 1990;33:179–186. doi: 10.1021/jm00163a030. [DOI] [PubMed] [Google Scholar]
- 31.Biggadike K, Borthwick AD, Exall AM, Kirk BE, Ward RA. J. Chem. Soc. Chem. Commun. 1988:898–890. [Google Scholar]
- 32.Tann CH, Brodfuehrer PR, Brundidge SP, Sapino C, Howell HG. J. Org. Chem. 1985;50:3644–3647. [Google Scholar]
- 33 a).Juaristi E, Cuevas G. Tetrahedron. 1992;48:5019–5087. [Google Scholar]; b) Box VGS. Heterocycles. 1990;31:1157. [Google Scholar]; c) Box VGS. Heterocycles. 1991;32:795–807. [Google Scholar]
- 34.Wright JA, Taylor NF, Fox JJ. J. Org. Chem. 1969;34:2632–2636. doi: 10.1021/jo01261a031. [DOI] [PubMed] [Google Scholar]
- 35 a).Chu CK, Matulic-Adamic J, Huang J-T, Chou T-C, Burchenal JH, Fox JJ, Watanabe KA. Chem. Pharm. Bull. 1989;37:336–338. doi: 10.1248/cpb.37.336. [DOI] [PubMed] [Google Scholar]; b) Marquez VE, Tseng CK—H, Mitsuya H, Aoki S, Kelley JA, Ford H, Roth JS, Broder S, Johns DG, Driscoll JS. J. Med. Chem. 1990;33:978–985. doi: 10.1021/jm00165a015. [DOI] [PubMed] [Google Scholar]
- 36.Krzeminski J, Nawrot B, Pankiewicz KW, Watanabe KA. Nucleosides Nucleotides. 1991;10:781–786. [Google Scholar]
- 37.Sivetsa GG, Kalinichenkoa EN, Mikhailopulo IA. Lett. Org. Chem. 2006;3:402–405. [Google Scholar]
- 38.Ma T, Pai SB, Zhu YL, Lin JS, Shanmuganathan K, Du J, Wang C, Kim H, Newton MG, Cheng YC, Chu CK. J. Med. Chem. 1996;39:2835–2843. doi: 10.1021/jm960098l. [DOI] [PubMed] [Google Scholar]
- 39.Du J, et al. Nulceosides Nucleotides. 1999;18:187–192. doi: 10.1080/15257779908043066. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui MA, Driscoll JS, Marquez VE, Roth JS, Shirasaka T, Mitsuya H, Barchi JJ, Kelleyt JA. J. Med. Chem. 1992;35:2195–2201. doi: 10.1021/jm00090a008. [DOI] [PubMed] [Google Scholar]
- 41 a).Van Aerschot A, Herdewijn P, Balzarini J, Pauwels R, De Clercq E. J. Med. Chem. 1989;32:1743–1749. doi: 10.1021/jm00128a013. [DOI] [PubMed] [Google Scholar]; b) Barchi JJ, Marquez VE, Driscoll JS, Ford H, Mitsuya H, Shirasaka T, Aoki S, Kelleyf JA. J. Med. Chem. 1991;34:1647–1655. doi: 10.1021/jm00109a018. [DOI] [PubMed] [Google Scholar]; c) Bhattacharya BK, Ojwang JO, Rando RF, Huffman JH, Revankar GR. J. Med. Chem. 1995;38:3957–3966. doi: 10.1021/jm00020a009. [DOI] [PubMed] [Google Scholar]; d) Izawa K, Takamatsu S, Katayama S, Hirose N, Kozai S, Maruyama T. Nucleosides Nuceotides Nucleic Acids. 2003;22:507–517. doi: 10.1081/NCN-120021951. [DOI] [PubMed] [Google Scholar]
- 42 a).Shanmuganathan K, Koudriakova T, Nampalli S, Du J, Gallo JM, Schinazi RF, Chu CK. J. Med. Chem. 1994;37:821–827. doi: 10.1021/jm00032a017. [DOI] [PubMed] [Google Scholar]; b) Kotra LP, Wang P, Bartlett MG, Shanmuganathan K, Xu Z, Cavalcanti S, Newton MG, Chu CK. J. Org. Chem. 1997;62:7267–7271. doi: 10.1021/jo970761t. [DOI] [PubMed] [Google Scholar]; c) Xiang Y, Cavalcanti S, Chu CK, Schinazi RF, Pal SB, Zhu Y-L, Cheng Y-C. Bioorg. Med. Chem. Lett. 1995;5:877–880. [Google Scholar]
- 43.Okabe M, Sun R-C, Zenchoff GB. J. Med. Chem. 1991;56:4392–4396. [Google Scholar]
- 44 a).Takamatsu S, Katayama S, Hirose N, De Cock E, Schelkens G, Demillequand M, Brepoels J, Izawa K. Nucleosides Nuceotides Nucleic Acids. 2002;21:849–862. doi: 10.1081/NCN-120016572. [DOI] [PubMed] [Google Scholar]; b) Takamatsu S, Maruyama T, Katayama S, Hirose N, Naito M, Izawa K. J. Org. Chem. 2001;66:7469–7477. doi: 10.1021/jo0158985. [DOI] [PubMed] [Google Scholar]
- 45 a).Siddiqui MA, Driscoll JS, Abushanab E, Kelley JA, Barchi JJ, Jr., Marquez VE. Nucleosides Nuceotides Nucleic Acids. 2000;19:1–10. doi: 10.1080/15257770008032993. [DOI] [PubMed] [Google Scholar]; b) Woltermann CJ, Lapin YA, Kunnen KB, Tueting DR, Sanchez IH. Tetrahedron. 2004;60:3445–3449. [Google Scholar]
- 46.Hertel LW, Kroin JS, Misner JW, Tustin JM. J. Org. Chem. 1988;53:85–88. [Google Scholar]
- 47.Kotra LP, Xiang Y, Newton MG, Schinazi RF, Cheng Y-C, Chu CK. J. Med. Chem. 1997;40:3635–3644. doi: 10.1021/jm970275y. [DOI] [PubMed] [Google Scholar]
- 48.Kotra LP, Newton MG, Chu CK. Carbohydr. Res. 1998;306:69–80. doi: 10.1016/s0008-6215(97)00275-9. [DOI] [PubMed] [Google Scholar]
- 49 a).Choi Y, Lee K, Hong JH, Schinazi RF, Chu Chung K. Tetrahedron Lett. 1998;39:4437–4441. [Google Scholar]; Lee K, Choi Y, Gullen E, Schlueter-Wirtz S, Schinazi RF, Cheng Y-C, Chu CK. J. Med. Chem. 1999;42:1320–1328. doi: 10.1021/jm980651u. [DOI] [PubMed] [Google Scholar]; b) Lee K, Choi Y, Gumina G, Zhou W, Schinazi RF, Chu CK. J. Med. Chem. 2002;45:1313–1320. doi: 10.1021/jm010418n. [DOI] [PubMed] [Google Scholar]; c) Choi Y, Choo H, Chong Y, Lee S, Olgen S, Schinazi RF, Chu CK. Org. Lett. 2002;2:305–307. doi: 10.1021/ol0171665. [DOI] [PubMed] [Google Scholar]; d) Chong Y, Choo H, Choi Y, Mathew J, Schinazi RF, Chu CK. J. Med. Chem. 2002;45:4888–4898. doi: 10.1021/jm020246+. [DOI] [PubMed] [Google Scholar]; e) Choo H, Chong Y, Choi Y, Mathew J, Schinazi RF, Chu CK. J. Med. Chem. 2003;46:389–398. doi: 10.1021/jm020376i. [DOI] [PubMed] [Google Scholar]; f) Wang J, Jin Y, Rapp KL, Bennett M, Schinazi RF, Chu CK. J. Med. Chem. 2005;48:3736–3748. doi: 10.1021/jm050096d. [DOI] [PubMed] [Google Scholar]
- 50.Marquez VE, Lim BB, Barchi JJ, Nicklaus MC. In: Nucleosides and Nucleotides as Antitumor and Antiviral Agents. Chu CK, Baker DC, editors. Plenum; New York: 1993. pp. 265–284. [Google Scholar]
- 51.Herdewijn P, Balzarini J, De Clercq E, Pauwels R, Baba M, Broder S, Vanderhaeghe H. J. Med. Chem. 1987;30:1270–1278. doi: 10.1021/jm00391a003. [DOI] [PubMed] [Google Scholar]
- 52 a).Balzarini J, Baba M, Pauwels R, Herdewijn J, De Clercq E. Biochem. Pharmacol. 1988;37:2847–2856. doi: 10.1016/0006-2952(88)90049-4. [DOI] [PubMed] [Google Scholar]; b) Mathes E, Lehmann CH, Scholz D, von Janta-Lipinski M, Gaertner K, Rosenthal HA, Langen P. Biochem. Biophys. Res. Commun. 1987;148:78–85. doi: 10.1016/0006-291x(87)91078-3. [DOI] [PubMed] [Google Scholar]
- 53 (a).Blzarini J, Aerschot A, Pauwels R, Baba M, Scholz D, Herdewijn P, De Clercq E. Mol. Phamacol. 1989;35:571–577. [PubMed] [Google Scholar]; (b) Daluge S, Purifoy DJM, Savina PM, Clair MH, Parry NR, Dev IK, Novak P, Ayers KM, Reardon JE, Roberts GB, Eyfe JA, Blum MB, Averett DR, Dornsife RE, Domin BA, Ferone R, Lewis DA, Krenitsky TA. Antimicrob. Agents Chemother. 1994;38:1591–1603. doi: 10.1128/aac.38.7.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chun BK, Schinazi RF, Cheng Y-C, Chu CK. Carbohydr. Res. 2000;328:49–59. doi: 10.1016/s0008-6215(99)00312-2. [DOI] [PubMed] [Google Scholar]
- 55.Baumgarten H, Bodenteich M, Griengl H. Tetrahedron Lett. 1988;29:5745–5749. [Google Scholar]
- 56 a).Kumar P, Ohkura K, Balzarini J, De Clercq E, Seki K, Wiebe LI. Nucleosides Nucleotides Nucleic Acids. 2004;23:7–29. doi: 10.1081/ncn-120027814. [DOI] [PubMed] [Google Scholar]; b) Carangio A, McGuigan C, Andrei G, Snoeck R, De Clercq E, Balzarini J. Nucleosides Nucleotides Nucleic Acids. 2003;22:935–937. doi: 10.1081/NCN-120022689. [DOI] [PubMed] [Google Scholar]; c) Marchand A, Mathe C, Imbach J-L, Gosselin G. Nucleosides Nucleotides Nucleic Acids. 2000;19:205–217. doi: 10.1080/15257770008033004. [DOI] [PubMed] [Google Scholar]; d) von Janta-Lipinski M, Costisella B, Ochs H, Hubscher U, Hafkemeyer P, Matthes E. J. Med. Chem. 1998;41:2040–2046. doi: 10.1021/jm9704210. [DOI] [PubMed] [Google Scholar]
- 57.Torii T, Onishi T, Izawa K, Maruyama T, Demizu Y, Neyts J, De Clercq E. Nucleosides Nucleotides Nucleic Acids. 2006;25:655–665. doi: 10.1080/15257770600686394. [DOI] [PubMed] [Google Scholar]
- 58 a).Chun BK, Schinazi RF, Cheng Y-C, Chu CK. Carbohydr. Res. 2000;328:49–59. doi: 10.1016/s0008-6215(99)00312-2. [DOI] [PubMed] [Google Scholar]; b) Herdewijn P, Pauwels R, Baba M, Balzarini J, De Clercq E. J. Med. Chem. 1987;30:2131–2137. doi: 10.1021/jm00394a034. [DOI] [PubMed] [Google Scholar]; c) Puech F, Gosselin G, Imbach J-L. Tetrahedron Lett. 1989;30:3171–3175. [Google Scholar]; d) Van Aerschot A, Herdewijn P, Janssen G, Cools M, De Clercq E. Antiviral Res. 1989;12:133–150. doi: 10.1016/0166-3542(89)90047-8. [DOI] [PubMed] [Google Scholar]; e) Mort CJW, Migaud ME, Galioneb A, Pottera BVL. Bioorg. Med. Chem. 2004;12:475–487. doi: 10.1016/j.bmc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Pierra C, Imbach J-L, De Clercq E, Balzarini J, Aerschot AV, Herdewijn P, Faraj A, Loi AG, Sommadossi J-P, Gosselin G. Antiviral Res. 2000;45:169–183. doi: 10.1016/s0166-3542(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 60 a).Robins MJ, Nowak I, Wnuk SF, Hansske F, Madej D. J. Org. Chem. 2007;72:8216–8221. doi: 10.1021/jo071102b. [DOI] [PubMed] [Google Scholar]; b) Kawana M, Kuzuhara H. Carbohydr. Res. 1989;189:181–193. [Google Scholar]; c) Timoshchuk Victor A., Hogrefe Richard I., Vaghefi Morteza M. Nucleosides Nucleotides Nucleic Acids. 2004;23:171–181. doi: 10.1081/ncn-120027826. [DOI] [PubMed] [Google Scholar]; d) Torii T, Onishi T, Izawa K, Maruyama T, Demizu Y, Neyts J, De Clercq E. Nucleosides Nucleotides Nucleic Acids. 2006;25:655–665. doi: 10.1080/15257770600686394. [DOI] [PubMed] [Google Scholar]
- 61 a).Takamatsu S, Katayama S, Naito M, Yamashita K, Ineyama T, Izawa K. Nucleosides Nucleotides Nucleic Acids. 2003;22:711–713. doi: 10.1081/NCN-120022616. [DOI] [PubMed] [Google Scholar]; b) Torii T, Onishi T, Tanji S, Izawa K. Nucleosides Nucleotides Nucleic Acids. 2005;24:1051–1054. doi: 10.1081/ncn-200060053. [DOI] [PubMed] [Google Scholar]; c) Katayama S, Takamatsu S, Naito M, Tanji S, Ineyama T, Izawa K. J. Fluorine Chem. 2006;127:524–528. [Google Scholar]
- 62 a).Gumina G, Schinazi RF, Chu CK. Org. Lett. 2001;3:4177–4181. doi: 10.1021/ol0168059. [DOI] [PubMed] [Google Scholar]; b) Chong Y, Gumina G, Mathew JS, Schinazi RF, Chu CK. J. Med. Chem. 2003;46:3245–3256. doi: 10.1021/jm0300274. [DOI] [PubMed] [Google Scholar]; c) Zhou W, Gumina G, Chong Y, Wang J, Schinazi RF, Chu CK. J. Med. Chem. 2004;47:3399–3408. doi: 10.1021/jm040027j. [DOI] [PubMed] [Google Scholar]
- 63.Zhu W, Chong Y, Choo H, Mathews J, Schinazi RF, Chu CK. J. Med. Chem. 2003;46:1631–1640. doi: 10.1021/jm0303148. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Jin Y, Rapp KL, Schinazi RF, Chu CK. J. Med. Chem. 2007;50:1828–1839. doi: 10.1021/jm061304k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65 a).Zhang X, Xia H, Dong X, Jin J, Meng W-D, Qing F-L. J. Org. Chem. 2003;68:9026–9033. doi: 10.1021/jo034512i. [DOI] [PubMed] [Google Scholar]; b) Xu X-H, Qiu X-L, Zhang X, Qing F-L. J. Org. Chem. 2006;71:2820–2824. doi: 10.1021/jo052652h. [DOI] [PubMed] [Google Scholar]
- 66.De Clercq E. J. Med. Chem. 2005;48:1297–1313. doi: 10.1021/jm040158k. [DOI] [PubMed] [Google Scholar]
- 67.Choo H, Chong Y, Chu CK. Bioorg. Med. Chem. Lett. 2003;13:1993–1996. doi: 10.1016/s0960-894x(03)00330-5. [DOI] [PubMed] [Google Scholar]
- 68 a).Waga T, Ohrui H, Meguro H. Nucleosides Nucleotides. 1996;15:287–304. [Google Scholar]; b) Yamaguchi T, Tomikawa A, Hirai T, Kawaguchi T, Ohrui H, Saneyoshi M. Nucleosides Nucleotides. 1997;16:1347. doi: 10.1080/07328319808005193. [DOI] [PubMed] [Google Scholar]; c) Waga T, Nishizaki T, Ohrui H, Meguro H. Biosci. Biotechnol. Biochem. 1993;57:1433–1438. doi: 10.1271/bbb.57.1433. [DOI] [PubMed] [Google Scholar]
- 69 a).Chen MS, Suttmann RT, Wu JC, Prisbe EJ. J. Biol. Chem. 1992;267:257–260. [PubMed] [Google Scholar]; b) Chen MS, Suttmann RT, Papp E, Cannon PD, McRobierts MJ, Bach C, Copeland WC, Wang T. Biochemistry. 1993;32:6002–6005. doi: 10.1021/bi00074a011. [DOI] [PubMed] [Google Scholar]
- 70 a).Ohrui H, Kohgo S, Kitano K, Kodama E, Yoshimura K, Matsuoka M, Shigeta S, Mitsuya H. J. Med. Chem. 2000;43:4516–4525. doi: 10.1021/jm000209n. [DOI] [PubMed] [Google Scholar]; b) Summerer D, Marx A. Bioorg. Med. Chem. Lett. 2005;15:869–871. doi: 10.1016/j.bmcl.2004.12.072. [DOI] [PubMed] [Google Scholar]; c) Kodama E, Kohgo S, Kitano K, Machida H, Gatanaga H, Shigeta S, Matsuoka M, Ohrui H, Mitsuya H. Antimicrob. Agents Chemother. 2001;45:1539–1546. doi: 10.1128/AAC.45.5.1539-1546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X, Zhou W, Schinazi RF, Chu CK. J. Org. Chem. 2004;69:6034–6041. doi: 10.1021/jo049597h. [DOI] [PubMed] [Google Scholar]
- 72.Maag H, Rydzewski RM, McRoberts MJ, Crawford-Ruth D, Verheyden JPH, Prisbe EJ. J. Med. Chem. 1992;35:1440–1451. doi: 10.1021/jm00086a013. [DOI] [PubMed] [Google Scholar]
- 73.Liu P, Sharon A, Chu CK. Tetrahedron Assymetry. 2006:3304–3314. [Google Scholar]
- 74 a).Jenkins ID, Verheyden JPH, Moffatt JC. J. Am. Chem. Soc. 1971;93:4323–4324. doi: 10.1021/ja00746a056. [DOI] [PubMed] [Google Scholar]; b) Jenkins ID, Verheyden JPH, Moffatt JG. J. Am. Chem. Soc. 1976;98:3346–3357. doi: 10.1021/ja00427a049. [DOI] [PubMed] [Google Scholar]; c) Owen GR, Verheyden JPH, Moffatt JG. J. Org. Chem. 1976;41:3010–3017. doi: 10.1021/jo00880a018. [DOI] [PubMed] [Google Scholar]
- 75.Hong JH, Lee K, Choi Y, Chu CK. Tetrahedron Lett. 1998;39:3443–3447. [Google Scholar]
- 76.Gumina G, Chong Y, Choi Y, Chu CK. Org. Lett. 2000;2:1229–1232. doi: 10.1021/ol005665k. [DOI] [PubMed] [Google Scholar]; Chong Y, Gumina G, Chu CK. Tetrahedron Asymmetry. 2000;11:4853–4875. [Google Scholar]
- 77 a).Xiang Y, Kotra LP, Chu CK, Schinazi RF. Bioorg. Med. Chem. Lett. 1995;5:743–747. [Google Scholar]; Xiang Y, Cavalcanti S, Chu CK, Schinazi RF, Pai SB, Zhu Y-L, Cheng Y-C. Bioorg. Med. Chem. Lett. 1995;5:877–881. [Google Scholar]; b) Pai SB, Liu S-H, Zhu Y-L, Chu CK, Cheng Y-C. Antimicrob. Agents Chemother. 1996;40:380–386. doi: 10.1128/aac.40.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ma T, Pai SB, Zhu YL, Lin J-S, Shanmuganathan K, Du J, Wang C, Kim H, Newton MG, Cheng Y-C, Chu CK. J. Med. Chem. 1996;39:2835–2843. doi: 10.1021/jm960098l. [DOI] [PubMed] [Google Scholar]; d) Ma T, Lin J-S, Newton MG, Cheng Y-C, Chu CK. J. Med. Chem. 1997;40:2750–2754. doi: 10.1021/jm970233+. [DOI] [PubMed] [Google Scholar]; Kotra LP, Xiang Y, Newton MG, Schinazi RF, Cheng Y-C, Chu CK. J. Med. Chem. 1997;40:3635–3644. doi: 10.1021/jm970275y. [DOI] [PubMed] [Google Scholar]; e) Choi Y, Lee K, Hong JH, Schinazi RF, Chu CK. Tetrahedron Lett. 1998;39:4437–4441. [Google Scholar]; f) Lee K, Choi Y, Gullen E, Schlueter-Wirtz S, Schinazi RF, Cheng Y-C, Chu CK. J. Med. Chem. 1999;7:1320–1328. doi: 10.1021/jm980651u. [DOI] [PubMed] [Google Scholar]; g) Lee K, Choi Y, Hong JH, Schinazi RF, Chu CK. Nucleosides Nucleotides. 1999;18:537–540. doi: 10.1080/15257779908041489. [DOI] [PubMed] [Google Scholar]; h) Marquez VE, Tseng CK-H, Mitsuya H, Aoki S, Kelley JA, Ford H, Jr., Driscoll JS. J. Med. Chem. 1990;33:978–985. doi: 10.1021/jm00165a015. [DOI] [PubMed] [Google Scholar]
- 78.Blackburn GM, England DE, Kolkmann F. J. Chem. Soc., Chem. Commun. 1981:930–936. [Google Scholar]
- 79.Yang Y-Y, Meng W-D, Qing F-L. Org. Lett. 2004;6:4257–4261. doi: 10.1021/ol0482947. [DOI] [PubMed] [Google Scholar]
- 80.Wachtmeister J, Classon B, Samuelsson B. Tetrahedron. 1997;53:1861–1872. [Google Scholar]
- 81.McClinton MA, McClinton DA. Tetrahedron. 1992;48:6555–6666. [Google Scholar]
- 82.Sharma PK, Nair V. Nucleosides Nucleotides Nucleic Acids. 2000;19:757–762. doi: 10.1080/15257770008035023. [DOI] [PubMed] [Google Scholar]
- 83.Jeannot F, Gosselin G, Mathé C. Org. Biomol. Chem. 2003;1:2096–2102. doi: 10.1039/b303093h. [DOI] [PubMed] [Google Scholar]
- 84 a).Serafinowski PJ, Brown CA. Tetrahedron. 2000;56:333–339. [Google Scholar]; b) Serafinowski PJ, Brown CA, Barnes CL. Nucleosides Nucleotides Nucleic Acids. 2001;20:921–926. doi: 10.1081/NCN-100002459. [DOI] [PubMed] [Google Scholar]
- 85.McCarthy JR, Jarvi ET, Matthews DP, Edwards ML, Prakash NJ, Bowlin TL, Mehdi S, Sunkara PS, Bey P. J. Am. Chem. Soc. 1989;111:1127–1128. doi: 10.1021/jm00106a028. [DOI] [PubMed] [Google Scholar]
- 86.Ye J-D, Liao X, Piccirilli JA. J. Org. Chem. 2005;70:7902–7910. doi: 10.1021/jo0507542. [DOI] [PubMed] [Google Scholar]
- 87 a).McCarthy JR, Matthews DP. J. Am. Chem. Soc. 1991;113:7439–7440. [Google Scholar]; b) van der Donk WA, Gerfen GJ, Stubbe J. J. Am. Chem. Soc. 1998;120:4252–4253. [Google Scholar]; c) van der Donk WA, Yu G, Silva DJ, Stubbe J. Biochem. 1996;35:8381–8383. doi: 10.1021/bi960190j. [DOI] [PubMed] [Google Scholar]
- 88 a).Yamamoto Y, Nishiyama Y, Kimura N, Ishikawa S, Okuda M, Bandoh S, Kanaji N, Asakura M, Ohkawa M. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:236–245. doi: 10.1007/s00259-007-0613-0. [DOI] [PubMed] [Google Scholar]; b) Manta S, Agelis G, Botic T, Cencic A, Komiotis D. Eur. J. Med. Chem. 2008;43:420–428. doi: 10.1016/j.ejmech.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 89 a).Murakami E, Niu C, Bao H, Steuer H. M. Micolochick, Whitaker T, Nachman T, Sofia MA, Wang P, Otto MJ, Furman PA. Antimicrob. Agents Chemother. 2008;52:458–464. doi: 10.1128/AAC.01184-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li W, Yin X, Schneller SW. Bioorg. Med. Chem. Lett. 2008;18:220–222. doi: 10.1016/j.bmcl.2007.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bégué J-P, Bonnet-Delpon D. J. Fluorine Chem. 2006;127:992–1012. [Google Scholar]
- 91 a).Cihlar T, Ray AS, Boojamra CG, Zhang L, Hui H, Laflamme G, Vela JE, Grant D, Chen J, Myrick F, White KL, Gao YY, Lin KY, Douglas JL, Parkin NT, Carey A, Pakdaman R, Mackman RL. Antimicrob. Agents. Chemother. 2008;52:655–665. doi: 10.1128/AAC.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Manta S, Agelis G, Botic T, Cencic A, Komiotis D. Bioorg. Med. Chem. 2007;15:980–987. doi: 10.1016/j.bmc.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 92 a).Kore AR, Shanmugasundaram M, Charles I, Cheng AM, Barta TJ. Bioorg. Med. Chem. Lett. 2007;17:5295–5299. doi: 10.1016/j.bmcl.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Seela F, Xu K, Chittepu P, Ming X. Nucleosides Nucleotides Nucleic Acids. 2007;26:607–610. doi: 10.1080/15257770701490357. [DOI] [PubMed] [Google Scholar]
- 93 a).Lee K, Chong Y, Chu CK. Nucleosides Nucleotides Nucleic Acids. 2001;20:385–389. doi: 10.1081/NCN-100002311. [DOI] [PubMed] [Google Scholar]; b) Lee K, Chu CK. Antimicrob. Agents. Chemother. 2001;45:138–144. doi: 10.1128/AAC.45.1.138-144.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Chong Y, Akula N, Chu CK. Bioorg. Med. Chem. Lett. 2003;13:4019–4022. doi: 10.1016/j.bmcl.2003.08.052. [DOI] [PubMed] [Google Scholar]; d) Choo H, Chong Y, Chu CK. Bioorg. Med. Chem. Lett. 2003;13:1993–1996. doi: 10.1016/s0960-894x(03)00330-5. [DOI] [PubMed] [Google Scholar]; e) Chong Y, Chu CK. Front Biosci. 2004;9:164–165. doi: 10.2741/1220. [DOI] [PubMed] [Google Scholar]
- 94.Huang H, Chopra R, Verdine GL, Harrison SC. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 95.Bartholomeusz A, Tehan BG, Chalmers DK. Antivir. Ther. 2004;9:149–152. [PubMed] [Google Scholar]
- 96.Sabini E, Hazra S, Konrad M, Burley SK, Lavie A. Nucl. Acids Res. 2007;35:186–192. doi: 10.1093/nar/gkl1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97 a).Chu CK, Boudinot FD, Peek SF, Hong JH, Choi Y, Korba BE, Gerin JL, Cote PJ, Tennant BC, Cheng YC. Antivir. Ther. 1998;3:113–121. [PubMed] [Google Scholar]; b) Hui CK, Lau GK. Expert Opin Investig. Drugs. 2005;14:1277–1284. doi: 10.1517/13543784.14.10.1277. [DOI] [PubMed] [Google Scholar]
- 98.De Clercq E. Int. J. Antimicrob. Agents. 1999;12:81–95. doi: 10.1016/s0924-8579(99)00060-6. [DOI] [PubMed] [Google Scholar]
- 99.Liu SH, Grove KL, Cheng YC. Antimicrob. Agents Chemother. 1998;42:833–839. doi: 10.1128/aac.42.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu R, Li L, Degreve B, Dutschman GE, Lam W, Cheng YC. Antimicrob. Agents Chemother. 2005;49:2044–2049. doi: 10.1128/AAC.49.5.2044-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krishnan P, Gullen EA, Lam W, Dutschman GE, Grill SP, Cheng YC. J. Biol. Chem. 2003;278:36726–36732. doi: 10.1074/jbc.M307052200. [DOI] [PubMed] [Google Scholar]
- 102.Pai S. Balakrishna, Liu SH, Zhu YL, Chu CK, Cheng YC. Antimicrob. Agents Chemother. 1996;40:380–386. doi: 10.1128/aac.40.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aguesse-Germon S, Liu SH, Chevallier M, Pichoud C, Jamard C, Borel C, Chu CK, Trepo C, Cheng YC, Zoulim F. Antimicrob. Agents Chemother. 1998;42:369–376. doi: 10.1128/aac.42.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peek SF, Cote PJ, Jacob JR, Toshkov IA, Hornbuckle WE, Baldwin BH, Wells FV, Chu CK, Gerin JL, Tennant BC, Korba BE. Hepatology. 2001;33:254–266. doi: 10.1053/jhep.2001.20899. [DOI] [PubMed] [Google Scholar]
- 105 a).Marcellin P, Mommeja-Marin H, Sacks SL, Lau GK, Sereni D, Bronowicki JP, Conway B, Trepo C, Blum MR, Yoo BC, Mondou E, Sorbel J, Snow A, Rousseau F, Lee HS. Hepatology. 2004;40:140–150. doi: 10.1002/hep.20257. [DOI] [PubMed] [Google Scholar]; b) Lee HS, Chung YH, Lee K, Byun KS, Paik SW, Han JY, Yoo K, Yoo HW, Lee JH, Yoo BC. Hepatology. 2006;43:982–988. doi: 10.1002/hep.21166. [DOI] [PubMed] [Google Scholar]
- 106.Yoo BC, Kim JH, Chung YH, Lee KS, Paik SW, Ryu SH, Han BH, Han JY, Byun KS, Cho M, Lee HJ, Kim TH, Cho SH, Park JW, Um SH, Hwang SG, Kim YS, Lee YJ, Chon CY, Kim BI, Lee YS, Yang JM, Kim HC, Hwang JS, Choi SK, Kweon YO, Jeong SH, Lee MS, Choi JY, Kim DG, Kim YS, Lee HY, Yoo K, Yoo HW, Lee HS. Hepatology. 2007;45:1172–1178. doi: 10.1002/hep.21629. [DOI] [PubMed] [Google Scholar]
- 107.Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, Litwin S, Furman PA, Jilbert AR, Mason WS. J. Virol. 2001;75:311–322. doi: 10.1128/JVI.75.1.311-322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elion GB. Science. 1989;244:41–47. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- 109.Heidelberger C, Danenberg PV, Moran RG. Adv. Enzymol. Relat. Areas Mol. Biol. 1983;54:58–119. [PubMed] [Google Scholar]
- 110.Harbers E, Chaudhuri NK, Heidelberger C. J. Biol. Chem. 1959;234:1255–1262. [PubMed] [Google Scholar]
- 111.Huang P, Robertson LE, Wright S, Plunkett W. Clin. Cancer. Res. 1995;1:1005–1013. [PubMed] [Google Scholar]
- 112.Montgomery JA, Shortnacy-Fowler AT, Clayton SD, Riordan JM, Secrist JA., 3rd J. Med. Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- 113.Faderl S, Gandhi V, Kantarjian HM. Curr. Opin Hematol. 2008;15:101–107. doi: 10.1097/MOH.0b013e3282f46e94. [DOI] [PubMed] [Google Scholar]
- 114.Duschinsky R, Pleven E, Heidelberger C. J. Am. Chem. Soc. 1957;79:4559–4560. [Google Scholar]; Isanbor C, O’Hagan D. J. Fluorine Chem. 2006;127:303–319. [Google Scholar]
- 115.Xie C, Plunkett W. Cancer Res. 1995;55:2847–2852. [PubMed] [Google Scholar]
- 116.Gidwani P, Ramesh KH, Liu Y, Kolb EA. Chemotherapy. 2008;54:120–124. doi: 10.1159/000118664. [DOI] [PubMed] [Google Scholar]
- 117.Cariveau MJ, Stackhouse M, Cui XL, Tiwari K, Waud W, Secrist JA, 3rd, Xu B. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:213–220. doi: 10.1016/j.ijrobp.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 118.Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Nature. 1957;179:663–664. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 119.De Clercq E, Descamps J, De Somer P, Barr PJ, Jones AS, Walker RT. Proc. Nat. Acad. Sci. U. S. A. 1979;76:2947–2948. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]; Heidelberger C, King DH. Pharmacol. Therap. 1979;6:427–442. [Google Scholar]
- 120.Hertel LW, Kroin JS, Grossman CS, Grindey GB, Dorr AF, Storniolo AMV, Plunkett W, Ganghi V, Huang P. In: Biomedical Frontiers of Fluorine Chemistry. Ojima I, McCarthy J, Welch JT, editors. Washington, DC: 1996. pp. 265–278. (ACS Symposium Series 639). [Google Scholar]
- 121.Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Semin Oncol. 1995;22:3–10. [PubMed] [Google Scholar]
- 122.Rieger J, Durka S, Streffer J, Dichgans J, Weller M. Eur. J. Pharmacol. 1999;365:301–308. doi: 10.1016/s0014-2999(98)00883-8. [DOI] [PubMed] [Google Scholar]
- 123.Fehlauer F, Muench M, Smid EJ, Slotman B, Richter E, Van der Valk P, Sminia P. Oncol Rep. 2006;15:97–105. [PubMed] [Google Scholar]
- 124.McCarthy R, Sunkara PS, Matthews DP, Bitonti AJ, Jarvi EJ, Sabol JS, Resvick RJ, Huber EW, van der Donk WA, Yu G, Stubbe JA. In: Biomedical Frontiers of Fluorine Chemistry. Ojima I, McCarthy J, Welch JT, editors. Washington, DC: 1996. pp. 246–264. (ACS Symposium Series 639). [Google Scholar]
- 125.Nishida H, Murase T, Ueno H. Leukemia Res. 2006;30:1589. doi: 10.1016/j.leukres.2006.02.011. [DOI] [PubMed] [Google Scholar]; Anderson VR, Perry CM. Drugs. 2007;67:1633–1644. doi: 10.2165/00003495-200767110-00008. [DOI] [PubMed] [Google Scholar]
- 126.Apparaju SK, Gudelsky GA, Desai PB. Cancer Chemother Pharmacol. 2008;61:223–229. doi: 10.1007/s00280-007-0464-1. [DOI] [PubMed] [Google Scholar]