Abstract
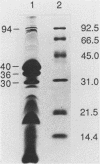
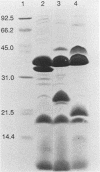
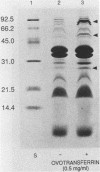
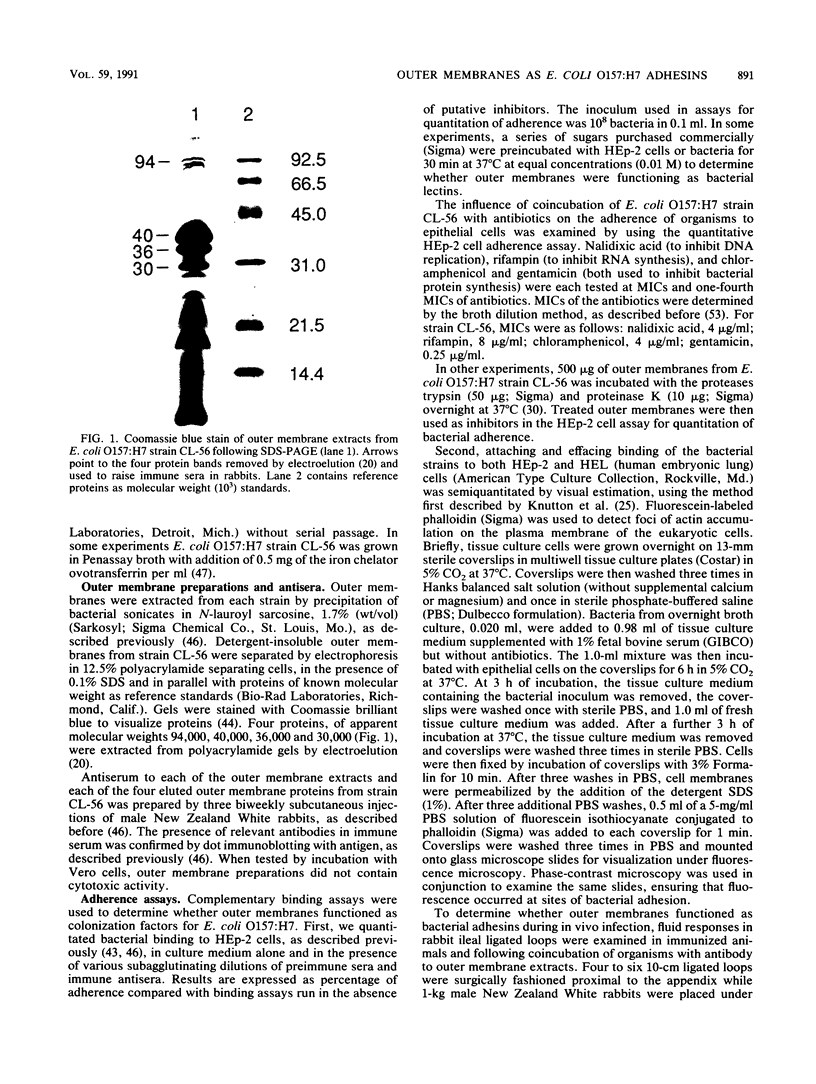
Escherichia coli of serotype O157:H7 are Vero cytotoxin-producing enteric pathogens that have been associated recently with sporadic cases and outbreaks of hemorrhagic colitis and with the hemolytic-uremic syndrome. Adherence of many enteropathogenic bacteria to mucosal surfaces is a critical step in the pathogenesis of diarrheal disease. We showed previously that adherence of E. coli O157:H7 strain CL-56 to epithelial cells in vitro is inhibited by outer membranes. In this study we examined whether outer membranes from a series of E. coli O157:H7 strains mediated competitive inhibition of bacterial binding to epithelial cells grown in tissue culture. We also determined which constituents of the outer membrane mediated inhibition of CL-56 adherence. Binding of six O157:H7 strains to HEp-2 cells was determined by quantitating the number of adherent bacteria in the presence and absence of outer membranes which were extracted from each strain with N-lauroyl sarcosinate (1.7%, wt/vol). After separation of outer membranes by gel electrophoresis, four bands (94, 40, 36, and 30 kDa) were collected by electroelution. Immune sera were raised in rabbits to each of the four eluted bands. Outer membrane extracts from each of the six O157:H7 strains inhibited binding of homologous organisms to the HEp-2 cells. At dilutions which did not cause bacterial agglutination, antiserum raised against the 94-kDa outer membrane protein showed maximal inhibition of bacterial adherence (17.0 +/- 7.3% adherence of control levels). Growth of bacteria in iron-depleted broth did not affect their binding to HEp-2 cells, suggesting that iron-regulated outer membranes were not involved. Fluid accumulation in ileal ligated loops of rabbits in response to E. coli O157:H7 challenge was diminished following both parenteral immunization with outer membranes extracted from the homologous strain and coincubation of organisms with immune serum which contained antibodies to outer membrane extracts. These data indicate that outer membranes are competitive inhibitors of E. coli O157:H7 adherence. Specific constituents of the outer membrane may function as bacterial attachment factors (i.e., adhesins) for E. coli O157:H7 adherence to epithelial cell surfaces.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Baldwin T. J., Brooks S. F., Knutton S., Manjarrez Hernandez H. A., Aitken A., Williams P. H. Protein phosphorylation by protein kinase C in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1990 Mar;58(3):761–765. doi: 10.1128/iai.58.3.761-765.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beery J. T., Doyle M. P., Schoeni J. L. Colonization of chicken cecae by Escherichia coli associated with hemorrhagic colitis. Appl Environ Microbiol. 1985 Feb;49(2):310–315. doi: 10.1128/aem.49.2.310-315.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Bauer K. Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Review on bacterial porins. Eur J Biochem. 1988 Sep 1;176(1):1–19. doi: 10.1111/j.1432-1033.1988.tb14245.x. [DOI] [PubMed] [Google Scholar]
- Brumfitt W., Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989 May 4;320(18):1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Lushbaugh W. B., Inman L. R. Attachment of bacteria to intestinal epithelial cells in diarrhea caused by Escherichia coli strain RDEC-1 in the rabbit: stages and role of capsule. J Infect Dis. 1981 Feb;143(2):219–230. doi: 10.1093/infdis/143.2.219. [DOI] [PubMed] [Google Scholar]
- Chan R., Acres S. D., Costerton J. W. Use of specific antibody to demonstrate glycocalyx, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrum-fed calves. Infect Immun. 1982 Sep;37(3):1170–1180. doi: 10.1128/iai.37.3.1170-1180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanter N., Hall G. A., Bland A. P., Hayle A. J., Parsons K. R. Dysentery in calves caused by an atypical strain of Escherichia coli (S102-9). Vet Microbiol. 1986 Sep;12(3):241–253. doi: 10.1016/0378-1135(86)90053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Rowe B. The outer membrane protein of enteropathogenic Escherichia coli, described as the 'localised adherence factor', is OmpF and probably not involved in adhesion to HEp-2 cells. FEMS Microbiol Lett. 1989 Oct 15;52(3):291–295. doi: 10.1016/0378-1097(89)90213-9. [DOI] [PubMed] [Google Scholar]
- Chart H., Scotland S. M., Willshaw G. A., Rowe B. HEp-2 adhesion and the expression of a 94 kDa outer-membrane protein by strains of Escherichia coli belonging to enteropathogenic serogroups. J Gen Microbiol. 1988 May;134(5):1315–1321. doi: 10.1099/00221287-134-5-1315. [DOI] [PubMed] [Google Scholar]
- Chart H., Stevenson P., Griffiths E. Iron-regulated outer-membrane proteins of Escherichia coli strains associated with enteric or extraintestinal diseases of man and animals. J Gen Microbiol. 1988 Jun;134(6):1549–1559. doi: 10.1099/00221287-134-6-1549. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Donohue-Rolfe A., Keusch G. T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989 Sep;160(3):452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- Fauchère J. L., Kervella M., Rosenau A., Mohanna K., Véron M. Adhesion to HeLa cells of Campylobacter jejuni and C. coli outer membrane components. Res Microbiol. 1989 Jul-Aug;140(6):379–392. doi: 10.1016/0923-2508(89)90014-4. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Francis D. H., Collins J. E., Duimstra J. R. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect Immun. 1986 Mar;51(3):953–956. doi: 10.1128/iai.51.3.953-956.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. A., Reynolds D. J., Chanter N., Morgan J. H., Parsons K. R., Debney T. G., Bland A. P., Bridger J. C. Dysentery caused by Escherichia coli (S102-9) in calves: natural and experimental disease. Vet Pathol. 1985 Mar;22(2):156–163. doi: 10.1177/030098588502200210. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Determinants for thermoinducible cell binding and plasmid-encoded cellular penetration detected in the absence of the Yersinia pseudotuberculosis invasin protein. Infect Immun. 1989 Jul;57(7):1998–2005. doi: 10.1128/iai.57.7.1998-2005.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Heesemann J., Laufs R., O'Brien A. D., Tacket C. O., Levine M. M. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infect Immun. 1987 Feb;55(2):455–461. doi: 10.1128/iai.55.2.455-461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
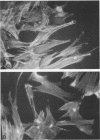
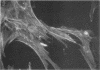
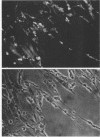
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxley R. A., Francis D. H. Natural and experimental infection with an attaching and effacing strain of Escherichia coli in calves. Infect Immun. 1986 Aug;53(2):339–346. doi: 10.1128/iai.53.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Holmes R. K. Shiga and Shiga-like toxins. Microbiol Rev. 1987 Jun;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam S., March S. B., Ahmed R., Bezanson G. S., Kasatiya S. Characterization of Escherichia coli serotype O157:H7. J Clin Microbiol. 1988 Oct;26(10):2006–2012. doi: 10.1128/jcm.26.10.2006-2012.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Libaek L. B., Schoolnik G. K. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect Immun. 1987 Sep;55(9):2052–2056. doi: 10.1128/iai.55.9.2052-2056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Remis R. S., Helgerson S. D., McGee H. B., Wells J. G., Davis B. R., Hebert R. J., Olcott E. S., Johnson L. M., Hargrett N. T. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983 Mar 24;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Riley L. W. The epidemiologic, clinical, and microbiologic features of hemorrhagic colitis. Annu Rev Microbiol. 1987;41:383–407. doi: 10.1146/annurev.mi.41.100187.002123. [DOI] [PubMed] [Google Scholar]
- Scaletsky I. C., Milani S. R., Trabulsi L. R., Travassos L. R. Isolation and characterization of the localized adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1988 Nov;56(11):2979–2983. doi: 10.1128/iai.56.11.2979-2983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli D. M., Beachey E. H. Bacterial adhesion: modulation by antibiotics which perturb protein synthesis. Antimicrob Agents Chemother. 1988 Nov;32(11):1603–1608. doi: 10.1128/aac.32.11.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferli D. M., Beachey E. H. Bacterial adhesion: modulation by antibiotics with primary targets other than protein synthesis. Antimicrob Agents Chemother. 1988 Nov;32(11):1609–1613. doi: 10.1128/aac.32.11.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D., Datta-Roy K., Banerjee K., Ghose A. C. Identification of some antigenically related outer-membrane proteins of strains of Vibrio cholerae O1 and non-O1 serovars involved in intestinal adhesion and the protective role of antibodies to them. J Med Microbiol. 1989 May;29(1):33–39. doi: 10.1099/00222615-29-1-33. [DOI] [PubMed] [Google Scholar]
- Sherman P. M., Houston W. L., Boedeker E. C. Functional heterogeneity of intestinal Escherichia coli strains expressing type 1 somatic pili (fimbriae): assessment of bacterial adherence to intestinal membranes and surface hydrophobicity. Infect Immun. 1985 Sep;49(3):797–804. doi: 10.1128/iai.49.3.797-804.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Soni R. Adherence of Vero cytotoxin-producing Escherichia coli of serotype O157:H7 to human epithelial cells in tissue culture: role of outer membranes as bacterial adhesins. J Med Microbiol. 1988 May;26(1):11–17. doi: 10.1099/00222615-26-1-11. [DOI] [PubMed] [Google Scholar]
- Sherman P., Soni R., Karmali M. Attaching and effacing adherence of Vero cytotoxin-producing Escherichia coli to rabbit intestinal epithelium in vivo. Infect Immun. 1988 Apr;56(4):756–761. doi: 10.1128/iai.56.4.756-761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Soni R., Petric M., Karmali M. Surface properties of the Vero cytotoxin-producing Escherichia coli O157:H7. Infect Immun. 1987 Aug;55(8):1824–1829. doi: 10.1128/iai.55.8.1824-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Soni R., Yeger H. Characterization of flagella purified from enterohemorrhagic, vero-cytotoxin-producing Escherichia coli serotype O157:H7. J Clin Microbiol. 1988 Jul;26(7):1367–1372. doi: 10.1128/jcm.26.7.1367-1372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I., Cohen M. L., Rumschlag H. S., Riley L. W., White E. H., Carr J. H., Bond W. W., Wachsmuth I. K. Influence of the 60-megadalton plasmid on adherence of Escherichia coli O157:H7 and genetic derivatives. Infect Immun. 1990 May;58(5):1223–1231. doi: 10.1128/iai.58.5.1223-1231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Karch H., Wachsmuth K. I., Robins-Browne R. M., O'Brien A. D., Lior H., Cohen M. L., Smithers J., Levine M. M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987 Dec;55(12):3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Wachsmuth I. K., Chapman C., Birden R., Brittingham J., Jackson C., Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986 Oct;154(4):712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Wachsmuth K. I., Smithers J., Jackson C. Studies in gnotobiotic piglets on non-O157:H7 Escherichia coli serotypes isolated from patients with hemorrhagic colitis. Gastroenterology. 1988 Mar;94(3):590–597. doi: 10.1016/0016-5085(88)90228-4. [DOI] [PubMed] [Google Scholar]
- Udhayakumar V., Muthukkaruppan V. R. Protective immunity induced by outer membrane proteins of Salmonella typhimurium in mice. Infect Immun. 1987 Mar;55(3):816–821. doi: 10.1128/iai.55.3.816-821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. K., Andrews G. P., Fritz D. L., Sjogren R. W., Jr, Boedeker E. C. Characterization of the plasmid from Escherichia coli RDEC-1 that mediates expression of adhesin AF/R1 and evidence that AF/R1 pili promote but are not essential for enteropathogenic disease. Infect Immun. 1988 Aug;56(8):1846–1857. doi: 10.1128/iai.56.8.1846-1857.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafriri D., Oron Y., Eisenstein B. I., Ofek I. Growth advantage and enhanced toxicity of Escherichia coli adherent to tissue culture cells due to restricted diffusion of products secreted by the cells. J Clin Invest. 1987 Apr;79(4):1210–1216. doi: 10.1172/JCI112939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva M. L., Mortara R. A., Barros H. C., de Souza W., Trabulsi L. R. Aggregation of membrane-associated actin filaments following localized adherence of enteropathogenic Escherichia coli to HeLa cells. J Cell Sci. 1989 Jul;93(Pt 3):439–446. doi: 10.1242/jcs.93.3.439. [DOI] [PubMed] [Google Scholar]
- de Melo M. A., Pechère J. C. Identification of Campylobacter jejuni surface proteins that bind to Eucaryotic cells in vitro. Infect Immun. 1990 Jun;58(6):1749–1756. doi: 10.1128/iai.58.6.1749-1756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]