SUMMARY
Transgenic expression of activated AKT1 in the murine prostate induces Prostatic Intraepithelial Neoplasia (PIN) that does not progress to invasive prostate cancer (CaP). In luminal epithelial cells of Akt-driven PIN we show the concomitant induction of p27kip1 and senescence. Genetic ablation of p27Kip1 led to down regulation of senescence markers and progression to cancer. In humans, p27Kip1 and senescence markers were elevated in PIN not associated with CaP, but were decreased and absent, respectively in cancer-associated PIN and in CaP. Importantly, p27Kip1 up-regulation in mouse and human in situ lesions did not depend upon mTOR or Akt activation but was instead specifically associated with alterations in cellular polarity, architecture and adhesion molecules. These data suggest that a p27Kip1-driven checkpoint limits progression of PIN to CaP.
Keywords: CaP, prostate cancer, senescence, PIN, Prostate intraepithelial neoplasia, Akt and p27Kip1
SIGNIFICANCE
Most human epithelial cancers progress from dysplastic in situ lesions to invasive and ultimately metastatic disease. Prostatic intraepithelial neoplasia (PIN) is a precursor of invasive prostate cancer, while the molecular mechanisms underpinning this transition are largely unknown. Here we define loss of p27Kip1 expression as a key event in this progression of PIN to invasive cancer. We further show p27Kip1 upregulation in PIN correlates with senescence in both murine and human prostate tissues. Up-regulation of p27Kip1 in pre-invasive lesions is not dependent on mTOR or AKT activation, but is secondary to loss of cellular polarity and disruption of cell-cell adhesions. We suggest that the p27Kip1-dependent checkpoint limits the progression of PIN to invasive cancers.
INTRODUCTION
Activation of AKT through deregulated PI3K signaling resulting from genetic inactivation of PTEN, mutational activation of PI3K or through the activation of upstream oncogenic tryosine kinases is a frequent molecular event in human cancer (Brugge et al., 2007; Lee et al., 2007) We have previously shown that transgenic expression of activated AKT in the murine prostate induces a uniform and highly penetrant PIN phenotype confined to the ventral prostate (Majumder et al., 2003). Despite long-term follow-up these mice do not develop invasive cancers. However, in this model, there is robust and predictable time-dependent regression of the PIN with the mTOR inhibitor RAD001(Majumder et al., 2004). This model thus provides a robust system in which to define transition steps leading from PIN to invasive cancer.
In human epithelial cancers, reduced levels of p27Kip1 expression is frequently observed (Slingerland and Pagano, 2000) and is correlated with tumor progression and poor survival (Loda et al., 1997; Porter et al., 1997; Yang et al., 1998). p27Kip1 functions primarily as a negative regulator of cyclin-CDK activity and thus likely participates in tumor suppression by inhibiting cell-cycle progression (reviewed in (Chu et al., 2008). Targeted disruption of p27Kip1 (Cdkn1b−/−) in the mouse leads to prostatic hyperplasia (Cordon-Cardo et al., 1998) and development of pituitary adenomas (Fero et al., 1998; Fero et al., 1996) as the mice age. However, Cdkn1b−/−mice do not typically develop other spontaneous tumors (Fero et al., 1996; Kiyokawa et al., 1996; Nakayama et al., 1996).
In many human cancer cells, oncogene induced senescence (OIS) is associated with known tumor suppressor pathways such as p53, VHL and RB (Serrano et al., 1997) (Young et al., 2008) It has been reported that OIS occurs in many human and mouse precursors of cancer and this phenomenon can be reversed by the inactivation of tumor suppressor pathways (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005). Recently, Young et al have reported that senesence induced by loss of VHL in renal cancer cells is RB, p27Kip1 and SKP2 dependent but p53 independent (Young et al., 2008).
Here, we have investigated the role of p27kip1 in tumor suppression in prostate cancer. Using both genetically engineered mice and human prostate samples, we have identified a relationship among senescence induction, p27kip1 expression and PIN that support the notion that p27Kip1 induction in the context of early neoplastic lesions may represent a novel pre-invasive checkpoint linked to cellular senescence.
RESULTS
p27Kip1 protein and markers for cellular senescence are increased in AKT1-transgenic prostate
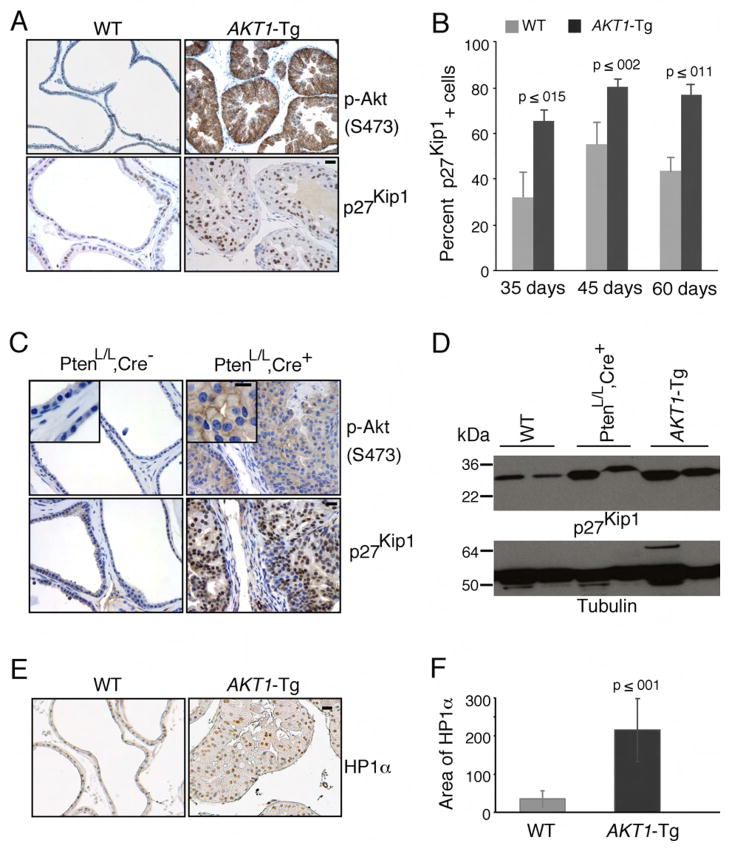
Transgenic animals expressing activated Akt (AKT1-Tg) in the ventral prostate uniformly develop intra-ductal/intra-acinar lesions consistent with PIN. However, progression to invasive cancer has not been seen after two years of observation (data not shown). Moreover, despite the strong proliferative signal delivered by activated Akt in vitro, AKT1-Tg prostates show only a modest increase in BrdU incorporation suggesting a possible up-regulation of cyclin dependent kinase inhibitors (Majumder et al., 2004). Thus to determine the level of p27Kip1 in the ventral prostate of AKT1-Tg mice, we performed immunohistochemical analysis using anti-p27Kip1. While activation of Akt is associated with down regulation of p27Kip1 in a number of in vitro systems (Liang et al., 2002; Shin et al., 2002; Viglietto et al., 2002), surprisingly p27Kip1 protein levels were significantly higher in prostate epithelial cells expressing myr-AKT1 when compared to littermate wild-type mice (Figure 1A and D). Similarly, significant levels of elevated p27Kip1 protein were observed in the VP of AKT1-Tg mice at the 35, 45 and 60 days (Figure 1B).
Figure 1. Induction of p27Kip1 and senescence in prostatic intraepithelial neoplasia of AKT1-Tg and PtenL/L;Cre+ mice.
(A) VPs from wild-type (WT) and AKT1-Tg mice were stained by immunohistochemisty using antibodies directed against phospho-Akt (S473) (upper panel) or against p27Kip1 (lower panel). Scale bar, 50 μM
(B) The number of p27kip1 positively staining cells was determined in the VPs of wild type (WT) and AKT1-Tg mice of the indicated ages. Data are presented as mean ± SEM.
(C) Wild type (PtenL/L;Cre−) and Pten conditional knock out (prostate specific) (PtenL/L;Cre+) VPs were stained with phospho-Akt (S473) (upper panel) or anti-p27Kip1 (lower panel). Scale bar, 50 μM; insert 100μM
(D) Western blot analysis of p27Kip1 and Tubulin in whole cell lysates from ventral prostates of Wild type (WT), AKT1-Tg and Pten conditional knock out (PtenL/L;Cre+) mice of 6 weeks old
(E) VPs from wild type and AKT1-Tg mice were stained with antibody against HP1α. Scale bar,50μM.
(F) Area of HP1α staining were measured both in wild type and AKT1-Tg prostates. Data are presented as mean ± SD.
We previously observed that PIN lesions in AKT1-Tg and Pten heterozygous (+/−) mice are histologically similar (Majumder et al., 2003). In the latter, activation of Akt is seen in conjunction with PTEN loss in PIN lesions (Majumder et al., 2003). Thus, we next examined mice harboring two floxed alleles of PTEN and a transgene driving the expression of prostate restricted Cre (ARR2PB-Cre) (referred to as PtenL/L;Cre+) (Wang et al., 2003). We found that the development of PIN at 6 weeks of age was accompanied by phosphorylation and activation of Akt as well as a concomitant increase in p27Kip1 protein when compared to littermate controls (Figure 1C and D). These data suggest that p27Kip1 is upregulated in the context of PIN driven either by myristoylation-dependent Akt activation or by loss of PTEN.
Cellular senescence is commonly seen in the early or precursor stages of invasive cancer (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005). To determine whether cells in the PIN lesions found in the AKT1-Tg mice were senescent we stained tissue sections for senescence-associated β-gal in frozen tissue samples, or antibodies against HP1α and HP1γ in paraffin-embedded tissue (Bartkova et al., 2006). We found that the stabilization of p27Kip1 protein was associated with the increased level of HP1α, HP1γ and β-gal activity in the PIN lesions of the AKT1-Tg mice (Figure 1 E–F, Figure 2A–B and data not shown).
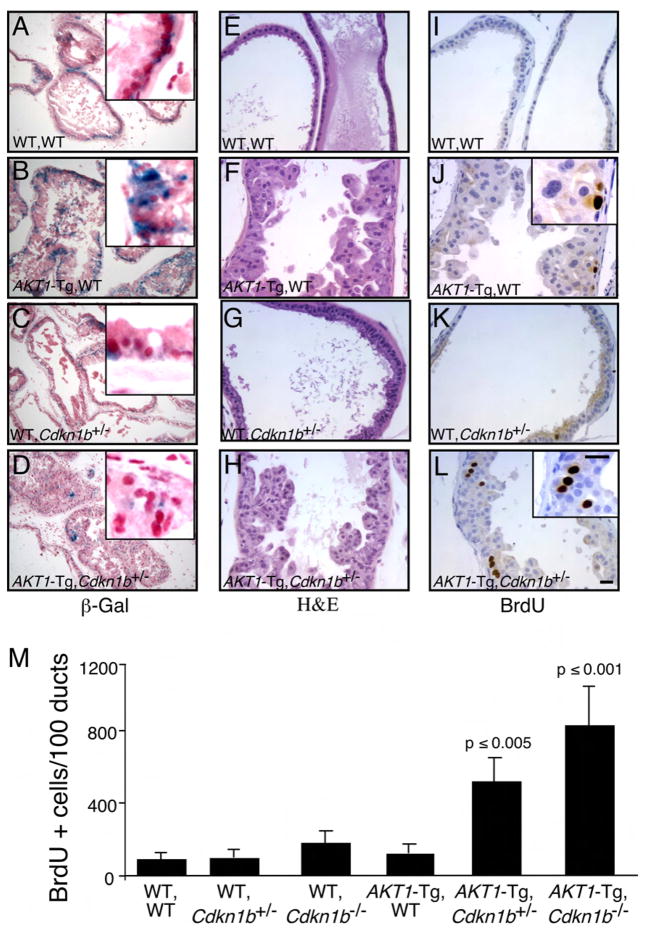
Figure 2. Genetic inactivation of Cdkn1b rescues cell from senescence and increases proliferation in AKT1-Tg mice.
(A–L) Twelve hours after BrdU administration, mice were sacrificed and the VPs of wild-type (A, E, I), AKT1-Tg (B, F, J), Cdkn1b+/− (C, G, E) and AKT1-Tg, Cdkn1b+/− (D, H, L) mice were stained with either β-Gal (A– D), H&E (E–H) or with anti-BrdU antibody (I–L). Scale bar, 50 μM; insert 100μM.
(M) The numbers of BrdU stained cells per 100 ducts was determined by manual counting of BrdU positive cells in all lobes of VP. Data are presented as mean ± SD.
Loss of p27Kip1 in AKT1-Tg mice rescued cells from senescence and increased proliferation in prostate epithelial cells
It has been reported that oncogenes induce senescence in different human and murine precursors of cancer as well as in tumors harboring deletion in the Pten tumor suppressor (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005). Senescence induced by PTEN deficiency has been reported to be dependent on p53. Inactivation of Pten causes cellular senescence and combined inactivation of Pten and p53 causes a lethal form of invasive prostate cancer (Chen et al., 2005). To test the hypothesis that the senescence observed in the PIN lesions of the AKT1-Tg mice could be reversed by the inactivation of the tumor suppressor p27Kip1, compound mice were generated where Myr-AKT1 was expressed in both the Cdkn1b heterozygous and homozygous null settings. PIN in the ventral prostate of AKT1-Tg mice displayed high β-Gal activity (Figure 2B) and both HP1α and HP1γ were elevated (Figure 1D–E and data not shown). However, β-Gal activity as well as expression of HP1α, HP1γ were decreased in the PIN lesions of mice in which AKT1 was expressed in the context of the Cdkn1b heterozygous or homozygous background (Figure 2C–D and data not shown). These data suggest that the PIN-induced cellular senescence checkpoint is dependent on p27kip1.
These findings also raised the possibility that p27Kip1 induced senescence results in a block in cell-cycle progression and consequently a failure to progress beyond the PIN phenotype. After the administration of BrdU, the VPs from 10–16 week-old offspring were harvested, and the rate of proliferation was determined by immunohistochemical detection of incorporated BrdU. Indeed, VPs from AKT1-Tg/Cdkn1b+/− and AKT1-Tg/Cdkn1b−/− mice showed significantly increased rates of BrdU incorporation (Figure 2L & M) as compared with AKT1-Tg/Cdkn1b+/+, AKT1-WT/Cdkn1b+/−, AKT1-WT/Cdkn1b−/− and AKT1-WT/Cdkn1b+/+ prostates (Figure 2I–K & M and data not shown). At 10–16 weeks of age, the PIN phenotype was exacerbated, but no additional cancer phenotype was observed at this time point (Figure 2E–H). Thus, at early time points loss of Cdkn1b abrogated the induction of senescence and resulted in increased prostate epithelial cell proliferation in the setting of Akt activation.
AKT1-Tg mice harboring loss of either one or both Cdkn1b alleles develop invasive prostate cancer
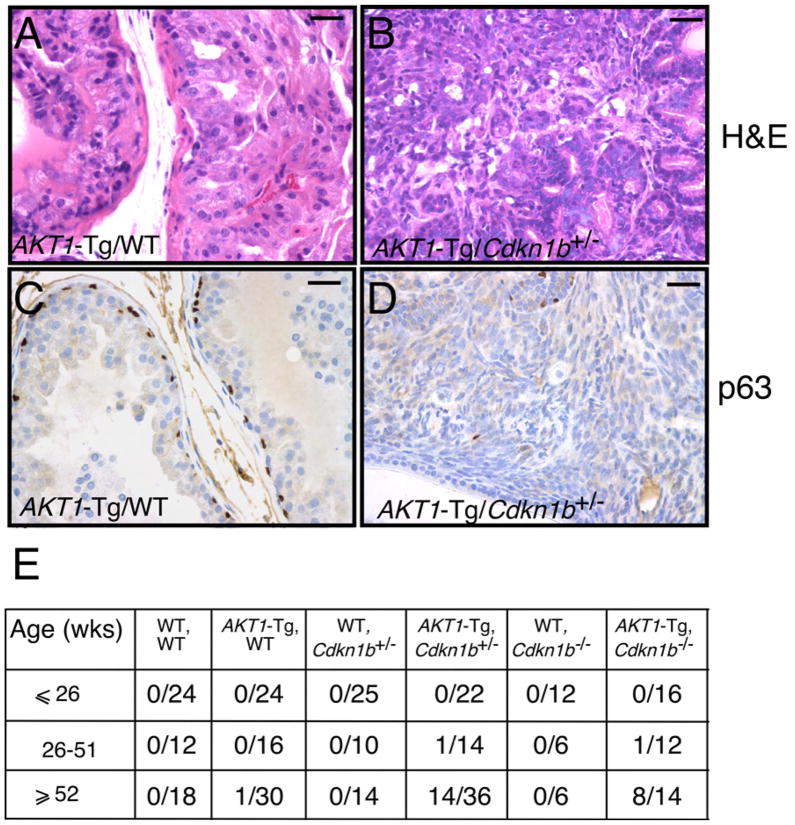
These observations are consistent with the notion that p27kip1 acts as a checkpoint that limits hyperplastic proliferation and malignant transformation. To determine whether p27Kip1 loss also results in the progression from PIN to invasive cancer, we examined the VPs of all genotypes resulting from the intercross of AKT1-Tg/Cdkn1b+/− mice as the mice aged. As predicted, the AKT1-Tg/Cdkn1b+/− and AKT1-Tg/Cdkn1b−/− mice but not littermate controls or AKT1-Tg/Cdkn1b+/+ developed invasive cancer in the VP (Figure 3A–B, E and data not shown). Thirty-eight percent of AKT1-Tg/Cdkn1b+/− mice and 57% of AKT1-Tg/Cdkn1b−/− mice developed invasive prostate cancer at the age of 1 year or more as compared with their control littermates (Figure 3E). One AKT1-Tg/Cdkn1b+/− and one AKT1-Tg/Cdkn1b−/− mouse developed a tumor in the VP prior to one year (Figure 3E and data not shown).
Figure 3. Genetic inactivation of Cdkn1b in AKT1-Tg mice results in the development of invasive prostate cancer.
(A) Prostatic intraepithelial neoplasia in a representative section from the VP of AKT1-Tg mouse (~52 weeks old). Scale bar, 100 μM
(B) Invasive prostate cancer in representative sections from AKT1-Tg/Cdkn1b+/−. Sections were stained with H&E. Scale bar, 100 μM
(C) VP from AKT1-Tg mice were stained with antibodies directed against p63. Scale bar, 100 μM
(D) VP from AKT1-Tg/Cdkn1b+/− mice were stained with antibodies directed against p63. Scale bar, 100 μM
(E) Summary of tumor incidence by age and genotype.
Loss of the basal epithelial cell layer and hence loss of expression of the basal cell-specific marker p63 is a distinguishing feature of the transition from PIN (p63+) to invasive CaP (p63−) in humans (Signoretti et al., 2000). We therefore examined p63 expression in the murine prostate tumors by IHC. While basal cells were present in the PIN lesions in the VP of AKT1-Tg (Figure 3C), prostate tumors from AKT1-Tg/Cdkn1b+/− and AKT1-Tg/Cdkn1b−/− mice (Figure 3D and data not shown) lacked the basal cell layer, confirming the invasiveness of these tumors. To further characterize the tumor phenotype, we examined the expression of the androgen receptor (AR) and the luminal cytokeratin-19 by IHC and found that the tumors expressed readily detectable AR and cytokeratin-19 (data not shown and Figure S1A–B) but failed to express cytokeratin-14 and p63 (data not shown and Figure 3D). These observations demonstrate the luminal nature of these tumors which is similar to human prostate cancer. Finally, BrdU incorporation assays showed that, as was the case in the PIN lesions arising in AKT1-Tg/Cdkn1b+/− and AKT1-Tg/Cdkn1b−/− mice, the resulting tumors were also highly proliferative (Figure S1C–F). IHC and immunoblot analysis of p27Kip1 suggested that these tumors retained the second allele of p27Kip1 though protein levels were markedly reduced (Figure S2D–SF, SJ). We noted that Akt expression was reduced in these tumors due to variegated expression of the transgene rather than reduction of Akt on a per cell basis (data not shown).
Stabilization of p27Kip1 is dependent on the PIN phenotype
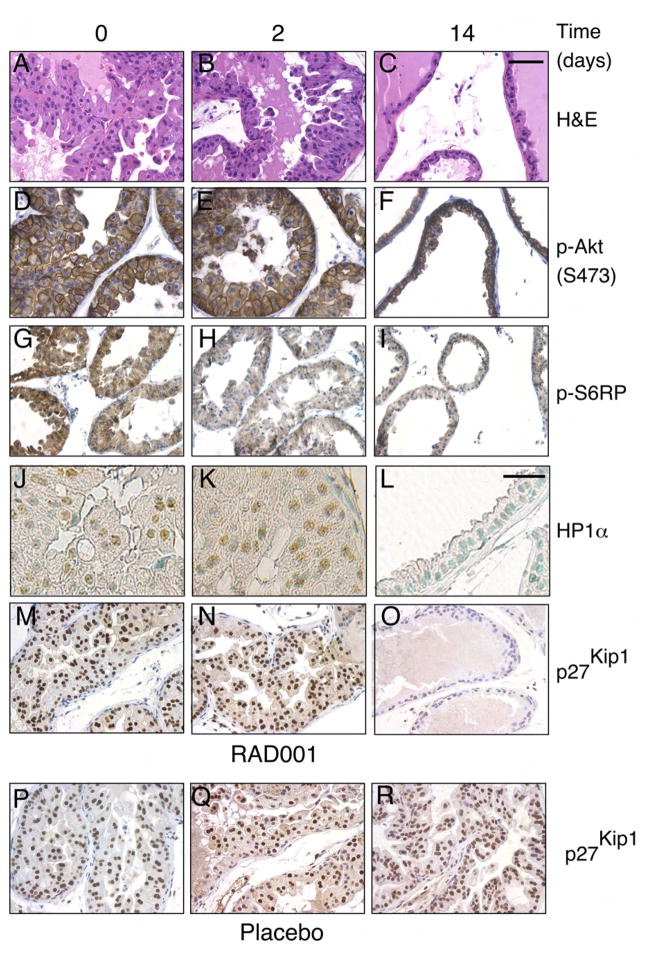
The finding that p27Kip1 levels were increased in the AKT1-Tg mice raised the possibility that Akt activity might directly lead to elevated levels of p27Kip1. Alternatively, p27Kip1 elevation might be associated with activation of the mTOR kinase or p70S6K pathways further downstream of Akt. To distinguish among these possibilities we took advantage of the mTOR dependence of this PIN phenotype. Specifically, inhibition of mTOR activity using the rapamycin-derivative RAD001 (everolimus), leads to the resolution of the PIN phenotype by day 14 of treatment (Majumder et al. 2004). By comparing RAD001 or placebo treated AKT1-Tg or WT mice, we hoped to distinguish the contribution of three possible effectors of p27Kip1 induction namely, the PIN phenotype itself (present or absent), mTOR (active or inactive) and Akt (active or inactive). To this end, 8–12 week old AKT1-Tg and littermate wild-type (WT) mice, were treated with placebo or RAD001 for 2 and 14 days. Consistent with our previous findings, 14 days after treatment the VP histology of RAD001 treated AKT1-Tg mice reverted to normal (Figure 4A–C and data not shown) while PIN persisted in placebo treated AKT1-Tg mice (data not shown) and in mice treated with RAD001 for 2 days (Figure 4B).
Figure 4. Induction of p27Kip1 and increases of senescence markers do not require mTOR activation and is correlated with the PIN phenotype.
(A–O) AKT1-Tg mice were treated with RAD001 at 10mg/kg/QD/PO for 0, 2, and 14 days
(P–R) AKT1-Tg mice were treated with placebo for 0, 2, and 14 days.
(A–C) Tissue sections from the VPs were stained with H&E,
(D–F) Tissue sections were stained with antibody directed against phospho-Akt (S473)
(G–I) Tissue sections were satained with antibody directed against phospho-S6RP
(J–L) Immunohistochemical analysis was done in tissue sections with antibody against HP1α Scale bar, 200μM
(M–R) Tissue sections were stained with antibody directed against p27Kip1. Shown are sections representative of the results obtained in at least 12 animals evaluated after each specific treatment period. Scale bar, 100 μM (A–I and M–R),
Next, we examined p27Kip1 in the VPs of AKT1-Tg mice treated with placebo and RAD001. Elevated level of p27Kip1 was observed in the VPs of AKT1-Tg mice treated with placebo (Figure 4 P–R) and in AKT1-Tg mice treated with RAD001 for 2 days (Figure 4M–N). However, p27Kip1 levels reverted to normal after 14 days of treatment concomitant with the disappearance of the PIN phenotype (Figure 4O and C). In AKT1-Tg animals, mTOR inhibition leads to the loss of phosphorylation of both eiF4G and ribosomal S6 protein (S6RP). In these experiments, S6RP phosphorylation was inhibited after 2 days of treatment with RAD001 (Figure 4 H–I) however, p27Kip1 levels were unaffected (compare Figure 4M and 4N). As we have previously shown, the activation of Akt is unaffected by treatment with RAD001 and persisted on day 14 at which time both the PIN phenotype and the induction of p27Kip1 had resolved (Majumder et al., 2004)(Figure 4 D–F). Thus, Akt oncogenic activity, by itself was not sufficient for p27Kip1 induction (compare Figure 4F and Figure 4O).
Finally, we have used expression profiling to identify two classes of transcripts indicative of either mTOR pathway activation (Majumder et al., 2004) or a distinct set of transcripts correlated with the presence or absence of the PIN phenotype Aldolase 3 (a Hif-1 target) and Prostate Stem Cell Antigen (PSCA) are prototypical members of each class of transcripts respectively. Consistent with the notion that p27Kip1 protein induction is linked to the PIN phenotype, elevated p27Kip1 levels correlated best with the pattern of mRNA induction seen with the phenotype marker PSCA and senescence markers rather than with the pharmacodynamic marker of mTOR activity, aldolase 3 (Figure S3, upper panel). Together these data, summarized in Figure S3 (lower panel), suggest that the cellular or morphological alterations secondarily arising during the formation of the PIN lesion either alone or together with activation of Akt leads to the induction of p27kip1 followed by cellular senescence and a resulting block in both cell proliferation and phenotype progression.
A prediction that follows from these findings is that this putative p27Kip1 checkpoint might be activated in PIN lesions that are induced independently of PI3K pathway activation. Indeed, we also found significant elevation of p27Kip1 in the PIN phenotype of VPs of c-MYC transgenic mice as compared to wild-type controls (Figure S4, A–B). The c-MYC-transgenic mice develop invasive cancers at the age of 12 months, and in these cancers p27Kip1 appeared to be down regulated as reported in human CaP (Figure S4A, B and Figure 5 F–G). Thus, the p27Kip1 checkpoint correlates best with the appearance of PIN lesions.
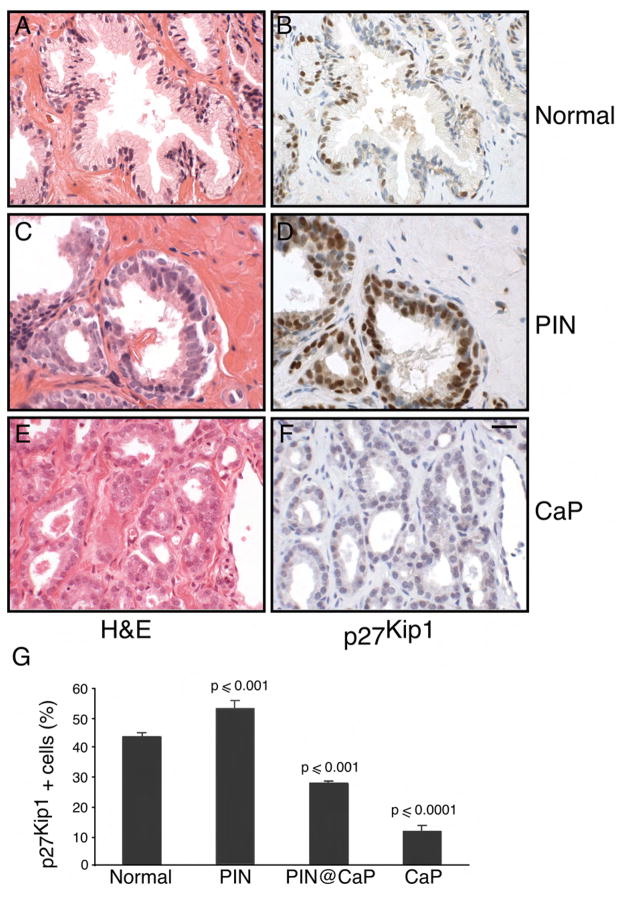
Figure 5. Stabilization of p27Kip1 in human prostate intraepithelial neoplasia.
(A) Tissue sections from normal human prostate were stained with H&E
(B) Normal prostate tissue sections were stained with antibody directed against p27Kip1.
(C) Prostatic intraepithelial neoplasia (PIN) tissue sections were stained with H&E
(D) Tissue sections from human PIN were stained with antibody directed against p27Kip1
(E) Tissue sections from human prostate cancer (CaP) were stained with H&E
(F) Human prostate cancer (CaP) tissue sections were stained with antibody directed against p27Kip1. Scale bar, 50 μM (A–F).
(G) The number of p27kip1 positive cells was determined in the normal, PIN, PIN adjacent to invasive cancer and CaP. Data are presented as mean ± SEM.
The relevance of p27kip1 elevation in human PIN
The level of p27Kip1 in representative foci of human benign prostatic epithelium, PIN and invasive cancer was evaluated by immunohistochemical analysis. The level of p27Kip1 was significantly reduced in invasive cancer compared with benign epithelium (Figure 5D, F and G) as previously reported (Guo et al., 1997; Thomas et al., 2000). Foci of PIN adjacent to invasive cancer consistently showed both a reduced intensity of staining and a reduced number of p27Kip1 positive cells compared with benign epithelium (Data not shown and Figure 5G) as previously reported (De Marzo et al., 1998). In contrast, in 60% of the cases (15 of 25) of isolated PIN without adjacent invasive cancer, p27Kip1 staining was of similar intensity to adjacent benign epithelium (Figure 5B) and was present in a higher percentage of cells compared to benign epithelium (Figure 5D and G). The mean percentage of cells stained positively for p27Kip1 was 52.4% (range 38–82%) in this subset of PIN and 42.9% (range 32–50) in the secretory cells of adjacent benign epithelium (p < 0.001) (Figure 5G). Thus, we confirmed that the increase in p27Kip1 in murine models of prostate cancer also occurs in primary human prostate cancers.
Human prostate intraepithelial neoplasia displays the hallmark of senescence
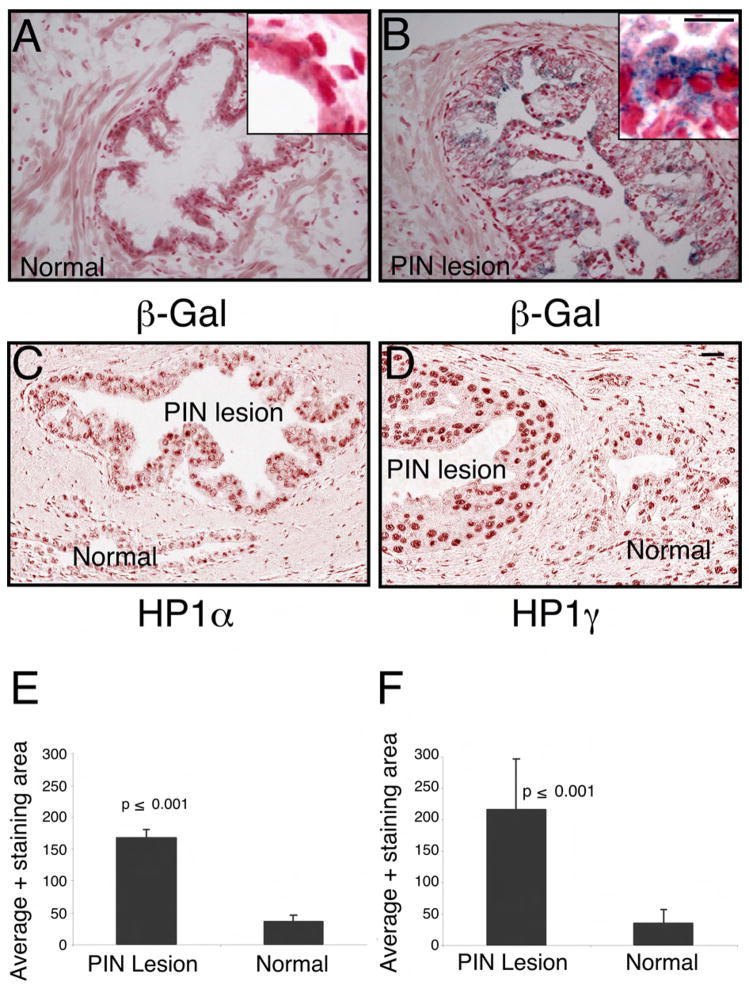
It has been reported that senescence is a hall mark of precursor lesions in many different human tumors (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005). In order to determine whether a senescence checkpoint can be identified in human PIN lesions we performed pathological analysis of 21 frozen tissue samples from prostates with documented PIN. The PIN lesions were documented in seven of them and senescence was investigated by β-Gal staining. Indeed, four of the seven identified PIN lesions showed considerably more β-Gal activity when compared to non-dysplastic epithelial cells in the same tissue sections (Figure 6A–B). In addition, we characterized 44 human PIN lesions obtained from specimens without adjacent cancer, with normal counterparts from paraffin embedded tissue sections. The level of HP1α and HP1γ as determined by IHC and quantitative analysis by spectral imaging showed increased level of both markers in PIN lesions when compared to normal ducts (Figure 6C–F and Figure S5 D).
Figure 6. Markers of cellular senescence are elevated in human PIN.
(A) Frozen sections of normal human prostate were subjected to β-Gal and hematoxylin/eosin staining. The blue staining indicates the β-Gal activity, haematoxylin and eosin was used as a counter stain
(B) Frozen sections of humn Prostate Intraepithelial Neoplasia (PIN) were subjected to β-Gal and hematoxylin/eosin staining. The blue staining indicates the β-Gal activity, haematoxylin and eosin was used as a counter stain to visualize the PIN lesion
(C) Tissue sections from paraffin embedded normal human prostate and PIN lesions were stained with antibodies against HP1α
(D) Tissue sections from paraffin embedded normal human prostate and PIN lesions were also stained with antibodies against HP1γ. Scale bar, 50μM (A–D); insert 100 μM.
(E) Average area of HP1α positive stain was measured in both normal and PIN lesion Data are presented as mean ± SD.
(F) Average area of HP1γ positive stain was measured in both normal and PIN lesion Data are presented as mean ± SD.
Induction of p27Kip1 during detachment and inhibition of cell-cell contact in human primary prostate epithelial cells
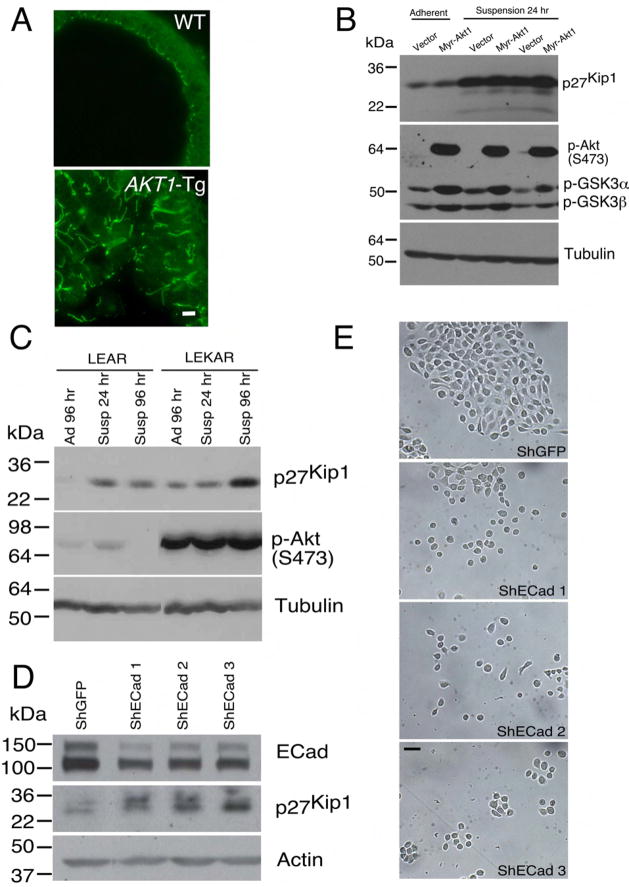
The PIN phenotype in AKT1-Tg mice is characterized by disorganization of luminal epithelial cells including loss of appropriate cell polarization (see (Majumder et al., 2004) and Figure 7A). In addition, in PIN lesions numerous prostate epithelial cells are no longer in tight contact with the basement membrane or with nearby basal cells. These observations raised the possibility that epithelial cell detachment may trigger upregulation of p27Kip1 protein. To address this possibility, we turned to primary human prostate epithelial cells (PrEC) engineered to express SV40 Large T antigen, the catalytic subunit of human telomerase (hTERT) and the androgen receptor (AR) (Berger et al., 2004) (Garraway et al., 2003). These immortalized cells, PrEC-LEAR, were then transduced with a retrovirus encoding myr-Akt to create PrEC-LEKAR cells. Next, these cells were plated either on standard culture plates or on low-adhesion coated plates or in suspension and protein extracts were prepared after 24, 48 and 96 hours. In both cell lines cell detachment led to a significant increases in p27Kip1 which was more notable in Akt expressing cells (Figure 7C). These data support the notion that detachment of human prostate epithelial cells, either with or without Akt expression, leads to the induction of p27Kip1. Similar results were also obtained in Rat1 fibroblasts transformed with myr-AKT1 (Figure 7B).
Figure 7. Disruption of cellular polarization and adhesion is associated with upregulation of p27Kip1 level.
(A) Ventral prostates of both wild type (WT) and AKT1-Tg mice stained with Z0-1 antibody and imaged by confocal microscope. Scale bar, 50μM..
(B) Rat embryonic fibroblast cells stably transfected with either vector or Myr-AKT1 were cultured under adherent and suspension condition. Protein lysates were prepared and immunoblotted with antibodies directed against p27Kip1 (upper panel), phospho-Akt (S473), phosho-GSK3 (α and β) (middle panel) and Tubulin (lower panel).
(C) Protein lysates were made from primary human epithelial cells (LEAR and LEKAR) cultured under suspension (Susp) or adherent (Ad) conditions. Immunoblot analysis using antibodies directed against p27Kip1(upper panel), phospho-Akt (S473) (middle panel), and Tubulin (lower panel)
(D) Primary human epithelial cells (LEAR) were transduced with three different shRNAs against E-Cadherin and shGFP as control. Cell were harvested 2 days after post selection and total protein lysates were prepared. E-Cadherin (upper panel), p27Kip1 (middle panel) and Actin (lower panel) western blot analysis was performed
(E) LEAR cells with E-Cadherin ShRNAs and control (ShGFP) were grown in plate and pictures were taken 2 days after post selection. Scale bar, 100 μM.
E-Cadherin, a member of cadherin family mediates epithelial cell-cell interaction and maintains normal architecture and polarity of epithelial cells in tissue. It also plays an important role in the progression of many human cancers including prostate cancer. We asked whether inhibition of cell-cell contact is sufficient to induce p27Kip1 stabilization in human primary prostate cancer cells. Suppression of E-Cadherin expression in PrEC cells resulted an inhibition of the normal architecture of cell-cell contact (Figure 7E) and increased the level of p27Kip1 protein (Figure 7D).
DISCUSSION
Cancer is characterized by a series of transitions from pre-neoplastic lesions to invasive cancer and finally metastatic disease. A great deal of emphasis has been placed on defining these events and to understand the disease progression at the molecular level in metastatic settings. However, less attention has been placed on the earlier transition points. It is not clear whether overcoming certain phenotypic transitions is only linked to checkpoints triggered by specific oncogenic mechanisms, or whether such phenotype transitions intrinsically raise specific checkpoint barriers that must be overcome. The angiogenic-switch (as an example) or the epithelial mesenchymal transition might represent such barriers or checkpoints to phenotypic transitions where tumors must acquire new properties in order to progress (Hanahan and Folkman, 1996)
We have studied the phenotypic transition between PIN and invasive cancers in a model of prostatic intraepithelial neoplasia resulting from transgenic activation of AKT. Here we show that in Akt transgenic and Pten homozygous mice the PIN phenotype is associated with increased levels of p27Kip1 and the induction of markers of senescence. In order to determine whether the induction of p27Kip1 was causally related to the block in phenotype progression we studied the results of genetic inactivation of Cdkn1b in the context of the AKT1-Tg mice. Loss or down-regulation of p27Kip1 led to increased proliferative rates, loss of senescence markers and to a progression of the PIN phenotype to invasive cancer. These latter data are consistent with prior data showing that genetic inactivation of Cdkn1b in the context of the PTEN+/− mice produces an invasive cancer phenotype (Di Cristofano et al., 2001).
Cellular senescence opposes neoplastic transformation triggered by activation of oncogenic pathways both in vitro and in vivo (Chen et al., 2005; Collado et al., 2005). Inhibition of cellular proliferation in pre-malignant lesion in mouse and human might be the result of oncogene induced senescence (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Michaloglou et al., 2005). Chen et al have shown that cells undergo senescence in preneoplastic lesions in Pten deficient prostate (Chen et al., 2005). In addition, myristoylated Akt induces cellular senescence in primary MEFs (Miyauchi et al., 2004). These published data suggested the senescence induced by loss of function of Pten is a result of Akt activation but require p53. In contrast to these findings, more recent data demonstrate that cellular senescence due to the loss of another tumor suppressor gene, VHL, is p53 independent (Young et al., 2008).Thus, the cooperating events that contribute to the induction of senescence in preneoplastic lesions that occurs as a result of loss of a tumor suppressor gene or activation of an oncogene, may be diverse.
In our model we asked whether the p27Kip1 and senescence checkpoint were specifically associated with oncogene activation or with a phenotype induced checkpoint. Careful dissection of the phenotype in response to mTOR inhibition coupled to measures of pathway activation both for AKT and for mTOR demonstrated that increased p27Kip1 (Figure 4M–O) and cellular senescence (Figure 4J–L) are dependent on the preneoplastic lesions and do not depend directly upon either Akt or mTOR activation. Similarly we did not find any correlation between mTOR activation and level of p27Kip1 or senescence markers in a subset of clinical samples with PIN. However, correlation between loss of PTEN staining and activation of mTOR in those clinical PIN specimens was observed (data not shown and Figure S5). Thus, it appears that a PIN-dependent p27Kip1 checkpoint rather than an oncogene-induced checkpoint is enacted in the AKT1-transgenic mice. Moreover, in MYC-Tg mice p27kip1 elevation was also seen in the PIN lesions. Thus, the checkpoint is apparently induced as a consequence of phenotypic perturbation that can be brought about by multiple upstream mechanisms. The apparent phenotype-dependence of the p27kip1 checkpoint may, at least in part, explain the synergy noted when Cdkn1b is genetically inactivated in the context of multiple other oncogenic or tumor suppressor manipulations and the high frequency of down-regulation of p27kip1 in human tumors.
It is not yet clear specifically what perturbation associated with the PIN phenotype might trigger accumulation of p27kip1. Among many possibilities including loss of matrix association, loss of polarity or loss of luminal-basal cell interactions could be considered. Mammoto et. al. previously found that restricting extra-cellular matrix adhesion and inhibiting cell spreading were associated with induction of p27Kip1 and G1 arrest (Mammoto et al., 2004). In keeping with this notion, we found that in human prostate epithelial cells p27kip1 was induced under conditions of contact free growth (Figure 7B–C). Recent data suggest that when E-Cadherin is lost, cells fail to polarize (Capaldo and Macara, 2007). Here we showed that down regulation of E-Cadherin expression by shRNAs in primary human prostate cancer cells increased the level of p27Kip1 and disrupted cell-cell interactions (Figure 7 D–E). We speculate that similar conditions might be recapitulated by loss of contact between epithelial cells and the basement membrane in vivo as E-cadherin has been shown to be downregulated in human PIN as well as subsequent carcinoma (Jaggi et al., 2005). In addition, we have previously shown that the PIN lesions in the AKT1-Tg mice lose oriented zona occludens-1 localization and thus lose normal apical-basal cell polarity (Majumder et al., 2004). In fact, loss of polarity and detachment from basal cells and the basement membrane are characteristic of PIN. Links between the regulation of polarity and cell-cycle control are increasingly recognized particularly in tying cell orientation to oriented cell division [reviewed in (Wodarz, 2001)]. Intriguingly, disruption of the polarized cytoskeleton and of bud formation in Sacchromyces cerevisiae is linked to delays in cell-cycle progression, dependent at least in part, on the SweP1, a CDK-inhibitory kinase [reviewed in (Lew, 2003)]. Whether regulation of cell polarity is tied to a senescence checkpoint remains to be determined.
Importantly, these data are highly relevant to human prostate cancer. Indeed, we show that p27Kip1 is overexpressed in human PIN not associated with invasive cancer, presumably representing the earliest phase of neoplastic transformation. In contrast, PIN adjacent to invasive cancer, where checkpoint loss may have already occurred, is associated with low levels of p27Kip1. The role of p27Kip1 in this process is further supported by a body of data showing that loss of p27Kip1 is commonly found in human cancers (Chu et al., 2008) and that invasive tumor cells specifically degrade p27kip1. In turn, this results in increased CDK2 activity (Loda et al., 1997).
As many as 30% of men with a diagnosis of PIN on biopsy subsequently are found to harbor an invasive prostatic adenocarcinoma on repeat biopsy (Gokden et al., 2005). Our findings therefore suggest that CDK inhibitors might have utility in preventing cancer progression from in situ dysplasia to invasion. Indeed, our group has previously shown that flavopiridol can in fact reduce the prevalence of esophageal cancer in a murine surgical model of Barrett’s dysplasia and esophageal carcinoma in Cdkn1b−/− mice (Lechpammer et al., 2005). We have also shown that pre-invasive lesions of metaplastic esophageal mucosa in humans (Barrett’s associated dysplasias) also show marked over-expression of p27Kip1 with subsequent loss as invasion ensued, suggesting that up-regulation of p27kip1 may be a checkpoint in other tissues as well (Singh et al., 1998). CDK inhibitors are in clinical trials in a number of human cancers. If safety and efficacy can be established, prevention trials with these agents in high-risk patients harboring pre-invasive lesions could be considered.
EXPERIMENTAL PROCEDURES
Generation of compound heterozygous mice
Cdkn1b heterozygous mice (B6.129S4-Cdkn1btm1Mlf/J), generated by the laboratory of James Roberts were obtained from Jackson Laboratory (Fero et al., 1996). The heterozygous mice were bred to AKT1-Tg heterozygous mice (FVB-Tg(Pbsn-Akt1wrs9) to generate F1 AKT1-Tg, Cdkn1b heterozygous mice. The compound AKT1-Tg heterozygous and Cdkn1b heterozygous mice were intercrossed to generate the F2 colony and the resulting offspring were used in this study. All procedures were performed according to protocols approved by Institutional Animal Care and Use Committee of Dana Farber Cancer Institute.
Genotyping, Dissections and Preparation of Tissues
Isolation of genomic DNA from tail cuts or ear punches, PCR-based genotyping, prostate and genitourinary tract dissections, tissue fixation and H&E stains were performed as described previously (Majumder et al., 2003) (Xu et al., 2007). Cdkn1b heterozygous and homozygous mice were genotyped as previously described (Di Cristofano et al., 1998).
Administration of RAD001 or BrdU
10mg/kg/day of RAD001 (40-O-(2-hydroxyethyl)-rapamycin) was administered as a microemulsion (Taesch and Niese, 1994) (2% w/w) diluted in ddH20 by oral gavage as previously described (Majumder et al., 2004). Mice receiving BrdU were injected intra-peritoneally with 50mg/kg of BrdU in PBS 12 hours prior to euthanasia and sacrificed after immediate ventricular perfusion with 4% buffered formaldehyde.
Immunohistochemical and immunoblot analysis
Mounted tissue sections (both human and murine) were hydrated, incubated for 30 min with 3% H2O2 in methanol at RT, washed with ddH2O and PBS and heated in a microwave to 199°F in 1 mM EDTA (pH 8.0) for 25 min (anti-p27Kip1, anti-CK-19, anti-phospho-AKT(S473) and anti-Androgen Receptor (AR) and anti-BrdU staining), or in 10mM citrate buffer (pH 6.0) for 30 min (anti-p63, anti-HP1α and anti-HP1γ staining). Sections were blocked in 10% goat serum (Vector) (30 min), incubated with anti-Akt-pS473 (1:400), anti-AR (1:400) (Cell Signaling), anti-CK-19 (1:200), anti-p27Kip1 (1:200), anti-p63 (1:100) or anti-BrdU (1:200) (BD Pharmingen) or anti-HP1α (1:1000) (clone 15.19s2, Upstate), or anti-HP1γ (1:1000) (clone 42s2, Upstate) in 1% BSA for 12h at 4°C, washed with PBS and incubated with secondary antibody (1:200) (Vector Laboratories) for 30 min. Antigen-antibody complexes were detected with the ABC kit (Vector Laboratories) or by DAB followed by methyl green counter staining (for HP1α and HP1γ). Five μm thick frozen sections of ventral prostates of different genotypes and human frozen prostate specimens were stained with β-Gal (Calbiochem) and CK14. Protein extracts and immunoblots were prepared as described (Majumder et al., 2003). Anti-phospho-Akt (S473), anti-phospho-GSK3 (Cell Signaling), anti-p27Kip1 (BD PharMingen), E-Cadherin (BD PharMIngen) was used at 1:1000 and anti-tubulin (B-5-1-2) (Sigma) were used at 1:1000.
Scoring of p27Kip1 immunohistochemistry in human prostate specimens
Sixty nine formalin-fixed cases of PIN were including 44 cases of PIN with adjacent invasive CaP and 25 cases in patients without the histologic diagnosis of invasive cancer. All human tissues were collected from the Brigham and Women’s Hospital (Boston. MA) using a protocol approved by the Instituitional Review Boards. Sections were stained with anti-p27kip1 antibody as described above. 300 cells from representative PIN foci, adjacent benign prostatic epithelium and invasive cancer were counted, and the number of cells with nuclear p27Kip1 staining was determined. Only cells where the staining intensity was equal to or greater than the adjacent normal secretory prostatic epithelium were considered positive.
Twenty one frozen human prostate biopsy tissues were stained with β-Gal and found presence of PIN in seven samples. Higher level of β-Gal positivity, compared to the adjacent normal cells, was present in four of these seven.
Scoring of HP1α and HP1γ staining in mouse and human prostate specimens
Both human (n = 29) and mouse (n = 20) prostate tissues were stained with anti-HP1α and HPγ. Since HP1 differential staining was low, localization and relative intensity were assessed using spectral imaging methods similar to those previously described (Byers et al., 2007). Spectral imaging and digital spectral cube deconvolution was performed using a CRI Nuance spectral analyzer (CRI Inc., Woburn, MA) and associated software package. Image analysis and stain intensity quantitation was performed on the appropriate spectra using ImageJ (National Institutes of Health, Bethesda, MD). Images were taken at ×20 objective magnification and image intensities were normalized to a threshold value. Staining above the threshold intensity was considered positive, and the positive area within fields of 20 nuclei was measured.
Human prostate cell lines, rat fibroblast cells and E-Cadherin knockdown
Human primary prostate epithelial cells (PrEC) expressing SV40 Large T antigen, hTERT and androgen receptor (AR) without (PrEC LEAR) or with myristoylated-flag-Akt1 (PrEC LEKAR) (Berger et al., 2004) were plated on ultralow attachment plates (Corning) at a density of 200,000 viable cells/mL in serum-free media (PrEGM) as previously described (Berger et al., 2004). Spheres were collected by gentle centrifugation (800 rpm) after 1, 2 and 4 days and dissociated by incubation for 10 min in 0.05% trypsin, 0.53 mM EDTA (Invitrogen). LEAR cells were infected with three independent ShRNAs against E-Cadherin (shECad1: 5′-CCAGTGAACAACGATGGCATT-3′ shECad2: 5′-CCAAGCAGA ATTGCTCACATT-3′, shECad3: 5′-CGATTCAAAGTGGGCACAGAT-3′) and control ShGFP. Cells were harvested after 2 days post selection. PrEC cells were lysed by sonication in 1.25% SDS, 0.0125 NaPO4 (pH 7.2), 50 mM NaF, 2 mM EDTA, 1.25% Nonidet P-40, 1 mM sodium vanadate with protease inhibitors (Roche).
Rat embryonic fibroblast cells were infected with Myr-AKT1 and a stable cell line was established by single cell dilution. Expression of Myr-Akt was determined by immunoblot analysis using an antibody against phospho-Akt (S473). These cells were grown either in adherent (cells culture dishes) or in suspension for 24 hours. Cells were lysed and protein was isolated as described before.
Statistical analysis
ONE WAY ANNOVA was used to test for differences in cell proliferation and IHC scores between different genotypes.
Supplementary Material
Acknowledgments
We thank Dr. Erguen Sahin, M.D. for the generous gift of mouse specific cytokeratin-19 antibody, Michelangelo Fiorentino, M.D., Ph.D. for IHC analysis of p27Kip1, HP1 in human PIN and Heidi Lane, Ph.D. of the Novartis Institute for Biomedical Research, Oncology, Basel, Switzerland for RAD001. We thank Nandita Bhattacharya, Yeonju Shim and Jennifer Kum for excellent technical support. This work was supported the Linda and Arthur Gelb Center for Translational Research, by the National Cancer Institute (PO1CA89021 WRS, K01 CA94223 WCH), by the Prostate Cancer Foundation (WRS, WCH, ML, RB), by the Damon-Runyon Cancer Research Foundation (W.R.S.), and by a Career Development Award from the DF/HCC SPORE in Prostate Cancer (P.K.M).
Footnotes
COMPETING INTEREST STATEMENT
W.R.S., W.C.H and M.L. declare that they receive research support from and are consultants for the Novartis Institute For Biomedical Research. WRS is now an employee of the Novartis Institutes For Biomedical Research. P.K.M. and K.E.Y. are employees of Merck Research Laboratories-Boston.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Berger R, Febbo PG, Majumder PK, Zhao JJ, Mukherjee S, Signoretti S, Campbell KT, Sellers WR, Roberts TM, Loda M, et al. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 2004;64:8867–8875. doi: 10.1158/0008-5472.CAN-04-2938. [DOI] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–107. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Byers RJ, Di Vizio D, O’Connell F, Tholouli E, Levenson RM, Gossage K, Twomey D, Yang Y, Benedettini E, Rose J, et al. Semiautomated multiplexed quantum dot-based in situ hybridization and spectral deconvolution. J Mol Diagn. 2007;9:20–29. doi: 10.2353/jmoldx.2007.060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massague J, Scher HI. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90:1284–1291. doi: 10.1093/jnci/90.17.1284. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Meeker AK, Epstein JI, Coffey DS. Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. Am J Pathol. 1998;153:911–919. doi: 10.1016/S0002-9440(10)65632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Garraway LA, Lin D, Signoretti S, Waltregny D, Dilks J, Bhattacharya N, Loda M. Intermediate basal cells of the prostate: in vitro and in vivo characterization. Prostate. 2003;55:206–218. doi: 10.1002/pros.10244. [DOI] [PubMed] [Google Scholar]
- Gokden N, Roehl KA, Catalona WJ, Humphrey PA. High-grade prostatic intraepithelial neoplasia in needle biopsy as risk factor for detection of adenocarcinoma: current level of risk in screening population. Urology. 2005;65:538–542. doi: 10.1016/j.urology.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Guo Y, Sklar GN, Borkowski A, Kyprianou N. Loss of the cyclin-dependent kinase inhibitor p27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res. 1997;3:2269–2274. [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Jaggi M, Johansson SL, Baker JJ, Smith LM, Galich A, Balaji KC. Aberrant expression of E-cadherin and beta-catenin in human prostate cancer. Urol Oncol. 2005;23:402–406. doi: 10.1016/j.urolonc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Lechpammer M, Xu X, Ellis FH, Bhattacharaya N, Shapiro GI, Loda M. Flavopiridol reduces malignant transformation of the esophageal mucosa in p27 knockout mice. Oncogene. 2005;24:1683–1688. doi: 10.1038/sj.onc.1208375. [DOI] [PubMed] [Google Scholar]
- Lee JY, Engelman JA, Cantley LC. Biochemistry. PI3K charges ahead. Science. 2007;317:206–207. doi: 10.1126/science.1146073. [DOI] [PubMed] [Google Scholar]
- Lew DJ. The morphogenesis checkpoint: how yeast cells watch their figures. Curr Opin Cell Biol. 2003;15:648–653. doi: 10.1016/j.ceb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci U S A. 2003;100:7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. Embo J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Porter PL, Malone KE, Heagerty PJ, Alexander GM, Gatti LA, Firpo EJ, Daling JR, Roberts JM. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Lipman J, Goldman H, Ellis FH, Jr, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett’s associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Taesch S, Niese D. Safety and tolerability of a new oral formulation of cyclosporin A, Sandimmun Neoral, in renal transplant patients. Transpl Int. 1994;7(Suppl 1):S263–266. doi: 10.1111/j.1432-2277.1994.tb01363.x. [DOI] [PubMed] [Google Scholar]
- Thomas GV, Schrage MI, Rosenfelt L, Kim JH, Salur G, deKernion JB, Dorey F, Said J, Reiter RE. Preoperative prostate needle biopsy p27 correlates with subsequent radical prostatectomy p27, Gleason grade and pathological stage. J Urol. 2000;164:1987–1991. [PubMed] [Google Scholar]
- Viglietto G, Motti ML, Bruni P, Melillo RM, D’Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- Wodarz A. Cell polarity: no need to reinvent the wheel. Curr Biol. 2001;11:R975–978. doi: 10.1016/s0960-9822(01)00578-4. [DOI] [PubMed] [Google Scholar]
- Xu Q, Majumder PK, Ross K, Shim Y, Golub TR, Loda M, Sellers WR. Identification of prostate cancer modifier pathways using parental strain expression mapping. Proc Natl Acad Sci U S A. 2007;104:17771–17776. doi: 10.1073/pnas.0708476104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang RM, Naitoh J, Murphy M, Wang HJ, Phillipson J, deKernion JB, Loda M, Reiter RE. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol. 1998;159:941–945. [PubMed] [Google Scholar]
- Young AP, Schlisio S, Minamishima YA, Zhang Q, Li L, Grisanzio C, Signoretti S, Kaelin WG., Jr VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.