Abstract
Acute flaccid polio-like paralysis occurs during natural West Nile virus (WNV) infection in a subset of cases in animals and humans. To evaluate the pathology and the possibility for therapeutic intervention, the authors developed a model of acute flaccid paralysis by injecting WNV directly into the sciatic nerve or spinal cord of hamsters. By directly injecting selected sites of the nervous system with WNV, the authors mapped the lesions responsible for hind limb paralysis to the lumbar spinal cord. Immunohistochemical analysis of spinal cord sections from paralyzed hamsters revealed that WNV-infected neurons localized primarily to the ventral motor horn of the gray matter, consistent with the polio-like clinical presentation. Neuronal apoptosis and diminished cell function were identified by TUNEL (terminal deoxynucleotidyl transferase–mediated BrdUTP nick end labeling) and choline acetyltransferase staining, respectively. Administration of hE16, a potently neutralizing humanized anti-WNV monoclonal antibody, 2 to 3 days after direct WNV infection of the spinal cord, significantly reduced paralysis and mortality. Additionally, a single injection of hE16 as late as 5 days after WNV inoculation of the sciatic nerve also prevented paralysis. Overall, these experiments establish that WNV-induced acute flaccid paralysis in hamsters is due to neuronal infection and injury in the lumbar spinal cord and that treatment with a therapeutic antibody prevents paralysis when administered after WNV infection of spinal cord neurons.
Keywords: West Nile virus, paralysis, therapy, hE16, antibody, spinal cord, neuron
Introduction
Symptoms of West Nile neurological disease (WNND) in human patients include acute flaccid paralysis, tremors, seizures, ataxia, limb atrophy, cognitive impairment, and autonomic dysfunction (Sejvar et al, 2003, 2006; Fratkin et al, 2004). Pathologic analyses suggest these clinical manifestations are likely caused by focal neurological lesions (Bhangoo et al, 2005; Cao et al, 2005). Focal infection of the gray matter of the anterior horn of the spinal cord is a proposed etiology for acute flaccid paralysis in West Nile virus (WNV)-infected patients (Sejvar et al, 2003; Fratkin et al, 2004; Bouffard et al, 2004; Davis et al, 2006), as patients show asymmetric weakness, areflexia, yet no sensory abnormalities. Postmortem histopathological analysis supports a poliomyelitis-like pathologic syndrome in human patients after WNV infection. One case report suggests direct viral infection of the spinal cord and nerve roots as a mechanism of the WNV-induced flaccid paralysis (Bouffard et al, 2004). Additional studies suggest that WNV can cause a poliomyelitis-like syndrome, because injury to spinal and sympathetic ganglia mirrored the damage to the spinal gray matter (Fratkin et al, 2004).
The development of a poliomyelitis-like syndrome in humans is supported by animal studies. The anterior horn of the spinal cord of monkeys injected intracranially with WNV shows injury and cell death (Manuelidis, 1956). Similarly, naturally infected horses develop multifocal poliomyelitis with the ventral and lateral horns of the gray matter of the thoracic and lumbar spinal one cord staining positive for WNV antigen. There were no other apparent lesions in the peripheral nerves or ganglia (Cantile et al, 2001). Analogously, in infected birds, the gray matter of the spinal cord contains pathologic lesions and WNV-infected cells (Steele et al, 2000). Hamsters infected with WNV also display neurological disease signs (Morrey et al, 2004), such as hind limb paralysis and front limb tremors that occur when viremia has concluded and after the virus is known to infect neurons in the central nervous system (CNS) (Morrey et al, 2006).
A humanized monoclonal antibody specific for the envelope protein (E), hE16, reduces mortality of hamsters and mice challenged subcutaneously (s.c.) with WNV, even when treatment is initiated after the virus has infected neurons in the brain (Morrey et al, 2006; Oliphant et al, 2005, 2006). However, morbidity is far more common than mortality with the human infection, so an animal model in which therapies can be evaluated for treatment of disease signs other than mortality also is important. As in the human infection (Sejvar et al, 2003, 2006; Fratkin et al, 2004), WNV-infected hamsters exhibit variable disease signs during the course of the infection (Morrey et al, 2004). Consequently, the evaluation of therapies for the treatment of neurological signs with variable presentation, such as acute flaccid paralysis, requires an animal model with more uniform expression of a disease phenotype. As such, we recently established a sciatic nerve injection model that demonstrates that WNV undergoes axonal transport, resulting in acute flaccid paralysis (Samuel et al, 2007).
In this study, we further develop the hamster model of acute flaccid paralysis to study the pathological steps in detail, and identify possible stages for therapeutic antibody administration. By inoculating WNV into peripheral neurons or the spinal cord, we investigate the kinetics and histopathology associated with paralysis. We find that limb paralysis in hamsters is directly associated with infection and injury of anterior horn motor neurons in the lumbar section of the spinal cord. Surprisingly, administration of a therapeutic neutralizing monoclonal antibody as late as 5 days after inoculation and infection of neurons prevented the development of the acute flaccid paralysis caused by WNV.
Results
Defining the pathogenesis of WNV paralysis
After subcutaneous infection of hamsters with WNV, disease signs occurred only after 6 days post infection (d.p.i.) when the virus is known to infect neurons in the CNS (Morrey et al, 2006) (data not shown). Some animals died early or late in the time course with no other apparent signs of disease. Others had single or combinations of disease signs, which occurred at somewhat unpredictable times after initial infection. Hind limb paralysis occurred in animals (<10%) between 8 and 18 days (data not shown).
To begin to identify the anatomic and pathologic basis for WNV-induced paralysis, using differential injection, we mapped the CNS region that correlated infection and paralysis. Mice or hamsters injected into the olfactory bulb or the caudal pontine reticular nucleus in the mid-brain did not display paralysis before death (Table 1). Animals injected in the cervical spinal cord developed both front and hind limb paralysis, which was rarely observed (<1%) in hundreds of animals infected subcutaneously. In contrast, injection of the thoracic or lumbar spinal cord caused hind limb, not front limb, paralysis in 94% or 100% of animals, respectively; this hind limb paralysis was similar to that observed with subcutaneous injection, only at a much greater frequency. Hind limb paralysis only occurred on the ipsilateral side, and not contralateral side, with respect to the side of spinal cord injection. Thus, it appeared that paralysis after spinal cord injection was attributable to local spinal cord infection. As sham-injected animals did not develop paralysis, the phenotype was due to viral infection and not iatrogenic complications of the surgery.
Table 1.
Mapped lesions causing paralysis of WNV-infected rodents to the spinal cord
| Species | Virus | Injection locationa | % Hind limb paralysis (pos/total) | % Front limb paralysisb (pos/total) |
|---|---|---|---|---|
| Hamster | WNV | C6 | 100% (2/2) | 100% (2/2) |
| Mouse | WNV | C6 | 100% (1/1) | 100% (1/1) |
| C6 summary | 100% (3/3) | 100% (3/3) | ||
| Hamster | WNV | T8 | 87% (7/8) | 0% (0/8) |
| Mouse | WNV | T8 | 100% (10/10) | 0% (0/10) |
| T9 summary | 94% (17/18) | 0% (0/18) | ||
| Hamster | WNV | L2–L4 | 100% (1/1) | 0% (0/1) |
| Mouse | WNV | L2–L4 | 100% (1/1) | 0% (0/1) |
| L2–L4 summary | 100% (2/2) | 0% (0/2) | ||
| Hamster | WNV | Olfactoryc | 0% (0/4) | 0% (0/4) |
| Hamster | WNV | Pontinec | 0% (0/3) | 0% (0/3) |
| Hamster | Sham | T8 | 0% (0/3) | 0% (0/3) |
| Mouse | Sham | T8 | 0% (0/1) | 0% (0/1) |
| Hamster | Sham | C6 | 0% (0/2) | 0% (0/2) |
| Mouse | Sham | C6 | 0% (0/1) | 0% (0/1) |
| Hamster | Sham | L2–L4 | 0% (0/2) | 0% (0/2) |
| Mouse | Sham | L2–L4 | 0% (0/2) | 0% (0/2) |
| Hamster | Sham | Olfactory | 0% (0/2) | 0% (0/2) |
| Hamster | Sham | Pontine | 0% (0/3) | 0% (0/3) |
Note. Mice or hamsters were injected precisely into specific anatomical locations of the CNS with 1 μl of virus. The proportions of animals with hind limb or front limb paralysis were determined.
C-cervical, T-thoracic, L-lumbar vertebrae, olfactory bulb, caudal pontine reticular nucleus.
Never observed in animals injected s.c. with WNV.
Animals injected into the olfactory bulb or pontine did not have paralysis but exhibited fatal neurological disease signs such as nonresponsiveness and hunched backs.
Histopathology in subcutaneously challenged paralyzed hamsters
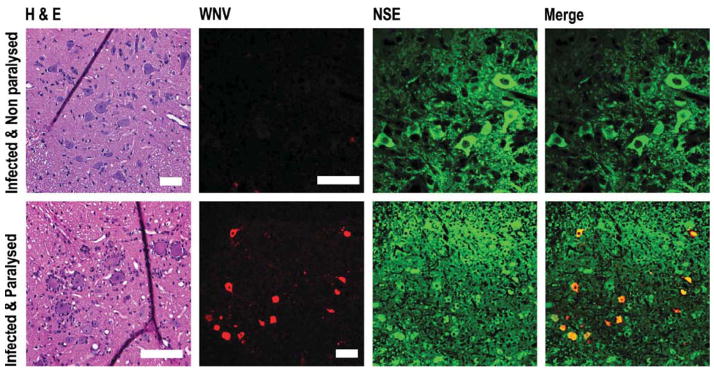
Hamsters that were injected subcutaneously with WNV and did not develop paralysis showed no histopathological changes or WNV antigen in their lumber spinal cord (Figure 1). In contrast, hamsters that developed paralysis after subcutaneous injection showed evidence of necrosis with satellitosis and chromatolysis of ventral horn neurons. Moreover, WNV antigen colocalized with ventral horn cells expressing neuron-specific enolase (NSE) only in paralyzed animals.
Figure 1.
Hematoxylineosin (H&E) staining, and confocal costaining, of WNV envelope and neuron-specific enolase (NSE) antigens of coronal spinal cord sections at L2–L4 vertebrae the first day of paralysis (9 d.p.i.) after subcutaneous injection with 105.1 50% cell culture infectious doses of WNV. The ventral horn was shown. Scale bar = 20 μm.
Histopathology of intra–spinal cord challenged paralyzed hamsters
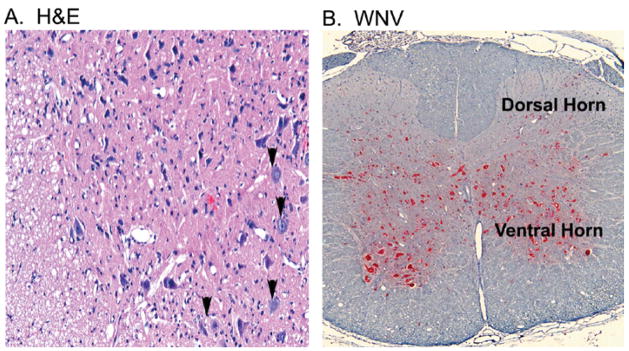
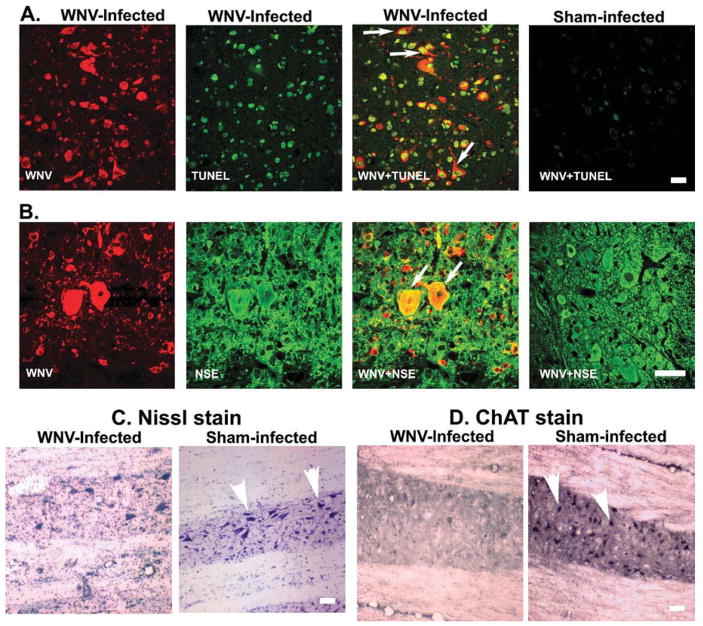
Although the pathologic lesions in the spinal cord of subcutaneously injected hamsters correlated with paralysis, this was a relatively infrequent occurrence. Because a model with greater incidence of paralysis could provide additional insight into pathogenesis, hamsters were injected directly into the spinal cord at the T8 level: this resulted in uniform hind-limb paralysis. Immediately after paralysis was observed, spinal cord tissues were harvested for pathologic analysis. Throughout the spinal cord, mild gliosis was observed in ventral gray matter, particularly in areas around the central canal (Figure 2A), and few hypereosinophilic, necrotic neurons were observed. In contrast to hamsters paralyzed after subcutaneous challenge, satellitosis or central chromatolysis was not apparent (Figure 1). WNV antigen staining was present in ventral horn of the gray matter, and to a lesser extent, in the dorsal horns (Figure 2B). Because WNV structural and non-structural proteins are reported to induce apoptosis (Yang et al, 2002; Chu and Ng, 2003; Samuel et al, 2006), confocal analysis was performed to define colocalization of ventral horn neurons with WNV antigen and TUNEL (terminal deoxynucleotidyltransferase-mediated BrdUTP nick end labeling) staining (Figure 3A and B). By scoring double-positive cells for NSE and TUNEL staining, we determined that ventral horn neurons underwent apoptosis at a high frequency (53% in spinal cord challenged animals compared to 0% of uninfected animals; p ≤ .001). By scoring double-positive cells for WNV antigen and NSE, we determined that approximately 20% of the ventral horn neurons were infected by WNV, which suggested that 33% of cells were damaged by bystander effects of WNV infection. Consistent with this, Nissl staining of viable neurons in infected spinal cords was markedly reduced in infected versus uninfected tissue (Figure 3C). Also, the choline acetylesterase (ChAT) was greatly diminished in infected tissue (Figure 3D).
Figure 2.
Coronal section of spinal cord at L2–L4 vertebrae from a hamster at the first day of paralysis (8 d.p.i.) challenged by injection of WNV (101.8 p.f.u.) in the cord at T8 verbetrae. (A) H&E staining on the first day of paralysis at 8 days after challenge. (B) Alkaline phosphatase detection of WNV env antigen (red) at 5 days after challenge.
Figure 3.
Coronal section of spinal cord at L2–L4 vertebrae from a hamster at the first day of paralysis (8 d.p.i.) when challenged by injection of WNV (101.8 p.f.u.) in the cord at T8 verbetrae. (A) Confocal TUNEL assay double-stained (arrow) with WNV envelope antigen. (B) WNV envelope antigen double-stained (arrow) with NSE at 8 days after challenge (coronal sections). (C) Nissl stain in longitudinal sections. (D) Immunoperoxidase staining of choline acetyltransferase (ChAT) (arrowheads) in longitudinal sections. Scale bar = 20 μm.
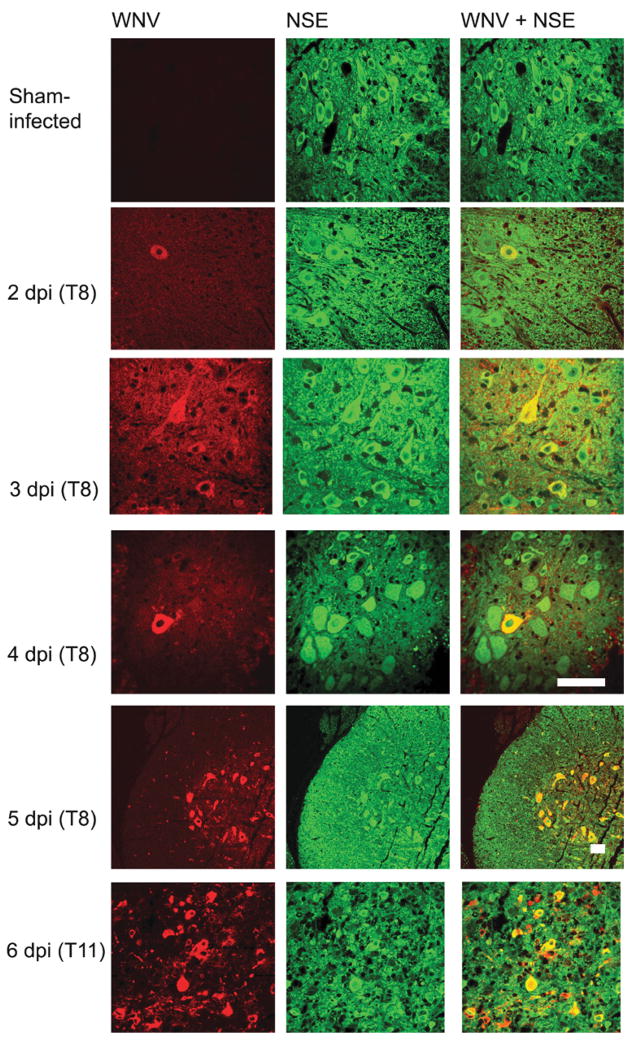
To determine the time point when WNV infection spread among neurons occurs after initial inoculation, a detailed temporal study was performed. Although no WNV-infected neurons were detected by immunofluorescence within hours after injection (data not shown), by day 2, rare infected neurons were observed at the site of spinal cord injection (T8) (Figure 4). By days 3 and 4, the frequency and intensity of WNV antigen in neurons was modestly increased. However, by day 5, the numbers of infected neurons rose abruptly, not only at the site of injection, but throughout the spinal cord. By day 6, neuronal infection was widespread, and paralysis became apparent in the animals.
Figure 4.
Temporal expression of WNV using confocal analysis of WNV envelope antigen in spinal cord neurons at 2, 3, 4, 5, and 6 days after WNV (101.8 p.f.u.) was injected into the spinal cord at T8 vertebra. T8 and T11 indicate the vertebrae from which the cord was obtained. Samples were coimmunostained for WNV and NSE. Scale bar = 20 μm.
hE16 treatment
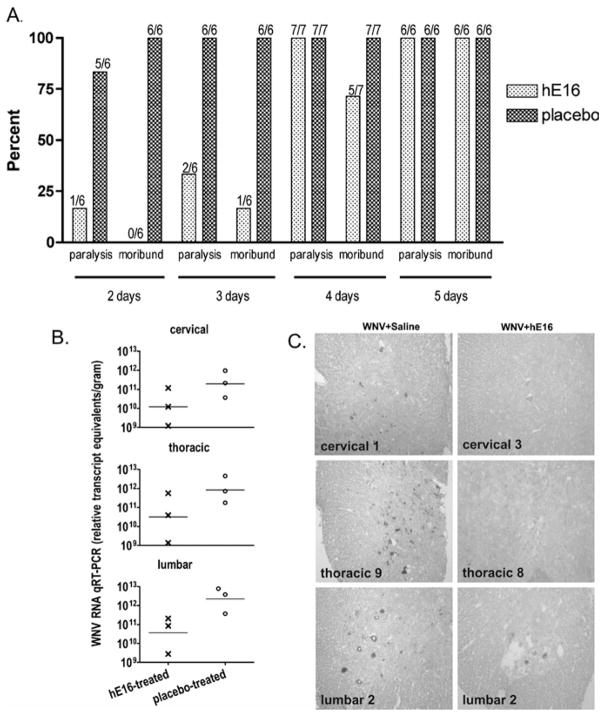
To expand the utility of hE16 to treat CNS infection, hamsters injected with WNV into the spinal cord at the T8 level were treated intraperitoneally on 2, 3, 4, or 5 d.p.i. with hE16 (32 mg/kg). Treatment at 2 d.p.i. reduced paralysis and mortality effectively (Figure 5A). Five of 6 placebo-treated animals developed paralysis, and the one placebo-treated animal that did not become paralyzed was moribund early at 6 d.p.i. All placebo-treated animals succumbed to disease after spinal cord infection with 100% mortality by day 10. One of the 6 hE16-treated animals became paralyzed, but not as seriously as the paralysis of placebo-treated animals. This animal developed only ipsilateral hind limb paralysis, which did not progress to bilateral paralysis or death as with the placebo-treated animals. Moreover, the paralysis of this hE16-treated animal improved over time (data not shown). In contrast to animals treated with placebo, all animals treated with hE16 at 2 d.p.i. survived infection.
Figure 5.
Effect of hE16 (32 mg/kg) administered i.p. after injection of 101.8 p.f.u. of WNV into the spinal cord at the T8 vertebra of hamsters. (A) Hind limb paralysis and mortality were monitored when administered with hE16 for 2, 3, 4, or 5 days. The numbers of affected/total animals are indicated in the figure above the bars. (B) WNV RNA in the cervical, thoracic, and lumbar spinal cord of 3 hamsters treated i.p. with hE16 (32 mg/kg) and 3 hamsters treated with placebo on day 3 when assayed at 6 days after injection of WNV. (C) Alkaline phosphatase staining of WNV antigen in spinal cord at cervical, thoracic, and lumbar vertebrae from hamsters 6 days after injection of WNV. Hamsters were treated i.p. with 0.1 ml of sterile physiological saline or with 32 mg/kg of hE16 administered 3 days after viral challenge. Scale bar = 20 μm.
Treatment with hE16 intraperitoneally (i.p.) at day 3 after spinal cord infection was also effective. As expected, in this group, all hamsters treated with placebo became paralyzed and moribund (Figure 5A). In contrast, of the 6 animals treated with hE16, only 2 became paralyzed and only 1 became moribund. By day 4 after infection of the spinal cord, the therapeutic benefit of hE16 was reduced; 2/7 hamsters were protected from lethal infection, whereas again, all placebo-treated animals died of WNV infection. One of the surviving hE16-treated hamsters treated at 4 days had partial paresis, and was able to move extremities but not support body weight. Thus, analogous to studies with subcutaneous infection (Morrey et al, 2007), treatment of direct spinal cord infection with hE16 had a therapeutic window, after which little benefit was observed.
To understand the cellular basis for antibody protection, WNV RNA and antigen levels were measured in the spinal cords of hamsters after virus injection at T8 and hE16 i.p. treatment 3 days later. At 6 days after challenge, WNV RNA in the spinal cord of hE16-treated animals was reduced in the cervical, thoracic, and lumbar cords compared to animals receiving the placebo treatment (Figure 5B). Similarly, WNV-antigen staining of neurons was markedly reduced in hE16-treated hamsters (Figure 5C). Although antibody treatment 3 days after viral challenge in the spinal cord significantly reduced paralysis and mortality (Figure 5A), it did not completely eliminate infected cells (Figure 5C).
To confirm that hE16 was acting locally in the spinal cord, we measured its concentration in spinal cord homogenates two days after i.p. treatment. As expected, the levels of hE16 were below the levels of detection in the spinal cords of 2 placebo-treated animals (<0.03 hE16 μg per g tissue). The concentrations of hE16 in the spinal cords of 5 hE16-treated animals were 0.1, 0.46, 0.16, 0.27, 0.21 μg of hE16 per gram of spinal cord tissue. This amount exceeds the neutralizing potential of the antibody, which has been measured at approximately 0.01 μg/ml in cell culture (Oliphant et al, 2005).
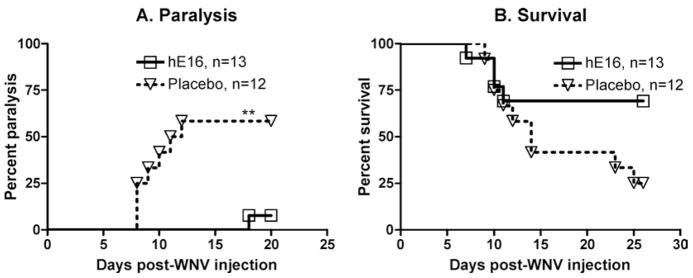
To verify that hE16 could reduce paralysis after spinal cord neurons were infected and without having to disrupt the lamina for direct spinal cord injection, hE16 was administered after intra–sciatic nerve injection and axonal spread of the virus into the spinal cord (Samuel et al, 2007). We expanded this earlier study from hE16 treatment at 1 day to 5 days after intra–sciatic nerve injection when the virus had infected neurons in the spinal cord. Only 1/13 of the hE16-treated animals treated on day 5 became paralyzed, whereas 9/12 animals treated with placebo became paralyzed (Figure 6A) (p ≤ .01). The 1 animal treated that became paralyzed recovered the next day. The day 5 treatment showed a trend toward improved survival (Figure 6D), but the difference did not reach statistical significance (p = .07). The efficacy of hE16 when administered at day 5 is important because neurons in the spinal cord at the L2–L4 levels were infected as evidenced by strong WNV antigen staining at day 5 (data not shown).
Figure 6.
Effect of hE16 on paralysis and survival of hamsters injected in the sciatic nerve with 1 μL of 101.8 p.f.u. of WNV. The sciatic nerve was injected above (proximal) two constricting sutures and then cut between the sutures. The hamsters were treated i.p. on day 5 after viral injection with 32 mg/kg of hE16. Hind limb paralysis (A) and survival (B) were monitored daily through 30 days. The number of hamsters in each group is stated in the figure. **P ≤ .01, compared to placebo using logrank analysis.
Discussion
Acute flaccid polio-like paralysis occurs after West Nile virus (WNV) infection in a subset of cases in animals and humans. Using existing small animal models in rodents, the pathogenesis of paralysis has been difficult to study because the phenotype occurs at a frequency of ~10% or less after subcutaneous inoculation. Using direct neuron inoculation models, we observed acute flaccid paralysis at increased frequencies and mapped the lesions responsible for hind limb paralysis to the anterior horn motor neurons of the lumbar spinal cord. Peripheral administration of a neutralizing humanized anti-WNV monoclonal antibody several days after direct spinal cord or sciatic nerve infection significantly reduced paralysis and mortality.
As hind limb paralysis is a neurological sign that is clinically relevant in humans, we investigated its pathogenesis in detail. The neuropathologic lesion was identified after direct stereotactic injection of WNV into specific locations of the CNS. Infection of the olfactory bulb or pons/midbrain did not cause paralysis, and infection of the cervical spinal cord caused atypical paralysis of both hind limbs and fore-limbs. Such quadriplegia or quadraparesis has been rarely observed (<1%) in hundreds of animals infected via the subcutaneous route (data not shown). In contrast, WNV injection into the lumbar region caused hind limb paralysis that was typical of subcutaneous inoculation, but occurring in 100% of the animals. As the lumbar spinal cord innervates the hind limbs, we injected the spinal cord at the T8 vertebral level to avoid direct needle-tract injury to the lumbar cord and allow infection to occur after neuronal spread. Animals injected at T8 spinal cord uniformly developed hind limb paralysis; this was not caused by peripheral virus spread, as ipsilateral paralysis was exclusively observed.
The synchronized quality of the paralysis provided the opportunity to investigate presymptomatic infection and the very earliest stages of symptomatic disease. The injury from the needle injection was not responsible for the paralysis as sham- and WNV-infected animals had the same local histopathology at the needle injection site. Moreover, none of the sham-injected animals developed paralysis. At areas in the spinal cord distal from the injection site, particularly in the lumbar spinal cord, we observed infiltration of lymphocytes, perineuronal gliosis, and individual necrotic neurons. The more extensive gliosis and inflammation that has been reported in human or animal autopsy spinal cord samples after peripheral inoculation (Fratkin et al, 2004) may reflect the time at which tissues are sampled after the onset of paralysis. In our study, tissues were harvested at a very early time, within 1 day of the development of signs of paralysis.
The observations that WNV directly causes neuronal death, in part by an apoptotic mechanism (Yang et al, 2002; Shrestha et al, 2003; Oh et al, 2006), supports our finding that ~53% of ventral horn neurons in paralyzed animals costained with TUNEL and showed impaired metabolic activity. Thus, as has been observed with cultured cells in vitro (Chu and Ng, 2003; Samuel et al, 2006; Oh et al, 2006), WNV infection of anterior horn spinal cord neurons in vivo is highly cytopathic and likely linked directly to impaired function and clinical phenotype.
Electromyelography and postmortem histological analysis of WNV patients have suggested that the acute flaccid paralysis syndrome is caused by a pathology resembling poliomyelitis (Sejvar et al, 2003; Fratkin et al, 2004; Bhangoo et al, 2005; Bouffard et al, 2004). In humans, acute flaccid paralysis is often asymmetric, and associated with infection of anterior horn motor neurons of the gray matter in the spinal cord (Fratkin et al, 2004). The paralysis in the hamsters after sciatic nerve or thoracic spinal cord inoculation was initially asymmetrical, and sometimes progressed to bilateral paralysis. Immunohistochemical staining for WNV antigen in the spinal cord at 5 days after injection and just before onset of paralysis showed staining predominantly in the ventral horn of the gray matter. Thus, the poliomyelitis-like syndrome in hamsters appears to be related to infection and injury of anterior horn motor neurons in the lumbar spinal cord.
This study provided additional proof that hE16 can improve neurological disease. Prior studies showed that hE16 treatment improved survival and virological outcome of mice and hamsters infected subcutaneously with WNV when administered at a time when WNV RNA, antigen, and infectious virus were present in the brain (Morrey et al, 2006, 2007). Because the WNV-induced lesions of the brain responsible for the death of the animal had not been conclusively identified, the survival benefit afforded by hE16 in these prior studies, in theory, could have been affected by the improvement in systemic disease and not neurological disease. In this study, hind limb paralysis was caused by direct injection of the virus into the spinal cord or the sciatic nerve, because paralysis occurred on the ipsilateral side of injection. This is in contrast to a predicted outcome of peripheral spread of the virus where either contralateral or ipsilateral paralysis might occur. Therefore, our data show that the therapeutic benefit of hE16 was attributed to improvement of the neurological disease in the spinal cord. Our data also extend the limit of the therapeutic benefit observed by a related study, in which paralysis was prevented by systemic hE16 administration only 1 day after sciatic nerve injection of WNV (Samuel et al, 2007). Indeed, we show convincingly that even 5 days after sciatic nerve inoculation, systemic administration of hE16 improved neurological disease to prevent acute flaccid limb paralysis.
Using the spinal cord inoculation and paralysis model, we analyzed day-by-day progression of WNV pathogenesis. Prior to day 5, small numbers of neurons were infected after direct spinal cord or sciatic nerve inoculation. By day 5, significant spreading throughout the spinal cord became apparent. The kinetics of spinal cord spread are consistent with axonal transport, because studies in compartmentalized chambers in vitro suggest that 3 to 4 day transport times are necessary for retrograde or anterograde neuronal spread of WNV infection over a distance of several centimeters (Samuel et al, 2007). In a previous study (Samuel et al, 2007), we estimated that 20% of the neurons were stained for WNV antigen in a paralyzed animal. Although exact quantifications were not made in this study, it appeared that <1% of the neurons were stained for WNV at day 4 after viral challenge, which was the latest time-point for which hE16 was efficacious. Beyond this time-point when a higher percentage of neurons were stained for WNV, hE16 was not effcacious. Thus, the efficacy of systemically administered hE16 may be limited to when CNS viral loads are relatively low. It remains possible that higher or targeted dosing of hE16 could improve efficacy. Indeed, direct administration of hE16 into the mid-brain increased the therapeutic window of WNV-challenged hamsters (Morrey et al, 2007).
In summary, these studies characterize the pathogenic basis of acute flaccid paralysis in hamsters, and point to an important role for direct infection and injury to anterior horn motor neurons in the lumbar region of the spinal cord. They also establish that in hamsters, hE16 can be used to treat WNV neurological disease if administered after spinal cord neurons are infected, but before high viral load occurs. Clinical studies are needed to determine whether neutralizing antibody therapy will also reduce the severity or frequency of acute flaccid paralysis in humans after WNV infection.
Materials and methods
Animals and virus
Adult female Syrian golden hamsters and C57BL/6 mice were obtained from Charles River Laboratories. Animals were held at least one week at the USU facility before initiating the experiment. The NY99 WNV strain (Lanciotti et al, 2002; Lanciotti and Kerst, 2001) was propagated in MA-104 cells and diluted in minimal essential medium (MEM) immediately prior to injection. In most studies, hamsters or mice were injected with 101.8plaque-forming units (p.f.u.) of WNV into the spinal cord or sciatic nerve in a volume of 1 μl. A challenge of 101.8 p.f.u. was used to determine the incidence of paralysis in hamsters injected into the sciatic nerve that was either transected or not transected. Hamsters were also injected subcutaneously at the femoral triangle near the leg and abdomen. Paralysis was identified when the animal dragged a hind limb while walking forward. Animals were considered moribund if they did not respond to touch by the handler, upon which they were euthanized. Animal use was in compliance with the Utah State University Institutional Animal Care and Use Committee in an AAALAC-accredited facility.
Humanized antibodies
hE16, the humanized monoclonal antibody (immnunoglobulin G1 [IgG1]) specific for domain III of the WNV E protein (Oliphant et al, 2005), was obtained from MacroGenics (Rockville, MD) as described previously (Morrey et al, 2006, 2007). An isotype-matched antibody was used in a previous hE16 therapeutic study (Morrey et al, 2006) to establish that it has no therapeutic value as does the hE16.
Viral assay
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was used to determine the levels of WNV-specific RNA (Morrey et al, 2006; Julander et al, 2005). Fresh tissues were homogenized in Trizol RNA purification reagent (Sigma-Aldrich Chemical). Primer-pairs and qRT-PCR algorithms for measuring WNV RNA and mouse glyceraldehyde phosphate de-hydrogenase (mGAPDH) from tissues have been described previously (Julander et al, 2006). A standard curve, generated from WNV RNA transcribed from PCR product cloned into a plasmid, was used. The data were reported as relative WNV transcript equivalents/gram of tissues.
Dorsal laminectomy
Laminectomy was performed on animals (Ellegala et al, 1996) at the 8th thoracic or 2nd to 4th lumbar vertebrae (T8, L2–L4, respectively). Animals were anesthetized with ketamine and isoflurane inhalation anesthesia (2% isoflurane, 1 L O2/min). After shearing the hair, disinfecting the surgical site, and incising the skin, the subcutaneous tissues were retracted laterally and the paraspinal muscle was incised immediately lateral to the spinous process. Muscle adhering to the desired vertebra was scraped clear until the underlying bone was revealed. Under 5× magnification, the intact dura mater was exposed by removing the spinal process and lamina using a Mico Friedman Rongeur. To prevent respiratory depression, 1% lidocaine was applied directly to the spinal cord with the excess wiped away with a cotton swab. An initial dural opening was made with a 30-gauge needle held at a 20-degree angle from the horizontal axis of the spine. Tension was provided to hold the dura open by suturing the dural flaps to the adjacent muscle. One microliter of virus (101.8 p.f.u.) was injected into one side of the spinal cord with a Hamilton syringe held at the arm of stereotactic apparatus. The needle was kept in situ for 3 min after injection to minimize leakage of the inoculum. Control animals were sham-infected with viral diluent (minimal essential medium). The dura was sutured with interrupted 4-0 absorbable suture with a tapered needle (Ethicon). The paraspinal muscles were sutured with 3-0 absorbable sutures, and the skin was closed with wound clips. Buprenorphine analgesia was administered. The animals were kept on a heating pad until they regained consciousness.
Stereotaxic injection of WNV into the pontine or olfactory bulb
The procedure used for injection of the caudal pon-tine reticular nucleus (PnC) of hamsters has been described (Mori et al, 2002). For injection of the olfactory bulb in mice, the following stereotactic coordinates in the parietal cortex were used: from the bregma, anterior-posterior: −1.5 mm; mediolateral: ±1.5 mm; dorsoventral: + 0.75 mm.
Intra–sciatic nerve injection and transection
This inoculation procedure was based on earlier feline and rat studies (Ma, 2001; Branner et al, 2001). Adult animals (6 to 7 weeks old) were anesthetized with ketamine and maintained by isoflurane inhalation. The hair on the back of the leg was sheared and a skin incision was made under aseptic conditions. The biceps femoris muscles were separated, and retracted to expose the sciatic nerve. The sciatic nerve was injected 1 cm deep at ~30-degree angle with 1 μl of WNV using a Hamilton syringe and a 30-gauge needle. In the case of transection, the nerve was cut between two constricting sutures close to each other. The incision was closed with suture 4-0 non-absorbable. Animals were monitored for hind limb paralysis and lethality for 30 days.
Humanized monoclonal antibody detection
Thoracic and lumbar spinal cords were weighed and homogenized in MEM at 1:1. Dilutions of homogenized tissues were incubated in wells coated with 100 ng goat anti-human IgG (Fc-specific) (Jack-son ImmunoResearch Laboratories). After incubation and washing, bound hE16 was detected using alkaline phosphatase–conjugated goat anti-human Kappa (1/10,000 dilution) (Jackson) and then developed using the fluorogenic substrate 4-methylumbelliferyl phosphate (Sigma). Plates were read using a fluorometric plate reader and concentrations calculated from a standard curve using a nonlinear four-parameter fit.
Histochemistry
Immunohistochemistry for detection of WNV and neuron-specific enolase (NSE) antigens or apoptosis by the terminal deoxynucleotidyl transferase–mediated BrdUTP nick end labeling using TUNEL assay kit (Molecular Probes, Eugene, OR) was performed on paraformaldehyde-fixed spinal cord tissues from hamsters as described (Morrey et al, 2006). Deparafinized sections were incubated in (1:10 dilution) DakoCytomation Target Retrieval Solution (DakoCytomation, Carpinteria, CA) in distilled water and boiled in a microwave or a Decloaking chamber (Bio-care Medical, Walnut Creek CA). WNV antigen staining was performed with 7H2 anti-WNV mouse monoclonal antibody (mAb) (BioReliance, Invitrogen Bioservices, Rockville, MD), and Alexa-fluor 568 goat anti-mouse IgG secondary antibody. For double-staining with NSE, rabbit polyclonal anti-neuron specific enolase (NSE) (Chemicon, Temecula, CA) and Alexafluor 488 goat anti-rabbit IgG antibody (Molecular Probes, Eugene, OR) were used. Stained slides were visualized using a Nikon Eclipse TE300 microscope (Nikon) attached with Lambda DG4 (Sutter Instrument Company, Novato, CA) and a BioRad MRC 1024 confocal microscope (BioRad, Hercules, CA). Captured images were processed using Confocal assistant software from BioRad.
For alkaline phosphatase localization of WNV envelope antigen, tissue sections were processed similar to the immunofluorescence protocol up through the antigen retrieval step. Using the Ventana NexES IHC Full System (N750-NXIHC-FS; Ventana Medical Systems, Tucson, AR), the sections were subsequently stained with 7H2 MAb for WNV envelope and detected using Ventana Basic AEC Detection kit (Ventana Medical Systems) according to the manufacurer’s instructions and counter-stained with hematoxylin.
Cryostat sections (20 μm), blocked with goat serum, were stained for choline acetylesterase (ChAT) (Darman et al, 2004] with polyclonal rabbit anti-choline acetyltransferase antibody (Chemicon AB5042; 1:2000) at 4°C overnight and avidinbiotin-peroxidase complex detection system using biotinylated goat anti-rabbit IgG (1:200; BA-1000, Vector Lab) and ABC (1:100; Vectastain ABC kit, Vector Lab). Chromogen reaction used 0.3% hydrogen peroxide and 3,3′-diaminobenzidine (DAB) with 0.15 mg/ml nickel-ammonium sulfate. Each step was washed with phosphate-buffered saline (PBS).
For the Nissl stain, cryostat sections were incubated with cresyl violet solution (FD Neuro Technologies) for 5 to 10 min, rinsed briefly in distilled water, then 95% ethanol (0.1% glacial acetic acid), followed by 100% ethanol, xylene, and mounting medium (Permount, Fisher Scientific).
Statistical analysis
Survival data were analyzed using the Wilcoxon logrank survival analysis, and other data were evaluated by one-way analysis of variance (JMP Software; The Statistical Discovery Software, SAS Institute).
Acknowledgments
Funding. NIH NO1-AI-15435 (J.D.M.) Virology Branch, NIAID, NIH; 1-U54 AI06357-01 Rocky Mountain Regional Centers of Excellence (J.D.M.); U01-AI061373 (M.S.D.), and NIAID, NIH, DPHS Contract No. HHSN266200600013C (MacroGenics).
Footnotes
Conflict of interest. V.S., A.L.O., H.W., J.O.H., R.T.S. do not have any conflict of interest. S.K., S.J., and J.L.N. are employees of MacroGenics Inc., and the company has rights to commercialize the monoclonal antibody described in this article. M.S.D. and J.D.M. have consulting agreements with MacroGenics Inc.
References
- Bhangoo S, Chua R, Hammond C, Kimmel Z, Semenov I, Videnovic A, Kessler J, Borsody M. Focal neurological injury caused by West Nile virus infection may occur independent of patient age and premorbid health. J Neurol Sci. 2005;234:93–98. doi: 10.1016/j.jns.2005.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffard JP, Riudavets MA, Holman R, Rushing EJ. Neuropathology of the brain and spinal cord in human West Nile virus infection. Clin Neuropathol. 2004;23:59–61. [PubMed] [Google Scholar]
- Branner A, Stein RB, Normann RA. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J Neurophysiol. 2001;85:1585–1594. doi: 10.1152/jn.2001.85.4.1585. [DOI] [PubMed] [Google Scholar]
- Cantile C, Del Piero F, Di Guardo G, Arispici M. Pathologic and immunohistochemical findings in naturally occuring West Nile virus infection in horses. Vet Pathol. 2001;38:414–421. doi: 10.1354/vp.38-4-414. [DOI] [PubMed] [Google Scholar]
- Cao NJ, Ranganathan C, Kupsky WJ, Li J. Recovery and prognosticators of paralysis in West Nile virus infection. J Neurol Sci. 2005;236:73–80. doi: 10.1016/j.jns.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Chu JJ, Ng ML. The mechanism of cell death during West Nile virus infection is dependent on initial infectious dose. J Gen Virol. 2003;84:3305–3314. doi: 10.1099/vir.0.19447-0. [DOI] [PubMed] [Google Scholar]
- Darman J, Backovic S, Dike S, Maragakis NJ, Krishnan C, Rothstein JD, Irani DN, Kerr DA. Viral-induced spinal motor neuron death is non-cell-autonomous and involves glutamate excitotoxicity. J Neurosci. 2004;24:7566–7575. doi: 10.1523/JNEUROSCI.2002-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- Ellegala DB, Tassone JC, Avellino AM, Pekow CA, Cunningham ML, Kliot M. Dorsal laminectomy in the adult mouse: a model for nervous system research. Lab Anim Sci. 1996;46:86–89. [PubMed] [Google Scholar]
- Fratkin JD, Leis AA, Stokic DS, Slavinski SA, Geiss RW. Spinal cord neuropathology in human West Nile virus infection. Arch Pathol Lab Med. 2004;128:533–537. doi: 10.5858/2004-128-533-SCNIHW. [DOI] [PubMed] [Google Scholar]
- Julander JG, Winger QA, Olsen AL, Day CW, Sidwell RW, Morrey JD. Treatment of West Nile virus-infected mice with reactive immumoglobulin reduces fetal titers and increases dam survival. Antiviral Res. 2005;65:79–85. doi: 10.1016/j.antiviral.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Julander JG, Winger QA, Rickords LF, Shi PY, Tilgner M, Gavin HM, Sidwell RW, Morrey JD. West Nile virus infection of the placenta. Virology. 2006;347:175–182. doi: 10.1016/j.virol.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Ebel GD, Deubel V, Kerst AJ, Murri S, Meyer R, Bowen M, McKinney N, Morrill WE, Crabtree MB, Kramer LD, Roehrig JT. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298:96–105. doi: 10.1006/viro.2002.1449. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J Clin Microbiol. 2001;39:4506–4513. doi: 10.1128/JCM.39.12.4506-4513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP. The expression of bradykinin B(1) receptors on primary sensory neurones that give rise to small caliber sciatic nerve fibres in rats. Neuroscience. 2001;107:665–673. doi: 10.1016/s0306-4522(01)00387-6. [DOI] [PubMed] [Google Scholar]
- Manuelidis EE. Neuropathology of experimental West Nile virus infection in monkeys. J Neuropathol Exp Neurol. 1956;15:448–460. doi: 10.1097/00005072-195610000-00007. [DOI] [PubMed] [Google Scholar]
- Mori I, Liu B, Hossain MJ, Takakuwa H, Daikoku T, Nishiyama Y, Naiki H, Matsumoto K, Yokochi T, Kimura Y. Successful protection by amantadine hydrochloride against lethal encephalitis caused by a highly neurovirulent recombinant influenza A virus in mice. Virology. 2002;303:287–296. doi: 10.1006/viro.2002.1601. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Olsen AL, Sidwell RW, Cheney CD, Blatt LM. Modeling hamsters for evaluating West Nile virus therapies. Antiviral Res. 2004;63:41–50. doi: 10.1016/j.antiviral.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Roper GY, Wang HC, Baldwin TJ, Koenig S, Johnson S, Nordstrom JL, Diamond MS. Humanized monoclonal antibody against West Nile virus E protein administered after neuronal infection protects against lethal encephalitis in hamsters. J Infect Dis. 2006;194:1300–1308. doi: 10.1086/508293. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Wang H, Julander JG, Hall JO, Li H, Nordstrom JL, Koenig S, Johnson S, Diamond MS. Defining the limit of effective treatment for West Nile virus neurological infection with a humanized neutralizing monoclonal antibody. Antimicrob Agents Chemother. 2007;51:2396–2402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W, Yang MR, Lee EW, Park KM, Pyo S, Yang JS, Lee HW, Song J. Jab1 mediates cytoplasmic localization and degradation of west nile virus capsid protein. J Biol Chem. 2006;281:30166–30174. doi: 10.1074/jbc.M602651200. [DOI] [PubMed] [Google Scholar]
- Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. Antibody recognition and neutralization determinants on domains I and II of West Nile virus envelope protein. J Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Morrey JD, Diamond MS. Caspase-3 dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J Virol. 2006;81:2614–2623. doi: 10.1128/JVI.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Wang H, Siddharthan V, Morrey JD, Diamond MS. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc Natl Acad Sci U S A. 2007;104:17140–17145. doi: 10.1073/pnas.0705837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ, Bode AV, Marfin AA, Campbell GL, Pape J, Bigger-staff BJ, Petersen LR. West Nile Virus-associated flaccid paralysis outcome. Emerg Infect Dis. 2006;12:514–516. doi: 10.3201/eid1203.050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ, Leis AA, Stokic DS, Van Gerpen JA, Marfin AA, Webb R, Haddad MB, Tierney BC, Slavinski SA, Polk JL, Dostrow V, Winkelmann M, Petersen LR. Acute flaccid paralysis and West Nile virus infection. Emerg Infect Dis. 2003;9:788–793. doi: 10.3201/eid0907.030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele KE, Linn MJ, Schoepp RJ, Komar N, Geisbert TW, Manduca RM, Calle PP, Raphael BL, Clippinger TL, Larsen T, Smith J, Lanciotti RS, Panella NA, McNamara TS. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet Pathol. 2000;37:208–224. doi: 10.1354/vp.37-3-208. [DOI] [PubMed] [Google Scholar]
- Yang JS, Ramanathan MP, Muthumani K, Choo AY, Jin SH, Yu QC, Hwang DS, Choo DK, Lee MD, Dang K, Tang W, Kim JJ, Weiner DB. Induction of inflammation by West Nile virus Capsid through the caspase-9 apoptotic pathway. Emerg Infect Dis. 2002;8:1379–1384. doi: 10.3201/eid0812.020224. [DOI] [PMC free article] [PubMed] [Google Scholar]