Abstract
Monodispersed cobalt nanoparticles (NPs) with controllable size (8–14 nm) have been synthesized using thermal decomposition of dicobaltoctacarbonyl in organic solvent. The as-synthesized high magnetic moment (125 emu/g) Co NPs are dispersible in various organic solvents, and can be easily transferred into aqueous phase by surface modification using phospholipids. However, the modified hydrophilic Co NPs are not stable as they are quickly oxidized, agglomerated in buffer. Co NPs are stabilized by coating the MFe2O4 (M = Fe, Mn) ferrite shell. Core/shell structured bimagnetic Co/MFe2O4 nanocomposites are prepared with tunable shell thickness (1–5 nm). The Co/MFe2O4 nanocomposites retain the high magnetic moment density from the Co core, while gaining chemical and magnetic stability from the ferrite shell. Comparing to Co NPs, the nanocomposites show much enhanced stability in buffer solution at elevated temperatures, making them promising for biomedical applications.
Keywords: Nanocomposites, Cobalt nanoparticles, Core/shell structure, Ferrite, Biomedical applications, Synthesis, Surface modification
1. Introduction
The fabrication of nanomaterials with high magnetic moment density has attracted increasing research interests in the past due to their potential biomedical applications such as bio-separation, bio-sensing, magnetic imaging, drug delivery and magnetic fluid hyperthermia [1]. Cobalt is a class of ferromagnetic material with high magnetic moment density (160 emu/g or 1422 emu/cc) and is magnetically soft. However, cobalt-related high moment nanomaterials are chemically and magnetically unstable in ambient conditions due to their rapid oxidation [2]. Many methods have been developed to synthesize monodispersed superparamagnetic Co nanoparticles (NPs) in the size range below 20 nm with high moment density [3–8]. But to produce stable aqueous dispersions of Co NPs for biomedical applications has been very challenging. Attempts to stabilize Co NPs via Au, Pt, CdSe and SiO2 coating often yield polydispersed particles with non-uniform coatings and dramatic reduction of magnetic moment [9–12].
We recently modified the thermal decomposition process of dicobaltoctacarbonyl (Co2(CO)8) based on a published recipe [3], and synthesized monodisperse Co NPs with controllable size of 8–14 nm [13]. The as prepared Co NPs are superparamagnetic with moment density achieving ~125 emu/g Co. To stabilize these NPs, we developed a facile synthesis to coat the Co NPs with a layer of MFe2O4 (M = Fe, Mn) ferrite. The Co/MFe2O4 core/shell nanocomposites show much enhanced chemical and magnetic stability. These Co/MFe2O4 composites are promising candidates for biomedical applications.
2. Experimental section
All syntheses were carried out using commercially available reagents. Oleic acid (OA, 90%), oleylamine (O-NH2, 70%), dioctylamine (DOA, 98%), benzyl ether (99%), 1,2,3,4-tetrahydronaphthalene (tetralin, 97%), manganese(II) acetylacetonate (Mn(acac)2, 98%) and 1,2-hexadecanediol (90%) were purchased from Sigma Aldrich. Iron(III) acetylacetonate (Fe(acac)3, 99%), dicobaltoctacarbonyl (Co2(CO)8, stabilized with 1–5% hexane) was purchased from STREM chemicals. PEGylated phospholipids DSPE-PEG(2000)carboxylic acid were purchased from Avanti Polar Lipids.
In a typical synthesis of 10 nm Co NPs, a mixture containing 0.35 mL of OA, 0.5 mL of DOA, and 18 mL of tetralin was heated at 110°C for 30 min under nitrogen (N2) atmosphere. After the mixture was cooled down to room temperature (r.t.), 0.54 g of Co2(CO)8 was quickly added into the solution. The reaction flask was rapidly re-sealed and purged with fast N2 flow for 5 min at r.t. The solution was re-heated at 100°C under a constant N2 flow (~20mL/min) for 30 min. The reaction system was then protected with a N2 blanket and temperature was ramped to 208°C at 15°C/min. The reaction mixture was heated at 208°C for 30 min before the heating source was removed and the mixture was cooled down to r.t. A black dispersion of Co NPs was precipitated by adding 30 mL of anhydrous ethanol. The product was collected using centrifuge (8500 rpm, 8 min), and re-dispersed in 10 mL hexane. The dispersion was centrifuged to remove any undispersed species and the product was precipitated out by adding 20 ml of anhydrous ethanol and re-dispersed in 10 ml hexane and stored under N2. The Co NPs could also be dispersed in chloroform and toluene.
By carefully controlling the amount of OA used in the synthesis, we could tune the size of the Co NPs. For example, 0.3 mL of OA gave 12 nm, 0.4 mL of OA yielded 8 nm Co NPs. In this way, monodisperse Co NPs with tunable size of 8–14 nm were synthesized.
The ferrite coating over Co is similar to the seed mediate process reported in the synthesis of MFe2O4 NPs [14]. In a typical synthesis of 10 nm/3 nm Co/Fe3O4 nanocomposites, 1 mmol of Fe(acac)3, 5 mmol of 1,2-hexadecanediol, 1 mmol of OA, 1 mmol of O-NH2 and 20 mL of benzyl ether were was mixed and magnetically stirred under N2. The mixture was heated to 150°C for 30 min. A seed dispersion containing 60 mg of 10 nm Co NPs in 3 mL of hexane was injected to the mixture at 150°C and kept at that temperature for another 30 min to remove the hexane completely. The reaction solution was then raised to 200°C and kept at that temperature for 1 h followed by ramping up to 300°C at 10°C/min. The solution was refluxed for 30 min before it was cooled down to r.t. The purification process was similar to what was described in the synthesis of Co NPs. The Co/Fe3O4 nanocomposites could be dispersed in hexane, chloroform and toluene. Co/MnFe2O4 nanocomposites were synthesized in the same way except that 0.5 mmol Mn(acac)2 was added as the Mn-source. By tuning the precursor/seed ratio, core/shell composites with different shell thickness were obtained. For example, 10 nm/5 nm Co/Fe3O4 nanocomposites were from 50 mg, instead of 60 mg, of Co NP seeds were used.
The as-synthesized Co NPs and Co/MFe2O4 nanocomposites are transferred from organic phase to aqueous phase via ligand addition of PEGylated phospholipids to the NPs. The addition forms a double layer structure over the surface of the NPs and the original coating is intact [15–17]. Taking DSPE-PEG(2000)carboxylic acid as an example, 1 mL of NP chloroform dispersion containing 3 mg of NPs was added dropwise into 10 mg of the phospholipids in 1 mL of chloroform. The mixture was shaken for 3 h at r.t. before drying under N2. 2 mL of distilled water or PBS buffer was added and the mixture was shaken to form aqueous dispersion. A small portion of agglomerations in the product was excluded by filtering through a 0.2 μm syringe filter. Free phospholipids were removed by dialysis against water at r.t. for 24–48 h.
Stability of Co NPs and Co/MFe2O4 nanocomposites were examined in air and in aqueous solutions. Stability tests in air were carried out by evaporating NP hexane dispersion on glass substrates (5 mm×5 mm) at r.t. The samples were then baked in air at 70°C and taken out at time intervals for r.t. magnetic measurements. Stability tests in aqueous phase were carried out by incubating NP PBS dispersion (~ 1mg/mL) in air at 70°C in a water bath. Aliquots of the solution were taken out at time intervals and were dried on glass substrates for r.t. magnetic measurements.
Samples for transmission electron microscopy (TEM) analyses were prepared by drying the NP hexane dispersion on an amorphous carbon coated copper grid. Particles were imaged using a Philips EM 420 (120 kV). X-ray powder diffraction (XRD) patterns of the particle assemblies were collected on a Bruker AXS D8-Advanced diffractometer with Cu Ka radiation (λ = 1.5418 Å). Magnetic studies were carried out using a Lakeshore 7404 high sensitivity vibrating sample magnetometer (VSM) with fields up to 1.5 tesla.
3. Results and discussion
During the synthesis of Co NPs, the solution color was dark red upon the addition of Co2(CO)8 precursor, and gradually changed to dark yellow during the temperature increase to 100°C, and further to dark green, indicating partial decomposition of Co2(CO)8 and the formation of Co cluster complex [18,19]. Upon further heating, the reaction mixture became black around 130~140°C, indicating the formation of Co cluster. After collecting the black product of Co NPs using centrifugation, a light-purple supernatant was left behind. The supernatant was deep colored as less amount of OA was used to synthesize larger Co NPs. This result is consistent with what has been observed in OA ligand effect on Co NP synthesis [19]. However, our synthesis does not need injection, nor does it require the size selection process, to prepare monodisperse NPs.
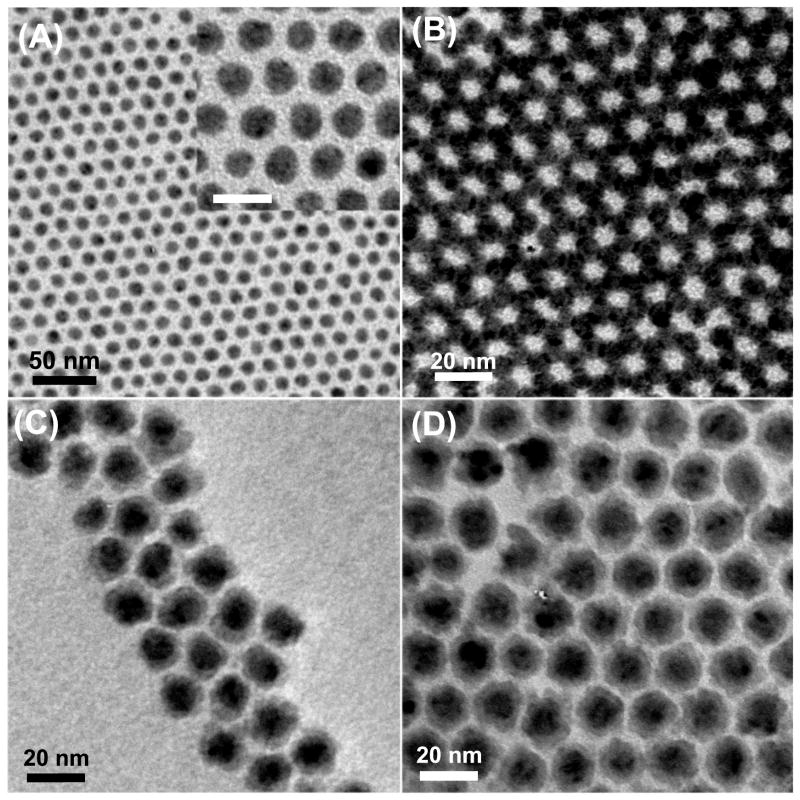
TEM study shows that the as-synthesized Co NPs are monodispersed with standard deviation (in diameter) less than 7%. Monolayer and multilayer assemblies can be easily made by controlling the dispersion concentration and the solvent evaporation rate on the copper coated TEM grid, as shown in Fig. 1 A–B. The core/shell structured Co/Fe3O4 nanocomposites have a core size of 10 nm in diameter and a shell thickness of 3 nm, as shown in Fig. 1 C–D. The Co size remains unchanged before and after coating. The Co/MnFe2O4 particles resemble the Co/Fe3O4 ones.
Fig. 1.
TEM images of (A) a monolayer of the 10 nm Co NPs (the inset shows higher magnification, scale bar is 20 nm), (B) self-assembled superlattice of the 10 nm Co NPs, (C) the 10 nm/3 nm Co/Fe3O4 NPs and (D) the 10 nm/3 nm Co/MnFe2O4 NPs.
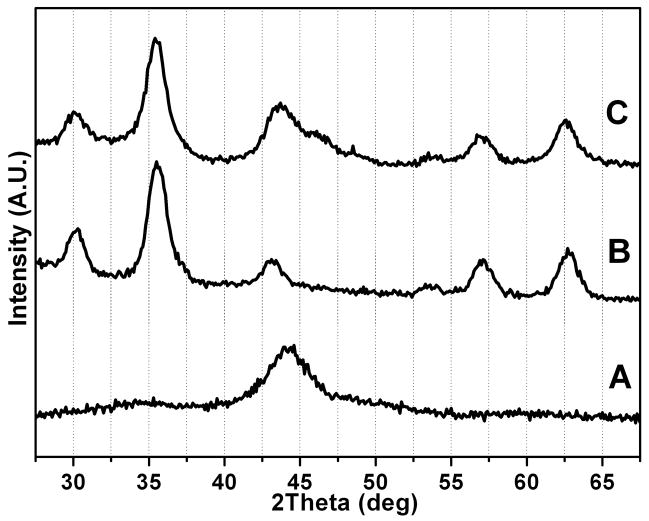
XRD pattern (Fig. 2A) of the as-synthesized Co NPs shows only a broad peak around 44°, indicating the Co NPs are polycrystalline. No significant peaks from CoO are observed. The diffraction pattern of 6 nm Fe3O4 NPs, synthesized by the decomposition of Fe(acac)3 without Co NP seeds, is shown in Fig. 2B as a reference [14]. Fig. 2C shows the pattern of 10 nm/3 nm Co/Fe3O4 core/shell nanocomposites. It can be seen that the pattern contains both Co and Fe3O4 components.
Fig. 2.
XRD patterns of (A) the 10 nm Co NPs, (B) the 6 nm Fe3O4 NPs, (C) the 10 nm/3 nm Co/Fe3O4 nanocomposite particles.
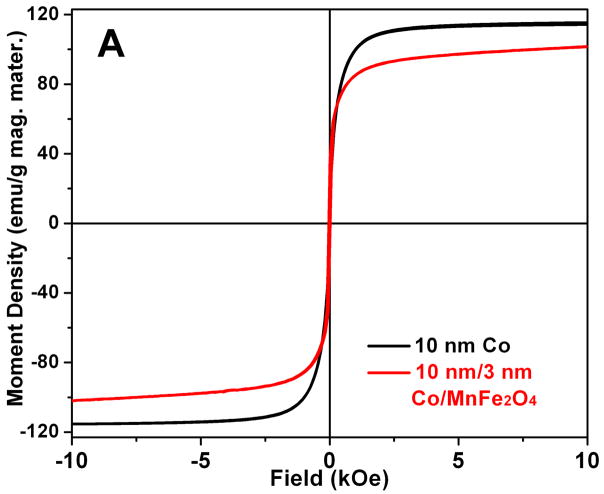
Magnetic measurement of the 10 nm Co NPs shows a superparamagnetic hysteresis loop with saturation moment of 58 emu/g nanoparticles, corresponding to ~115 emu/g Co [20], while a slightly higher moment of 125 emu/gCo is obtained from the 14 nm Co NPs. Comparing to the bulk saturation moment of Co which is ~160emu/g, our Co NPs reach about 80% of that value. This reduction is probably caused by thermal agitation and uncontrolled surface oxidation of the Co NPs. The magnetic moment density of the 10 nm/3 nm Co/MFe2O4 (M = Fe, Mn) nanocomposites reaches to 57 emu/g nanocomposites, which is almost the same as the value of 58emu/g Co NPs for the uncoated Co NPs. This corresponds to ~103 emu/g magnetic materials [20], which is slightly lower than that of the Co NPs, as shown in Fig. 3A. However, the core/shell composites show much higher moment density comparing with the previously reported Co composites with nonmagnetic coatings [9–12].
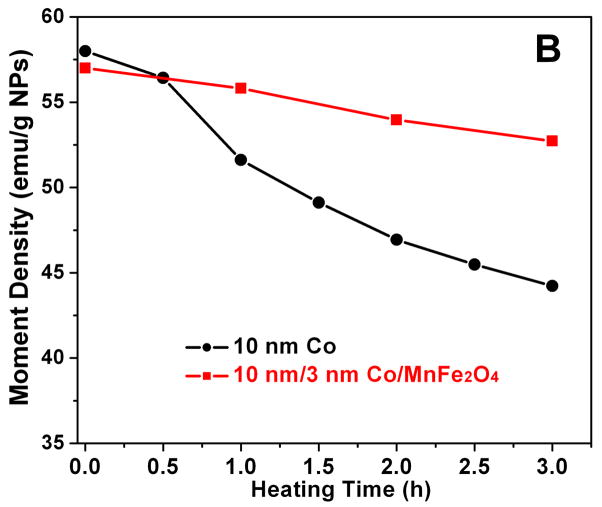
Fig 3.
(A) Hysteresis loops of the 10 nm Co NPs and the 10 nm/3 nm Co/MnFe2O4 nanocomposites; (B) Moment density change of the 10 nm Co NPs and 10 nm/3 nm Co/MnFe2O4 nanocomposites upon heating in air at 70°C.
Solid state stability of the Co NPs and Co/MFe2O4 nanocomposites was investigated by baking NP assemblies in air at 70°C. As shown in Fig. 3B, for Co NPs, the moment density quickly drops to ~70% of its saturated value, while for Co/MFe2O4 nanocomposites, the moment density decreases in a much slow pace –after 12 h heating, their magnetic moment is stabilized at 90% of its original value. This indicates that the ferrite coating does protect the Co core from fast oxidation.
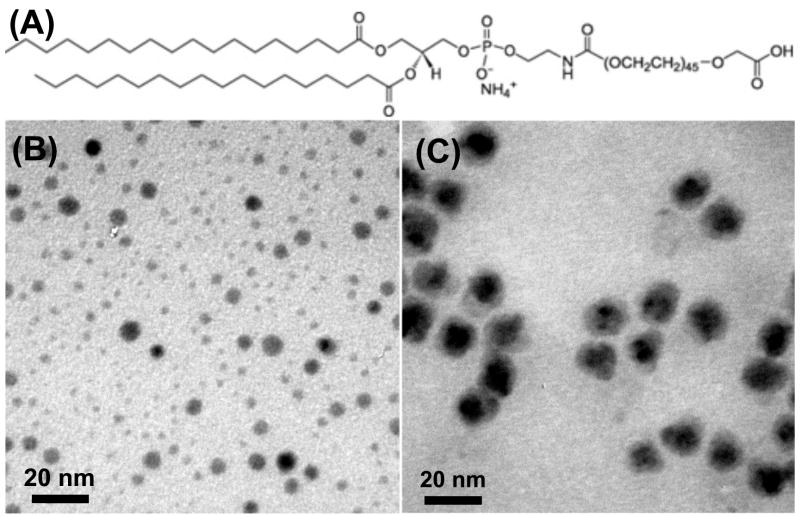
The as-synthesized NPs, coated with oleic acid and dioctylamine (oleylamine), are hydrophobic. To make them water soluble, we modified their surface with PEGylated phospholipids. Taking DSPE-PEG(2000)carboxylic acid as an example (Fig. 4A), the ligand is composed of a hydrophobic hydrocarbon “tail” and a hydrophilic PEG “head”, and has a carboxylic group on the end of the PEG “head”. Once mixed with the hydrophobic NPs, the phospholipids assemble onto the NP surface to form a double layer structure with the original surfactant molecules through hydrophobic-hydrophobic interactions [15–17]. With the PEG “head” pitching outward, the phospholipids are water soluble and the functional “head” are suitable for bio-conjugation. TEM study shows that, Co NPs are subject to morphology change upon the ligand addition and become polydispersed (Fig. 4B). This may be caused by the oxidation/etching of the metallic species in the presence of excess of COOH in the phospholipids. On the contrary, Co/MFe2O4 nanocomposites retain their morphology after the same modification (Fig. 4C), and their aqueous dispersion can be kept under ambient environment for months without noticeable color change or agglomeration.
Fig. 4.
(A) Structure of phospholipid DSPE-PEG(2000)carboxylic acid; (B) TEM image of the Co NPs modified with (A) in aqueous phase; (C) TEM image of the Co/Fe3O4 nanocomposites modified with (A) in aqueous phase.
Magnetic measurement of the Co NPs immediately after their transferring into aqueous phase shows a reduced moment density at 55 emu/g NPs, while the value for Co/MFe2O4 drops from 57 emu/g particle to 53 emu/g particle. This is because phospholipids take up more weight percentage in the particle. Aqueous phase stability of the NPs was checked by incubating the NP PBS buffer dispersion in air at 70°C. As shown in Fig. 5A, the dark color of the NP dispersion disappears within 30 mins and the moment density of the NPs from the dispersion drops to zero. As a comparison, the color of the Co/Fe3O4 dispersion remains unchanged and the moment density of the Co/Fe3O4 is stabilized at around 50 emu/g particle. This further proves that the stability of the composite NPs is greatly enhanced by the coating of the oxide shell.
Fig. 5.

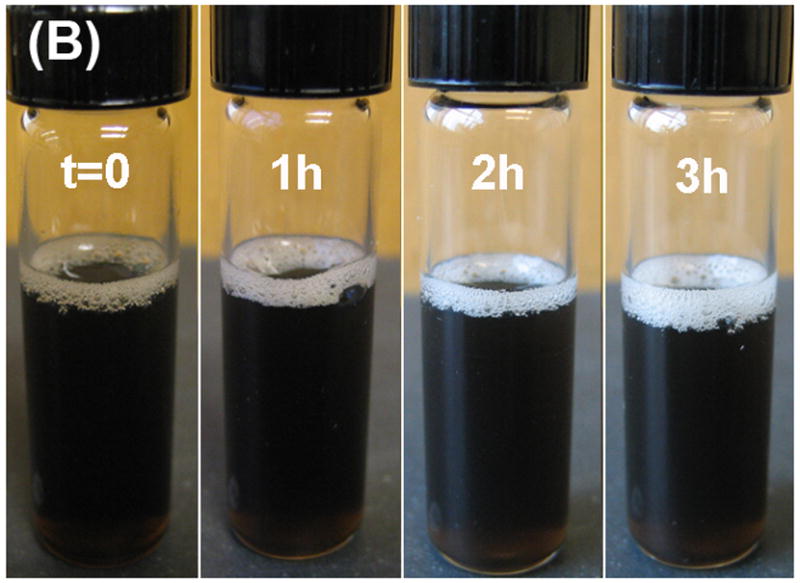
Solution color change of the heated (at 70°C in air) PBS solution of (A) Co NPs and (B) Co/Fe3O4 nanocomposite.
4. Conclusions
We successfully synthesized monodispersed Co NPs and core/shell structured Co/MFe2O4 (M = Fe, Mn) nanocomposites with tunable Co size from 8–14 nm and shell thickness from 1–5 nm. The hydrophobic NPs were transferred into aqueous phase via a simple ligand addition of bipolar phospholipids. The core/shell composite NPs gain much enhanced chemical and magnetic stability in solid state, organic solution and aqueous phase. The surface modified Co/MFe2O4 nanocomposites with high moment density are promising candidates for applications in bioseparation and biosensing.
Acknowledgments
The work was supported in part by NIH/NCI 1R21CA12859-01, NSF/DMR 0606264, ONR/MURI under grant Nos N00014-05-1-0497 and DOE/EPSCoR DE-FG02-07ER36374.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pankhurst QA, Connolly J, Jones SK, Dobson J. J Phys D: Appl Phys. 2003;36:R167. [Google Scholar]
- 2.Desvaux C, Amiens C, Fejes P, Renaud P, Respaud M, Lecante P, Snoeck E, Chaudret B. Nat Mater. 2005;4:750. doi: 10.1038/nmat1480. [DOI] [PubMed] [Google Scholar]
- 3.Puntes VF, Krishnan KM, Alivisatos PA. Science. 2001;291:2115. doi: 10.1126/science.1057553. [DOI] [PubMed] [Google Scholar]
- 4.Sun S, Murray CB. J Appl Phys. 1999;85(8):4325. [Google Scholar]
- 5.Park JI, Kang NJ, Jun YW, Oh SJ, Ri HC, Cheon J. ChemPhysChem. 2002;3:543. doi: 10.1002/1439-7641(20020617)3:6<543::AID-CPHC543>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Petit C, Taleb A, Pileni MP. J Phys Chem B. 1999;103:1805. [Google Scholar]
- 7.Giersig M, Hilgendorff M. J Phys D: Appl Phys. 1999;32:L111. [Google Scholar]
- 8.Grass RN, Stark WJ. J Mater Chem. 2006;16:1825. [Google Scholar]
- 9.Bao Y, Krishnan KM. J Magn Magn Mater. 2005;293:15. [Google Scholar]
- 10.Lee W-r, Kim MG, Choi J-r, Park J-I, Ko SJ, Oh SJ, Cheon J. J Am Chem Soc. 2005;127(46):16090. doi: 10.1021/ja053659j. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Achermann M, Balet LP, Hollingsworth JA, Klimov VI. J Am Chem Soc. 2005;127:544. doi: 10.1021/ja047107x. [DOI] [PubMed] [Google Scholar]
- 12.Vadala ML, Zalich MA, Fulks DB, Pierre TGS, Dailey JP, Riffle JS. J Magn Magn Mater. 2005;293:162. [Google Scholar]
- 13.Hou Y, Xu Z, Peng S, Rong C, Liu JP, Sun S. Adv Mater. 2007;19:3349. [Google Scholar]
- 14.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. J Am Chem Soc. 2004;126:273. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 15.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. Science. 2002;298:1759. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 16.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, Peng S, Brower N, Pourmand N, Wang SX, Sun S. Pure Appl Chem. 2005;78:1003. [Google Scholar]
- 18.Hütten A, Sudfeld D, Ennen I, Reiss G, Wojczykowski K, Jutzi P. J Magn Magn Mater. 2005;293:93. [Google Scholar]
- 19.Samia ACS, Hylzer K, Schlueter JA, Qin CJ, Jiang S, Bader SD, Lin XM. J Am Chem Soc. 2005;127:4126. doi: 10.1021/ja044419r. [DOI] [PubMed] [Google Scholar]
- 20.Thermal annealing of the nanoparticle powder at 750°C under Ar for 1 hour was used to measure the weight percentage of the inorganic core. The as synthesized 10 nm Co nanoparticles have 51% of Co, while the 10 nm/3 nm Co/MFe2O4 ones have 55% of Co and Fe3O4.