Abstract
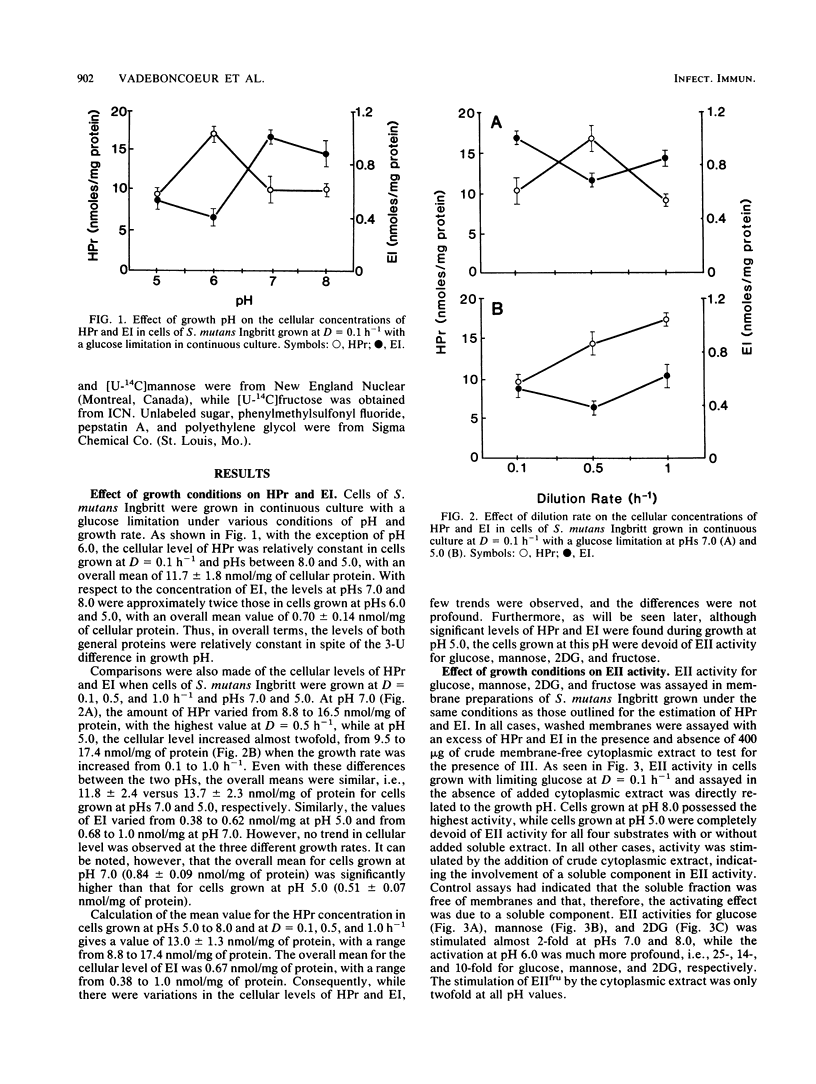
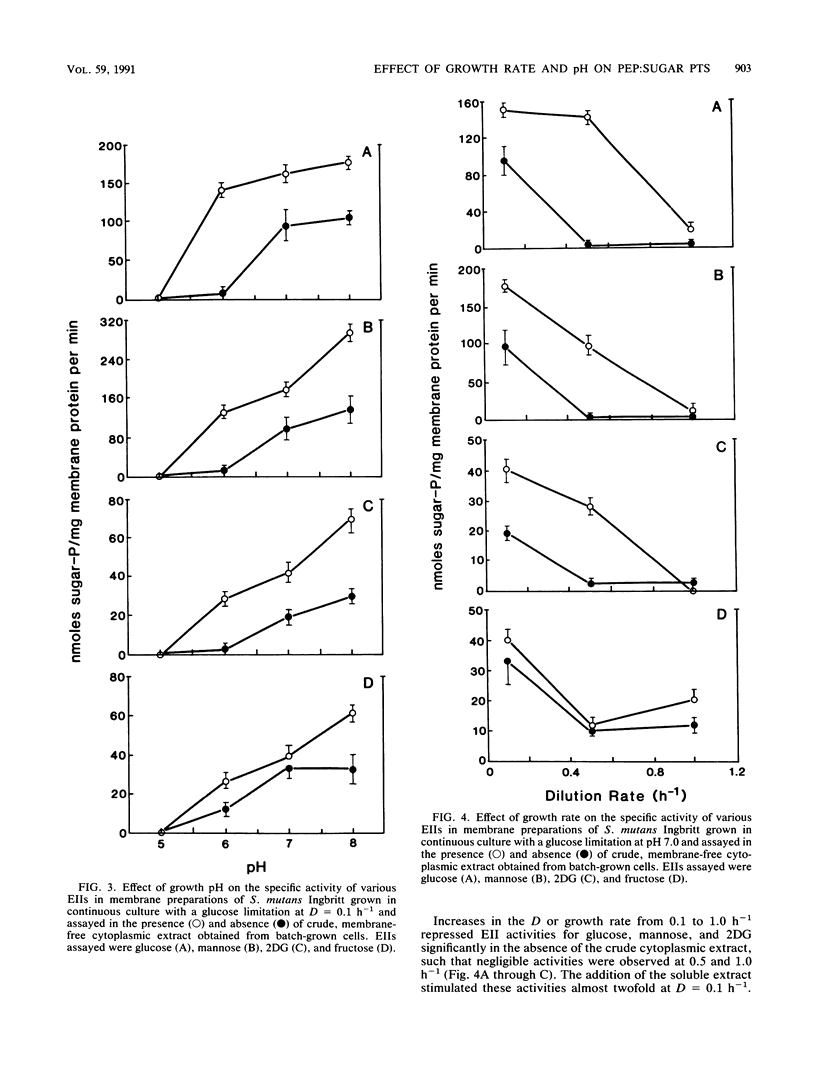
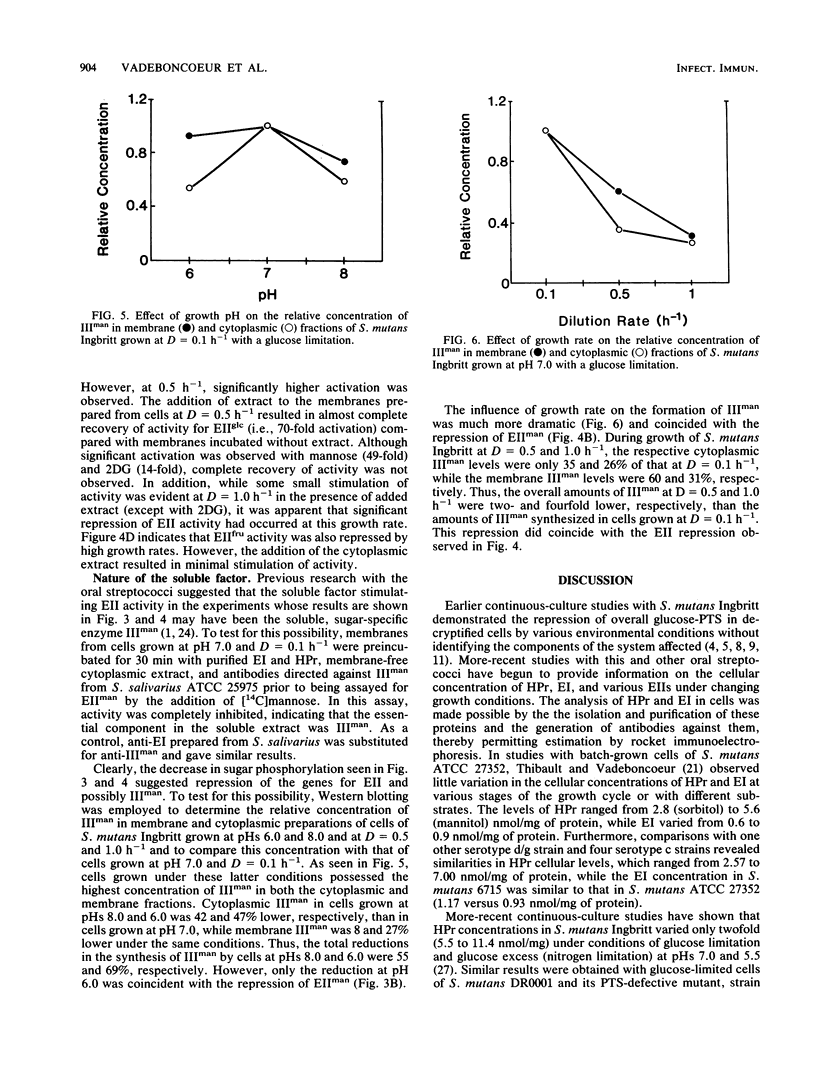
The growth of Streptococcus mutants Ingbritt in continuous culture at low pH or high growth rates repressed the biosynthesis of the components of the phosphoenolpyruvate:sugar phosphotransferase system (PTS). The cellular concentrations of the soluble components HPr, enzyme I (EI), and EIII for mannose (IIIman) and EII activity for glucose, mannose, 2-deoxyglucose (2DG), and fructose were determined in membrane preparations from cells grown at pHs from 8.0 to 5.0 and at dilution (D) or growth rates from 0.1 to 1.0 h-1. The cellular levels of HPr and EI varied less than threefold under all of the growth conditions tested. On the other hand, EII activity in membranes from cells grown at D = 0.1 h-1 was repressed by growth at pHs below 8.0, with cells grown at pH 5.0 completely devoid of EII activity. In addition, cells grown at D = 0.5 and 1.0 h-1 exhibited little PTS activity for glucose, mannose, and 2DG and twofold-lower activity for fructose. These activities were stimulated by the addition of a membrane-free cytoplasmic fraction, and this activating activity was shown to be due to the presence of IIIman. Estimation of the cellular content of IIIman indicated that the synthesis of this factor was repressed by growth above and below pH 7.0 and was particularly sensitive to growth at high rates. These results indicate that with S. mutans Ingbritt, both pH and growth rate regulate the genes for the synthesis of EIIs involved in the phosphorylation of glucose, mannose, 2DG, and fructose and the gene for the formation of IIIman.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourassa S., Gauthier L., Giguère R., Vadeboncoeur C. A IIIman protein is involved in the transport of glucose, mannose and fructose by oral streptococci. Oral Microbiol Immunol. 1990 Oct;5(5):288–297. doi: 10.1111/j.1399-302x.1990.tb00427.x. [DOI] [PubMed] [Google Scholar]
- Bowden G. H., Hardie J. M., Fillery E. D. Antigens from Actinomyces species and their value in identification. J Dent Res. 1976 Jan;55:A192–A204. doi: 10.1177/002203457605500112011. [DOI] [PubMed] [Google Scholar]
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Hamilton I. R. Properties of Streptococcus mutans Ingbritt growing on limiting sucrose in a chemostat: repression of the phosphoenolpyruvate phosphotransferase transport system. Infect Immun. 1982 May;36(2):576–581. doi: 10.1128/iai.36.2.576-581.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier L., Mayrand D., Vadeboncoeur C. Isolation of a novel protein involved in the transport of fructose by an inducible phosphoenolpyruvate fructose phosphotransferase system in Streptococcus mutans. J Bacteriol. 1984 Nov;160(2):755–763. doi: 10.1128/jb.160.2.755-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Bowden G. H. Response of freshly isolated strains of Streptococcus mutans and Streptococcus mitior to change in pH in the presence and absence of fluoride during growth in continuous culture. Infect Immun. 1982 Apr;36(1):255–262. doi: 10.1128/iai.36.1.255-262.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Gauthier L., Desjardins B., Vadeboncoeur C. Concentration-dependent repression of the soluble and membrane components of the Streptococcus mutans phosphoenolpyruvate: sugar phosphotransferase system by glucose. J Bacteriol. 1989 Jun;171(6):2942–2948. doi: 10.1128/jb.171.6.2942-2948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R. Synthesis and degradiation of intracellular polyglucose in Streptococcus salivarius. Can J Microbiol. 1968 Jan;14(1):65–77. doi: 10.1139/m68-011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Role of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Streptococcus mutans GS5 in the regulation of lactose uptake. Infect Immun. 1984 Feb;43(2):536–542. doi: 10.1128/iai.43.2.536-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Transport of glucose and mannose by a common phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus mutans GS5. Infect Immun. 1984 Mar;43(3):1106–1109. doi: 10.1128/iai.43.3.1106-1109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue L., Lacoste L., Trahan L., Vadeboncoeur C. Effect of nutritional constraints on the biosynthesis of the components of the phosphoenolpyruvate: sugar phosphotransferase system in a fresh isolate of Streptococcus mutans. Infect Immun. 1988 Feb;56(2):518–522. doi: 10.1128/iai.56.2.518-522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault L., Vadeboncoeur C. Phosphoenolpyruvate-sugar phosphotransferase transport system of Streptococcus mutans: purification of HPr and enzyme I and determination of their intracellular concentrations by rocket immunoelectrophoresis. Infect Immun. 1985 Dec;50(3):817–825. doi: 10.1128/iai.50.3.817-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Gauthier L. The phosphoenolpyruvate: sugar phosphotransferase system of Streptococcus salivarius. Identification of a IIIman protein. Can J Microbiol. 1987 Feb;33(2):118–122. doi: 10.1139/m87-020. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Proulx M. Lactose transport in Streptococcus mutans: isolation and characterization of factor IIIlac, a specific protein component of the phosphoenolpyruvate-lactose phosphotransferase system. Infect Immun. 1984 Oct;46(1):213–219. doi: 10.1128/iai.46.1.213-219.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Proulx M., Trahan L. Purification of proteins similar to HPr and enzyme I from the oral bacterium Streptococcus salivarius. Biochemical and immunochemical properties. Can J Microbiol. 1983 Dec;29(12):1694–1705. doi: 10.1139/m83-260. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C. Structure and properties of the phosphoenolpyruvate: glucose phosphotransferase system of oral streptococci. Can J Microbiol. 1984 Apr;30(4):495–502. doi: 10.1139/m84-073. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Thibault L., Neron S., Halvorson H., Hamilton I. R. Effect of growth conditions on levels of components of the phosphoenolpyruvate:sugar phosphotransferase system in Streptococcus mutans and Streptococcus sobrinus grown in continuous culture. J Bacteriol. 1987 Dec;169(12):5686–5691. doi: 10.1128/jb.169.12.5686-5691.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadeboncoeur C., Trahan L. Glucose transport in Streptococcus salivarius. Evidence for the presence of a distinct phosphoenolpyruvate: glucose phosphotransferase system which catalyses the phosphorylation of alpha-methyl glucoside. Can J Microbiol. 1982 Feb;28(2):190–199. doi: 10.1139/m82-025. [DOI] [PubMed] [Google Scholar]



