Abstract
Background
Levels of marinobufagenin (MBG), an endogenous bufadienolide Na/K-ATPase (NKA) inhibitor, increase in preeclampsia and in NaCl-sensitive hypertension.
Methods
We tested a 3E9 monoclonal anti-MBG antibody (mAb) for the ability to lower blood pressure (BP) in NaCl-sensitive hypertension and to reverse the preeclampsia-induced inhibition of erythrocyte NKA. Measurements of MBG were performed via immunoassay based on 4G4 anti-MBG mAb.
Results
In hypertensive Dahl-S rats, an intraperitoneal administration of 50 μg/kg 3E9 mAb lowered BP by 40 mmHg and activated Na/K-pump in thoracic aorta by 51%. NaCl supplementation of pregnant rats (n = 16) produced a 37 mmHg increase in BP, a 3.5-fold rise in MBG excretion, and a 25% inhibition of the Na/K-pump in the thoracic aorta, compared with pregnant rats on a normal NaCl intake. In eight pregnant hypertensive rats, 3E9 mAb reduced the BP (25 mmHg) and restored the vascular Na/K-pump. In 14 patients with preeclampsia (mean BP, 126 ± 3 mmHg; 26.9 ± 1.4 years; gestational age, 37 ± 0.8 weeks), plasma MBG was increased three-fold and erythrocyte NKA was inhibited compared with that of 12 normotensive pregnant women (mean BP, 71 W 3 mmHg)(1.5 ± 0.1 vs. 3.1 ± 0.2 μmol Pi/ml/h, respectively; P < .01). Ex-vivo 3E9 mAb restored NKA activity in erythrocytes from patients with preeclampsia. As compared with 3E9 mAb, Digibind, an affinity-purified antidigoxin antibody, was less active with respect to lowering BP in both hypertensive models and to restoration of NKA from erythrocytes from patients with preeclampsia.
Conclusion
Anti-MBG mAbs may be a useful tool in the studies of MBG in vitro and in vivo and may offer treatment of preeclampsia.
Keywords: marinobufagenin, monoclonal antibody, Na/K-ATPase, preeclampsia, salt-sensitive hypertension
Introduction
Preeclampsia complicates from 5 to 10% of pregnancies and it is the number one cause of maternal and fetal morbidity and mortality worldwide [1]. Still, the cause of preeclampsia remains unclear and approaches to its treatment have not changed significantly in many years [2]. One of the hypotheses attributes the endogenous digitalis-like Na/K-ATPase (NKA) inhibitors, that is, endogenous cardiotonic steroids (CTS), an important role in the pathogenesis of preeclampsia [3].
The mammalian CTS includes a cardenolide (endogenous ouabain) [4], and bufadienolides, bufalin [5], marinobufagenin (MBG) [6], and telocinobufagin [7] (Fig. 1a,b). Renal sodium retention stimulates CTS to promote natriuresis via inhibition of renotubular NKA [8-10]. In salt-sensitive hypertension, an excessive production of CTS contributes to hypertension via inhibition of NKA in vascular smooth muscle cells [8,10]. MBG in vivo acts as a vasoconstrictor and a natriuretic [11-13],and α-1 NKA, the main isoform in the vascular smooth muscle and an exclusive isoform in the kidney, exhibits high sensitivity to low, physiologically relevant concentrations of MBG [14,15]. Levels of MBG increase during states associated with plasma volume expansion and sodium retention, for example, in patients with essential hypertension [16], in Dahl-S rats on a high NaCl intake [11], in chronic renal failure [16,17], in congestive heart failure [18], during normal pregnancy [19,20], and in NaCl-induced hypertension in pregnant rats [20].
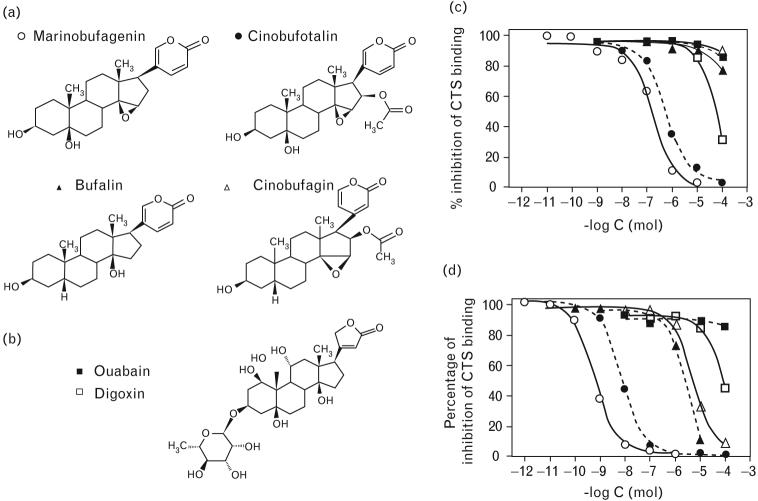
Fig. 1.
Chemical structures of bufadienolide (a) and cardenolide (b) CTS. Displacement of binding of 3E9 (c) and 4G4 (d) anti-MBG mAbs to MBG–thyroglobulin conjugates by MBG (○), cinobufotalin (●), bufalin (▲), cinobufagin (△), ouabain (■), and digoxin (□) in DELFIA competitive fluoroimmunoassay. CTS, cardiotonic steroids; DELFIA, dissociation-enhanced fluoroimmunoassay; mAb, monoclonal anti-marinobufagenin antibody; MBG, marinobufagenin.
In normal pregnancy, moderate elevations of MBG induced by fluid retention are not sufficient to produce hypertension [20]. In patients with preeclampsia, elevations of arterial pressure are associated with markedly increased plasma levels of MBG and with a more moderate elevation of endogenous ouabain levels [19,21]. Pregnant rats on a high NaCl intake exhibit preeclampsia-like symptoms, including elevations of MBG levels [20]. Administration of polyclonal anti-MBG antibody to pregnant NaCl-supplemented rats lowers the arterial pressure and is associated with an increase in the vascular sodium pump activity [20].
Convincing evidence in favor of the role of CTS in preeclampsia comes from studies in which intravenously administered Digibind (ovine antidigoxin antibody; GlaxoSmithKline, King of Prussia, Pennsylvania, USA), due to its ability to immunoneutralize with CTS, lowered the blood pressure in patients with preeclampsia. In 1988, Goodlin [3] reported a decrease in blood pressure in a 25.5-week preeclampsia patient following two intravenous infusions of Digibind. Later, Adair et al. [22] reported another case of successful use of Digibind in preeclampsia. Subsequently, the same group, in a placebo-controlled double-blinded study [23], demonstrated that Digibind lowered the blood pressure in 13 patients with postpartum preeclampsia. Importantly, Digibind did not exert adverse effects in these studies.
Despite its therapeutic promise, the wide use of Digibind in patients with preeclampsia may be problematic because the quantities of polyclonal antibodies are limited and Digibind exhibits low cross-reactivity with endogenous CTS [21,24]. The goal of our study was to develop monoclonal anti-MBG antibodies (mAbs) that could be used to measure levels of this substance and to block its effects in vivo.
Methods
Development of characterization of monoclonal antibody
The immunogen, MBG–bovine serum albumin (BSA) conjugate, was prepared as described previously in detail [25]. Balb/c mice were immunized with 100 μg of MBG–BSA conjugate four times and within 3–5 days after the last boost; spleen cells were fused with a mouse myeloma Sp2/0 cell line. Spleen cells were dispersed into a single cell suspension and 4.5 × 107 cells were fused with 4.5 × 107 myeloma cells in the presence of polyethylene glycol and dimethyl sulfoxide (PEG/ DMSO) solution. Fused cells were centrifuged and resuspended in RPMI-1640 medium (Cellgro, Herndon, Virginia, USA) containing 20% fetal bovine serum, Hypoxanthine–Aminopterin–Thymidine (HAT) media supplement, and macrophage conditioned media. The cells were dispensed in 0.2 ml aliquots in 96-well cell culture plates and were incubated in 5% CO2 at 37°C. Hybridomas that produced anti-MBG mAbs were cultured in RPMI 1640 (Cellgro) with 10% fetal calf serum. Positive clones were selected using dissociation-enhanced fluoroimmunoassay (DELFIA) based on the binding of anti-MBG antibody to a MBG-thyroglobulin conjugate (Immunoassays, below). Culture media were dialyzed with a Spectra-Por dialysis membrane with 100 000 Da molecular weight cut-off (Spectrum Laboratories Inc., Rancho Dominiguez, California, USA) against Tris-buffered saline (50 nmol/l Tris–HCl; 140 mmol/l NaCl; pH 7.4), and immunoglobulins were concentrated using Centricon YM-100 columns (Millipore Co., Bedford, Massachusetts, USA). Immunoglobulin types were determined using Mouse Monoclonal Antibody Isotyping Kit (Sigma Chemicals, Saint Louis, Missouri, USA). Concentration of IgG was determined using mouse IgG enzyme-linked immunosorbent assay (ELISA) quantitation kits (Roche Applied Science, Mannheim, Germany). Cross-reactivity of anti-MBG mAbs was determined in a competitive DELFIA fluoroimmunoassay (below). The reagents were obtained from the American Type Culture Collection (ATCC; Manassas, Virginia, USA), unless otherwise indicated.
Na/K-ATPase
The in-vitro ability of anti-MBG mAbs to reverse NKA inhibition has been studied in membranes purified from pig renal outer medulla and rat renal outer medulla, as described by Jorgensen [26] with modifications reported recently in detail [27]. Medullary slices were homogenized in a solution containing (in mmol/l) sucrose 250, histidine 30, imidazole 5, ethylenediaminetetraacetic acid (EDTA) 1 (4°C; pH 7.4), and then centrifuged (6000 g, 15min, 4°C). The supernatant was respun at 15 000 g for 30 min at 4°C, and the resultant supernatant centrifuged at 148 000 g for 90 min at 4°C. The pellet (membranes) was suspended in a homogenizing medium, applied to discontinuous sucrose gradients, consisting of 0.32–1.2 mol/l layers of sucrose buffered with 30 mmol/l histidine and 5 mmol/l imidazole (pH 7.4), and centrifuged at 148 000 g for 90 min. The pellet appearing at the 0.8 mol/l fraction was aspirated, resedimented at 148 000 g for 90 min, and resuspended in a homogenizing medium to a protein concentration 3–4 mg/ml, and stored in liquid nitrogen. NKA activity was determined as reported recently in detail [27].
Dahl-S rats on a high NaCl diet
The animal protocol was approved by the Animal Care and Use Committee of the Intramural Research Program, National Institute on Aging. Five-week-old male Dahl-S (SS/JrHsd) rats (n=20) were obtained from Harlan Sprague–Dawley Inc (Indianapolis, Indiana, USA). After adaptation for a week on a low-salt diet (0.2% NaCl; ICN Biochemicals, Irvine, California, USA), rats were placed on an 8% NaCl diet (ICN Biochemicals) and water ad libitum for 4 weeks. During this period, water consumption and urine output were measured weekly. Systolic blood pressure (SBP) was recorded by tail-cuff plethysmography. The effect of anti-MBG antibodies on SBP was studied in Dahl-S rats following 4 weeks of 8% NaCl intake. Anti-MBG 3E9 mAb (n=10) was administered intraperitoneally at a concentration that in vitro reverses the IC75 of MBG-induced inhibition of rat kidney NKA, as described recently in detail (50 μg/kg) [11,12,19]. Mouse isotype IgG1 control (50 μg/kg; clone 11711, R&D Systems, Inc, Minneapolis, Minnesota, USA) was used as a vehicle (n=10). Following antibody administration, SBP was measured hourly for 3 h. Thereafter, animals were anesthetized with ketamine (100 mg/kg) and sacrificed by exsanguination. Blood samples were collected for measurements of MBG and endogenous ouabain. The serum titers of anti-MBG antibody were determined via solid-phase fluoroimmunoassay as reported previously [20]. Thoracic aortae were excised for measurements of sodium pump activity (below).
Pregnant rats on a high NaCl intake
The aim of the experiment was to define whether anti-MBG 4G4 mAb detects heightened MBG levels and whether 3E9 mAbs lowers blood pressure in NaCl-supplemented pregnant rats. Experiments were performed on female Sprague–Dawley rats (225–250 g) purchased from Charles River (Wilmington, Massachusetts, USA) on day 5 of pregnancy (n=26) and on virgin, age-matched controls (n=10). Sixteen pregnant rats were NaCl-supplemented during days 12–19 of gestation and drank 1.8% NaCl. Ten pregnant rats and 10 virgin rats on a normal NaCl intake comprised the control groups. The effect of anti-MBG antibodies (n=8) and vehicle (n=8) on SBP and sodium pump in thoracic aortae was studied in pregnant rats following NaCl supplementation for 7 days (day 19 of gestation) as described above for Dahl-S rats and reported previously in detail [20].
Comparison of 3E9 monoclonal anti-marinobufagenin antibody with Digibind in hypertensive rats
In separate subsets of hypertensive Dahl-S and of NaCl-supplemented pregnant rats, we compared anti-hypertensive activity of 3E9 mAb and Digibind. In 16 Dahl-S rats rendered hypertensive by a 4-week administration of 8% NaCl diet, we compared effects of anti-MBG 3E9 mAb (n=8) and Digibind (0.5 mg/kg) following single intraperitoneal administration. Dose of 3E9 mAb was determined as described above (Dahl-S on a high NaCl diet), and dose of Digibind was comparable to that effectively administered to patients with preeclampsia in previous reports (0.1–1.5 mg/kg) [3,22,23]. Following administration of antibodies, SBP was measured in 3 h after the treatment and every 12 h thereafter for 48 h. Animals were placed in the metabolic chambers and 24-h urine samples were collected for determination of urinary sodium excretion. Thereafter, animals were anesthetized with ketamine (100 mg/kg) and sacrificed by exsanguination.
The effect of anti-MBG 3E9 mAb (n=6; 50 μg/kg), Digibind (n=6; 0.5 mg/kg), and vehicle (n=6) on SBP and on the activity of NKA in erythrocytes was studied in pregnant rats following NaCl supplementation for 7 days (day 19 of gestation) as described above (pregnant rats on a high NaCl intake). Following administration of antibodies, SBP was measured hourly for 3 h after the treatment. Thereafter, animals were anesthetized with ketamine (100 mg/kg) and sacrificed by exsanguination. Activity of NKA in erythrocytes was determined in pregnant NaCl-supplemented rats treated with vehicle, Digibind, and 3E9 mAb, and in six virgin female rats, as reported previously in detail [11].
Patients with preeclampsia
The protocol for the human study was approved by the Research Council of St Petersburg School of Pediatric Medicine and by the Institutional Review Board of Medstar Research Institute, Washington, DC. Consecutive patients with preeclampsia (gestational age 37–39 weeks) admitted to Kolpino Obstetric Hospital and Snegirev Obstetric Hospital (St Petersburg, Russia) were enrolled in the study. Preeclampsia was diagnosed according to the criteria of American College of Obstetrics and Gynecology [28]. This definition includes diastolic blood pressure of at least 90 mmHg or a SBP of at least 140 mmHg, or a rise in the former of at least 15 mmHg or in the latter of 30 mmHg on at least two occasions 6 h or more apart; proteinuria (presence of 300 mg or more of protein in a 24-h urine collection or a protein concentration of 1 g or more per liter in at least two random urine specimens collected 6 h or more apart) or edema (a generalized accumulation of fluid of greater than 1+ pitting edema after 12 h of bed rest or weight gain of 5 pounds or more in 1 week), or both, induced by pregnancy after the 20th week of gestation, and sometimes earlier. Twelve consecutive gestational age-matched subjects with uncomplicated pregnancies served as controls. None of the subjects studied had ever taken digitalis drugs or had a history of disease known to be associated with increased plasma concentrations of CTS (severe hypertension, renal and hepatic diseases, and endocrine dysfunctions). Aliquots of plasma (1 ml) were stored at −70°C for measurements of MBG and endogenous ouabain via fluoroimmunoassay.
Activity of the NKA in the erythrocytes of patients with preeclampsia and in the control pregnant subjects, in the presence and in the absence of anti-MBG mAbs, anti-ouabain polyclonal antibody (serum anti-OU-Spb05), and Digibind (GlaxoSmithKline) was determined as reported previously in detail [11,21]. Anti-MBG or antiouabain antibodies were used at concentrations that, in vitro, reversed the MBG-induced 75% inhibition of rat kidney NKA (α-1 isoform) or ouabain-induced 75% inhibition of NKA from rat fetal brain (α-3 isoform), respectively [11]. Digibind (GlaxoSmithKline) was used at concentrations 1 and 10 μg/ml, which are in the same range as doses of Digibind previously used to treat patients with preeclampsia [3,22,23].
High performance liquid chromatography purification of cardiotonic steroids from preeclamptic placentae
Placentae were collected from a subset of subjects with normal pregnancies and from a subset of patients with preeclampsia. The placentae were perfused with a solution containing (in mmol/l) NaCl 120; KCl 4; CaCl2 2.5; MgCl2 2.0; NaH2PO4 1.1; NaHCO3 24; and glucose 5.6 until complete removal of blood, minced, and homogenized. The homogenate was extracted with chloroform and dried under vacuum. Dried extract was sonicated in water (1 : 5 w/v) and applied on reverse-phase C-18 SepPak ‘long body’ cartridges, eluted with 80% acetonitrile, and dried in SpeedVac centrifuge (Savant, Hicksville, New York, USA). Levels of MBG and endogenous ouabain were determined in the prepurified extract as described below (Immunoassays). Ouabain and MBG standards, and the combined C-18 prepurified extracts from preeclamptic placentae were fractionated on Agilent 1100 series liquid chromatographic system using Agilent Zorbax Eclipse XDB-C18 (Agilent Technologies, Palo Alto, California, USA), 4.6 × 150 mm, 5 μm particle size, 80 Å column, flow rate 1 ml/min, in linear (10–85.5%) gradient of acetonitrile against 0.1% trifluoroacetic (TFA) for 45 min. Thirty 1.5-min fractions were collected and analyzed for ouabain-immunoreactivity and MBG-immunoreactivity.
Sodium pump activity
The transport activity of the sodium pump was estimated in the rings of rat thoracic aorta by measurement of ouabain-sensitive 86Rb uptake as reported previously [20,27]. Vascular rings of 2–2.5 mm diameter were equilibrated for 60 min in 5-ml flasks in a medium containing (in mmol/l) NaCl 120; KCl 4; CaCl2 2.5; MgCl2 2.0; NaH2PO4 1.1; NaHCO3 24; and glucose 5.6 gassed with 95% O2 and 5% CO2 at pH 7.4 and t = 32°C. 86Rb (185 MBq; 0.1 μCi/sample, NEN Life Science Products, Boston, Massachusetts, USA) was then added and the vessels were incubated for 60 min at 37°C in the absence or presence of 1 mmol/l ouabain. The samples were then rinsed in ice-cold medium, blotted with filter paper, weighed, and counted in a gamma counter (Cherenkov radiation). Total 86Rb uptake was determined on a wet weight basis. The activity of the sodium pump was measured as the difference between the total uptake of 86Rb and the uptake in the presence of 1 mmol/l ouabain and expressed in nanomoles of 86Rb per 1 mg of wet weight of tissue per min.
Immunoassays
For measurement of MBG and endogenous ouabain, samples of plasma and urine were extracted on SepPak C-18 cartridges (Waters, Milford, Massachusetts, USA) as described previously in detail [11]. The MBG DELFIA fluoroimmunoassays based on anti-MBG 3E9 and 4G4 mAbs were performed as previously described for rabbit anti-MBG polyclonal antibodies [11]. The assay is based on competition between immobilized antigen (MBG-glycoside-thyroglobulin) and MBG, other cross-reactants, or endogenous CTS within the sample for a limited number of binding sites on an anti-MBG mAbs. Secondary (goat antimouse) antibody labeled with non-radioactive europium was obtained from Perkin-Elmer (Waltham, Massachusetts, USA).
The endogenous ouabain assay was based on a similar principle utilizing an ouabain–ovalbumin conjugate and ouabain antiserum (anti-OU-M-2005; 1 : 20 000) obtained from rabbits immunized with a ouabain-BSA conjugate [20]. The cross-reactivity of this ouabain antibody is (%) ouabain, 100; ouabagenin, 52, digoxin, 1.8; digitoxin, 0.47; progesterone, 0.002; prednisone, 0.001; proscillaridin, 0.03; bufalin, 0.10; aldosterone, 0.04; telocinobufagin, 0.02; resibufagin, 0.15; marinobufotoxin, 0.06; cinobufagin, 0.02; and MBG, 0.036.
Statistical analyses
The results are presented as means ± SEM. Data were analyzed using one-way analysis of variance (ANOVA) followed by Newman–Keuls test (intragroup analyses), by repeated measures ANOVA followed by Newman–Keuls test (intergroup analyses), and by two-tailed t-test (when applicable; GraphPad Prism software, San Diego, California, USA). A two-sided P value of less than 0.05 was considered to be statistically significant.
Results
Characterization of monoclonal antibody
Anti-MBG mAbs (IgG1A class of immunglobulins) were purified from two clones, 3E9 and 4G4. Data on specificity of 3E9 and 4G4 mAbs and on their ability to neutralize effect of MBG in vitro are presented in Figs 1 and 2 and in Table 1. In DELFIA fluoroimmunoassay, 3E9 and 4G4 mAbs exhibited 0.17 and 2.8 nmol/l affinity to MBG, respectively. Neither antibody reacted with cardenolides, steroid hormones, BSA (used for conjugation of MBG), and thyroglobulin used for separation of phases in a competitive immunoassay. 3E9 and 4G4 mAbs exhibited cross-reactivity with cinobufotalin, a steroid very closely related to MBG, and 4G4 mAb crossreacted with marinobufatoxin (MBG–suberylaginine conjugate) and less with telocinobufagin, a possible precursor of MBG [7].
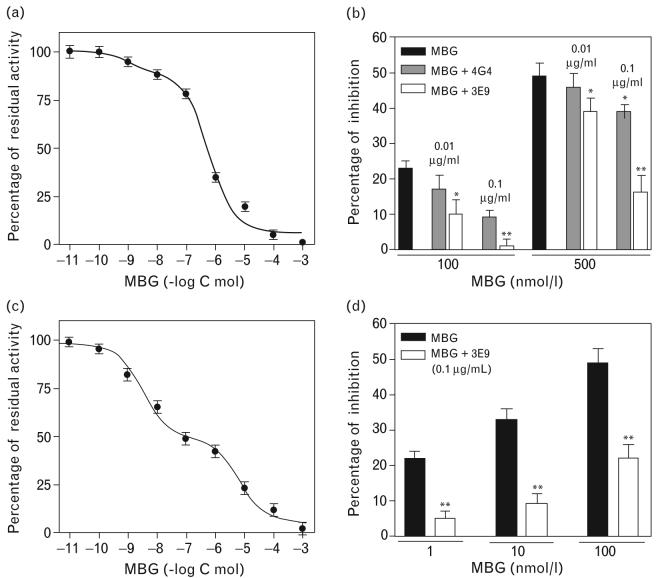
Fig. 2.
Inhibition of NKA from pig renal outer medulla by MBG (a) and effect of 3E9 and 4G4 anti-MBG mAbs on MBG-induced inhibition of NKA from pig kidney (b). Inhibition of NKA from rat renal medulla by MBG (c), and effect of 3E9 anti-MBG mAb on MBG-induced inhibition of NKA from rat renal medulla (d). Concentration-response curves were determined five to eight times. These were averaged to give means SEM. In pig renal medulla, the fit of the curve converged with inhibition occurring at the levels of low-affinity binding only (IC50 = 0.46 ± 0.23 μmol/l). In rat renal medulla, the fit of the curve converged with inhibition occurring at the level of higher-affinity and lower-affinity binding sites (IC50 = 1.9 ± 1.3 nmol/l and 4.3 ± 2.9 μmol/l, respectively). Each bar represents means ± SEM from five measurements. By one-way ANOVA followed by Newman–Keuls test: *P < 0.05; **P < 0.01 vs. effect of MBG. NKA, Na/K-ATPase; mAb, monoclonal anti-marinobufagenin antibody; MBG, marinobufagenin.
Table 1.
Cross-immunoreactivity of monoclonal anti-marinobufagenin antibodiesy
| mAbs cross-immunoreactivity (%) |
||
|---|---|---|
| Cross-reactants | 3E9 | 4G4 |
| Marinobufagenin | 100 | 100 |
| Marinobufotoxin | 4 | 43 |
| Telocinobufagin | 7 | 14 |
| Cinobufotalin | 44 | 40 |
| Cinobufagin | 1.4 | 0.07 |
| Resibufagenin | 0.45 | 0.5 |
| Bufalin | 0.25 | 0.08 |
| Proscillaridin A | 3 | <0.001 |
| Ouabain | 0.02 | 0.005 |
| Ouabagenin | <0.01 | <0.001 |
| Digoxin | 1.8 | 0.03 |
| Digoxigenin | 1 | 0.004 |
| Digitoxin | 0.7 | <0.001 |
| Aldosterone | <0.001 | <0.001 |
| Progesterone | <0.01 | <0.001 |
| Prednisone | <0.01 | <0.001 |
| Corticosterone | <0.01 | <0.001 |
| BSA | <0.001 | <0.001 |
| Thyroglobulin | <0.001 | <0.001 |
BSA, bovine serum albumin; mAb, monoclonal anti-marinobufagenin antibody; MBG, marinobufagenin.
The screening of 3E9 and 4G4 mAbs was performed using NKA purified from pig outer medulla (Fig. 2a,b), and the activity of 3E9 mAb was further assessed using NKA purified from rat outer medulla (Fig. 2c,d). In agreement with our previous results [15,29], NKA from rat kidney exhibited much greater sensitivity to MBG as compared with NKA from pig outer medulla (Fig. 2a,c). Thus, 1 nmol/l MBG, a concentration which is identical to that present in plasma of hypertensive humans [16] and rats [11,18,20], induced 22% inhibition of rat renal NKA. Despite its lower affinity for MBG in a competitive immunoassay, 3E9 mAb substantially exceeded 4G4 mAb in its functional ability to reverse MBG-induced inhibition of NKA purified from pig outer medulla (Fig. 2b). In rat outer medulla, 3E9 mAb reversed inhibition of NKA induced by 1–10 nmol/l MBG and significantly attenuated effect of 100 nmol/l MBG (Fig. 2d). Due to its higher capacity for neutralization of MBG activity, 3E9 mAb has been used in the subsequent in-vivo experiments. As sensitivity of immunoassay based on 4G4 mAb was 0.05 nmol/l, this antibody has been used further for determination of MBG levels.
Dahl-S rats on a high NaCl intake
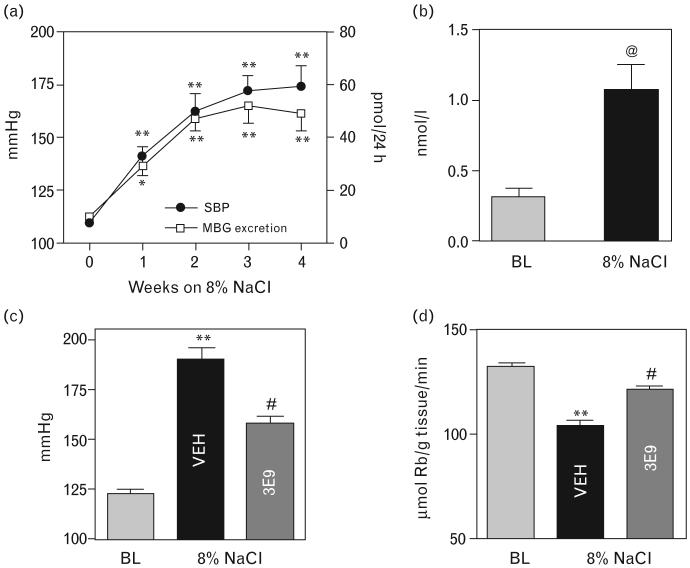
Chronic administration of an 8% NaCl diet to Dahl-S rats for 4 weeks induced a progressive increase in SBP (Fig. 3a). Renal MBG excretion, measured by assay using 4G4 mAb, rose in NaCl-loaded rats in parallel to changes in SBP. As illustrated in Fig. 3b, after 4 weeks of high NaCl intake, plasma MBG levels in Dahl-S rats rose 3.5-fold. As presented in Fig. 3d, in NaCl-loaded Dahl-S rats treated with vehicle (50 μg/kg mouse isotype IgG1 control), sodium pump in thoracic aorta was inhibited by 30% compared with that in rats on low NaCl intake. Intraperitoneal administration of 50 μg/kg 3E9 mAb to hypertensive Dahl-S rats lowered SBP from 190 to 158 mmHg within 3 h (Fig. 3c), which was associated with restoration of the vascular sodium pump activity (Fig. 3d). Serum titer of 3E9 mAb following its intraperitoneal administration exceeded 1 : 10 000.
Fig. 3.
(a) Changes in systolic blood pressure and renal MBG excretion in Dahl-S rats during 4 weeks of administration of 8% NaCl diet. (b) Plasma levels of MBG in Dahl-S rats during normal NaCl intake (baseline) and following 4 weeks of administration of 8% NaCl diet. (c) Systolic blood pressure and (d) activity of the sodium pump in thoracic aortae in Dahl-S rats at baseline and after 4 weeks of administration of 8% NaCl diet 3 h following intraperitoneal administration of vehicle (VEH), or anti-MBG mAbs, 3E9. Each point or bar represents means ± SEM from 10 observations. By one-way ANOVA followed by Newman–Keuls test: *P < 0.05; **P < 0.01 vs. baseline or time 0; #P < 0.05 vs. VEH. By two-tailed t-test: @P < 0.01 vs. baseline. BL, baseline; MBG, marinobufagenin; SBP, systolic blood pressure.
NaCl supplemented pregnant Sprague–Dawley rats
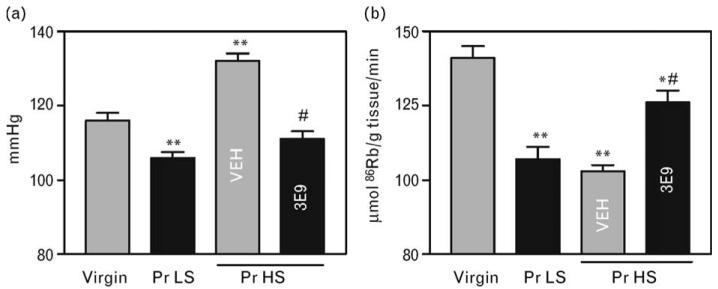
Data on the effect of 3E9 mAb in pregnant hypertensive rats are summarized in Figs 4 and 5. In accord with recently reported results, in rats, normal pregnancy was associated with moderate elevations in plasma levels and renal excretion of MBG (Fig. 4a,b), with a drop in SBP (Fig. 5a), and with reduction in the sodium pump activity in the thoracic aorta (Fig. 5b). NaCl supplementation of rats during days 12–19 of gestation resulted in a progressive increase in MBG levels (Fig. 4a,b), increase in renal protein excretion (Fig. 4c), decrease in fetal weight (Fig. 4d), and a 26 mmHg increase in SBP (Fig. 5a). Within 3 h of a single intraperitoneal administration of 50 μg/kg 3E9 mAb, the in-vivo titer of the antibody exceeded 1 : 10 000, SBP decreased by 21 mmHg, and activity of the sodium pump in thoracic aorta exhibited a 25% increase.
Fig. 4.
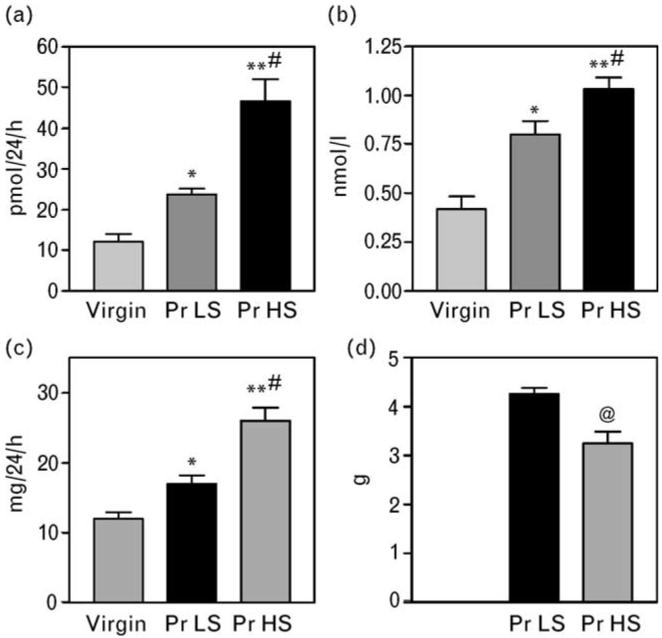
Renal excretion (a) and plasma concentration (b) of MBG and renal protein excretion (c) in virgin rats, and in pregnant rats with (Pr HS) or without (Pr LS) NaCl supplementation (day 19 of gestation). Means± SEM from 8–10 observations. By one-way ANOVA followed by Newman–Keuls test: *P < 0.05; **P < 0.01 vs. virgin rats. #P < 0.01 vs. Pr LS. (d) Mean weight of pups from Pr LS and Pr HS; @P < 0.01 vs. Pr LS, two-tailed t-test. MBG, marinobufagenin; Pr HS, pregnant rats with high-salt intake; Pr LS, pregnant rats with low-salt intake.
Fig. 5.
Systolic blood pressure (a) and activity of the sodium pump in the thoracic aorta (b) in virgin rats, in pregnant rats (day 19 of gestation) without NaCl supplementation (Pr LS) and in NaCl-supplemented pregnant rats (Pr HS) treated with 3E9 anti-MBG mAb or with vehicle (VEH). Means ± SEM from 8–10 observations. By one-way ANOVA followed by Newman–Keuls test: *P < 0.05; **P < 0.01 vs. virgin rats. #P < 0.01 vs. Pr HS, VEH. Pr HS, pregnant rats with high-salt intake; Pr LS, pregnant rats with low-salt intake; mAb, monoclonal anti-marinobufagenin antibody; MBG, marinobufagenin.
Comparison of 3E9 monoclonal anti-marinobufagenin antibody with Digibind in hypertensive rats
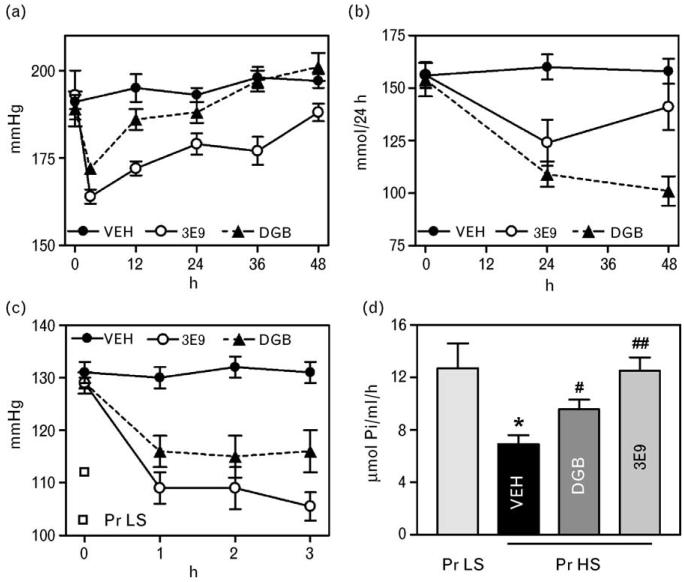
As presented in Fig. 6a, within 3 h of a single intraperitoneal administration of 50 μg/kg 3E9 mAb and 0.5 mg/kg Digibind to hypertensive Dahl-S rats, SBP decreased by 30 and 17 mmHg, respectively. Within 12 h following administration of Digibind, SPB rose to the levels that did not differ from that in vehicle-treated rats. In rats treated with 3E9 mAb, although SBP increased, it remained significantly lower than that in vehicle-treated rats for the duration of experiments. Prior to treatment, heart rate in Dahl-S was 412 ± 16. No changes in heart rate was observed within 3 h following administration of either antibody. Within 24 h following administration, effects of both 3E9 and Digibind were associated with marginally significant increases in heart rate (460 ± 16 and 447 ± 17, respectively; by repeated measures ANOVA; P = 0.05 vs. vehicle). As demonstrated in Fig. 6b, both 3E9 mAb and Digibind, within 24 h after administration, reduced renal sodium excretion by 21 and 29%, respectively. Within 48 h following administration of Digibind, renal sodium excretion remained reduced as compared with that in vehicle-treated rats. In 3E9 mAb-treated rats, renal sodium excretion increased to baseline levels within 48 h following administration of the antibody.
Fig. 6.
Systolic blood pressure (a) and renal sodium excretion (b) in hypertensive Dahl-S rats following single intraperitoneal administration of vehicle (VEH), 3E9 mAb, and Digibind (DGB). Repeated measures ANOVA followed by Newman–Keuls test. Systolic blood pressure: VEH vs. 3E9; P < 0.001; VEH vs. DGB; P < 0.05; 3E9 vs. DGB; P < 0.001. Renal sodium excretion: VEH vs. 3E9; P < 0.05; VEH vs. DGB; P < 0.01; 3E9 vs. DGB; P < 0.05. Systolic blood pressure (c) and activity of NKA in erythrocytes in NaCl-supplemented rats following single intraperitoneal administration of vehicle (VEH), 3E9 mAb, and DGB. Repeated measures ANOVA followed by Newman–Keuls test. Systolic blood pressure: VEH vs. 3E9; P < 0.001; VEH vs. DGB; P < 0.05; 3E9 vs. DGB; P < 0.001. One-way ANOVA followed by Newman–Keuls test: *P < 0.01 vs. pregnant rats on a low NaCl intake (PrLS); #P < 0.05, ##P < 0.01 vs. vehicle (VEH). DS, Dahl-S rats Pr HS, pregnant rats with high-salt intake; Pr LS, pregnant rats with low-salt intake.
Data on the comparison of effects of 50 μg/kg 3E9 mAb and 0.5 mg/kg Digibind in NaCl-supplemented pregnant rats are summarized in Fig. 6c and d. Within 3 h following administration, 3E9 mAb and Digibind lowered SPB by 24 and 13 mmHg, respectively (Fig. 6c; P < 0.01 by repeated measures ANOVA, followed by Newman–Keuls test; P < 0.05 3E9 mAb vs. Digibind). In pregnant NaCl-loaded rats, administration of both 3E9 mAb and Digibind did not cause significant changes in heart rate. The effect of treatment with both antibodies on the activity of NKA in erythrocytes was consistent with their effect on SBP (Fig. 6d). In NaCl-supplementated vehicle-treated pregnant rats erythrocyte NKA was inhibited by 50%, treatment with 3E9 mAb restored NKA activity to baseline levels, whereas Digibind exhibited partial but significant NKA restoration.
Human preeclampsia
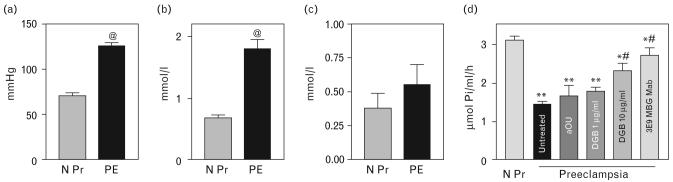
Fourteen consecutive patients with preeclampsia (age, 26.9 ± 1.4 years, gestation age 37.0 ± 0.8 weeks) and 12 consecutive participants with uncomplicated pregnancy (age, 25.9 ± 1.3 years, gestation age 36.0 ± 0.7 weeks) were enrolled in the study. The results are summarized in Fig. 7. As compared with normotensive pregnant participants, preeclampsia was associated with a 55 mmHg elevation of mean arterial pressure (Fig. 7a), with a three-fold increase in plasma levels of MBG (Fig. 7b), and a 54% inhibition of the NKA in the erythrocytes (Fig. 7d). Plasma levels of endogenous ouabain in patients with preeclampsia did not differ from those in participants with normotensive pregnancy (Fig. 7c). As presented in Fig. 7d, the ex-vivo treatment of preeclampsia erythrocytes from patients with preeclampsia, with antiouabain antiserum and with Digibind at concentration comparable to its dose used to treat patients with preeclampsia, did not affect NKA activity. A high concentration of Digibind (10 μg/ml) partially reversed NKA inhibition. By contrast, 3E9 mAb restored NKA to control levels.
Fig. 7.
Mean blood pressure (a) and plasma concentrations of MBG (b) and endogenous ouabain (c) in subjects with uncomplicated pregnancy (N Pr) and with preeclampsia. Means ± SEM. @ - P < 0.01 vs. N Pr by two-tailed t-test. (d) Activity of NKA in erythrocytes from normotensive pregnant subjects (N Pr) and from patients with preeclampsia treated ex vivo by antiouabain antibody (aOU), Digibind (DGB), and 3E9 anti-MBG mAb. Means ± SEM. By one-way ANOVA followed by Newman–Keuls test: *P < 0.05, **P < 0.01 vs. N Pr; #P < 0.05, ##P < 0.01 vs. untreated preeclampsia erythrocytes. BP, blood pressure; mAb, monoclonal anti-marinobufagenin antibody; MBG, marinobufagenin; NKA, Na/K-ATPase; PE, preeclampsia; EO, endogenous ouabain.
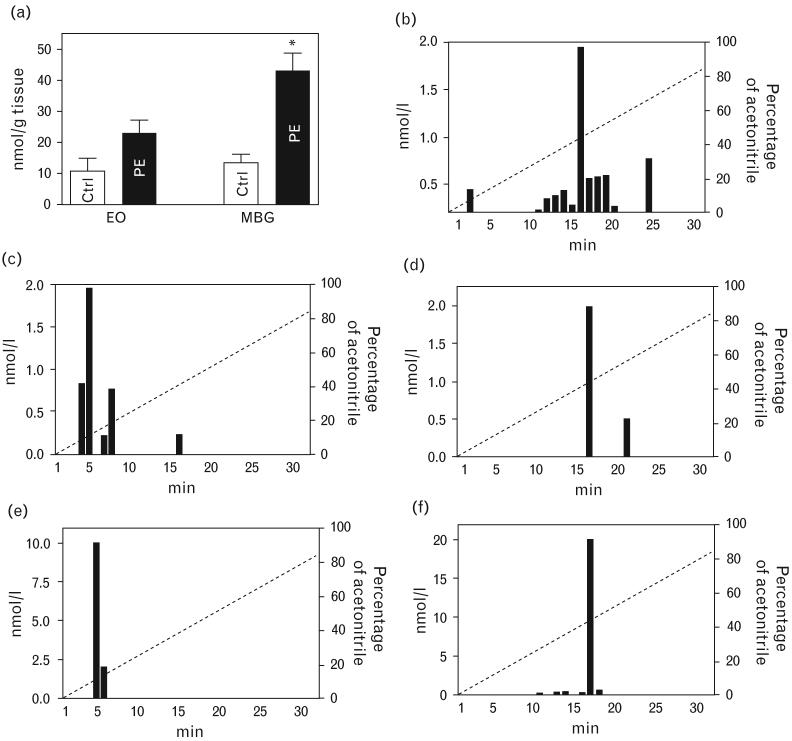
Purification of marinobufagenin-immunoreactive material from preeclamptic placentae
After the delivery placentae were collected from seven healthy pregnant participants (28 ± 1 years, blood pressure, 155 ± 9/95 ± 3 mmHg) and from nine patients with preeclampsia (27 ± 1 years, blood pressure, 115 ± 3/70 ± 4 mmHg). As presented in Fig. 8a, levels of MBG in preeclamptic placentae were increased three-fold compared with that in control placentae (P < 0.001), while endogenous ouabain in preeclamptic placentae exhibited an increase in a borderline statistical significance (P = 0.05). Figure 8b-d illustrates distribution of MBG-immunoreactivity and ouabain-immunoreactivity between high performance liquid chromatography (HPLC) fractions when extract from preeclamptic placentae was fractionated on Agilent Zorbax Eclipse XDB-C18 HPLC column (Agilent Technologies). Maximum of ouabain-immunoreactive material was eluted in 5 min (18% acetonitrile), which was similar to the elution time of ouabain standard (Fig. 8e). Maximum of MBG-immunoreactivity determined by both mAbs, 4G4 and 3E9, was eluted in 16 min (42% acetonitrile), which was identical to the elution time of MBG standard (Fig. 8f).
Fig. 8.
Purification of cardiotonic steroids from preeclamptic placentae. (a) Levels of MBG and endogenous ouabain in control (Ctrl) and preeclamptic placentae. *P < 0.001 vs. Ctrl. (b–d) Pattern of elution of MBG-immunoreactivity and ouabain-immunoreactivity following HPLC fractionation of placental extracts on Zorbax Eclipse XDB-C18 HPLC. (e,f) Elution of ouabain (1 μmol/l; 50 μl) and MBG (1 μmol/l; 50 μl) standards from Zorbax Eclipse XDB-C18 HPLC. CTS, cardiotonic steroids; EO, endogenous ouabain; HPLC, high performance liquid chromatography; mAb, monoclonal anti-marinobufagenin antibody; MBG, marinobufagenin; PE, preeclampsia.
Discussion
Major findings of the present study are that highly specific anti-MBG mAbs detect elevated levels of MBG in rats with NaCl-sensitive hypertension and in patients with preeclampsia. They lower SBP in vivo and reactivate the vascular sodium pump in Dahl-S rats and in pregnant rats with NaCl-induced hypertension, and ex vivo, they restore activity of the NKA in erythrocytes from patients with preeclampsia. These results further support a causative role for MBG in NaCl-sensitive hypertension and in preeclampsia and suggest that MBG represents a potential target for immunoneutralization in preeclampsia and NaCl-sensitive hypertension.
In Dahl-S rats on a chronic high NaCl intake, using an immunoassay based on 4G4 anti-MBG mAb, we observed that a progressive increase in blood pressure was paralleled by elevations in the levels of MBG renal excretion. Plasma levels and renal excretion of MBG in hypertensive Dahl-S rats in the present study were similar to those in our previous experiments utilizing immunoassays based on polyclonal anti-MBG antibodies [11,30]. The fact that in-vivo administration of 3E9 mAb to hypertensive Dahl-S rats not only exhibited an anti-hypertensive effect, but also restored activity of the sodium pump in thoracic aorta, suggests at the minimum a strong correlation between heightened levels of MBG, inhibition of vascular sodium pump, and enhanced vascular tone in this model of salt-sensitive hypertension.
In rats and humans, normal pregnancy is characterized by increased circulatory volume, by sodium retention [30] and by moderate elevations in MBG levels [19,20]. However, due to systemic peripheral vasodilation, arterial pressure in pregnancy usually progressively decreases [31]. When pregnant rats are given 1.8% NaCl supplements during the last week of gestation, rats develop hypertension and exhibit some of the symptoms of preeclampsia, that is, fetal growth retardation and proteinuria [32]. In accord with our previous results [20], in the present study, immunoassay based on 4G4 mAb detected a three-fold elevation in renal MBG excretion, and in-vivo immunoneutralization of MBG with 3E9 mAb was associated with a drop in SBP and with a simultaneous restoration of the vascular sodium pump activity.
In patients with preeclampsia, elevation of arterial pressure was associated with a three-fold increased plasma MBG and with a 54% inhibition of the NKA in erythrocytes, in the absence of significant changes in plasma levels of endogenous ouabain. Likewise, antiouabain antibody ex vivo did not alter activity of NKA in preeclampsia erythrocytes. In previous reports, in preeclampsia, Digibind exhibited antihypertensive effects at doses ranging from 0.1 to 1.5 mg/kg [3,23,24]. In the present work, a lower concentration of Digibind (1 μg/ml in vitro), which is comparable to clinically effective concentrations of this drug, did not significantly alter the activity of NKA in the erythrocytes from patients with preeclampsia. However, a high concentration of Digibind (10 μg/ml) induced partial but significant restoration of NKA activity, whereas anti-MBG 3E9 mAb reversed the preeclampsia-induced inhibition of NKA completely to the levels observed in subjects with non-complicated pregnancy. In a competitive immunoassay, Digibind exhibited low cross-immunoreactivity with both MBG (0.11%) and ouabain (0.39%) [21]. Because in preeclampsia, levels of endogenous ouabain did not increase in plasma, and were modestly elevated in placentae, and antiouabain antibody ex vivo did not restore NKA activity in the erythrocytes, MBG appears to be a primary target for Digibind in these patients.
In the present study, similar to that in plasma, levels of MBG in preeclamptic placentae exhibited a three-fold increase compared with that in placentae from normotensive pregnant subjects. The elution pattern of MBG and endogenous ouabain from Zorbax Eclipse XDB-C18 HPLC column (Agilent Technologies) was similar to that observed in our previous work when endogenous CTS was purified from human urine [6] and plasma [19]. Ouabain-immunoreactivity was eluted from HPLC column earlier than MBG-immunoreactive material, which is in accord with the higher and lower polarity of ouabain and MBG, respectively [6]. When placental material was fractionated on HPLC columns, immunoassays based on two anti-MBG mAbs, 4G4 and 3E9, detected maximum of MBG-immunoreactive material in a fraction that coeluted with MBG standard.
In accord with clinical data demonstrating that 3E9 anti-MBG mAb exceeds Digibind with respect to ex-vivo restoration of NKA from preeclamptic erythrocytes, our present experimental observations also demonstrate that in both hypertensive Dahl-S rats and in pregnant NaCl-supplemented rats, 3E9 mAb exhibited greater antihypertensive effect as compared with that of Digi-bind. Previously, we demonstrated that immunoneutralization of MBG in NaCl-loaded Sprague–Dawley rats resulted in a decrease in renal sodium excretion [13]. In the present study, however, Digibind, but not 3E9 mAb, produced a greater and longer lasting reduction of renal sodium excretion in hypertensive Dahl-S rats. We do not yet have explanation for this observation and studies of the mechanisms of renal effects of both antibodies deserve further attention. Similar to that in Dahl-S rats, antihypertensive activity of 3E9 mAb exceeded that of Digibind in NaCl-supplemented rats. Likewise, the effect of 3E9 was associated with greater restoration of NKA activity in erythrocytes. These findings parallel our clinical observations and establish a causative link between elevated levels of MBG and inhibition of NKA in salt-sensitive hypertension and preeclampsia. Furthermore, our observations that, in both experimental models, in hypertensive Dahl-S rats and in NaCl-supplemented pregnant rats, the in-vivo immunoneutralization of elevated MBG levels was associated with restoration of vascular sodium pump activity, indicates that MBG exhibits its pressor effects via inhibition of NKA in vascular smooth muscle.
Previously, we demonstrated that low, nanomolar concentrations of MBG induce 20% inhibition of vascular NKA [14], exhibit vasoconstriction in vitro [19], and induce hypertension following chronic in-vivo administration to rats [17]. Polyclonal anti-MBG antibodies were shown to reduce blood pressure and to restore the sodium pump activity in aorta in NaCl-supplemented pregnant rats [20]. Furthermore, active immunization against MBG has been shown to reduce arterial pressure and cardiac hypertrophy in hypertensive rats with subtotal nephrectomy [17]. Our present findings, together with previous observations, suggest that MBG fits the criteria for a putative endogenous vasoconstrictive natriuretic hormone and make MBG a potential target for antihypertensive therapy.
In conclusion, our results provide further evidence for a causative role for MBG in the pathogenesis of preeclampsia and NaCl-sensitive hypertension. The 4G4 anti-MBG mAb establishes a specific and sensitive competitive MBG immunoassay, whereas the 3E9 mAb holds promise as physiologic tool to elucidate CTS action in vivo and may offer an effective novel therapy for preeclampsia.
Acknowledgements
These studies were supported by Intramural Research Program, National Institute on Aging, NIH. Fragments of the studies were supported by Russian Foundation for Fundamental Science (grant 06-04-48956). Authors gratefully acknowledge excellent technical assistance by Irina V. Averina, MD, Ekaterina P. Fadeeva, MD, Alexandra (Namikas) Newman, Danielle Joseph, and Chad Boily.
References
- 1.Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, Harlap S. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–215. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Preeclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Goodlin RC. Antidigoxin antibodies in eclampsia. N Engl J Med. 1988;318:518–519. doi: 10.1056/NEJM198802253180815. [DOI] [PubMed] [Google Scholar]
- 4.Hamlyn JM, Hamilton BP, Manunta P. Endogenous ouabain, sodium balance and blood pressure: a review and a hypothesis. J Hypertens. 1996;14:151–167. doi: 10.1097/00004872-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lichtstein D, Gati I, Samuelov S, Berson D, Rozenman Y, Landau L, Deutsch J. Identification of digitalis-like compounds in human cataractous lenses. Eur J Biochem. 1993;216:261–268. doi: 10.1111/j.1432-1033.1993.tb18141.x. [DOI] [PubMed] [Google Scholar]
- 6.Bagrov AY, Fedorova OV, Dmitrieva RI, Howald WN, Hunter AP, Kuznetsova EA, Shpen VM. Bufodienolide nature of endogenous inhibitor of Na/K ATPase in the urine from patients with acute myocardial infarction. Hypertension. 1998;31:1097–1103. doi: 10.1161/01.hyp.31.5.1097. [DOI] [PubMed] [Google Scholar]
- 7.Komiyama Y, Dong XH, Nishimura N, Masaki H, Yoshika M, Masuda M, Takahashi H. A novel endogenous digitalis, telecinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Haddy FJ. Role of dietary salt in hypertension. Life Sci. 2006;79:1585–1592. doi: 10.1016/j.lfs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Gruber KA, Whitaker JM, Buckalew VM. Endogenous digitalis-like substance in plasma of volume-expanded dogs. Nature. 1980;287:743–745. doi: 10.1038/287743a0. [DOI] [PubMed] [Google Scholar]
- 10.Blaustein MP. Sodium ions, calcium ions, blood pressure regulation and hypertension: a reassessment and a hypothesis. Am J Physiol. 1977;232:C167–C173. doi: 10.1152/ajpcell.1977.232.5.C165. [DOI] [PubMed] [Google Scholar]
- 11.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. An endogenous ligand of α-1 sodium pump, marinobufagenin, is a novel mediator of sodium chloride dependent hypertension. Circulation. 2002;105:1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 12.Fedorova OV, Agalakova NI, Talan MI, Lakatta EG, Bagrov AY. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl loaded Dahl-S rats. J Hypertens. 2005;23:1515–1523. doi: 10.1097/01.hjh.0000174969.79836.8b. [DOI] [PubMed] [Google Scholar]
- 13.Periyasamy SM, Liu J, Tanta F, Kabak B, Wakefield B, Malhotra D, et al. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney Int. 2005;67:1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 14.Fedorova OV, Bagrov AY. Inhibition of Na/K ATPase from rat aorta by two endogenous Na/K pump inhibitors, ouabain and marinobufagenin. Evidence of interaction with different alpha-subunit isoforms. Am J Hypertens. 1997;10:929–935. doi: 10.1016/s0895-7061(97)00096-4. [DOI] [PubMed] [Google Scholar]
- 15.Fedorova OV, Lakatta EG, Bagrov AY. Differential effects of acute NaCl loading on endogenous ouabain-like and marinobufagenin-like ligands of the sodium pump in Dahl hypertensive rats. Circulation. 2000;102:3009–3014. doi: 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- 16.Gonick HC, Ding Y, Vaziri ND, Bagrov AY, Fedorova OV. Simultaneous measurement of marinobufagenin, ouabain and hypertension-associated protein in various disease state. Clin Exp Hypertens. 1998;20:617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, et al. Central role for the cardiotonic steroid, marinobufagenin, in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 18.Fridman AI, Matveev SA, Agalakova NI, Fedorova OV, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous ligand of α-1 Na/K-ATPase, is a marker of congestive heart failure severity. J Hypertens. 2002;20:1189–1194. doi: 10.1097/00004872-200206000-00032. [DOI] [PubMed] [Google Scholar]
- 19.Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, Bagrov AY. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17:1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 20.Fedorova OV, Kolodkin NI, Agalakova NI, Namikas AR, Bzhelyansky A, St-Louis J, Lakatta EG, et al. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J Hypertens. 2005;23:835–842. doi: 10.1097/01.hjh.0000163153.27954.33. [DOI] [PubMed] [Google Scholar]
- 21.Averina IV, Tapilskaya NI, Reznik VA, Frolova EV, Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na/K-ATPase inhibitors in patients with preeclampsia. Cell Mol Biol. 2006;52:19–23. [PubMed] [Google Scholar]
- 22.Adair CD, Buckalew V, Taylor K, Ernest JM, Frye AH, Evans C, Veille JC. Elevated endoxin-like factor complicating a multifetal second trimester pregnancy: treatment with digoxin-binding immunoglobulin. Am J Nephrol. 1996;16:529–531. doi: 10.1159/000169054. [DOI] [PubMed] [Google Scholar]
- 23.Adair D, Hinshaw A, Russell G, Rose J, Veille J, Buckalew V. Effects of Fab digoxin-specific antibodies on mean arterial pressure in severe preeclampsia. Am J Hypertens. 1997;10:11A. [Google Scholar]
- 24.Pullen MA, Brooks DP, Edwards RM. Characterization of the neutralizing activity of digoxin-specific Fab toward ouabain-like steroids. J Pharmacol Exp Ther. 2004;310:319–325. doi: 10.1124/jpet.104.065250. [DOI] [PubMed] [Google Scholar]
- 25.Bagrov AY, Fedorova OV, Dmitrieva RI, French AW, Anderson DE. Plasma marinobufagenin-like and ouabain-like immunoreactivity during acute saline volume expansion in anesthetized dogs. Cardiovasc Res. 1996;206:296–305. [PubMed] [Google Scholar]
- 26.Jorgensen PL. Purification and characterization of the Na-K ATPase from outer medulla of mammalian kidney. Biochem Biophys Acta. 1974;336:36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- 27.Fedorova OB, Agalakova NI, Morrell CH, Lakatta EG, Bagrov AY. ANP differentially modulates marinobufagenin-induced sodium pump inhibition in kidney and aorta. Hypertension. 2006;48:1160–1168. doi: 10.1161/01.HYP.0000248129.20524.d0. [DOI] [PubMed] [Google Scholar]
- 28.National Institutes of Health Working Group on Hypertension in Pregnancy . Classification of hypertensive disorders of pregnancy. US Department of Health and Human Services; Bethesda, MD: 1991. [Google Scholar]
- 29.Liu J, Periyasamy SM, Gunning W, Fedorova OV, Bagrov AY, Malhotra D, et al. Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney Int. 2002;62:2118–2125. doi: 10.1046/j.1523-1755.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 30.Fedorova OV, Talan MI, Agalakova NI, Droy-Lefaix M-T, Lakatta EG, Bagrov AY. Reduction in myocardial PKC β2, Na/K-ATPase sensitivity to marinobufagenin and blood pressure in response to cicletanine. Hypertension. 2003;41:505–511. doi: 10.1161/01.HYP.0000053446.43894.9F. [DOI] [PubMed] [Google Scholar]
- 31.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med. 2006;119(7 Suppl 1):S47–S53. doi: 10.1016/j.amjmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Wilson M, Morganti AA, Zervoudakis U, Letcher RL, Romney BM, Von Oyeon P, et al. Blood pressure, the renin–angiotensin aldosterone system and sex steroids throughout normal pregnancy. Am J Med. 1980;86:97–104. doi: 10.1016/0002-9343(80)90178-3. [DOI] [PubMed] [Google Scholar]