Abstract
Previous studies have demonstrated that treating cultured cells with cisplatin (CDDP) upregulated the expression of glutathione (GSH) and its de novo rate-limiting enzyme, glutamate-cysteine ligase (GCL), which consists of a catalytic (GCLC) and a modifier (GCLM) subunits. It has also been shown that many CDDP-resistant cell lines exhibit high levels of GCLC/GCLM and GSH. Since GSH system is the major intracellular regulator of redox conditions that serve as an important detoxification cytoprotector, these results have been taken into considerations that elevated levels of GCL/GSH are responsible for the CDDP resistance. In contrast to this context, we demonstrated here that overexpression of GSH by transfection with expression plasmid containing the GCLC cDNA conferred sensitization to CDDP through upregulation of human copper transporter 1 (hCtr1), which is also a transporter for CDDP. Depleting GSH levels in these transfected cells reversed CDDP sensitivity with concomitant reduction of hCtr1 expression. While rates of Cu transport were also upregulated in the transfected cells, these cells exhibited biochemical signature of Cu deficiency, suggesting that GSH functions as an intracellular Cu-chelator and that overexpression of GSH can alter Cu metabolism. More importantly, our results reveal a new role of GSH in the regulation of CDDP sensitivity. Overproduction of GSH depletes bioavailable Cu pool, leading to upregulation of hCtr1 and sensitization of CDDP transport and cell killing. These findings also have important implications that modulation of intracellular Cu pool may be a novel strategy for improving chemotherapeutic efficacy of platinum-based antitumor agents.
Introduction
Cisplatin (CDDP) has been recognized as an important antitumor agent because of its activity against many human malignancies, including testicular, ovarian, cervical, bladder, head and neck, and small cell lung cancers (SCLC) (Prestayko et al., 1979; Kollmannsberger et al., 2006). However, many patients eventually relapse and develop resistance to the treatment. It is well known that CDDP acts on multiple cellular targets representing diverse signal transduction pathways. It is therefore conceivable that multiple mechanisms have been proposed for CDDP resistance, including reduction of drug transport and increased DNA adduct tolerance and repair (for reviews, see ref. (Giaccone, 2000; Siddik, 2003; Wang and Lippard, 2005; Kartalou and Essigmann, 2001; Kelland, 2007)).
Another CDDP-resistance mechanism that has been widely described in the literature is the detoxification through glutathione (GSH) system. GSH is the most abundant thiol-containing antioxidant (1 ∼ 10 mM). De novo biosynthesis of GSH is controlled by the rate-limiting enzyme, glutamate-cysteine ligase (GCL) which consists of a catalytic (GCLC) and a modifier (GCLM) subunits. Previous studies demonstrated that exposure of cultured cells to CDDP led to the development of CDDP resistance that were closely correlated with increased cellular GSH levels. Moreover, GSH depletion by buthionine sulfoximine (BSO) is associated with increased sensitivity to CDDP. In many cases when GCL mRNA contents were measured, elevated levels of GCL mRNA were also correlated with CDDP resistance. These studies have been widely taken to suggest that intracellular GSH levels play an important role in regulating CDDP resistance (see reviews (Perez, 1998; Rabik and Dolan, 2007; Kartalou and Essigmann, 2001; Kelland, 2007; Stewart, 2007; Siddik, 2003) and references therein in). However, these studies frequently used CDDP-treated cells and the observations were mostly correlation in nature. To investigate the cause-effect relationships between elevated GSH and CDDP sensitivity, we used GCLC-overexpressing cell lines established by stably transfected with GCLC cDNA plasmid. We observed that the transfected cells, which displayed elevated levels of GSH, in fact, exhibited elevated sensitivity to CDDP due to upregulation of its transporter, human Cu transporter (hCtr1) (Ishida et al., 2002; Song et al., 2004). Thus, our results provide a previously undiscovered new role of GSH in the regulation of CDDP sensitivity.
Materials and Methods
Cell Cultures and Transduction with GCLM Inducible Recombinant Adenovirus
SR3A and the development of its GCLC- stably transfected cell lines, SR3A-13, SR3A-14, and SR3A-15, have been described previously (Yamane et al., 1998). The cell lines have been kept in liquid N2. The cells were grown in DMEM containing 10 % fetal calf serum at 37 °C in 5% CO2 atmosphere except otherwise described and 400 μg/ml of G418 for the transfected cell lines. Construction and preparation of recombinant adenovirus, AdE1.tTA.GCLC (previously referred to as AdE1.tTA.γ-GCSh), were described previously (Savaraj et al., 2005).
Determination of Drug Sensitivity
Cells grown in 96-well plates (104 cells/ml medium) were continuously exposed to various concentrations of drugs for 72 hr, 200 μl of MTT (0.5 mg/ml, Sigma) was added to each well, and the plate was incubated for 4 hr. The medium was removed and the formazan products were solubilized with 120 μl DMSO. The cell contents were measured by the absorbance at 570 nm. The IC50 value (μM) was calculated by the Hill plot method with linear regression.
Measurements of Cu and CDDP Uptake
Measurements of 64Cu and CDDP uptake followed the procedures previously described (Song et al., 2004). In brief, 106 cells were plated in a 12-well plate. After 24 hr, cells were treated with 20 μM CDDP or 2 μM 64CuSO4 in the culture medium for various time intervals every hr from 0 to four hours. Cells were harvested for CDDP and 64CuSO4 measurements as follows. Plates were placed on ice and rinsed three times with 3 ml of ice cold PBS. Cell lysis buffer (0.1 % Triton-X100 and 1 % SDS in PBS) in a volume of 800 μl was added to the well, and the radioactivity of cell lysates was determined by scintillation counter. For measurement of CDDP contents, cell lysates were acidified with 200 μl of 0.3N HCl and determined in an atomic absorption spectrometer (SpectrAA300, Varian, Palo Alto, CA). Rates of uptake were calculated from the slopes of plot using amounts of 64Cu of CDDP contents vs time (in hour).
RNase Protection Assay and Immunoblots
The procedures for preparation of RNA and RNase protection assay have been described previously (Song et al., 2004;Yamane et al., 1998). Western blottings were carried out according to the procedures previously described (Song et al., 2004) using the following antibodies: rabbit polyclonal antibodies against hCtr1 (Klomp et al., 2002), ATP7A (Orbigen, San Diego, CA, 1:500), ATP7B (Orbigen, 1:500), Cu/Zn SOD (Calbiochem, Darmstadt, Germany, 1:5,000) and mouse monoclonal β-actin (Pierce, Rockford, IL) antibody.
Small interfering RAN (siRNA) Transfection
Cells (5-10 × 106) were transfected with hCtr1 specific siRNA, a control (scrambled) sequence at a 100 nM concentration or without any siRNA by using Lipofectamine 2000 (Invitrogen). Transfected cells were maintained in the regular culture medium for 2 days and transfection was repeated once more as described earlier. After 24 hr of post-transfection cells were divided in to 96-well plates. Different concentrations of CDDP were added to each well the following day. Cell killing was measured by MTT assay three days thereafter.
Measurements of Cytochrome C Oxidase (CCO) and Superoxide dismutase (SOD1) Activities and Ceruloplasmin (Cp) Enzyme Profile
CCO activity was determined using an assay kit obtained from Sigma according to the procedure provided by the vendor. SOD1 activity was measured by the procedure described by Chen et al (Chen et al., 2001). Measurements of Cp holoenzyme and apoenzyme by nonreducing gel electrophoresis followed the procedures described previously (Hellman et al., 2002; Nose et al., 2006).
Results
Elevated Expression of GSH in GCLC-Transfected Cells Sensitizes Cells to CDDP But Not to Copper
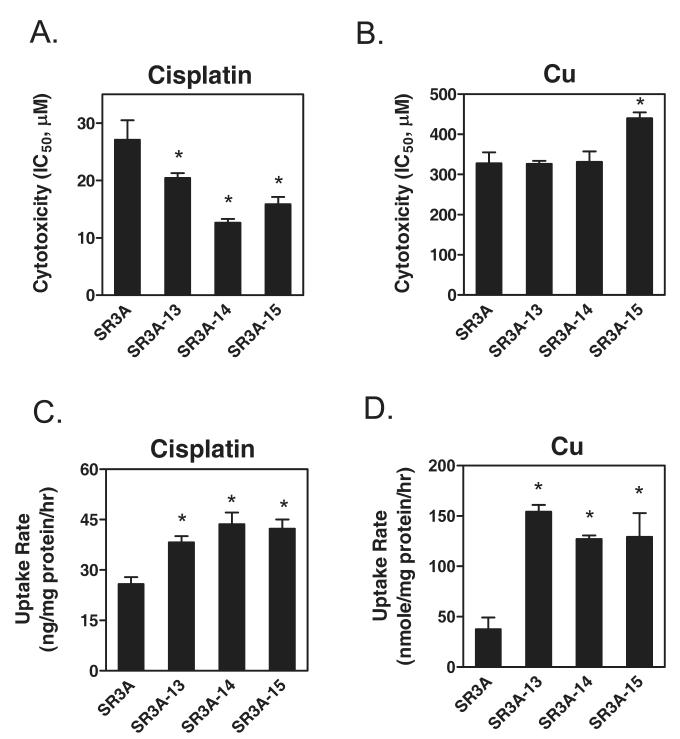
We previously performed transfection of GCLC recombinant cDNA into a human SCLC cell line, SR3A. Several GCLC-overproducing cell lines were obtained. Among them, SR3A-13, SR3A-14, and SR3A-15, exhibited 5.1-, 9.6- and 2.2-fold, respectively, increased GCLC mRNA levels, corresponding to 2.3-, 3.0- and 2.1-fold increases of GSH levels (Yamane et al., 1998) as compared with those in SR3A cell line and the SR3A cells transfected with empty vector. As consistence with previous study (Mulcahy et al., 1995), all the transfected cells exhibited resistance to the alkylating agents, melphalan, and chlorambucil (data not shown). Interestingly, these cells exhibited elevated sensitivity to the toxicity of CDDP (Fig. 1A), but not to Cu except SR3A-15 (Fig. 1B). Similar results were observed in GCLC-transfected rat hepatoma cell lines, H9 and H17 (Yamane et al., 1998) (data not shown). It may be important to note that although SA3A-13 and SR3A-15 exhibited similar levels of elevated GSH, yet the IC50 values were not comparable. This may reflect clonal variation in the context of multiple mechanisms of CDDP resistance as alluded above (Giaccone, 2000; Siddik, 2003; Wang and Lippard, 2005; Kartalou and Essigmann, 2001; Kelland, 2007). These results demonstrated that elevated expression of GSH by transfection enhanced cellular sensitivity to CDDP treatment.
Fig. 1.
Measurements of sensitivities to CDDP (A) and Cu (B) and uptake of CDDP (C) and Cu (D) in SR3A and the GCLC-transfected (SR3A-13, SR3A-14, and SR3A-15) cell lines. Measurements of sensitivities to CDDP and CuSO4, and rates of uptakes of CDDP and 64Cu are described in Experimental procedures. Each bar represents mean ± S.D. *: P<0.01, significantly different from SR3A cells when tested by Student’ t-test.
GCLC-Transfected Cells Showed Increased Transport Activities of CDDP and Cu
To investigate whether enhanced CDDP sensitivity was due to increased drug accumulation, we determined the rates of drug uptake. SR3A and its three transfected lines were treated with 20 μM CDDP or 2 μM 64Cu. At different time intervals, the cellular CDDP and Cu contents were measured by atomic absorption spectroscopy and scintillation counting, respectively. The rates of CDDP (Fig. 1C) and 64Cu (Fig. 1D) transport were both significantly increased in the transfected cells as compared with those in the untransfected SR3A cells. The rates of CDDP efflux were not significantly different among these cells (data not shown).
Recent studies have demonstrated that many copper transporters, including import transporter (hCtr1) and export transporters (ATP7A and ATP7B), are also involved in CDDP transports (for review, see ref. (Kuo et al., 2007)). To investigate the roles of these CDDP transporters in CDDP accumulation in the transfected cells, we first performed RNase protection assay to determine the steady-state mRNA levels encoding these transporters. Fig. 2A shows that levels of hCtr1 mRNA were elevated in the transfected cells, whereas the levels of ATP7A and ATP7B mRNA show no significant difference as compared with those in SR3A cells. Elevated expression of hCtr1 (Fig. 2B), but not ATP7A and ATP7B, was also evidenced by immunoblottings (Fig. 2C). Although it has been demonstrated that hCtr1 exits in oligomeric conformation and previous report showed multiple species of hCtr1 signal in the SDS-PAGE gels (Puig and Thiele, 2002; Kuo et al., 2007), in our hands, we only detected a single band in the SDS gels (Fig. 2B). The elevated expression hCtr1 but not ATP7A, and ATP7B could be detected by RNase protection and by western blotting. The increases of hCtr1 expression in the three transfected cells did not correlate precisely with increase rate of CDDP and Cu uptake, these may due to clonal variations among the clones.
Fig. 2.
Analyses of the expression of various copper transporters in SR3A and GCLC-transfected variants. Messenger RNA (A) and protein (B, C, D) levels of hCtr1, ATP7A, and ATP7B were determined by the RNase protection assay and by immunoblots using 18S RNA and β-actin as loading controls, respectively. Numbers underneath are fold increased estimated by phosphimager analyses. Panel D, Analysis of hCtr1 expression in AdE1.tTA.GCLC-transduced SR3A cells. The cells were treated with recombinant adenovirus at 50 MOI for 24 and 48 hr as indicated either in the presence (+) or absence (-) of 1 μg/ml tet. Total cell lysates were prepared and probed by using anti-GCLC, anti-hCtr1, and anti-β-actin antibodies.
To validate these results, we utilized tetracycline (tet)-inducible GCLC expression system. Recombinant adenoviral vector AdE1.tTA.GCLC (Savaraj et al., 2005) contains two expression cassettes: one constitutively expresses tet-regulatable transactivator (rTA) and the other contains a GCL-expression cassette whose expression is under the control of rTA. In the absence of tet, the constitutively expressed rTA binds to the promoter of GCLC and activates the transcription of GCLC; whereas in its presence, expression of GCLC was inhibited. SR3A cells were transduced with AdE1.tTA.GCLC in the presence (+) or absence (-) of tet for 24 or 48 hrs. Levels of GCLC expression were increased approximately 11- and 30-fold in cells grown in the absence of tet for 24 and 48 hr, respectively, as compared with those treated with tet. Comparable levels of elevated GSH were found in the GCLC-overexpressing samples (Savaraj et al., 2005). Levels of hCtr1 expression were elevated in the GCLC-overexpressing cells (Fig. 2D). These results confirmed that elevated GCLC levels induce the expression of hCtr1. Low levels of hCtr1 upregulation were also observed in cells treated with tet which suppress the induced expression of GCLC. The precise mechanism of this induction is not known, perhaps due to adenoviral transduction-associated oxidative stress which induces the expression of GCLC because similar low levels of GCLC expression were also increased when the cells were transduced with recombinant adenovirus encoding β-globin gene (Savaraj et al., 2005). Alternatively, incomplete repression of GCLC by tet could not be entirely ruled out.
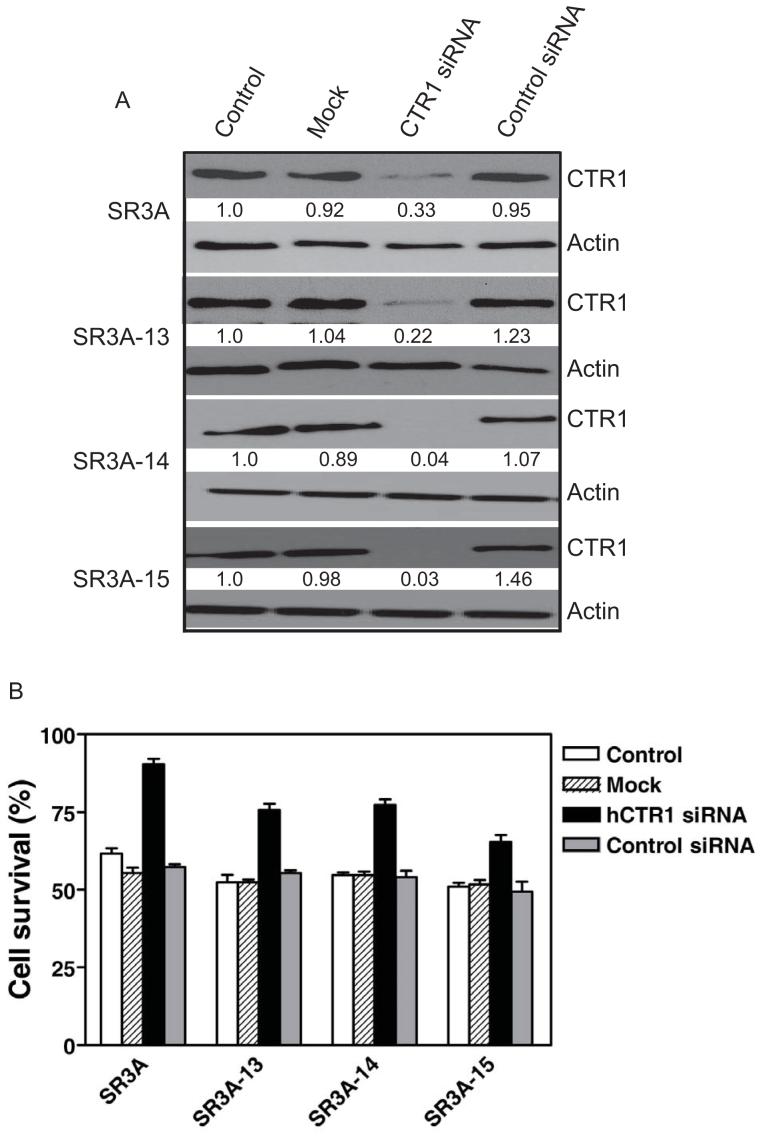
Down Regulation of the Overexpressed hCtr1 mRNA in the GCLC-transfected Cells by siRNA Resulted in Enhanced Resistance to CDDP
While we have previously showed that overexpression of hCtr1 in SCLC cells and their CDDP-resistant variants (SR2 line) enhanced the transport of CDDP and enhanced cell killing by CDDP to these cells (Song et al., 2004). To further demonstrate that the causal link between increased expression of hCtr1 and enhanced sensitivity to CDDP in the GCLC-transfected cells, we used siRNA approach to downregulate the elevated expression of hCtr1. Figure 3 shows that downregulation of hCtr1 in SR3A-13, SR-3A-14, and SR3A-15 by hCtr1 siRNA increased levels of resistance to CDDP, as compared with untreated, mock-treated, and control siRNA-treated cells (Fig. 3B). Similar results were obtained in SR-3A cells. These results demonstrated that the hCtr1 is responsible for the elevated sensitivities to CDDP.
Fig. 3.
hCtr1 knockdown decreases sensitivity against CDDP. A. Cell extracts prepared from cells treated with hCTR1-specific siRNA (100 nM), or mock, scrambled siRNA, and were subjected to 12% SDS-polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and immunoblotted by anti-hCtr1-specific antibody. Anti-β-actin antibody was used as a loading control. Bands were visualized with SuperSignal West Femto western-blot detection kit. B. Reduction of sensitivity of GCLC-transfected cells to CDDP by knockdown of hCtr1. All results are expressed as the means ± SD of the results of at least three experiments.
Elevated GSH Levels Reduces Bioavailable Pool of Cu That Upregulates hCtr1 Expression
Glutathione can form a GSH-Cu(I) complex by directly interacting with its internal cysteine-SH residue. The formation of this complex is almost a spontaneous reaction and requires no enzymatic involvement (Harris, 2000; Freedman et al., 1989), resulting in reduced intracellular bioavailability of Cu. It has been well-established that Ctr1 expression is regulated by intracellular available Cu levels (Kuo et al., 2007). Expression of hCtr1 is upregulated in Cu-depleted cultured cells but downregulated under Cu-replete condition (Song et al., 2008). To investigate whether upregulation of hCtr1 in the GCLC-transfected cells is due to the reduction of available Cu, we analyzed three biochemical markers associated with Cu deficiency. (i) We examined the enzymatic activity of the well-characterized Cu-dependent enzyme, SOD1, which is reduced in Cu-deficient animals (Reeves et al., 2004; Prohaska et al., 2003). As shown in Fig. 4A (open bars), the activity of SOD1 was significantly reduced in the SR3A-13, SR3A-14, and SR3A-15 cells as compared with that in SR3A cells. (ii) The mitochondrial CCO requires Cu for its activity. It has been demonstrated that reduced CCO activity is associated with Cu depletion (Cobine et al., 2006). We found that CCO activity was significantly reduced in the GCLC-transfected cells as compared with that in the non-transfected cells (Fig. 4B). (iii) Ceruloplasmin is a copper-containing ferroxidase that plays an important role in mammalian iron homeostasis. This protein utilizes the bound Cu ions to couple iron oxidation with four-electron reduction of dioxygen. Cultured cells grown in Cu-depleted medium severely comprise the incorporation of seven Cu atoms into the Cp as it transverses the secretary pathway (Hellman et al., 2002), resulting in increased apo-Cp at the expense of holo-Cp. This phenomenon is also observed in the serum from animals of intestinal epithelial cell-specific ctr1 knockout mice (Nose et al., 2006). We performed immunoblot analysis of Cp from cultured media of GCLC-transfected cells following SDS-PAGE under non-reducing conditions which resolve the apo- and holo-enzymes into molecular mass corresponding to 135 kDa and 85 kDa, respectively (Hellman et al., 2002;Nose et al., 2006). As shown in Fig. 4C (control), the majority of Cp secreted into medium from cultured SR3A cells was holoenzyme (apoCP:holoCp is 1:2.5). However, in the GCLC-transfected cells, almost all the secreted Cp was in apo-Cp form. Thus, the analyses of three Cu-containing enzymes strongly suggested that elevated GSH levels reduced intracellular available Cu and led to the induction of hCtr1 expression, resulting in increased uptake of CDDP and the sensitization of cells to CDDP toxicity.
Fig. 4.
Measurements of biochemical signature for Cu availability in the GCL- transfected cells either in the presence of absence of BSO. (A) SOD1 activities; (B) CCO activities; and (C) western blotting analysis of ceruloplasmin. Numbers in Panel C denote the relative band intensity determined by phosphoimerger. *: P<0.05, significantly different from SR3A cells when tested by Student’ t-test.
Reduction of GSH Levels in the GCLC-Transfected Cells Reverses CDDP Sensitivity and hCtr1 Expression
To validate the above results, we used BSO to deplete intracellular GSH levels and analyzed the sensitivities of the treated cells to Cu and CDDP. Fig. 4A and Fig. 4B show that BSO treatment restored the reduced SOD and CCO activities in the GCLC-transfected cells as compared with their respective untransfected cells. Moreover, almost all the secreted Cp in the BSO-treated, GCLC-transfected cells were in the holo-form; whereas the nontransfected SR3A cells contain about 50% holoenzyme (Fig. 4C). These results demonstrated BSO treatments enhanced the bioavailable Cu pools in the GCLC-transfected cells. Taken together, these results showed, for the first time, that intracellular Cu availability is inversely regulated by GSH levels.
Figure 5 shows that treatment of SR3A cells with BSO greatly sensitized SR3A cells to CDDP (90% decrease in IC50 value as compared with those in the untreated cells). Increased CDDP sensitivity is correlated with increased expression of hCtr1 (Fig. 5E). The precise mechanisms underlying the induction of hCtr1 expression by BSO treatment is not known but could be due to a consequence of oxidative stress, as suggested from the results shown in Fig. 2D. Paradoxically, increased hCtr1 expression in the BSO-treated SR3A cells did not show increased uptakes of CDDP and 64Cu (Fig. 5C and Fig. 5D). In contrast, the IC50 values of CDDP in the BSO-treated SR3A-13, SR3A-14, and SR3A-15 cells increased to the level comparable to that of untreated SR3A (Fig. 5A). Strikingly, expression levels of hCtr1 were reduced in the BSO-treated transfected cells as determined by western blotting (Fig. 5E). Reduction of hCtr1 expression in the GCLC-transfected by BSO treatment was correlated with the reduced uptake rates of CDDP and 64Cu (Fig. 5C and 5D). Levels of ATP7A and ATP 7B were not changed in all the cell lines investigated, regardless whether BSO were used or not (Fig. 5E). These results demonstrated that treating the GCLC-transfected cells with GSH-depleting agent reversed the acquired CDDP and Cu sensitivity (Fig. 5A and 5B), further supporting the role of GSH in the regulation of hCtr1 expression and CDDP sensitivity.
Fig. 5.
Determinations of the effects of BSO treatments on the cytotoxicity of CDDP (A) and Cu (B), and rates of uptake of CDDP (C) and Cu (D), expression of hCtr1, ATP7A, and ATP7B by western blots (E) and hCtr1. For cytotoxicity assays, cells grown in 96-well plates (104 cells/well) were continuously exposed to various concentrations of copper and CDDP in the presence or absence of 100 μM BSO. After 72 hr incubation, cytotoxicity was measured by MTT assay. The IC50 value (μM) was calculated by the Hill plot method with linear regression. * p<0.01, significantly different from SR3A cells. #: p<0.01, significantly different from SR3A cell in the presence of BSO.
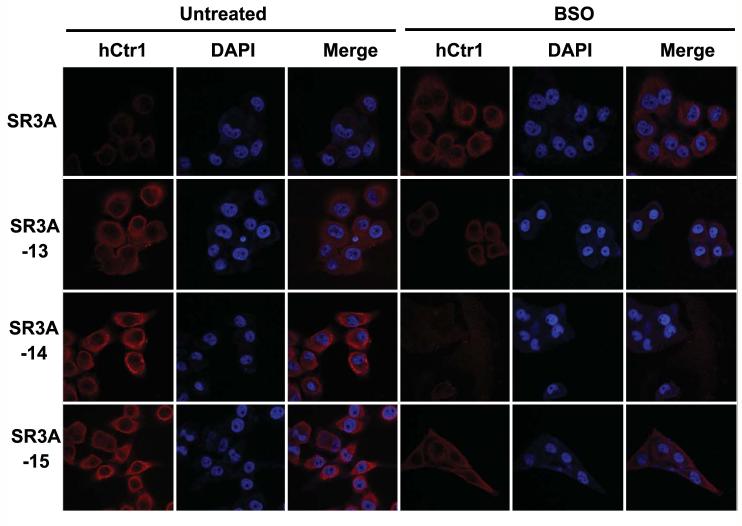
The elevated expression of hCtr1 in the GCLC-transfected cells and down-regulation of hCtr1 in the BSO treatment was also demonstrated by immunofluorescent microcopy (Fig. 6). While the majority of overexpressed hCtr1 was associated with membrane, as consistent with previous observation (Klomp et al., 2002), cytoplasmic location of hCtr1 was also evidenced (Fig. 6). Taken together, these results show that depleting GSH levels by BSO in the GCLC-transfected cells are associated with reduced hCtr1 expression and thus reverse the CDDP sensitivity.
Fig. 6.
Immunofluorescence staining in SR3A and GCLC-transfected cells. SR3A, SR3A-13, SR3A-14, SR3A-15 treated with or without BSO were stained with anti-hCtr1 anti-hCtr1 antibody and counterstained with DAPI for nucleus. Fluorescence signals were viewed by an Nikon Eclipse TE2000 Confocal Microscope.
Discussion
The discovery that hCtr1 can transport platinum (Pt)-based antitumor agents (Ishida et al., 2002) underscores the importance of this metal transporter in cancer chemotherapy. In this communication, we report that overexpression of GSH sensitizes cancer cells to the treatment of CDDP by upregulating hCtr1 expression. While the rates of Cu transport were also elevated in these hCtr1-overproducing cells, no enhanced sensitization to Cu toxicity was found in these cells. These results suggest that the imported Cu was nontoxic, most likely because of detoxification either by direct metallation of GSH or by GSH-mediated sequestration of undefined compartments. Consistent with this notion, the bioavailable Cu was reduced in these cells.
Our results showed, for the first time, that GSH can interfere with intracellular Cu physiology by depleting the pool of bioavailable Cu (Fig. 7), leading to the increased import of CDDP, which enhances the cell killing effects of CDDP. This observation is in contrast to those previously described in the CDDP-treated cells, which showed elevated expression of GCL/GSH. Strikingly speaking, there is no direct proof that increased GCL/GSH is responsible for CDDP resistance, yet this association is commonly made and has almost become a running theme (see reviews in ref. (Siddik, 2003; Kartalou and Essigmann, 2001; Kelland, 2007; Rabik and Dolan, 2007; Stewart, 2007; Perez, 1998)). Several mechanisms have been proposed in previous studies to account for GSH-mediated CDDP resistance: (i) GSH system is a major cellular detoxification machinery by means of redox chemistry, increased CDDP resistance by elevated GSH levels is because of reduced redox conditions (Giaccone, 2000; Siddik, 2003; Wang and Lippard, 2005). However, the mechanisms by which redox regulates CDDP sensitivity has not been vigorously proven. (ii) CDDP may be detoxified by glutathionation and Pt-GSH complex is then eliminated by ATP-dependent efflux pump, MRP/GS-X pump (Ishikawa and li-Osman, 1993; Minamino et al., 1999). However, unlike redox-active metals, such as copper and cadmium ions, reaction of Pt-GSH formation is a very slow process (Ishikawa and li-Osman, 1993) and overexpression of MRP1 in cultured cells did not always confer resistance to CDDP (Cole et al., 1994). (iii) GSH may protect cells by maintaining proper nucleotide pool for DNA repairing system for CDDP-induced DNA damages (Lai et al., 1989).
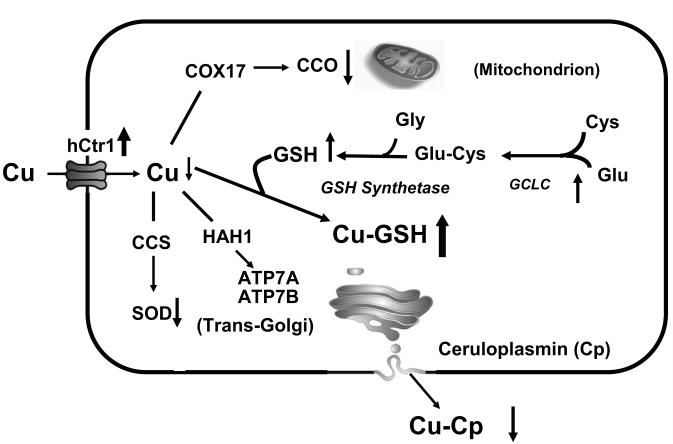
Fig. 7.
Schematic illustration showing the effects of GCLC overexpression on cellular Cu metabolism. Overexpression of GCLC, which catalyzes the ligation of cysteine (Cys) and glutamate (Glu), results in increased GSH levels. Excess GSH functions as a Cu depletor, as evidenced by the reduction of CCO and SOD activity, and holo-ceruloplasmin (Cu-Cp) contents. Intracellular Cu deficiency upregulates hCtr1 expression resulting in elevated sensitivity to CDDP treatment. CCS, HAH1, and COX17 are Cu chaperones that shuffle Cu to their respective targets as indicated by arrows.
It is important to note that all those previous studies were done prior to the discovery of hCtr1 as a CDDP transporter. The expression levels of hCtr1 in these CDDP-resistant variants were not measured. We recently analyzed the expression levels of hCtr1, ATP7A, and ATP7B in five pairs of CDDP-resistant human cell lines by the RNase protection assay. We found that four of these cell lines exhibited reduced levels of hCtr1 mRNA expression to various extents as compared with those in their respective parental cell lines; whereas levels of ATP7A, and ATP7B expression were not reduced (Song et al., 2004). These results demonstrated that reduced expression of hCtr1 mRNA is frequently observed in CDDP-resistant variants.
Normal cells contain millimolar concentrations of endogenous GSH. From stoichiometric considerations, one could argue that these abundant amounts of thiol compound may already be sufficient to neutralize the cytotoxic effects of CDDP which are in micromolar ranges. The several-fold increases of GSH content at most found in the CDDP-treated cells may not be necessarily to have a major impact in the detoxification mechanism of CDDP. We reason that elevated expression of GCL, and thus GSH, in CDDP-treated cells is an oxidative stress-induced phenomenon, because GCLC is a sensor/regulator of ROS imbalance; and its expression can be induced by a wide array of cytotoxic insults, including antitumor agents, carcinogens, metal ions, antioxidants, prooxidants, etc (see (Song et al., 2005), and references therein). In many cases, the elevated expression of GCLC and/or GSH did not confer CDDP resistance in the treated cells.
Our observations that the depleted pool of bioavailable Cu by GSH results in upregulation of hCtr1 expression is consistent with the well-documented phenomenon that intracellular Cu content plays an important role in regulating hCtr1 expression. Available information regarding mechanisms that regulate hCtr1 expression in response to copper concentrations suggests that the regulation is mainly controlled at the posttranslational levels, but the results are controversial (Petris et al., 2003; Eisses et al., 2005). Our results showing that the steady-state levels of hCtr1 mRNA were elevated in the GCLC-transfected cells (Fig. 2A) and downregulated in these cells treated with GSH clearly demonstrate that hCtr1 mRNA levels can be modulated in accordance to intracellular concentration fluctuation. These results are consistent with our recent finding showing upregulation of hCtr1 mRNA in SCLC cells treated with Cu depletor (bathocuproine disulfonic acid, 100 mM for 16 hr) and downregulation of hCtr1 mRNA in SCLC cells treated with Cu (Song et al., 2008). It is important to note that previous studies have also demonstrated that expression of Saccharomyces cerevisiae yCtr1 and yCtr3 [see review in ref (Rutherford and Bird, 2004)] and Drosophila melanogaster dCtrB (Selvaraj et al., 2005) was also regulated by intracellular Cu homeostasis. These findings, collectively, suggest that an evolutionarily conserved intracellular Cu pool-sensing mechanism is involved in the regulation of Ctr1 expression from yeast to mammals. While it has been reported that almost all the intracellular Cu is occupied and free Cu pool is limited to less than one molecule per cell (Rae et al., 1999). In light of current finding, it is important to investigate how intracellular Cu pool is mobilized in response to Cu stress challenges, leading to regulation of Ctr1 expression accordingly requires future investigations.
In summary, we have revealed a novel mechanism on regulation of CDDP sensitivity by GSH. Moreover, our results show that it is feasible to modulate cellular sensitivities to CDDP treatment by using the Cu chelator, GSH. Development of small molecules targeting the pool of intracellular available Cu may be a novel approach toward improving the efficacy of platinum-based antitumor agents. Such approaches have been used in the first line treatment of Cu toxicosis in Wilson’s disease (Das and Ray, 2006) and may be an effective strategy in cancer chemotherapy.
Abbreviations
- SCLC
small cell lung cancer
- GSH
glutathione
- GCL
glutamatecysteine ligase
- α-GCSh
catalytic (heavy) subunit of GCL
- hCtr1
human copper transporter 1
- CDDP
cisplatin
- BSO
buthionine sulfoximine
- CCO
cytrochrome C oxidase
- SOD
superoxide dismutase
- Cp
ceruloplasmin
Footnotes
This work was supported by National Cancer Institute grants CA72404 and CA79085 (to M. T. K.) and CA17762 (Institutional Core) and VA Merit Review Research fund (to N. S.).
References List
- Chen J, Liao C, Mao SJ, Chen T, Weng C. A Simple Technique for the Simultaneous Determination of Molecular Weight and Activity of Superoxide Dismutase Using SDS-PAGE. J Biochem Biophys Methods. 2001;47:233–237. doi: 10.1016/s0165-022x(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Cobine PA, Pierrel F, Winge DR. Copper Trafficking to the Mitochondrion and Assembly of Copper Metalloenzymes. Biochim Biophys Acta. 2006;1763:759–772. doi: 10.1016/j.bbamcr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Cole SP, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM, Deeley RG. Pharmacological Characterization of Multidrug Resistant MRP-Transfected Human Tumor Cells. Cancer Res. 1994;54:5902–5910. [PubMed] [Google Scholar]
- Das SK, Ray K. Wilson’s Disease: an Update. Nat Clin Pract Neurol. 2006;2:482–493. doi: 10.1038/ncpneuro0291. [DOI] [PubMed] [Google Scholar]
- Eisses JF, Chi Y, Kaplan JH. Stable Plasma Membrane Levels of HCTR1 Mediate Cellular Copper Uptake. J Biol Chem. 2005;280:9635–9639. doi: 10.1074/jbc.M500116200. [DOI] [PubMed] [Google Scholar]
- Freedman JH, Ciriolo MR, Peisach J. The Role of Glutathione in Copper Metabolism and Toxicity. J Biol Chem. 1989;264:5598–5605. [PubMed] [Google Scholar]
- Giaccone G. Clinical Perspectives on Platinum Resistance. Drugs. 2000;59(Suppl 4):9–17. doi: 10.2165/00003495-200059004-00002. [DOI] [PubMed] [Google Scholar]
- Harris ED. Cellular Copper Transport and Metabolism. Annu Rev Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
- Hellman NE, Kono S, Mancini GM, Hoogeboom AJ, De Jong GJ, Gitlin JD. Mechanisms of Copper Incorporation into Human Ceruloplasmin. J Biol Chem. 2002;277:46632–46638. doi: 10.1074/jbc.M206246200. [DOI] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the Anticancer Drug Cisplatin Mediated by the Copper Transporter Ctr1 in Yeast and Mammals. Proc Natl Acad Sci U S A. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, li-Osman F. Glutathione-Associated Cis-Diamminedichloroplatinum(II) Metabolism and ATP-Dependent Efflux From Leukemia Cells. Molecular Characterization of Glutathione-Platinum Complex and Its Biological Significance. J Biol Chem. 1993;268:20116–20125. [PubMed] [Google Scholar]
- Kartalou M, Essigmann JM. Mechanisms of Resistance to Cisplatin. Mutat Res. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Kelland L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- Klomp AE, Tops BB, Van D,I, Berger R, Klomp LW. Biochemical Characterization and Subcellular Localization of Human Copper Transporter 1 (HCTR1) Biochem J. 2002;364:497–505. doi: 10.1042/BJ20011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmannsberger C, Nichols C, Bokemeyer C. Recent Advances in Management of Patients With Platinum-Refractory Testicular Germ Cell Tumors. Cancer. 2006;106:1217–1226. doi: 10.1002/cncr.21742. [DOI] [PubMed] [Google Scholar]
- Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The Roles of Copper Transporters in Cisplatin Resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- Lai GM, Ozols RF, Young RC, Hamilton TC. Effect of Glutathione on DNA Repair in Cisplatin-Resistant Human Ovarian Cancer Cell Lines. J Natl Cancer Inst. 1989;81:535–539. doi: 10.1093/jnci/81.7.535. [DOI] [PubMed] [Google Scholar]
- Minamino T, Tamai M, Itoh Y, Tatsumi Y, Nomura M, Yokogawa K, Suzuki H, Sugiyama Y, Ohshima T, Miyamoto K. In Vivo Cisplatin Resistance Depending Upon Canalicular Multispecific Organic Anion Transporter (CMOAT) Jpn J Cancer Res. 1999;90:1171–1178. doi: 10.1111/j.1349-7006.1999.tb00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy RT, Bailey HH, Gipp JJ. Transfection of Complementary DNAs for the Heavy and Light Subunits of Human Gamma-Glutamylcysteine Synthetase Results in an Elevation of Intracellular Glutathione and Resistance to Melphalan. Cancer Res. 1995;55:4771–4775. [PubMed] [Google Scholar]
- Nose Y, Kim BE, Thiele DJ. Ctr1 Drives Intestinal Copper Absorption and Is Essential for Growth, Iron Metabolism, and Neonatal Cardiac Function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Perez RP. Cellular and Molecular Determinants of Cisplatin Resistance. Eur J Cancer. 1998;34:1535–1542. doi: 10.1016/s0959-8049(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Smith K, Lee J, Thiele DJ. Copper-Stimulated Endocytosis and Degradation of the Human Copper Transporter, HCtr1. J Biol Chem. 2003;278:9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- Prestayko AW, D’Aoust JC, Issell BF, Crooke ST. Cisplatin (Cis-Diamminedichloroplatinum II) Cancer Treat Rev. 1979;6:17–39. doi: 10.1016/s0305-7372(79)80057-2. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Geissler J, Brokate B, Broderius M. Copper, Zinc-Superoxide Dismutase Protein but Not MRNA Is Lower in Copper-Deficient Mice and Mice Lacking the Copper Chaperone for Superoxide Dismutase. Exp Biol Med (Maywood) 2003;228:959–966. doi: 10.1177/153537020322800812. [DOI] [PubMed] [Google Scholar]
- Puig S, Thiele DJ. Molecular Mechanisms of Copper Uptake and Distribution. Curr Opin Chem Biol. 2002;6:171–180. doi: 10.1016/s1367-5931(02)00298-3. [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. Molecular Mechanisms of Resistance and Toxicity Associated With Platinating Agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O’Halloran TV. Undetectable Intracellular Free Copper: the Requirement of a Copper Chaperone for Superoxide Dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Ralston NV, Idso JP, Lukaski HC. Contrasting and Cooperative Effects of Copper and Iron Deficiencies in Male Rats Fed Different Concentrations of Manganese and Different Sources of Sulfur Amino Acids in an AIN-93G-Based Diet. J Nutr. 2004;134:416–425. doi: 10.1093/jn/134.2.416. [DOI] [PubMed] [Google Scholar]
- Rutherford JC, Bird AJ. Metal-Responsive Transcription Factors That Regulate Iron, Zinc, and Copper Homeostasis in Eukaryotic Cells. Eukaryot Cell. 2004;3:1–13. doi: 10.1128/EC.3.1.1-13.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaraj N, Wei Y, Unate H, Liu PM, Wu CJ, Wangpaichitr M, Xia D, Xu HJ, Hu SX, Tien KM. Redox Regulation of Matrix Metalloproteinase Gene Family in Small Cell Lung Cancer Cells. Free Radic Res. 2005;39:373–381. doi: 10.1080/10715760400029694. [DOI] [PubMed] [Google Scholar]
- Selvaraj A, Balamurugan K, Yepiskoposyan H, Zhou H, Egli D, Georgiev O, Thiele DJ, Schaffner W. Metal-Responsive Transcription Factor (MTF-1) Handles Both Extremes, Copper Load and Copper Starvation, by Activating Different Genes. Genes Dev. 2005;19:891–896. doi: 10.1101/gad.1301805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: Mode of Cytotoxic Action and Molecular Basis of Resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. Role of Human Copper Transporter Ctr1 in the Transport of Platinum-Based Antitumor Agents in Cisplatin-Sensitive and Cisplatin-Resistant Cells. Mol Cancer Ther. 2004;3:1543–1549. [PubMed] [Google Scholar]
- Song IS, Tatebe S, Dai W, Kuo MT. Delayed Mechanism for Induction of Gamma-Glutamylcysteine Synthetase Heavy Subunit MRNA Stability by Oxidative Stress Involving P38 Mitogen-Activated Protein Kinase Signaling. J Biol Chem. 2005;280:28230–28240. doi: 10.1074/jbc.M413103200. [DOI] [PubMed] [Google Scholar]
- Song IS, Chen HHW, Aiba I, Hossain A, Liang ZD, Klomp LWJ, Kuo MT.Transcription Factor Sp1 Plays an Important Role in the Regulation of Copper Homeostasis in Mammalian Cells Mol Pharmacol(in press).2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ. Mechanisms of Resistance to Cisplatin and Carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Wang D, Lippard SJ. Cellular Processing of Platinum Anticancer Drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Yamane Y, Furuichi M, Song R, Van NT, Mulcahy RT, Ishikawa T, Kuo MT. Expression of Multidrug Resistance Protein/GS-X Pump and Gamma-Glutamylcysteine Synthetase Genes Is Regulated by Oxidative Stress. J Biol Chem. 1998;273:31075–31085. doi: 10.1074/jbc.273.47.31075. [DOI] [PubMed] [Google Scholar]