Abstract
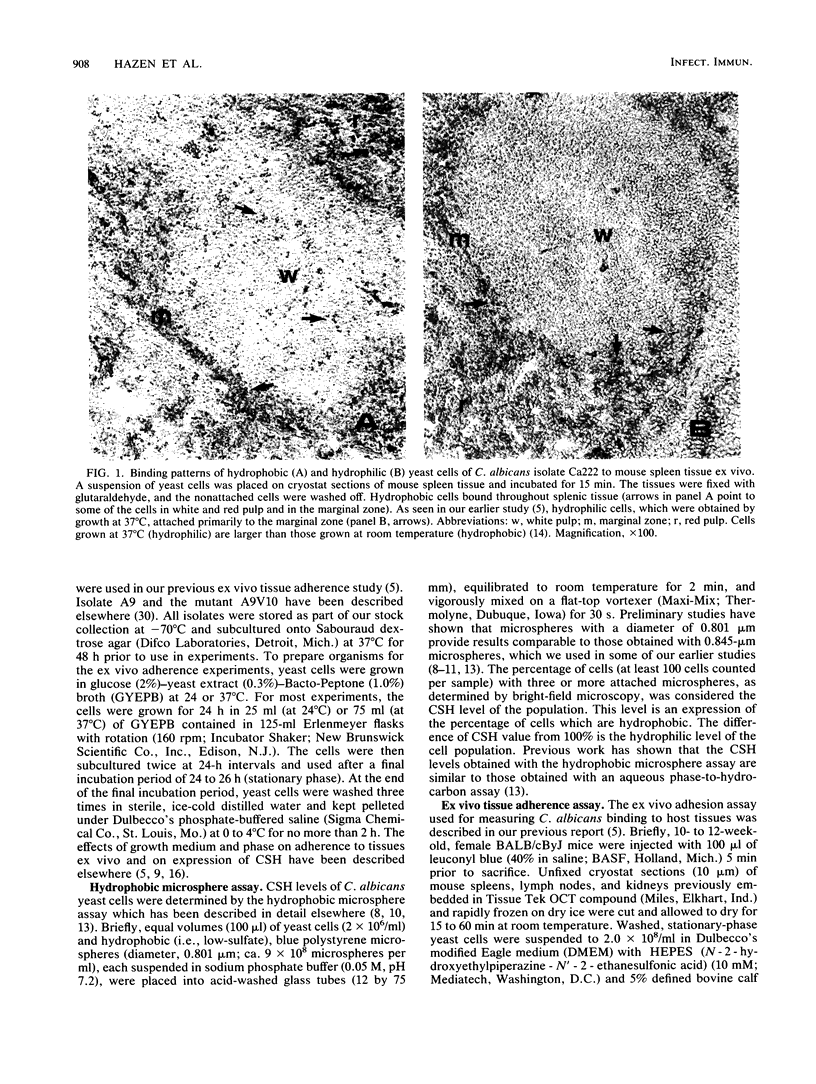
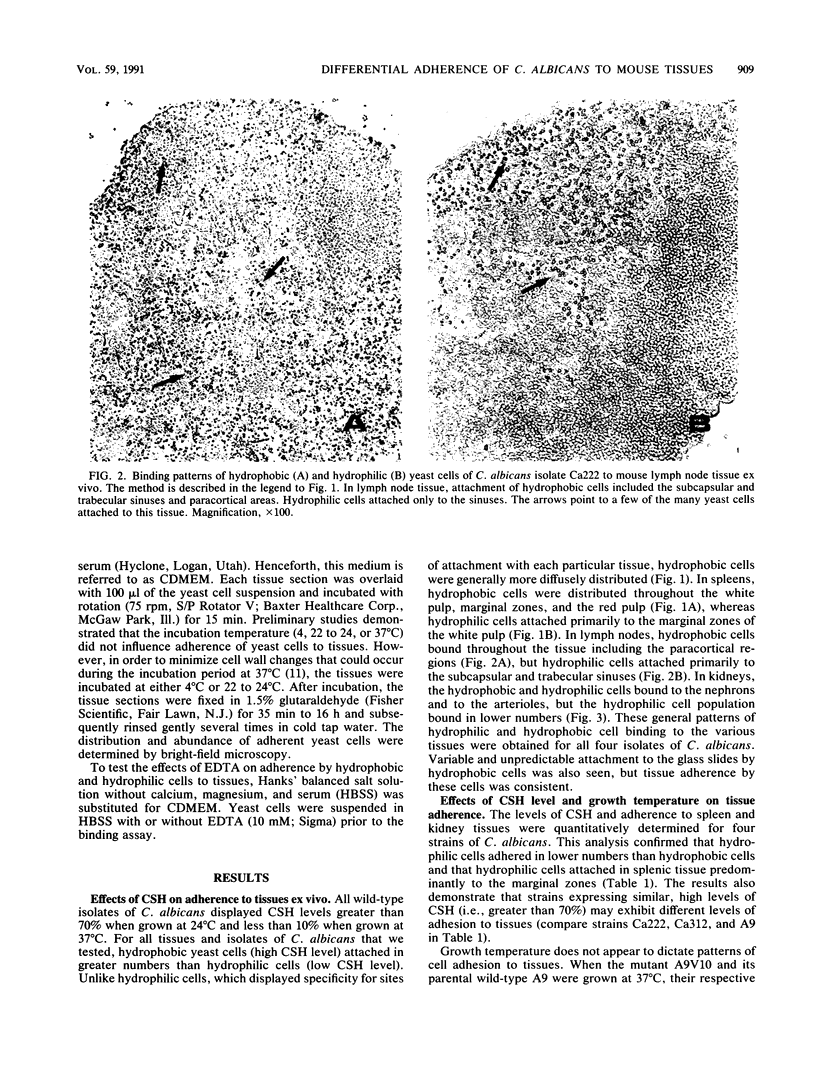
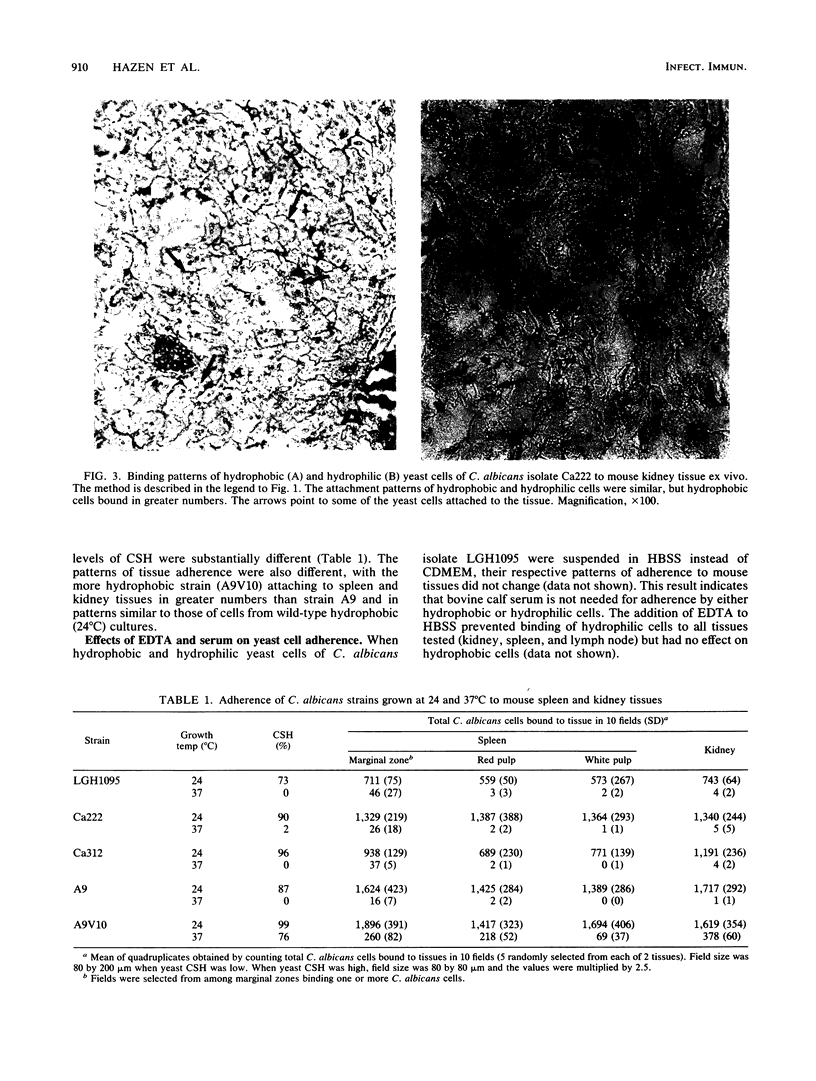
Using an ex vivo binding assay, we previously demonstrated that yeast cells grown at 37 degrees C display binding specificity in mouse spleen, lymph node, and kidney tissues. In spleen and lymph node tissues, binding was predominantly in regions rich in macrophages. Here, we tested the possibility that hydrophobic and hydrophilic cells bind differentially to host tissues. Hydrophobic and hydrophilic yeast cells of four Candida albicans strains were incubated for 15 min at 4 degrees C with cryostat sections of organs that had been rapidly frozen after removal from BALB/cByJ mice. Unattached cells were removed by washing, and the sections were examined. Hydrophobic cells bound diffusely and abundantly to all tissues, while hydrophilic cell attachment was restricted to specific sites. For example, hydrophobic cells bound to the white and red pulp and the marginal zones in spleens, whereas hydrophilic cells attached primarily to the marginal zones. Hydrophobic yeast cells attached throughout lymph node tissue including paracortical areas, but hydrophilic cell attachment occurred primarily at the subcapsular and trabecular sinuses, EDTA inhibited the adherence of hydrophilic cells but not hydrophobic cells to mouse tissues. Hydrophobic C. albicans strains displaying similar levels of hydrophobicity differed quantitatively in their levels of attachment to kidney and spleen tissues, confirming our earlier observation that surface hydrophobicity is not the sole determinant in adherence to host cells. Other studies have shown that hydrophobic and hydrophilic cells display different virulence characteristics related to their surface properties and that hydrophobic cells are more virulent than hydrophilic cells. Taken together, the present results suggest that the enhanced virulence of hydrophobic cells over hydrophilic cells may be due, in part, to the potential of hydrophobic cells to bind throughout various organs following clearance from the bloodstream.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antley P. P., Hazen K. C. Role of yeast cell growth temperature on Candida albicans virulence in mice. Infect Immun. 1988 Nov;56(11):2884–2890. doi: 10.1128/iai.56.11.2884-2890.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., Goldstein L. A., Jutila M. A., Nakache M., Picker L. J., Streeter P. R., Wu N. W., Zhou D., Butcher E. C. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989 Apr;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of a cell surface determinant on Candida albicans as evidenced by an agglutinating monoclonal antibody. Infect Immun. 1984 Mar;43(3):966–972. doi: 10.1128/iai.43.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C., Scollay R. G., Weissman I. L. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979 Nov;123(5):1996–2003. [PubMed] [Google Scholar]
- Cutler J. E., Brawner D. L., Hazen K. C., Jutila M. A. Characteristics of Candida albicans adherence to mouse tissues. Infect Immun. 1990 Jun;58(6):1902–1908. doi: 10.1128/iai.58.6.1902-1908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D. Fungal surfaces: effects of interactions with phagocytic cells. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S428–S431. doi: 10.1093/cid/10.supplement_2.s428. [DOI] [PubMed] [Google Scholar]
- Eigentler A., Schulz T. F., Larcher C., Breitwieser E. M., Myones B. L., Petzer A. L., Dierich M. P. C3bi-binding protein on Candida albicans: temperature-dependent expression and relationship to human complement receptor type 3. Infect Immun. 1989 Feb;57(2):616–622. doi: 10.1128/iai.57.2.616-622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen B. W., Hazen K. C. Dynamic expression of cell surface hydrophobicity during initial yeast cell growth and before germ tube formation of Candida albicans. Infect Immun. 1988 Sep;56(9):2521–2525. doi: 10.1128/iai.56.9.2521-2525.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen B. W., Hazen K. C. Modification and application of a simple, surface hydrophobicity detection method to immune cells. J Immunol Methods. 1988 Mar 16;107(2):157–163. doi: 10.1016/0022-1759(88)90214-1. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Hazen B. W. Temperature-modulated physiological characteristics of Candida albicans. Microbiol Immunol. 1987;31(6):497–508. doi: 10.1111/j.1348-0421.1987.tb03112.x. [DOI] [PubMed] [Google Scholar]
- Hazen K. C., Lay J. G., Hazen B. W., Fu R. C., Murthy S. Partial biochemical characterization of cell surface hydrophobicity and hydrophilicity of Candida albicans. Infect Immun. 1990 Nov;58(11):3469–3476. doi: 10.1128/iai.58.11.3469-3476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen K. C., Plotkin B. J., Klimas D. M. Influence of growth conditions on cell surface hydrophobicity of Candida albicans and Candida glabrata. Infect Immun. 1986 Oct;54(1):269–271. doi: 10.1128/iai.54.1.269-271.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N., McGuiggan P. M. Forces between surfaces in liquids. Science. 1988 Aug 12;241(4867):795–800. doi: 10.1126/science.241.4867.795. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Butcher E. C. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2244–2248. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Parkkinen J., Hacker J., Finne J., Pere A., Rhen M., Holthöfer H. Binding of Escherichia coli S fimbriae to human kidney epithelium. Infect Immun. 1986 Nov;54(2):322–327. doi: 10.1128/iai.54.2.322-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Virkola R., Westurlund B., Holthöfer H., Parkkinen J. Tissue tropism of Escherichia coli adhesins in human extraintestinal infections. Curr Top Microbiol Immunol. 1990;151:115–127. doi: 10.1007/978-3-642-74703-8_6. [DOI] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Marshall K. C. Adsorption and adhesion processes in microbial growth at interfaces. Adv Colloid Interface Sci. 1986 Jun;25(1):59–86. doi: 10.1016/0001-8686(86)80002-1. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Holthöfer H., Saraneva T., Rhen M., Väisänen-Rhen V., Korhonen T. K. Location of adhesion sites for P-fimbriated and for 075X-positive Escherichia coli in the human kidney. Microb Pathog. 1986 Apr;1(2):169–180. doi: 10.1016/0882-4010(86)90019-7. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Prieto J., Rincon J., Timonen T., Lundberg C., Lindbom L., Asjö B., Gahmberg C. G. Leukocyte-cell adhesion: a molecular process fundamental in leukocyte physiology. Immunol Rev. 1990 Apr;114:67–108. doi: 10.1111/j.1600-065x.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Delga J. M., Wadsworth E., Walsh T. J., Kwon-Chung K. J., Calderone R., Lipke P. N. Isolation and characterization of cell surface mutants of Candida albicans. Infect Immun. 1990 Jun;58(6):1552–1557. doi: 10.1128/iai.58.6.1552-1557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock T. A., Stoolman L. M., Rosen S. D. Phosphomannosyl-derivatized beads detect a receptor involved in lymphocyte homing. J Cell Biol. 1987 Mar;104(3):713–723. doi: 10.1083/jcb.104.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]






