Abstract
The rostral fastigial nucleus (RFN) of the cerebellum is thought to play an important role in postural control, and recent studies in conscious nonhuman primates suggest that this region also participates in the sensory processing required to compute body motion in space. The goal of the present study was to examine the dynamic and spatial responses to sinusoidal rotations in vertical planes of RFN neurons in conscious cats, and determine if they are similar to responses reported for monkeys. Approximately half of the RFN neurons examined were classified as graviceptive, since their firing was synchronized with stimulus position and the gain of their responses was relatively unaffected by the frequency of the tilts. The large majority (80%) of graviceptive RFN neurons were activated by pitch rotations. Most of the remaining RFN units exhibited responses to vertical oscillations that encoded stimulus velocity, and approximately 50% of these velocity units had a response vector orientation aligned near the plane of a single vertical semicircular canal. Unlike in primates, few feline RFN neurons had responses to vertical rotations that suggested integration of graviceptive (otolith) and velocity (vertical semicircular canal) signals. These data indicate that the physiological role of the RFN may differ between primates and lower mammals. The RFN in rats and cats in known to be involved in adjusting blood pressure and breathing during postural alterations in the transverse (pitch) plane. The relatively simple responses of many RFN neurons in cats are appropriate for triggering such compensatory autonomic responses.
Keywords: Vestibular, Semicircular canal, Otolith organ, Vestibulo-autonomic reflex
Introduction
The fastigial nucleus of the cerebellum comprises two distinct regions, both of which include neurons that respond robustly to vestibular stimulation (Gardner and Fuchs, 1975; Büttner et al., 1991; Shaikh et al., 2005). The caudal fastigial nucleus contains neurons with eye movement-related activity, and coordinates oculomotor responses (Gardner and Fuchs, 1975; Büttner et al., 1991; Robinson and Fuchs, 2001; Brettler and Fuchs, 2002; Shaikh et al., 2005). In contrast, neurons in the rostral fastigial nucleus (RFN) lack firing related to eye movements, and are believed to participate in the control of gait and posture (Büttner et al., 1991; Thach et al., 1992; Siebold et al., 1997; Mori et al., 1998; 2004), presumably through the extensive projections of these cells to the lateral vestibular nucleus and the medial medullary reticular formation (Batton et al., 1977; Carleton and Carpenter, 1983). Furthermore, recent studies in nonhuman primates indicate that the RFN plays an important role in computing body motion in space and determining spatial orientation (Kleine et al., 2004; Shaikh et al., 2004; 2005). In addition, evidence from experiments in rats, cats, and goats suggests that the RFN participates in regulating breathing (Huang et al., 1977; Lutherer and Williams, 1986; Xu and Frazier, 1995; 1997; 2000; 2002; Martino et al., 2006a; 2006b) and blood distribution in the body (Doba and Reis, 1972; 1974; Huang et al., 1977) during postural alterations.
The first experiments considering the responses of RFN neurons to vestibular stimulation were conducted using the decerebrate cat preparation (Ghelarducci, 1973; Ghelarducci et al., 1974; Erway et al., 1978; Favilla et al., 1980; Stanojevic et al., 1980; Stanojevic, 1981). About a quarter of the cells responded to static ear-down tilt (Ghelarducci, 1973), although the activity of two-thirds of the units was modulated by low-frequency sinusoidal roll rotations that presumably were more effective in activating otolith afferents and additionally provided a minor stimulus to the vertical semicircular canals (Stanojevic et al., 1980). In addition, 65% of RFN cells were activated by horizontal rotations (Favilla et al., 1980). However, since the variety of rotational stimuli used during these studies was limited (e.g., pitch rotations were not employed) and the animals were not conscious, it is difficult to draw firm conclusions regarding the processing of vestibular signals by feline RFN neurons from these data.
A more extensive analysis of the responses of anterior vermis Purkinje cells to vestibular stimulation has been conducted in decerebrate cats (Manzoni et al., 1995; Pompeiano et al., 1997); many Purkinje cells in the anterior vermis have projections to the RFN. These studies employed off-vertical axis rotations that selectively activate otolith organs (Manzoni et al., 1995) or constant-amplitude tilts whose direction moves around the animal at a constant speed (“wobble” stimuli), which stimulate both the otolith organs and vertical semicircular canals (Pompeiano et al., 1997). Anterior vermis Purkinje cells responded to a wide array of tilt directions, and a majority of the neurons were only activated by rotations in the clockwise (CW) or counterclockwise (CCW) direction or had unequal responses to the two directions of movement. These data suggest that the neurons receive vestibular inputs with different spatial and temporal properties, and thus can be classified as spatiotemporal convergence (STC) units. However, it is yet to be determined whether neurons in the feline RFN display analogous STC behavior.
RFN neuronal responses to vestibular stimulation have recently been characterized extensively in conscious nonhuman primates (Büttner et al., 1991; Siebold et al., 1997; Büttner et al., 1999; Kleine et al., 1999; Siebold et al., 1999; 2001; Zhou et al., 2001; Wilden et al., 2002; Büttner et al., 2003; Kleine et al., 2004; Shaikh et al., 2004; 2005). Many RFN neurons in this animal model were determined to receive otolith organ inputs, in accordance with their responses to vertical tilts (Siebold et al., 1997; Büttner et al., 1999) or linear translation (Zhou et al., 2001; Shaikh et al., 2004; 2005). Furthermore, based on the dynamics of RFN neuronal responses to vertical rotations (Siebold et al., 1997; 2001) or translations combined with yaw rotations (Shaikh et al., 2005), it was concluded that convergence of semicircular canal and otolith inputs onto the cells is common. Moreover, many RFN neurons exhibited STC behavior in response to vestibular stimulation (Kleine et al., 1999; Siebold et al., 1999; Zhou et al., 2001; Wilden et al., 2002). During vertical rotations, most units in the primate RFN had response vector orientations near the roll plane or the planes of the vertical semicircular canals; only 13% were best activated by oscillations in the transverse (pitch) plane (Siebold et al., 1997). In particular, few cells that responded to pitch rotations were characterized as receiving otolith inputs, based on having responses synchronized with stimulus position (Siebold et al., 1997). Studies using linear translation as a stimulus also showed that only a small fraction of RFN neurons in monkeys is best activated by fore-aft accelerations (Shaikh et al., 2005).
Nonhuman primates differ from other mammals in their typical postural orientation: monkeys usually remain semi-erect in a vertical stance, whereas other species such as cats are normally in a horizontal position. Thus, comparing vestibular processing by the RFN in nonhuman primates and felines is likely to provide insights about the physiological role of this nucleus. The goal of the present study was to determine the dynamic and spatial responses to rotations in vertical planes of RFN neurons in conscious cats, and to contrast these findings to those previously obtained in monkeys (e.g., (Siebold et al., 1997).
Experimental Procedures
All procedures on animals performed in this study were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee, and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were conducted on three purpose-bred adult female cats obtained from Liberty Research (Waverly, NY, USA). Animals were spayed prior to being included in this study to eliminate cyclic changes in hormonal levels.
Animals underwent an aseptic surgery that employed standard techniques and incorporated anesthetic and post-surgical procedures we have employed in many previous studies (e.g., Wilson et al., 2006; Arshian et al., 2007). A fixation plate was mounted on the skull so that the head could subsequently be immobilized during recordings. Silver/silver-chloride electrodes were implanted adjacent to each eye for monitoring the electrooculogram (EOG). A craniotomy with a diameter of 1 cm was performed at the midline of the posterior aspect of the skull, and a recording chamber (David Kopf Instruments, Tujunga, CA, USA) was lowered using a microdrive to stereotaxic coordinates that would permit access to the RFN, and attached to the skull adjacent to the craniotomy using Palacos® bone cement (Zimmer, Warsaw, IN, USA) and stainless steel screws. The chamber was tilted at an 8° angle relative to the frontal stereotaxic plane so that electrodes would course slightly rostrally as they were lowered.
Prior to recordings, the animals were trained over a period of 1–2 months to be restrained on a tilt table during sinusoidal rotations in vertical planes at frequencies of 0.02–2 Hz and maximal amplitudes ranging from 5° at high frequencies to 20° at low frequencies. The head was immobilized by inserting a screw into the nut fixed to the animal’s skull; the head was pitched 15° down from the stereotaxic plane to bring the plane of the vertical canals close to vertical and minimize horizontal canal stimulation during vertical rotations. The torso was enclosed in a cylindrical tube, and straps placed around the animal’s body ensured that its position on the table did not change during rotations.
All recordings were conducted in a dimly lit room; the visual field available to the animal was rotated with its body, such that no visual cues regarding body position in space were available. An X-Y positioner was attached to the recording chamber and used to maneuver an 8–10 MΩ epoxy-insulated tungsten microelectrode (Frederick Haer, Bowdoin, ME, USA), which was inserted through the dura via a 25-gauge guide tube, and lowered into the RFN using a David Kopf model 650 hydraulic microdrive. Neural activity was amplified by a factor of 10,000, filtered with a bandpass of 300–10,000 Hz, and led into a window discriminator for the delineation of spikes from single units. The output of the window discriminator was led into a 1401-plus data collection system (Cambridge Electronic Design, Cambridge, UK) and Macintosh G4 computer (Apple Computer, Cupertino, CA, USA) running Spike-2 software (Cambridge Electronic Design); the sampling rate was 10,000 Hz.
When a unit was encountered, its spontaneous firing was recorded along with the EOG, which was amplified by a factor of 1000 and sampled at 1000 Hz. After a unit was verified to lack activity correlated with voluntary eye movements, we recorded its responses to tilting the entire animal about the pitch and roll axes using a servo-controlled hydraulic tilt table (Neurokinetics, Pittsburgh, PA, USA), as in our previous studies (e.g., Jian et al., 2002). The plane of tilt that produced maximal modulation of a unit’s firing rate (response vector orientation) was first determined with the use of the wobble stimulus, a constant-amplitude tilt whose direction moves around the animal at a consistent speed (Schor et al., 1984). The direction of the response vector orientation lies midway between the maximal response directions to CW and CCW wobble stimulation, because the phase differences between stimulus and response are reversed during the two directions of rotation (Schor et al., 1984). Thus, by consideration of both responses, these phase differences could be accounted for. Subsequently, the response vector orientation was confirmed by comparing the gain of responses to tilts in the roll and pitch planes. After a unit’s response vector orientation was established, planar tilts at or near this orientation were used to study the dynamics of the vestibular response (i.e., response gain and phase across stimulus frequencies). Response dynamics were routinely determined over the frequency range of 0.1–1 Hz or 0.05–0.5 Hz; the amplitude of rotations was usually 2.5–10° at frequencies ≥ 0.5 Hz, and 10°–20° at frequencies ≤ 0.2 Hz. The following number of sweeps were typically averaged, depending on the magnitude of responses: 3–5 at 0.02 Hz, 5–25 at 0.05–0.1Hz, 15–30 at 0.2 Hz, 25–50 at 0.5 Hz, and 75–150 at 1 Hz.
For each unit, spontaneous firing rate and coefficient of variation of firing rate (CV; standard deviation of interval between spikes divided by mean interval between spikes) were determined from recordings performed in the absence of stimulation. Neural activity recorded during rotations in vertical planes was binned (1000 bins/cycle) and averaged over the sinusoidal stimulus period. Sine waves were fitted to responses with the use of a least-squares minimization technique (Schor et al., 1984). The response sinusoid was characterized by two parameters: phase shift from the stimulus sinusoid (subsequently referred to as phase) and amplitude relative to the stimulus sinusoid (subsequently referred to as gain). Gain and phase measurements were then corrected for the dynamics of the tilt table. Responses were considered to be significant if the signal-to-noise ratio (see (Schor et al., 1984) for method of calculation) was > 0.5 and only the first harmonic was prominent (see Fig. 1 and Fig. 2 for examples of significant responses). Statistical analyses were performed using Prism 5 software (GraphPad Software, San Diego, CA, USA). Pooled data are presented as means ± one standard error.
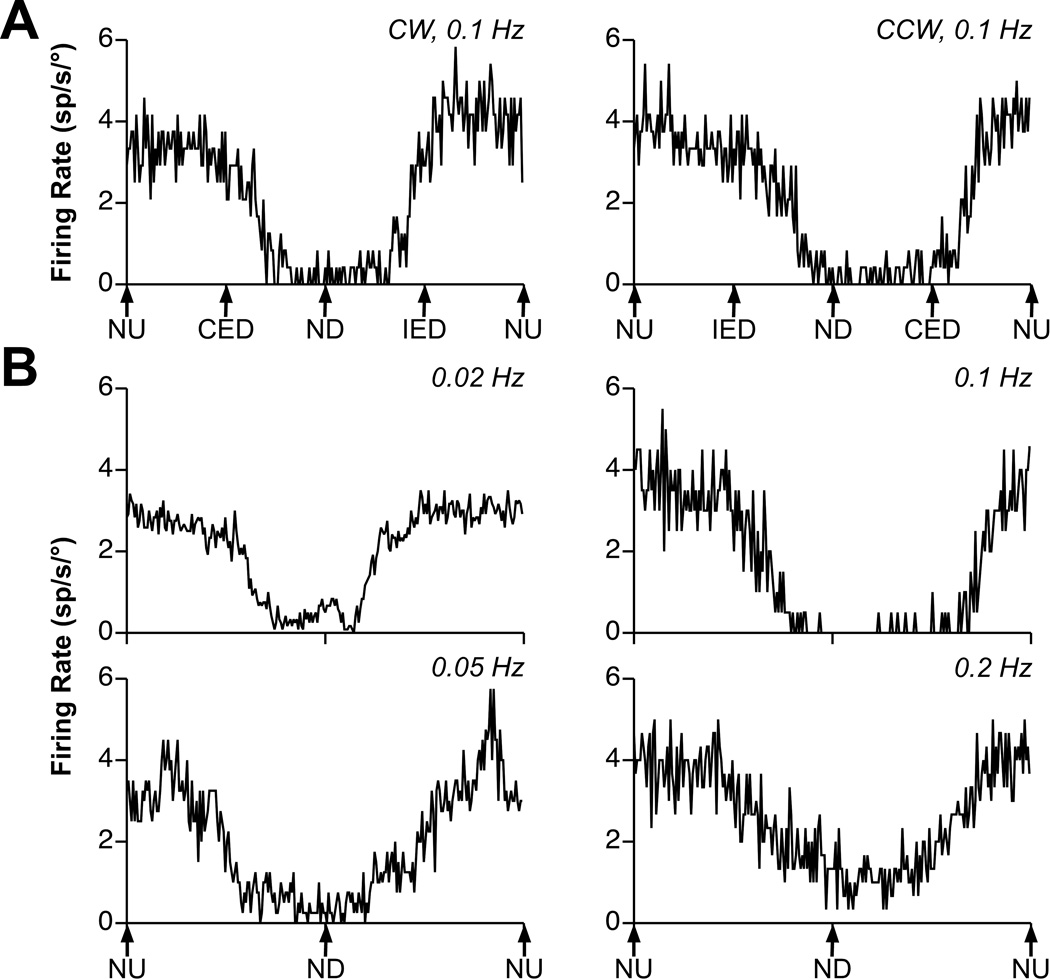
Fig. 1. Examples of averaged responses of a graviceptive RFN neuron to vertical vestibular stimulation.
A: responses to clockwise (CW) and counterclockwise (CCW) wobble stimuli delivered at a frequency of 0.1 Hz and amplitude of 10°. Traces represent the average of 6 sweeps. During wobble stimuli, the animal’s body is always tilted from upright by the same amount, but the direction of tilt moves around the animal at a consistent speed. For example, during CW wobble, the body is first tilted nose-up (NU), then contralateral ear-down (CED), then nose-down (ND), and then ipsilateral ear-down (IED). Note that the gains of the responses to CW and CCW wobble were nearly identical (~2 spikes/sec/°). B: responses to rotations in the pitch plane delivered at 0.02–0.2 Hz. The tilt amplitude was 15° at 0.02 Hz and 10° at other frequencies. The number of sweeps averaged to produce each trace increased with advancing stimulus frequency, with 4 being included at 0.02 Hz, 5 at 0.05–0.1 Hz, and 15 at 0.2 Hz. The responses at every frequency were near stimulus position: there was a 22° phase lead with respect to stimulus position at 0.02 Hz, a 7° phase lead at 0.05 Hz, a 28° phase lag at 0.1 Hz, and a 10° phase lag at 0.2 Hz.
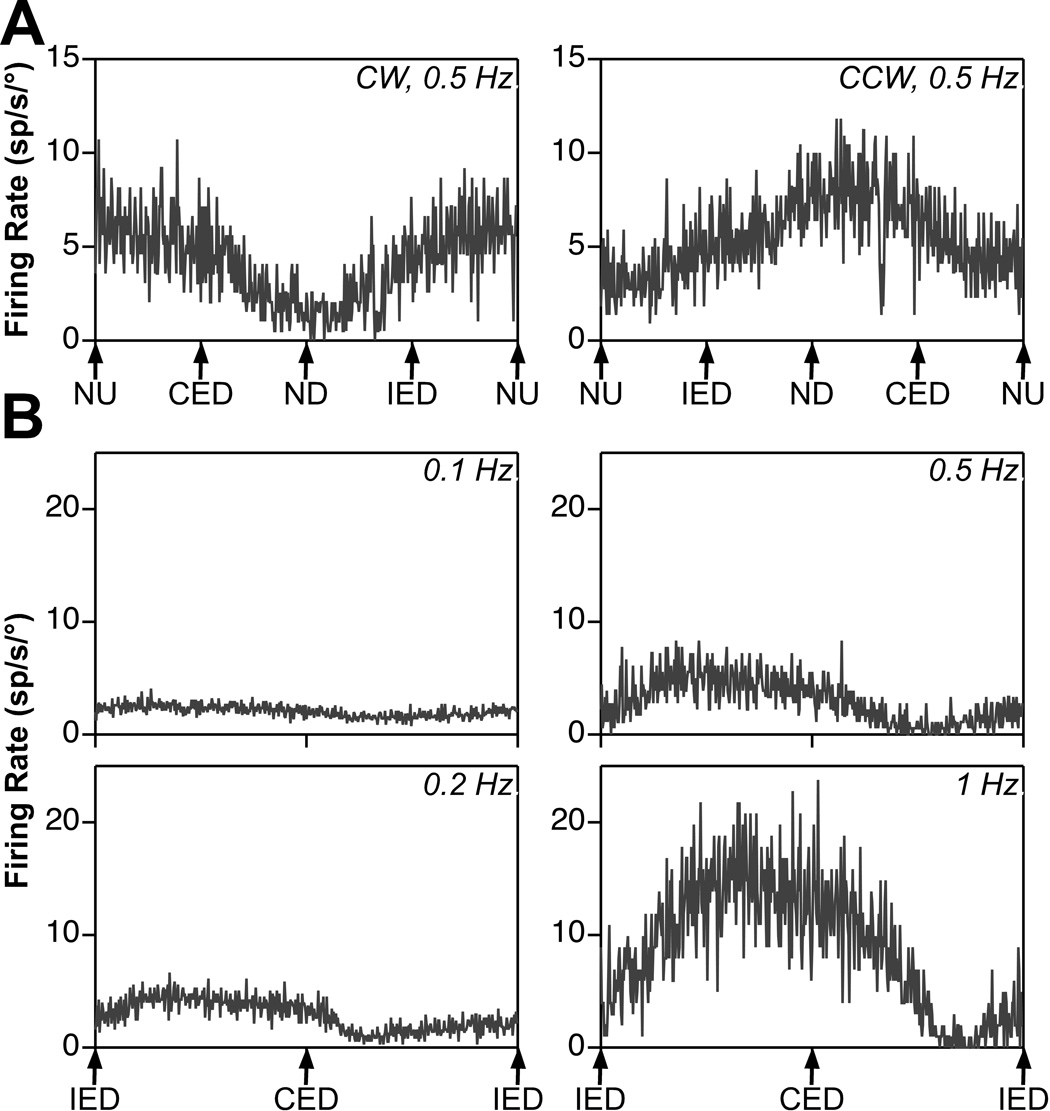
Fig. 2. Examples of averaged responses of an RFN neuron with velocity-related activity during rotations in vertical planes.
A: responses to clockwise (CW) and counterclockwise (CCW) wobble stimuli delivered at a frequency of 0.5 Hz and amplitude of 10°. The gains of the two responses were nearly identical: 2.6 spikes/sec/°. Traces reflect the average of ~50 sweeps. B: responses to rotations in the roll plane delivered at 0.1–1 Hz. The tilt amplitude was 20° at 0.1 Hz, 15° at 0.2 Hz, 10° at 0.5 Hz, and 5° at 1 Hz. The number of sweeps averaged to produce each trace increased with advancing stimulus frequency, with 25 being included at 0.1 Hz and 100 at 1 Hz. The responses at every frequency were near stimulus velocity (i.e., led stimulus position by ~90°); there was a 112° phase lead with respect to stimulus position at 0.1 Hz, a 96° phase lead at 0.2 Hz, a 92° phase lead at 0.5 Hz, and a 115° phase lead at 1 Hz. Abbreviations: CED, contralateral ear down roll; IED, ipsilateral ear down roll; ND, nose down pitch; NU, nose up pitch.
After experimental procedures were completed for an animal, electrolytic lesions were made at defined coordinates by passing a 20 µA negative current for 60 sec through a 0.5 MΩ tungsten electrode, so that recording locations could be reconstructed. Approximately one week later, the animals were deeply anesthetized by injecting 40 mg/kg pentobarbital sodium i.p., and perfused transcardially with 10% formalin. Sections of the brainstem and cerebellum (50 µm thick) were made in the transverse plane and stained with thionine. Locations of recorded neurons were reconstructed on camera lucida drawings of sections with reference to placement of electrolytic lesions, relative locations of electrode tracks, and microelectrode depth.
Results
Neural activity was recorded in 3 animals, from 94 neurons in the left and right RFN whose firing was modulated by whole-body rotations in vertical planes. The response dynamics of 47 of the units were characterized in detail, whereas the other cells were lost before our standard stimulus battery could be completed (“uncharacterized” cells). At minimal, however, all neurons included in the sample were tested using both CW and CCW wobble stimuli, usually at 0.2 or 0.5 Hz, and the response vector orientation was confirmed by considering responses to rotations in single planes. None of the neurons included in the data sample or cells in their vicinity either had firing related to eye movements or exhibited complex spike activity, indicating that recordings occurred in the RFN instead of more caudal parts of the nucleus or the overlying cerebellar cortex. The mean spontaneous firing rate of the RFN neurons sampled was 32±2 spikes/s, and the mean CV of firing rate was 0.8±0.05.
Fig. 1A shows examples of the responses of one neuron to the “wobble” stimulus, a constant-amplitude tilt whose direction moves around the animal at consistent speed. The response to CW wobble was maximal when the body was tilted NU, whereas the response to CCW wobble was maximal when the body was tilted in a direction between NU and IED. Based on these responses, the response vector orientation was calculated to be −72° (18° from the NU and 72° from the IED directions). Fig. 1B illustrates the responses of the cell to rotations in the pitch plane at frequencies of 0.02–0.2 Hz. The response gain remained nearly consistent over this range of frequencies, and the response phase was near stimulus position during all trials.
Data recorded from another unit with different responses to vertical vestibular stimulation are illustrated in Fig. 2. Fig 2A shows the responses of the cell to “wobble” rotations. During both CW and CCW rotations, the response occurred in advance of contralateral ear down roll. From these data, the response vector orientation was determined to be just 10° displaced from the roll plane (−170°). Fig. 2B illustrates the responses of the neuron to rotations in the roll plane at frequencies of 0.1–1 Hz. The response gain with respect to stimulus position increased 11.6-fold over this range of frequencies, and the response phase was near stimulus velocity (led stimulus position by ~90°) during all trials.
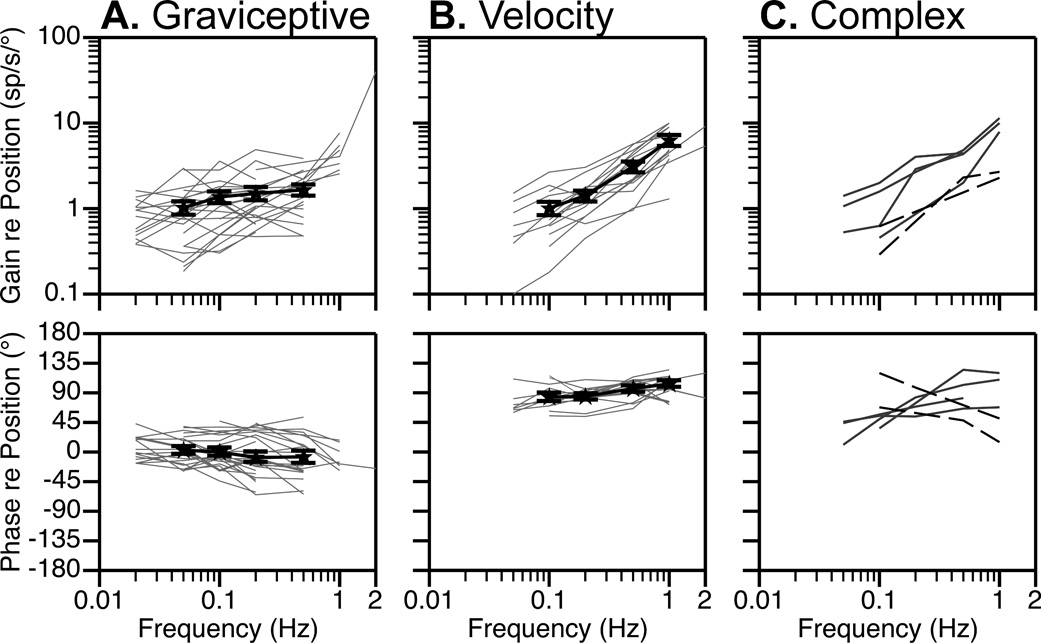
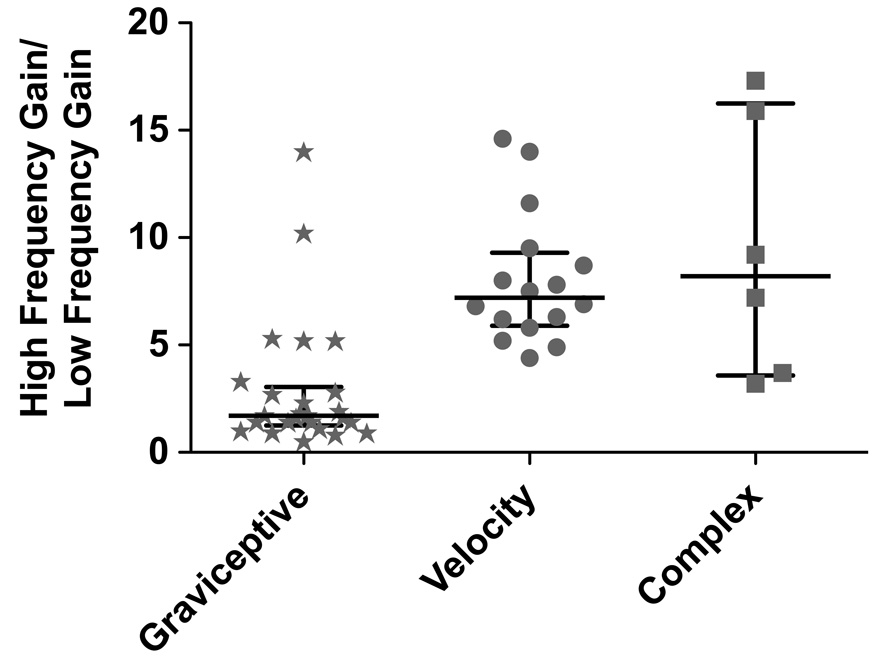
Fig. 3 contains Bode plots that illustrate the dynamic properties of RFN neuronal responses to rotations in fixed planes near the response vector orientation. Both response gain and phase are plotted with respect to stimulus position. Fig. 3A shows data for the 25 of the 47 well-characterized cells (53%) that we identified as “graviceptive” by having response phases that led stimulus position by no more than 45° at any frequency; examples of graviceptive responses are shown in Fig. 1B. A small subset of these neurons (4/25) developed modest phase lags (45°–65°) at higher stimulus frequencies. The relative increase in response gain per stimulus decade (usually determined for the frequency range of 0.1–1 Hz or 0.05–0.5 Hz) for the neurons is shown in Fig. 4. For most graviceptive cells, the gain increased < 2-fold per stimulus decade; a median 1.7-fold relative gain increase occurred.
Fig. 3. Bode plots illustrating the dynamic properties of RFN neuronal responses to rotations at multiple frequencies.
Response gain and phase are plotted relative to stimulus position. In panels A and B, thin gray lines designate responses of individual neurons, whereas thick black lines show averaged data for all units. Error bars designate one standard error. A: graviceptive neurons with response phases that led stimulus position by no more than 45° at any frequency. B: velocity units whose response phases were within 35° of stimulus velocity at all frequencies. C: neurons with more complex response dynamics. The responses of 4 of these neurons (indicated by solid lines) were in phase with stimulus position when low rotation frequencies were employed, but were in phase with stimulus velocity when high frequencies were delivered. The responses of the other two cells (indicated by dashed lines) were near stimulus velocity when low-frequency tilts were provided, but developed a >50° phase lag when the stimulus frequency was increased.
Fig. 4. The relative increase in response gain per stimulus decade (usually determined for the frequency range of 0.1–1 Hz or 0.05–0.5 Hz) of different types of RFN neurons.
Symbols indicate the ratio of the gain of the response to the high frequency stimulus to the gain of the response to the low frequency stimulus for each neuron. Horizontal lines indicate the median relative gain per decade for each neuronal type; error bars designate the interquartile range (i.e., the difference between the 75th and 25th percentile).
Fig. 3B contains Bode plots for the 16 of the 47 well-characterized cells (34%) that we deemed to have “velocity” responses due to the presence of response phases that were within 35° of stimulus velocity at all frequencies. The velocity responses of one neuron are illustrated in Fig. 2B. The gain of the responses of these units increased substantially when higher stimulus frequencies were employed; the median relative gain increase per stimulus decade was 7.2, as indicated in Fig. 4. The relative response gain per decade was shown to be significantly different for the graviceptive and velocity units by the use of the Mann Whitney test (p<0.0001). Fig. 3C includes Bode plots for the 6 units that did not fall into either the graviceptive or velocity categories. The responses of 4 of these neurons (indicated by solid lines) were in phase with stimulus position when low rotation frequencies were employed, but were in phase with stimulus velocity when high frequencies were delivered. The responses of the other two cells (indicated by dashed lines) were near stimulus velocity when low-frequency tilts were provided, but developed a >50° phase lag when the stimulus frequency was increased.
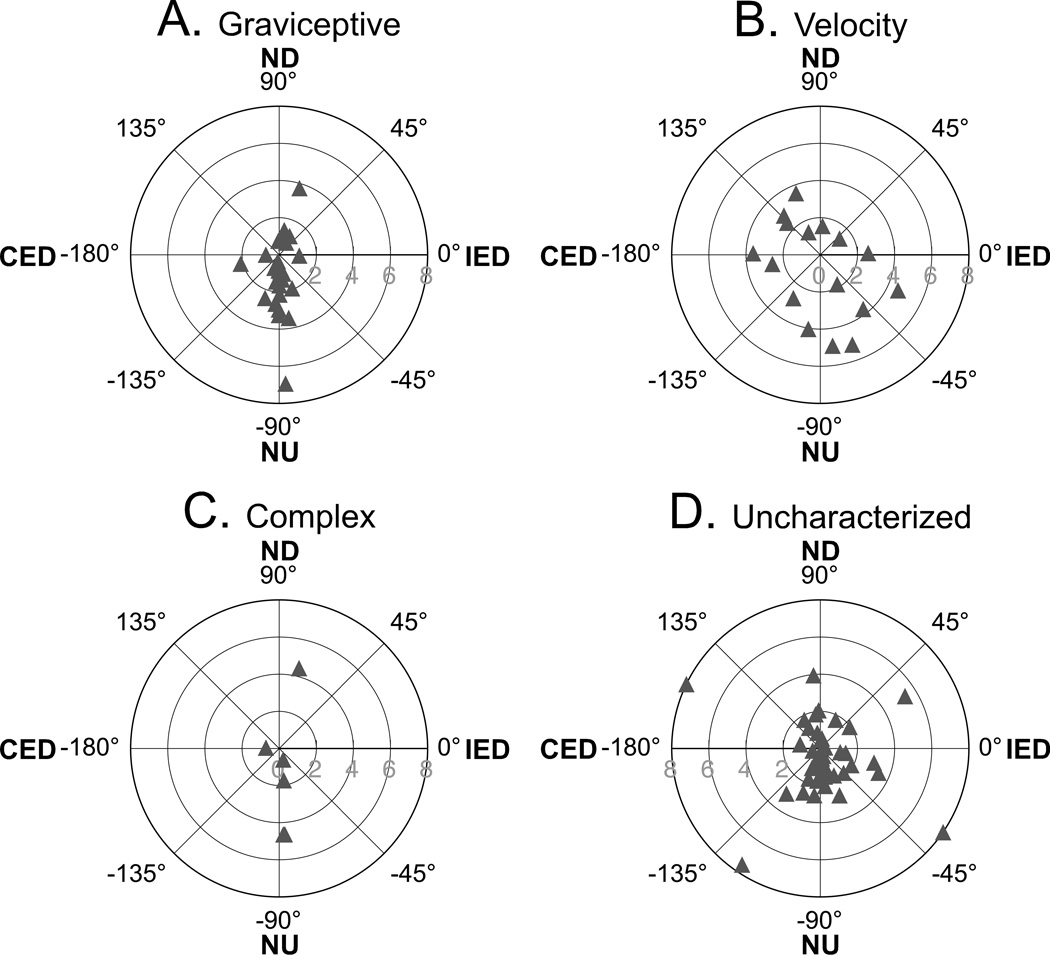
The response vector orientations of RFN neurons are indicated in Fig. 5, whereas Table 1 summarizes whether these orientations were closest (i.e., within 22°) to the roll plane, the pitch plane, or the plane of one of the vertical semicircular canals. For the population of RFN cells as a whole, more than half (57%) were best activated by pitch rotations; 15% and 28% respectively responded preferentially to roll tilts and tilts near the planes of the vertical canals. Neurons with graviceptive responses were particularly likely to exhibit response vector orientations that were close to the pitch plane: 20/25 or 80% of the cells had such a characteristic. In contrast, half of the neurons with velocity responses (8/16) were best activated by rotations near the plane of one of the vertical canals, although most of the remaining velocity cells (5/16 or 31%) responded preferentially to pitch oscillations.
Fig. 5. Polar plots showing the response vector orientations and gains for RFN neurons, determined using wobble stimuli that were usually delivered at 0.2 or 0.5 Hz.
A, graviceptive neurons; B, velocity neurons; C, neurons with complex response dynamics; D, uncharacterized neurons that were lost before response dynamics could be characterized in detail. Numbers along the radius of each plot indicate gain (spikes/s/°). Abbreviations: CED, contralateral ear down roll; IED, ipsilateral ear down roll; ND, nose down pitch; NU, nose up pitch.
Table 1.
Number (%) of RFN units of different types with response vector orientations closest (i.e., within 22°) to roll, pitch, or vertical canal planes. The response dynamics for “uncharacterized” units were not ascertained.
| Unit Type | Plane of Response Vector Orientation | ||
|---|---|---|---|
| Roll | Pitch | Vertical Canal | |
| Graviceptive (n=25) |
3 (12%) | 20 (80%) | 2 (8%) |
| Velocity (n=16) |
3 (19%) | 5 (31%) | 8 (50%) |
| Complex (n=6) |
1 (17%) | 4 (67%) | 1 (17%) |
| Uncharacterized (n=47) |
7 (15%) | 25 (53%) | 15 (32%) |
|
All Types Combined (n=94) |
14 (15%) | 54 (57%) | 26 (28%) |
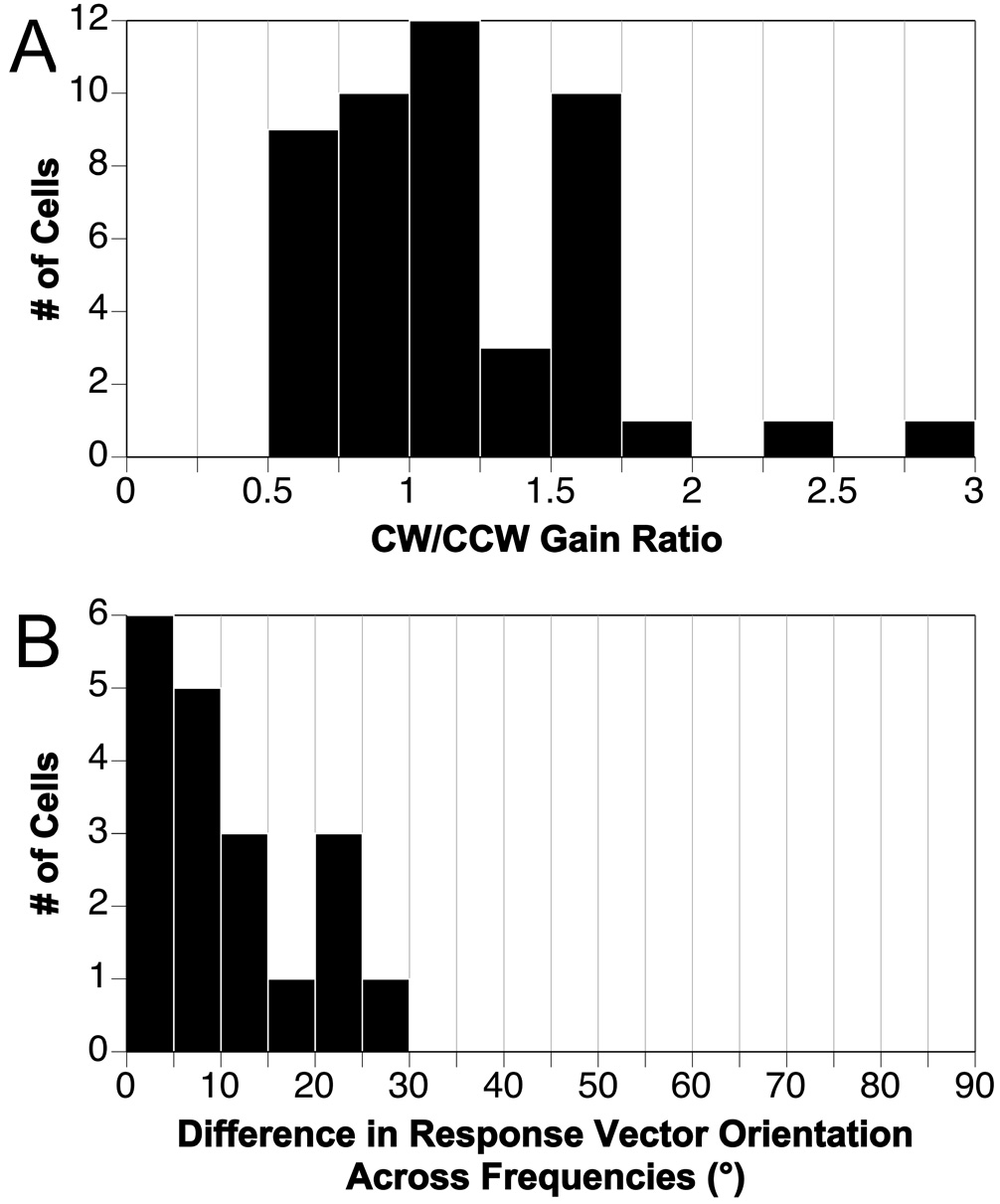
For units exhibiting robust STC behavior, the gains of responses to CW and CCW wobble rotations are different (Wilson et al., 1986; Kasper et al., 1988; Schor and Angelaki, 1992), because the misaligned inputs to the cell add during one direction of rotation but subtract when rotations are delivered in the opposite direction. To determine the prevalence of STC responses in the feline RFN, the ratio of the gain of the responses to CW and CCW wobble stimulation was ascertained for the highest frequency rotations employed for a unit (usually 0.2 or 0.5 Hz), where an STC response is expected to be evident if it is present. These ratios are shown in Fig. 6A for the 47 units whose response dynamics were characterized in detail. For the large majority of cells (45/47), the ratios were < 2:1 (i.e., no less than 0.5 and no larger than 2.0). For example, the gains of responses to CW and CCW wobble illustrated in Fig. 1A and Fig. 2A are nearly identical. The response vector orientations of STC cells also vary as a function of tilt frequency (Baker et al., 1984; Kasper et al., 1988; Schor and Angelaki, 1992; Angelaki, 1993), as one input predominates during low frequency rotations whereas another that is spatially misaligned predominates during high frequency rotations. Accordingly, for 19 neurons we determined the frequency-related variability in response vector orientations by delivering wobble stimuli at two or more frequencies between 0.05 and 0.5 Hz. As indicated in Fig. 6B, these values changed no more than 30° for any cell, and typically the variability in response vector orientation was much smaller: the median was 7°. These data suggest that few cells in the feline RFN have strong STC responses.
Fig. 6. Determination of whether RFN neurons exhibit spatiotemporal convergence (STC) behavior.
A: Ratio of the gains of responses to clockwise (CW) and counterclockwise (CCW) wobble stimulation, usually delivered at 0.2 or 0.5 Hz. Data were included for all neurons whose response dynamics were characterized. B: the maximal variability in response vector orientations for the 19 neurons tested using wobble stimuli delivered at two or more frequencies between 0.05 and 0.5 Hz.
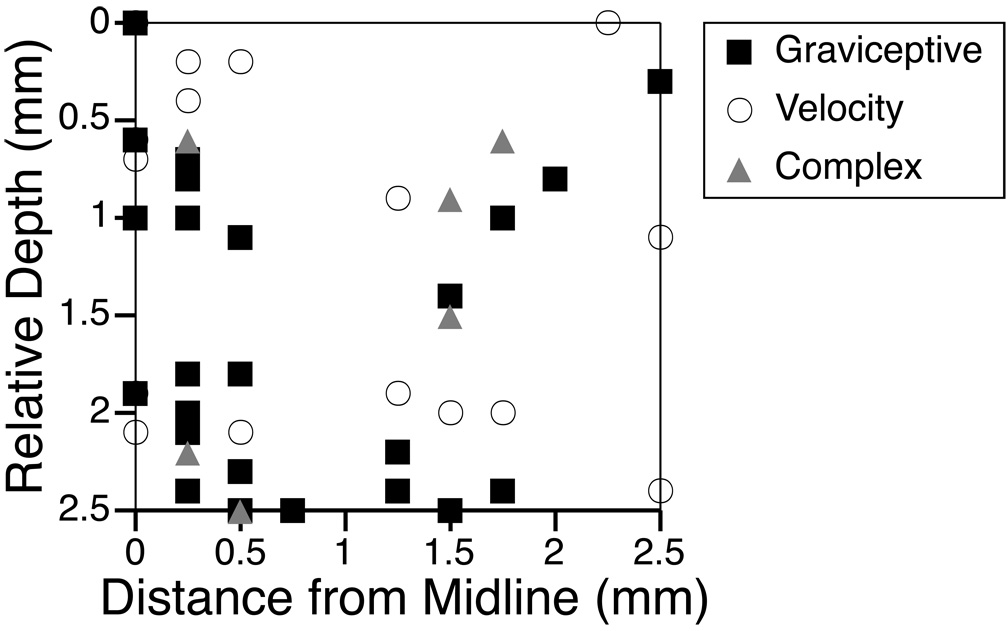
The relative depths and lateralities of the RFN neurons whose response dynamics were characterized in detail are shown in Fig. 7. Neurons with particular response characteristics did not appear to be clustered in a particular region of the nucleus.
Fig. 7. Relative depths and distances from the midline of RFN neurons whose response dynamics were characterized.
Each neuronal type is represented by a different symbol.
Discussion
The major finding of this study is that RFN neurons in conscious felines have relatively simple responses to rotations in vertical planes. Approximately half of the RFN neurons examined were classified as graviceptive, since their activity was synchronized with stimulus position and the gain of their responses was relatively unaffected by the frequency of the rotations. Such characteristics are appropriate for indicating orientation in space. Since 80% of the graviceptive RFN neurons were preferentially activated by rotations near the pitch plane, these cells appear to mainly signal the presence of head-up or head-down positioning of the body axis. Most of the remaining RFN units exhibited responses to vertical rotations that encoded stimulus velocity. Approximately 50% of these velocity units had a response vector orientation aligned near the plane of a single vertical semicircular canal, suggesting that the cells predominantly received inputs from just that canal and not from multiple labyrinthine receptors. Less than one-third of feline RFN neurons had response dynamics consistent with a complex processing of signals from multiple receptors. In contrast, in nonhuman primates the responses of RFN neurons to natural vestibular stimulation appear to be much more elaborate, as the majority of cells integrate signals from both semicircular canals and otolith organs and/or exhibit STC behavior (Siebold et al., 1997; Kleine et al., 1999; Siebold et al., 1999; 2001; Zhou et al., 2001; Wilden et al., 2002; Shaikh et al., 2005). Furthermore, the preponderance of RFN neurons in nonhuman primates were activated by tilts in the roll or vertical canal planes (Siebold et al., 1997) or translations in the analogous directions (Shaikh et al., 2005). Conversely, most RFN neurons in cats, particularly those with graviceptive responses, responded preferentially to pitch rotations. It is thus apparent that sensorimotor integration by the RFN is considerably different in felines and monkeys.
The lack of STC behavior exhibited by RFN neurons in this study is at odds with findings in decerebrate cats showing that many anterior vermis Purkinje cells display such complex responses to vertical rotations (Manzoni et al., 1995; Pompeiano et al., 1997). Since Purkinje cells provide only a subset of afferents to RFN neurons, a possibility is that the additional inputs to the cells are relatively simple, and that the integrated responses of RFN cells to vestibular stimulation are largely governed by signals arising from sources other than the cerebellar cortex. An alternative is that the complex responses of anterior vermis neurons to vertical rotations reported previously in decerebrate animals are an epiphenomenon due to the removal of descending cortical and subcortical inputs to the brainstem. To make this determination, it will be necessary to conduct additional studies in conscious felines comparing the effects of natural vestibular stimulation on the activity of RFN neurons and vermis Purkinje cells.
The RFN has historically been thought to play an important role in postural control (Büttner et al., 1991; Thach et al., 1992; Siebold et al., 1997; Mori et al., 1998; 2004). Consequently, it could be postulated that the discrepancies in RFN responses in felines and nonhuman primates relate to the fact that the two species typically maintain different postures. However, the body geometry of felines is such that the animals are unstable about the longitudinal axis. Accordingly, vestibular reflexes acting on the limbs (Wilson et al., 1986) as well as the firing of vestibulospinal (Kasper et al., 1988; Iwamoto et al., 1996) and reticulospinal (Bolton et al., 1992) neurons are preferentially modulated by roll rotations in this species. Although the subset of cat RFN neurons that was activated by tilts in the roll or vertical canal planes had response characteristics appropriate to contribute to postural regulation, the functional significance of the larger population of neurons whose firing was modulated by pitch rotations is more difficult to surmise.
Physiological studies conducted in the rat, cat, and goat indicated that the RFN participates in control of the cardiovascular system and breathing. Lesions of this region attenuate compensatory corrections in blood pressure during head-up rotations of the body (Doba and Reis, 1972; 1974; Huang et al., 1977). In addition, stimulation or lesions of the RFN alter respiratory muscle activity as well as adjustments in breathing during hypoxia and altered blood CO2 levels (Huang et al., 1977; Lutherer and Williams, 1986; Xu and Frazier, 1995; 1997; 2000; 2002; Martino et al., 2006a; 2006b). Furthermore, the RFN has been suggested to trigger compensatory respiratory responses during alterations in body position, at least in felines and rodents (Huang et al., 1977; Lutherer and Williams, 1986; Xu and Frazier, 1995; 1997; 2000; 2002; Martino et al., 2006a; 2006b); such breathing adjustments are required during head-up rotations of the body axis (Yates et al., 2002). Thus, pitch-activated RFN neurons could signal changes in body position in the transverse plane that challenge the maintenance of stable blood pressure (Doba and Reis, 1972; 1974; Huang et al., 1977) and breathing (Yates et al., 2002). As such, the present data support previous findings indicating that the RFN participates in regulation of homeostasis in cats (Doba and Reis, 1972; 1974; Huang et al., 1977; Lutherer and Williams, 1986; Xu and Frazier, 1995).
In addition to providing inputs to vestibulospinal and reticulospinal neurons, the RFN of the cat has projections to brainstem regions that control autonomic functions (Homma et al., 1995). If our notions regarding the role of the RFN in autonomic regulation are correct, then it would be expected that the axons of many RFN neurons that respond to pitch rotations terminate in brainstem areas involved in respiratory and cardiovascular regulation. In contrast, we predict that RFN cells that are preferentially activated by tilts in the roll or vertical canal planes supply inputs to vestibulospinal and reticulospinal neurons that influence limb and trunk muscles. Future studies will be needed to test these hypotheses.
Considerable evidence is mounting to suggest that the RFN in nonhuman primates participates in the sensory processing required to compute body motion in space and to perceive spatial orientation (Kleine et al., 2004; Shaikh et al., 2004; 2005).It is presently unclear whether the relatively simple responses of most feline RFN neurons to vertical rotations are adequate to permit such computations. Thus, the present data raise the possibility that the physiological role of the fastigial nucleus differs between mammals, and that the RFN has assumed a unique role in spatial cognition in primates. Further comparative studies, particularly those that examine the physiological and behavioral consequences of ablation of fastigial nucleus neurons in multiple species, will be needed to make this determination.
Acknowledgements
The authors thank Joseph Troupe III, Jason Draper, and James Lois for assistance with some of the experiments. Funding was provided by Grants R01-DC00693 and R01-DC03732 from the National Institutes of Health (USA). Core support was provided by NIH grant P30-DC05205.
List of Abbreviations
- CW
Clockwise
- CCW
Counterclockwise
- CV
Coefficient of variation
- EOG
Electrooculogram
- RFN
Rostral fastigial nucleus
- STC
Spatiotemporal convergence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelaki DE. Generation of 2-dimensional spatial and temporal properties through spatiotemporal convergence between one-dimensional neurons. IEEE Trans Biomed Eng. 1993;40:686–692. doi: 10.1109/10.237698. [DOI] [PubMed] [Google Scholar]
- Arshian M, Holtje RJ, Cotter LA, Rice CD, Cass SP, Yates BJ. Consequences of postural changes and removal of vestibular inputs on the movement of air in and out of the lungs of conscious felines. J Appl Physiol. 2007;103:347–352. doi: 10.1152/japplphysiol.00211.2007. [DOI] [PubMed] [Google Scholar]
- Baker J, Goldberg J, Hermann G, Peterson BW. Spatial and temporal response properties of secondary neurons that receive convergent input in vestibular nuclei of adult cats. Brain Res. 1984;294:138–143. doi: 10.1016/0006-8993(84)91318-0. [DOI] [PubMed] [Google Scholar]
- Batton RR, 3rd, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol. 1977;174:281–305. doi: 10.1002/cne.901740206. [DOI] [PubMed] [Google Scholar]
- Bolton PS, Goto T, Schor RH, Wilson VJ, Yamagata Y, Yates BJ. Response of pontomedullary reticulospinal neurons to vestibular stimuli in vertical planes. Role in vertical vestibulospinal reflexes of the decerebrate cat. J Neurophysiol. 1992;67:639–647. doi: 10.1152/jn.1992.67.3.639. [DOI] [PubMed] [Google Scholar]
- Brettler SC, Fuchs AF. Role of caudal fastigial neurons during head-free gaze shifts in the monkey. Ann N Y Acad Sci. 2002;978:505–506. doi: 10.1111/j.1749-6632.2002.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Büttner U, Fuchs AF, Markert-Schwab G, Buckmaster P. Fastigial nucleus activity in the alert monkey during slow eye and head movements. J Neurophysiol. 1991;65:1360–1371. doi: 10.1152/jn.1991.65.6.1360. [DOI] [PubMed] [Google Scholar]
- Büttner U, Glasauer S, Glonti L, Guan Y, Kipiani E, Kleine J, Siebold C, Tchelidze T, Wilden A. Multimodal signal integration in vestibular neurons of the primate fastigial nucleus. Ann N Y Acad Sci. 2003;1004:241–251. doi: 10.1196/annals.1303.021. [DOI] [PubMed] [Google Scholar]
- Büttner U, Glasauer S, Glonti L, Kleine JF, Siebold C. Otolith processing in the deep cerebellar nuclei. Ann N Y Acad Sci. 1999;871:81–93. doi: 10.1111/j.1749-6632.1999.tb09177.x. [DOI] [PubMed] [Google Scholar]
- Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res. 1983;278:29–51. doi: 10.1016/0006-8993(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Doba N, Reis DJ. Cerebellum: role in reflex cardiovascular adjustment to posture. Brain Res. 1972;39:495–500. doi: 10.1016/0006-8993(72)90451-9. [DOI] [PubMed] [Google Scholar]
- Doba N, Reis DJ. Role of the cerebellum and vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res. 1974;34:9–18. doi: 10.1161/01.res.40.4.9. [DOI] [PubMed] [Google Scholar]
- Erway LC, Ghelarducci B, Pompeiano O, Stanojevic M. Responses of cerebellar fastigial neurons to stimulation of contralateral macular labyrinthine receptors. Arch Ital Biol. 1978;116:205–224. [PubMed] [Google Scholar]
- Favilla M, Ghelarducci B, Hill CD, Spyer KM. Vestibular inputs to the fastigial nucleus; evidence of convergence of macular and ampullar inputs. Pflugers Arch. 1980;384:193–201. doi: 10.1007/BF00584553. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Fuchs AF. Single-unit responses to natural vestibular stimuli and eye movements in deep cerebellar nuclei of the alert rhesus monkey. J Neurophysiol. 1975;38:627–649. doi: 10.1152/jn.1975.38.3.627. [DOI] [PubMed] [Google Scholar]
- Ghelarducci B. Responses of the cerebellar fastigial neurones to tilt. Pflugers Arch. 1973;344:195–206. doi: 10.1007/BF00588460. [DOI] [PubMed] [Google Scholar]
- Ghelarducci B, Pompeiano O, Spyer KM. Distribution of the neuronal responses to static tilts within the cerebellar fastigial nucleus. Arch Ital Biol. 1974;112:126–141. [PubMed] [Google Scholar]
- Homma Y, Nonaka S, Matsuyama K, Mori S. Fastigiofugal projection to the brainstem nuclei in the cat: an anterograde PHA-L tracing study. Neurosci Res. 1995;23:89–102. [PubMed] [Google Scholar]
- Huang TF, Carpenter MB, Wang SC. Fastigial nucleus and orthostatic reflex in cat and monkey. Am J Physiol. 1977;232:H676–H681. doi: 10.1152/ajpheart.1977.232.6.H676. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Perlmutter SI, Baker JF, Peterson BW. Spatial coordination by descending vestibular signals. 2. Response properties of medial and lateral vestibulospinal tract neurons in alert and decerebrate cats. Exp Brain Res. 1996;108:85–100. doi: 10.1007/BF00242906. [DOI] [PubMed] [Google Scholar]
- Jian BJ, Shintani T, Emanuel BA, Yates BJ. Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp Brain Res. 2002;144:247–257. doi: 10.1007/s00221-002-1042-8. [DOI] [PubMed] [Google Scholar]
- Kasper J, Schor RH, Wilson VJ. Response of vestibular neurons to head rotations in vertical planes. I. Response to vestibular stimulation. J Neurophysiol. 1988;60:1753–1764. doi: 10.1152/jn.1988.60.5.1753. [DOI] [PubMed] [Google Scholar]
- Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Büttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol. 2004;91:2090–2100. doi: 10.1152/jn.00849.2003. [DOI] [PubMed] [Google Scholar]
- Kleine JF, Wilden A, Siebold C, Glasauer S, Büttner U. Linear spatio-temporal convergence in vestibular neurons of the primate nucleus fastigii. NeuroReport. 1999;10:3915–3921. doi: 10.1097/00001756-199912160-00035. [DOI] [PubMed] [Google Scholar]
- Lutherer LO, Williams JL. Stimulating fastigial nucleus pressor region elicits patterned respiratory responses. Am J Physiol. 1986;250:R418–R426. doi: 10.1152/ajpregu.1986.250.3.R418. [DOI] [PubMed] [Google Scholar]
- Manzoni D, Andre P, Pompeiano O. Responses of Purkinje cells in the cerebellar anterior vermis to off-vertical axis rotation. Pflugers Arch-Eur J Physiol. 1995;431:141–154. doi: 10.1007/BF00410185. [DOI] [PubMed] [Google Scholar]
- Martino PF, Davis S, Opansky C, Krause K, Bonis JM, Czerniak SG, Pan LG, Qian B, Forster HV. Lesions in the cerebellar fastigial nucleus have a small effect on the hyperpnea needed to meet the gas exchange requirements of submaximal exercise. J Appl Physiol. 2006a;101:1199–1206. doi: 10.1152/japplphysiol.00330.2006. [DOI] [PubMed] [Google Scholar]
- Martino PF, Hodges MR, Davis S, Opansky C, Pan LG, Krause K, Qian B, Forster HV. CO2/H+ chemoreceptors in the cerebellar fastigial nucleus do not uniformly affect breathing of awake goats. J Appl Physiol. 2006b;101:241–248. doi: 10.1152/japplphysiol.00968.2005. [DOI] [PubMed] [Google Scholar]
- Mori S, Matsui T, Kuze B, Asanome M, Nakajima K, Matsuyama K. Cerebellar-induced locomotion: reticulospinal control of spinal rhythm generating mechanism in cats. Ann N Y Acad Sci. 1998;860:94–105. doi: 10.1111/j.1749-6632.1998.tb09041.x. [DOI] [PubMed] [Google Scholar]
- Mori S, Nakajima K, Mori F, Matsuyama K. Integration of multiple motor segments for the elaboration of locomotion: role of the fastigial nucleus of the cerebellum. Prog Brain Res. 2004;143:341–351. doi: 10.1016/S0079-6123(03)43033-1. [DOI] [PubMed] [Google Scholar]
- Pompeiano O, Andre P, Manzoni D. Spatiotemporal response properties of cerebellar Purkinje cells to animal displacement: A population analysis. Neurosci. 1997;81:609–626. doi: 10.1016/s0306-4522(97)00201-7. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Ann Rev Neurosci. 2001;24:981–1004. doi: 10.1146/annurev.neuro.24.1.981. [DOI] [PubMed] [Google Scholar]
- Schor RH, Angelaki DE. The algebra of neural response vectors. Ann N Y Acad Sci. 1992;656:190–204. doi: 10.1111/j.1749-6632.1992.tb25209.x. [DOI] [PubMed] [Google Scholar]
- Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol. 1984;51:136–146. doi: 10.1152/jn.1984.51.1.136. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Ghasia FF, Dickman JD, Angelaki DE. Properties of cerebellar fastigial neurons during translation, rotation, and eye movements. J Neurophysiol. 2005;93:853–863. doi: 10.1152/jn.00879.2004. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Meng H, Angelaki DE. Multiple reference frames for motion in the primate cerebellum. J Neurosci. 2004;24:4491–4497. doi: 10.1523/JNEUROSCI.0109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold C, Anagnostou E, Glasauer S, Glonti L, Kleine JF, Tchelidze T, Büttner U. Canal-otolith interaction in the fastigial nucleus of the alert monkey. Exp Brain Res. 2001;136:169–178. doi: 10.1007/s002210000575. [DOI] [PubMed] [Google Scholar]
- Siebold C, Glonti L, Glasauer S, Büttner U. Rostral fastigial nucleus activity in the alert monkey during three-dimensional passive head movements. J Neurophysiol. 1997;77:1432–1446. doi: 10.1152/jn.1997.77.3.1432. [DOI] [PubMed] [Google Scholar]
- Siebold C, Kleine JF, Glonti L, Tchelidze T, Büttner U. Fastigial nucleus activity during different frequencies and orientations of vertical vestibular stimulation in the monkey. J Neurophysiol. 1999;82:34–41. doi: 10.1152/jn.1999.82.1.34. [DOI] [PubMed] [Google Scholar]
- Stanojevic M. Responses of cerebellar fastigial neurons to neck and macular vestibular inputs. Pflugers Arch. 1981;391:267–272. doi: 10.1007/BF00581505. [DOI] [PubMed] [Google Scholar]
- Stanojevic M, Erway L, Ghelarducci B, Pompeiano O, Willis WD., Jr A comparison of the response characteristics of cerebellar fastigial and vermal cortex neurons to sinusoidal stimulation of macular vestibular receptors. Pflugers Archiv. 1980;385:95–104. doi: 10.1007/BF00588687. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Ann Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Wilden A, Glasauer S, Kleine JF, Büttner U. Modelling transfer characteristics of vestibular neurons in the fastigial nucleus of the behaving monkey on the basis of canal-otolith interaction. NeuroReport. 2002;13:799–804. doi: 10.1097/00001756-200205070-00013. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Cotter LA, Draper JA, Misra SP, Rice CD, Cass SP, Yates BJ. Effects of postural changes and removal of vestibular inputs on blood flow to the head of conscious felines. J Appl Physiol. 2006;100:1475–1482. doi: 10.1152/japplphysiol.01585.2005. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Schor RH, Suzuki I, Park BR. Spatial organization of neck and vestibular reflexes acting on the forelimbs of the decerebrate cat. J Neurophysiol. 1986;55:514–526. doi: 10.1152/jn.1986.55.3.514. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Medullary respiratory neuronal activity modulated by stimulation of the fastigial nucleus of the cerebellum. Brain Res. 1995;705:53–64. doi: 10.1016/0006-8993(95)01138-2. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Involvement of the fastigial nuclei in vagally mediated respiratory responses. J Appl Physiol. 1997;82:1853–1861. doi: 10.1152/jappl.1997.82.6.1853. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum. 2002;1:35–40. doi: 10.1080/147342202753203078. [DOI] [PubMed] [Google Scholar]
- Xu FD, Frazier DT. Modulation of respiratory motor output by cerebellar deep nuclei in the rat. J Appl Physiol. 2000;89:996–1004. doi: 10.1152/jappl.2000.89.3.996. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Billig I, Cotter LA, Mori RL, Card JP. Role of the vestibular system in regulating respiratory muscle activity during movement. Clin Exp Pharmacol Physiol. 2002;29:112–117. doi: 10.1046/j.1440-1681.2002.03612.x. [DOI] [PubMed] [Google Scholar]
- Zhou W, Tang BF, King WM. Responses of rostral fastigial neurons to linear acceleration in an alert monkey. Exp Brain Res. 2001;139:111–115. doi: 10.1007/s002210100747. [DOI] [PubMed] [Google Scholar]