Abstract
Previous studies by our group have shown that peripheral inflammatory insult, using the λ-carrageenan inflammatory pain (CIP) model, induced alterations in the molecular and functional properties of the blood-brain barrier (BBB). The question remained whether these changes were mediated via an inflammatory and/or neuronal mechanism. In this study, we investigated the involvement of neuronal input from pain activity on alterations in BBB integrity by peripheral inhibition of nociceptive input. A perineural injection of 0.75% bupivacaine into the right hind leg prior to CIP was used for peripheral nerve block. Upon nerve block, there was a significant decrease in thermal allodynia induced by CIP, but no effect on edema formation 1 h post CIP. BBB permeability was increased 1 h post CIP treatment as determined by in situ brain perfusion of [14C] sucrose; bupivacaine nerve block of CIP caused an attenuation of [14C] sucrose permeability, back to saline control levels. Paralleling the changes in [14C] sucrose permeability, we also report increased expression of three tight junction (TJ) proteins, zonula occluden-1 (ZO-1), occludin and claudin-5 with CIP. Upon bupivacaine nerve block, changes in expression were prevented. These data show that the λ-carrageenan induced changes in [14C] sucrose permeability and protein expression of ZO-1, occludin and claudin-5 are prevented with inhibition of nociceptive input. Therefore, we suggest that nociceptive signaling is in part responsible for the alteration in BBB integrity under CIP.
Keywords: Pain, tight junction, blood-brain barrier, permeability, allodynia, λ-carrageenan
1. Introduction
The BBB is a dynamic, physiological and metabolic barrier separating the blood from the CNS and is essential for maintaining brain homeostasis and enabling proper neuronal function (Rubin and Staddon, 1999). The BBB is characterized by the presence of tight junctions (TJ) which are formed via a complex interaction of specific transmembrane, accessory and cytoskeleton proteins. The primary seal of the TJ is formed by the transmembrane proteins occludin and the claudins (Fricker and Miller, 2004; Hawkins and Davis, 2005). These proteins are linked to the actin cytoskeleton via dynamic interactions with accessory proteins, zonula occluden (ZO)-1, 2 and 3 (Tsukamoto and Nigam,1997). In addition, the ZO proteins act as a scaffold for a number of signaling networks which can modulate this interaction and are involved in regulation of TJ function and paracellular permeability (Haskins et al., 1998). The TJ proteins of the BBB interact with each other in a dynamic manner, where occludin and claudins can bind to each of the ZO proteins, their primary link to the cytoskeleton is via ZO-1 binding.
There are several central nervous system conditions with pain and/or inflammatory components, including multiple sclerosis, Alzheimer’s disease, human immunodeficiency virus (HIV) dementia, and meningitis where decreased blood-brain barrier (BBB) function has been described (Hawkins and Davis, 2005).
We have recently demonstrated significant changes in BBB permeability to [14C] sucrose in three models of inflammatory pain (Huber et al., 2001b). Using the λ-carrageenan inflammatory pain (CIP) model, in which a sub-planter injection of λ-carrageenan is made into the rat hind paw, the permeability of the BBB showed a biphasic increase in BBB paracellular permeability to [14C] sucrose at 1 h to 6 h and at 48 h. These changes in permeability were paralleled by altered expression of the TJ proteins occludin and ZO-1. However, intravenous administration of λ-carrageenan did not lead to changes in the BBB permeability, thus indicating that the change in [14C] sucrose permeability was due to either CIP induced inflammatory or neuronal modulation of the TJ (Huber et al., 2002).
Brain endothelial cells can be modulated by a range of inflammatory mediators (Abbott, 2000). The endothelial intercellular adhesion molecule-1 (ICAM-1, CD54), an immunoglobulin surface receptor, has been shown to induce changes in the endothelial cytoskeleton, transcription and interendothelial junctions, factors which may modulate endothelial disposition to infiltrating leukocytes (Turowski et al., 2005). Using CIP, Huber et al. (2006) showed an increase in ICAM mRNA and protein expression at the BBB. Additionally, a number of circulating, systemic, pro-inflammatory mediators were not detected in the early response phase of increased BBB permeability (1–6 h); however, there were brain region specific increases in microglial activation suggesting a potential role for a central-mediated response to CIP induced BBB changes.
Central-mediated changes in BBB permeability is supported by work from a number of groups. Bondy and Purdy (1974) demonstrated an increase in permeability to tyrosine after sensory input deprivation. The authors attributed this to a compensatory mechanism in order to maintain a constant supply of nutrients to the brain during variations in cerebral blood flow. Yarnitsky et al., 2004 showed that stimulation of parasympathetic nerve fibers arising from the sphenopalatine ganglion, resulted in a reversible increase in BBB permeability, with enhanced entry of macromolecules such as FITC–dextran, Evans blue dye–albumin and the chemotherapeutic agents anti-HER2 mAb and etoposide into the brain. Using a neuropathic pain model of peripheral chronic nerve lesion, Gordh and Sharma, 2006, demonstrated a loss of blood-spinal cord barrier integrity, with increased permeability and activation of astrocytes in the spinal cord. Disruption of the blood-spinal cord barrier reflected a widespread alteration in the fluid microenvironment and exhibited similarities with precipitating glial cell reaction to peripheral nerve stimulation, thus providing evidence of barrier function alteration as a result of nociceptive input.
The aim of this study was to elucidate the contribution that central-mediated nociception has on the modulation of BBB permeability and on TJ protein expression. We induced peripheral inflammation with λ-carrageenan and examined BBB integrity 1 h post, with and without bupivacaine-induced peripheral inhibition of nociceptive input. We show for the first time the effects of inhibition of neuronal signaling on BBB permeability and changes in three integral TJ proteins during acute inflammatory pain.
2. Results
2.1. Thermal allodynia and edema formation following λ-carrageenan treatment
The response to noxious thermal stimulation in animals treated with λ-carrageenan, was significantly (p<0.05; F7,56=25.45) increased compared to the saline alone treated group (Fig. 1A). A perineural pre-injection of 0.75% bupivacaine reduced the λ-carrageenan induced allodynia, as shown by the maintenance of paw withdraw latency pre- versus post-injection at a level comparable to that seen in the saline paw injected control group. There was a trend towards a decrease in sensibility in the groups treated with bupivacaine, as demonstrated with increased paw removal latency, however this decrease was not statistically significant (bupivacaine with saline group p=0.062; bupivacaine with λ-carrageenan group p=0.068).
Fig. 1.
λ-carrageenan-induced hind paw thermal allodynia and edema formation and effect of bupivacaine injection. (A) Thermal allodynia based on paw removal latency from an infrared heat source was measured (s) pre versus post injection of either λ-carrageenan or saline into the right hind paw in rats pretreated with either saline or bupivacaine. (B) Edema formation in the right hind paw measured as the percent change in paw volume 1 h post-injection of saline or λ-carrageenan in rats pretreated with either saline or bupivacaine. Bars represent mean ± S.E., n=8 for each group, *=p<0.05.
One hour post-paw injection, administration of λ-carrageenan induced a significant (p<0.01, F3,12=9.49) hind paw edema compared to saline paw injected treatment groups increasing the paw volume three fold (Fig. 1B). A perineural nerve blockade using bupivacaine did not prevent the λ-carrageenan induced paw edema formation. For comparison purposes, the left paw was measured and demonstrated no volume change among all groups studied.
2.2. Effects of nociceptive inhibition on locomotion
To determine animal mobility following pretreatment with bupivacaine nociceptive inhibition, an assessment of locomotion was performed for each group. Among the four treatment groups, there was no statistically significant difference in locomotion, indicating that bupivacaine nociceptive inhibition did not prevent or alter locomotive function.
2.3. λ-carrageenan-induced changes in BBB permeability to [14C] sucrose
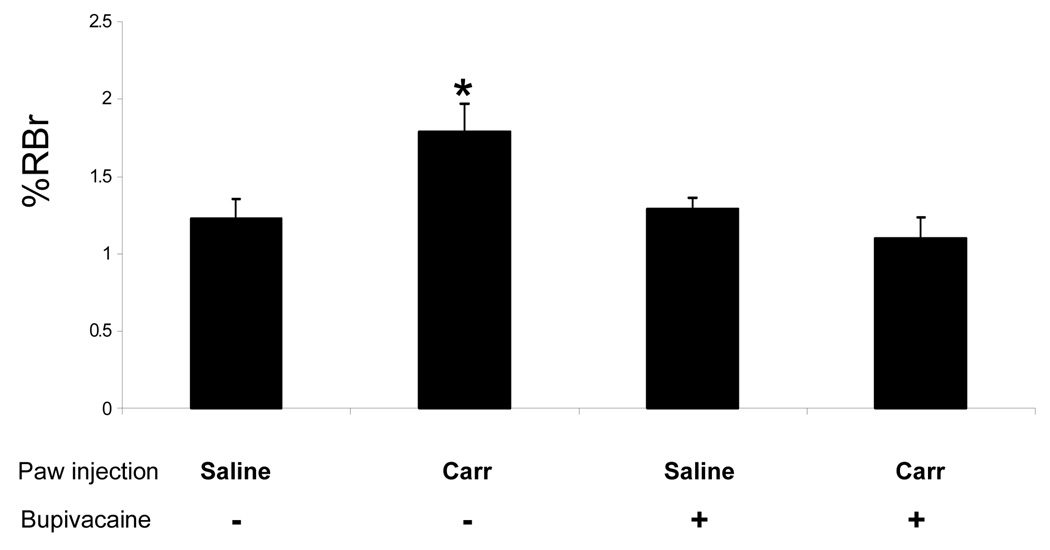
The effect of CIP on BBB permeability was assessed at 1 h post CIP induction using [14C] sucrose. In control animals, the ratio of radioactivity in the brain compared to that in the perfusate (Rbr) was 1.23±0.3%. This value is representative of vascular volume and can be converted to a vascular space of 18 µl/g brain tissue, which is consistent with previous studies (3–20 µl/g brain) (Blasberg et al., 1983). One hour post λ-carrageenan treatment, there was a significantly (p<0.05, F3,19=5.66) higher content of [14C] sucrose (1.79±0.3% Rbr) associated with the brain when compared to saline paw injected brain content (Fig. 2). Upon bupivacaine inhibition of nociception the λ-carrageenan treated animals showed a RBr value of 1.10±0.3% Rbr, indicating no increase in [14C] sucrose permeability relative to control values. A visual assessment of the brain parenchyma post-in situ perfusion showed no Evans blue albumin influx, indicating the BBB was morphologically intact.
Fig. 2.
Changes in paracellular permeability to [14C] sucrose following λ-carrageenan-induced inflammatory hyeralgesia and with bupivacaine inhibition. Graph shows the percent of radioactivity detected in the brain as compared to that in the perfusate (% RBr) for the four groups following 1 h injection of either λ-carrageenan or 0.9% saline into the right hind paw with either saline or bupivacaine pre injection. Bars represent mean ± S.E., n=6 each group, *=p<0.05.
2.4. Changes in ZO-1, claudin-5 and occludin protein expression
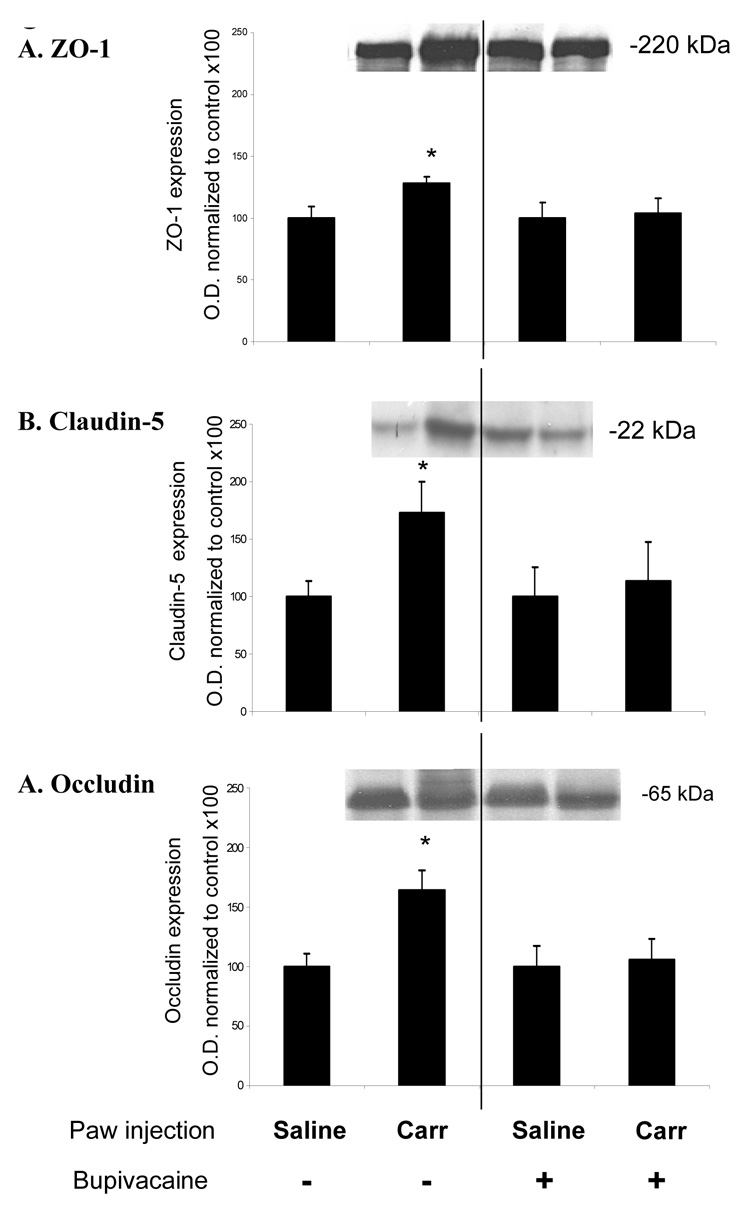
The expression of tight junction proteins ZO-1, claudin-5 and occludin were examined for changes 1 h post induction of CIP, with a pretreatment of either bupivacaine nociceptive inhibition or saline vehicle control. In λ-carrageenan treated animals, ZO-1 showed an increase in expression of 28% (p<0.05) compared to saline controls (Fig. 3A). In λ-carrageenan animals pretreated with bupivacaine, there was no increase in ZO-1 protein expression compared to date matched saline control.
Fig. 3.
λ-carrageenan-induced changes in ZO-1, claudin-5 and occludin protein expression. Western blot analysis of cerebral microvessels isolated 1 h post saline or λ-carrageenan injection. There is a significant (p<0.05) increase in (A) ZO-1, (B) claudin-5 and (C) in animals exhibiting allodynia versus animals not exhibiting allodynia. Data based on optical densities and normalized to saline control values from the same experimental day. Bars represent mean ± S.E., n=6–8 each group, *=p<0.05.
In λ-carrageenan treated animals, there was 73% increase (p<0.05) in expression of claudin-5 compared to saline control (Fig. 3B). In λ-carrageenan animals pretreated with bupivacaine, the expression of claudin-5 did not show any significant increase in protein expression compared to date matched saline controls.
The expression of transmembrane protein occludin in λ-carrageenan animals showed a statistically significant increase of 64% compared to that obtained in saline controls (Fig. 3C). In CIP animals pretreated with bupivacaine, there was no significant increase in protein expression compared to date matched saline control based on quantitated optical densities.
3. Discussion
There is a growing body of research characterizing BBB paracellular permeability changes to various stimuli such as systemic inflammation and pharmacological manipulations (Hawkins and Davis, 2005). However, the mechanism associated with BBB regulation under in vivo pathophysiological conditions, such as inflammatory pain, remains to be clarified. The present study, demonstrates the influence of peripheral nociceptive input on CIP-induced changes in BBB permeability to [14C] sucrose. Using the local anesthetic bupivacaine, we inhibited the allodynia input associated with CIP and measured paracellular permeability changes of the BBB to [14C] sucrose. CIP induced allodynia, edema formation, a significant increase in paracellular permeability to [14C] sucrose and increased expression of the TJ proteins, ZO-1, claudin-5 and occludin. Inhibition of peripheral nociceptive input using bupivacaine prevented λ-carrageenan-induced allodynia, the increase in [14C] sucrose permeability, as well as changes in TJ protein expression, without significantly affecting edema formation.
CIP Has been shown to elicit a biphasic increase in BBB permeability at 1–6 h and at 48 h (Huber et al., 2002). Characterization of circulating markers of inflammation indicated no difference in total white blood cell counts compared to controls over a 0–72 h time course without a significant change in plasma concentrations of the cytokines TNFα, IL 1β, and IL 6 at the time points studied. There was a region specific response of activated microglia in the parietal and frontal cortices and the thalamus. These brain regions have been implicated in the recognition of pain signaling (Wager et al., 2004). These observations led Huber and colleagues (2006) to propose that the perturbations in the BBB at the earlier time point were not due to systemic effects, but rather a centrally mediated response to CIP stimuli via efferent signaling through the spinothalamic tracts.
In the present study, we investigated the role that centrally mediated response to painful stimuli has on the modulation of BBB permeability after 1 h CIP. The time point of 1 h was chosen as it is the earliest time point of the 0–72 h time course of CIP treatment that demonstrated a functional changes in paracellular permeability to [14C] sucrose, with associated changes in the TJ proteins ZO-1, claudin-5 and occludin. Using bupivacaine to elicit a peripheral nerve inhibition of the saphenous, tibial and common peroneal nerves, we were able to inhibit CIP-induced allodynia over the one hour experimental period, as evidenced by the significantly diminished allodynic response (Fig. 1A). There was a trend towards a decrease in sensibility upon treatment with bupivacaine; however, this decrease was not statistically different from the control saline injections. This trend towards a decrease suggests that there was sufficient inhibition of nociceptive input. Using the automated TruScan loco-motor boxes to quantify the animal’s mobility, we also showed that there was no decrease in locomotion with bupivacaine treatment (at the dose used), suggesting the rat was capable of moving its limb away from an applied stimulus. Therefore, motor paralysis was not a confounding variable to the allodynia assessment. Bupivacaine did not inhibit the characterized paw edema formation resultant from λ-carrageenan injection as measured by plethysmography (Fig. 1B).
Bupivacaine is used clinically for the induction of local analgesia in procedures involving limb surgery, dental and oral surgery and obstetrical procedures (Wildsmith, 1986). The Primary action of bupivacaine is through decreasing or preventing the large transient increase in permeability to sodium ions that follows membrane depolarization. This stabilizing effect on excitable membranes, lends to the weak neuromuscular blocking effect caused by bupivacaine. Clinically, a solution of 0.5% in 5 ml physiologic saline is used for a peripheral nerve block, with a reported moderate motor blockade (Dollery, 1999). A dose of 0.75% in 0.65 ml was selected for our work based on earlier dose optimization studies. This dose of bupivacaine is not sufficient to cause neuronal degeneration, given the exposure time and injection method (Foster and Carlson, 1980). The method of injection is an adaptation previously described in dog where a perineural injection of bupivacaine was applied to the saphenous, tibial and common peroneal nerves for analgesia below the thigh (Rasmussen et al., 2006), the injection sites were adjusted for use in rats. The four experimental groups were as follows: saline perineural pre-injection with saline injected into the paw (saline control); saline perineural preinjection with λ-carrageenan injected into the paw (CIP group); bupivacaine perineural pre-injection with saline injected into the paw (nociceptive inhibition control); bupivacaine perineural pre-injection, with λ-carrageenan injected into the paw (nociception inhibited CIP group).
In this study, functional paracellular permeability of the BBB was assessed in all groups using the method of in situ perfusion using [14C] sucrose. This method is a modification of that originally developed to study transport of neuroactive peptides across the BBB (Zlokovic et al., 1985; Zlokovic et al., 1987; Zlokovic et al., 1989). The BBB is only slightly permeant to sucrose (Bhattacharjee et al., 2001) and as such, permeability to sucrose has been used as a method to assess the integrity the BBB in several studies (Zlokovic et al., 1987; Brown et al., 2004; Hawkins and Davis, 2005) It has been shown that CIP leads to a reorganization of the TJ proteins ZO-1 and occludin, with an associated increase in BBB permeability to [14C] sucrose (Huber, 2002b). Capillary depletion studies were conducted to rule out the possibility of vascular trapping of labeled sucrose within the brain capillaries so the observed increase in BBB permeability was due to increased paracellular diffusion between brain microvascular endothelial cells (Huber et al., 2001a). In the present study λ-carrageenan treatment increased BBB permeability to [14C] sucrose within 1 h post CIP as shown by the increased ratio of radioactivity in the brain to that in the perfusate of 1.79±0.3% compared to control values of 1.23±0.3%. Upon inhibition of nociceptive input with bupivacaine, λ-carrageenan-induced increase in BBB permeability was not seen, as [14C] sucrose permeability was similar to control levels. These results indicate that upon blockade of nociceptive input, there is prevention of functional changes in BBB permeability seen under inflammatory pain conditions.
The brain microvasculature is composed of endothelial cells connected by TJs (Kniesel and Wolburg, 2000). The TJ consists of transmembrane proteins (occludin and claudins) that interact on adjacent endothelial cells to form a physical barrier to paracellular diffusion (Fanning et al., 1999) with accessory proteins such as ZO-1 and ZO-2 also playing an integral role (Anderson et al., 1995). Permeability changes have been correlated with alterations in expression and localization of ZO-1, claudin-5 and occludin ((Brooks et al., 2006); (Huber et al., 2001a); (Lee et al., 2004)). In this study we examined the expression of ZO-1, occludin and claudin-5 under conditions of peripheral nerve block of CIP to help clarify the association between nociceptive input and changes in BBB integrity. CIP animals exhibiting nociception showed an increase in ZO-1 protein expression 28% over saline injection controls, consistent with a previous report from our laboratory (Huber et al., 2002), where an increase of 377% was observed. Inhibiting nociceptive input prevents this increase in ZO-1 from occurring. ZO-1 may act as a signaling molecule that communicates the state of the TJ to the interior of the cell, or vice versa (Gonzalez-Mariscal et al., 2000). The increase in ZO-1 protein expression seen in this study may be an endothelial cellular response to peripheral nociceptive activity arising from CIP since when nociceptive input was blocked by bupivacaine there was no increase in ZO-1 expression. The discrepancy in percentage of increase in expression seen with the current study and that previously reported may be due to a difference in the treatment paradigm used in this study as compared to that used by Huber et al. 2002. In the Huber et al. study animals were anesthetized with pentobarbital Na+ (60 mg/kg) prior to hindpaw injection, where as hindpaw injection in the current study had to be performed in the wake state, without pentobarbital pre-dosing. This is a crucial difference, as it has been demonstrated that BBB properties and metabolism in anesthetized as compared to non-anesthetized rats differs (Saija et al., 1989). This is an important point with regards to our occludin protein expression data. Here we show a significant increase in occludin expression at 1 h post-CIP. This change in expression is not seen in the nociception inhibited CIP group. This increase in expression is in contrast to what has been previously reported under similar treatment conditions (CIP; 1 h) (Huber et al., 2002), and may be a result of the differences in BBB properties and metabolism in anesthetized as compared to non-anesthetized rats as described above. This difference is an interesting finding warrants further investigation.
The transmembrane protein claudin-5 also showed an increase in protein expression of 73% in CIP animals, versus saline injection controls. An increased expression of claudin-5 has been shown to result in a loss of barrier function with increased permeability and paracellular volume of distribution ((Coyne et al., 2003); (Kojima et al., 2002);(Brooks et al., 2005)). Similar to both ZO-1 and occludin, inhibiting nociceptive input prevented increased claudin-5 expression.
In summary, the present study shows that CIP results in allodynia, increased paracellular BBB permeability to [14C] sucrose and increased ZO-1, claudin-5 and occludin expression. All of the changes seen under CIP are prevented upon peripheral inhibition of nociceptive input using bupivacaine. These results show for the first time that inflammatory based nociceptive signaling is involved in perturbations of the BBB. The data suggest a systemically-mediated modulation in BBB integrity, particularly nociceptive influence on protein signaling states, leading to the conclusion that nociception may play a role in systemically-mediated modulations in BBB properties. Understanding how changes in BBB structure and function occur in response to inflammatory pain will provide further insight into more effective and safer therapeutic approaches to treating inflammatory pain.
4. Experimental Procedures
4.1. Materials
The [14C] sucrose (0.44 Ci/mmol) was purchased from ICN Pharmaceuticals (specific activity 462 mCi/mol; Irvine, CA). TS-2 tissue solublizer were purchased from Research Products International (Mt. Prospect, IL), and the Optiphase SuperMix from Perkin Elmer (Boston, MA). The protease inhibitor (Complete Mini tablet; 10 ml) used in all buffers was purchased from Roche Biochemicals (St. Louis, MO). Nitrocellulose and Tris·HCl Criterion gels were purchased from Bio-Rad Laboratories (Hercules, CA). Western Lightning Chemiluminescence Reagent Plus was purchased from New England Nuclear/Perkin Elmer Life Sciences (Boston, MA). Mouse anti-claudin-5, rabbit antioccludin, and rabbit anti-ZO-1 were purchased from Zymed Laboratories (San Francisco, CA). Anti-mouse and anti-rabbit peroxidase conjugated secondary antibodies were obtained from Amersham Biosciences (Piscataway, NJ). Bupivacaine, λ-carrageenan, and all other chemicals and supplies were purchased from Sigma (St. Louis, MO).
4.2. Animals and treatments
Female Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 225–250 g were housed under standard 12:12-h light-dark conditions and received food ad libitum. All protocols involving animals were approved by the University of Arizona Institutional Animal Care and Use Committee and abide by National Institutes of Health guidelines. In order to minimize stress, animals were acclimated to handling prior to injections using a modification of the method of quick habituation (Dobrakovova et al., 1993) through repeated handling by the same investigator who performed the injections one day prior to experimentation and day of experimentation. Handling consisted of gently catching and picking up the animal from the cage and returning it back to its home cage after 1 min. To inhibit neuronal signaling and induce analgesia below the right hind knee, a perineural pre-injection of 0.65 ml bupivacaine (0.75% in 0.9% sterile saline) was applied to the saphenous, tibial, and common peroneal nerves. Control animals received a 0.65 ml injection of 0.9% saline in the same location. Ten minutes post bupivacaine nerve block, 0.1 ml of either 3% λ-carrageenan (CIP group) or 0.9% saline (saline control group) was injected into the plantar surface of the right hind paw. All experiments were performed 1 h post paw injection. There were four experimental groups: saline perineural pre-injection with saline injected into the paw (saline control); saline perineural pre-injection with λ-carrageenan injected into the paw (CIP group); bupivacaine perineural pre-injection with saline injected into the paw (nociceptive inhibition control); bupivacaine perineural pre-injection, with λ-carrageenan injected into the paw (nociception inhibited CIP group).
4.3 Edema formation and thermal allodynia
Hind paw edema was measured using a plethysmometer (model 7141; Ugo Basile, Comerio Varese, Italy). Each animal served as an internal control, with initial paw volume measurements taken prior to injection and compared with paw volume measurements taken 1 h post paw injection. Thermally evoked paw withdrawal latency was assessed using an infrared (IR) heat stimulus (Hargreaves et al.; Montagne-Clavel and Oliveras, 1996) using a Plantar Analgesia Instrument (model 7375; Ugo Basile). This applies a linearly increasing IR heat source from 23.5°C at time zero to an automatic cut off of 37°C at 15 s. To insure uniformity of testing the rats were first habituated to individual boxes on an elevated glass table for 20 min prior to exposure of the plantar surface of the right hind paw to IR heat source. Paw withdrawal latencies were defined as the time (s) taken for the rat to remove its hind paw from the heat source. Three consecutive measurements were performed on each hind paw, with 5-min intervals between measurements. Thermal paw withdrawal latencies were calculated as the average of three consecutive measurements, and converted to ratio of change pre-versus post paw injection of λ-carrageenan.
4.4. Locomotor activity assay
Locomotor activity assay was performed under low-light conditions in a quiet, temperature- and humidity-controlled environment to determine the motor effects of bupivacaine. All measurements were quantified using eight automated TruScan devices (Coulbourn Instruments, Allentown, PA). These computer-controlled devices utilize a floor plane photobeam array (0.5 inch beam spacing) over a smooth black 16 × 16 inch arena to calculate averaged body location, total movements, velocity, thigmotaxis, duration (1 s sample interval) and absolute distance traveled. Rats were randomly assigned to each sample group and allowed to acclimatize to the test chambers for 1 h on day one. Baseline activity was assessed on day two, paying particular attention to closely approximate the same time-of-day for each assessment period. Raw coordinate data was processed using TruScan 2.0 software to track total movement distance over time, measured in centimeters. All data was compared to controls where no anesthetic was used.
4.5. In situ brain perfusion with [14C] sucrose
One hour post paw injection, rats underwent 20 min in situ brain perfusion of [14C] sucrose as previously described (Hawkins et al., 2004). Rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and heparinized (10,000 U/kg i.p.) immediately prior to surgery. Body temperature was maintained at 37°C using a heating pad. The common carotid arteries were cannulated with silicone tubing connected to a perfusion circuit. The perfusate was an erythrocyte-free modified mammalian Ringer's solution made of (117 mM NaCl; 4.7 mM KCl; 0.8 mM MgSO4; 1.2 mM KH2PO4; 2.5 mM CaCl2, 10 mM D-glucose, 3.9 % dextran (mol. wt. 60,000), and 1 g/l bovine serum albumin (type IV), warmed to 37°C and oxygenated with 95% O2–5% CO2.). Evans blue (55 mg/l) was added to the Ringer’s solution to serve as a gross visual marker of BBB integrity. After cannulation, once the desired perfusion pressure of 85–95 mm Hg and flow rate of 3.1 ml/min were achieved, jugular veins were sectioned to allow drainage. Using a slow-drive syringe pump (0.5 ml/min per hemisphere; Model 22; Harvard Apparatus, Holliston, MA) [14C] sucrose (10 µCi/20 ml Ringer) was added to the inflowing perfusate. Following a 20-min perfusion, the rat was decapitated, brain removed and the perfusate containing the radiolabeled marker was collected from each carotid cannula to serve as a reference. The choroid plexus and meninges were excised and cerebral hemispheres sectioned and homogenized. One milliliter of TS2 tissue solubilizer was added and the samples allowed to solubilized for 2 days at room temperature. To eliminate chemiluminescence, 100 µl of 30% glacial acetic acid was added along with 2.5 ml liquid Optiphase SuperMix (Perkin Elmer, Boston, MA) scintillation cocktail. The samples were then measured for radioactivity on a liquid scintillation counter (model 1450 LSC & Luminescence Counter; Perkin Elmer). Results were reported as the ratio of radioactivity in the brain to that in the perfusate (Rbr), which is equal to the total amount of radiolabeled isotope in the whole brain, (Cbrain, in disintegrations/min/g) divided by amount of radiolabeled isotope in perfusate (Cperfusate, in disintegrations/min/ml).
4.6. Microvessel isolation
At 1 h after paw injection, rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.), decapitated and the brains removed. Meninges and choroid plexus were excised and cerebral hemispheres homogenized in 5 ml of microvessel isolation buffer (103 mM NaCl; 4.7 mM KCl; 2.5 mM CaCl2; 1.2 mM KH2PO4; 1.2 mM MgSO4; 15 mM HEPES; pH 7.4), with a Complete-mini protease inhibitor tablet (1 tablet per 10 ml; Roche). Six ml of 26% dextran at 4°C were added to the homogenate and vortexed. Homogenates were centrifuged (5,600 g; 4°C) for 10 min and supernatant aspirated. Pellets were resuspended in 10 ml of microvessel isolation buffer and passed through a 70 µm filter (Becton Dickinson, Franklin, NJ). Filtered homogenates were pelleted by centrifugation at 3,000 g for 10 min. The pellet was re-suspended in 0.3 ml 6M urea buffer urea lysis buffer (6M urea, 0.1% Triton X, 10mM Tris, pH 8.0, 1mM dithiothreitol, 5mM MgCl2, 5mM EGTA, 150mM NaCl) with Complete Protease Inhibitor for protein expression analysis. Protein concentrations were determined using bicinchoninic acid protein assay (Pierce; Rockford, IL) and protein samples were either immediately used or stored at − 40°C.
4.7. Western blot analysis
Protein isolated from isolated cerebral microvessels was analyzed for expression of the TJ proteins ZO-1, claudin-5 and occludin. Microvessel protein samples (40 µg) were resolved on a 4–20% Tris–glycine gel (Bio-rad, Hercules, CA) for 1 h at 200 V and transferred to a nitrocellulose membrane (Bio-rad) for 2 h at 30 V. Gel-Code Blue (Pierce, Rockford, IL) was used to stain the gels to ensure proper loading of protein. The nitrocellulose membranes were incubated in 5% non-fat milk/TBST blocking buffer (20 mM Tris Base, 137 mM NaCl, 2 M HCl, 0.1% Tween 20; pH 7.6) overnight at 4°C. Membranes were then incubated with primary antibody of either mouse anti-claudin-5 (1:1000), rabbit anti-occludin (1:2000) or rabbit anti-ZO-1 (1:1000) in 5% non-fat milk/TBST blocking buffer at 4°C overnight. Membranes were washed in 5% non-fat milk/TBST blocking buffer (3x 15 min) and then finally incubated with secondary antibodies of either HRP-conjugated anti-mouse (1:1000 for claudin-5 ) or HRP-conjugated anti-rabbit (1:2000 for ZO-1; 1:1500 for occludin) at room temperature for 45 min. Membranes were developed using enhanced chemiluminescence (ECL+, Amersham, Piscataway, NJ) and analyzed using Scion Image (Scion, Frederick, MD). All data was normalized to saline control values that are matched to the treated animals from the same experimental day.
4.8. Statistical analysis
In all experiments, data were presented as mean ± S.E. Allodynia, plethysmography and % protein expression data were analyzed using one-way analysis of variance (ANOVA) to determine significance among treatment groups. To determine significance of changes in BBB uptake of [14C] sucrose, calculated %Rbr values for each treatment group were analyzed using a two-way ANOVA. Pairwise comparisons for all significant groupings were performed using Tukey's HSD post-hoc test. Significance was defined as p<0.05. All analyses were performed using the Sigma Stat 2.03 statistical software package (SPSS, San Rafael, CA).
Acknowledgements
This work was supported by NIH grants DA11271, NS39592 and NS42652 to TPD.
Abbreviations used
- BBB
blood-brain barrier
- TJ
tight junction
- ICAM-1
integrin leukocyte function-associated molecule-1
- CIP
λ-carrageenan inflammatory pain
- CBF
cerebral blood flow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20(2):131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Fanning AS, Lapierre L, Van Itallie CM. Zonula occludens (ZO)-1 and ZO-2: membrane-associated guanylate kinase homologues (MAGuKs) of the tight junction. Biochem Soc Trans. 1995;23(3):470–475. doi: 10.1042/bst0230470. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. Quantification of early blood-brain barrier disruption by in situ brain perfusion technique. Brain Res Brain Res Protoc. 2001;8(2):126–131. doi: 10.1016/s1385-299x(01)00094-0. [DOI] [PubMed] [Google Scholar]
- Blasberg RG, Fenstermacher JD, Patlak CS. Transport of alpha-aminoisobutyric acid across brain capillary and cellular membranes. J Cereb Blood Flow Metab. 1983;3(1):8–32. doi: 10.1038/jcbfm.1983.2. [DOI] [PubMed] [Google Scholar]
- Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289(2):H738–H743. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks TA, Ocheltree SM, Seelbach MJ, Charles RA, Nametz N, Egleton RD, Davis TP. Biphasic cytoarchitecture and functional changes in the BBB induced by chronic inflammatory pain. Brain Res. 2006;1120(1):172–182. doi: 10.1016/j.brainres.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Egleton RD, Davis TP. Mannitol opening of the blood-brain barrier: regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res. 2004;1014(1–2):221–227. doi: 10.1016/j.brainres.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285(5):L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- Dobrakovova M, Kvetnansky R, Oprsalova Z, Jezova D. Specificity of the effect of repeated handling on sympathetic-adrenomedullary and pituitary-adrenocortical activity in rats. Psychoneuroendocrinology. 1993;18(3):163–174. doi: 10.1016/0306-4530(93)90001-2. [DOI] [PubMed] [Google Scholar]
- Dollery CT. Therapeutic drugs. Edinburgh; New York: Churchill Livingstone; 1999. [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10(6):1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Foster AH, Carlson BM. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg. 1980;59(10):727–736. [PubMed] [Google Scholar]
- Fricker G, Miller DS. Modulation of drug transporters at the blood-brain barrier. Pharmacology. 2004;70(4):169–176. doi: 10.1159/000075545. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11(4):315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- Gordh T, Sharma HS. Chronic spinal nerve ligation induces microvascular permeability disturbances, astrocytic reaction, and structural changes in the rat spinal cord. Acta Neurochir Suppl. 2006;96:335–340. doi: 10.1007/3-211-30714-1_70. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141(1):199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027(1–2):48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001a;24(12):719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP. Blood- brain barrier tight junctions are altered during a 72-h exposure to lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol. 2002;283(4):H1531–h1537. doi: 10.1152/ajpheart.00027.2002. [DOI] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001b;280(3):H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20(1):57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Rahner C, Peng S, Rizzolo LJ. Claudin 5 is transiently expressed during the development of the retinal pigment epithelium. J Membr Biol. 2002;186(2):81–88. doi: 10.1007/s00232-001-0137-7. [DOI] [PubMed] [Google Scholar]
- Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res. 2004;68(3):231–238. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL. The "plantar test" apparatus (Ugo Basile Biological Apparatus), a controlled infrared noxious radiant heat stimulus for precise withdrawal latency measurement in the rat, as a tool for humans? Somatosens Mot Res. 1996;13(3–4):215–223. doi: 10.3109/08990229609052577. [DOI] [PubMed] [Google Scholar]
- Rasmussen LM, Lipowitz AJ, Graham LF. Development and verification of saphenous, tibial and common peroneal nerve block techniques for analgesia below the thigh in the nonchondrodystrophoid dog. Vet Anaesth Analg. 2006;33(1):36–48. doi: 10.1111/j.1467-2995.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- Saija A, Princi P, De Pasquale R, Costa G. Modifications of the permeability of the blood-brain barrier and local cerebral metabolism in pentobarbital- and ketamine-anaesthetized rats. Neuropharmacology. 1989;28(9):997–1002. doi: 10.1016/0028-3908(89)90202-5. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J Biol Chem. 1997;272(26):16133–16139. doi: 10.1074/jbc.272.26.16133. [DOI] [PubMed] [Google Scholar]
- Turowski P, Adamson P, Greenwood J. Pharmacological targeting of ICAM-1 signaling in brain endothelial cells: potential for treating neuroinflammation. Cell Mol Neurobiol. 2005;25(1):153–170. doi: 10.1007/s10571-004-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wildsmith JA. Peripheral nerve and local anaesthetic drugs. Br J Anaesth. 1986;58(7):692–700. doi: 10.1093/bja/58.7.692. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D, Gross Y, Lorian A, Shalev A, Lamensdorf I, Bornstein R, Shorer S, Mayevsky A, Patel KP, Abbott NJ, et al. Blood-brain barrier opened by stimulation of the parasympathetic sphenopalatine ganglion: a new method for macromolecule delivery to the brain. J Neurosurg. 2004;101(2):303–309. doi: 10.3171/jns.2004.101.2.0303. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336(1):125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Lipovac MN, Begley DJ, Davson H, Rakic L. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J Neurochem. 1987;49(1):310–315. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Mackic JB, Djuricic B, Davson H. Kinetic analysis of leucine-enkephalin cellular uptake at the luminal side of the blood-brain barrier of an in situ perfused guinea-pig brain. J Neurochem. 1989;53(5):1333–1340. doi: 10.1111/j.1471-4159.1989.tb08522.x. [DOI] [PubMed] [Google Scholar]