Abstract
Objective
Fibromyalgia (FMS) is characterized by chronic pain, high psychiatric comorbidity, and the absence of observable pathology. Our objective was to examine positive and negative affective indices, both at the trait and contextual levels, in FMS compared to a chronic pain control group, osteoarthritis (OA).
Methods
The sample consisted of 126 female FMS (87) and OA (39) patients from the community. Participants answered a self-report questionnaire assessing demographic and personality variables and were interviewed regarding average pain, affect, anxiety, and depression. Participants were then interviewed weekly for up to 12 weeks regarding pain, affect, fatigue, perceived interpersonal stress (IS), and positive interpersonal events (PE).
Results
FMS participants reported lower levels of positive affect (p < .01) and extraversion (p < .01) than OA participants. There were no significant differences between groups in negative affect, depression, anxiety, or neuroticism after controlling for age and average pain. At the weekly level, FMS participants reported lower levels of positive affect (p < .01), but not negative affect. Furthermore, during weeks of elevated IS, FMS participants evidenced steeper declines in positive affect than OA participants (p = .01).
Conclusions
Despite the predominance of literature focusing on psychological disturbance in FMS, these analyses identified dysfunctional positive affect regulation as a key feature of FMS. FMS status was uniquely characterized by lower levels of positive affect, especially during stressful weeks. These findings challenge current conceptualizations of FMS and point to new directions for interventions that focus on improving positive affective resources, especially during times of stress.
Keywords: fibromyalgia, positive affect, interpersonal stress, osteoarthritis, chronic pain
Chronic pain is a feature in many health conditions, and in some illnesses, it is presumed to be the defining characteristic. Symptoms of psychological distress often accompany chronic pain conditions and include elevations in depression and anxiety, as well as other negative affective states. Indeed, the link between pain and negative affect is well established across a range of chronic pain conditions including osteoarthritis (OA), Fibromyalgia (FMS), and rheumatoid arthritis (1, 2). In comparison to non-pain groups, both those with FMS and those with joint pain without FMS show greater psychological distress across a range of indicators (3, 4). Though most investigations have neglected the study of positive affect, there is evidence that FMS patients may also suffer from a relative absence of positive emotional resources (5, 6). It would be particularly useful to examine both positive and negative aspects of emotional functioning across different pain conditions in order to identify the distinctive features of each condition as well as to help plan psychosocial interventions targeted to meet the specific needs of each group. This study investigated how measures of positive and negative affective conditions distinguish FMS patients from OA patients who also have chronic pain.
A comparison of positive and negative indicators of emotional well-being between FMS and OA participants would appear particularly warranted given the nature of these two conditions. OA is considered a wear-and-tear disease of predominantly weight-bearing joints. Joints of OA patients show physical signs of swelling and tenderness, and x-rays confirm damage to cartilage and surrounding tissue as a result of disease processes (7). Patients with FMS, on the other hand, show no outward manifestations of disease processes and complain of substantial pain in soft tissue rather than in the joints (8). This pain is widespread, often disabling, and is frequently accompanied by negative mood. In fact, the presence of widespread pain in the absence of observable pathological signs, along with relatively high rates of psychological symptoms, has led some investigators to propose that an affective disorder underlies the unexplained symptom profile (e.g., 4). Others have suggested that FMS might be a manifestation of neuroticism (9), and Charles, Gatz, Pederson, and Dahlberg (10) reported that higher levels of neuroticism increased the risk for later self-reported joint pain.
Missing in these formulations is an appreciation of those factors thought valuable in the restoration of well-being. Positive emotions, in particular, have been shown to play an important role as resources that foster resilience, aiding in recovery following episodes of high pain, over and above their role in modulating negative affect on low pain days (2). On balance, however, positive affective states have received far less attention in the FMS research literature. In fact, hidden in the overattention to negative states may be an inattention to the positive. It may be that a key problem of patients with FMS, in comparison with other chronic and painful illnesses, is an inability to mobilize sufficient positive affective resources to neutralize the experience of pain and the associated negative affect effectively.
Deficits in positive affective responding may have several origins, and an examination of psychosocial variables in the context of everyday life might reveal the mechanisms involved. One potential source of differences in positive affect between groups might be fewer positive interpersonal interactions, and other activities that engender greater positive affect. FMS patients may also differ from other chronic pain patients in their responsiveness to these events. Consistent with the view that FMS difficulties stem, at least in part, from depressive features, deficits in responsiveness to positive events may underlie the FMS condition. An assessment of weekly reports of events and their associations with positive affect for the two groups would have the potential to reveal evidence of a relatively greater difficulty in experiencing positive emotion in response to everyday events.
A second potential mechanism for chronically low positive affect in FMS may be derived from a “stress-diathesis” model of adaptation (11, 12). The losses in positive emotion may accumulate over time from a failure to recover positive affect following stressful life events. The lower levels of positive affect observed in FMS may be due to a failure of processes that appear to confer what may be referred to as “psychological immunity”: a deployment of positive affective resources that serve to neutralize distress (13). Prior research has documented an increase in the capacity of positive affect to down regulate negative affective states during times of stress (2, 14). Such a capacity may be lacking in patients with FMS in comparison to other groups.
This investigation of the differences between FMS and OA in positive and negative affect was conducted in two sets of analyses. The first analyses examined mean differences between diagnostic groups in their levels of positive and negative affective states including differences in personality features and more stable trait estimates of positive and negative emotional health. We predicted that FMS patients would exhibit a deficit in positive affective resources in comparison to OA patients.
The second set analyzed weekly reports of events, affect, and pain conducted on the same sample. We predicted that the FMS sample would show greater stress reactivity than OA patients. Specifically, we predicted that FMS patients would evidence steeper declines in positive affective states than OA patients during stressful weeks. We tested, but made no predictions about differences in pain and negative affect between groups that may arise as a consequence of stressful weeks. We also examined an alternative causal mechanism for the PA deficit: anhedonic responses to everyday positive events, which were reported during the weekly interviews along with the stressful events. This dual approach provided a broad-band investigation of the role of positive affective states in differentiating FMS patients from a sample with similar pain levels, OA patients. Moreover, these questions hold important implications for clinical interventions. If positive events do not benefit both groups equally, or if social stress leads to greater psychological consequences for the FMS group, then it may be warranted to focus on increasing positive social events or improving strategies to boost positive affect during times of stress (respectively) in interventions for FMS patients.
Method
Participants
Participants were 126 women with FMS (N = 87) and/or OA (N = 39) Diagnoses of FMS were based on a modification of the FMS Self-report Screening Instrument (Bradley, personal communication, 1997). Further, if participants had illnesses other than FMS or OA, they ranked their FMS or OA as causing them the most difficulty of all their illnesses. We also contacted participants’ physicians in order to confirm diagnoses of FMS or OA. At the start of the study, each participant was not currently involved in any health-related litigation, and was living with a romantic partner. Participants were recruited through a variety of means including flyers placed in physicians’ offices and other public locations, newspaper ads, and mass mailings to members of the Arthritis Foundation. Ninety-Five percent of participants were Caucasian, and the average income for both groups was in the $50,000 − $59,000 range. Other demographic data, as well as chi-square tests for differences between groups, are presented in Table 1.
Table 1.
Average Scores for Participants with Osteoarthritis and Fibromyalgia on Demographic Characteristics, Personality Dimensions, Pain, and Affective States
| |
Osteoarthritis (N = 39) |
Fibromyalgia (N = 87) |
|
|
||
|---|---|---|---|---|---|---|
| Variables | M/% | SD | M/% | SD | F/χ2 | p |
| Demographics | ||||||
| Age | 58.87 | 9.18 | 52.68 | 7.33 | 4.05 | < .01 |
| Percent Married | 89.7% | 89.5% | .001 | .97 | ||
| % Completing High School | 97.4% | 97.7% | .008 | .93 | ||
| % 4 yrs. College or Post College | 20.5% | 35.6% | 2.88 | .09 | ||
| Percent Employed | 41.0% | 37.2% | .16 | .68 | ||
| Trait Measures | ||||||
| Neuroticism | 3.00 | 0.85 | 3.35 | 0.75 | 5.48 | .02 |
| Extraversion | 3.69 | 0.88 | 3.25 | 0.80 | 7.75 | < .01 |
| State Measures | ||||||
| Average Pain | 50.23 | 24.81 | 60.47 | 19.52 | 2.50 | .01 |
| Negative Affect | 1.57 | 0.52 | 1.84 | 0.72 | 4.28 | .04 |
| Anxiety | 2.18 | 0.91 | 2.64 | 0.97 | 6.18 | .01 |
| Depression | 1.93 | 0.72 | 2.38 | 0.98 | 6.54 | .01 |
| Positive Affect | 3.33 | 0.65 | 2.67 | 0.77 | 21.56 | < .01 |
| Joviality | 3.18 | 0.82 | 2.43 | 0.84 | 21.35 | < .01 |
| Self-Assurance | 2.94 | 0.77 | 2.30 | 0.74 | 19.68 | < .01 |
Procedure
Upon receipt of informed consent forms, participants were mailed an initial questionnaire, followed by a home interview by a research assistant. After the home visit, participants were assigned an interviewer, a research assistant trained to perform a standardized 45-minute telephone interview once a week for 10 weeks (or extended to 12 weeks for participants with few interpersonal stressors). Participants completing all aspects of the study (including the initial phase) were paid a total of $100.
Of the 126 participants providing data for the first set or analyses, 124 provided weekly interviews. The average number of weekly interviews for each subject was 9.0, with 5.8% of weekly interviews missing.
Measures
Extraversion/neuroticism
Extraversion and neuroticism were assessed in the initial questionnaire. The neuroticism/extraversion items (8 of each) from the Big Five Inventory (BFI; 15) were used. Examples of extraversion items include “Is talkative,” and “Is outgoing, sociable.” Examples of neuroticism items included “Can be moody,” and “Gets nervous easily.” Cronbach's alpha was .81 for neuroticism and .83 for extraversion in the present study.
Depression/anxiety
Depression and anxiety (over the previous week) were assessed during the initial visit using ten depression items and nine anxiety items from the Mental Health Inventory (MHI; 16), which has been used in prior work with chronic pain populations (17). The depression subscale included face valid items such as “Did you feel depressed,” and the anxiety subscale included items such as “Have you been anxious or worried.” Cronbach's alpha was .90 for the anxiety scale and .92 for the depression scale in this sample.
Pain
During the initial visit and weekly interviews, participants were asked to rate the average level of pain they experienced due to their Fibromyalgia or Osteoarthritis during the past week on a scale from 0 to 100 with zero indicating “no pain” and 100 indicating “pain as bad as it can be.” In order to estimate reliability for this single item measure, test-retest reliabilities were conducted across the weeks. This yielded an average week-to-week correlation of .69.
Positive affect (PA) and negative affect (NA)
PA and NA were measured during the initial visit and in the weekly interviews using the original 20-item Positive and Negative Affect Schedule plus the joviality and self-assurance scales from the Positive and Negative Affect Schedule (18). Participants were asked to indicate on a 5-point scale from 1 (very slightly or not at all) to 5(extremely) the extent to which they had experienced each affect during the past week. The PA scale included items such as “interested,” “excited,” and “proud,” and the NA scale included items such as “distressed,” “nervous,” and irritable.” PA and NA scores were obtained by computing the mean for the 10 items in each scale. Cronbach's alpha was .88 for the PA scale and .84 for the NA scale the present study. Two additional partially-overlapping sub-scales of positive affect were constructed following Watson and Clark (18). “Joviality” included items such as “happy,” “joyful,” and “delighted,” and “Self-assurance” included items such as “strong,” “confident,” and “bold.” Cronbach's alpha was .92 for joviality and .84 for self-assurance.
With repeated observations of each participant in the weekly interview phase, we were also able to estimate within-subject internal consistency reliability separately from between-person reliability by transforming item scores into z-scores representing deviations from each participant's own mean score (across the weeks) on each item in the scale. The within-subject alpha was .85 for PA and .86 for NA. For the estimation of the reliability of the scale across participants, we computed averages of each person's scores (at the item-level) across weeks, resulting in a mean score for each subject for each item. The between-subject alpha for PA was .94 and .91 for NA.
Fatigue index
Fatigue was measured during each weekly interview using four items from the SF-36 vitality subscale (19) and one item asking participants to rate, “Did you feel fatigued?” The within-subject alpha was .82 and the between-subject alpha was .96.
Perceived interpersonal stress
Perceived interpersonal stress (IS) was measured during each weekly interview within four interpersonal domains: (1) friends and acquaintances, (2) spouse or live-in partner, (3) family members, and (4) coworkers. This measure has been utilized with chronic pain populations, and has been correlated with negative social events and negative affect (20,2,6). Following established methods (e.g., 20), the interviewer read a series of items that identified stressful and non-stressful events within each interpersonal domain, and asked participants to report on the frequency of those events over the past week. This procedure was used to provide an event-related context for ratings of stressfulness. Following the reports of events, the interviewer asked the participant to rate the stressfulness of relationships in each domain by asking, “Overall, how stressful were your relations with (friends/spouse/family members/coworkers) this past week?” If items from one domain were missing (e.g., because they were not employed), then the average of the remaining items was taken. Cronbach's alpha was not computed because the items were designed to measure non-overlapping interpersonal domains. Positive interpersonal events. Positive social events (PE) were also measured using the Inventory of Small Life Events (ISLE) for older adults (21). Participants provided frequency counts of the weekly occurrence of 29 positive events (e.g., “played a sport, game, or cards with friends”) gathered from the same four domains of the ISLE as mentioned above for IS, and a total sum score was obtained.
Results
Analysis of Initial Data
The question we wished to address with the first set of analyses was the extent to which key personality features and affective states distinguished FMS from OA participants. Through the use of an OA control group, we could control in part for the presence of chronic pain when examining personality and affective conditions unique to FMS. Table 1 provides summary statistics for demographic, personality, and affective indices for the two groups. The OA sample was older, on average, than the FMS sample (59 versus 53 years old; t (124) = 4.05, p < 0.01). FMS participants also reported somewhat higher average pain than the OA sample (approximately 10 points on a 0 to 100 numeric scale; t (124) = 2.50, p = 0.01).
A multivariate analyses of variance (MANOVA) was run using diagnosis (FMS versus OA) as the independent variable, with age as a covariate. Neuroticism and extroversion were included as global personality attributes, and depression and anxiety symptoms were included as measures of stable affective disturbance. Overall negative affect and three measures of positive affect (overall positive affect, self-assurance, and joviality) taken during the initial assessment were also included. As shown in Table 1, FMS participants scored higher on all of the negative indices and lower on all of the positive indices compared to the OA participants when no covariates were employed. However, the results of the first MANCOVA run, with only age as a covariate, revealed differences between the two diagnostic groups that were significant only for the positive affect variables (positive affect, self-assurance, joviality, extraversion), but not for the negative affect variables (negative affect, neuroticism, depression, anxiety). Diagnosis accounted for between 1 and 3% of the variance in the negative affect variables, but accounted for between 5 and 10% of the variance in the positive affect variables.
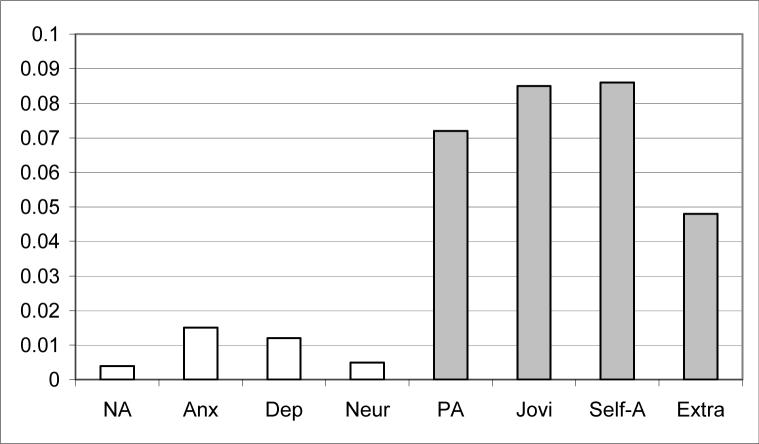
The data were also analyzed with a second MANOVA, controlling for pain as well as age differences between groups. As in the previous analysis, FMS patients reported lower levels of positive affect [F(1,122) = 9.45, p < 0.01], self-assurance [F(1,122) = 11.44, p < 0.01], joviality [F(1,122) = 11.31, p < 0.01], and extraversion [F(1,122) = 6.20, p = .014] than the OA patients. On the other hand, the FMS participants did not differ from the OA participants in negative affect [F(1,122) = 0.43, p = 0.51], depression [F(1, 122) = 1. 53, p = 0.22], anxiety [F = (1, 122) = 1.81, p = 0.18], or neuroticism [F(1, 122) = 0.57, p = 0.45]. Figure 1 below displays the findings in terms of variance accounted for between groups for each of the variables. Three variables accounted for 7% or more of the variance between groups: overall positive affect, and both the self-assurance and joviality sub-scales. Of interest also was the relative absence of effects for negative affect, depression, anxiety, and neuroticism. Subsequent analyses controlling for personality features did not affect these findings.
Figure 1.
R-squared values of predictors of FMS versus OA status covarying age and pain.
Analysis of Weekly Data
Average levels of weekly variables were also compared between the FMS and OA groups, and are shown in Table 2. Compared to the OA sample, the FMS sample had significantly higher scores on weekly pain, weekly fatigue, and weekly IS, and significantly lower scores on weekly PA. There were no significant differences between groups on measures of weekly NA or in frequencies of weekly PE.
Table 2.
Differences between Participants with Osteoarthritis and Fibromyalgia on Key Weekly Variables
| |
Osteoarthritis (N = 37) |
Fibromyalgia (N = 87) |
|
|
||
|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | t | p |
| Weekly Pain | 48.30 | 19.32 | 55.75 | 16.71 | −2.17 | .03 |
| Fatigue | 3.56 | 0.89 | 4.35 | 0.78 | −4.87 | < .01 |
| Interpersonal Stress | 0.44 | 0.40 | 0.60 | 0.34 | −2.23 | .03 |
| PA | 3.16 | 0.55 | 2.78 | 0.58 | 3.36 | < .01 |
| NA | 1.66 | 0.47 | 1.83 | 0.51 | −1.74 | .08 |
| Weekly Positive Events | 36.31 | 12.03 | 33.94 | 11.67 | 1.02 | .31 |
Multi-level modeling was used as the primary data-analytic tool to analyze the weekly data. This method is particularly useful for the analysis of data that have a nested hierarchical structure such as in the present study, with up to 12 observations nested within each of the study participants. All multi-level analyses were conducted using SAS PROC MIXED software (22).
Weekly positive affect was the primary criterion variable to be predicted in these analyses. There were two basic prediction equations: A level 1 equation which examined the influence of within person variations of key variables on positive affect, and a level 2 equation which tested the effects of between person differences. The level 1 analyses took the following form: When a person has higher stress, do they also report lower positive affect? Level 2 variables addressed questions regarding between-person differences, such as whether people who score higher on the predictor (e.g., stress) also have less positive affect. Finally, cross-level interactions allow us to combine the questions of when and whom, such as whether FMS participants may be particularly vulnerable to losses in positive affect when they have weeks with more stress. Unlike traditional least squares regression analyses, multi-level models allow for partitioning of variance into within and between components to address these different types of questions, and allow for stronger causal inferences by studying within-person changes over time (23).
For level 1 analyses, weekly deviation scores on IS and PE were computed by subtracting each participant's average score on those variables across all weeks from her weekly report on each variable, yielding weekly person-centered scores. The equation was initially specified at level 1 as follows:
β0 yields an estimate of the average weekly PA and β1-β2 provide slope estimates of the effects of weekly changes in IS and PE on weekly positive affect. In addition to PE and IS, initial models also included the week number in the study to test for any effects of the week of assessment on these prediction equations. The linear or fixed effect of week was non-significant and was later dropped from the prediction equation.
Individual differences in the average level of the weekly variables were probed through analyses at level 2. The between-person variables of diagnosis and neuroticism were also added to further examine individual differences. These variables were used as predictors of variance in level 1 weekly PA (the level 1 intercept: β0) and slopes of the relationships between deviation scores and PA (β1-β2 in the level 1 equation above). The first level 2 equation is as follows:
where each person's level 1 intercept is predicted by an intercept, the two between-persons variables of interest, a random error component, and the mean levels of the level 1 independent variables.
Level 2 equations were then specified to predict between-persons differences in the level 1 slopes for the effects of positive events and stress on PA. The equations for predicting the slopes were written as follows:
| (1) |
| (2) |
such that each person's level 1 slopes are predicted by an intercept, the between-persons variables, and a random error component. There were no significant effects of neuroticism on the level 1 slopes, and these effects were later dropped from the final equations.
The other specifications for this model were selected following Singer (24) to identify the best fitting model of the variances and covariances of the variables under study. Goodness of fit tests were employed to examine whether the weekly deviations in interpersonal stress and/or positive events also varied randomly across participants. PE showed significant random effects in the mixed models predicting positive affect and was therefore specified as a random effects variable. The correlation of the slope (between PE and PA) and the intercept was −.43, indicating that those with lower levels of PA were more greatly affected by changes in positive events. PE did not show random effects in the models predicting fatigue, and IS did not show significant random effects in either model. A first-order autoregressive variance-covariance matrix was also chosen to model the within-subjects covariance on the dependent variable. When these analyses were repeated on negative affect, pain, and fatigue as dependent variables, the same analysis framework was employed.
There were two levels of variability in this study suitable for analysis in the multilevel model: The level of individual differences between people and the within-person level assessed through the weekly observations. Our focus was on what is sometimes referred to as cross-level interactions. We predicted differences in the downward slopes of the regression lines that describe the relations between weekly IS and positive affective states. FMS participants should show a significantly steeper decline in PA during weeks of increased IS than OA patients. This result would amount to finding a significant difference in the β2 coefficient defining the degree of relationship between IS and PA, for OA and FMS groups. The interaction term, IS by diagnosis, carries this effect.
The results of this analysis for PA are shown in Table 3. What is apparent from the Level 2 results is a difference between diagnostic groups in PA, with lower scores for the FMS group (β = −.23, p = .03). Participants with higher neuroticism scores also showed lower PA (β = −.20, p < .01). There was no overall decrease in PA on more stressful weeks, as evidenced by the non-significant Level 1 effect of ΔIS (β = −.02, p = .75). Nor was there a significant effect of average IS on PA; participants who had higher average levels of IS did not show lower levels of PA (β = −.02, p = .88). As predicted, there was a significant interaction effect of ΔIS by diagnosis, indicating that the effects of weekly stress on lowering PA were confined to the FMS group (β = −.24, p < .01).
Table 3.
Multilevel Regressions Predicting Weekly Positive Affect
|
Random Effects: Prediction of Positive Affect | ||||||
|---|---|---|---|---|---|---|
| Covariance Parameter Estimates | Subject | Estimate | Std. Error | Z | P | |
| Variance of intercepts | UN (1,1) | ID | 0.22 | 0.03 | 6.52 | < .01 |
| Covariance btwn. slope and intercept | UN (2,1) | ID | −.002 | 0.001 | −2.06 | .04 |
| Variance of slopes | UN (2,2) | ID | 1.01*10−4 | 4.9*10−5 | 2.02 | .02 |
| Autoregressive component | AR (1) | ID | 0.22 | 0.04 | 5.88 | < .01 |
| Level 1 residual | Residual | 0.23 | 0.01 | 19.01 | < .01 | |
|
Fixed Effects | ||||||
|---|---|---|---|---|---|---|
| Predictor Variables | β | Std. Error | DF | T | P | |
| Level 1 | ||||||
| Δ in Interpersonal Stress (ΔIS) | −0.02 | 0.08 | 1044 | −0.32 | .75 | |
| Δ in Positive Events (ΔPE) | 0.01 | 0.003 | 1044 | 4.08 | < .01 | |
| Level 2 | ||||||
| Neuroticism | −0.20 | 0.06 | 119 | −3.23 | < .01 | |
| Diagnosis | −0.23 | 0.10 | 119 | −2.20 | .03 | |
| Average PE | 0.01 | 0.004 | 119 | 3.15 | < .01 | |
| Average IS | −0.02 | 0.14 | 119 | −0.16 | .88 | |
| Level 1 × Level 2 | ||||||
| ΔIS × Diagnosis | −0.24 | 0.09 | 1044 | −2.62 | < .01 | |
| ΔPE × Diagnosis | 0.001 | 0.004 | 1044 | 0.15 | .88 | |
Note: Positive Events (Δ PE) was treated as a random effect in this model.
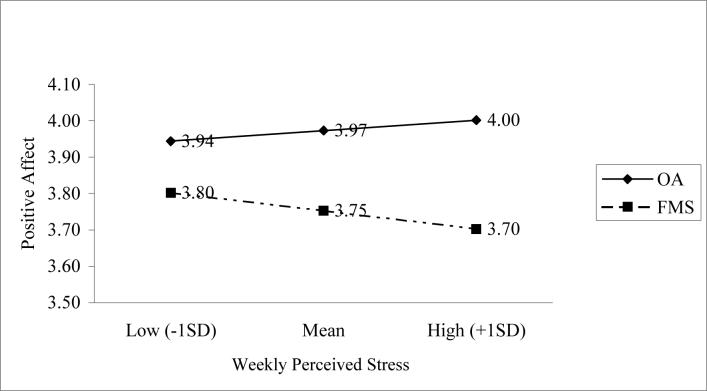
Figure 2 displays the shape of this interaction effect. During weeks when IS is elevated, there is a steeper decline in PA for the FMS group than there is for the OA group. Also included in these equations were tests for the influence of positive interpersonal events on positive affect for FMS and OA samples. These results are also shown in Table 3, along with the findings for stress reactivity. A main effect for ΔPE on mood was observed (β = .01, p < .01), but there was no differential influence of diagnosis on the PE-PA relationships (β = .001, p = .82). Patients in both groups appeared to benefit from increments in positive events when they occurred. There was also a significant main effect for average PE, indicating that participants from both groups who reported more frequent PE across the weeks had higher levels of PA (β = .01, p < .01)
Figure 2.
Plot of the interaction of diagnosis and weekly perceived stress on positive affect.
We next examined the evidence for changes in levels of fatigue. For these analyses we examined weekly scores on the fatigue index and tested the effects of weekly variations in PE and IS, FMS versus OA diagnosis, and their interaction in the prediction of fatigue. These results, shown in Table 4, were a mirror image to the findings for PA. As expected, FMS patients showed greater fatigue overall (β = .61, p < .01). There was no main effect of ΔIS (β = .09, p = .44) or average IS (β = .26, p = .22) on fatigue, but there was an interaction effect between ΔIS and diagnosis in the prediction of weekly variations in fatigue. FMS participants showed greater elevations in fatigue (β = .33, p = .01) during stressful weeks than OA participants. There was a main effect for average PE (β = −.01, p = .03), indicating that participants from both groups who reported more frequent PE also reported lower levels of fatigue across the weeks. Changes in positive interpersonal events were only marginally related to less fatigue (β = −.01 p = .06) and there were no differences between groups in the strength of this relationship (β = −.003, p = .53).
Table 4.
Multilevel Regression Predictions of Weekly Fatigue
|
Random Effects: Prediction of Weekly Fatigue | ||||||
|---|---|---|---|---|---|---|
| Covariance Parameter Estimates | Subject | Estimate | Std. Error | Z | P | |
| Variance of intercepts | UN (1,1) | ID | 0.50 | 0.08 | 6.48 | < .01 |
| Autoregressive component | AR (1) | ID | 0.26 | 0.04 | 7.11 | < .01 |
| Level 1 residual | Residual | 0.53 | 0.03 | 19.46 | < .01 | |
|
Fixed Effects | ||||||
|---|---|---|---|---|---|---|
| Predictor Variables | β | Std. Error | DF | T | p | |
| Level 1 | ||||||
| Δ in Interpersonal Stress (ΔIS) | 0.09 | 0.11 | 1046 | 0.78 | .44 | |
| Δ in Positive Events (ΔPE) | −0.007 | 0.004 | 1046 | −1.87 | .06 | |
| Level 2 | ||||||
| Neuroticism | 0.18 | 0.10 | 119 | 1.81 | .07 | |
| Diagnosis | 0.61 | 0.16 | 119 | 3.89 | < .01 | |
| Average PE | −0.01 | 0.006 | 119 | −2.14 | .03 | |
| Average IS | 0.26 | 0.21 | 119 | 1.23 | .22 | |
| Level 1 × Level 2 | ||||||
| ΔIS × Diagnosis | 0.33 | 0.13 | 1046 | 2.49 | .01 | |
| ΔPE × Diagnosis | −0.003 | 0.005 | 1046 | −0.64 | .53 | |
Finally, we examined whether the differences in stress reactivity for PA were also present in the prediction of NA and weekly pain. Analyses parallel to PA were run using weekly ratings of NA and weekly pain reports. On weeks of greater interpersonal stress, patients from both groups reported more negative affect (β = .50, p < .01) but not more pain (β = .10, p = .97). There were no differences between groups in the extent of the relationship between IS and NA (β = .10, p = .22), and there were no differences between groups in how changes in perceived stress covaried with pain (β = 2.8, p = .30). Further, positive events had no effect on pain (β = −.05, p = .42) or NA (β = −.002, p = .50) for either group. The reactivity differences between groups were specific to PA following the occurrence of stressful events. FMS patients were not less responsive to positive events. FMS was distinct from OA patients only in the relatively greater vulnerability to loss of positive affect following interpersonally stressful experiences.
Discussion
The purpose of this paper has been to test whether FMS patients showed affective profiles that were unique from those profiles of another chronic pain group: women with chronic pain from Osteoarthritis. After controlling for chronic pain levels, we found little evidence that these FMS women had greater difficulty in the management of negative emotion than their OA counterparts. Contrary to expectation from the literature on FMS, differences between groups on neuroticism, depression, and anxiety were not substantial. Furthermore, FMS women did not report greater negative affect than the OA women. The unique problems these FMS patients had in emotion regulation did not arise primarily from the negative affective domain.
We did find substantial differences between groups in the regulation of positive affective states. FMS patients reported lower levels of positive affect, joviality, and self-assurance compared to OA patients. In addition to finding differences between groups in their retrospective accounts of positive affect, we also found key deficits in the FMS sample's everyday life experiences with positive emotion. These differences were not due to fewer social engagements in the FMS sample versus the OA sample. The quantity of positive interpersonal events was comparable between groups, and when these positive events occurred, both groups showed significant increases in positive emotion.
This last point is important because it rules out anhedonia as a possible explanation for the current findings, and diminishes the likelihood that a simple information processing bias, where patients may not be able to attain positive emotions from positive events, often observed in depressed patients, might account for the differences we observed. Both frequency of positive interpersonal events and their influence on positive affect were undisturbed in FMS patients in all comparisons with OA controls.
The weekly interviews revealed a surprising difference in how participants responded to stressful interpersonal events. Contrary to what may have been expected from the literature, FMS participants did not report more pain or negative affect in the face of stress compared to OA participants. Instead, when reporting more stress, FMS patients could not sustain positive affect as well as the OA sample. This response to everyday stressors aggregated over time provides us with the potential mechanism underlying the low positive affect and high fatigue commonly observed in this population: A unique vulnerability of the positive affect system to interpersonal stress for women with FMS.
How might we understand the nature of this deficit in emotional regulation? This form of stress reactivity is unusual. In fact, the authors do not know of any previous studies of this phenomenon in the context of chronic pain or, for that matter, with any chronically ill population. However prior research does provide some directions for future study. Zautra and colleagues (25) have shown previously that stress can narrow affective space, increasing the degree of negative correlation between positive and negative states. FMS participants may find it more difficult to mount a resilient affective response to stressful events, if the force of negative affect compromises their resources of positive affect. Over time, the inability to maintain emotional homeostasis during times of stress may make FMS patients more likely to perceive future events as stressors, resulting in a perpetuation of this cycle. Such a pattern may be supported by our finding that FMS participants reported more perceived stress than OAs.
Earlier, we alluded to a phenomenon referred to as psychological immunity: A natural psychobiological response to stress that boosts positive feeling states as a means of hastening recovery from stressful events. A relative lack of these affective resources appears to characterize FMS patients. It is interesting in this regard to note that one of the common pharmacological treatments for this condition involves opioid preparations. Though the explanation for the effectiveness of these medications has been the reduction in pain, we may speculate that pain itself may be prolonged by a failure of these underlying processes to mount an adequate countervailing positive affective response that will restore well-being following stress.
Another possible biological mechanism for restoration of well-being following stress involves the mammalian neuropeptide oxytocin (26). A study by Anderberg and Uvnas-Moberg (27) found that oxytocin levels were negatively correlated with perceptions of severe pain, depression, stress, and anxiety in FMS patients, and were positively correlated with perceptions of happiness in both the FMS and control sample.
Social conflict may also play a significant role in vulnerabilities of the positive affect system among these patients. Davis, Zautra, and Reich (5) found evidence of a narrowing of affective responses to social interactions for patients with FMS such that increases in perceived stress brought more withdrawal from positive exchanges among FMS patients than a comparison group (see 28 for a review). Problems in the management of unpredictable pain episodes that have no known cause or treatment are likely to provoke extensive challenges to interpersonal relationships as well. Indeed, multiple causes for this disorder are likely given the heterogeneity in the symptom presentation. From the current data, we cannot discriminate between biological and psychosocial mechanisms for FMS vulnerabilities to loss of PA following interpersonal stress. Future research should address this specific question using both psychological and physiological methods to resolve the issue.
How important is this deficit in PA to understanding a primary symptom of FMS: widespread pain? We controlled for pain level in this study when examining how FMS participants were different from OAs. In so doing, we ruled out the study of direct effects of positive emotional states on pain levels, and focused instead on additional features beyond the pain reports that distinguished FMS from OA. We suspect, however, that the stress-related loss of positive affect does have major implications for the relatively slow recovery from stressful events and the central sensitization that characterizes patients with FMS. More research is needed to bridge the gap in knowledge between two apparent facts about the condition: The generally low levels of PA found among FMS patients, especially during stress, and the abnormally high levels of widespread pain that they report.
Our analyses also found that FMS participants responded to stress with increased fatigue compared to the OA sample. Fatigue is a common feature of FMS, and future investigations should attempt to clarify the links between the regulation of positive affect and fatigue in FMS.
A limitation in this study may be a somewhat unrepresentative sample. In order to examine the effects of intimate relationships, as well as to ensure that all participants were similar in terms of pertinent social relationships, inclusion criteria required for a spouse or live-in partner. FMS participants with significant others may not be representative of FMS patients in the population. This selection factor may limit the external validity of the current findings.
Learning more about these mechanisms that may maintain FMS may be critical to our understanding of how to intervene to treat this poorly understood condition. If indeed, the lack of positive affect contributes to the maintenance or worsening of this chronic health condition, then treatments that assist FMS patients in broadening their emotional repertoire and increasing their capacity for positive emotion, especially during stressful times, may be particularly effective as a means of improving their condition.
Acknowledgements
This research was supported by an Arthritis Foundation Clinical Sciences Grant and a grant from NIAMS to the first author.
Acronyms used in text
- FMS
fibromyalgia syndrome
- OA
osteoarthritis
- PA
positive affect
- NA
negative affect
- IS
perceived interpersonal stress
- PE
positive interpersonal events
References
- 1.Affleck G, Tennen H, Urrows S, Higgins P. Neuroticism and the pain-mood relation in rheumatoid arthritis: Insights from a prospective daily study. J Consult Clin Psychol. 1992;60:119–26. doi: 10.1037//0022-006x.60.1.119. [DOI] [PubMed] [Google Scholar]
- 2.Zautra A, Smith B, Affleck G, Tennen H. Examinations of chronic pain and affect relationships: applications of a dynamic model of affect. J Consult Clin Psychol. 2001;69:786–95. doi: 10.1037//0022-006x.69.5.786. [DOI] [PubMed] [Google Scholar]
- 3.Aaron LA, Bradley LA, Alarcon GS, Alexander RW, Triana-Alexander M, Martin MY, Alberts KR. Psychiatric diagnoses in patients with fibromyalgia are related to health-care seeking behavior rather than to illness. Arthritis Rheum. 1996;39:436–45. doi: 10.1002/art.1780390311. [DOI] [PubMed] [Google Scholar]
- 4.Hudson JI, Pope HG., Jr. Fibromyalgia and psychopathology: Is fibromyalgia a form of “affective spectrum disorder? J Rheumatol Suppl 19. 1989;16:15–22. [PubMed] [Google Scholar]
- 5.Davis MC, Zautra AJ, Reich JW. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis. Ann Behav Med. 2001;23:215–26. doi: 10.1207/S15324796ABM2303_9. [DOI] [PubMed] [Google Scholar]
- 6.Zautra AJ, Hamilton N, Burke HM. Comparison of stress responses in women with two types of chronic pain: Fibromyalgia and Osteoarthritis. Cognit Ther Res. 1999;23:209–30. [Google Scholar]
- 7.Kraus VB. Pathogenesis and treatment of osteoarthritis. Med Clin North Am. 1997;81:85–112. doi: 10.1016/s0025-7125(05)70506-x. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 9.Netter P, Hennig J. The fibromyalgia syndrome as a manifestation of neuroticism? Z Rheumatol Suppl 2. 1998;57:105–8. doi: 10.1007/s003930050248. [DOI] [PubMed] [Google Scholar]
- 10.Charles ST, Gatz M, Pedersen NL, Dahlberg L. Genetic and behavioral risk factors for self-reported joint pain among a population-based sample of Swedish twins. Health Psychol. 1999;18:644–54. doi: 10.1037//0278-6133.18.6.644. [DOI] [PubMed] [Google Scholar]
- 11.Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull. 1996;119:95–110. [Google Scholar]
- 12.Monroe SM. The social environment and psychopathology. J Soc Pers Relat. 1988;5:347–66. [Google Scholar]
- 13.Gilbert DT, Pinel EC, Wilson TD, Blumberg SJ, Wheatley TP. Immune neglect: A source of durability bias in affective forecasting. J Pers Soc Psychol. 1998;75:617–38. doi: 10.1037//0022-3514.75.3.617. [DOI] [PubMed] [Google Scholar]
- 14.Zautra AJ. Emotions, stress, and health. 1st ed. Oxford University Press; New York: 2003. [Google Scholar]
- 15.John OP, Donahue EM, Kentle RL. The “Big Five” Inventory - Versions 4a and 5a. University of California, Berkeley, Institute of Personality and Social Research; Berkeley: 1991. [Google Scholar]
- 16.Veit CT, Ware JE. The structure of psychological distress and well-being in general populations. J Consult Clin Psychol. 1983;51:730–42. doi: 10.1037//0022-006x.51.5.730. [DOI] [PubMed] [Google Scholar]
- 17.Zautra AJ, Reich JW, Guarnaccia CA. Some everyday life consequences of disability and bereavement for older adults. J Pers Soc Psychol. 1990;59:550–61. doi: 10.1037//0022-3514.59.3.550. [DOI] [PubMed] [Google Scholar]
- 18.Watson D, Clark LA. The PANAS-X manual for the positive and negative affect schedule—Expanded form. 1999 Retrieved September 24, 2003 http://www.psychology.uiowa.edu/Faculty/Watson/PANAS-X.pdf. from the University of Iowa Web Site:
- 19.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 20.Zautra AJ, Smith BW. Depression and reactivity to stress in older women with rheumatoid arthritis and osteoarthritis. Psychosom Med. 2001;63:687–96. doi: 10.1097/00006842-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Zautra AJ, Guarnaccia CA, Dohrenwend BP. Measuring small life events. Am J Community Psychol. 1986;14:629–55. doi: 10.1007/BF00931340. [DOI] [PubMed] [Google Scholar]
- 22.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute; Cary, N.C.: 1996. [Google Scholar]
- 23.Affleck G, Tennen H. Daily processes in coping with chronic pain: method and analytic strategies. In: Zeidner M, Endler N, editors. Handbook of Coping. Wiley; New York: 1996. pp. 151–181. [Google Scholar]
- 24.Singer JD. Using SAS PROC MIXED to fit multilevel models hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24:323–55. [Google Scholar]
- 25.Zautra AJ, Berkhof J, Nicolson NA. Changes in affect interrelations as a function of stressful events. Cognition and Emotion. 2002;16:309–18. [Google Scholar]
- 26.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 27.Anderberg UM, Uvnas-Moberg K. Plasma oxytocin levels in female fibromyalgia syndrome patients. Z Rheumatol. 2000;59:373–79. doi: 10.1007/s003930070045. [DOI] [PubMed] [Google Scholar]
- 28.Davis MC, Zautra AJ, Smith B. Chronic pain, stress, and the dynamics of affective differentiation. J Pers. doi: 10.1111/j.1467-6494.2004.00293.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]